Abstract
The nonobese diabetic (NOD) mouse is an animal model of human type I diabetes with a strong genetic component that maps to the major histocompatibility complex (MHC) of the genome. We have identified in NOD lymphocytes a specific proteasome defect that results from the lack of the LMP2 subunit. The pronounced proteasome defect results in defective production and activation of the transcription factor NF-κB, which plays an important role in immune and inflammatory responses as well as in preventing apoptosis induced by tumor necrosis factor alpha. The defect in proteasome function in NOD mouse splenocytes was evident from impaired NF-κB subunit p50 and p52 generation by proteolytic processing and impaired degradation of the NF-κB-inhibitory protein IκBα. An obligatory role of MHC-linked proteasome subunits in transcription factor processing and activation has been established in a spontaneous-disease model and mutant cells similarly lacking the MHC-encoded subunit. These data suggest that NOD proteasome dysfunction is due to a tissue- and developmental-stage-specific defect in expression of the MHC-linked Lmp2 gene, resulting in altered transcription factor NF-κB activity, and that this defect contributes to pathogenesis in NOD mice. These observations are consistent with the diverse symptomatology of type I diabetes and demonstrate clear sex-, tissue-, and age-specific differences in the expression of this error which parallel the initiation and disease course of insulin-dependent (type I) diabetes mellitus.
Type I diabetes is an autoimmune disease with genetic risk factors that map to the major histocompatibility complex (MHC) region of the genome. In both human type I diabetes and animal models of the disease (the nonobese diabetic [NOD] mouse and the BB rat), the insulin-producing β cells in the pancreatic islets of Langerhans are selectively destroyed by the abnormal immune responses (15, 63). The MHC region of the genome contains immune response genes that are important in T-cell education and in antigen presentation through either MHC class I or class II molecules. Two such genes, encoding the proteasome components LMP2 and LMP7, are located in this genomic region.
The proteasome is a multiprotein complex that catalyzes the ATP-dependent processing or degradation of intracellular proteins in eukaryotic cells (14, 31). Proteasome-mediated protein cleavage plays important roles in the regulation of cell growth, metabolism, and function. Cleavage of endogenous proteins by the proteasome generates small self-peptide fragments that contribute to T-cell education after presentation by MHC class I molecules. Although, in general, the proteasome exhibits minimal variability in substrate selectivity and subunit composition, gamma interferon (IFN-γ) induces the expression of the LMP2 and LMP7 subunits. Incorporation of these subunits into the proteasome alters its specificity for self-proteins in such a manner in that suitability of the generated peptides for presentation in the peptide-binding groove of molecules is increased (7, 29).
The proteasome also plays an important role in the processing and activation of the transcription factor nuclear factor κB (NF-κB). NF-κB is activated in response to various extracellular stimuli, including interleukin (IL-1), lipopolysaccharide (LPS), and tumor necrosis factor alpha (TNF-α) (3, 4, 78, 82). Activated NF-κB, in turn, has been implicated in the regulation of genes that contribute to cytokine generation, expression of cell surface adhesion epitopes, lymphocyte maturation, protection from apoptosis, and MHC class I antigen processing and presentation (5, 9, 16, 76, 80).
Active NF-κB exists predominantly as a heterodimer composed of either a p50 or p52 subunit and p65 (RelA). The p50 and p52 subunits of NF-κB are the products of processing of p105 and p100 precursors, respectively, by the proteasome (13, 19, 61, 67, 68). Additionally, cotranslational processing by the ubiquitin-proteasome pathway is an alternative mechanism for p50 generation (50). In the cytoplasm of resting cells, NF-κB associates with the inhibitory protein IκBα. Cell stimulation results in the degradation of phosphorylated IκBα by the proteasome, thereby allowing the p50-p65 or p52-p65 dimers to translocate to the nucleus and initiate the transcription of target genes. The p105, p100, and IκBα proteins are thought to typically undergo phosphorylation and ubiquitination prior to proteasome degradation (8, 51, 61). Studies of knockout mice lacking IκB kinase revealed that phosphorylation of IκBα requires the IκB kinase IKK-2 (18, 54, 64, 83). Recent reports demonstrate that Rel complexes sequestered by p105 or p100 are not rapidly mobilized to the nucleus in response to these same signals (50, 74).
Insights into the various biological functions of NF-κB have been obtained by the generation of knockout mice lacking NF-κB subunits or linked regulatory proteins in all tissues (6, 10–12, 24, 41, 44, 79, 88). For example, mice lacking the p50 subunit exhibit multifocal defects in immune responses, including defects specific to B lymphocytes. B cells derived from p50−/− mice do not proliferate in response to either CD40L (L indicates ligand) or bacterial LPS, and they exhibit differentiation defects, an altered pattern of cytokine secretion, and abnormal germ line immunoglobulin class switching (70, 72). The phenotype of the IKK-1 knockout mouse is principally a defect in epidermal skin development; the limb defect in IKK-1−/− mice is believed to be a product of this epidermal abnormality (38, 48, 75). In contrast, ablation of IKK-2 causes severe TNF-α sensitivity , as well as liver abnormalities due to apoptosis, and mimics the phenotype of p65-deficient mice (6, 37, 47, 59, 77).
Humans and animals with autoimmune diabetes exhibit symptomatology indicative of possible NF-κB and proteasome dysfunction, although there is no evidence supporting a generalized proteasome defect or implicating NF-κB dysregulation. In both affected humans and NOD mice, impaired antigen presentation by MHC class I molecules is due to a defect in the generation of self-peptides, suggestive of altered immune peptide generation or transport (21, 27, 45). Errors in lymphocyte development, often characterized by an overabundance of naive-lymphocyte subsets, are also characteristic of humans with diabetes and of rodent (BB rat and NOD mouse) models of autoimmune diabetes (15, 20, 22, 40, 66, 69, 71). Moreover, humans with type I diabetes and NOD mice appear to be defective in the production of LMP2 mRNA, possibly implying deranged immune peptide generation by the proteasome (28, 91). Humans and rodents with autoimmune diabetes also exhibit defects in cytokine gene regulation and secretion (63).
We have now investigated the activity and subunit composition of NF-κB as an indicator of general proteasome function and diverse protein processing errors in NOD mice. The activity of NF-κB in NOD mouse lymphocytes was shown to be markedly impaired because of a pronounced defect in the proteolytic processing of p50 and p52 and degradation of phosphorylated IκBα by the ubiquitin-proteasome pathway. The virtual inability of TNF-α to activate NF-κB in NOD mouse spleen cells was associated with an increased susceptibility to TNF-α-induced apoptosis. The defect in proteasome function in NOD mouse splenocytes was cellular and developmentally specific and resulted from the lack of the LMP2 subunit protein. Studies of mutant cell lines confirmed that LMP proteins are obligatory for diverse protein processing events beyond peptide presentation for class I antigen processing. We propose that proteasome dysfunction caused by a defect in the expression of the MHC-linked Lmp2 gene is an important factor predisposing NOD mice to autoimmune diabetes. Deranged immune and nonimmune protein processing by the NOD proteasome is tissue and developmental stage specific for cells and likely contributes to the diverse symptomatology that relates to disease expression. The NOD mouse represents a novel spontaneous-disease model of disrupted intracellular protein production and processing. These observations provide an explanation for the sex and age differences observed at the onset of insulin-dependent diabetes mellitus (type I diabetes).
MATERIALS AND METHODS
Preparation of nuclear and cytosolic extracts.
Nuclear and cytosolic extracts were prepared from lymphoid cells isolated from lung cells (enriched macrophages [Kupffer cells]) and spleen cells of 6-week-old BALB/c NOD and Lmp2−/− mice. Spleen cells were harvested, centrifuged for 15 min at 1,500 × g, washed in 10 ml of ice-cold phosphate-buffered saline, and collected by centrifugation for 15 min at 1,500 × g. The resulting pellets were resuspended in 4-ml volumes of solution A (10 mM HEPES-NaOH [pH 7.8], 10 mM KCl, 2 mM MgCl2, 1 mM dithiothreitol [DTT], 0.1 mM EDTA, 0.1 mM phenylmethylsulfonyl fluoride) and incubated for 15 min at 4°C. Frozen lung tissue was partially thawed, homogenized in 5 ml of solution A as described previously (30), and then incubated for 15 min at 4°C. After the addition of 250 or 320 μl of 10% (vol/vol) NP-40, the cell suspensions and homogenate were vigorously mixed, incubated for 30 min at 4°C, and centrifuged for 15 min at 1,500 × g. The resulting supernatants were saved as the cytosolic extracts (protein concentration, 35 μg/μl). The nuclear pellets were resuspended in 1.5 ml of a solution containing 50 mM HEPES-NaOH (pH 7.8), 50 mM KCl, 300 mM NaCl, 0.1 mM EDTA, 1 mM DTT, 0.1 mM phenylmethylsulfonyl fluoride, and 10% (vol/vol) glycerol; mixed for 30 min at 4°C; and centrifuged for 15 min at 1,500 × g. The resulting supernatants were saved as the nuclear extracts (protein concentration, 20 μg/μl).
Oligonucleotides and EMSA.
Double-stranded oligodeoxynucleotides were synthesized on a DNA synthesizer by the phosphoramidate method and purified with the use of an open-column cartridge (Life Technologies, Grand Island, N.Y.). The oligomers corresponded to the κB-binding motifs of human immunodeficiency virus type 1 (5′-GATCTAGGGACTTTCCGCTGGGGACTTTCCAG; κB1). The oligonucleotides were end labeled with [α-32P]dCTP and the Klenow fragment of DNA polymerase (Promega, Madison, Wis.). For electrophoretic mobility shift assays (EMSAs) of κB-binding activity, nuclear extract was incubated at 37°C for 30 min in a total volume of 10 μl containing 10 mM HEPES-NaOH (pH 7.9), 50 mM KCl, 5 mM Tris-HCl (pH 7.0), 1 mM DTT, 15 mM EDTA, 10% glycerol, 1.0 μg of poly(dI:dC), and 4 ng of 32P-labeled κB oligonucleotide. The DNA-protein complexes were resolved by electrophoresis on nondenaturing 8% polyacrylamide gels with 0.5× Tris-borate-EDTA buffer at 4°C. For competition experiments, nuclear extract was incubated for 15 min at 4°C with a 100-fold molar excess of unlabeled κB oligonucleotide before addition of the radioactive probe. Cytosolic extracts were treated with 1.2% (vol/vol) NP-40 and 0.8% (wt/vol) deoxycholate to induce dissociation of IκB from NF-κB before incubation with the 32P-labeled probe (34, 35). For supershift assays, nuclear extracts were incubated with specific antibodies for 1 h at 4°C before addition of DNA probes.
In vitro assay of p105 processing.
The processing reaction was performed as described by Fan and Maniatis (19). In brief, the pcDNA1p105 and p60Tth vectors were subjected to transcription and translation in vitro with wheat germ extract (Promega) in the presence of [35S]methionine. The 35S-labeled p105 and p60Tth proteins were immunoprecipitated with polyclonal antibodies to p50 and purified for use as substrates. Each substrate protein was incubated for 90 min at 30°C with spleen cytosolic extract (20 or 40 μg of protein) in a final volume of 25 μl in the absence or presence of 10 mM ATP (61). The proteasome inhibitor MG115 was also added to the reaction mixture where indicated. The processed proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 10% gel and visualized by autoradiography.
Cell survival assay.
Spleen cells prepared from BALB/c, NOD, and Lmp2−/− mice were cultured in RPMI 1640 medium containing 10% fetal bovine serum and exposed to mouse TNF-α (R&D Systems, Minneapolis, Minn.) for various periods of time. Embryonic macrophages were prepared from BALB/c, NOD, or Lmp2−/− 13.5-day fetal livers essentially as described elsewhere (5). Colony-formed macrophages were treated with TNF-α (10 ng/ml) for various time periods or were treated with TNF-α at various concentrations (up to 20 ng/ml) for 24 h. The number of viable cells was determined by trypan blue exclusion as described elsewhere (5).
Immunoblot analysis.
Nuclear or cytosolic extracts of spleen cells were subjected to SDS-PAGE on a 12.5% gel under nonreducing conditions. The separated proteins were transferred electrophoretically to a polyvinylidene difluoride membrane, which was then incubated for 2 h at room temperature with TBS-T (20 mM Tris-HCl [pH 7.6], 137 mM NaCl, 0.05% [vol/vol] Tween 20) containing 8% (wt/vol) bovine serum albumin. The membrane was then incubated for 12 h at 4°C with TBS-T containing the appropriate polyclonal antibodies, washed four times with TBS-T for 15 min each time at room temperature, incubated for 2 h at room temperature with TBS-T containing alkaline phosphatase-conjugated secondary antibodies, washed five times with TBS-T, and subjected to the alkaline phosphatase color reaction.
Granulocyte-macrophage colony formation culture (GM-CFC) assay.
Spleen cells (105) derived from 6-week-old BALB/c or NOD mice were mixed with 1.3% methylcellulose gel dissolved in culture medium (1× Dulbecco modified Eagle medium containing 30% fetal calf serum, 1% bovine serum albumin, 100 μM β-mercaptoethanol, and 20 ng of granulocyte-macrophage colony-stimulating factor [GM-CSF] per ml) and layered onto a bed composed of 0.53% agarose and culture medium. Spleen cells were cultured in 1.3% methylcellulose gel dissolved in culture medium containing or lacking TNF-α (10 ng/ml). Colonies were scored 3 weeks after cell plating. Each experiment was done in duplicate.
RESULTS
DNA-binding activity of NF-κB is reduced in NOD lymphoid cells.
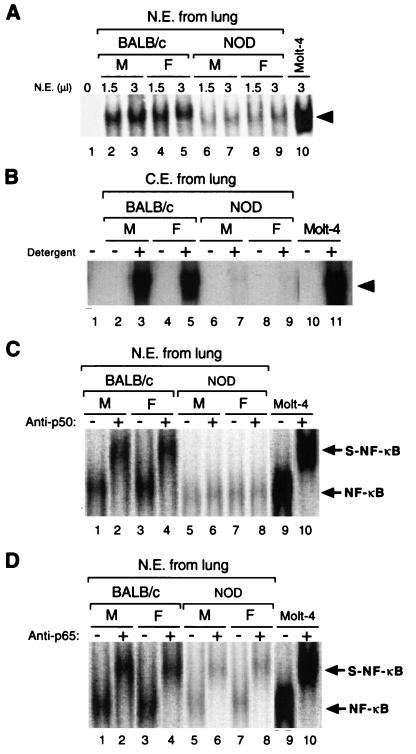
Nuclear extracts of human T-cell lymphoma Molt-4 cells as well as lymphocyte-enriched lung cell preparations from BALB/c and NOD mice were subjected to EMSA. The analysis was done with a 32P-labeled DNA probe (κB1) containing the κB binding sequence. The DNA-binding activity of NF-κB in the nuclear extracts of lymphocytes enriched from lungs from NOD mice (male or female) was markedly reduced relative to that in nuclear extracts of BALB/c or TNF-α-treated Molt-4 cells (Fig. 1A). The specificity of the DNA-binding activity in the lymphocyte nuclear extracts from both BALB/c and NOD mice was confirmed by “cold” competition assays with unlabeled wild-type κB and mutant κB oligonucleotide. NF-κB binding to the κB probe was prevented by preincubation of the nuclear extracts with a 100-fold molar excess of unlabeled wild-type κB oligonucleotide, but not by preincubation of the nuclear extracts with mutant κB oligonucleotide (data not shown). We conclude that the DNA-binding activities are due to the activity of NF-κB.
FIG. 1.
Comparison of the DNA-binding activities of NF-κB in lymphocyte-enriched lung cells of NOD mice and control BALB/c Mice. (A) NF-κB DNA-binding activity in the indicated amounts of nuclear extract (N.E.) of lymphocyte-enriched lung cells from male (M) and female (F) NOD and BALB/c mice, as well as that in nuclear extract of TNF-α-treated Molt-4 cells, was analyzed by EMSA with a 32P-labeled oligonucleotide (κB1) containing the κB binding motif. Lane 1 corresponds to a negative control in which nuclear extract was not added to the reaction mixture. The arrowhead indicates the putative NF-κB–DNA complexes. (B) NF-κB DNA-binding activity in cytosolic extract (C.E.) prepared from lung lymphocytes of male and female NOD and BALB/c mice, as well as that in cytosolic extract of Molt-4 cells, was analyzed by EMSA with 32P-labeled κB1 oligonucleotide after incubation with (+) or without (−) NP-40 and deoxycholate detergents. Lane 1 corresponds to a negative control in which extract was not added. (C and D) Supershift analysis of the κB-binding proteins in lung cell nuclear extracts from NOD and BALB/C mice. Nuclear extracts were incubated in the absence (−) or presence (+) of polyclonal antibodies to p50 (C) or p65 (D) before EMSA analysis with the κB1 oligonucleotide. Original DNA-protein complexes (NF-κB) and supershifted DNA-protein complexes (S-NF-κB) are indicated by arrows. Lanes 9 and 10 correspond to control incubations performed with nuclear extract of TNF-α-treated Molt-4 cells.
The DNA-binding activity of NF-κB in cytosolic extracts was examined by EMSA after treatment of the extracts with the detergents NP-40 and deoxycholate to induce a physical dissociation of IκB from NF-κB (30, 34, 35). The κB-binding activity in cytosolic extracts of lymphocytes of male or female NOD mouse lung origin was markedly reduced relative to that apparent in cytosolic extracts of BALB/c mouse lymphocytes or of Molt-4 cells (Fig. 1B). The specificity of the defect in NF-κB DNA-binding activity in NOD mice also was investigated by EMSA of the DNA-binding activities of the transcription factors SP1 and AP1. The DNA-binding activities of SP1 and AP1 for nuclear extracts of BALB/c mice did not differ from those for NOD mouse extracts (data not shown).
To identify the NF-κB subunits present in the DNA-protein complexes detected by EMSA, we performed supershift assays. Preincubation of nuclear extracts prepared from BALB/c mouse lymphocytes with polyclonal antibodies to p50 resulted in a shift in the DNA-protein complex to a position of slower mobility (Fig. 1C). Similar results were obtained with nuclear extracts of TNF-α-treated Molt-4 cells. However, no such shift in mobility was apparent with the DNA-protein complex formed by nuclear extracts prepared from NOD (male or female) lymphocytes of lung origin (Fig. 1C). In contrast, pretreatment of nuclear extracts from enriched lung lymphocytes of BALB/c or NOD mice or from TNF-α-treated Molt-4 cells with polyclonal antibodies to p65 reduced the mobility of the DNA-protein complex in each instance (Fig. 1D). Polyclonal antibodies to a transcription factor negative control, C/EBP, had no effect on the mobility of the DNA-protein complex formed by nuclear extracts of BALB/c or NOD mouse lymphoid cells or TNF-α-treated Molt-4 cells (data not shown). We conclude that DNA-protein complexes are composed of p65 and other proteins, but not p50, in the nuclear extracts prepared from NOD mouse (male or female) enriched lung lymphocytes.
NF-κB DNA-binding activity of NOD spleen cells is not restored by TNF-α treatment.
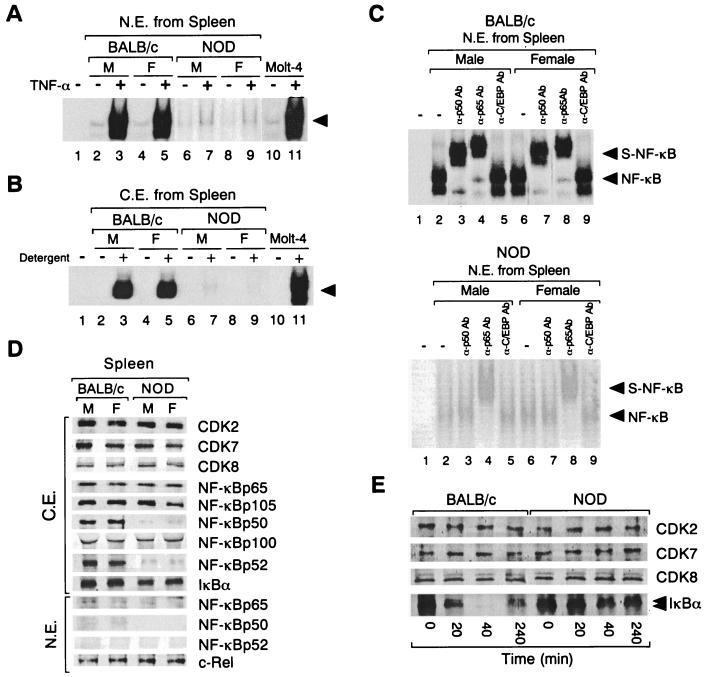
The effect of TNF-α on the DNA-binding activity of NF-κB was investigated with Molt-4 cells and with spleen cells derived from BALB/c and NOD mice. Incubation of Molt-4 cells or BALB/c mouse spleen cells with TNF-α (10 ng/ml) for 4 h resulted in a marked increase in nuclear NF-κB DNA-binding activity in the nuclear extracts as determined by EMSA (Fig. 2A). In contrast, TNF-α at a concentration of 10 ng/ml had little effect on NF-κB activity in the nuclear extract of spleen cells from NOD mice (male or female) (Fig. 2A). The specificity of the DNA-binding activity in the nuclear extracts from TNF-α-treated lymphocyte cells from both BALB/c and NOD mice was confirmed by cold competition assays with unlabeled wild-type κB and mutant κB oligonucleotide. NF-κB binding to the κB probe was prevented by preincubation of the nuclear extracts with a 100-fold molar excess of unlabeled wild-type κB oligonucleotide, but not by preincubation of the nuclear extracts with mutant κB oligonucleotide (data not shown). We concluded that the DNA-binding activities are due to the activity of NF-κB.
FIG. 2.
Comparison of basal (−) and TNF-α-induced (+) NF-κB DNA-binding activities of and expression of NF-κB subunits and degradation of IκBα by BALB/c and NOD mouse spleen cells. (A) NF-κB DNA-binding activity was examined by EMSA using the κB1 oligonucleotide and nuclear extracts (N.E.) prepared from NOD and BALB/c mouse (male [M] and female [F]) spleen cells after incubation of cells for 4 h in the absence (−) or presence (+) of TNF-α (10 ng/ml). The results of an identical experiment with Molt-4 cells are also shown (lanes 10 and 11). Lane 1 corresponds to a negative control in which no nuclear extract was added to the reaction mixture. The arrowhead indicates specific DNA-protein complexes. The X-ray film was exposed for 12 h at −70°C. (B) NF-κB DNA-binding activity in cytosolic extracts (C.E.) of male and female BALB/c and NOD mouse spleen cells (unstimulated) or of Molt-4 cells was analyzed by EMSA with the κB1 oligonucleotide after incubation of extracts with (+) or without (−) NP-40 and deoxycholate detergent. Lane 1 corresponds to a negative control in which cytosolic extract was not added to the reaction mixture. (C) Spleen cells from male or female BALB/c (upper panel) or NOD (lower panel) mice were treated with TNF-α (10 ng/ml) for 4 h. Nuclear extracts were then prepared and incubated in the absence (−) or presence (+) of polyclonal antibodies to p50 (α-p50Ab), to 65 (α-p65Ab), or to C/EBP (α-C/EBPAb) before EMSA with the κB1 oligonucleotide. Lanes 1 represent negative controls in which nuclear extract was not added to the reaction mixtures. Original DNA-protein complexes (NF-κB) and supershifted complexes (S-NF-κB) are indicated by arrowheads. The X-ray films (upper and lower panels) were exposed for 12 h at −70°C. (D) Immunoblot analysis of NF-κB subunits (p50, p52, p105, p65, and p100), c-Re1, IκBα, and CDKs in unstimulated BALB/c and NOD mouse spleen cells. Cytosolic and nuclear extracts of spleen cells from male or female BALB/c or NOD mice were subjected to immunoblot analysis with antibodies to the indicated proteins. (E) Effect of TNF-α on the abundance of IκBα in spleen cells of BALB/c and NOD mice. Spleen cells isolated from BALB/c or NOD mice were incubated with TNF-α (10 ng/ml) for the indicated periods of time, after which cytosolic extracts were prepared and subjected to immunoblot analysis with antibodies to IκBα or to CDKs.
As with cytosolic extracts prepared from lung lymphocytes, NF-κB DNA-binding activity in detergent-treated cytosolic extracts of NOD mouse spleen cells was markedly reduced compared with that observed with such extracts of BALB/c mouse spleen cells (Fig. 2B). Again, the DNA-binding activities of SP1 and AP1 for nuclear extracts of BALB/c mouse spleen cells did not differ from those for nuclear extracts of NOD mouse spleen cells (data not shown). Furthermore, in a supershift analysis performed with nuclear extracts of TNF-α-treated spleen cells, antibodies to p50 or p65 reduced the mobility of the DNA-protein complexes formed by the nuclear extracts of TNF-α-treated BALB/c spleen cells and the κB1 oligonucleotide, whereas antibodies to p65, but not those to p50, had a similar effect on the nuclear extracts of TNF-α-treated NOD spleen cells (Fig. 2C). Antibodies to C/EBP had no effect on the DNA-protein complexes formed by the nuclear extracts of either mouse strain (Fig. 2C).
Abnormal p52 proteins can be produced in lymphocytes as a result of chromosome rearrangements affecting the human NFKB2 locus (56). To investigate whether p52 binds to the κB1 oligonucleotide probe, we performed supershift assays with polyclonal antibodies to p52. These antibodies had no effect on the mobility of the DNA-protein complexes formed by the nuclear extracts of TNF-α-treated spleen cells from either BALB/c or NOD mice or by those of TNF-α-treated Molt-4 cells with labeled κB1 oligonucleotide (data not shown). The anti-p52 antibodies did reduce the mobility of the DNA-protein complex formed by the nuclear extract of TNF-α-treated Molt-4 cells and an oligonucleotide probe (H2TF1) corresponding to the κB-binding motif of the MHC class I gene enhancer (data not shown).
Reduced expression of p50 and p52 and impaired degradation of IκBα in NOD spleen cells.
The basal expression of NF-κB subunits in the cytosolic and nuclear extracts of BALB/c and NOD mouse spleen cells was examined by immunoblot analysis (Fig. 2D). The abundances of p65, the precursor protein p105, and the precursor protein p100, as well as those of IκBα and the cyclin-dependent kinases CDK8, CDK7, and CDK2 (assayed as internal controls), in cytosolic extracts of BALB/c mice did not differ markedly from those of NOD mice in unstimulated cells (Fig. 2D). However, the expression of p50 and p52 in the cytosolic extract of unstimulated spleen cells from NOD mice (male or female) was markedly reduced relative to that in cytosolic extracts of unstimulated spleen cells from BALB/c mice (Fig. 2D). Similarly, whereas the amounts of p65 and c-Rel were similar in the nuclear extracts of the two mouse strains, the amounts of p50 and p52 were again reduced in NOD mouse nuclear extract (Fig. 2D). Northern blot analysis also revealed that the abundances of both p65 and p105 mRNAs in cytosolic extracts of BALB/c mouse lymphoid cells of spleen or lung origin did not differ from those of NOD mouse extracts (data not shown).
We also investigated the dynamics of IκBα protein expression during TNF-α-induced lymphocyte activation with spleen cells from BALB/c and NOD mice. IκBα had virtually disappeared from the cytosol of BALB/c spleen cells after exposure to TNF-α for 40 min (Fig. 2E); this decrease in cytosolic IκBα was not accompanied by an increase in the amount of protein in the nucleus (data not shown). The abundance of IκBα in the cytosol of BALB/c spleen cells had begun to recover after treatment with TNF-α for 4 h (Fig. 2E). In contrast, the amount of IκBα in the cytosol of NOD mouse spleen cells was not markedly affected by TNF-α at 4 h or at later time points (Fig. 2E). The phosphorylated form of IκBα was detected as the upper band of two immunoreactive bands for TNF-α-treated spleen cells from both BALB/c and NOD mice. The basal expression of NF-κB-inducing kinase protein in cytosolic extract of spleen cells from BALB/c mice was similar to that of NOD mouse cytosolic extract (data not shown). A recent report demonstrated that Rel complexes sequestered by p105 and p100 are not rapidly mobilized to the nucleus in response to these same signals (50, 74). These results suggest that NF-κB accumulates in the cytosol when NOD lymphocytes are stimulated by TNF-α treatment.
NF-κB DNA-binding activity is reduced in Lmp2−/− lymphocytes.
The p50 subunit of NF-κB is generated by the ubiquitin-proteasome processing pathway (19, 50, 60, 61). Furthermore, proteasome inhibitors block activation of NF-κB and reduce cell survival after exposure to TNF-α (17). Published data documents down-regulation of transcriptional activation of the Lmp2 gene in NOD lymphocytes (91). To investigate whether the apparent proteasome dysfunction in NOD mice is attributable to down-regulation of LMP2, one of the β subunits of the 20S proteasome (43, 91), we examined the DNA-binding activity of NF-κB in Lmp2-negative lymphocytes derived from Lmp2−/− mouse spleen cells.
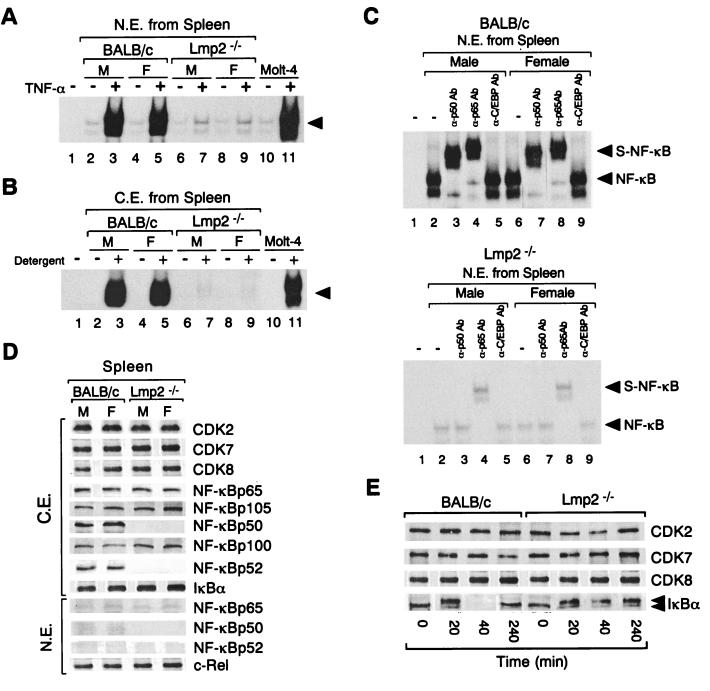
The effect of TNF-α on the DNA-binding activity of NF-κB was investigated with Molt-4 cells as well as spleen cells derived from BALB/c and Lmp2−/− mice. Incubation of Molt-4 cells or BALB/c mouse spleen cells with TNF-α (10 ng/ml) for 4 h resulted in a marked increase in NF-κB DNA-binding activity in nuclear extracts as determined by EMSA (Fig. 3A). In contrast, TNF-α at a concentration of 10 ng/ml had no significant effect on the NF-κB activity in spleen cell nuclear extract from Lmp2−/− mice (male or female) (Fig. 3A). The specificity of the DNA-binding activity in the nuclear extracts of TNF-α-treated lymphocyte cells from both BALB/c and Lmp2−/− mice was confirmed by cold competition assays with unlabeled wild-type κB and mutant κB oligonucleotide. NF-κB binding to the κB probe was prevented by preincubation of the nuclear extracts with a 100-fold molar excess of unlabeled wild-type κB oligonucleotide, but not by preincubation of the extracts with mutant κB oligonucleotide (data not shown). We concluded that the DNA-binding activities are due to the activity of NF-κB.
FIG. 3.
Comparison of TNF-α-induced NF-κB DNA-binding activities of and expression of NF-κB subunits and degradation of IκBα by BALB/c and Lmp2−/− mouse spleen cells. (A) NF-κB DNA-binding activity was examined by EMSA using the κB1 oligonucleotide and nuclear extracts (N.E.) prepared from Lmp2−/− and BALB/c mouse (male [M] and female [F]) spleen cells after incubation of cells for 4 h in the absence (−) or presence (+) of TNF-α (10 ng/ml). The results of an identical experiment with Molt-4 cells are also shown (lanes 10 and 11). Lane 1 corresponds to a negative control in which no nuclear extract was added to the reaction mixture. The arrowhead indicates specific DNA-protein complexes. (B) NF-κB DNA-binding activity in cytosolic extracts (C.E.) of male or female BALB/c and Lmp2−/− mouse spleen cells (unstimulated) or of Molt-4 cells was analyzed by EMSA with the κB1 oligonucleotide after incubation of extracts with (+) or without (−) NP-40 and deoxycholate detergent. Lane 1 corresponds to a negative control in which cytosolic extract was not added to the reaction mixture. (C) Spleen cells from male or female BALB/c (upper panel) or Lmp2−/− (lower panel) mice were treated with TNF-α (10 ng/ml) for 4 h. Nuclear extracts were then prepared and incubated in the absence (−) or presence (+) of polyclonal antibodies to p50 (α-p50Ab), to p65 (α-p65Ab), or to C/EBP (α-C/EBPAb) before EMSA with the κB1 oligonucleotide. Lanes 1 represent negative controls in which nuclear extract was not added to the reaction mixtures. Original DNA-protein complexes (NF-κB) and supershifted complexes (S-NF-κB) are indicated by arrowheads. (D) Immunoblot analysis of NF-κB subunits (p50, p52, p105, p65, and p100), c-Rel, IκBα, and CDKs in unstimulated BALB/c and NOD mice spleen cells. Cytosolic and nuclear extracts of spleen cells from male or female BALB/c or Lmp2−/− mice were subjected to immunoblot analysis with antibodies to the indicated proteins. (E) Effect of TNF-α on the abundance of IκBα in spleen cells of BALB/c and Lmp2−/− mice. Spleen cells isolated from BALB/c and Lmp2−/− mice were incubated with TNF-α (10 ng/ml) for the indicated periods of time, after which cytosolic extracts were prepared and subjected to immunoblot analysis with antibodies to IκBα or to CDKs.
The cytosolic NF-κB–IκB complexes in Lmp2−/− lymphocytes were similarly tested by EMSA. NF-κB DNA-binding activity in detergent-treated cytosolic extracts of Lmp2−/− mouse spleen cells was markedly reduced compared with that observed for cytosolic extracts of BALB/c mouse spleen cells (Fig. 3B). Again, the DNA-binding activities of SP1 and AP1 in nuclear extracts of BALB/c and Lmp2−/− mouse spleen cells did not differ (data not shown). Furthermore, in a supershift analysis performed with nuclear extract of TNF-α-treated spleen cells, antibodies to p50 or p65 reduced the mobility of the DNA-protein complexes formed by the nuclear extracts of TNF-α-treated BALB/c spleen cells and the κB1 oligonucleotide. In marked contrast, antibodies to p65, but not those to p50, had an effect on the nuclear extracts of TNF-α-treated Lmp2−/− spleen cells (Fig. 3C). Antibodies to C/EBP had no effect on the DNA-protein complexes formed by the nuclear extract of either mouse strain (Fig. 3C).
To investigate whether p52 binds to the κB1 oligonucleotide probe, we performed supershift assays with polyclonal antibodies to p52. These antibodies had no effect on the mobility of the DNA-protein complexes formed by nuclear extracts of TNF-α-treated spleen cells from either BALB/c or Lmp2−/− mice or by those of TNF-α-treated Molt-4 cells and the κB1 oligonucleotide probe (data not shown). The anti-p52 antibodies did reduce the mobility of the DNA-protein complex formed by the nuclear extract of TNF-α-treated Molt-4 cells and an oligonucleotide probe (H2TF1) corresponding to the κB-binding motif of the MHC class I gene enhancer (data not shown).
The basal expression of NF-κB subunits in the cytosolic and nuclear extracts of BALB/c and Lmp2−/− mouse spleen cells was examined by immunoblot analysis (Fig. 3D). The abundances of p65, the precursor protein p105, and the precursor protein p100, as well as those of IκBα and the cyclin-dependent kinases (CDKs) CDK8, CDK7, and CDK2 (assayed as internal controls), in cytosolic extracts of BALB/c mice did not differ markedly from those of Lmp2−/− mouse cytosolic extracts (Fig. 3D). However, the levels of p50 and p52 in the cytosolic extracts prepared from Lmp2−/− mouse (male or female) spleen cells were markedly reduced relative to those in extracts from BALB/c mice (Fig. 3D). Similarly, whereas the amounts of p65 and c-Rel were similar in nuclear extracts from the two mouse strains, the amounts of p50 and p52 were greatly reduced in Lmp2−/− mice (Fig. 3D). Northern blot analysis also revealed that the abundances of both p65 and p105 mRNAs in cytosolic extracts of spleen cells of BALB/c mice did not differ from those of Lmp2−/− mouse cytosolic extracts (data not shown).
We also investigated the dynamics of IκBα protein expression during TNF-α-induced lymphocyte activation with spleen cells from BALB/c and Lmp2−/− mice. IκBα had virtually disappeared from the cytosol of BALB/c spleen cells after exposure to TNF-α for 40 min (Fig. 3E); this decrease in cytosolic IκBα was not accompanied by an increase in the amount of the protein in the nucleus (data not shown). The abundance of IκBα in the cytosol of BALB/c spleen cells had begun to recover by hour 4 of TNF-α treatment (Fig. 3E). In contrast, the amount of IκBα in the cytosol of Lmp2−/− mouse spleen cells was not markedly affected by TNF-α (Fig. 3E). The phosphorylated form of IκBα was detected as the upper of two immunoreactive bands for TNF-α-treated spleen cells from both BALB/c and Lmp2−/− mice. The basal expression of NF-κB-inducing kinase protein in cytosolic extract of spleen cells from BALB/c mice was similar to that for NOD mouse extract (data not shown).
Impaired proteasomal processing of p105 to p50 by NOD mouse cell extract.
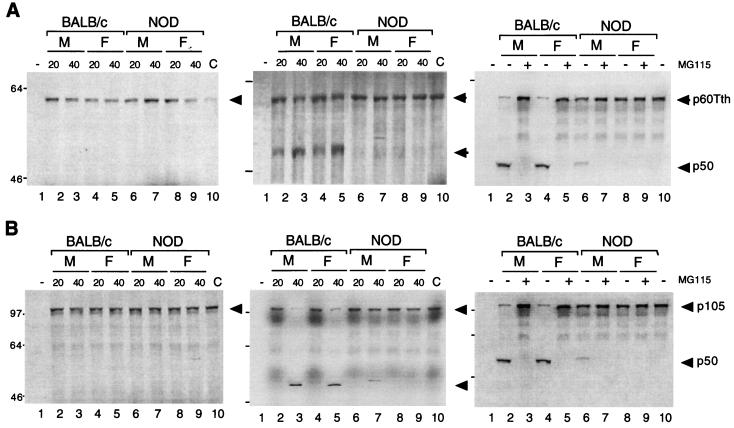
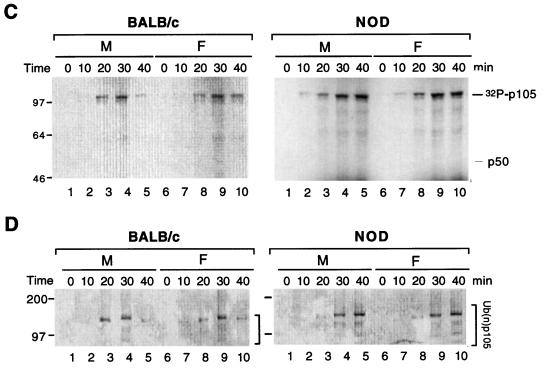
To investigate whether the reduced expression of p50 in NOD mouse spleen cells is attributable to defective p50 generation by the proteasome, we examined p50 generation by cytosolic extracts by using an in vitro assay in which 35S-labeled recombinant p105, or the truncated version, p60Tth, was used as the substrate (19, 61). Incubation of p60Tth with the cytosolic extract of BALB/c or NOD mouse spleen cells in the absence of ATP did not result in the generation of p50 (Fig. 4A, left panel). However, when p60Tth was incubated with cytosolic extract of BALB/c cells in the presence of 10 mM ATP, substantial amounts of p50 were produced (Fig. 4A, center panel). The generation of p50 has previously been shown to occur via an ATP-dependent pathway (19, 61). Similar results were obtained with p105 as a substrate, although the extent of processing was less than that observed with p60Tth (Fig. 4B, left and center panels). The extent of ATP-dependent p50 generation from both p60Tth and p105 with cyotosolic extracts of NOD mouse spleen cells was greatly reduced compared with that apparent with BALB/c cell extracts, and the defect appeared more pronounced for NOD females than for NOD males (Fig. 4A and B). A clear sex difference was observed with respect to the onset of diabetes. In NOD mice, diabetes penetrance typically exceeds 85% in females but typically is <10 to ca. 30% in males at 40 weeks of age (52). To confirm that the formation of p50 in this in vitro assay was mediated by the ubiquitin-proteasome pathway, we examined the effect of MG115, a potent inhibitor of the chymotryptic site on the 20S proteasome particle, which has previously been shown to reduce the degradation of ubiquitin-conjugated proteins in cell extracts and, at a concentration of 50 μM, to prevent generation of p50 from p105 (61). In the present study, the processing of p105 and p60Tth was also completely inhibited by MG115 at a concentration of 50 μM (Fig. 4A and B, right panels).
FIG. 4.
Proteolysis of p105 mediated by cytosolic extracts of BALB/c and NOD mice in vitro. (A and B) Purified 35S-labeled recombinant p60Tth (A) or p105 (B) was incubated in a reaction mixture containing cytosolic extract of male (M) or female (F) BALB/c or NOD mouse spleen cell (20 or 40 μg of protein in left and center panels and 40 μg of protein in right panel) in the absence (left panels) and presence (center and right panels) of 10 mM ATP. Incubations in the right panels were also performed in the absence (−) and presence (+) of 50 μM MG115. Lanes 1 correspond to reaction mixtures without substrate; lanes 10 correspond to negative controls without added extract. Incubations were at 30°C for 90 min, after which SDS-PAGE and autoradiography were performed. (C) Recombinant p105 was incubated for various periods of time at 30°C in a reaction mixture containing [γ-32P]ATP cytosolic extract (40 μg of protein) of spleen cells from male or female BALB/c or NOD mice, after which p105 was immunoprecipitated with antibodies to p50 and subjected to SDS-PAGE and autoradiography. The positions of phosphorylated p105 and unphosphorylated p50 are indicated. (D) Recombinant p105 was incubated for various time periods at 30°C in a reaction mixture containing cytosolic extract (40 μg of protein) of spleen cells from male or female BALB/c or NOD mice, after which complexes were cross-linked with glutaraldehyde, immunoprecipitated with antibodies to p50, and detected by immunoblot analysis with antibodies to ubiquitin. The positions of ubiquitinated p105 [Ub(n)-p105] and of molecular size standards (in kilodaltons) are indicated. (E) Immunoblot analysis of proteasome subunits in BALB/c and NOD mice. Cytosolic extract of spleen cells from male or female BALB/c or NOD mice was subjected to immunoblot analysis with 20S proteasome subunit antibodies and control CDK and TAFII250 antibodies.
Phosphorylation of a PEST-rich domain downstream of the ankyrin repeats of p105 is induced by exposure of cells to TNF-α (8, 51, 55). We therefore examined the phosphorylation status of recombinant p105 after incubation with [γ-32P]ATP and cytosolic extracts of spleen cells from BALB/c or NOD mice. The phosphorylation of p105 by cytosolic extracts of BALB/c spleen cells reached a maximum at 30 min and thereafter decreased, presumably because the ubiquitin-proteasome pathway degraded the phosphorylated protein (Fig. 4C, left panel). In contrast, the phosphorylation of p105 by cytosolic extracts of spleen cells from NOD mice (male and female) continued to increase for up to 40 min, presumably because the phosphorylated protein did not undergo proteolysis (Fig. 4C, right panel). Thus, the activity of the p105 kinase in cytosolic extracts of NOD mouse spleen cells appears to be normal.
Ubiquitination of the ankyrin repeats of p105 is also observed and may be required for proteolytic processing of this protein (58, 60, 61). We therefore examine the ubiquitination of recombinant p105 after incubation with cytosolic extracts of BALB/c and NOD mouse spleen cells. Cross-linking of ubiquitin-p105 complexes with glutaraldehyde, followed by their immunoprecipitation with antibodies to p50 and immunoblot analysis with antibodies to ubiquitin, revealed a temporal pattern for ubiquitination similar to that for phosphorylation of p105 (Fig. 4D). Whereas the ubiquitination of p105 by cytosolic extracts of BALB/c cells reached a maximum at 30 min and thereafter decreased, that mediated by extracts of NOD mouse (male or female) cells continued to increase for up to 40 min (Fig. 4D). Thus, ubiquitination activity appeared not to be down-regulated in cytosolic extracts of NOD mouse spleen cells. Overall, these data localize the defect in p105 processing in NOD mouse cells to the proteasome function.
We also examined the proteasomal processing of recombinant p105 by cytosolic extracts of lymphocytes from Lmp2−/− mouse spleens. Lmp2−/− lymphocytes did not catalyze the processing of p105 to p50 in an ATP-dependent pathway or in an MG115-sensitive manner (data not shown). Immunoblot analysis of cytosolic extracts of the Lmp2−/− lymphocytes confirmed that Lmp2−/− lymphocytes lacked LMP2. However, the expression of the 20S proteasome subunits HC9 and LMP10 did not differ among BALB/c mice (data not shown).
NOD spleen cells lacks the LMP2 proteasome subunit.
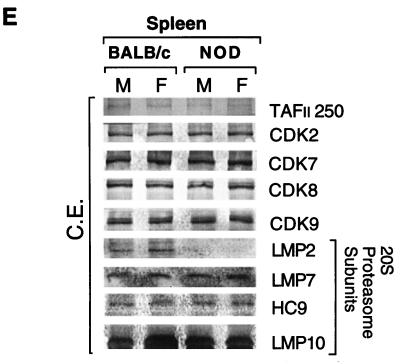
Immunoblot analysis revealed the presence of the LMP2 proteasome subunit in cytosolic extracts of spleen cells derived from BALB/c mice, but not in extracts of NOD spleen cells (Fig. 4E). LMP2 protein was virtually undetectable in the NOD spleen cells. Basal expression of the proteasome subunits LMP7, LMP10, and HC9 in cytosolic extract of spleen cells from BALB/c mice was similar to that in NOD mouse extract (Fig. 4E). The C9 antibody recognizes most precursor proteasomes and mature proteasomes. Immunoblot analysis of whole-cell lysates of mouse embryonic fibroblasts (MEFs) derived from BALB/c and NOD 13.5-day embryos revealed similar amounts of NF-κB p65, p50, p52, p100, and p105 as well as of the proteasome subunits LMP2, LMP7, LMP10, and HC9 (data not shown). The tissue specificity of the MHC-encoded LMP2 protein defect was further established. Freshly isolated pancreatic islets of Langerhans from NOD mice and BALB/c mice had normal levels of LMP2 protein, evidence that the proteasome error is restricted to the lymphoid lineage (data not shown).
NOD mouse spleen cells selectively show increased sensitivity to TNF-α-induced apoptosis.
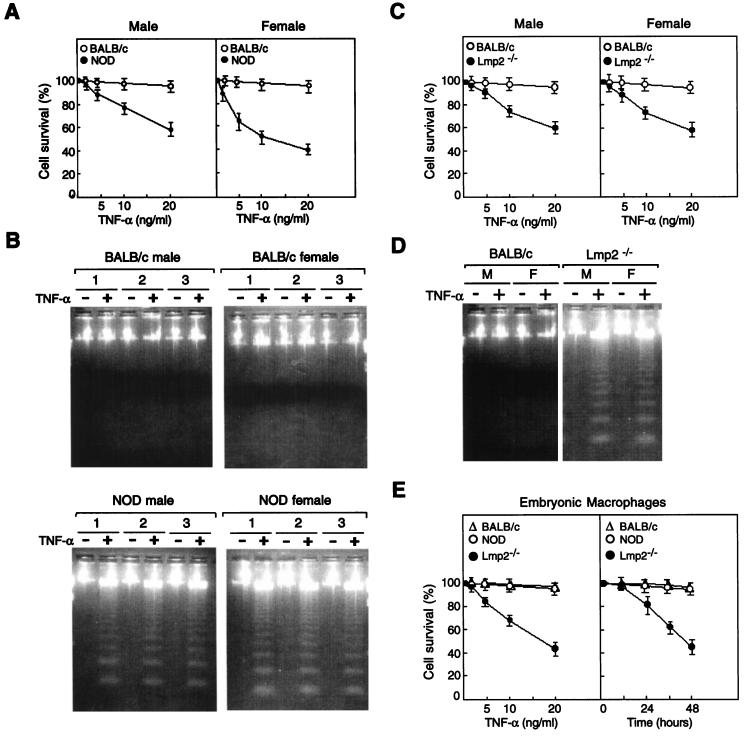
The activation of NF-κB via the ubiquitin-proteasome pathway appears to protect cells from TNF-α-induced cell death (5, 80, 87, 90). Furthermore, inhibition of the nuclear translocation of NF-κB enhances the apoptotic effect of TNF-α. We therefore investigated the effect of TNF-α treatment on the viability of spleen cells derived from NOD mice, in which we have shown impairment of TNF-α-induced NF-κB activation. Whereas incubation of BALB/c spleen cells with various concentrations of TNF-α for 24 h had virtually no effect on cell survival, TNF-α induced a dose-dependent decrease in the survival of spleen cells from male or female NOD mice (Fig. 5A). Similarly, whereas incubation of BALB/c spleen cells with TNF-α at 10 ng/ml for up to 48 h had no effect on cell viability, the survival of NOD mouse spleen cells was already markedly reduced after incubation with TNF-α (10 ng/ml) for only 12 h (data not shown). The toxic effect of TNF-α on NOD mouse spleen cells appeared more pronounced for female than for male animals. Treatment of Lmp2−/− lymphocytes with TNF-α resulted in marked cell death (Fig. 5C). Thus, it is likely that the toxicity of TNF-α to NOD spleen cells is attributable to the defect in activation of the proteasome and NF-κB in these cells. Analysis of DNA fragmentation by agarose gel electrophoresis confirmed that TNF-α induced a pattern of internucleosomal fragmentation characteristic of apoptosis in NOD and Lmp2−/− mouse spleen cells but not in BALB/c spleen cells (Fig. 5B and D). Furthermore, TNF-α also mildly induced a dose- and time-dependent decrease in the viability of spleen cells derived from 7-day-old NOD mice but had no such effect on spleen cells from 7-day-old BALB/c mice (data not shown). In contrast, and as shown in Fig. 5E, TNF-α had no effect on the viability of cultured macrophages derived from BALB/c or NOD 13.5-day-embryo livers; TNF-α induced a dose-and time-dependent decrease in the viability of cultured macrophages derived from Lmp2−/− 13.5-day-embryo livers (Fig. 5E). Also, TNF-α had no effect on the viability of cultures of either NOD or BALB/c MEFs, whereas treatment of Lmp2−/− MEFs with TNF-α resulted in prominent cell death (data not shown). Disruption of the p65 subunit of NF-κB in knockout mice is associated with marked problems with liver development. Hematoxylin-eosin-stained liver sections from 6-week-old NOD mice showed normal liver development relative to that of BALB/c mice (data not shown).
FIG. 5.
Effects of TNF-α on survival of spleen cells derived from BALB/c, NOD, and Lmp2−/− mice. (A) Spleen cells from male or female BALB/c or NOD mice were incubated with various concentrations of TNF-α for 24 h, after which cell viability was assessed by the trypan blue exclusion method. Data are means ± standard deviations (SD) of four replicates from a representative experiment and are expressed as percentages of the survival values for the corresponding non-TNF-α-exposed cells. (B) Spleen cells from three different male or female BALB/c or NOD mice were incubated for 24 h with (+) or without (−) TNF-α (10 ng/ml), after which DNA fragmentation was analyzed by agarose gel electrophoresis and ethidium bromide staining. (C) Spleen cells from male or female BALB/c or Lmp2−/− mice were incubated with various concentrations of TNF-α for 24 h, after which cell viability was assessed by the trypan blue exclusion method. Data are means ± SD of four replicates from a representative experiment and are expressed as percentages of the survival values for the corresponding cells not exposed to TNF-α. (D) Spleen cells from three different male (M) or female (F) BALB/c or Lmp2−/− mice were incubated for 24 h with or without TNF-α (10 ng/ml), after which DNA fragmentation was analyzed by agarose gel electrophoresis and ethidium bromide staining. (E) Embryonic macrophages obtained from BALB/c, NOD, or Lmp2−/− 13.5-day fetal livers were treated with various concentrations of TNF-α for 24 h or were treated with TNF-α (10 ng/ml) for various time periods, after which cell viability was assessed by the trypan blue exclusion method. Data are means ± SD of four replicates from a representative experiment and are expressed as percentages of the survival values for the corresponding cells not exposed to TNF-α.
We have thus shown that the phosphorylation and ubiquitination of p105 in NOD mouse spleen cells appear normal but that the proteolytic processing of p105 to p50 by the proteasome is impaired. Furthermore, lymphocytes from Lmp2−/− mice are more sensitive to apoptosis in response to TNF-α due to the lack of sufficient NF-κB activity (Fig. 3 and 5C and D). Thus, the defect in proteasome function in these cells is associated with impaired TNF-α-induced NF-κB activation and increased susceptibility to TNF-α-induced apoptosis.
Immature granulocyte-macrophage colony formation and TNF-α-induced apoptosis in NOD spleen cells.
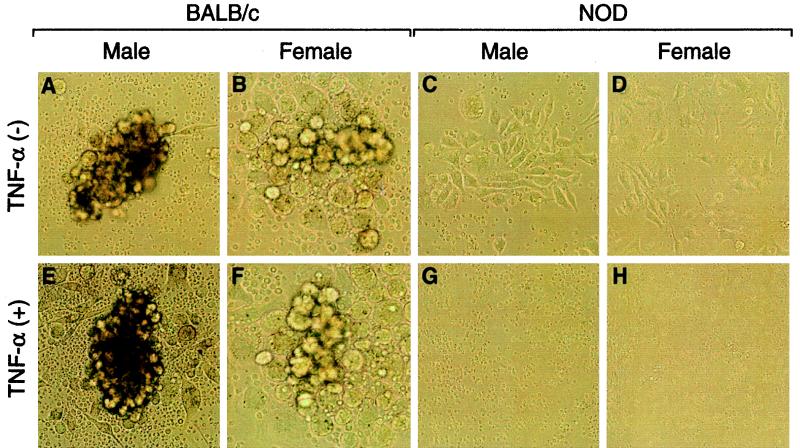
NF-κB has a known role in lymphocyte/monocyte maturation as well as in protection from TNF-α-induced apoptosis. To specifically address both of these issues, we examined the development of the granulocyte-macrophage cell lineage of 6-week-old NOD mice and the ability of this cell population to resist TNF-α-induced cell death. GM-CFCs were established. The GM-CFC assay revealed the failure of NOD-derived spleen cells to adequately form clusters when stimulated with GM-CSF (Fig. 6C and D). In marked contrast, mature GM-CFCs were prominently observed with spleen cells from BALB/c male or female mice (Fig. 6A to D). Treatment of BALB/c GM-CFCs with TNF-α had no effect on cell viability or colony development; however, TNF-α treatment dramatically induced NOD cell death (Fig. 6E to H). To verify the specificity of TNF-α toxicity for the granulocyte-macrophage lineage cells, CFUs of erythrocytes were similarly established with spleen cells derived from 6-week-old BALB/c and NOD mice. In contrast to the granulocyte-macrophage cell lineage, erythrocyte colony formation was normal in erythropoietin-treated cultures of either BALB/c or NOD spleen cells (data not shown). Additionally, TNF-α treatment had no effect on establishment of CFUs of erythrocytes for either BALB/c or NOD spleen cells, in which NF-κB plays an established role in erythropoiesis (data not shown). These research results show the lack of Lmp2 expression as well as the lack of NF-κB activation in granulocytes and macrophages derived from NOD mice at 6 weeks of age. Furthermore, TNF-α mildly induced a dose- and time-dependent decrease in the viability of spleen cells derived from 7-day-old NOD mice, whereas TNF-α had no significant effect on the viability of spleen cells derived from 7-day-old BALB/c mice (data not shown). In contrast, other age-matched cell lineages such as erythrocytes appear to have functional NF-κB-afforded protection and intact cellular development. Since TNF-α had no effect on the viability of cultured macrophages derived from BALB/c or NOD 13.5-day-embryo livers (Fig. 5E), the deranged biological function of the proteasome and processing of proteins may be dependent on the cell type, i.e., macrophages, and is furthermore developmentally regulated.
FIG. 6.
Immature granulocyte-macrophage colony formation and TNF-α-induced apoptosis in NOD spleen cells. Spleen cells (105) derived from 6-week-old BALB/c (A, B, E, and F) and NOD (C, D, G, and H) mice were mixed with 1.3% methylcellulose gel dissolved in culture medium and layered onto a bed composed of 0.53% agarose and culture medium. Colonies were scored 3 weeks after cell plating. Spleen cells were cultured in 1.3% methylcellulose gels containing (+) (lower panels) or not containing (−) (upper panels) TNF-α (10 ng/ml). Each experiment was done in duplicate.
DISCUSSION
In this study, we have identified a marked proteasome defect in NOD mice. Spleen cells, specifically granulocytes and macrophages, of these animals lack the LMP2 proteasome subunit, with the consequence that degradation of intracellular proteins is impaired. Generalized proteasome processing defects can now be attributed to the selective deletion of specific MHC-encoded subunits.
The defect in proteasome function in NOD mouse splenocytes was evident from the impaired NF-κB subunit p50 and p52 generation by proteolytic processing as well as from the lack of degradation of phosphorylated IκBα (Fig. 2 and 4). The increased sensitivity of NOD mouse spleen cells to TNF-α-induced apoptosis was one consequence of the failure of TNF-α to activate NF-κB in these cells (Fig. 5). The role of LMP2 in NF-κB activation was confirmed by two approaches. First, the use of the Lmp2−/− splenocytes, similarly lacking the MHC-encoded proteasome subunit, demonstrated the obligatory role of LMP2 in generalized proteasome-mediated NF-κB activation (Fig. 3). Second, only NOD tissues lacking LMP2 protein exhibited impaired NF-κB activation and no protection from TNF-α-induced apoptosis. Our findings suggest that the defect in LMP2 protein production in NOD mice is both developmental (age) and tissue specific. Macrophages and fibroblasts from 13.5-day-old NOD mouse embryos had normal levels of LMP2 protein and intact resistance to TNF-α exposure (Fig. 5E). In contrast, 6- to 8-week old adult NOD mouse splenocytes, granulocytes-macrophages, and enriched lung Kupffer cells lacked LMP2 protein and/or produced dysfunctional NF-κB, with TNF-α-induced cell death evident (Fig. 1, 2, 4E, 5A, and 6). Adult NOD islet cells, erythrocytes, and liver cells properly expressed the LMP2 protein. Dysfunction of a gene in the MHC region thus virtually abolishes the activity of a transcription factor that plays important roles in immune and nonimmune functions. Significant proteasome dysfunction occurs only in select tissues. The NOD mouse is a newly defined model of developmental-stage- and tissue-specific mosaic defects of discordant MHC gene expression.
The ubiquitin-proteasome pathway plays an essential role in a number of key biological processes, including cell cycle progression, transcription, and signal transduction (53). Degradation by the ubiquitin-proteasome pathway generates peptides for presentation by MHC class I antigens and activates or inactivates transcription factors, proteins involved in biological functions. In general, proteasome subunits of eukaryotic cells differ minimally. However, IFN-γ increases expression of the LMP2 and LMP7 proteins, both LMP2 and LMP7 are encoded by genes located in the MHC region of the genome, and most cells express basal amounts of both of these proteins in the absence of interferon induction (26, 32, 36, 81). Prior to this report, proteasome expression of LMP2 and LMP7 was viewed as an immune function that promoted only the generation of endogenous peptides compatible with the peptide-binding clefts of MHC class I molecules (1, 7). The new data suggests that MHC-encoded proteasome subunits play an obligatory role in generalized proteasome function, including NF-κB processing.
Our findings support immune and nonimmune proteasome processing errors in the NOD mouse and a role for MHC-linked proteasome subunits in altered cleavage patterns. If deranged proteasome processing represents a central step linked to murine disease, similar errors should be uncovered in autoimmune patients. Indirect evidence suggests that human autoimmune disease may be associated with protein processing errors controlled by the MHC. Cytosolic extracts of lymphocytes from individuals with type I diabetes exhibit altered proteasome cleavage of test substrates, resulting in the generation of peptides poorly suited for assembly with MHC class I molecules (28). Furthermore, humans with diverse autoimmune diseases, including type I diabetes, exhibit decreased expression of peptide-loaded MHC class I molecules on the surface of lymphocytes, suggesting the existence of altered peptide delivery or proteasome-generated proteins for immune functions (21, 27, 46). Intracellular immune peptide processing defects associated with human autoimmune diseases map to the MHC region of the genome (28), suggesting the existence of a similar MHC-mediated defect in humans. Furthermore, random human peripheral blood lymphocytes from genetically diverse type I diabetes patients uniformly demonstrate decreased survival when exposed to TNF-α (33a). The impaired TNF-α signaling pathway with MHC-linked proteasome defects may play similar roles in human and murine autoimmune diseases.
The experiments described here were carefully gender controlled. This analysis is relevant to the NOD mouse because close to 100% of female mice in most colonies have diabetes by 40 weeks; less than 30% of male NOD mice have diabetes at the same time. Most human autoimmune diseases are also preferentially expressed in females. Two gender-specific proteasome/NF-κB errors were uncovered in the NOD mouse study. First, a very small amount of incompletely generated p50 protein is consistently produced in vitro by male NOD mouse proteasomes (Fig. 4A and B); female NOD mice do not generate detectable p50 protein in this assay. Second, in both dose- and time-dependent experiments, TNF-α treatment prominently increases female NOD mouse splenocyte sensitivity and apoptosis (Fig. 5A). These studies identify clear TNF-α susceptibility and NF-κB dysfunction related to gender and disease expression.
The transcription factor NF-κB requires complex processing by the proteasome for functional activity. Once activated, NF-κB protects cells from TNF-α-induced apoptosis, promotes lymphocyte maturation and antigen processing, and regulates the expression of various cytokine genes. The symptomatology characteristics of knockout mice lacking Rel family proteins or LMP2 show partial overlap with those of NOD mice (6, 10, 44, 81, 88). However, Lmp2 mice do not develop diabetes by 32 weeks of age (22a), confirming the well-established genetic requirements of multiple chromosomal regions governing disease penetrance in NOD mice and humans. Importantly, the homogeneous nature of the gene defects in all tissues in Lmp2−/− mice does not mirror the mosaic nature of developmental-stage- and tissue-specific dysregulation of the NOD proteasome cleavage errors. Significantly, discordance in developmental-stage- and tissue-specific errors in the NOD proteasome could confer target selection and disease expression. Restoration or continuous normal expression of endogenous peptide presentation by cell surface MHC class I molecules on select NOD tissues could elicit an ensuing response of the immature and improperly educated immune system. Indeed, cultured T cells from humans with type I diabetes kill syngeneic target cells from disease-free identical twins that maintain presentation of class I and peptide complexes (21). Lmp2−/− or other knockout mice with defects in the assembly of class I molecules and self-peptide destroy transplanted syngeneic tissues from control animals (25, 49, 84). NOD mouse islets express normal levels of the LMP2 protein and have intact NF-κB activation (data not shown). Lymphocyte-specific interruption of proteasome processing of endogenous peptides in the NOD mouse could permit an autologous T-cell attack of islet parenchymal cells with intact proteasome function. Based on these data, target cell loss would preferentially occur via direct attack by cytotoxic T lymphocytes in the early stages of autoimmune disease.
In contrast to cytotoxic-T-lymphocyte-mediated β-cell death in the insulin-secreting islets, the dramatic TNF-α-induced apoptosis of NOD mouse lymphocytes might logically indicate an in situ role for TNF-α in early β-cell destruction in NOD mice. For this pathogenesis to occur, NOD mouse islets, similar to NOD lymphocytes, would require matched proteasome processing defects, with resultant low levels of LMP2 protein, defective NF-κB activation, and rapid apoptosis upon TNF-α treatment, and would show diminished peptide-filled class I surface complexes. The published data do not support this hypothesis. One of the early pathogenic lesions of autoimmune diabetes in both humans and rodent models is hyperexpression of correctly assembled MHC class I molecules on the surface of β cells, a step requiring intact proteasome function (23, 33, 42, 57, 73, 85, 89). Early up-regulation of LMP2 or the TAP1 protein, a transporter protein delivering proteasome-cleaved fragments to MHC class I molecules, is similarly observed in target tissues affected by autoimmune thyroid disease or diabetes (86). Furthermore, immortalized NOD mouse islet cell lines overexpress NF-κB (73), the processing of which we have now shown to be dependent on LMP2 and which is required for resistance to apoptosis. In sum, the NOD mouse islets express LMP2 protein and do not appear to have a heightened sensitivity to TNF-α. Instead, NOD mouse islets and human autoimmunity-related tissues apparently exhibit normal or heightened proteasome-mediated peptide processing, which is likely to protect them from TNF-α-mediated apoptosis. Unfortunately, intact or heightened proteasome activity in islets could, in contrast, permit toxicity through other cytokines requiring proteasome-dependent activation steps (65). The data from humans and animals with diabetes or other autoimmune diseases suggest that discordance in the regulation of MHC-linked genes among tissues might confer target specificity by classic CTL attack or IL-1β toxicity. We propose that developmental-stage- or tissue-specific dysregulation of MHC genes is a key determinant in target selection and disease penetrance.
Macrophages and MEFs derived from 13.5-day NOD mouse embryos demonstrate normal cell growth and normal resistance to TNF-α exposure. The experiments with both cell types obtained from Lmp2−/− mice suggest that both of these NOD embryonic cell characteristics are dependent on LMP2 protein expression and normal proteasome processing of NF-κB. TNF-α also mildly induced a dose- and time-dependent decrease in the viability of spleen cells derived from 7-day-old newborn NOD mice but had no such effect on spleen cells from 7-day-old newborn BALB/c mice (data not shown). In marked contrast, 6- to 8-week-old NOD mouse lymphoid cells of splenic origin, macrophages isolated from lungs (Kupffer cells), and granulocytes-macrophages have ablated TNF-α protection and NF-κB activity and/or diminished LMP2 protein levels (Fig. 1, 2, and 6). Furthermore, consistent with the role of the proteasome, as well as that of NF-κB, in maintaining normal cell growth, the macrophage-granulocyte cell lineage from 6-week-old NOD mice failed to properly expand or exhibit normal morphology when exposed to GM-CSF (Fig. 6). In contrast, 6- to 8-week-old NOD mouse islets of Langerhans, liver, and erythrocytes appeared normal. Published data also demonstrate that NOD macrophages and type I diabetic antigen-presenting cells both exhibit an impaired ability to activate regulatory T cells in an autologous mixed-lymphocyte reaction (2, 21). In sum, the MHC-controlled proteasome defect is developmentally controlled and probably explains some essential features of autoimmune disease. First, NOD mice exhibit no signs of autoimmunity up to 3 weeks of age. At 5 weeks of age and older, insulitis starts to appear, and by 8 weeks of age, autoantibodies become detectable. The insulitis continues to increase in intensity, reaching 100% by 30 weeks of age (52). Second, for many interventions and treatments of NOD mice, age-related effects and treatment outcomes are prominent. For instance, the administration of cytokines to NOD mice older than 6 weeks has been reported to prevent the development of type I diabetes, but these same cytokines are useless for NOD mice younger than 4 weeks of age (92). Therefore, our findings indicate that both the time course of histopathology of autoreactivity and the paradoxical responses to TNF-α in NOD mice parallel the altered developmental regulation of the LMP2 gene and NF-κB activity.
NF-κB has an established role in the development and maturation of lymphoid cells. We propose that the NOD mouse’s immune system is probably intact until approximately 6 weeks of age, at which time LMP2 protein almost totally disappears and NF-κB activation is obliterated. LMP2 ablation directly interrupts the preparation of peptides for MHC class I education of CD8+ T cells, and both the NOD mouse and diabetic human exhibit partial ablation of this pathway (21). NF-κB disruption then culminates in antigen-presenting cells that poorly process class II antigens for proper CD4+ cell selection, a trait of immature cells (62). Autoreactive T cells escape from proper immune selection and are destructive to organs with intact or high levels of expression of LMP2, a situation that permits self-antigen display.
The impaired intracellular processing pathways we described for the NOD mouse are apparently not an isolated phenomenon of this spontaneous animal model, suggesting that they are of central importance in autoimmunity. The immune system of the diabetic human is similarly immature, and this phenotype tracks with disease expression (20, 22, 40, 66, 69, 71). Random human peripheral blood lymphocytes from genetically diverse type I diabetic patients uniformly demonstrate decreased survival when exposed to TNF-α (33a).
Published studies have reported the phenotypes of knockout mice lacking the NF-κB subunits or LMP2 proteasome subunits. Remarkably, the lymphocyte-specific delayed-maturation and cytokine errors of the spontaneous NOD mouse model are mirrored, in part, by the clinical phenotypes of the p50−/−, p100/p52−/−, p50−/− p52−/−, and Lmp2−/− knockout models with both homogeneous gene interruptions (Table 1). The clinical relevance of the NOD and knockout mouse phenotypes to disease course is further enforced by nearly identical cytokine and lymphocyte maturation errors in the immune systems of humans with type I diabetes (15, 20, 22, 63).
TABLE 1.
Clinical phenotypes of NOD mice and mice lacking NF-κB subunits and a proteasome
| Murine knockout model [reference(s)] | Phenotypesa | NOD symptomatology |
|---|---|---|
| NF-κB | ||
| p50−/− (70, 72) | Low IL-6 levels | − |
| High IFN-β levels | +++ | |
| Defective LPS stimulation | +++ | |
| Impaired antibody production | − | |
| Increased susceptibility to bacterial infections | +++ | |
| Normal resistance to viral infections | +++ | |
| Abnormal Ig class switching | +++ | |
| p65−/− (5, 6, 37, 59) | Embryonic lethality | − |
| Liver apoptosis | − | |
| Low GM-CSF levels | − | |
| TNF-α-induced apoptosis | +++ | |
| Inactivation of CD40 | +++ | |
| p100/p52−/− (12) | Defective immunological response by B cells | +++ |
| Abnormal spleen architecture | +++ | |
| Defective B-cell education | +++ | |
| p50−/− p52−/− (24, 39) | Inactivation of GM-CSF | − |
| Inactivation of M-CSF | − | |
| Inactivation of IL-12 | − | |
| Inactivation of IL-10 | +++ | |
| High-level expression of IL-6 | +++ | |
| High-level expression of IFN-β | +++ | |
| High-level expression of IFN-γ | +++ | |
| Defective B-cell maturation | +++ | |
| Proteasome (LMP2−/−) (81) | Defective MHC class I antigen presentation | +++ |
| Defective CD8+-T-cell education | +++ |
Abbreviations: Ig, immunoglobulin; M-CSF, macrophage colony-stimulating factor.
In conclusion, we have demonstrated marked proteasome dysfunction in lymphocytes from autoimmune-diabetes-prone NOD mice. This dysfunction results from a lack of LMP2, which is encoded by a gene located in the MHC region of the genome. Defective proteasome activation results in the inability to activate NF-κB. The proteasome error and NF-κB error are selectively expressed in lymphoid cells such as granulocytes and macrophages and at select developmental stages; this is proposed to confer target specificity in autoimmunity. Abnormal processing of intracellular proteins thus may contribute to the pathogenesis of type I diabetes.
ACKNOWLEDGMENTS
We graciously thank C. Sear and T. Maniatis for the pcDNA1p105 and p60Tth constructs. We additionally thank T. Maniatis for critical review of the manuscript and data. We thank J. J. Monaco for the proteasome antibodies. We also sincerely appreciate the generous donation of LMP2−/− breeding mice by L. Van Kaer and S. Tonegawa.
This work was supported by the Iacocca Foundation and National Institutes of Health grant RO1 DE11151 (to D.F.).
REFERENCES
- 1.Akiyama K Y, Yokota K, Kagawa S, Shimbara N, Tamura T, Akioka H, Nothwang H G, Noda C, Tanaka K, Ichihara A. cDNA cloning and interferon γ down-regulation of proteasomal subunits X and Y. Science. 1994;265:1231–1234. doi: 10.1126/science.8066462. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson M A, Leiter E H. The NOD mouse model of type 1 diabetes: as good as it gets? Nat Med. 1999;5:601–604. doi: 10.1038/9442. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin A S. The NF-κB and IκB proteins: new discoveries and insights. Annu Rev Immunol. 1996;12:141–179. [Google Scholar]
- 4.Baltimore D. NF-κB: ten years after. Cell. 1996;87:13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- 5.Beg A A, Baltimore D. An essential role for NF-κB in preventing TNF-α-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 6.Beg A A, Sha W C, Bronson R T, Ghosh S, Baltimore D. Embryonic lethality and liver degeneration in mice lacking the relA component of NF-κB. Nature. 1995;376:167–170. doi: 10.1038/376167a0. [DOI] [PubMed] [Google Scholar]
- 7.Belich M P, Glynne R J, Senger G, Sheer D, Trowsdale J. Proteasome components with reciprocal expression to that of the MHC-encoded LMP proteins. Curr Biol. 1994;4:769–776. doi: 10.1016/s0960-9822(00)00174-3. [DOI] [PubMed] [Google Scholar]
- 8.Belich M P, Salmeron A, Johnston L T, Ley S C. TPL-2 kinase regulates the proteolysis of the NF-κB inhibitory protein NF-κB1p105. Nature. 1999;397:363–368. doi: 10.1038/16946. [DOI] [PubMed] [Google Scholar]
- 9.Bohnlein E, Lowenthal J W, Siekevitz M, Franza B R, Greene W C. The same inducible nuclear protein regulates mitogen activation of both the interleukin-2 receptor-α gene and type 1 HIV. Cell. 1988;53:827–836. doi: 10.1016/0092-8674(88)90099-2. [DOI] [PubMed] [Google Scholar]
- 10.Burkly L, Hession C, Ogata L, Reilly C, Marconi L A, Olson D, Tizard R, Cate R, Lo D. Expression of RelB is required for the development of thymic medulla and dendritic cells. Nature. 1995;373:531–536. doi: 10.1038/373531a0. [DOI] [PubMed] [Google Scholar]
- 11.Bushdid P B, Brantley D M, Yull F E, Blaeuer G L, Hoffman L H, Niswander L, Kerr L D. Inhibition of NF-κB activity results in disruption of the apical ectodermal ridge and aderrant limb morphogenesis. Nature. 1998;392:615–618. doi: 10.1038/33435. [DOI] [PubMed] [Google Scholar]
- 12.Caamano J H, Rizzo C A, Durham S K, Barton D S, Raventos-Suarez C, Snapper C M, Bravo R. Nuclear factor (NF)-κB2 (p100/p52) is required for normal splenic microarchitecture and B cell-mediated immune responses. J Exp Med. 1998;187:185–196. doi: 10.1084/jem.187.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coux O, Goldberg A L. Enzymes catalyzing ubiquitination and proteolytic processing of the p105 precursor of nuclear factor κB1. J Biol Chem. 1998;273:8820–8828. doi: 10.1074/jbc.273.15.8820. [DOI] [PubMed] [Google Scholar]
- 14.Coux O, Tanaka K, Goldberg A L. Structure and functions of the 20S and 26S proteasomes. Annu Rev Biochem. 1996;65:801–847. doi: 10.1146/annurev.bi.65.070196.004101. [DOI] [PubMed] [Google Scholar]
- 15.Crisa L, Mordes J P, Rossini A A. Autoimmune diabetes mellitus in the BB rat. Diabetes Metab Rev. 1992;8:9–37. [PubMed] [Google Scholar]
- 16.Cross S L, Halden N F, Lenardo M, Leonard W J. Functionally distinct NF-κB binding sites in the immunoglobulin-κ and IL-2 receptor α chain genes. Science. 1989;244:466–468. doi: 10.1126/science.2497520. [DOI] [PubMed] [Google Scholar]
- 17.Cui H, Matusi K, Omura S, Schaver S L, Matulka R A, Sonenshein G E, Ju S T. Proteasome regulation of activation-induced T cell death. Proc Natl Acad Sci USA. 1997;94:7515–7520. doi: 10.1073/pnas.94.14.7515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.DiDonato J A, Hayakawa M, Rothwarf D M, Zandi E, Karin M. A cytokine-responsive IκB kinase that activates the transcription factor NF-κB. Nature. 1997;388:548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 19.Fan C M, Maniatis T. Generation of p50 subunit of NF-κB by processing of p105 through an ATP-dependent pathway. Nature. 1991;354:395–398. doi: 10.1038/354395a0. [DOI] [PubMed] [Google Scholar]
- 20.Faustman D, Eisenbarth G, Daley J, Breitmeyer J. Abnormal T lymphocyte subsets in type I diabetes mellitus: analysis with anti-2H4 and anti-4B4 antibodies. Diabetes. 1989;38:1462–1468. doi: 10.2337/diab.38.11.1462. [DOI] [PubMed] [Google Scholar]
- 21.Faustman D, Li X, Lin H Y, Fu Y, Eisenbarth G, Avruch J, Guo J. Linkage of faulty major histocompatibility complex class I to autoimmune diabetes. Science. 1991;254:1756–1761. doi: 10.1126/science.1763324. [DOI] [PubMed] [Google Scholar]
- 22.Faustman D L. Occult CD45 T cell developmental defect in type I diabetes. Diabetes Metab. 1993;19:446–457. [PubMed] [Google Scholar]
- 22a.Faustman, D. L. Unpublished observation.
- 23.Foulis A K. C. L. Oakley Lecture 1987. The pathogenesis of β cell destruction in type I (insulin-dependent) diabetes mellitus. J Pathol. 1987;152:141–148. doi: 10.1002/path.1711520302. [DOI] [PubMed] [Google Scholar]
- 24.Franzo G, Carlson L, Xing L, Poljak L, Shores E W, Brown K D, Leonardi A, Tran T, Boyce B F, Siebenlist U. Requirement for NF-κB in osteoclast and B-cell development. Genes Dev. 1997;11:3482–3496. doi: 10.1101/gad.11.24.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freland S, Chambers B J, Andersson M, Van Kaer L, Ljunggren H G. Rejection of allogenic and syngeneic but not MHC class I-deficient tumor grafts by MHC class I-deficient mice. J Immunol. 1998;160:572–579. [PubMed] [Google Scholar]
- 26.Fruh K, Yang Y, Arnold D, Chambers J, Wu L, Waters J B, Spies T, Peterson P A. Alternative exon usage and processing of the major histocompatibility complex-encoded proteasome subunits. J Biol Chem. 1992;267:22131–22140. [PubMed] [Google Scholar]
- 27.Fu Y, Nathan D M, Li F, Li X, Faustman D L. Defective major histocompatibility complex class I expression on lymphoid cells in autoimmunity. J Clin Investig. 1993;91:2301–2307. doi: 10.1172/JCI116459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu Y, Yan G, Shi L, Faustman D. Antigen processing and autoimmunity. Evaluation of mRNA abundance and function of HLA-linked genes. Ann NY Acad Sci. 1998;842:138–155. doi: 10.1111/j.1749-6632.1998.tb09642.x. [DOI] [PubMed] [Google Scholar]
- 29.Gaczynska M, Goldberg A L, Tanaka K, Hendil K B, Rock K L. Proteasome subunits X and Y alter peptidase activities in opposite ways to the interferon-gamma-induced subunits LMP2 and LMP7. J Biol Chem. 1996;271:17275–17280. doi: 10.1074/jbc.271.29.17275. [DOI] [PubMed] [Google Scholar]
- 30.Ghosh S, Baltimore D. Activation in vitro of NF-κB by phosphorylation of its inhibitor IκB. Nature. 1990;344:678–682. doi: 10.1038/344678a0. [DOI] [PubMed] [Google Scholar]
- 31.Goldberg A L. Functions of the proteasome: the lysis at the end of the tunnel. Science. 1995;268:522–523. doi: 10.1126/science.7725095. [DOI] [PubMed] [Google Scholar]
- 32.Griffin T A, Nandi D, Cruz M, Fehling H J, Van Kaer L, Monaco J J, Colbert R A. Immunoproteasome assembly: cooperative incorporation of interferon γ (IFN-γ)-inducible subunits. J Exp Med. 1998;187:97–104. doi: 10.1084/jem.187.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hanafusa T, Miyazaki A, Miyagawa J, Tamura S, Inada M, Yamada K, Shinji Y, Katsura H, Yamagata K, Itoh N. Examination of islets in the pancreas biopsy specimens from newly diagnosed type I (insulin-dependent) diabetic patients. Diabetologia. 1990;33:105–111. doi: 10.1007/BF00401048. [DOI] [PubMed] [Google Scholar]
- 33a.Hayashi, T., and D. Faustman. Unpublished data.
- 34.Hayashi T, Sekine T, Okamoto T. Identification of a new serine kinase that activates NF-κB by direct phosphorylation. J Biol Chem. 1993;268:26790–26795. [PubMed] [Google Scholar]
- 35.Hayashi T, Ueno Y, Okamoto T. Oxireductive regulation of nuclear factor κB. Involvement of a cellular reducing catalyst thioredoxin. J Biol Chem. 1993;268:11380–11388. [PubMed] [Google Scholar]
- 36.Hisamatsu H, Shimbara N, Saito Y, Kristensen P, Hendil K B, Fujiwara T, Takahashi E, Tanahashi N, Tamura T, Ichihara A, Tanaka K. Newly identified pair of proteasomal subunits regulated reciprocally by interferon-γ. J Exp Med. 1996;183:1807–1816. doi: 10.1084/jem.183.4.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Horwitz B H, Scott M L, Cherry S R, Bronson R T, Baltimore D. Failure of lymphopoiesis after adoptive transfer of NF-κB-deficient fetal liver cells. Immunity. 1997;6:765–772. doi: 10.1016/s1074-7613(00)80451-3. [DOI] [PubMed] [Google Scholar]
- 38.Hu Y, Baud V, Delhase M, Zhang P, Deerinck T, Ellisman M, Johnson R, Karin M. Abnormal morphogenesis but intact IKK activation in mice lacking the IKKα subunit of IκB kinase. Science. 1999;284:316–320. doi: 10.1126/science.284.5412.316. [DOI] [PubMed] [Google Scholar]
- 39.Iotsova V, Caamano J, Loy J, Yang Y, Lewin A, Bravo R. Osteopetrosis in mice lacking NF-κB1 and NF-κB2. Nat Med. 1997;3:1285–1289. doi: 10.1038/nm1197-1285. [DOI] [PubMed] [Google Scholar]
- 40.Jansen A, Van Hagen M, Drexhage H A. Defective maturation and function of antigen-presenting cells in type I diabetes. Lancet. 1995;345:491–492. doi: 10.1016/s0140-6736(95)90586-3. [DOI] [PubMed] [Google Scholar]
- 41.Kanegae Y, Tavares A T, Belmonte J C I, Verma I M. Role of Rel/NF-κB transcription factors during the outgrowth of the vertebrate limb. Nature. 1998;392:611–614. doi: 10.1038/33429. [DOI] [PubMed] [Google Scholar]
- 42.Kay T W H, Campbell I L, Oxbrow L, Harrison L C. Overexpression of class I major histocompatibility complex accompanies insulitis in the nonobese diabetic mouse and is prevented by anti-interferon-γ antibody. Diabetologia. 1991;34:779–785. doi: 10.1007/BF00408350. [DOI] [PubMed] [Google Scholar]
- 43.Kishi F, Suminami Y, Monaco J J. Genomic organization of the mouse Lmp2 gene and characteristic structure of its promoter. Gene. 1993;133:243–248. [PubMed] [Google Scholar]
- 44.Kontgen F, Grumont R J, Strasser A, Metcalf D, Li R, Tarlinton D, Gerondakis S. Mice lacking the c-rel proto-oncogene exhibit defects in lymphocyte proliferation, humoral immunity, and interleukin-2 expression. Genes Dev. 1995;9:1965–1977. doi: 10.1101/gad.9.16.1965. [DOI] [PubMed] [Google Scholar]
- 45.Li F, Guo J, Fu Y, Yan G, Faustman D. Abnormal class I assembly and peptide presentation in the diabetic NOD mouse. Proc Natl Acad Sci USA. 1994;91:11128–11132. doi: 10.1073/pnas.91.23.11128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li F, Hauser S L, Linan M J, Stein M C, Faustman D L. Reduced expression of peptide-loaded HLA class I molecules on multiple sclerosis lymphocytes. Ann Neurol. 1995;38:147–154. doi: 10.1002/ana.410380205. [DOI] [PubMed] [Google Scholar]
- 47.Li Q, Van Antwerp D, Mercurio F, Lee K F, Verma I M. Severe liver degeneration in mice lacking the IκB kinase 2 gene. Science. 1999;284:321–325. doi: 10.1126/science.284.5412.321. [DOI] [PubMed] [Google Scholar]
- 48.Li Q, Lu Q, Hwang J Y, Buscher D, Lee K F, Izpisua-Belmonte J C, Verma I M. Ikk1-deficient mice exhibit abnormal development of skin and skeleton. Genes Dev. 1999;13:1322–1328. doi: 10.1101/gad.13.10.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li X, Faustman D. Use of donor β2-microglobulin-deficient transgenic mouse liver cells for isografts, allografts, and xenografts. Transplantation. 1993;55:940–946. doi: 10.1097/00007890-199304000-00046. [DOI] [PubMed] [Google Scholar]
- 50.Lin L, DeMartino G N, Greene W C. Co-translational biogenesis of NF-κB p50 by the 26S proteasome. Cell. 1998;92:819–828. doi: 10.1016/s0092-8674(00)81409-9. [DOI] [PubMed] [Google Scholar]
- 51.MacKichan M L, Logeat F, Israel A. Phosphorylation of p105 PEST sequences via a redox-insensitive pathway up-regulates processing of p50 NF-κB. J Biol Chem. 1996;271:6084–6091. doi: 10.1074/jbc.271.11.6084. [DOI] [PubMed] [Google Scholar]
- 52.Makino S, Kunimoto K, Muraoka Y, Mizushima Y, Katagiri K, Tochino Y. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu. 1980;29:1–13. doi: 10.1538/expanim1978.29.1_1. [DOI] [PubMed] [Google Scholar]
- 53.Maniatis T. A ubiquitin ligase complex essential for the NF-κB, Wnt/Wingless, and Hedgehog signaling pathways. Genes Dev. 1999;13:505–510. doi: 10.1101/gad.13.5.505. [DOI] [PubMed] [Google Scholar]
- 54.Mercurio F, Zhu H, Murray B W, Shevchenko A, Bennett B L, Li J, Young D B, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2: cytokine-activated IκB kinases essential for NF-κB activation. Science. 1997;278:860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 55.Naumann N, Scheidereit C. Activation of NF-κB in vivo is regulated by multiple phosphorylations. EMBO J. 1994;13:4597–4607. doi: 10.1002/j.1460-2075.1994.tb06781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Neri A, Chang C C, Lombardi L, Salina M, Corrandini P, Maiolo A T, Chaganti R S, Dalla-Favera R. B cell lymphoma-associated chromosomal translocation involves candidate oncogene lyt-10, homologous to NF-κB p50. Cell. 1991;67:1075–1087. doi: 10.1016/0092-8674(91)90285-7. [DOI] [PubMed] [Google Scholar]
- 57.Ono S J, Issa-Chergui B, Colle E, Guttmann R D, Seemayer T A, Fuks A. IDDM in BB rats. Enhanced MHC class I heavy-chain gene expression in pancreatic islets. Diabetes. 1988;37:1411–1418. [PubMed] [Google Scholar]
- 58.Orian A, Whiteside S, Israel A, Stancovski I, Schwartz A L, Ciechanover A. Ubiquitin-mediated processing of NF-κB transcriptional activator precursor p105. J Biol Chem. 1995;270:21707–21714. doi: 10.1074/jbc.270.37.21707. [DOI] [PubMed] [Google Scholar]
- 59.Ouaaz F, Li M, Beg A A. A critical role for the RelA subunit of nuclear factor κB in regulation of multiple immune-response genes and in Fas-induced cell death. J Exp Med. 1999;189:999–1004. doi: 10.1084/jem.189.6.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pahl H L, Baeuerle P A. Control of gene expression by proteolysis. Curr Opin Cell Biol. 1996;8:340–347. doi: 10.1016/s0955-0674(96)80007-x. [DOI] [PubMed] [Google Scholar]
- 61.Palombella V, Rando O J, Goldberg A L, Maniatis T. The ubiquitin-proteasome pathway is required for processing the NF-κB1 precursor protein and the activation of NF-κB. Cell. 1994;78:773–785. doi: 10.1016/s0092-8674(94)90482-0. [DOI] [PubMed] [Google Scholar]
- 62.Pierre P, Turley S J, Gatti E, Hull M, Meltzer J, Mirza A, Inaba K, Steinman R M, Mellman I. Developmental regulation of MHC class II transport in mouse dendritic cells. Nature. 1999;388:787–792. doi: 10.1038/42039. [DOI] [PubMed] [Google Scholar]
- 63.Rabinovitch A, Skyler J S. Prevention of type 1 diabetes. Med Clin N Am. 1998;82:739–755. doi: 10.1016/s0025-7125(05)70022-5. [DOI] [PubMed] [Google Scholar]
- 64.Regnier C H, Song H Y, Gao X, Goeddel D V, Cao Z, Rothe M. Identification and characterization of an IκB kinase. Cell. 1997;90:373–383. doi: 10.1016/s0092-8674(00)80344-x. [DOI] [PubMed] [Google Scholar]
- 65.Reimers J I, Rasmussen A K, Karlsen A E, Bjerre U, Liang H, Morin O, Andersen H U, Mandrup-Poulsen T, Burger A G, Feldt-Rasmussen U, Nerup J. Interleukin-1 β inhibits rat thyroid cell function in vivo and in vitro by a NOD-independent mechanism and induces hypothyroidism and accelerated thyroiditis in diabetes-prone BB rats. J Endocrinol. 1996;151:147–157. doi: 10.1677/joe.0.1510147. [DOI] [PubMed] [Google Scholar]
- 66.Schatz D A, Lang F, Cantor A B, Riley W J, Maclaren N K, Sleasman J W, Barrett D J. CD5+ B lymphocytes in high-risk islet cell antibody-positive and newly diagnosed IDDM subjects. Diabetes. 1991;40:1314–1318. doi: 10.2337/diab.40.10.1314. [DOI] [PubMed] [Google Scholar]
- 67.Schmid R M, Perkins N D, Duckett C S, Andrews P C, Nabel G J. Cloning of an NF-κB subunit which stimulates HIV transcription in synergy with p65. Nature. 1991;352:733–736. doi: 10.1038/352733a0. [DOI] [PubMed] [Google Scholar]
- 68.Sears C, Olesen J, Rubin D, Finley D, Maniatis T. NF-κB p105 processing via the ubiquitin-proteasome pathway. J Biol Chem. 1998;273:1409–1419. doi: 10.1074/jbc.273.3.1409. [DOI] [PubMed] [Google Scholar]
- 69.Sempe P, Ezine S, Marvel J. Role of CD4+CD45RA+ T cells in the development of autoimmune diabetes in the nonobese (NOD) mouse. Int Immunol. 1993;5:479–489. doi: 10.1093/intimm/5.5.479. [DOI] [PubMed] [Google Scholar]
- 70.Sha W C, Liou H C, Tuomanen E I, Baltimore D. Targeted disruption of the p50 subunit of NF-κB leads to multifocal defects in immune responses. Cell. 1995;80:321–330. doi: 10.1016/0092-8674(95)90415-8. [DOI] [PubMed] [Google Scholar]
- 71.Smerdon R A, Peakman M, Hussain M J, Alviggi L, Watkins P J, Leslie D G, Vergami D. Increase in simultaneous coexpression of naïve and memory lymphocyte markers at diagnosis of IDDM. Diabetes. 1993;42:127–133. doi: 10.2337/diab.42.1.127. [DOI] [PubMed] [Google Scholar]
- 72.Snapper C M, Zelazowski P, Rosas F R, Kehry M R, Tian M, Baltimore D, Sha W C. B cells from p50/NF-κB knockout mice have selective defects in proliferation, differentiation, germ-line CH transcription, and Ig class switching. J Immunol. 1996;156:183–191. [PubMed] [Google Scholar]
- 73.Stephens L A, Thomas H E, Kay T W. Protection of NIT-1 pancreatic β-cells from immune attack by inhibition of NF-κB. J Autoimmun. 1997;10:293–298. doi: 10.1006/jaut.1997.0133. [DOI] [PubMed] [Google Scholar]
- 74.Sun S C, Ganchi P A, Beraud C, Ballard D W, Greene W C. Autoregulation of the NF-κB transactivator RelA (p65) by multiple cytoplasmic inhibitors containing ankyrin motifs. Proc Natl Acad Sci USA. 1994;91:1346–1350. doi: 10.1073/pnas.91.4.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takeda K, Takeuchi O, Tsujimura T, Itami S, Adachi O, Kawai T, Sanjo H, Yoshikawa K, Terada N, Akira S. Limb and skin abnormalities in mice lacking IKKα. Science. 1999;284:313–316. doi: 10.1126/science.284.5412.313. [DOI] [PubMed] [Google Scholar]
- 76.Tan T-H, Huang G P, Sica A, Ghosh P, Young H A, Longo D L, Rice N R. κB site-dependent activation of the interleukin-2 receptor α-chain gene promoter by human c-Rel. Mol Cell Biol. 1992;12:4067–4075. doi: 10.1128/mcb.12.9.4067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tanaka M, Fuentes M E, Yamaguchi K, Durnin M H, Dalrymple S A, Hardy K L, Goeddel D V. Embryonic lethality, liver degeneration, and impaired NF-κB activation in IKK-β-deficient mice. Immunity. 1999;10:421–429. doi: 10.1016/s1074-7613(00)80042-4. [DOI] [PubMed] [Google Scholar]
- 78.Thanos D, Maniatis T. NF-κB: a lesson in family values. Cell. 1995;80:529–532. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 79.Tickle C. Worlds in common through NF-κB. Nature. 1998;392:547–549. doi: 10.1038/33276. [DOI] [PubMed] [Google Scholar]
- 80.Van Antwerp D J, Martin S J, Kafri T, Green D R, Verma I M. Suppression of TNF-α-induced apoptosis by NF-κB. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 81.Van Kaer L, Ashton-Rickardt P G, Eichelberger M, Gaczynska M, Nagashima K, Rock K L, Goldberg A L, Doherty P C, Tonegawa S. Altered peptidase and viral-specific T cell response in LMP-2 mutant mice. Immunity. 1994;1:533–541. doi: 10.1016/1074-7613(94)90043-4. [DOI] [PubMed] [Google Scholar]
- 82.Verma I M, Stevenson J K, Schwarz D V, Van Antwerp S, Miyamoto S. Rel/NF-κB/IκB family: intimate tales of association and dissociation. Genes Dev. 1995;9:2723–2735. doi: 10.1101/gad.9.22.2723. [DOI] [PubMed] [Google Scholar]
- 83.Verma I M, Stevenson J. IκB kinase: beginning, not the end. Proc Natl Acad Sci USA. 1997;94:11758–11760. doi: 10.1073/pnas.94.22.11758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vidal-Puig A, Faustman D L. Tolerance to peripheral tissue is transient and maintained by tissue specific class I expression. Transplant Proc. 1994;26:3314–3316. [PubMed] [Google Scholar]
- 85.Vives-Pi M, Armengol M P, Alcalde L, Costa M, Somoza N, Vargas F, Jaraquemada D, Pujol-Borrell R. Expression of transporter associated with antigen processing-1 in the endocrine cells of human pancreatic islets: effects of cytokines and evidence of hyperexpression in IDDM. Diabetes. 1996;45:779–788. doi: 10.2337/diab.45.6.779. [DOI] [PubMed] [Google Scholar]
- 86.Vives-Pi M, Vargas F, James R F, Trowsdale J, Costa M, Sospedra M, Somoza N, Obiols G, Tampe R, Pujol-Borrel R. Proteasome subunits, low-molecular-mass polypeptides 2 and 7 are hyperexpressed by target cells in autoimmune thyroid disease but not in insulin-dependent diabetes mellitus: implications for autoimmunity. Tissue Antigens. 1997;50:153–163. doi: 10.1111/j.1399-0039.1997.tb02854.x. [DOI] [PubMed] [Google Scholar]
- 87.Wang C Y, Mayo M W, Baldwin A S. TNF- and cancer therapy-induced apoptosis potentiation by inhibition of NF-κB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 88.Weih F, Carrasco D, Durham S K, Barton D S, Rizzo C A, Ryseck R P, Lira S A, Bravo R. Multiorgan inflammation and hematopoietic abnormalities in mice with a targeted disruption of RelB, a member of the NF-κB/Rel family. Cell. 1995;80:331–340. doi: 10.1016/0092-8674(95)90416-6. [DOI] [PubMed] [Google Scholar]
- 89.Weringer E J, Like A A. Identification of T cell subsets and class I and class II antigen expression in islet grafts and pancreatic islets of diabetic BioBreeding/Worcester rats. Am J Pathol. 1988;132:292–303. [PMC free article] [PubMed] [Google Scholar]
- 90.Wu M, Lee H, Bellas R E, Schauer S L, Arsura M, Katz D, Fitzgerald M J, Rothstein T L, Sherr D H, Sonenshein G E. Inhibition of NF-κB/Rel induces apoptosis of murine B cells. EMBO J. 1996;15:4682–4690. [PMC free article] [PubMed] [Google Scholar]
- 91.Yan G, Fu Y, Faustman D L. Reduced expression of Tap1 and Lmp2 antigen processing genes in the nonobese diabetic (NOD) mouse due to a mutation in their shared bidirectional promoter. J Immunol. 1997;159:3068–3080. [PubMed] [Google Scholar]
- 92.Yang X D, Tisch R, Singer S M, Cao Z A, Liblau R S, Schriber R D, McDevitt H O. Effect of tumor necrosis factor alpha on insulin-dependent diabetes mellitus in NOD mice. I. The early development of autoimmunity and the diabetogenic process. J Exp Med. 1994;180:995–1004. doi: 10.1084/jem.180.3.995. [DOI] [PMC free article] [PubMed] [Google Scholar]