Abstract
Purpose of Review:
In this narrative review, we discuss the indications for elective and therapeutic neck dissections and the postoperative surveillance and treatment options for recurrent nodal disease in patients with well-differentiated thyroid cancer.
Recent Findings:
Increased availability of advanced imaging modalities has led to an increased detection rate of previously occult nodal disease in thyroid cancer. Nodal metastasis are more common in young patients, large primary tumors, specific genotypes and certain histological types. While clinically evident nodal disease in the lateral neck compartments has a significant oncological impact, particularly in the older age group, microscopic metastases to the central or the lateral neck in well differentiated thyroid cancer do not significantly affect outcome.
Summary:
As patients with clinically evident nodal disease are associated with worse outcomes, they should be treated surgically in order to reduce rates of regional recurrence and improve survival. The benefit of elective neck dissection remains unverified as the impact of microscopic disease on outcomes is not significant.
Keywords: Lymphatic metastasis, neck dissection, thyroid neoplasms
Introduction
Well-differentiated thyroid cancer (WDTC), which includes papillary and follicular cancer, comprises the vast majority (>90%) of all thyroid cancers [1]. Thyroid cancer frequently metastasizes to the central and lateral nodal compartments of the neck. The rate of occult nodal metastases in elective central neck dissections performed for papillary thyroid carcinoma has been reported to be as high as 80% by some authors [2–3]. Patients with clinically positive nodes in the lateral neck, have an 83-86% rate of occult positive nodes in the ipsilateral central neck and 34% occult positivity rate in the contralateral central neck compartment [4–6].
Increased availability of advanced imaging modalities and widespread use of thyroglobulin assays have led to an increased detection rate of occult nodal disease in thyroid cancer. Nodal metastasis are more common in young patients, large primary tumors, specific genotypes (e.g. BRAF mutations) and certain histological types (Table 1). Regional nodal metastases are more common in papillary and medullary carcinoma whereas follicular carcinoma infrequently metastasize to regional lymphatics.
Table 1.
Factors associated with nodal disease in well differentiated thyroid carcinoma.
| Tumor/patient factors | Impact on nodal metastases |
|---|---|
| Patient age | More common in younger patients |
| Primary tumor size | More common in larger tumors |
| Histology | More common in papillary>medullary>follicular carcinoma |
| Genotype | More common in papillary thyroid cancer with positive BRAF mutations |
The clinical importance of nodal metastases on the overall survival of patients with WDTC is minimal. Important factors to take into consideration when determining this effect are the patient’s age, number and size of nodes and presence of extranodal extension. Cady et al. in the 1970s reported that clinical nodal metastases exert a paradoxically positive effect on prognosis [7]. These surprising findings were later explained by the high rate of nodal disease in younger patients whose survival was superior to older patients. In the 1990s, a matched-pair analysis from Memorial Sloan-Kettering Cancer Center showed that clinical nodal involvement in patients older than 45 years of age was associated with poor outcomes [8]. Adam et al. analyzed a large cohort of approximately 50,000 patients to demonstrate that patients younger than 45 years with nodal metastases have a significantly increased mortality risk compared to young patients without neck disease. They also found that having more metastatic nodes (up to six) resulted in an increased risk of death [9]. Ito et al. and Sugitani et al. from Japan have reported that lymph node metastases larger than 3cm is associated with negative outcomes [10,11].
Yamashita et al. reported a correlation between extranodal extension and high recurrence rates with poor overall survival in a large series of patients with PTC [12]. Similarly, in 2012, the American Thyroid Association (ATA)’s Surgical Affairs Committee’s Taskforce on Thyroid Cancer Nodal Surgery reported a 24% median risk of recurrence in patients with extranodal extension [13]. Urken et al. agreed that extranodal extension is a negative prognosticator but they emphasized that there is significant variation among pathologists reporting this finding [14].
In a multivariable analysis of 600 patients treated with a therapeutic central neck dissection, Wang et al. showed that the only statistically significant predictor of neck recurrence was the presence of extranodal extension [15]. In another multivariate analysis of 438 patients treated with a therapeutic lateral neck dissection, lateral lymph node burden greater than 17% (one positive node in six lateral neck nodes excised) was predictive for recurrence in all age groups. Extranodal extension was also prognostic for recurrence in patients aged forty-five years of age or older [16]. Similarly, in a study of 2,542 patients with PTC who underwent neck dissection, a lymph node density greater than 19% was independently associated with an adverse disease-specific survival and overall survival [17].
In an international cohort analysis of over 9,000 patients from multiple institutions, changing the cut off age for staging of differentiated thyroid cancer from 45 to 55 years in the AJCC/UICC staging system, was found to be a more reliable prognosticator regarding positive lymph nodes [18]. While macroscopic disease is now well recognized to impact prognosis, this is not the case for occult nodal disease. Microscopic nodal metastases have no influence on survival and at most, a limited impact on recurrence [13,19].
Recently the concept of clinically apparent nodal disease has been increasingly recognized as important in the optimal management of patients with PTC nodal metastasis. Clinically apparent nodes are those which are radiographically apparent preoperatively on US or CT or clearly identified as abnormal during surgical exploration. Surgical management of clinically evident metastatic nodal disease has been shown to be beneficial in WDTC in relation to local recurrence and survival [20]. Treatment however is contentious in the setting of clinically non-apparent, occult nodal disease when observation of the neck is a well-accepted management option [21].
The purpose of this review is to encompass the thoughts of several authors from different institutions around the world and form a unified philosophy on the management of nodal metastases in patients with WDTC. In this article we discuss the lymphatic drainage of the thyroid gland and the identification and significance of regional nodal metastases. We also review the indications for elective and therapeutic neck dissections and discuss the postoperative surveillance and treatment options for recurrent nodal disease in patients with WDTC. All evidence for this article was drawn from PUBMED articles cross-referenced with international guideline statements.
Lymphatic Drainage of the Thyroid Gland
Regional metastases from thyroid carcinoma most frequently involve the central compartment lymph nodes such as the prelaryngeal (Delphian), pretracheal and paratracheal nodes [22]. The first echelon lymph nodes of the thyroid gland are in the central compartment (level VI) of the neck followed by second echelon nodal drainage to the lower jugular chain (level IV) and the upper mediastinum (level VII). Occasionally however, upper pole lesions can metastasize to lateral neck nodes without any evidence of central neck nodal metastases. Park et al. have shown that the rate of “skip metastases” can be as high as 22% in patients with small upper pole primary thyroid tumors [23]. WDTC can even rarely metastasize to retropharyngeal nodes in the setting of recurrent or persistent disease [24].
Evaluation of Nodal Metastases
The initial approach to patients with thyroid cancer is to obtain a detailed history and perform a thorough clinical examination. This should always include visualization of the larynx and assessment of vocal cord movements. Ultrasound of the thyroid gland is the most widely used imaging modality as it can evaluate the thyroid gland and both the central and lateral neck lymphatic compartments. Loss of fatty hilum, calcifications, peripheral vascularity, hyperechogenicity, rounded rather than oval shape, cystic changes and large size are all characteristics of metastatic lymph nodes on ultrasound. Leboulleux et al. reported that among these features, peripheral vascularity has the highest sensitivity [25]. Previous studies by Frasoldati et al. and Kuna et al. have shown that absence of fatty hilum and the presence of microcalcifications have the highest sensitivity and specificity, respectively [26–27]. Hwang et al. reported significantly lower diagnostic accuracy of ultrasound in detecting occult disease in the central neck compared to the lateral neck compartment [28].
Thyroglobulin measurement increases the diagnostic accuracy of fine needle aspiration cytology (FNAC) when evaluating metastatic lymph nodes in WDTC. Chung et al. have shown that thyroglobulin measurement in FNAC fluid aspirate can be helpful in the diagnosis of metastatic lateral neck nodes in patients with papillary thyroid carcinoma (PTC) [29]. This can also be a useful tool for the evaluation of cystic lateral neck masses, where aspiration cytology is often paucicellular, particularly when the clinical behavior is consistent with WDTC [30,31].
The major advantage of cross-sectional imaging (CT or MRI) with IV contrast over ultrasound is that the former is not operator dependent, is superior at visualizing deep anatomical structures such as the mediastinum and the retropharyngeal or parapharyngeal space, and provides the surgeon with a preoperative “roadmap”. In a cohort of 162 patients with PTC, Lesnik et al. showed that preoperative CT combined with ultrasound was superior to ultrasound alone in the evaluation of the central neck compartment [32]. Ultrasound has been shown to be inferior to CT in investigating the central neck compartment particularly when the thyroid gland is still present [28,32]. An additional advantage of obtaining a preoperative CT scan is the identification of an aberrant right retroesophageal subclavian artery (arteria lusoria), which indicates a non-recurrent laryngeal nerve [33].
It is known that as iodine avidity decreases, fluorodeoxyglucose-positron emission tomography (FDG-PET) avidity increases as tumor dedifferentiation progresses from well differentiated to poorly differentiated and anaplastic thyroid carcinoma. However, Jeong et al. showed that 18FDG-PET combined with CT does not provide any additional diagnostic benefit when compared to ultrasound or CT in the assessment of cervical lymph nodes in patients with PTC [34]. Routine preoperative 18FDG-PET scanning is therefore not recommended in the investigation of nodal metastases in WDTC [35].
8th Edition AJCC/TNM Staging System – Revision on nodal status
As it became evident that the prognostic impact of small volume nodal metastases on younger patients is less significant, in 2016 the American Joint Committee on Cancer (AJCC) updated the AJCC/tumor, lymph node, metastasis (TNM) staging system. For patients younger than 55 years of age, N1 disease is no longer upstaged to stage III and is now stage I. In patients older than 55 years of age, N1 disease is now stage II [36]. In the previous edition, level VII nodal metastasis were identified as N1b and grouped as stage IV. However, in the 8th edition, the level VII nodes were not considered as N1b and the stage was reduced from stage IV to stage I or II.
Therapeutic Neck Dissection in WDTC
A therapeutic neck dissection is defined as the removal of metastatic nodes that are evident either clinically (preoperatively or intraoperatively) or radiologically (clinically N1). It is standard practice to perform a therapeutic neck dissection for all patients with clinically evident central or lateral neck metastases [35,37]. Every effort should be made in these cases to preserve function while ensuring complete oncological removal of all nodal disease.
The ATA in a consensus statement in 2009, specified that a central neck dissection involves a compartment-oriented removal of the prelaryngeal, pretracheal nodes and at least one paratracheal lymph node basin [38]. The superior and inferior borders of a central neck dissection are the thyroid notch and the innominate artery. It is not necessary to perform a thymus resection since the thymus hardly ever contains metastatic disease [39]. The lateral borders of the dissection are the carotid arteries (levels VI and VII). The procedure should be performed by experienced surgeons as complication rates, hypocalcemia and laryngeal nerves injury, can be unacceptably high in low volume centers. Of note, the surgeon should be aware of the 3% incidence of a right non-recurrent inferior laryngeal nerve, as indicated by the presence of an arteria lusoria on the CT scan [33,40].
Similarly, if there is metastatic disease in the lateral neck then a therapeutic neck dissection should be done at the time of the primary surgery. Lateral neck metastases are rarely found in level I or superior to the accessory nerve. Therefore, to minimize risk to the marginal mandibular and accessory nerves, lateral lymphadenectomy should include levels IIa, III, IV and Vb [13].
Elective Neck Dissection in WDTC – Central Compartment
Prophylactic or elective central neck dissection (CND) for WDTC in the absence of clinical evidence of nodal disease (cN0) is controversial due to questionable clinical benefit and the potential of morbidity related to the recurrent laryngeal nerves and parathyroid glands (Figure 1). Incidence of nodal metastases in patients with follicular thyroid cancer (FTC) is low (1-8%) and hence, a prophylactic central neck dissection is not indicated [41]. There have been no prospective randomized trials directly comparing thyroidectomy with or without elective neck dissection, such that current evidence is based on retrospective studies and meta-analyses of these retrospective studies.
Figure 1.
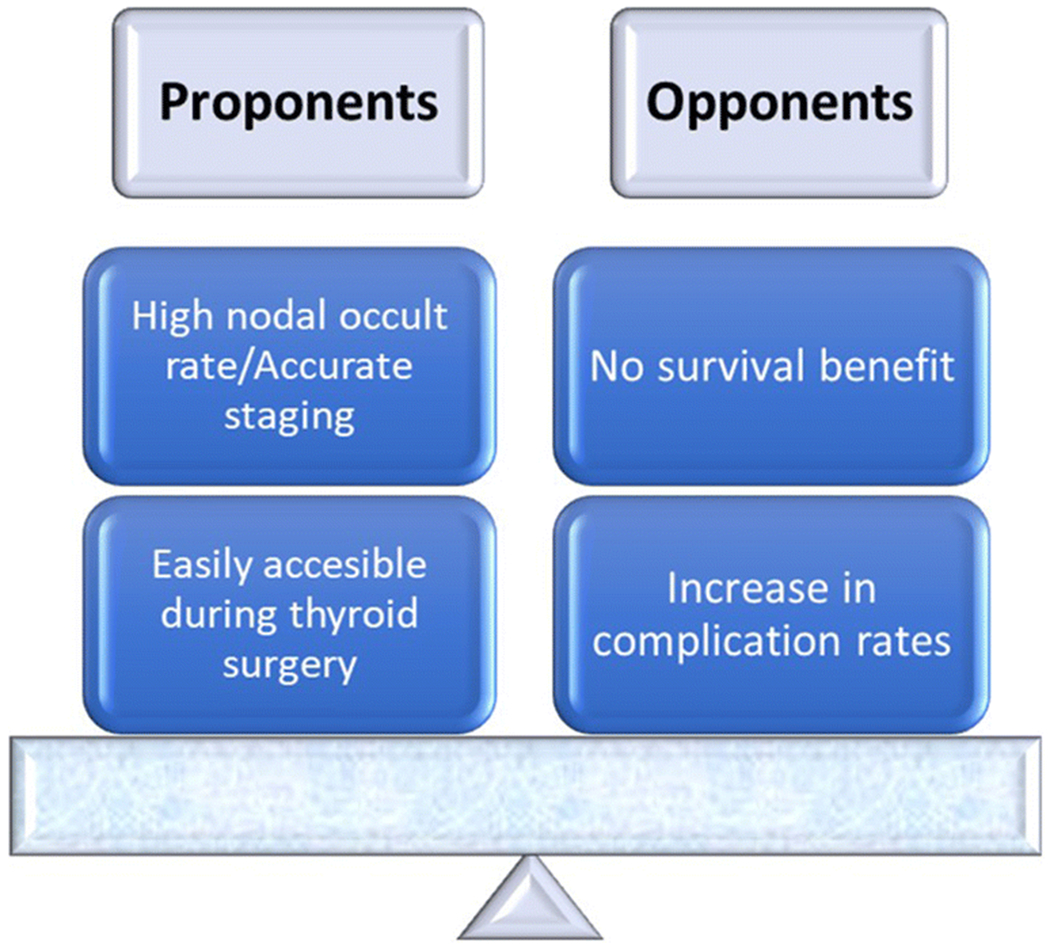
Arguments for and against performing elective central neck dissection in patients with papillary thyroid carcinoma. Most agree currently that elective central neck dissections are generally not indicated.
Proponents of Prophylactic Elective CND
In a retrospective analysis of 640 patients with PTC, Barczynski et al. reported that prophylactic central neck dissection during total thyroidectomy improves disease-specific survival and locoregional control without increasing morbidity [42]. However, their results have not been reproduced by other groups [43]. The main argument for performing a central neck dissection is the finding of significant occult disease in a clinically negative central neck (cN0pN+) [2,3]. Hughes et al. reported an occult positivity rate of 60% in patients undergoing prophylactic CND [43]. As the central compartment of the neck is accessible without significantly lengthening the thyroidectomy incision, pathologic analysis of the central neck can be used to refine staging [44]. In the current staging system, only patients aged 55 years or older will be upstaged and consequently offered adjuvant radioactive iodine ablation (RAI) [43,44]. There are however several centers around the world where all patients with nodal disease will be offered RAI regardless of age and stage.[45,46] Another potential benefit of performing a prophylactic CND, is that it can improve biochemical cure rates. Sywak et al. reported lower thyroglobulin levels with prophylactic CND compared to thyroidectomy alone. This effect however was not sustained and became non-significant after six months [47]. A further retrospective study found that prophylactic neck dissection decreased the rate of re-treatment with radioactive iodine and revision surgery [48]. Two recent meta-analyses have shown decreased locoregional recurrence (OR 0.65-0.66) and central compartment recurrence rates (OR 0.4) with prophylactic central compartment dissection [49,50].
Opponents of Prophylactic Elective CND
The main argument against a prophylactic central neck dissection in patients with PTC is that patients without clinical nodal disease (cN0) have excellent outcomes when managed with thyroidectomy and observation of their necks. Ito et al. from Japan reported recurrence rates of 1% and 3% at 5 and 10 years, respectively, in patients who did not have a prophylactic central neck dissection [51]. Nixon et al. showed a 5-year recurrence-free survival of 97% and central neck recurrence free survival of 99% in patients who underwent observation of their central neck compartments. The 5-year disease-specific survival in this large cohort of patients with PTC was 100% [21]. Similarly, Monchik et al. reported less than 2% emergence of central nodal disease in low risk patients who had their central necks observed after total thyroidectomy [52]. In a large meta-analysis of over 1,200 patients, Zetoune et al. reported no benefit of loco-regional control nor overall survival with a prophylactic central neck dissection in patients with PTC [53]. Up to date, there are several systematic reviews with contradictory results on the role of prophylactic CND due to differences in inclusion criteria, size of the tumor and heterogeneity in the interventions [50,54–59]. As there is a lack of evidence to support any benefit from prophylactic central neck dissection in low risk patient groups, the concept of aggressive neck surgery to upstage and facilitate RAI seems illogical.
In addition to the lack of oncologic benefit from prophylactic central neck dissection, patients undergoing additional surgery are at risk of complications. Although data from high volume centers report low surgical complication rates [44,60], national database analyses report higher rates when prophylactic central dissection is performed. The Scandinavian Quality Register for Thyroid and Parathyroid Surgery study showed higher rates of hypocalcemia and wound infection with neck dissection compared to thyroidectomy alone [61]. Similarly, two recent meta-analyses reported a significant increase in temporary (OR 2.23-2.28) and permanent hypoparathyroidism (OR 1.84-2.22) and temporary recurrent laryngeal nerve palsy (OR 1.53-2.03) with prophylactic CND [49,50].
In addition to the above arguments against performing a routine prophylactic central neck dissection, surgical experience is also an important factor. Clayman et al. reported that 60% of recurrent central neck nodes were found dorsal to the recurrent laryngeal nerve [62]. This is an area that would not be routinely dissected in the prophylactic central neck dissection setting and would likely be unnoticed by an inexperienced surgeon.
As it has become more apparent that prophylactic central neck dissection increases the risk of complications without significant benefit, the recent ATA guidelines have deviated from their previous recommendation that it should be considered in all cases. The 2015 ATA guidelines suggest (weak recommendation) that prophylactic central neck node dissection should be considered in patients with PTC with advanced primary tumors (T3 or T4) or clinically positive lateral neck nodes (cN1b), or if the information will be used to plan further management. Thyroidectomy without prophylactic central neck dissection is strongly recommended for small (T1 or T2), non-invasive, clinically negative (cN0) PTC and for most follicular cancers [35]. This is a subtle but important modification from the 2009 ATA guidelines which recommended that prophylactic central neck dissection (ipsilateral or bilateral) may be performed in patients with PTC with clinically uninvolved central neck lymph nodes, especially for advanced primary tumors (T3 or T4) [63].
Controversy remains over the role of prophylactic central neck surgery in PTC. In some health care settings, adjuvant RAI remains the standard of care for all but the lowest risk cases. Clinicians may therefore perform a prophylactic central neck dissection to demonstrate pN0 disease and avoid the need for total thyroidectomy or adjuvant RAI [46,64]. Internationally there has also been a move away from aggressive treatment for PTC which includes lower rates of prophylactic central neck dissection.
Prophylactic/Elective Neck Dissection in WDTC – Lateral Compartment
Patients with PTC have much lower rates (23%) of occult nodal metastases in the lateral than in the central neck compartment [18]. Furthermore, prophylactic lateral neck dissection can result in high risk of surgical complications with some groups reporting of 25-50% complications after total thyroidectomy and lateral neck dissection [32,44]. Even proponents of a prophylactic central neck dissection do not recommend a prophylactic lateral neck dissection as there is no clinical benefit in terms of disease recurrence [44,65]. The 2009 ATA guidelines only support therapeutic, not prophylactic lateral neck dissection, a recommendation that did not change in the 2015 guidelines [35]. A summary of the surgical approach to the management of nodal disease in PTC is shown in Figure 2.
Figure 2.
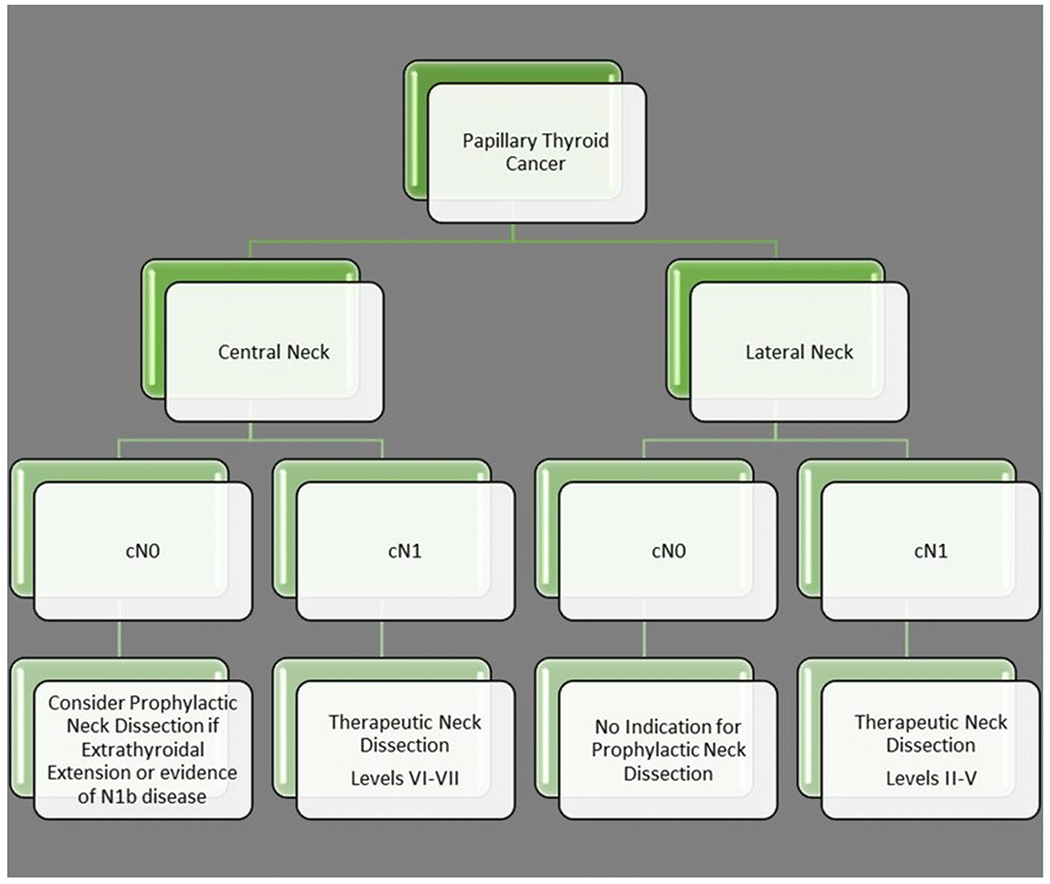
Surgical management of nodal metastases in patients with papillary thyroid cancer.
Sentinel Node Biopsy in cN0 Thyroid Cancer
Sentinel node biopsy is employed for many types of cancers as a means of precise lymph node staging in clinically N0 patients for treatment intensification and prognostication. Sentinel node biopsy using radiotracers, dyes or colloidal nanoparticles is feasible in thyroid cancer, with highest rates of sentinel node detection seen with radiotracer techniques [66]. However, false negative rates in the range of 7-20% have been reported [67,68]. There is lack of high-level evidence associating sentinel node biopsy with improvement in oncologic outcomes in thyroid cancer [69].
Complications of Neck Dissection – Central Compartment
Operating in the central neck nodal compartment increases the risk of parathyroid and recurrent laryngeal nerve injury. Large studies such as the Scandinavian Quality Register for Thyroid and Parathyroid Surgery report higher hypocalcemia rates when performing CND compared to thyroidectomy alone [61]. However, this is not the case for high volume centers who report low complication rates when performing central neck surgery concomitantly with thyroidectomy. A French group practicing routine prophylactic CND for PTC in 317 patients, reported rates of both permanent hypoparathyroidism and vocal cord paralysis of 0.6% [44]. Sywak et al. reported permanent hypocalcemia and temporary hypocalcemia rates of 1.8% and 18%, respectively,after prophylactic CND [47]. Popodich et al. in a retrospective, multicenter cohort study of 259 patients undergoing elective CND, reported permanent and temporary hypocalcemia rates of 0.8% and 9.7%, respectively [60]. Both groups however, report higher rates of parathyroid autotransplantation during prophylactic CND compared to thyroidectomy alone. If a parathyroid is devascularized during thyroidectomy, it should be implanted preferably into the contralateral sternomastoid muscle [70]. The surgical specimen should always be carefully examined for any inadvertently removed parathyroid glands. Frozen section histopathological examination should be performed prior to autotransplantation to ensure that the excised tissue is not a metastatic lymph node.
Complications of Neck Dissection – Lateral Compartment
Performing a lateral neck dissection in patients with DTC is only indicated in the therapeutic setting. During the consent process, the patient should be informed about potential complications from a lateral neck dissection. Temporary or permanent injury to the accessory nerve has been reported to be as high as 20%. This is more common when the lymphatics superior to the accessory nerve (levels IIB and VA) are dissected. Chronic neck pain can be secondary to injury of the accessory nerve or the cervical root branches during dissection in the posterior triangle [71]. Roh et al. reported that up to 11% of patients undergoing lateral neck dissection during thyroidectomy develop chronic pain [72]. In the presence of an anatomically intact accessory nerve, traction injury and devascularization are thought to be the most common modes of nerve injury. Injury to the motor branches of the cervical plexus which provide a significant amount of neural input to the trapezius muscle can also result in significant shoulder dysfunction. Intraoperative nerve monitoring can be used in routine neck dissections to detect these branches [73]. Intensive physiotherapy can improve shoulder function in most patients [74].
The thoracic duct is located at the junction of the left subclavian vein and the left internal jugular vein. As the incidence of nodal metastasis at level IV in thyroid carcinoma is high, injury to the lymphatics is common. In a large Japanese cohort of approximately 1,500 patients undergoing lateral neck dissection during total thyroidectomy, Ito et el. reported a chyle leak rate of 2.2%. The rate of repairing the leak with neck exploration was 0.6% [10]. In a smaller cohort from Korea, Roh et al. reported a 3.6% rate of chyle leak in patients undergoing neck dissection during total thyroidectomy [72]. If a chyle leak was identified intraoperatively, it should be repaired by ligation using nonabsorbable ties or hemoclips [75]. If the leak is still not controlled, then transfixion sutures should be considered, with a sternomastoid muscle buttress [74]. Low output chyle leaks identified postoperatively are best managed conservatively. Pressure dressing, antibiotics and diet modification with medium-chain triglycerides will suffice for most patients. Neck exploration should be considered only if the chyle output is high i.e. more than 500 cc per day. This can be challenging as the soft tissues are irritated by chyle and placement of sutures can cause further damage to the lymphatics. Biological sealants in such circumstances may be helpful. Video-assisted thoracoscopic surgery (VATS) [76] to ligate the thoracic duct or thoracic duct embolization [77] can be considered as treatment option in high output cases.
Less common complications of lateral neck dissection occur in less than 1% of patients. These include injury to the phrenic nerve and the sympathetic chain [10]. Horner’s syndrome characterized by miosis, ptosis and anhidrosis can result from damage to the sympathetic chain. Although neck paresthesia following lateral neck dissection is common, it is not widely reported in literature. Preservation of the cervical plexus rootlets and the great auricular nerve during dissection in the posterior neck and subplatysmal flap elevation, respectively, may reduce the intensity of paresthesia.
Technical Pearls in Neck Dissection in WDTC
The thyroid surgeon performing neck dissection should be aware of the following points:
Ultrasound scan: careful preoperative evaluation and identification of all nodal disease as a surgical mapping plan.
CT scan: used to supplement ultrasound to further identify nodal disease in the superior mediastinum and retropharyngeal areas. Can be used as a surgical roadmap in patients with extensive regional disease. It also demonstrates the presence of an aberrant right retroesophageal subclavian artery indicating a non-recurrent laryngeal nerve.
- Critical areas of nodal disease: Nodal metastases from thyroid cancer can be found in areas not necessarily addressed by a neck dissection as described for other types of head and neck cancer; meticulous intraoperative assessment should concentrate on the following areas for evidence of occult nodal disease:
- Central compartment:
- Paratracheal nodes
- Pretracheal nodes and prelaryngeal nodes (near the cricothyroid membrane)
- Right paraesophageal nodes (lateral to the right recurrent laryngeal nerve)
- Nodes deep to the right recurrent laryngeal nerve (common location for residual disease missed during initial surgery as the nerve is more superficial on the right side)
- Paralaryngeal nodes (along the superior thyroid vascular pedicle and the laryngeal vascular pedicle, medial to the carotid bifurcation)
- Low-lying nodes adjacent to the brachiocephalic vessels, below the sternal notch (this area used to be defined as superior mediastinal nodes or level VII, but is accessible in most patients through the classic cervical approach [78]; these nodes now are considered part of level VI)
- Lateral neck compartment:
- Retrocarotid and retrojugular nodes: full exposure of the carotid sheath should be performed to allow palpation around the carotid artery and internal jugular vein, particularly at level IV
- Level II nodes
- Supraclavicular nodes
- Retropharyngeal nodes
- Nodes below the superior border of the clavicle
- Retrojugular nodes near the lymphatic ducts
- Lymph nodes near the lateral cervical vessels
Postoperative Surveillance and Management of the Neck in WDTC
Following surgical management and histopathological analysis of the primary thyroid tumor and excised lymph nodes, post surgical risk stratification should be performed. This allows the clinician to evaluate the risk of disease recurrence after initial surgical treatment. With regard to pathological nodal status, the 2015 ATA guidelines consider high risk features to be the presence of any metastatic lymph node greater than 3 cm and intermediate risk as the presence of palpable disease or more than five involved nodes less than 3 cm in size. Patients with such features should be considered for adjuvant RAI especially when post-operative serum thyroglobulin levels remain elevated after surgery [35].
Historically, patients would then be followed with clinical examination and palpation of the neck. Recent advances in ultrasound imaging and biochemical surveillance with thyroglobulin levels will allow early detection of small volume recurrence in the neck. Although ultrasound is highly accurate in diagnosing lateral neck disease, it can overlook disease in the central neck compartment [28]. Any significant rise in serum thyroglobulin levels without evidence of lateral neck disease on ultrasound, should prompt the clinician to obtain a CT scan to rule out disease in the central neck, mediastinum and lung. Although iodinated contrast causes a stunning effect that may imply delay of radioactive iodine treatment, if early RAI is not deemed essential, consideration should be given for the use of iodinated contrast. This will provide superior imaging for detecting lymphadenopathy in the neck and mediastinum. Thyroglobulin levels have been found to positively correlate with disease volume. In WDTC, thyroglobulin levels correlate with the site of metastases. Levels less than 10 ng/ml are suggestive of persistent local or recurrent nodal disease, levels 20-500 ng/ml suggest pulmonary metastases and levels over 1000 ng/ml are suggestive of bony metastases [79].
FDG-PET scanning is rarely indicated during postoperative surveillance in WDTC unless there is a consistent rapid rise in thyroglobulin levels that cannot be identified on conventional axial neck imaging and there is suspicion of primary tumor dedifferentiation into a poorly differentiated variety. In addition to this, FDG-PET is also indicated in the postoperative surveillance of Hurthle cell thyroid carcinoma [80].
Surgical Management of Nodal Recurrence in WDTC
According to the 2015 ATA guidelines, persistent or recurrent nodal disease with central neck nodes greater than 8 mm and lateral neck nodes greater than 10mm detected with or without adjuvant RAI, should be treated surgically [35]. In a retrospective analysis of 210 patients with PTC and recurrent or persistent central neck disease, Clayman et al. demonstrated that 90% of central neck disease could be controlled after the first salvage central neck dissection. Overall control rate in the central neck was reported to be 98% when further recurrences were surgically salvaged [62]. Al-Saif et al. reported biochemical cure rates of 27% in patients with persistent disease in both central and lateral neck compartments after multiple operations. Despite achieving low biochemical control rates, there were no clinical recurrences, distant metastases, or deaths in their patient cohort [81].
Revision neck dissection can be more challenging in the setting of recurrent nodal disease compared to the previously non-operated neck [70]. Microclips or black silk suture ties can resemble tumor nodules or disease in the previously dissected central neck compartment. Strap muscles are often found to be adherent to the cricoid and trachea. The carotid artery and the internal jugular vein are often medially located due to scarring from previous surgery. In such cases, a lateral neck approach is advised in order to gain safe access to the central neck. Persistent disease is more likely to be found in areas that were not previously explored. Failure to clear disease behind the clavicular heads and by the right recurrent laryngeal nerve are common sites for disease recurrence. The recurrent laryngeal nerve might be more challenging to identify because of scar tissue formation or nerve displacement from nodal recurrence. It is helpful to identify the nerve inferiorly near the thoracic inlet where previous dissection did not occur. The role of laryngeal nerve monitoring (IONM) is of paramount importance in such circumstances [82]. There have been great advances in IONM in the last several years and guidelines has now delineated optimal definition of loss of signal and how a surgeon should incorporate that information into the surgical strategy [83]. In addition IONM information is critical in the management of recurrent laryngeal nerves invaded by thyroid cancer [84]. Identification and preservation of the parathyroid glands may also be challenging during revision surgery. As discussed previously, autotransplantation should be highly considered, if a parathyroid gland is felt to be devascularized.
It is generally recommended that recurrence in a region not already addressed by compartment-oriented selective neck dissection should be treated with a complete dissection of that neck level. Lymph node “picking” is only reserved for nodal recurrences in regions having already been completely dissected. Intraoperative identification of the lesion can be aided using intraoperative ultrasound or preoperative ultrasound guided tattooing of the lesion with colloidal charcoal or other dyes [85,86].
Non-Surgical Management of Nodal Recurrence in WDTC
The 2015 ATA guidelines recommend that recurrent nodal disease measuring less than 8 mm in the central neck and less than 10 mm in the lateral neck can be monitored with serial imaging, reserving needle biopsy for patients with evidence of disease progression [35]. Other factors that may affect clinician’s decision to surgically intervene are the proximity of recurrent nodal disease to vital neurovascular or aerodigestive structures and the patient’s symptoms and preferences.
Non-operative management options of nodal metastases include percutaneous ethanol injections. 95% to 99.9% ethanol is injected directly into the nodal lesion under ultrasound guidance. Hay et al. from the Mayo Clinic reported 84% to 100% complete response of nodal metastases on ultrasound imaging with alcohol ablation [87,88]. Radiofrequency ablation has also been used to treat recurrent nodal metastases. High-frequency alternating current at 350 to 500 kHz is introduced through a needle into the nodal mass under ultrasound guidance [89]. Despite showing promising initial results, injection techniques are limited to small cohorts with poor long-term follow-up. It is premature to draw any conclusions on their ultimate impact on nodal recurrence and overall prognosis.
Conclusions
Well differentiated thyroid cancer, particularly PTC, frequently metastasizes to regional lymph nodes. Recent evidence highlights the importance of the patient’s age, size, number and extent of nodal metastases in the most recent staging and risk stratification systems. Our current recommendations for management of the central and lateral neck in WDTC are clearly depicted in Figure 2.
Thyroid surgeons must be familiar with the indications and techniques of compartmental lymph node neck dissections. As patients with clinically evident nodal disease are associated with worse outcomes, they should be treated surgically in order to reduce rates of regional recurrence and improve survival. The benefit of elective neck dissection remains unverified as the impact of microscopic disease on outcomes is not significant. Recent guidelines have therefore moved away from routine prophylactic central neck dissection in every patient with PTC.
The aim of the modern thyroid surgeon should be to perform a comprehensive and oncologically safe neck dissection when indicated, while ensuring minimal morbidity for the patient. As complication rates are low with experienced surgeons, such patients should be treated at high volume centers to optimize both functional and oncological outcomes.
Funding:
No funding or sponsorship was received for this study or publication of this article.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
This article was written by members and invitees of the International Head and Neck Scientific Group (www.IHNSG.com).
Conflicts of interest/Competing interests: All authors declare that they have no conflict of interest.
Ethics approval: This article is based on previously conducted studies and does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate: Not applicable.
Consent for publication: All named authors have given their approval for this article to be published.
Availability of data and material: The authors confirm that the data supporting the findings of this study are available within the article.
Code availability: Microsoft Word for Microsoft 365MSO 32-bit
References
- 1.Sherman SI. Thyroid carcinoma. Lancet. 2000;361:501–11. [DOI] [PubMed] [Google Scholar]
- 2.Yan DG, Zhang B, An CM, Zhang ZM, Li ZJ, Xu ZG, et al. Cervical lymph node metastasis in clinical N0 papillary thyroid carcinoma. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2011;46:887–91. [PubMed] [Google Scholar]
- 3.Wada N, Duh QY, Sugino K, Iwasaki H, Kameyama K, Mimura T, et al. Lymph node metastasis from 259 papillary thyroid microcarcinomas: frequency, pattern of occurrence and recurrence, and optimal strategy for neck dissection. Ann Surg. 2003;237:399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roh JL, Park JY, Rha KS, Park CI. Is central neck dissection necessary for the treatment of lateral cervical nodal recurrence of papillary thyroid carcinoma? Head Neck. 2007;29:901–6. [DOI] [PubMed] [Google Scholar]
- 5.Koo BS, Choi EC, Park YH, Kim EH, Lim YC. Occult contralateral central lymph node metastases in papillary thyroid carcinoma with unilateral lymph node metastasis in the lateral neck. J Am Coll Surg. 2010;210:895–900. [DOI] [PubMed] [Google Scholar]
- 6.Khafif A, Ben-Yosef R, Abergel A, Kesler A, Landsberg R, Fliss DM. Elective paratracheal neck dissection for lateral metastases from papillary carcinoma of the thyroid: is it indicated? Head Neck. 2008;30:306–10. [DOI] [PubMed] [Google Scholar]
- 7.Cady B, Sedgwick CE, Meissner WA, Bookwalter JR, Romagosa V, Werber J. Changing clinical, pathologic, therapeutic, and survival patterns in differentiated thyroid carcinoma. Ann Surg. 1976;184:541–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hughes CJ, Shaha AR, Shah JP, Loree TR. Impact of lymph node metastasis in differentiated carcinoma of the thyroid: a matched-pair analysis. Head Neck. 1996;18:127–32. [DOI] [PubMed] [Google Scholar]
- 9.Adam MA, Pura J, Goffredo P, Dinan MA, Reed SD, Scheri RP, et al. Presence and number of lymph node metastases are associated with compromised survival for patients younger than age 45 years with papillary thyroid cancer. J Clin Oncol. 2015;33:2370–5. [DOI] [PubMed] [Google Scholar]
- 10.Ito Y, Higashiyama T, Takamura Y, Miya A, Kobayashi K, Matsuzuka F, et al. Risk factors for recurrence to the lymph node in papillary thyroid carcinoma patients without preoperatively detectable lateral node metastasis: validity of prophylactic modified radical neck dissection. World J Surg. 2007;31:2085–91. [DOI] [PubMed] [Google Scholar]
- 11.Sugitani I, Kasai N, Fujimoto Y, Yanagisawa A. A novel classification system for patients with PTC: addition of the new variables of large (3 cm or greater) nodal metastases and reclassification during the follow-up period. Surgery. 2004;135:139–48. [DOI] [PubMed] [Google Scholar]
- 12.Yamashita H, Noguchi S, Murakami N, Toda M, Uchino S, Watanabe S, et al. Extracapsular invasion of lymph node metastasis. A good indicator of disease recurrence and poor prognosis in patients with thyroid microcarcinoma. Cancer. 1999;86:842–9. [DOI] [PubMed] [Google Scholar]
- 13.Randolph GW, Duh QY, Heller KS, LiVolsi VA, Mandel SJ, Steward DL, et al. The prognostic significance of nodal metastases from papillary thyroid carcinoma can be stratified based on the size and number of metastatic lymph nodes, as well as the presence of extranodal extension. Thyroid. 2012;22:1144–52. [DOI] [PubMed] [Google Scholar]
- 14.Urken ML, Haser GC, Likhterov I, Wenig BM. The impact of metastatic lymph nodes on risk stratification in differentiated thyroid cancer: Have We Reached a Higher Level of Understanding? Thyroid. 2016;26:481–8. [DOI] [PubMed] [Google Scholar]
- 15.Wang LY, Palmer FL, Nixon IJ, Thomas D, Shah JP, Patel SG, et al. Central lymph node characteristics predictive of outcome in patients with differentiated thyroid cancer. Thyroid. 2014;24:1790–5. [DOI] [PubMed] [Google Scholar]
- 16.Wang LY, Palmer FL, Nixon IJ, Tuttle RM, Shah JP, Patel SG, et al. Lateral neck lymph node characteristics prognostic of outcome in patients with clinically evident N1b papillary thyroid cancer. Ann Surg Oncol. 2015;22:3530–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.∎.Amit M, Tam S, Boonsripitayanon M, Cabanillas, Busaidy NL, Grubbs EG, et al. Association of lymph node density with survival of patients with papillary thyroid cancer. JAMA Otolaryngol Head Neck Surg. 2018;144:108–14. [DOI] [PMC free article] [PubMed] [Google Scholar]; This large single-institute cohort study demonstrated worse overall and disease specific survival in patients with lymph node density greater than 19%.
- 18.Nixon IJ, Wang LY, Migliacci JC, Eskander A, Campbell MJ, Aniss A, et al. An international multi-institutional validation of age 55 Years as a cutoff for risk stratification in the AJCC/UICC staging system for well-differentiated thyroid cancer. Thyroid. 2016;26:373–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bozec A, Dassonville O, Chamorey E, Poissonnet G, Sudaka A, Peyrottes I, et al. Clinical impact of cervical lymph node involvement and central neck dissection in patients with papillary thyroid carcinoma: a retrospective analysis of 368 cases. Eur Arch Otorhinolaryngol. 2011;268;1205–12. [DOI] [PubMed] [Google Scholar]
- 20.Podnos YD, Smith D, Wagman LD, Ellenhorn JD. The implication of lymph node metastasis on survival in patients with well-differentiated thyroid cancer. Am Surg. 2005;71:731–4. [DOI] [PubMed] [Google Scholar]
- 21.Nixon IJ, Ganly I, Patel SG, Morris LG, Palmer FL, Thomas D, et al. Observation of clinically negative central compartment lymph nodes in papillary thyroid carcinoma. Surgery. 2013;154:1166–72. [DOI] [PubMed] [Google Scholar]
- 22.Iyer NG, Kumar A, Nixon IJ, Patel SG, Ganly I, Tuttle RM, et al. Incidence and significance of Delphian node metastasis in papillary thyroid cancer. Ann Surg. 2011;253:988–91. [DOI] [PubMed] [Google Scholar]
- 23.Park JH, Lee YS, Kim BW, Chang HS, Park CS. Skip lateral neck node metastases in papillary thyroid carcinoma. World J Surg. 2012;36:743–7. [DOI] [PubMed] [Google Scholar]
- 24.Harries V, McGill M, Tuttle RM, Shaha AR, Wong RJ, Shah JP, Patel SG, et al. Management of retropharyngeal lymph node metastases in differentiated thyroid carcinoma. Thyroid. 2020;30:688–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leboulleux S, Girard E, Rose M, Travagli JP, Sabbah N, Caillou B, et al. Ultrasound criteria of malignancy for cervical lymph nodes in patients followed up for differentiated thyroid cancer. J Clin Endocrinol Metab. 2007;92:3590–4. [DOI] [PubMed] [Google Scholar]
- 26.Frasoldati A, Valcavi R. Challenges in neck ultrasonography: lymphadenopathy and parathyroid glands. Endocr Pract. 2007;10:261–8. [DOI] [PubMed] [Google Scholar]
- 27.Kuna SK, Bracic I, Tesic V, Kuna K, Herceg GH, Dodig D. Ultrasonographic differentiation of benign from malignant neck lymphadenopathy in thyroid cancer. J Ultrasound Med. 2006;25:1531–7. [DOI] [PubMed] [Google Scholar]
- 28.Hwang HS, Orloff LA. Efficacy of preoperative neck ultrasound in the detection of cervical lymph node metastasis from thyroid cancer. Laryngoscope. 2011;121: 487–91. [DOI] [PubMed] [Google Scholar]
- 29.Chung J, Kim EK, Lim H, Son EJ, Yoon JH, Youk JH, et al. Optimal indication of thyroglobulin measurement in fine-needle aspiration for detecting lateral metastatic lymph nodes in patients with papillary thyroid carcinoma. Head Neck. 2014;36:795–801. [DOI] [PubMed] [Google Scholar]
- 30.Kawamura S, Kishino B, Miyauchi A, Takai S, Tajima K, Mashita K, et al. The differential diagnosis of cystic neck masses by the determination of thyroglobulin concentrations in the aspirates. Clin. Endocrinol (Oxf) 1984;20:261–7. [DOI] [PubMed] [Google Scholar]
- 31.Benmoussa JA, Chen K, Najjar S, Applewhite M, Warshaw J. Lateral neck cystic mass: The role of thyroglobulin measurement in fine needle aspiration. Endocr Pract. 2018;24:767. [DOI] [PubMed] [Google Scholar]
- 32.Lesnik D, Cunnane ME, Zurakowski D, Acar GO, Ecevit C, Mace A. et al. Papillary thyroid carcinoma nodal surgery directed by a preoperative radiographic map utilizing CT scan and ultrasound in all primary and reoperative patients. Head Neck. 2014;36:191–202. [DOI] [PubMed] [Google Scholar]
- 33.Hong KH, Park HT, Yang YS. Characteristic travelling patterns of non-recurrent laryngeal nerves. J Laryngol Otol. 2014;128: 534–9. [DOI] [PubMed] [Google Scholar]
- 34.Jeong HS, Baek CH, Son YI, Choi JY, Kim HJ, Ko YH, et al. Integrated 18F-FDG PET/CT for the initial evaluation of cervical node level of patients with papillary thyroid carcinoma: comparison with ultrasound and contrast-enhanced CT. Clin Endocrinol (Oxf). 2006; 65:402–7. [DOI] [PubMed] [Google Scholar]
- 35.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 2016; 26:1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. AJCC Cancer Staging Manual. 8th ed. New York: Springer; 2017. [Google Scholar]
- 37.Bardet S, Malville E, Rame JP, Babin E, Samama G, De Raucourt D, et al. Macroscopic lymph-node involvement and neck dissection predict lymph-node recurrence in papillary thyroid carcinoma. Eur J Endocrinol. 2008;158:551–60. [DOI] [PubMed] [Google Scholar]
- 38.American Thyroid Association Surgery Working Group, American Association of Endocrine Surgeons, American Academy of Otolaryngology-Head and Neck Surgery, American Head and Neck Society, Carty SE, Cooper DS, et al. Consensus statement on the terminology and classification of central neck dissection for thyroid cancer. Thyroid. 2009;19:1153–58. [DOI] [PubMed] [Google Scholar]
- 39.El Khatib Z, Lamblin J, Aubert S, Arnalsteen L, Leteurtre E, Caiazzo R, et al. Is thymectomy worthwhile in central lymph node dissection for differentiated thyroid cancer? World J Surg. 2010;34:1181–1186. [DOI] [PubMed] [Google Scholar]
- 40.Hermans R, Dewandel P, Debruyne F, Delaere PR. Arteria lusoria identified on preoperative CT and nonrecurrent inferior laryngeal nerve during thyroidectomy: a retrospective study. Head Neck. 2003;25:113–7. [DOI] [PubMed] [Google Scholar]
- 41.Alfalah H, Cranshaw I, Jany T, Arnalsteen L, Leteurtre E, Cardot C, et al. Risk factors for lateral cervical lymph node involvement in follicular thyroid carcinoma. World J Surg. 2008;32:2623–6. [DOI] [PubMed] [Google Scholar]
- 42.Barczynski M, Konturek A, Stopa M, Nowak W. Prophylactic central neck dissection for papillary thyroid cancer. Br J Surg. 2013;100:410–8. [DOI] [PubMed] [Google Scholar]
- 43.Hughes DT, White ML, Miller BS, Gauger PG, Burney RE, Doherty GM. Influence of prophylactic central lymph node dissection on postoperative thyroglobulin levels and radioiodine treatment in papillary thyroid cancer. Surgery. 2010; 148:1100–6. [DOI] [PubMed] [Google Scholar]
- 44.Hartl DM, Leboulleux S, A1 Ghuzlan A, Baudin E, Chami L, Schlumberger M, et al. Optimization of staging of the neck with prophylactic central and lateral neck dissection for papillary thyroid carcinoma. Ann Surg. 2012; 255:777–83. [DOI] [PubMed] [Google Scholar]
- 45.Verburg FA, Aktolun C, Chiti A, Frangos S, Luca G, Hoffmann M, et al. Why the European Association of Nuclear Medicine has declined to endorse the 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer. Eur J Nucl Med Mol Imaging. 2016;43:1001–5. [DOI] [PubMed] [Google Scholar]
- 46.Filetti S, Durante C, Hartl D, Lebboulleux S, Locati LD, Newbold K, et al. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30:1856–83. [DOI] [PubMed] [Google Scholar]
- 47.Sywak M, Comford L, Roach P, Stalberg P, Sidhu S, Delbridge L. Routine ipsilateral level VI lymphadenectomy reduces postoperative thyroglobulin levels in papillary thyroid cancer. Surgery. 2006;140:1000–5. [DOI] [PubMed] [Google Scholar]
- 48.Hartl DM, Mamelle E, Borget I, Leboulleux S, Mirghani H, Schlumberger M. Influence of prophylactic neck dissection on rate of retreatment for papillary thyroid carcinoma. World J Surg. 2013;37:1951–8. [DOI] [PubMed] [Google Scholar]
- 49.∎∎.Chen L, Wu YH, Lee CH, Chen HA, Loh EW, Tam KW. Prophylactic Central Neck Dissection for Papillary Thyroid Carcinoma with Clinically Uninvolved Central Neck Lymph Nodes: A Systematic Review and Meta-analysis. World J Surg. 2018;42:2846–57. [DOI] [PubMed] [Google Scholar]; This meta-analysis reported that patients who underwent prophylactic central neck dissection had lower loco-regional recurrence but significantly higher incidence rates of RLN injury and hypocalcemia compared with patients without neck dissection.
- 50.Zhao WJ, Luo H, Zhou YM, Dai WY, Zhu JQ. Evaluating the effectiveness of prophylactic central neck dissection with total thyroidectomy for cN0 papillary thyroid carcinoma: An updated meta-analysis. Eur J Surg Oncol. 2017;43:1989–2000. [DOI] [PubMed] [Google Scholar]
- 51.Ito Y, Miyauchi A, Inoue H, Fukushima M, Kihara M, Higashiyama T, et al. An observational trial for papillary thyroid microcarcinoma in Japanese patients. World J Surg. 2010;34:28–35. [DOI] [PubMed] [Google Scholar]
- 52.Monchik JM, Simon CJ, Caragacianu DL, Thomay AA, Tsai V, Cohen J, et al. Does failure to perform prophylactic level VI node dissection leave persistent disease detectable by ultrasonography in patients with low-risk papillary carcinoma of the thyroid? Surgery. 2009;146:1182–7. [DOI] [PubMed] [Google Scholar]
- 53.Zetoune T, Keutgen X, Buitrago D, Aldailami H, Shao H, Mazumdar M, et al. Prophylactic central neck dissection and local recurrence in papillary thyroid cancer: a meta-analysis. Ann Surg Oncol. 2010;17:3287–93. [DOI] [PubMed] [Google Scholar]
- 54.Zhao W, You L, Hou X, Chen S, Ren X, Chen G, et al. The effect of prophylactic central neck dissection on locoregional recurrence in papillary thyroid cancer after total thyroidectomy: A systematic review and meta-analysis: pCND for the locoregional recurrence of papillary thyroid cancer. Ann Surg Oncol 2017;24:2189–98. [DOI] [PubMed] [Google Scholar]
- 55.Su H, Li Y. Prophylactic central neck dissection and local recurrence in papillary thyroid microcarcinoma: a meta-analysis. Braz J Otorhinolaryngol. 2019;85:237–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu H, Li Y, Mao Y. Local lymph node recurrence after central neck dissection in papillary thyroid cancers: A meta analysis. Eur Ann Otorhinolaryngol Head Neck Dis. 2019;136:481–7. [DOI] [PubMed] [Google Scholar]
- 57.Liang J, Li Z, Fang F, Yu T, Li S. Is prophylactic central neck dissection necessary for cN0 differentiated thyroid cancer patients at initial treatment? A meta-analysis of the literature. Acta Otorhinolaryngol Ital. 2017;37:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shan CX, Zhang W, Jiang DZ, Zheng XM, Liu S, Qiu M. Routine central neck dissection in differentiated thyroid carcinoma: a systematic review and meta-analysis. Laryngoscope. 2012;122:797–804. [DOI] [PubMed] [Google Scholar]
- 59.Zhu W, Zhong M, Ai Z. Systematic evaluation of prophylactic neck dissection for the treatment of papillary thyroid carcinoma. Jpn J Clin Oncol. 2013;43:883–8. [DOI] [PubMed] [Google Scholar]
- 60.Popadich A, Levin O, Lee JC, Smooke-Praw S, Ro K, Fazel M, et al. A multicenter cohort study of total thyroidectomy and routine central lymph node dissection for cN0 papillary thyroid cancer. Surgery. 2011;150:1048–57. [DOI] [PubMed] [Google Scholar]
- 61.Bergenfelz A, Jansson S, Kristoffersson A, Martensson H, Reihner E, Wallin G, et al. Complications to thyroid surgery: results as reported in a database from a multicenter audit comprising 3,660 patients. Langenbecks Arch Surg. 2008;393:667–73. [DOI] [PubMed] [Google Scholar]
- 62.Clayman GL, Agarwal G, Edeiken BS, Waguespack SG, Roberts DB, Sherman SI. Long-term outcome of comprehensive central compartment dissection in patients with recurrent/persistent papillary thyroid carcinoma. Thyroid. 2011;21:1309–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer, Cooper DS, Doherty GM, Haugen BR, Kloos RT, Lee SL, et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19:1167–1214. [DOI] [PubMed] [Google Scholar]
- 64.Bonnet S, Hartl D, Leboulleux S, Baudin E, Lumbroso JD, Ghuzlan A, et al. Prophylactic lymph node dissection for papillary thyroid cancer less than 2 cm: Implications for radioiodine treatment. J Clin Endocrinol Metab. 2009;94:1162–67. [DOI] [PubMed] [Google Scholar]
- 65.∎.Ito Y, Miyauchi A, Kudo T, Kihara M, Fukushima M, Miya A. The effectiveness of prophylactic modified neck dissection for reducing the development of lymph node recurrence of papillary thyroid carcinoma. World J Surg. 2017;41:2283–9. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study from a large single institution compared lymph node recurrence-free survival after change of practice of neck management over a twenty-year period. Abolishing routine prophylactic lateral neck dissections did not influence nodal neck recurrence.
- 66.Garau LM, Rubello D, Morganti R, Boni G, Volterrani D, Colletti PM, et al. Sentinel lymph node biopsy in small papillary thyroid cancer: A meta-analysis. Clin Nucl Med. 2019;44:107–18. [DOI] [PubMed] [Google Scholar]
- 67.Balasubramanian SP, Harrison BJ. Systematic review and meta-analysis of sentinel node biopsy in thyroid cancer. Br J Surg. 2011;98:334–44. [DOI] [PubMed] [Google Scholar]
- 68.Albers MB, Nordenström E, Wohlfahrt J, Bergenfelz A, Almquist M. Sentinel Lymph Node Biopsy in Thyroid Cancer. World J Surg. 2020;44:142–7. [DOI] [PubMed] [Google Scholar]
- 69.Roh JL, Koch WM. Role of sentinel lymph node biopsy in thyroid cancer. Expert Rev Anticancer Ther. 2010;10:1429–37. [DOI] [PubMed] [Google Scholar]
- 70.Scharpf J, Tuttle M, Wong R, Ridge D, Smith R, Hartl D, et al. Comprehensive management of recurrent thyroid cancer: An American Head and Neck Society consensus statement: AHNS consensus statement. Head Neck. 2016;38:1862–9. [DOI] [PubMed] [Google Scholar]
- 71.Van Wilgen CP, Dijkstra PU, Van der Laan BF, Plukker JT, Roodenburg JL. Shoulder and neck morbidity in quality of life after surgery for head and neck cancer. Head Neck. 2004;26:839–44. [DOI] [PubMed] [Google Scholar]
- 72.Roh JL, Park JY, Park CI. Total thyroidectomy plus neck dissection in differentiated papillary thyroid carcinoma patients: pattern of nodal metastasis, morbidity, recurrence, and postoperative levels of serum parathyroid hormone. Ann Surg. 2007;245:604–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Svenberg Lind C, Lundberg B, Hammarstedt Nordenvall L, Heiwe S, Persson JK, Hydman J. Quantification of trapezius muscle innervation during neck dissections: cervical plexus versus the spinal accessory nerve. Ann Otol Rhinol Laryngol. 2015;124:881–5. [DOI] [PubMed] [Google Scholar]
- 74.Shaha AR. Complications of neck dissection for thyroid cancer. Ann Surg Oncol. 2008;15:397–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roh JL, Kim DH, Park CI. Prospective identification of chyle leakage in patients undergoing lateral neck dissection for metastatic thyroid cancer. Ann Surg Oncol. 2008;15:424–9. [DOI] [PubMed] [Google Scholar]
- 76.Abdel-Galil K, Milton R, McCaul J. High output chyle leak after neck surgery: the role of video-assisted thoracoscopic surgery. Br J Oral Maxillofac Surg. 2009;47:478–80. [DOI] [PubMed] [Google Scholar]
- 77.Itkin M, Kucharczuk JC, Kwak A, Trerotola SO, Kaiser LR. Nonoperative thoracic duct embolization for traumatic thoracic duct leak: experience in 109 patients. J Thorac Cardiovasc Surg. 2010;139:584–90. [DOI] [PubMed] [Google Scholar]
- 78.Hartl DM, Breuskin I, Mirghani H, Berdelou A, Déandréis D, Pottier E, et al. Anatomic variability of the upper mediastinal lymph node level VII. World J Surg. 2016;40:1899–1903. [DOI] [PubMed] [Google Scholar]
- 79.Elisei R, Agate L, Viola D, Matrone A, Biagini A, Molinaro E. How to manage patients with differentiated thyroid cancer and a rising serum thyroglobulin level. Endocrinol Metab Clin North Am. 2014;43:331–44. [DOI] [PubMed] [Google Scholar]
- 80.Salvatori M, Biondi B, Rufini V. Imaging in endocrinology: 2-[18F]-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography in differentiated thyroid carcinoma: clinical indications and controversies in diagnosis and follow-up. Eur J Endocrinol. 2015;173: R115–130. [DOI] [PubMed] [Google Scholar]
- 81.Al-Saif O, Farrar WB, Bloomston M, Porter K, Ringel MD, Kloos RT. Long-term efficacy of lymph node reoperation for persistent papillary thyroid cancer. J Clin Endocrinol Metab. 2010;95:2187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Randolph GW, Dralle H, International Intraoperative Monitoring Study Group, Abdullah H, Barczynski M, Bellantone R, et al. Electrophysiologic recurrent laryngeal nerve monitoring during thyroid and parathyroid surgery: international standards guideline statement. Laryngoscope. 2011;121:S1–S16. [DOI] [PubMed] [Google Scholar]
- 83.∎∎.Schneider R, Randolph GW, Dionigi G, Wu CW, Barczynski M, Chiang FY, et al. International neural monitoring study group guideline 2018 part I: Staging bilateral thyroid surgery with monitoring loss of signal. Laryngoscope. 2018;128:S1–S17. [DOI] [PubMed] [Google Scholar]; This international guideline document outlines how adverse electrophysiologic events, especially loss of neural signal, are incorporated in surgical decision making.
- 84.Wu CW, Dionigi G, Barczynski M, Chiang FY, Dralle H, Schneider R, et al. International neuromonitoring study group guidelines 2018: Part II: Optimal recurrent laryngeal nerve management for invasive thyroid cancer-incorporation of surgical, laryngeal, and neural electrophysiologic data. Laryngoscope. 2018;128:S18–S27. [DOI] [PubMed] [Google Scholar]
- 85.Hartl DM, Chami L, Al Ghuzlan A, Leboulleux S, Baudin E, Schlumberger M, et al. Charcoal suspension tattoo localization for differentiated thyroid cancer recurrence. Ann Surg Oncol. 2009;16:2602–8. [DOI] [PubMed] [Google Scholar]
- 86.Harari A, Sippel RS, Goldstein R, Aziz S, Shen W, Gosnell J, et al. Successful localization of recurrent thyroid cancer in reoperative neck surgery using ultrasound-guided methylene blue dye injection. J Am Coll Surg. 2012;215:555–61. [DOI] [PubMed] [Google Scholar]
- 87.Hay ID, Lee RA, Davidge–Pitts C, Reading CC, Charboneau JW. Longterm outcome of ultrasound-guided percutaneous ethanol ablation of selected “recurrent” neck nodal metastases in 25 patients with TNM stages III or IVA papillary thyroid carcinoma previously treated by surgery and 131I therapy. Surgery. 2013; 154:1448–55. [DOI] [PubMed] [Google Scholar]
- 88.Lewis BD, Hay ID, Charboneau JW, McIver B, Reading CC, Goellner JR. Percutaneous ethanol injection for treatment of cervical lymph node metastases in patients with papillary thyroid carcinoma. AJR Am J Roentgenol. 2002; 178:699–704. [DOI] [PubMed] [Google Scholar]
- 89.Dupuy DE, Monchik JM, Decrea C, Pisharodi L. Radiofrequency ablation of regional recurrence from well differentiated thyroid malignancy. Surgery. 2001; 130:971–7. [DOI] [PubMed] [Google Scholar]


