Abstract
Background and Aims:
Intravenous analgesics and local infiltration are used for postoperative analgesia in patients undergoing mastoidectomy. No randomised controlled trial (RCT) has so far determined response rates of pain reduction after ultrasound-guided superficial cervical plexus block (SCPB) in adult patients undergoing modified radical mastoidectomy.
Methods:
This double-blind RCT was conducted in 30 adult patients of the American Society of Anesthesiologists (ASA) grade I/II undergoing modified radical mastoidectomy. The primary outcome was a reduction in the visual analogue scale (VAS) score. Secondary outcomes were postoperative diclofenac consumption, haemodynamics, and occurrence of any adverse events. All patients received general anaesthesia. At the end of the surgery, patients were randomised to either Group ‘Block’ (n = 15) ultrasound guided SCPB with 5 mL ropivacaine 0.5% or Group ‘No block’ (n = 15). All patients received intravenous (IV) paracetamol 1 g every 6 hourly and rescue analgesic IV diclofenac 75 mg if VAS score >4.
Results:
Patients in Group ‘Block’ reported lower VAS score at rest versus Group ‘No block’ at 1 h postoperatively (P = 0.012). VAS score on movement was lower in patients of Group ‘Block’ compared to Group ‘No block’ at 1 h (P = 0.010), 4 h (P = 0.035), 8 h (P = 0.027), and 12 h (*P = 0.003) postoperatively. Diclofenac consumption was lower in patients of Group ‘Block’ (P = 0.041). No adverse effects were reported.
Conclusion:
Postoperative ultrasound-guided SCPB produced higher response rates in terms of reduction in VAS score in patients undergoing modified radical mastoidectomy.
Keywords: Analgesia, diclofenac, mastoidectomy, pain, postoperative, visual analogue scale
INTRODUCTION
Mastoidectomy is one of the most common surgical procedures done in the middle ear for chronic suppurative otitis media. The most commonly reported postoperative problems are pain, vertigo, nausea and vomiting.[1] Post-operative pain still remains a major problem despite developments in pain management. Multimodal opioid-free postoperative analgesia reduces side effects and hospital stay. Intravenous analgesics and local infiltration are generally used for postoperative analgesia in patients undergoing mastoidectomy. Regional nerve blocks provide a longer duration of analgesia, prevent sensitisation of the central and peripheral nervous system and development of chronic pain.[2] Peripheral nerve blocks have opioid- sparing effects, reduced side-effects and better patient satisfaction.[3,4] In a case series of three patients, superficial cervical plexus block (SCPB) was performed using landmark technique. The indication was different in each patient. First patient had intractable ear pain due to acute otitis externa, second patient had thyroid cartilage pain and third patient had ear pain due to acute purulent mastoid infection alongside cholesteatoma and excessive granulations. SCPB was effective in avoiding excessive opioid use and reduced discomfort during aural toilet.[5] The reported mechanism of SCPB is that it blocks the cutaneous supply to the ear and neck, motor supply to neck muscles and is known to have connections with the cranial nerve and sympathetic trunk via ansa cervicalis.[6] With encouraging results of ultrasound-guided SCPB, we planned the present randomised controlled trial (RCT) in patients undergoing modified radical mastoidectomy.[7,8]
We hypothesised that SCPB has no role in postoperative pain relief in patients undergoing modified radical mastoidectomy. The aim of this study was to determine the response rates of pain reduction after SCPB in adult patients undergoing modified radical mastoidectomy. The primary outcome of the study was a reduction in visual analogue scale (VAS) score in patients undergoing mastoidectomy. The secondary outcomes of the study were requirement of total dose of rescue analgesia, time to first rescue analgesia, haemodynamics, postoperative nausea vomiting (PONV), vertigo, patient satisfaction score and any other side effects during postoperative 24 h.
METHODS
This study was a prospective, randomised, double-blinded, and placebo-controlled trial. We obtained approval from the institutional ethics committee, and registration with the Clinical Trials Registry-India. Patients were enroled between April 2019 and November 2019. Written informed consent was obtained from all patients. Adult patients of the American Society of Anesthesiologists (ASA) physical status I/II scheduled for modified radical mastoidectomy were included in the study. Exclusion criteria were history of alcohol abuse, PONV, motion sickness, migraine, history of seizures, malignancy, allergy to drugs used in the study, drugs for chronic pain management, pregnancy and lactating mothers.
There were no previous RCTs on this topic, so the sample size was calculated based on 10 pilot cases performed to calculate response rates in terms of reduction as reflected by VAS score. It was anticipated that about 80% of patients will get pain relief using SCPB block against only 20% when using no block. At 5% level of significance and 90% power of test, 95% confidence coefficient, 12 patients were required to be recruited in each group. To compensate for dropouts, we decided to include 15 patients per group. So, the total sample size of our study was 30 patients.
All patients received standardised general anaesthesia (GA) using intravenous (IV) propofol (2–2.5 mg/kg), morphine (0.1 mg/kg), vecuronium(0.1 mg/kg), propofol infusion (50–200 μg/kg/min) and oxygen in air (FiO2 0.50). At the end of the surgery during skin suturing, all patients received IV ondansetron 4 mg and paracetamol 1 g. The sequentially numbered opaque envelopes with group randomisation details were opened by an anaesthesiologist who did not participate in subsequent conduct of the study. Randomisation of the patients was done using a computer-generated random numbers table. The patients were divided into two groups:
Group ‘Block’ (n = 15): Patients received ultrasound-guided SCPB with 5 mL of 0.5% ropivacaine.[5,9,10]
Group ‘No block’ (n = 15): Patients received skin preparation for SCPB, but no block was given.
The patients were blinded to group allocation as the block was performed under GA. SCPB was performed by an anaesthesiologist performing ultrasound-guided blocks for more than 10 years. The SCPB was performed using all sterile aseptic precautions. The patient was kept in the supine position with the head turned slightly away from the side. At the midpoint of the sternocleidomastoid, a high frequency 5–10 Hz linear ultrasound probe (Esaote, Philips weg 1 6227 AJ Maastricht, Netherlands, Europe) was placed and then moved posteriorly until the tapering edge of the muscle was visualised. A 22 G ultrasound needle was inserted behind the mid-belly of sternocleidomastoid muscle. The end point of needle tip placement was spread of normal saline 1 mL between the posterior border of the sternocleidomastoid (SCM) with investing layer of deep cervical fascia and the prevertebral fascia. After negative aspiration, 5 mL of 0.5% ropivacaine was injected. Then, neuromuscular blockade was reversed, 100% oxygen was administered, trachea was extubated, and the patient was shifted to post anaesthesia care unit (PACU). In PACU, the sensory assessment for analgesia was not done as it could have resulted in assessor bias. Data observations were recorded by an anaesthesia resident in the study proforma.
All the patients received a standardised postoperative regimen. IV paracetamol 1 g was given every 6 h in the ward and rescue analgesia was provided with IV diclofenac 75 mg if the patient had a VAS score >4. Maximum diclofenac allowed was 150 mg in 24 h.
All patients were monitored for haemodynamics, VAS at rest, VAS on movement, total rescue (diclofenac) analgesic consumption, time to first rescue analgesia, nausea or vomiting, vertigo and any adverse events in the immediate postoperative period (at 5, 10, 15 min, 1 h) and then at 4, 6, 12,18 and 24 h postoperatively. Satisfaction score of the patients was noted at 24 h postoperatively.
In the VAS scale for pain assessment, 0 stands for no pain and 10 stands for worst imaginable pain. In nausea and vomiting scale, 0 stands for none and 3 stands for severe vomiting. In the vertigo scale, 0 stands for no vertigo and 10 stands for severe vertigo.[11]
All results were expressed as, n (%) mean, standard deviation (SD) and 95% confidence interval (CI). The normality of quantitative data was checked by measures of Kolmogorov–Smirnov tests of normality. Normally distributed t test was applied for statistical analysis of the two groups. For skewed data or ordered categorical data, non-parametric Mann–Whitney U test was used for statistical analysis of the two groups. For categorical data, comparisons were made by Pearson Chi-square or Fisher's exact test as appropriate. The Student's t test with Bonferroni correction was used for continuous data like haemodynamics. Mann–Whitney U test was used for skewed data (oxygen saturation, VAS at rest, VAS on movement, rescue analgesia, PONV and adverse effects) and Chi-square test was used for vertigo. A value of P < 0.05 was considered significant. All the statistical tests were two-sided and were performed at a significance level of α =0.05. The statistical analysis was conducted using International Business Machines (IBM) Statistical Package for the Social Sciences (SPSS) software program, version 22.0.
RESULTS
This study included 33 patients and of these, three patients were excluded as summarised in the CONSORT chart [Figure 1]. Patient demographics were similar in Group ‘Block’ versus Group ‘No block’ [Table 1].
Figure 1.
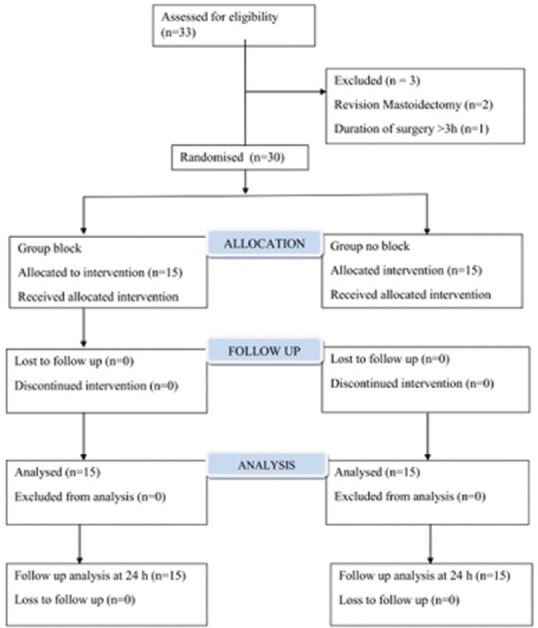
CONSORT diagram showing patient enrolment and randomisation
Table 1.
Demographics of patients receiving superficial cervical plexus block versus ‘No block’ in patients undergoing modified radical mastoidectomy
| Patient Characteristics | Group ‘Block’ (n=15) | Group ‘No block’ (n=15) | P |
|---|---|---|---|
| Age (years) | 37.73±13.44 | 29.93±7.39 | 0.059 |
| Males | 6 (40%) | 3 (20%) | 0.427 |
| Females | 9 (60%) | 12 (80%) | |
| ASA I | 11 (73.3%) | 15 (100.0%) | 0.100 |
| ASA II | 4 (26.7%) | 0 (0.0%) |
Values are represented as mean±standard deviation (SD) or n (%), ASA: American Society of Anesthesiologists, P<0.05 is considered statistically significant
Postoperative VAS score mean ± standard deviation (SD) [range] at rest at 1 h in Group ‘Block’ was 0.20 ± 0.56 [0–2], as compared to 1.87 ± 2.07 [0–5] in Group ‘No block’ (P = 0.012*) [Table 2 and Figure 2]. The postoperative VAS score mean ± SD [range] at movement was lower in Group ‘Block’ at 1 h (P = 0.010), 4 h (P = 0.035), 8 h (P = 0.027), and 12 h (*P = 0.003) as compared to Group ‘No block’ [Table 3 and Figure 3].
Table 2.
Postoperative comparison of VAS at rest in patients receiving superficial cervical plexus block versus ‘No block’ in patients undergoing modified radical mastoidectomy
| Time interval | Group ‘Block’ (n=15) | Group ‘No block’ (n=15) | P |
|---|---|---|---|
| 5 min | 0.20±0.77 [0-3] | 0.07±0.26 [0-1] | 0.962 |
| 10 min | 0.20±0.77 [0-3] | 0.20±0.41 [0-1] | 0.343 |
| 15 min | 0.47±1.36 [0-5] | 0.80±1.78 [0-6] | 0.406 |
| 1 h | 0.20±0.56 [0-2] | 1.87±2.07 [0-5] | 0.012* |
| 4 h | 0.20±0.41 [0-1] | 0.80±1.15 [0-4] | 0.085 |
| 8 h | 0.07±0.26 [0-1] | 0.73±1.44 [0-4] | 0.125 |
| 12 h | 0.00±0.00 [0-0] | 0.20±0.56 [0-2] | 0.150 |
| 18 h | 0.33±1.29 [0-5] | 0.07±0.26 [0-1] | 0.962 |
| 24 h | 0.07±0.26 [0-1] | 0.27±1.03 [0-4] | 0.962 |
Values are represented as mean±standard deviation (SD) [range]. VAS: Visual analogue scale, *P<0.05 is considered statistically significant
Figure 2.
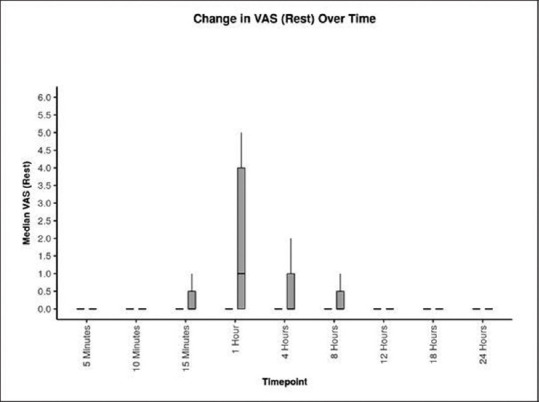
Postoperative comparison of visual analogue scale (VAS) at rest in Group ‘Block’(black colour) versus Group ‘No block’(grey colour) in patients undergoing modified radical mastoidectomy. Values are represented as box and whisker plot. The horizontal line is median, main box as inter-quartile range and the longitudinal lines are range
Table 3.
Postoperative comparison of VAS on movement in patients receiving superficial cervical plexus block versus ‘No block’ in patients undergoing modified radical mastoidectomy
| Time interval | Group ‘Block’ (n=15) | Group ‘No block’ (n=15) | P |
|---|---|---|---|
| 5 min | 0.53±1.12 [0-4] | 0.40±0.63 [0-2] | 0.837 |
| 10 min | 0.60±1.06 [0-4] | 0.33±0.72 [0-2] | 0.328 |
| 15 min | 0.87±1.81 [0-7] | 1.20±2.11 [0-7] | 0.902 |
| 1 h | 0.60±0.91 [0-3] | 2.73±2.43 [0-7] | 0.010* |
| 4 h | 0.53±0.83 [0-2] | 1.53±1.46 [0-5] | 0.035 |
| 8 h | 0.27±0.46 [0-1] | 1.33±1.68 [0-5] | 0.027* |
| 12 h | 0.00±0.00 [0-0] | 0.67±0.90 [0-3] | 0.003* |
| 18 h | 0.40±1.55 [0-6] | 0.27±0.59 [0-2] | 0.343 |
| 24 h | 0.13±0.52 [0-2] | 0.47±1.30 [0-5] | 0.309 |
Values are represented as mean±standard deviation (SD) [range]. VAS: Visual analogue scale, *P<0.05 is considered statistically significant
Figure 3.
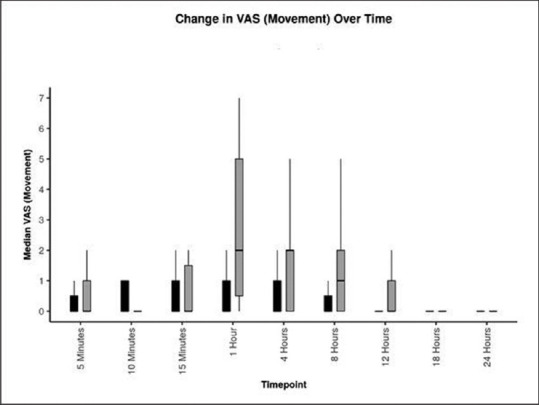
Postoperative comparison of visual analogue scale (VAS) on movement in Group ‘Block’(black colour) versus Group ‘No block’ (grey colour) in patients undergoing modified radical mastoidectomy surgery. Values are represented as box and whisker plot. The horizontal line is median, main box as inter-quartile range and the longitudinal lines are range
A reduction in postoperative diclofenac consumption at 1 h was recorded in the Group ‘Block'0.00 ± 0.00 [0–0] as compared to 0.33 ± 0.49 [0–1] mg in Group ‘No block', (P = 0.041). Patients in Group ‘No block’ consumed more diclofenac as compared to Group ‘Block', at the end of 24 h. Of these, eight patients in Group ‘No block’ and two patients in Group Block required rescue analgesia. The first rescue analgesia was required in the first 15 min in four patients in the Group ‘No block'. Haemodynamics between the two groups were statistically not significant and remained within physiological variables at all times. Only five patients had PONV in Group ‘No block’ and none in Group ‘Block'. PONV was observed in PACU at 10 min in two patients, 15 min in two patients and in one patient at 4 and 18 h in the ward. Vertigo was reported in two patients in Group ‘No block’ and in one patient in Group ‘Block'. In Group ‘No block', vertigo was observed in PACU in one patient at 10 min and 15 min and in another patient at 1 h, 4 h, 8 h and 18 h in the ward. In Group ‘Block', vertigo was observed in one patient at 18 h in the ward. No other adverse effects were reported during the entire study period.
DISCUSSION
In this study, use of ultrasound-guided SCPB with 5 mL of 0.5% ropivacaine was effective in blocking the superficial branches of cervical plexus and also effectively reduced analgesic requirement during the first 24 postoperative hours in patients undergoing modified radical mastoidectomy. Lower VAS scores at rest were reported in patients of Group ‘Block’ as compared to Group ‘No block’ at 1 h postoperatively. VAS scores on movement were lower in Group ‘Block’ at 1, 4, 8, and 12 h postoperatively as compared to Group ‘No block'. The patients in Group ‘No block’ consumed more diclofenac as compared to Group ‘Block', at the end of 24 h.
In this study, the technique used for performing ultrasound-guided SCPB anatomically corresponds to C4–C5 level and the end point was depositing local anaesthetic (LA) between the posterior surface of SCM muscle and prevertebral fascia.[5,10,11] Superficial and intermediate cervical plexus block showed a similar analgesic effect in patients undergoing carotid endarterectomy, rather intermediate was mentioned as part of superficial (intermediate) cervical plexus block.[12] The permeable nature of this prevertebral fascia can eventually affect the spread of LA to deeper tissues and can produce similar analgesic effects as deep cervical plexus block.[13,14,15] The superficial branches of cervical plexus arise from deep tissues, pass through this space after piercing the vertebral level and exit to the skin and superficial tissues via the posterior border of the SCM muscle.[16]
Volumes larger than 5 mL in SCPB can cause the unnecessary incidence of neurological problems and Horner's syndrome.[9] Use of landmark technique with larger volumes of LA (10-15 ml) has resulted in complications like phrenic nerve block, hoarseness of voice, Horner's syndrome, vertebral artery injury and total spinal block. Left upper limb paresis in one patient corresponding to C5–T1 dermatomes with neurological recovery after 12 h has been reported with theuse of 0.5% 6 mL ropivacaine for postoperative pain relief up to 24 h in patients undergoing thyroid surgery. The authors postulated that it was due to inadvertent leakage of LA into deep cervical space which blocked the roots.[10] The complications of SCPB under ultrasound guidance are rare as lower volumes of LA are required and placement of drug is under real time. The rarely reported adverse effects are voice hoarseness and partial hemi-diaphragmatic paresis which require no active intervention and are self-limiting.[5,6,9]
The cervical plexus (C2–C4) supplies sensory innervations to the SCM muscle, including proprioception, with variable anastomosis with the spinal accessory nerve at the posterior surface or inside of the SCM muscle. The cervical plexus (ansa cervicalis) is believed to constitute another source of motor innervation for the SCM muscle in addition to the spinal accessory nerve. Therefore, SCPB performed accurately at C 4 level (mid-belly of SCM), can block all four cutaneous branches of cervical plexus and sensory/motor branches of the cervical plexus supplying the SCM muscle simultaneously so that it provides adequate anaesthesia and analgesia.[6] The area of cranium, posterior auricular region of the scalp is supplied by C2, greater occipital nerve.[16] By performing SCPB, we provided a novel postoperative analgesic effect in patients undergoing mastoidectomy without causing any motor blockade which is a feature of deep cervical plexus block and any other related adverse effects.
The other modalities for pain relief in patients undergoing mastoidectomy include local infiltration and greater auricular nerve (GAN) block. Bhandari G et al.[17] administered 12 mL infiltration both after the incision and just before completion of modified radical mastoidectomy with a combination of bupivacaine and fentanyl 50 or 100 μg. The authors observed that patients receiving fentanyl 100 μg had lower VAS score as compared to patients requiring fentanyl 50 μg at 4 and 6 h postoperatively. Raghuwanshi et al.[18] reported improved pain scores in the first 6 h post-block in patients receiving 12 mL of 2% lignocaine with adrenaline and 30 μg of clonidine during infiltrative block given by the surgeon in chronic suppurative otitis media. In another study, higher tramadol consumption was reported in patients receiving GAN block with 5 mL of 0.25% bupivacaine versus ultrasound-guided SCPB with 10 mL of 0.25% bupivacaine.[19] However, the authors concluded that SCPB and GAN block provided similar pain control after tympanomastoid surgery. But in this study, SCPB with 5 mL of LA using real-time ultrasound guidance with a pre-defined end-point provided post-operative pain relief following modified radical mastoidectomy.
Postoperative vertigo occurs in patients undergoing middle ear surgery due to oedema following mastoidectomy surgery, residual cholesteatoma, use of opioids in post-operative period, previous history of motion sickness, migraine and dizziness. Only one patient experienced vertigo in Group ‘Block', that too in the late postoperative period as compared to two patients in Group ‘No block'. We standardised the enrolment of patients by excluding patients with history of motion sickness and migraine which can predispose patients to vertigo in the postoperative period. In this study, none of the patients had any baseline vertigo and all surgeries were performed by the same surgeon. Intraoperatively, the short-acting opioid fentanyl was used and opioid-free analgesia was used postoperatively. It is known that adequate pain relief and PONV are important for discharge and that they prevent readmissions in day-care ear surgery.[20] With this background evidence, we wanted to observe if adequate pain relief with multimodal analgesics reduced vertigo in patients undergoing middle ear surgery. As this was a secondary outcome in the current study, studies evaluating this aspect as primary outcome may be planned in the future.
Use of preoperative or postoperative peripheral nerve blocks has been evaluated. Infraclavicular brachial plexus blocks performed prior to incision have shown postoperative analgesia of longer duration as compared to block placed after surgery.[21] However, postoperative placement of interscalene block was also effective in reducing pain and opioid reduction in shoulder arthroscopic surgery.[22] As this is the first RCT of SCPB in patients undergoing mastoidectomy surgery under GA, a study comparing SCPB before incision versus at the end of surgery can be performed in future.
The limitations of this study are that this was a small sample size study. Secondly, we chose not to check sensory distribution, as this could have led to assessment bias by the assessor and patient blinding would not have been possible. Thirdly, patients were of ASA physical status I and II. In the future, RCTs with larger sample size and LA with an adjuvant in SCPB may be conducted.
CONCLUSION
In conclusion, postoperative ultrasound guided SCPB produced higher response rates in terms of reduction in VAS score in patients undergoing modified radical mastoidectomy under GA.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Tuli BS, Tuli I, Singh A, Kaur Tuli N. 1st ed. New Delhi: Jaypee Brothers Medical Publishers (P) Ltd; 2005. Chronic suppurative otitis media. Textbook of Ear, Nose and Throat; pp. 58–66. [Google Scholar]
- 2.Bajwa SJ. Managing acute post-operative pain: Advances, challenges and constraints. Indian J Anaesth. 2017;61:189–91. doi: 10.4103/ija.IJA_110_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Syal K, Chandel A, Goyal A, Sharma A. Comparison of ultrasound-guided intermediate vs subcutaneous cervical plexus block for postoperative analgesia in patients undergoing total thyroidectomy: A randomised double-blind trial. Indian J Anaesth. 2020;64:37–42. doi: 10.4103/ija.IJA_483_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wahal C, Kumar A, Pyati S. Advances in regional anaesthesia: A review of current practice, newer techniques and outcomes. Indian J Anaesth. 2018;62:94–102. doi: 10.4103/ija.IJA_433_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tahery J, Duodu J, Narayan S. Cervical plexus block in the management of acute otitis externa and severe laryngeal pain post trauma. Clin Otolaryngol. 2011;36:190–1. doi: 10.1111/j.1749-4486.2011.02283.x. [DOI] [PubMed] [Google Scholar]
- 6.Kim JS, Ko JS, Bang S, Kim H, Lee SY. Cervical plexus block. Korean J Anesthesiol. 2018;71:274–88. doi: 10.4097/kja.d.18.00143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ritchie MK, Wilson CA, Grose BW, Ranganathan P, Howell SM, Ellison MB. Ultrasound guided greater auricular nerve block as a sole anesthetic for ear surgery. Clin Pract. 2016;6:856. doi: 10.4081/cp.2016.856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ho B, De Paoli M. Use of ultrasound-guided superficial cervical plexus block for pain management in the emergency department. J Emerg Med. 2018;55:87–95. doi: 10.1016/j.jemermed.2018.04.030. [DOI] [PubMed] [Google Scholar]
- 9.Flores S, Riguzzi C, Herring AA, Nagdev A. Horner's syndrome after superficial cervical plexus block. West J Emerg Med. 2015;16:428–31. doi: 10.5811/westjem.2015.2.25336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoh SY, Doon YK, Chong SS, Ng KL. Randomized controlled trial comparing bilateral superficial cervical plexus block and local wound infiltration for pain control in thyroid surgery. Asian J Surg. 2019;42:1001–8. doi: 10.1016/j.asjsur.2019.01.017. [DOI] [PubMed] [Google Scholar]
- 11.Santosh BS, Mehandale SG. Does dexmedetomidine improve analgesia of superficial cervical plexus block for thyroid surgery? Indian J Anaesth. 2016;60:34–8. doi: 10.4103/0019-5049.174797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramchandran SK, Pixton P, Shanks A, Dorie J, Pandit JJ. Comparison of intermediate vs subcutaneous cervical plexus block for carotid endarterectomy. Br J Anaesth. 2011;107:157–63. doi: 10.1093/bja/aer118. [DOI] [PubMed] [Google Scholar]
- 13.Pandit JJ, Dutta D, Morris JF. Spread of injectate with superficial cervical plexus in humans: An anatomical study. Br J Anaesth. 2003;91:733–5. doi: 10.1093/bja/aeg250. [DOI] [PubMed] [Google Scholar]
- 14.Pandit JJ, Satya-Krishna R, Gration P. Superficial or deep cervical plexus block for carotid endarterectomy: A systematic review of complications. Br J Anaesth. 2007;99:159–69. doi: 10.1093/bja/aem160. [DOI] [PubMed] [Google Scholar]
- 15.Telford RJ, Stoneham MD. Correct nomenclature of superficial cervical plexus blocks. Br J Anaesth. 2004;92:775–6. doi: 10.1093/bja/aeh550. [DOI] [PubMed] [Google Scholar]
- 16.Choquet O, Dadure C, Capdevila X. Ultrasound-guided deep or intermediate cervical plexus block: The target should be the posterior cervical space. Anesth Analg. 2010;111:1563–4. doi: 10.1213/ANE.0b013e3181f1d48f. [DOI] [PubMed] [Google Scholar]
- 17.Bhandari G, Shahi KS, Parmar NK, Asad M, Joshi HK, Bhakuni R. Evaluation of analgesic effect of two different doses of fentanyl in combination with bupivacaine for surgical site infiltration in cases of modified radical mastoidectomy: A double blind randomized study. Anesth Essays Res. 2013;7:243–7. doi: 10.4103/0259-1162.118979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raghuwanshi SK, Chakravarty N, Asati DP, Bankwar V. Use of clonidine as an adjuvant to infiltration anaesthesia in tympanoplasty: A randomized double blind study. Indian J Otolaryngol Head Neck Surg. 2014;66:57–62. doi: 10.1007/s12070-013-0664-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okmen K, Metin Okmen B. Ultrasound guided superficial cervical plexus block versus greater auricular nerve block for postoperative tympanomastoid surgery pain: A prospective, randomized, single blind study. Agri. 2018;30:171–8. doi: 10.5505/agri.2018.60251. [DOI] [PubMed] [Google Scholar]
- 20.Khan MM, Parab SR. Day care ear surgery: Our experience of 4 years. Indian J Otolaryngol Head Neck Surg. 2012;64:280–4. doi: 10.1007/s12070-011-0303-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holmberg A, Sauter AR, Klaastad Ø, Draegni T, Raeder JC. Pre-operative brachial plexus block compared with an identical block performed at the end of surgery: A prospective, double-blind, randomised clinical trial. Anaesthesia. 2017;72:967–77. doi: 10.1111/anae.13939. [DOI] [PubMed] [Google Scholar]
- 22.Bosco L, Zhou C, Murdoch JAC, Bicknell R, Hopman WM, Phelan R, et al. Pre-or postoperative interscalene block and/or general anesthesia for arthroscopic shoulder surgery: A retrospective observational study. Can J Anaesth. 2017;64:1048–58. doi: 10.1007/s12630-017-0937-6. [DOI] [PubMed] [Google Scholar]


