Supplemental Digital Content is available in the text.
Key Words: metabolic dysfunction-associated fatty liver disease, nonalcoholic fatty liver disease, SARS-CoV-2, COVID-19, meta-analysis
Background:
The prevalence of metabolic dysfunction-associated fatty liver disease (MAFLD) in coronavirus disease-2019 (COVID-19) patients and whether it affects the outcomes of COVID-19 requires investigation.
Goals:
The aim was to determine the prevalence of MAFLD among COVID-19 patients and its influence on the outcomes of COVID-19 by meta-analysis.
Methods:
Our study protocol has been registered on PROSPERO (CRD42021242243). The studies published on PubMed, Embase, Cochrane Library, and Web of Science before March 11, 2021 were screened. The Newcastle-Ottawa scale (NOS) and Agency for Healthcare Research and Quality scale were used to assess the quality of the studies. Pooled analysis was conducted using the software RevMan version 5.3 and Stata version 15.0 SE. The stability of the results was assessed by sensitivity analysis. Publication bias was evaluated using funnel plots, Egger test, and trim-and-fill analysis.
Results:
Seven studies covering 2141 COVID-19 patients were included. It was confirmed that MAFLD increased the risk of severe COVID-19 (odds ratios: 1.80, 95% confidence interval: 1.53-2.13, P<0.00001). No association was found between the presence of MAFLD and the occurrence of COVID-19 death. The pooled prevalence of MAFLD among COVID-19 patients was 36% (95% confidence interval: 0.23-0.49, P<0.00001). Sensitivity analysis confirmed that the initial results were stable.
Conclusions:
MAFLD can increase the incidence of severe COVID-19, but the correlation between MAFLD and COVID-19 death has not been confirmed. Further investigation is needed to explore the possible mechanism of this association. Since MAFLD is common among patients infected with SARS-CoV-2, more care should be given to COVID-19 patients with underlying MAFLD.
Infection with SARS-CoV-2 was termed as coronavirus disease-2019 (COVID-19), which started in 2019, spread rapidly, and affected the whole world to become a major public health crisis, which led to it being characterized as a pandemic by the World Health Organization (WHO).1 The mortality rate for severe COVID-19 is up to 21% to 30%.2 Obesity has been shown to increase the incidence of severe illness by ∼3-fold.3 Hypertension, diabetes, and cardiovascular disease have also been demonstrated to be risk factors for severe disease.4
Nonalcoholic fatty liver disease, which was recently renamed as metabolic dysfunction-associated fatty liver disease (MAFLD), often combined with obesity and other metabolic diseases, has affected nearly 25% of the world’s population.5 Therefore, there is a high probability of overlap between MAFLD and COVID-19. Studies have demonstrated that 2% to 11% of COVID-19 patients have pre-existing liver disease, while liver injuries such as elevated transaminase occurred in 14% to 53% of patients without underlying liver disease.6
Some recent studies have confirmed that MAFLD could increase the incidence of severe COVID-19,7,8 while others have suggested that it does not affect COVID-19.9,10 To determine the prevalence of MAFLD among COVID-19 patients and its influence on the outcomes of the latter, we have herewith conducted a meta-analysis.
METHODS
Our protocol has been registered on PROSPERO (CRD42021242243).
Search Strategy
Studies published on PubMed, Embase, Cochrane Library, and Web of Science before March 11, 2021 were retrieved using the MeSH terms included “Non-alcoholic Fatty Liver Disease,” “Metabolic dysfunction-associated fatty liver disease,” “COVID-19,” “SARS-CoV-2” to identify potentially eligible studies. The specific search strategy is described in the supplementary information (Supplemental Digital Content 1, http://links.lww.com/JCG/A760). References of the included studies were manually screened to find additional suitable studies. Two reviewers separately screened the list of potential studies. If necessary, a third reviewer resolved disagreements regarding the eligibility of studies.
Selection Criteria
We included studies that explored the association between MAFLD and the occurrence of severe COVID-19 or death. COVID-19 was confirmed by real-time fluorescence RT-PCR, and the severity of COVID-19 was assessed according to the diagnostic criteria of the National Health Commission and National Administration of Traditional Chinese Medicine.11 In this meta-analysis, we have defined the severe cases and critical cases collectively as severe cases according to this guideline, which contains: (1) hypoxemia: low oxygen saturation (less than 93% at resting state) or arterial partial pressure of oxygen/fraction of inspired oxygen ≤300 mm Hg; (2) respiratory distress or respiratory failure requiring mechanical ventilation; (3) shock; (4) requirement of intensive care unit treatment for patients with failure of other organs.11 Patients meeting any of the above criteria were considered severe cases. Diagnosis of MAFLD conformed to the existing consensus diagnostic criteria.5 Only studies in the English language were included. Commentaries, case reports, reviews, and meta-analyses were excluded after screening the abstract and full-text. Because of the lack of relevant studies, high-quality letters, and conference proceedings were included.
Data Extraction
The studies were selected by 2 independent reviewers, and any disagreements would be resolved by a third reviewer. Information on first author name, year of publication, country, study design, age, gender distribution, number of patients with MAFLD, sample size, relevant odds ratios (ORs), and 95% confidence intervals (CIs) was extracted separately from each included study and collated in a standardized data table. Wherever the ORs were not marked, their value was calculated based on the existing data in the literature.
Quality Assessment
Two reviewers independently evaluated the quality of the included studies. Newcastle-Ottawa scale (NOS) was used for case-control studies and cohort studies, while the Agency for Healthcare Research and Quality scale was used for cross-sectional studies. Higher scores indicated the higher quality of the included studies.
Statistical Analysis
The data were analyzed by Review Manager version 5.3 (RevMan, the Cochrane Collaboration, Oxford, UK) and Stata version 15.0 SE (Stata Corp., College Station, TX). The pooled prevalence of MAFLD among COVID-19 patients, pooled ORs, and 95% CIs were calculated to evaluate the effect of MAFLD on COVID-19 outcomes. The final results were presented as forest plots. Two-sided P<0.05 indicated statistical significance.
Quantitative heterogeneity was evaluated by Q-based I 2, either I 2 >50% or P<0.05 indicated the existence of significant heterogeneity, after which, the random-effects model (DerSimonian-Laird method) was used,12 otherwise, the fixed-effects model would be used. Sensitivity analysis was conducted to investigate the stability of the original results, by removing each study in turn.
Publication bias was assessed through the visual examination of funnel plots for symmetry and Egger tests,13 with P<0.05 indicating significant publication bias, after which, Duval and Tweedie’s trim-and-fill analysis would be conducted to evaluate the influence of the bias on the results.14 If the conclusions were not significantly changed, no publication bias could be considered.
RESULTS
Characteristics of Included Studies
We identified 374 studies, of which 176 were duplicates. After carefully reading the titles and abstracts, 178 records were excluded. Of the remaining 20 full-text studies retrieved, 10 met the inclusion criteria. However, 5 of them analyzed the same COVID-19 patient group. Therefore, we only included the 1 with the largest sample size. Another study was identified through the reference review of eligible studies. Finally, a total of 7 studies was included in this meta-analysis. The process of retrieval and screening of the studies is presented in Figure 1.
FIGURE 1.
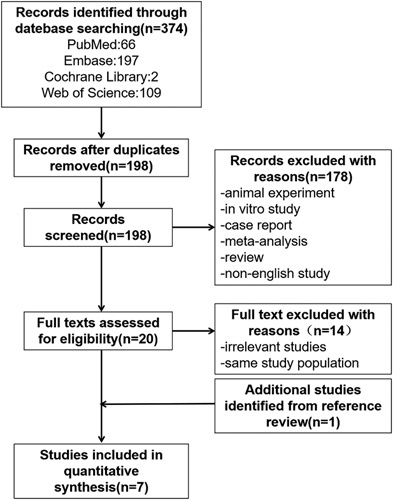
Flow diagram of the process of retrieval and selection of the studies.
The included studies were from 5 countries. Two studies were from China,15,16 2 from the United States,17,18 and 1 each from Qatar,10 the United Kingdom,9 and Israel.19 One study was a case-control study, 1 was a cohort study, and the remaining 5 were cross-sectional studies. A total of 2141 COVID-19 patients were included, of which, 840 also had underlying MAFLD. All the studies had a NOS score of 8. All the extracted data from the studies are presented in Table 1.
TABLE 1.
Main Characteristics of Included Studies
| References | Region | Study Design | Age | Male Sex | Sample Size | MAFLD% | NOS Score |
|---|---|---|---|---|---|---|---|
| Forlano et al9 | The United Kingdom | Cross-sectional study | — | — | 193 | 31.6 | 8 |
| Hashemi et al17 | The United States | Cohort study | 63.4±16.5 | 0.554 | 363 | 15.2 | 8 |
| Ji et al15 | China | Cross-sectional study | 44.5 | 0.559 | 202 | 37.6 | 8 |
| Mahamid et al19 | Israel | Case-control study | 51.0±21.7 | 0.282 | 71 | 31.0 | 8 |
| Mushtaq et al10 | Qatar | Cross-sectional study | 46.3 | 0.847 | 589 | 54.3 | 8 |
| Steiner et al18 | The United States | Cross-sectional study | 60.5 | 0.520 | 396 | 53.8 | 8 |
| Zhou et al16 | China | Cross-sectional study | — | — | 327 | 28.4 | 8 |
MAFLD indicates metabolic dysfunction-associated fatty liver disease; NOS, Newcastle-Ottawa scale.
Association Between MAFLD and Severe COVID-19
All the studies reported a relationship between MAFLD and COVID-19 severity. After verification using the software Revman version 5.3, the heterogeneity was I 2=26% (P=0.20). So, we selected the fixed-effects model for evaluation. The statistical relationship between the presence of MAFLD and severe COVID-19 was determined (pooled OR=1.80, 95% CI: 1.53-2.13, P<0.00001) (Fig. 2).
FIGURE 2.
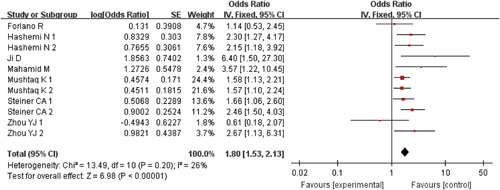
Forest plots for the association of metabolic dysfunction-associated fatty liver disease and severe coronavirus disease-2019. CI indicates confidence interval.
Association Between MAFLD and COVID-19 Death
Four studies explored the association between pre-existing MAFLD and COVID-19 death. The results revealed that the presence of MAFLD did not increase the risk of death in patients infected with SARS-CoV-2 (pooled OR=0.97, 95% CI: 0.69-1.36, P=0.85, I 2=0) (Fig. 3).
FIGURE 3.
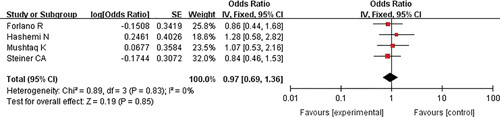
Forest plots for the association of metabolic dysfunction-associated fatty liver disease and coronavirus disease-2019 death. CI indicates confidence interval.
Prevalence of MALFD Among COVID-19 Patients
Pooled Prevalence
The prevalence of MAFLD among SARS-CoV-2 infected patients was reported in all the studies. The results collectively indicated that MAFLD was common among COVID-19 patients, with a pooled prevalence of 0.36 (95% CI: 0.23-0.49, P<0.00001, I 2=98%) (Fig. 4).
FIGURE 4.
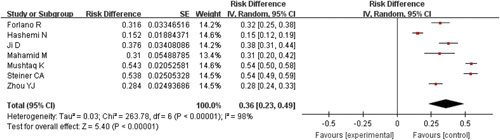
Forest plots for the prevalence of metabolic dysfunction-associated fatty liver disease among coronavirus disease-2019 patients. CI indicates confidence interval.
Sensitivity Analysis
By eliminating the studies one by one, we observed that the pooled prevalence and 95% CI were not changed significantly, which reflected the stability and reliability of the original results (Fig. 5).
FIGURE 5.
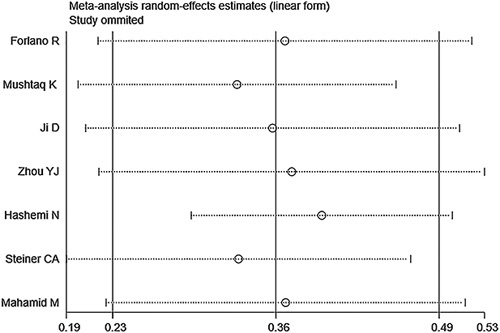
Sensitivity analysis for the prevalence of metabolic dysfunction-associated fatty liver disease among coronavirus disease-2019 patients.
Publication Bias
The funnel plots of both pooled prevalence (Fig. 6A) and pooled OR for severe COVID-19 (Fig. 6B) showed symmetry and further Egger test also showed nonsignificant (t=0.18, P=0.864; t=1.03, P=0.330, respectively). Because of the low number of studies included, the funnel plot was not applicable, we performed the Egger test to evaluate the publication bias of pooled OR for death (Fig. 7). The results confirmed the existence of publication bias (t=2.33, P=0.037), but the subsequent trim-and-fill analysis indicated that no trimming was performed and the data were unchanged (Fig. 8).
FIGURE 6.
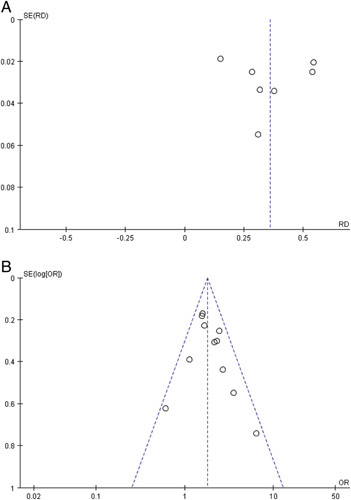
A, Funnel plot for the prevalence of metabolic dysfunction-associated fatty liver disease among coronavirus disease-2019 patients. B, Funnel plot for the association of metabolic dysfunction-associated fatty liver disease and severe coronavirus disease-2019. OR indicates odds ratio; RD, risk difference.
FIGURE 7.
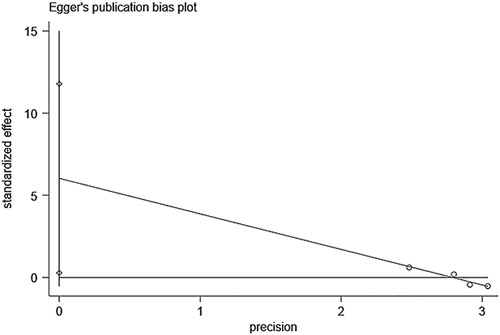
Egger test for the association of metabolic dysfunction-associated fatty liver disease and coronavirus disease-2019 death.
FIGURE 8.
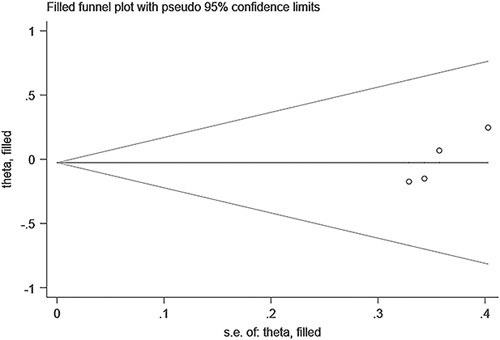
Trim-and-fill analysis for the association of metabolic dysfunction-associated fatty liver disease and coronavirus disease-2019 death.
DISCUSSION
This is the first meta-analysis that included studies from both Chinese and foreign countries to explore the association between MAFLD and the risk of severe COVID-19 and death. We observed that there was a 1.80-fold increase in the incidence of severe COVID-19 among patients with MAFLD, compared with those without MAFLD. However, no association was observed between the presence of MAFLD and the occurrence of COVID-19 death. We also observed that MAFLD had a remarkable prevalence among COVID-19 patients, with a pooled prevalence of 36%.
A meta-analysis has previously confirmed that MAFLD could increase the risk of disease progression of COVID-19. However, the extrapolated results were limited because of the small sample size and the fact that all the studies were of Chinese origin, with a majority of the included studies involving the same patient population.20 To make up for these limitations, we increased the number of studies, included foreign studies, and excluded those with the same patient population. We also investigated the relationship between MAFLD and COVID-19 death, which was not done before.
We found that MAFLD increases the risk of severe COVID-19, it is consistent with previous studies.21,22 However, the mechanism of this remains unclear. An excess of free fatty acids in MAFLD patients enter the liver and then initiate Kupffer cells. Because of the disordered hepatic innate immunity in MAFLD patients, the polarization status of Kupffer cells changes from anti-inflammatory M2 to proinflammatory M1, which then increases the production of tumor necrosis factor-α, interleukin-6, and other cytokines.23 Studies have demonstrated the involvement of interleukin-6 in cytokine ‘storm,’ and this ‘storm’ might be the main reason for the deterioration of COVID-19 disease.24 Thus, we hypothesize that this could be the mechanism by which MAFLD aggravates COVID-19.
Our study did not find the correlation between MAFLD and COVID-19 death. Since only 4 studies analyzing the correlation between MAFLD and COVID-19 death were included, and all of which were from non-Chinese countries, this conclusion may be drawn by the synthesis of several factors, such as ethnic and regional diversity, the discrepancy of liver function status when infected with SARS-CoV-2, and differences in severity of COVID-19. Therefore, it remains to be confirmed whether the nonsignificant relationship between MAFLD and COVID-19 deaths observed in our study is universal.
MAFLD is quite common among patients infected with SARS-CoV-2, but the results are considerably heterogeneous. Since the 7 studies that met the inclusion criteria were from 5 different countries and the number of Chinese studies was significantly lower than that of non-Chinese ones, we suggest that the regional differences might be the source of heterogeneity, although further verification is still needed.
We used meta-analysis techniques suggested by DerSimonian and Laird25 to calculated pooled estimates using RevMan version 5.3. The number of included studies was low, which made subgroup analysis difficult and affected the validity of the conclusions of the metaregression analysis. Therefore, we conducted a sensitivity analysis, which could assess the stability of the results and proved that the conclusions of our work were stable. Egger test, conducted to verify publication bias through Stata version 15.0 SE, revealed that only the publication bias of pooled OR for death was detected. We then used the trim-and-fill analysis and observed that the publication bias did not affect our results. Similar approaches have been used in some meta-analyses.26–28
Our study also has some limitations. First, the number of included studies, especially those from China, was not enough. Hence, the results of the analysis may not be representative. Nevertheless, the included studies were selected after a comprehensive search using multiple open databases. We will include new studies that meet the selection criteria to update our results in the future. Second, most of the included studies were cross-sectional, which is not as reliable as cohort studies. Considering that there are few relevant studies at present, more cohort studies are needed to confirm the results in the future. Third, the source of heterogeneity of the pooled prevalence was not identified, although the stability of the initial results was confirmed by sensitivity analysis.
CONCLUSION
We observed that MAFLD could increase the incidence of severe COVID-19, although the correlation between MAFLD and COVID-19 death has not been confirmed. Further investigation is needed to explore the possible mechanism of this association. Since MAFLD is quite common among patients infected with SARS-CoV-2, more care should be taken for COVID-19 patients with underlying MAFLD.
Supplementary Material
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.jcge.com.
Footnotes
The authors declare that they have nothing to disclose.
Contributor Information
Ziwen Tao, Email: 1802763495@qq.com.
Yueyue Li, Email: lyynqj@126.com.
Baoquan Cheng, Email: drcbq@163.com.
Tao Zhou, Email: zhoutao19720209@126.com.
Yanjing Gao, Email: gaoyanjing@sdu.edu.cn.
REFERENCES
- 1.Bedford J, Enria D, Giesecke J, et al. COVID-19: towards controlling of a pandemic. Lancet. 2020;395:1015–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tian W, Jiang W, Yao J, et al. Predictors of mortality in hospitalized COVID-19 patients: a systematic review and meta-analysis. J Med Virol. 2020;92:1875–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gao F, Zheng KI, Wang XB, et al. Obesity is a risk factor for greater COVID-19 severity. Diabetes Care. 2020;43:e72–e74. [DOI] [PubMed] [Google Scholar]
- 4.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eslam M, Newsome PN, Sarin SK, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–209. [DOI] [PubMed] [Google Scholar]
- 6.Zhang C, Shi L, Wang F-S. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5:428–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Targher G, Mantovani A, Byrne CD, et al. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut. 2020;69:1545–1547. [DOI] [PubMed] [Google Scholar]
- 8.Gao F, Zheng KI, Wang XB, et al. Metabolic associated fatty liver disease increases coronavirus disease 2019 disease severity in nondiabetic patients. J Gastroenterol Hepatol. 2021;36:204–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forlano R, Mullish BH, Mukherjee SK, et al. In-hospital mortality is associated with inflammatory response in NAFLD patients admitted for COVID-19. PLoS One. 2020;15:e0240400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mushtaq K, Khan MU, Iqbal F, et al. NAFLD is a predictor of liver injury in COVID-19 hospitalized patients but not of mortality, disease severity on the presentation or progression—the debate continues. J Hepatol. 2021;74:482–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.No authors listed. Diagnosis and treatment protocol for novel coronavirus pneumonia (Trial Version 7). Chin Med J (Engl). 2020;133:1087–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. [DOI] [PubMed] [Google Scholar]
- 15.Ji D, Qin E, Xu J, et al. Non-alcoholic fatty liver diseases in patients with COVID-19: a retrospective study. J Hepatol. 2020;73:451–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou YJ, Zheng KI, Wang XB, et al. Younger patients with MAFLD are at increased risk of severe COVID-19 illness: a multicenter preliminary analysis. J Hepatol. 2020;73:719–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hashemi N, Viveiros K, Redd WD, et al. Impact of chronic liver disease on outcomes of hospitalized patients with COVID-19: a multicentre United States experience. Liver Int. 2020;40:2515–2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steiner CA, Berinstein JA, Hsu CY, et al. Non-alcoholic fatty liver disease is associated with increased disease severity in patients with COVID-19. Am J Gastroenterol. 2020;115:S560–S561. [Google Scholar]
- 19.Mahamid M, Nseir W, Khoury T, et al. Nonalcoholic fatty liver disease is associated with COVID-19 severity independently of metabolic syndrome: a retrospective case-control study. Eur J Gastroenterol Hepatol. 2020. doi: 10.1097/MEG.0000000000001902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan L, Huang P, Xie X, et al. Metabolic associated fatty liver disease increases the severity of COVID-19: a meta-analysis. Dig Liver Dis. 2021;53:153–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng KI, Gao F, Wang XB, et al. Letter to the Editor: obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 2020;108:154244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou YJ, Zheng KI, Wang XB, et al. Metabolic-associated fatty liver disease is associated with severity of COVID-19. Liver Int. 2020;40:2160–2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma P, Kumar A. Metabolic dysfunction associated fatty liver disease increases risk of severe Covid-19. Diabetes Metab Syndr. 2020;14:825–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li G, Fan Y, Lai Y, et al. Coronavirus infections and immune responses. J Med Virol. 2020;92:424–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 26.Castillo JJ, Dalia S, Shum H. Meta-analysis of the association between cigarette smoking and incidence of Hodgkin’s lymphoma. J Clin Oncol. 2011;29:3900–3906. [DOI] [PubMed] [Google Scholar]
- 27.Moreno SG, Sutton AJ, Turner EH, et al. Novel methods to deal with publication biases: secondary analysis of antidepressant trials in the FDA trial registry database and related journal publications. BMJ. 2009;339:b2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gameiro S, Finnigan A. Long-term adjustment to unmet parenthood goals following ART: a systematic review and meta-analysis. Hum Reprod Update. 2017;23:322–337. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Digital Content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's website, www.jcge.com.


