Abstract
Background
Multiplex polymerase chain reaction (PCR) panels allow for rapid detection or exclusion of pathogens causing meningitis and encephalitis (ME). The clinical impact of rapid multiplex PCR ME panel results on the duration of empiric antibiotic therapy is not well characterized.
Methods
We performed a retrospective prepost study at our institution that evaluated the clinical impact of a multiplex PCR ME panel among adults with suspected bacterial meningitis who received empiric antibiotic therapy and underwent lumbar puncture in the emergency department. The primary outcome was the duration of empiric antibiotic therapy.
Results
The positive pathogen detection rates were similar between pre- and post-multiplex PCR ME panel periods (17.5%, 24 of 137 vs 20.3%, 14 of 69, respectively). The median duration of empiric antibiotic therapy was significantly reduced in the post-multiplex PCR ME panel period compared with the pre-multiplex PCR ME panel period (34.7 vs 12.3 hours, P = .01). At any point in time, 46% more patients in the post-multiplex PCR ME panel period had empiric antibiotic therapy discontinued or de-escalated compared with the pre-multiplex PCR ME panel period (sex- and immunosuppressant use-adjusted hazard ratio 1.46, P = .01). The median hospital length of stay was shorter in the post-multiplex PCR ME panel period (3 vs 4 days, P = .03).
Conclusions
The implementation of the multiplex PCR ME panel for bacterial meningitis reduced the duration of empiric antibiotic therapy and possibly hospital length of stay compared with traditional microbiological testing methods.
Keywords: antibiotics, FilmArray ME panel, meningitis, multiplex PCR
In this pre-post study, implementation of a multiplex polymerase chain reaction panel testing for agents of meningitis significantly reduced the duration of empiric antibiotic therapy, time to targeted therapy, and hospital length of stay compared with traditional microbiological testing methods.
Bacterial meningitis is a potentially fatal infection of the central nervous system (CNS) that is associated with significant complications including neurologic deficits and epilepsy in the majority of survivors [1]. Rapid diagnosis and treatment are critical to reducing morbidity and mortality; however, evaluation of suspected meningitis is complex and challenging due to its nonspecific presentation, the limited diagnostic utility of the clinical exam, and the requirement of cerebrospinal fluid (CSF) testing for diagnosis, which traditionally has long turnaround times for culture results and can be affected by antibiotic therapy initiated before performing lumbar puncture [2–5].
The recent development of the FilmArray meningitis/encephalitis (ME) panel (BioFire Diagnostics, LLC, Salt Lake City, UT), which has been US Food and Drug Administration-approved for use in community-acquired CNS infections, has permitted rapid identification (within 1 hour) of 14 different viral, bacterial, and fungal agents associated with ME in CSF using a multiplex polymerase chain reaction (PCR) system [6]. These pathogens include Escherichia coli K1, Haemophilus influenzae, Listeria monocytogenes, Neisseria meningitidis, Streptococcus pneumoniae, Streptococcus agalactiae, cytomegalovirus, enterovirus, herpes simplex virus 1 and 2, human herpesvirus 6, human parechovirus, varicella zoster virus, and Cryptococcus neoformans/Cryptococcus gattii. The multiplex PCR ME panel has demonstrated high overall agreement with culture-based methods [7, 8].
Although multiple studies have evaluated its diagnostic performance and clinical implementation in diagnostic pathways, few prior studies have assessed the impact of the multiplex PCR ME panel on the duration of empiric antibiotic therapy and time to targeted therapy, both of which have implications for patient safety and antibiotic stewardship [9, 10]. The objective of our study was to evaluate the clinical impact of the multiplex PCR ME panel on the duration of empiric antibiotic therapy for suspected bacterial meningitis.
METHODS
Study Design and Setting
We performed a retrospective pre-post intervention study at New York-Presbyterian/Weill Cornell Medical Center, an 860-bed academic medical center located in New York City. Manual chart reviews were conducted for a 3-year period before and after implementation of the multiplex PCR ME panel on February 14, 2017. The pre-multiplex PCR ME panel period was from March 1, 2014 to February 1, 2017. The post-multiplex PCR ME panel period was from March 1, 2017 to February 1, 2020. At the time of implementation of the multiplex PCR ME panel, clinicians and housestaff received education regarding use and interpretation of multiplex PCR ME panel results through institutional e-mails and a conference series. Recommended antimicrobial use based on multiplex PCR ME panel results were made available in the electronic health record system.
Multiplex PCR ME panel testing was available all hours of the day including weekends. The only restrictions to ordering the multiplex PCR ME panel were testing specimens collected via indwelling medical devices and orders placed >3 days into hospitalization, which required approval by the Clinical Microbiology Laboratory. The antibiotic stewardship program team members (eg, infectious diseases pharmacists and physicians) were not directly involved in receiving results from the Clinical Microbiology Laboratory or involved in communicating positive results to primary clinical teams. All positive results were immediately reported in the electronic health record and directly communicated from the Clinical Microbiology Laboratory to the clinician as a “critical value” by phone. There were no differences in the way results were communicated in the pre- and postintervention periods.
All the study procedures were conducted according to the regulation of our local ethical committee. This study was approved by the Weill Cornell Medicine Institutional Review Board (No. 20-02021441).
Study Population
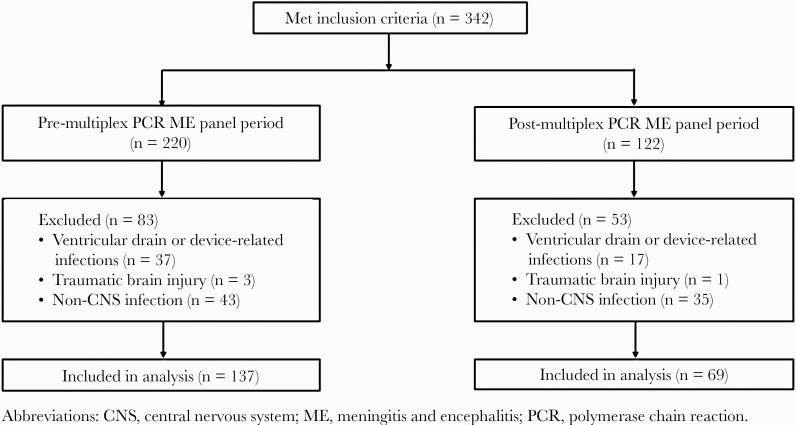
We included adults ≥18 years of age who presented to the emergency department with suspected bacterial meningitis defined by the following criteria: (1) received empiric antibiotic therapy for bacterial meningitis; (2) had lumbar puncture performed in the emergency department; and (3) underwent microbiological testing with CSF culture in the preintervention period and multiplex PCR ME panel testing plus CSF culture in the postintervention period. We excluded patients with evidence of ventricular drain or device-related infections, any history of traumatic brain injury, or documentation of a non-CNS infection at the time of data analysis upon retrospective review.
Study Outcomes and Definitions
The primary outcome was the duration of empiric antibiotic therapy in hours using drug administration times. If a patient received only a 1-time dose of 2 antibiotics (eg, vancomycin and ceftriaxone) administered at the same exact time, a duration of 0 hours was indicated. Other study outcomes included the time to targeted therapy (from the time of initiation of antibiotics), the duration of total antibiotic therapy during hospitalization, rate of hospitalization, hospital length of stay, and in-hospital mortality.
Empiric antibiotic therapy was defined as any 1 of the following regimens: (1) vancomycin plus ceftriaxone (or another third- or fourth-generation cephalosporin) with or without ampicillin; (2) vancomycin plus aztreonam and/or trimethoprim-sulfamethoxazole; (3) any other antibiotic regimen with clinician documentation of intended bacterial meningitis coverage. Targeted therapy was defined as any tailoring of antibiotic therapy based on positive microbiology results. Examples of targeted therapy might include switching to an antibiotic regimen directed towards a detected bacterial pathogen or discontinuation of antibiotic therapy in lieu of therapy directed towards a detected viral or fungal pathogen.
Data Collection
Review of clinical documentation in the electronic health record was performed by 4 clinician reviewers trained by the principal investigator (J.J.C.) using a standardized study instrument in REDCap (Research Electronic Data Capture), a secure, web-based software platform designed to support data capture for research studies [11,12]. Collected information included patient demographic information, clinical signs and symptoms of meningitis and encephalitis (fever ≥38.0°C, headache, neck stiffness, altered mental status, seizure, focal neurologic deficit), comorbid conditions, use of immunosuppressive drugs, microbiological testing, antibiotic drug administration times, hospital admission and discharge times, and vital status (alive or dead) upon discharge.
Data Analysis
The median and interquartile range (IQR) were calculated on all continuous variables. A bivariable analysis that compared baseline demographics, clinical characteristics, and study outcomes between the pre-multiplex PCR ME panel and post-multiplex PCR ME panel periods was performed using Mann-Whitney U tests, χ 2 tests, or Fisher’s exact tests, as appropriate. A P < .05 was considered statistically significant. Time-to-event analysis used the Kaplan-Meier method and log-rank statistics. Cox proportional hazards regression was used for the association between pre-/post-multiplex PCR ME panel period and the duration of empiric antibiotic therapy after adjustment for other patient characteristics. A post hoc decision was made based on the bivariable analysis to adjust the hazard ratio for sex and use of immunosuppressive drugs. There were no missing values for the analyzed baseline variables. All statistical analysis was performed using Stata version 17 software (StataCorp, College Station, TX).
RESULTS
Baseline Characteristics
The study cohort included 137 patients in the pre-multiplex PCR ME panel period and 69 patients in the post-multiplex PCR ME panel period in the final analysis (Figure 1). There were no statistically significant differences in baseline characteristics between groups (Table 1). The white blood cell count in CSF was not significantly different between the pre- and post-multiplex PCR ME panel periods (median 4 cells/mm3, IQR = 1–131 vs median 2 cells/mm3, IQR = 1–99 [P = .60], respectively).
Figure 1.
Study cohort flow diagram.
Table 1.
Baseline Characteristics for Patients With Suspected Bacterial Meningitis
| Characteristic | n (%) | ||
|---|---|---|---|
| Pre-multiplex PCR ME Panel (n = 137) |
Post-multiplex PCR ME Panel (n = 69) |
P Value | |
| Age, median (IQR), years | 42 (32–62) | 41 (32–57) | .80 |
| Female | 87 (63.5) | 35 (50.7) | .07 |
| Race | .31 | ||
| White | 62 (45.3) | 39 (56.5) | |
| Black | 19 (13.9) | 9 (13.0) | |
| Asian | 8 (5.8) | 4 (5.8) | |
| Other | 18 (13.1) | 4 (5.8) | |
| Not specified | 30 (21.9) | 10 (14.5) | |
| Comorbidities | |||
| Cancera | 11 (8.0) | 8 (11.6) | .40 |
| Coronary artery disease | 14 (10.2) | 4 (5.8) | .43 |
| Diabetes mellitus | 19 (13.9) | 7 (10.1) | .43 |
| Hypertension | 34 (24.8) | 15 (21.7) | .62 |
| Use of immunosuppressive drugs | 13 (9.5) | 12 (17.4) | .10 |
| Clinical Presentation | |||
| Fever | 75 (54.7) | 37 (53.6) | .88 |
| Headache | 84 (61.3) | 49 (71.0) | .17 |
| Neck stiffness | 46 (33.6) | 16 (23.2) | .13 |
| Altered mental status | 46 (33.6) | 24 (34.8) | .86 |
| Seizure | 10 (7.3) | 2 (2.9) | .34 |
| Focal neurologic deficit | 9 (6.6) | 6 (8.7) | .58 |
Abbreviations: ME, meningitis and encephalitis.
a Active cancer, defined by any of the following: diagnosed or receiving therapy within 6 months of presentation; recurrent or metastatic cancer.
Microbiology Testing
The positive detection rate of bacterial pathogens was 2.2% (3 of 137) by CSF culture in the pre-multiplex PCR ME panel period and 4.3% (3 of 69) by the multiplex PCR ME panel in the post-multiplex PCR ME panel period. There was no difference in the proportion of patients for whom any pathogen was detected by microbiological testing between pre- and post-multiplex PCR ME panel periods (17.5% vs 20.3%, P = .63). Among 24 patients in the pre-multiplex PCR ME panel period for whom a pathogen was identified, 3 patients had therapy targeted to bacterial pathogens (2 L monocytogenes, 1 S pneumoniae), 16 to viral pathogens (6 herpes simplex virus 2, 5 enterovirus, 4 varicella zoster virus, 1 herpes simplex virus 1), 2 to fungal pathogens (C neoformans), and 3 to spirochetes (Borrelia burgdorferi [Lyme disease] meningitis). Among 14 patients in the post-multiplex PCR ME panel period for whom a pathogen was identified, 3 patients had therapy targeted to bacterial pathogens (2 S pneumoniae, 1 H influenzae) and 11 to viral pathogens (5 enterovirus, 3 herpes simplex virus 2, 3 varicella zoster virus). The average turnaround time for the multiplex PCR ME panel was 2.6 hours. In the pre-multiplex PCR ME panel period, the average turnaround time for viral pathogen testing (performed by a viral encephalitis PCR panel on CSF samples) was 71.3 hours.
Study Outcomes
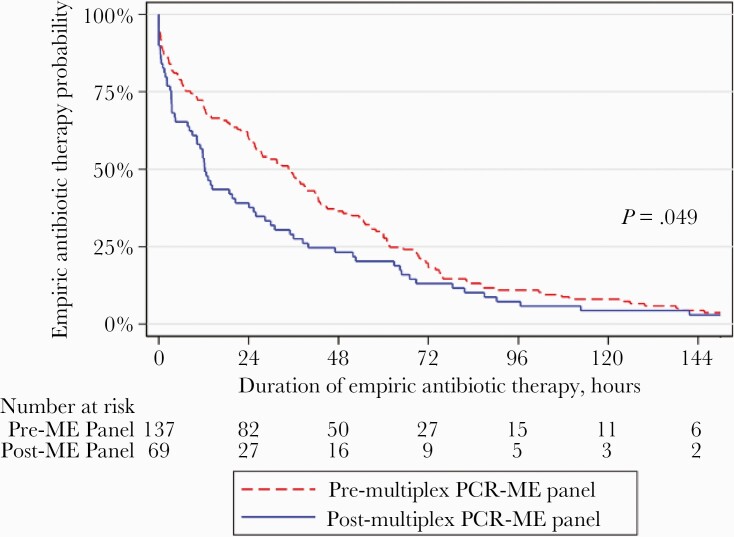
The duration of empiric antibiotic therapy significantly decreased after the implementation of the multiplex PCR ME panel (Table 2), from a median of 34.7 hours (IQR, 8.5–61.7) in the pre-multiplex PCR ME panel period to 12.3 hours (IQR, 3.3–40.0) in the post-multiplex PCR ME panel period (P = .01). At any point in time, 46% more patients in the post-multiplex PCR ME panel period had empiric antibiotic therapy discontinued or de-escalated compared with the pre-multiplex PCR ME panel period (sex- and immunosuppressant use-adjusted hazard ratio 1.46 [95% confidence interval, 1.08–1.97]; P = .01; log-rank test, P = .049) (Figure 2).
Table 2.
Antimicrobial Use and Hospitalization Outcomes
| Clinical Outcome | Pre-multiplex PCR ME Panel (n = 137) |
Post-multiplex PCR ME Panel (n = 69) |
P Value |
|---|---|---|---|
| Duration of empiric antimicrobial therapy, hours, median (IQR) | 34.7 (8.5–61.7) | 12.3 (3.3–40.0) | .01 |
| Duration of total antimicrobial therapy in hospital, hours, median (IQR) | 39.6 (10.3–86.0) | 14.3 (4.3–64.9) | .02 |
| Time to targeted therapy, hours, median (IQR) | 59.3 (36.5–74.6)a | 7.02 (0.9–12.4)b | <.001 |
| Patients hospitalized, n (%) | 126 (92.0) | 63 (91.3) | .87 |
| Hospital length of stay, days, median (IQR) | 4 (2–7) | 3 (1–5) | .03 |
| In-hospital mortality, n (%) | 3 (2.2) | 2 (2.9) | 1.00 |
Abbreviations: IQR, interquartile range; ME, meningitis and encephalitis.
aIn the pre-ME panel period, 24 of 137 (17.5%) patients had de-escalation of antibiotics to targeted therapy.
bIn the post-ME panel period, 14 of 69 (20.3%) patients had de-escalation of antibiotics to targeted therapy.
Figure 2.
Probability of empiric antibiotic therapy between pre-/post-multiplex polymerase chain reaction (PCR) meningitis and encephalitis (ME) panel periods. Kaplan-Meier analysis of the time from initiation of empiric antibiotic therapy to discontinuation or de-escalation of empiric antibiotic therapy between the pre- and post-ME panel periods. P value from log-rank test = 0.049 (n = 206). There was a significant difference in the time to discontinuation or de-escalation of empiric antibiotic therapy between the groups (sex- and immunosuppressant use-adjusted hazard ratio, 1.46 [95% confidence interval, 1.08–1.97]; P = .01).
Among patients with a pathogen detected, the time to targeted therapy was significantly reduced in the post-multiplex PCR ME panel period (median 7.0 hours; IQR, 0.9–12.4) compared with the pre-multiplex PCR ME panel period (median 59.3 hours; IQR, 36.5–74.6; P < .001). Among patients with no pathogen detected, the total duration of antibiotic therapy was significantly reduced in the post-multiplex PCR ME panel period (median 16.6 hours; IQR 4.4–65.3) compared with the pre-multiplex PCR ME panel period (median 39.6 hours; IQR, 10.3–86.0; P = .02).
Hospital length of stay was significantly reduced from a median 4 days (IQR, 2–7) to 3 days (IQR 1–5, P = .03) from before and after implementation of the multiplex PCR ME panel, respectively. The rates of hospitalization and in-hospital mortality were not significantly different between the pre- and post-multiplex PCR ME panel periods.
DISCUSSION
In this pre-post intervention study, our results show that implementation and use of the multiplex PCR ME panel for adult patients with suspected bacterial meningitis significantly reduces the duration of empiric antibiotic therapy (median 22 hours) and time to targeted therapy (median 52 hours) when compared with traditional CSF culture-based microbiology testing. To our knowledge, this is the first report of the multiplex PCR ME panel impacting the duration of empiric antibiotics and time to targeted therapy in adults with suspected bacterial meningitis compared with standard approaches utilizing CSF cultures. In addition, we found that total antibiotic duration was significantly reduced after implementation of the multiplex PCR ME panel among patients with negative ME panel results. This highlights the utility of negative rapid testing as an important approach to earlier discontinuation of antibiotics in patients whom bacterial meningitis has been effectively ruled out.
Our study builds on the mixed findings of prior studies that have assessed the impact of the multiplex PCR ME panel on the total duration of antibiotic therapy and hospital length of stay. One prior retrospective pre-post study of 97 total patients found no difference in the total duration of antibiotic therapy or hospital length of stay [9]. However, Moffa et al [10] found in a retrospective pre-post study of 160 patients hospitalized with a suspected community-acquired CNS infection that the total duration of antimicrobial therapy was significantly decreased by a median of 2 days, and hospital length of stay was significantly reduced by a mean of 2.2 days. However, in this study, antimicrobial therapy was still continued for a median of 5 days post-ME panel implementation.
In another retrospective pre-post study of 117 patients with suspected meningoencephalitis, DiDiodato and Bradbury [13] found a similar reduction in hospital length of stay of a mean of 1.5 days after implementation of the multiplex PCR ME panel; however, this study did not find a difference in duration of antimicrobial therapy. Our study adds to the literature as the largest study sample of patients evaluating the effectiveness of multiplex PCR ME panel implementation, finding significant reductions in both antibiotic exposure and hospital length of stay, while not compromising in-hospital mortality.
Our findings are clinically significant because each day of unnecessary broad-spectrum antibiotic exposure can be associated with unfavorable outcomes such as adverse drug events, development of antibiotic resistance, and adverse clinical outcomes [14–19]. Because most patients with suspected bacterial meningitis eventually receive an alternative diagnosis [20], we found that use of a multiplex PCR assay to rapidly exclude bacterial etiologies has the potential to mitigate the harms of prolonged and unnecessary exposure to broad-spectrum antibiotics. More importantly, these findings were observed in the absence of direct involvement from antimicrobial stewardship program team members, which suggests that the multiplex PCR ME panel itself influenced clinician behavior. This contrasts with the findings from other studies evaluating the impact of rapid multiplex PCR identification panels, such as for blood cultures [21], which have implied that antimicrobial stewardship program involvement is necessary to impact outcomes.
We found that although the rate of hospitalization was not reduced by implementation of the multiplex PCR ME panel, hospital length of stay was reduced by 1 day after implementation of the multiplex PCR ME panel. This suggests that the rapid identification or exclusion of pathogens in patients with suspected meningoencephalitis does not affect triage decisions for acutely ill patients in the emergency department; however, the need for continued hospitalization can be significantly reduced with earlier microbiological diagnosis and shorter time to de-escalation of empiric antibiotic therapy. A recent survey of clinical microbiologists and hospital epidemiologists found adoption of the multiplex PCR ME panel to range between 46% and 61% of surveyed hospitals, with cost being the most frequently cited barrier to adoption of rapid molecular diagnostic tests [22]. Our findings of reduced length of stay are consistent with the findings from several other centers who evaluated multiplex PCR ME panel implementation, and this suggests that laboratory costs can be offset by downstream healthcare system savings.
There remains some controversy with the use of multiplex PCR panels regarding concerns with false-positive and false-negative results, lack of clinical validation for most targets, need for confirmation testing for some targets, lack of adaptation for nosocomial infections or immunocompromised patients, and cost of such testing [23–25]. Multicenter studies and meta-analyses are needed to validate and elucidate the direct effect of multiplex PCR technologies on clinical management and cost effectiveness.
Our study has several limitations. First, it was conducted at a single institution, which may limit the generalizability of our findings to other hospital settings. Second, our study is prone to noncontemporaneous control bias given the absence of a contemporaneous control group during the post-multiplex PCR ME panel period. For example, temporal changes in length of stay could have influenced our findings. Third, we found an imbalance in the number of patients included between pre- and post-multiplex PCR ME panel periods, with fewer patients included in the post-multiplex PCR ME panel period. This may introduce a selection bias. The reason for this imbalance is unclear; however, possible explanations include a slow uptake due educational challenges reaching all providers at a large academic medical center and unfamiliarity with multiplex PCR ME panel testing among clinicians for a period of time after its implementation, or clinicians having a higher threshold to initiate empiric antibiotic therapy in the emergency department after implementation in patients with a relatively low clinical suspicion for meningitis knowing they would receive rapid microbiological results with the availability of the multiplex PCR ME panel. Our study did not compare baseline characteristics of patients who did and did not have multiplex PCR ME panel testing in the postintervention period; however, the fact that baseline characteristics were similar in the pre- and post-multiplex PCR ME panel periods is reassuring against a strong selection bias.
CONCLUSIONS
In conclusion, implementation of a multiplex PCR ME panel for testing of adult patients who present to the emergency department with suspected bacterial meningitis appears to reduce the duration of empiric antibiotic therapy, time to targeted therapy, and possibly hospital length of stay compared with traditional microbiological testing methods.
Acknowledgments
We acknowledge the support of Judith Hargrave, Yingzhe Kuang, Bulent Oral, and Sarah Russell for their technical assistance and expertise in the Clinical Microbiology Laboratory.
Financial support. This work was funded by New York-Presbyterian Hospital and Weill Cornell Medical College, including the Clinical and Translational Science Center (UL1 TR000457) and the National Institutes of Health/National Center for Advancing Translational Sciences (KL2-TR-002385; to J. J. C.).
Potential conflicts of interest. J. J. C. has received research support and consulting feeds from Roche Diagnostics and Allergan. M. J. G. has received research support from Gilead Sciences and Regeneron, served as a consultant to Enzychem, ReAlta Life Sciences, Regeneron, and Sobi, and received royalties from Springer and UpToDate. L. F. W. has received consulting fees from Roche Molecular Systems, Inc., Shionogi, Inc., and Talis Biomedical and research support from Accelerate Diagnostics, Inc., BioFire Diagnostics, LLC, and Roche Molecular Systems, Inc.
References
- 1. Edmond K, Clark A, Korczak VS, et al. Global and regional risk of disabling sequelae from bacterial meningitis: a systematic review and meta-analysis. Lancet Infect Dis 2010; 10:317–28. [DOI] [PubMed] [Google Scholar]
- 2. Tamune H, Kuki T, Kashiyama T, Uchihara T. Does this adult patient with jolt accentuation of headache have acute meningitis? Headache 2018; 58:1503–10. [DOI] [PubMed] [Google Scholar]
- 3. Tamune H, Takeya H, Suzuki W, et al. Absence of jolt accentuation of headache cannot accurately rule out meningitis in adults. Am J Emerg Med 2013; 31:1601–4. [DOI] [PubMed] [Google Scholar]
- 4. Iguchi M, Noguchi Y, Yamamoto S, et al. Diagnostic test accuracy of jolt accentuation for headache in acute meningitis in the emergency setting. Cochrane Database Syst. Rev. 2020; 6:CD012824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Straus SE, Thorpe KE, Holroyd-Leduc J. How do I perform a lumbar puncture and analyze the results to diagnose bacterial meningitis? JAMA 2006; 296:2012–22. [DOI] [PubMed] [Google Scholar]
- 6. Hanson KE. The first fully automated molecular diagnostic panel for meningitis and encephalitis: how well does it perform, and when should it be used? J Clin Microbiol 2016; 54:2222–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leber AL, Everhart K, Balada-Llasat JM, et al. Multicenter evaluation of BioFire FilmArray meningitis/encephalitis panel for detection of bacteria, viruses, and yeast in cerebrospinal fluid specimens. J Clin Microbiol 2016; 54:2251–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liesman RM, Strasburg AP, Heitman AK, et al. Evaluation of a commercial multiplex molecular panel for diagnosis of infectious meningitis and encephalitis. J Clin Microbiol 2018; 56:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dack K, Pankow S, Ablah E, et al. Contribution of the Biofire® Filmarray® meningitis/encephalitis panel: assessing antimicrobial duration and length of stay. Kans J Med 2019; 12:1–3. [PMC free article] [PubMed] [Google Scholar]
- 10. Moffa MA, Bremmer DN, Carr D, et al. Impact of a multiplex polymerase chain reaction assay on the clinical management of adults undergoing a lumbar puncture for suspected community-onset central nervous system infections. Antibiotics 2020; 9:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009; 42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harris PA, Taylor R, Minor BL, et al. ; REDCap Consortium. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019; 95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. DiDiodato G, Bradbury N. Cerebrospinal fluid analysis with the Biofire Filmarray meningitis/encephalitis molecular panel reduces length of hospital stay in patients with suspected central nervous system infections. Open Forum Infect Dis 2019; 6:ofz119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tamma PD, Avdic E, Li DX, et al. Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern Med 2017; 177:1308–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hagiya H, Kokado R, Ueda A, et al. Association of adverse drug events with broad-spectrum antibiotic use in hospitalized patients: a single-center study. Intern Med 2019; 58:2621–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rutter WC, Burgess DS. Incidence of acute kidney injury among patients treated with piperacillin-tazobactam or meropenem in combination with vancomycin. Antimicrob. Agents Chemother 2018; 62:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bellos I, Karageorgiou V, Pergialiotis V, Perrea DN. Acute kidney injury following the concurrent administration of antipseudomonal β-lactams and vancomycin: a network meta-analysis. Clin Microbiol Infect 2020; 26:696–705. [DOI] [PubMed] [Google Scholar]
- 18. Rhee C, Kadri SS, Dekker JP, et al. ; CDC Prevention Epicenters Program. Prevalence of antibiotic-resistant pathogens in culture-proven sepsis and outcomes associated with inadequate and broad-spectrum empiric antibiotic use. JAMA Netw Open 2020; 3:e202899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vaughn VM, Flanders SA, Snyder A, et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: a multihospital cohort study. Ann Intern Med 2019; 171:153–63. [DOI] [PubMed] [Google Scholar]
- 20. Hasbun R, Bijlsma M, Brouwer MC, et al. Risk score for identifying adults with CSF pleocytosis and negative CSF Gram stain at low risk for an urgent treatable cause. J Infect 2013; 67:102–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Timbrook TT, Morton JB, McConeghy KW, et al. The effect of molecular rapid diagnostic testing on clinical outcomes in bloodstream infections: a systematic review and meta-analysis. Clin Infect Dis 2017; 64:15–23. [DOI] [PubMed] [Google Scholar]
- 22. Kondo M, Simon MS, Westblade LF, et al. ; SHEA Research Network. Implementation of infectious diseases rapid molecular diagnostic tests and antimicrobial stewardship program involvement in acute-care hospitals. Infect Control Hosp Epidemiol 2021; 42:609–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fleischer E, Aronson PL. Rapid diagnostic tests for meningitis and encephalitis-BioFire. Pediatr Emerg Care 2020; 36:397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vetter P, Schibler M, Herrmann JL, Boutolleau D. Diagnostic challenges of central nervous system infection: extensive multiplex panels versus stepwise guided approach. Clin Microbiol Infect 2020; 26:706–12. [DOI] [PubMed] [Google Scholar]
- 25. Dien Bard J, Alby K. Point-counterpoint: meningitis/encephalitis syndromic testing in the clinical laboratory. J. Clin. Microbiol 2018; 56:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]