ABSTRACT
Background
To assess the efficacy and safety of corticosteroids in COVID-19 patients compared with standard care or placebo.
Methods
Electronic databases were searched to identify relevant studies. The mortality, adverse events, and other data from studies were pooled for statistical analysis.
Results
Ten randomized clinical trials were eligible for inclusion. Corticosteroid treatment in COVID-19 patients did not significantly reduce the risk of death (RR: 0.93; CI: 0.82, 1.05) and the need for mechanical ventilation (RR: 0.82; CI: 0.62, 1.08). No mortality reduction was also observed in the subgroup of patients requiring mechanical ventilation (RR: 0.90; CI: 0.79–1.03). The use of corticosteroids increased mortality in the subgroup of patients not requiring oxygen support (RR: 1.24; CI: 1.00–1.55). The survival benefit was observed in a low dosage of corticosteroids (RR: 0.90; CI: 0.84–0.97) and dexamethasone (RR: 0.90; 95% CI: 0.79–1.04). There was no difference in the rates of adverse events (RR: 1.13; CI: 0.58, 2.20) and secondary infections (RR: 0.87; CI: 0.66, 1.15).
Conclusion
Corticosteroid treatment did not convincingly improve survival in severe COVID-19 patients. Low-dose dexamethasone could be considered as a drug for the treatment of COVID-19 patients. More high-quality trials are needed to further verify this conclusion.
Expert Opinion: The effect of corticosteroids on patient survival highly depended on the selection of the right dosage and type and in a specific subgroup of patients. This meta-analysis, which included more RCTs, evaluated the safety and efficacy in severe COVID-19 patients and analyzed the effects of different types of corticosteroid treatments. Corticosteroid treatment did not convincingly improve survival in severe COVID-19 patients. But the low dose dexamethasone appear to have a role in the management of severe COVID-19 patients.
KEYWORDS: COVID-19, corticosteroids, dose, mortality, adverse events
1. Introduction
Since December 2019, coronavirus disease 2019 (COVID-19), a novel infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-COV-2), has affected the world [1,2]. Severe pulmonary or extrapulmonary symptoms and even death may present in different populations [3,4]. Up to July 2021, the SARS-COV-2 pandemic had resulted in approximately 4 million deaths worldwide [5], and the number is still rising. Nevertheless, there is currently no definitive and effective antiviral treatment for COVID-19 [6]. Therefore, it is of utmost urgency to determine drug treatment plans to address this severe disease.
Corticosteroids have been confirmed to have an excellent inhibitory effect on the expression of cytokines involved in the inflammatory response and are used as adjuvant drugs to treat viral pneumonia [7,8]. However, the value of corticoids in the treatment of COVID-19 has been widely debated with conflicting results [9–11]. Several trials have revealed that COVID-19 patients treated with corticosteroids had lower all-cause mortality than those not treated with corticosteroids [12,13]. Meanwhile, other randomized controlled trials (RCTs) showed no significant difference in all-cause mortality between COVID-19 patients treated with corticosteroids and those not treated with corticosteroids [10,14]. Previous meta-analyses mainly focused on the association between corticosteroids and COVID-19, and few of them explored the dose, the type of corticosteroids, and the disease severity of patients. In addition, most of the studies included in previous meta-analyses were retrospective or observational studies, or fewer RCTs, which provide low evidence grades to draw a scientific conclusion on the systemic use of corticosteroids in COVID-19 patients [15]. Our meta-analysis, which included more RCTs, aimed to evaluate the safety and efficacy in severe COVID-19 patients to provide a high level of evidence for clinical decision-making in treating severe COVID-19 patients.
2. Methods
This study-level systematic review and meta-analysis was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [16]. The PROSPERO registration ID is CRD42021229507.
2.1. Search strategy
An extensive search was conducted from December 2019 to 15 July 2021, in PubMed, EMBASE, the Cochrane Library, China National Knowledge Infrastructure (CNKI), and Wan Fang Data without language restriction. The search terms were ‘COVID-19’, ‘2019 novel coronavirus disease’, ‘SARS-CoV-2 infection’, ‘COVID-19 virus disease’, ‘2019 novel coronavirus infection’, ‘2019-nCoV infection’, ‘coronavirus disease 2019’, ‘2019-nCoV disease’, ‘corticosteroids’, ‘steroids’, ‘prednisolone’, ‘prednisone’, ‘dexamethasone’, ‘cortisol’, ‘hydrocortisone’, ‘glucocorticoid’, ‘methylprednisolone’. The reference lists from trials, review articles, and reports were also screened to identify additional eligible studies. The clinicaltrials.gov website was searched for RCTs that were also registered as completed but not yet published.
2.2. Inclusion and exclusion criteria
The inclusion criteria were as follows: (1)age>18 years; (2) hospitalized patients diagnosed with COVID-19; (3) patients treated with corticosteroids; (4) RCTs; (5) patients with SpO2 ≤ 94% at room air or the use of supplementary oxygen or mechanical ventilation; and (6) all languages available. The exclusion criteria were as follows: (1) lack of placebo or control group; (2) studies with missing data or outliers; (3) lack of dose control group or no fixed-dose strategery; and (4) repeated publication of literature or research using similar data.
2.3. Outcomes
The primary outcomes of this study included mortality and adverse events. The secondary outcomes included the need for invasive mechanical ventilation (for patients not intubated at inclusion) and secondary infections.
2.4. Data extraction
The two authors (Jiayuan T and Tian X) who screened studies according to the inclusion and exclusion criteria also independently extracted data from the included studies. Any differences were resolved by a third reviewer (Yun Liu) or by consensus. The extracted information included the first author, study design, median age, sex, intervention (including the type and dosage of corticosteroids), control, primary outcomes, and secondary outcomes.
2.5. Quality assessment
Two other reviewers independently assessed the quality of the RCTs with the Cochrane risk of bias tool, which contains seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias [17]. A third reviewer arbitrated any disagreements.
2.6. Statistical analysis
The data were analyzed using Review Manager 5.4. We derived risk ratios (RRs) and their 95% confidence intervals (CIs) for dichotomous outcomes. Depending on the presence of statistical heterogeneity, we used either fixed-effect or random-effects models. We quantified inconsistencies in associations among the trials using the I2 statistic and derived P values for heterogeneity using the Cochran Q statistic. P < 0.05 was considered statistically significant. Sensitivity analysis was conducted to assess the stability of the combined results.
3. Results
3.1. Study selection and characteristics
A total of 1647 articles were identified by searching the electronic databases. After the duplicates were removed, 1058 articles remained. A total of 573 articles were excluded after the title and abstract screening. After assessing the remaining 16 articles, six were excluded because they either did not report corticosteroid treatment or were not RCTs. Finally, 10 articles were included (Figure 1) [10,11,14,18–24].
Figure 1.
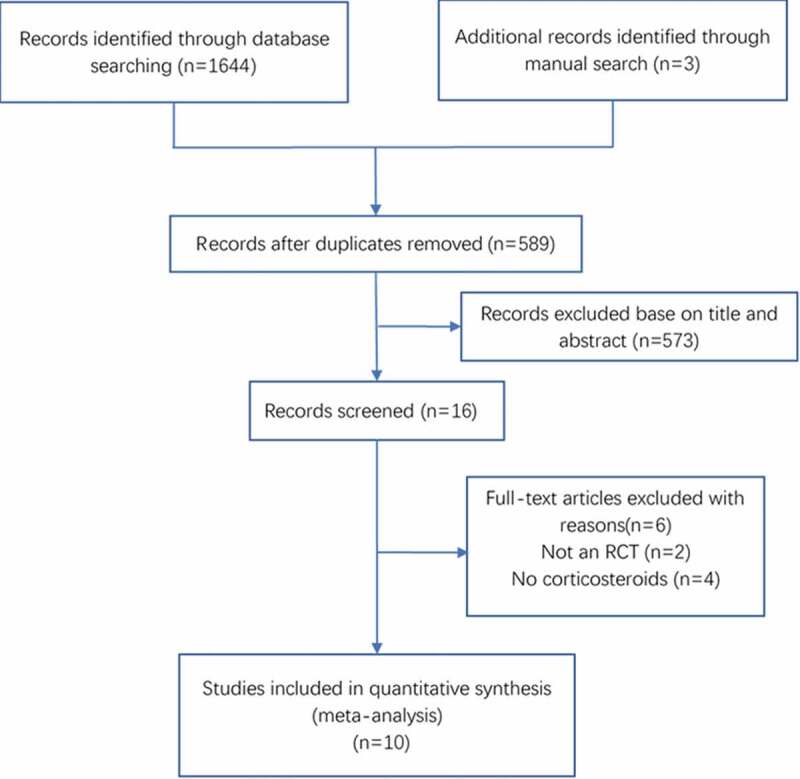
Flow diagram of the selection of studies for systematic review and meta-analysis
The main characteristics of the included studies are shown in Table 1. The studies originated from Brazil, Iran, the United Kingdom, China, Spain, and France with international cooperation, with varied sample sizes ranging from 50 to 6425 patients. The median ages of the patients in the enrolled studies ranged from 54 to 73 years, and the patients were predominantly male (46.5% to 72%). Clinical heterogeneity is mainly due to inclusion criteria, mechanical ventilation, type of corticosteroid, dosage and duration of administration, accompanying antiviral or anti-inflammatory drugs.
Table 1.
Characteristics of included randomized clinical trials
| Study | Country | Patients, total n | Male sex n(%) | Age, years | Intervention | Control | Primary outcomes | Secondary outcomes |
|---|---|---|---|---|---|---|---|---|
| Corral-Gudino L, et al | Spain | 64(35vs 29) | 23(66) vs 16(55) | 73 (11) vs 66 (12) | MP 40 mg bid for 3 Days and then 20 mg bid for 3 more days |
Standard of care |
All-cause mortality; escalation to ICU admission; progression of respiratory insufficiency that required noninvasive ventilation (NIV) | Adverse events; laboratory biomarkers; ndividual components of the composite endpoint |
| Dequin PF., et al | France | 149(76vs 73) | 54 (71.1) vs 50 (68.5) | 63.1(51.5–70.8) vs 66.3 (53.5–72.7) | “continuous intravenous infusion of hydrocortisone at an initial dose of 200 mg/duntil day 7 and then decreased to 100 mg/d for 4 days and 50 mg/d for 3 days, for a total of 14 days” |
Placebo (saline) | Treatment failure on day 21, defined as death or persistent dependency on mechanical ventilation or high-flow oxygen therapy | The use of tracheal intubation (for patients not intubated at inclusion use of prone position; extracorporealmembrane oxygenation or inhaled nitric oxide; the PaO2:FIO2 ratio; nosocomial infections; adverse events |
| Edalatifard M., et al | Iranian | 62(34 vs 28) | 24 (70.6%) vs 15 (53 · 5%) | 55.8(16.35) vs 61.7 (16.62) | Standard care with methylprednisolone pulse (intravenous injection, 250 mg/day for 3 days) |
Standard care alone | Discharge; mortality rate; time to improvement time; survival time |
Clinical signs BORG score; Laboratory test results; Adverse events |
| Jamaati H, et al | Iran | 50(25 vs 25) | 18 (72%) vs 18 (72%) | 62(14.07) vs 62(10.37) | Intravenous dexamethasone at a dose of 20 mg/day from day 1–5 and then at 10 mg/day from day 6–10 |
Not receivedexamethasone treatment | The need for invasive mechanical ventilation and death rate | Duration of clinical improvement; length of hospital stay; and radiological changes in the computed tomography (CT) scan. |
| Jeronimo CMP., et al | Brazil | 393(194 vs 199) | 126 (64.9) vs 128 (64.3) | 54(15) vs 57 (15) | Intravenous sodium succinate Methylprednisolone (0.5 mg/kg) twice daily for 5 days | Placebo (saline solution) twice daily for 5 days | 28-day mortality | Early mortality (Days 7 and 14); the need for orotracheal intubation by Day 7; the proportion of patients with an oxygenation index (PaO2 /FiO2) < 100 by Day 7 |
| RECOVERY | UnitedKingdom | 6425(2104 vs 4321) | 1338 (64) vs 2749 (64) | 66.9(15.4) vs65.8(15) | The usual standard of care plus oral or intravenous dexamethasone (at a dose of 6 mg once daily) for up to 10 days (or until hospital discharge if sooner) |
The usual standard of care alone | All-cause mortality within 28 days |
The time until discharge from the hospital; patients not receiving invasive mechanical ventilation at the time of randomization subsequent receipt of invasive mechanical ventilation or death; cause-specific mortality |
| RECOVERY2 | United Kingdom | 4716(1561 vs 3155) | 960(61.5) vs 1974(62.6) | 65.2(15.2) vs 65.4(15.4) | Patients re- ceived hydroxychloroquine sulfate in a loading dose of four tablets (total dose, 800 mg) at baseline and at 6 hours, which was followed by two tablets (total dose, 400 mg) start- ing at 12 hours after the initial dose and then every 12 hours for the next 9 days or until dis- charge |
Usual care | all-Cause mortality within 28 days; further anal- yses were specified at 6 months |
The time until discharge from the hospital and a composite of the initiation of invasive mechanical ventilation including extracorporeal membrane oxygenation or death among patients who were not receiving invasive mechanical ventilation at the time of randomization. |
| REMAP-CAP | Australia, Canada et al | 238(137 vs 101) | 98 (71.5) vs 72 (71.3) | 60.4 (11.6) vs 59.9 (14.6) | A fixed dose of intravenous hydrocortisone, 50 mg, every 6 hours for 7 days. | No hydrocortisone | Respiratory and cardiovascular organ support–free days up to day 21; death |
In-hospital mortality; ICU and hospital length of stay; respiratory support–free days; cardiovascular organ support–free days; a composite outcome of progression to invasive mechanical ventilation; extracorporeal membrane oxygenation (ECMO) or death among those not ventilated at baseline; the WHO ordinal scale; Adverse Events |
| Tang X, et al | China | 86(43 vs 43) | 21 (48.8) vs 20 (46.5) | 57(13.33) vs 55(20) | 1 mg/kg per day of methylprednisolone (produced by Pfizer Manufacturing Belgium NV) dissolved in 100 mL 0.9% normal saline was administered intravenously for 7 days | 100 mL 0.9% normal saline | The incidence of clinical deterioration 14 days after randomization | The incidence of clinical cure 14 days; the incidence of intensive care unit (ICU) admission; in-hospital mortality; the time from randomization to clinical cure; the time from the onset to virus shedding of SARS-CoV-2 in respiratory tract samples; hospitalization duration; complications including blood glucose abnormal; stress ulcer; secondary infections. |
| Tomazini BM., et al | Brazil | 299(151 vs 148) | 90 (59.6) vs 97 (65.6) | 60.1 (15.8) vs 62.7 (13.1) | Dexamethasone 20 mg intravenously once daily for 5 days, followed by 10 mg intravenously once daily for additional 5 days or until ICU discharge, whichever occurred first, plus standard care. |
Standard care only | Days alive and ventilator free at 28 d | 6-Point ordinal scale at day 15; 28-Day results; SOFA score; Serious adverse events; a New diagnosis of infection until day 28; Ventilator-associated pneumonia; Catheter-related bloodstream infection; Catheter-associated urinary tract infections; Other Bacteremia Insulin use for hyperglycemia |
3.2. Risk of bias
The risk of bias was assessed in all ten trials: 9 were deemed to have a moderate risk of bias [11,14,18–24], and 1 was deemed to have a low risk of bias [10]. Three trials were double-blind, randomized clinical trials [10,14,22], 3 trial was single-blinded [19,23,24], and 4 trials were open-label [11,18,20,21]. There was no evidence of reporting bias. The risk of bias for the studies is shown in Figure 2.
Figure 2.
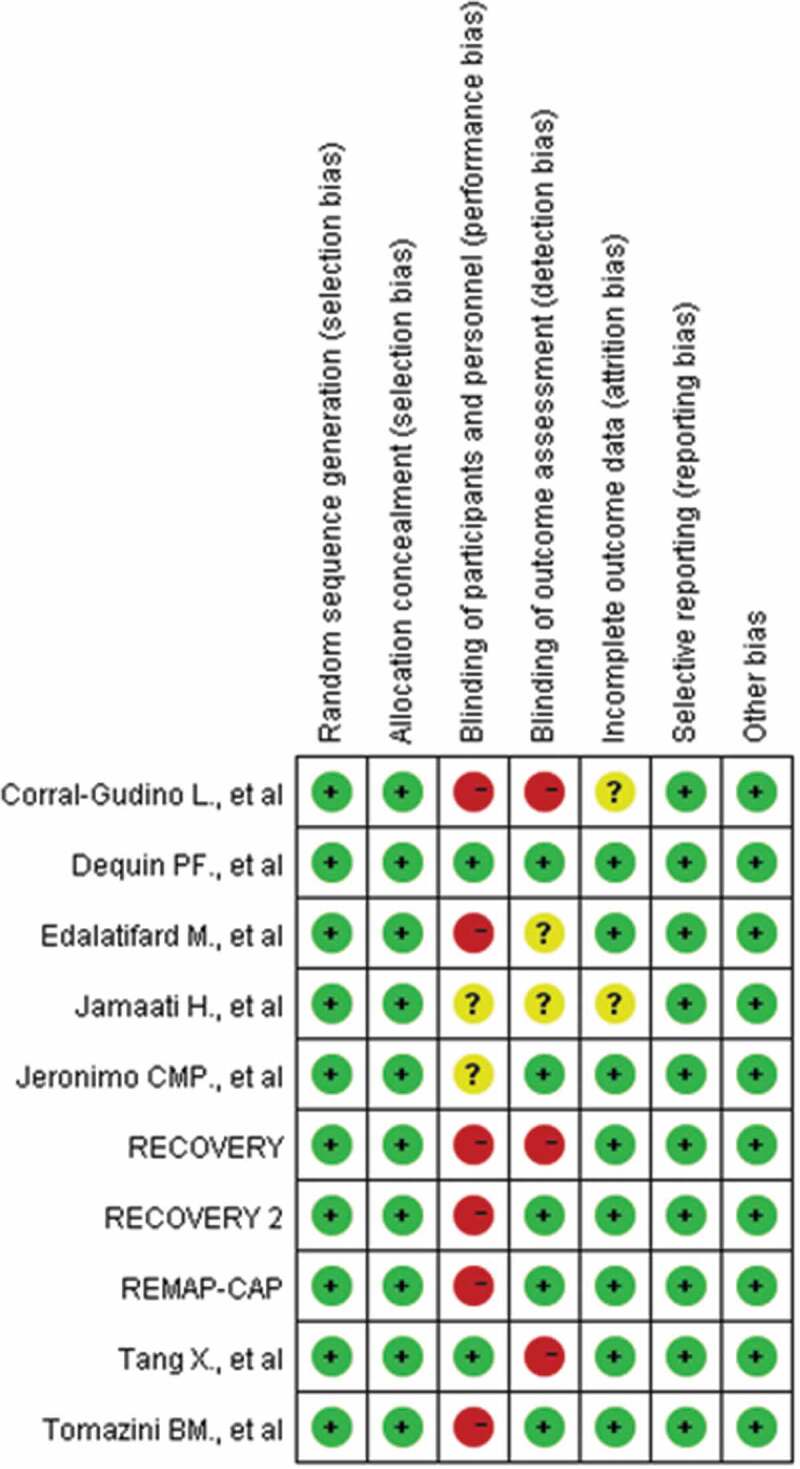
Risk of bias summary: review authors’ judgments about each risk of bias item for each included study
3.3. Outcomes
3.3.1. Mortality
A total of 12,473 participants in 10 studies were included in our meta-analysis [10,11,14,18–24]. A total of 1135 of 4354 patients died in the corticosteroid treatment group, and 2153 of 8119 patients died in the usual care or placebo group, which showed that corticosteroid did not significantly reduce the risk of death (RR: 0.93; CI: 0.82, 1.05; P = 0.26; I2 = 52%, Figure 3). To address the heterogeneity, we conducted subgroup analyses. No reduction in mortality also was observed in the subgroup of patients who required mechanical ventilation (355 of 815 [43.6%] in the corticosteroids group vs. 657 of 1441 [45.6%] in the control group, RR: 0.90; CI: 0.79–1.03; P = 0.13, I2 = 56%, Figure 4). However, the use of corticosteroids increased mortality in the subgroup of patients who do not require oxygen support, with results confirmed at sensitivity analyses (RR: 1.24; 95% CI: 1.00–1.55; P = 0.05; I2 = 0%, Figure 4). The benefit was observed in a low dosage of corticosteroids (RR: 0.90; 95% CI: 0.84–0.97; P = 0.007; I2 = 0%, Figure 5). Patients treated with dexamethasone had a significantly lower risk of mortality(RR: 0.90; 95% CI: 0.79–1.04; P = 0.14; I2 = 0%, Figure 5). No difference in mortality was found in the subgroups of hydrocortisone and methylprednisolone. However, these results were not stable, and if the RECOVERY trial was excluded [11], such survival benefit was absent.
Figure 3.
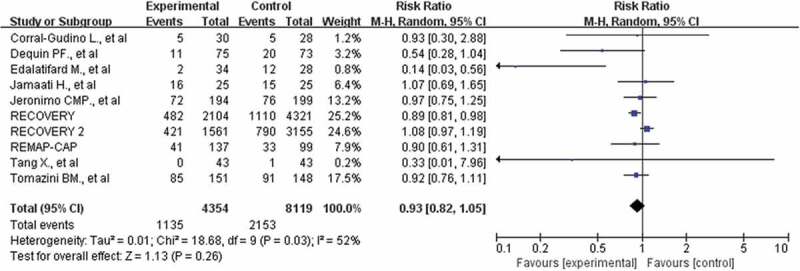
Forest plot of comparison: corticosteroids versus standard care or placebo, outcome: all-cause death
Figure 4.
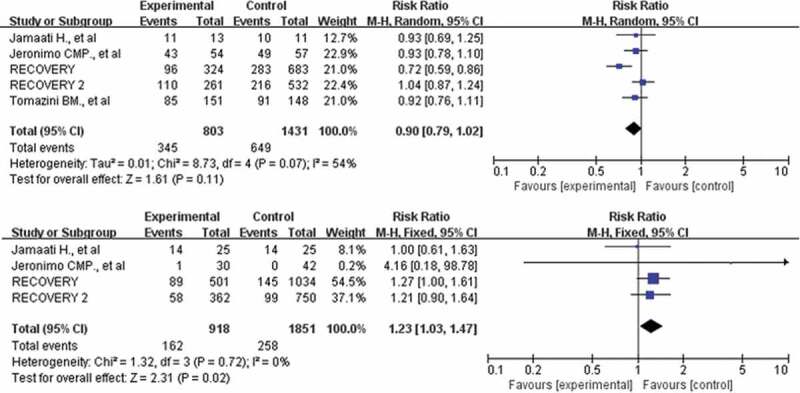
Forest plot of comparison: corticosteroids versus standard care or placebo, outcome: mortality in the subgroups of patients who required mechanical ventilation and patients who do not require oxygen support
Figure 5.
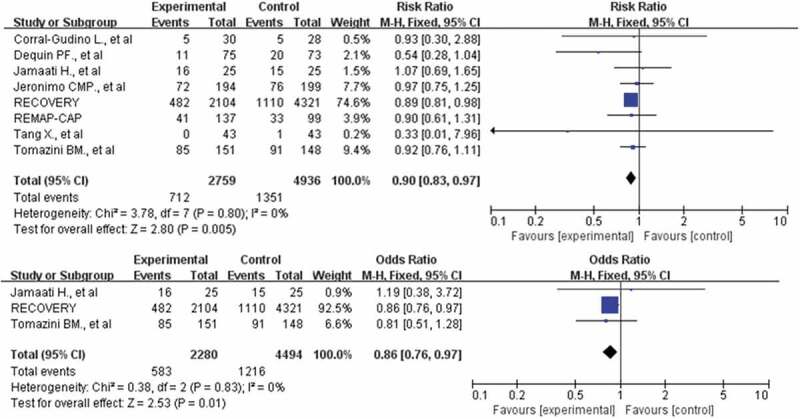
Forest plot of comparison: corticosteroids versus standard care or placebo, outcome: mortality in the subgroups of patients receiving low-dose corticosteroids and Patients treated with dexamethasone
3.3.2. Adverse events
A total of 3050 participants in 7 studies reported adverse events [10,18–21,23,24], including pulmonary embolism, edema, shock, intracranial hemorrhage, thrombocytopenia, ventricular tachycardia, and hypoglycemia, and so on. The incidence of adverse events was similar in both arms (7.0% vs 5.9%). There was no association between corticosteroids and adverse events (RR: 1.13; CI: 0.58, 2.20; P = 0.72; I2 = 41%, Figure 6), with results confirmed at sensitivity analyses.
Figure 6.
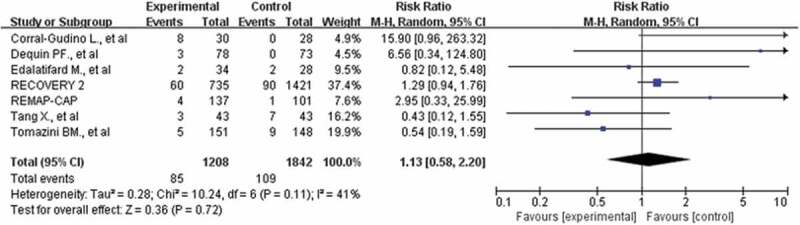
Forest plot of comparison: corticosteroids versus standard care or placebo, outcome: adverse events
3.3.3. Need for mechanical ventilation
Seven studies with 9771 participants were included in our meta-analysis [10,11,14.18,19,22,24]. 295(8.9%) patients in the corticosteroid group and 595(9.2%) patients in the control group required subsequent mechanical ventilation. There was no significant between-group difference (RR: 0.82; CI: 0.62, 1.08; P = 0.16; I2 = 71%, Figure 7). However, this result was not confirmed in the sensitivity analysis. If the RECOVERY2 trial excluded [24], there was a lower risk of the need for mechanical ventilation with corticosteroids in COVID-19 patients than with no corticosteroids or placebo treatment (RR: 0.74; CI: 0.58, 0.96; P = 0.02; I2 = 43%).
Figure 7.
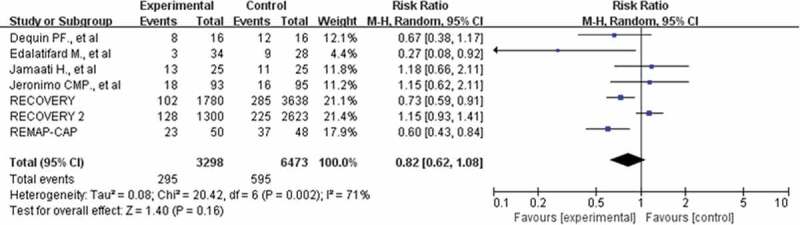
Forest plot of comparison: corticosteroids versus standard care or placebo, outcome: invasive mechanical ventilation
3.3.4. Secondary infections
Four studies with 567 participants who reported secondary infection were included in this meta-analysis [10,19–21]. The incidence of nosocomial infections was similar in both arms (22.8% vs 26.7%), and no significant differences were found among the studies (RR: 0.87; CI: 0.66, 1.15; P = 0.32; I2 = 0%, Figure 8). Sensitivity analysis showed that the result was stable.
Figure 8.
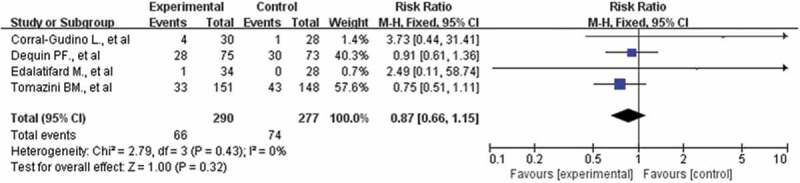
Forest plot of comparison: corticosteroids versus standard care or placebo, outcome: secondary infections
4. Discussion
In this study, a meta-analysis of 10 randomized clinical trials with a total of 12,473 severe COVID-19 patients showed corticosteroids treatment did not significantly reduce mortality. However, the subgroup analysis found the survival benefit was observed in both patients treated with a low dosage of corticosteroids and patients treated with dexamethasone. No increased risk of the need for mechanical ventilation, adverse events, or secondary infections were found. However, due to the great heterogeneity between trials, clear conclusions remain a challenge.
COVID-19 is an emerging infectious disease, and there is currently no optimal treatment [8]. Based on its in vitro SARS-CoV-2 antiviral activity and data from observational researches that effectively reduce viral load, corticosteroids have been widely used in severe COVID-19 patients [24], Whether the efficacy of this strategy has been controversial. Several meta-analysis studies have evaluated the efficacy of corticosteroids in COVID-19 patients with inconsistent results. In a meta-analysis based on retrospective studies, Pei et al. [25] reported that corticosteroids treatment might increase the risk of death (OR: 2.43; 95% CI: 1.44–4.1; P = 0.0001). Another meta-analysis of cohort studies shared a similar conclusion that corticosteroids were ineffective in reducing mortality, shortening the duration of symptoms, or virus clearance time [26]. However, both of these meta-analyses include non-RCTs, which may be biased and reduce the quality of conclusions. Conversely, Some recent meta-analyses of RCTs showed a mortality benefit in severe COVID-19 patients treated with corticosteroids [27,28]. Our meta-analysis included more RCTs and reached the different conclusions that 1135 (26.2%) patients died in the corticosteroid treatment group, 2153 (26.5%) patients died in the usual care or placebo group, corticosteroids did not significantly reduce the risk of death. But due to the different inclusion criteria, type of corticosteroid, dosage, this conclusion had great heterogeneity.
The effect of corticosteroids on patient survival highly depended on the selection of the right dosage and type and in a specific subgroup of patients [27]. Pasin et al. [29] found a reduction in mortality was observed in the subgroup of patients who required mechanical ventilation. It was recommended that severe patients could consider corticosteroids therapy. In our meta-analysis, the survival benefit of corticosteroid therapy was not observed in the subgroup of patients requiring mechanical ventilation. Evidence was mainly obtained from 5 trials, of which four trials showed no survival benefit [14,20,22,24]. One trial indicated that the use of dexamethasone reduced the 28-day mortality among those receiving either mechanical ventilation or oxygen alone [11]. Remarkably, our study found patients who have not received oxygen had a trend toward increased mortality when using corticosteroids. Jeronimo et al. [14] proposed a possible explanation that early use of corticosteroids in COVID-19 patients could lead to an increase in viral load, which needs to be confirmed by further studies. Therefore, caution is needed in the use of corticosteroids in mild subjects not receiving oxygen therapy. In addition, Patients over 60 years old receiving corticosteroids therapy had a lower mortality rate, while patients under 60 years old had a higher mortality rate [14]. However, Corral-Gudino et al. [21] found no evidence of interaction between treatment and patient age; corticosteroids have similar beneficial effects in the treatment of young and old patients.
Subgroup analyses of different dosages and types of corticosteroids were performed in our meta-analysis. The survival benefit was observed in a low dosage of corticosteroids but not in high-dose corticosteroids. Similar to Ma et al.’s findings [27]. 8 studies used low-dose corticosteroids (25–150 mg/d, methylprednisolone) and 2 studies used high-dose corticosteroids(>150 mg/d, methylprednisolone) [30]. Edalatifard et al. [19] used a higher dose of methylprednisolone(250 mg/d), and the results suggest it could be an efficient therapeutic agent for COVID-19 patients. On the contrary, Horby et al. [24] used 1200 mg/d hydroxychloroquine (equivalent dose was 240 mg/d methylprednisolone), and results suggest it had a longer duration of hospitalization and a higher risk of invasive mechanical ventilation or death. In previous research, the mortality rate was extremely high among COVID-19 patients treated with high-dose corticosteroids [31]. However, due to limited trials and great clinical heterogeneity, more data are required to elucidate the underlying clinical significance.
The survival benefit was also observed in treatment with dexamethasone. In our study, the main types of corticosteroids were hydrocortisone, dexamethasone, and methylprednisolone. No difference in mortality was found in the subgroups of hydrocortisone and methylprednisolone. A retrospective quasi-experimental study showed that dexamethasone is more effective in improving the PO2/FiO2 ratio of COVID-19 patients than methylprednisolone [32]. Another study also provided evidence that dexamethasone and betamethasone are effective for COVID-19 treatment because of their potential to inhibit the proteolytic activity of Mpro (a cysteine protease that plays a vital role in polyprotein processing and virus maturation) by comparing molecular docking studies of six corticosteroids (cortisone, hydrocortisone, prednisolone, methylprednisolone, betamethasone, and dexamethasone) and two repurposed drugs (darunavir and lopinavir) [33–36]. However, these survival benefits depended largely on the RECOVERY trial [11], which consisted of approximately 83.5% and 94.8%of the total number of patients in the analysis. if the RECOVERY trial excluded [11], these survival benefits were absent, more RCTs are needed in the future to draw definite conclusions.
The safety of corticosteroids in COVID-19 still is debated. Corticosteroid therapy attenuates the immune response, which increased the chance of infection and other adverse events [37]. In our study, seven studies reported the incidence rate of adverse events in COVID-19 patients (corticosteroid:7.0% vs control:5.9%) [10,18–21,23,24]. Four studies reported the incidence rate of nosocomial infections (corticosteroid:22.8% vs control:26.7%) [10,19–21]. There was no difference in the rates of adverse events and nosocomial infections between the corticosteroids group and the control group. One of 7 studies (GLUCOCOVID) showed that hyperglycemia (>180 mg/dl) was more frequent in the corticosteroid group in the ICU, with a significant difference [21]. Tomazini et al. [20] also reported unspecified hyperglycemia. Except for hyperglycemia, the incidence of adverse events was similar in either group. Similarly, a systematic review including peer-reviewed studies of any design reported that hyperglycemia was the most common adverse effect [38]. Therefore, when corticosteroids are used in clinical treatment, we need to pay more attention to blood sugar levels.
5. Limitations
The study has several limitations. First, only ten trials were included, and we were unable to obtain data for the ongoing unfinished studies, which may cause selection bias. Second, the presence of confounding variables (age, severity of disease, corticosteroids type, dosage, treatment duration, and so on) led to significant clinical heterogeneity and weakened the results of this meta-analysis. Therefore, the summary results need to be interpreted carefully. Third, the mortality rates in different periods were reported by multiple studies; 5 trials reported mortality at 28 days, 2 trials reported mortality at 21 days, 1 trial reported mortality at 14 days, and 1 trial did not mention the time of death, potentially leading to inconsistent experimental results. Fourth, Except for the RECOVERY trial and RECOVERY2 trial, most of the included studies have small sample sizes and may be biased. Small studies might have lower quality and a high risk of bias, which might contribute to the exaggerated intervention effects compared with large studies, so further exploration is needed [39,40].
6. Conclusions
In this meta-analysis of 10 RCTs and 12473 severe COVID-19 patients, pooled results suggested that corticosteroid therapy did not convincingly improve survival and reduce the need for mechanical ventilation in severe patients with COVID-19, and it is not recommended for patients who do not require oxygen support. A low dosage of dexamethasone could be considered as a drug for the treatment of COVID-19 patients. Due to significant clinical heterogeneity, more high-quality clinical trials are needed to further verify this conclusion.
Funding Statement
This paper was not funded.
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Ethics approval and consent to participate
Not applicable. The PROSPERO registration ID is CRD42021229507.
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
JY. Tu and T Xie conducted the literature search and selected studies. XQ. Mo and XD. Zhang assessed the methodologic quality of the studies. J Xie, Y Liu, and XS. Chen extracted data. JY. Tun conceived and planned the review, assessed the methodologic quality of the studies, verified the data, and drafted and revised the manuscript. T Xie provided methodologic advice, content expertise, and revised the manuscript. All authors contributed to writing the protocol. All authors read and approved the final manuscript.
References
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Y, Liu Q, Guo D.. Emerging coronaviruses: genome structure, replication, and pathogenesis. J Med Virol. 2020. Apr;924:418–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhong H, Wang Y, Zhang ZL, et al. Efficacy and safety of current therapeutic options for COVID-19 - lessons to be learnt from SARS and MERS epidemic: a systematic review and meta-analysis. Pharmacol Res. 2020Jul;157:104872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coronaviridae Study Group of the International Committee on Taxonomy of Viruses . The species severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat Microbiol. 2020. Apr;5(4):536–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coronavirus (COVID- 19) pandemic. [Accessed July 15 2021]. https://www.who.int/emergencies/diseases/novel-coronavirus–2019
- 6.Jean SS, Lee PI, Hsueh PR. Treatment options for COVID-19: the reality and challenges. J Microbiol Immunol Infect. 2020. Jun;53(3):436–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz-Topete D, Cidlowski JA. One hormone, two actions: anti- and pro-inflammatory effects of glucocorticoids. NeuroImmuno Modul. 2015;22(1–2):20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang Z, Liu J, Zhou Y, et al. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J Infect. 2020. Jul;81(1):e13–e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Keller MJ, Kitsis EA, Arora S, et al. Effect of systemic glucocorticoids on mortality or mechanical ventilation in patients with COVID-19. J Hosp Med. 2020. Aug;15(8):489–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dequin PF, Heming N, Meziani F, et al. Effect of hydrocortisone on 21-day mortality or respiratory support among critically Ill patients with COVID-19: a randomized clinical trial. JAMA. 2020. Oct 6;324(13):1298–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.RECOVERY Collaborative Group, Horby P, Lim WS, et al. Dexamethasone in hospitalized patients with Covid-19 - preliminary report. N Engl J Med. 2020. Jul 17;NEJMoa2021436. DOI: 10.1056/NEJMoa2021436. [DOI] [Google Scholar]; •• A large RCT which demonstrated mortality benefits with dexamethasone use in severe COVID-19 patients.
- 12.Salton F, Confalonieri P, Meduri GU, et al. Prolonged low-dose methylprednisolone in patients with severe COVID-19 pneumonia. Open Forum Infect Dis. 2020. Sep 12;7(10):ofaa421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernández-Cruz A, Ruiz-Antorán B, Muñoz-Gómez A, et al. A retrospective controlled cohort study of the impact of glucocorticoid treatment in SARS-CoV-2 infection mortality. Antimicrob Agents Chemother. 2020. Aug 20;64(9):e01168–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jeronimo CMP, Farias MEL, Val FFA, et al. Methylprednisolone as adjunctive therapy for patients hospitalized with COVID-19 (Metcovid): a randomised, double-blind, phase IIb, placebo-controlled trial. Clin Infect Dis. 2020. Aug 12;ciaa1177. DOI: 10.1093/cid/ciaa1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li H, Chen C, Hu F, et al. Impact of corticosteroid therapy on outcomes of persons with SARS-CoV-2, SARS-CoV, or MERS-CoV infection: a systematic review and meta-analysis. Leukemia. 2020Jun;346:1503–1511.Epub 2020 May 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions (Version 5.1.0). The Cochrane Collaboration, 2011. March. [Accessed March 16, 2021].http//www.cochrane-handbook.org
- 18.Angus DC, Derde L, Al-Beidh F, et al. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19 the REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. 2020. Oct 6;324(13):1317–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Edalatifard M, Akhtari M, Salehi M, et al. Intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: results from a randomised controlled clinical trial. Eur Respir J. 2020Sep;17:2002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020. Oct 6;324(13):1307–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.GLUCOCOVID investigators, Corral-Gudino L, Bahamonde A, Arnaiz-Revillas F, et al. Methylprednisolone in adults hospitalized with COVID-19 pneumonia: an open-label randomized trial (GLUCOCOVID). Wien Klin Wochenschr. 2021. Apr;133(7–8):303–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jamaati H, Hashemian SM, Farzanegan B, et al. No clinical benefit of high dose corticosteroid administration in patients with COVID-19: a preliminary report of a randomized clinical trial. Eur J Pharmacol. 2021. Apr 15;897:173947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang X, Feng YM, Ni JX, et al. Early use of corticosteroid may prolong SARS-CoV-2 shedding in non-intensive care unit patients with COVID-19 pneumonia: a multicenter, single-blind, randomized control trial. Respiration. 2021;100(2):116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.RECOVERY Collaborative Group, Horby P, Mafham M, et al. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020. Nov 19;383(21): 2030–2040. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Study with large sample size assessing effects of hydroxychloroquine in hospitalized patients with Covid-19.
- 25.Pei L, Zhang S, Huang L, et al. Antiviral agents, glucocorticoids, antibiotics, and intravenous immunoglobulin in 1142 patients with coronavirus disease 2019: a systematic review and meta-analysis. Pol Arch Intern Med. 2020. Sep 30;130(9):726–733. [DOI] [PubMed] [Google Scholar]
- 26.Cheng W, Li Y, Cui L, et al. Efficacy and safety of corticosteroid treatment in patients with COVID-19: a systematic review and meta-analysis. Front Pharmacol. 2020;11:571156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ma S, Xu C, Liu S, et al. Efficacy and safety of systematic corticosteroids among severe COVID-19 patients: a systematic review and meta-analysis of randomized controlled trials. Signal Transduct Target Ther. 2021. Feb 21;6(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pulakurthi YS, Pederson JM, Saravu K, et al. Corticosteroid therapy for COVID-19: a systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore). 2021. May 21;100(20):e25719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pasin L, Navalesi P, Zangrillo A, et al. Corticosteroids for patients with coronavirus disease 2019 (COVID-19) with different disease severity: a meta-analysis of randomized clinical trials. J Cardiothorac Vasc Anesth. 2021. Feb;35(2):578–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao B, Gao H, Zhou B, et al. Adjuvant corticosteroid treatment in adults with influenza A (H7N9) viral pneumonia. Crit Care Med. 2016. Jun;44(6):e318–28. [DOI] [PubMed] [Google Scholar]
- 31.Li X, Xu S, Yu M, et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J Allergy Clin Immunol. 2020. Jul;146(1):110–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rana MA, Hashmi M, Qayyum A, et al. Comparison of efficacy of dexamethasone and methylprednisolone in Improving PaO2/FiO2 ratio among COVID-19 patients. Cureus. 2020. Oct 12;12(10):e10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghosh R, Chakraborty A, Biswas A, et al. Potential therapeutic use of corticosteroids as SARS CoV-2 main protease inhibitors: a computational study. J Biomol Struct Dyn. 2020Oct;23:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dai W, Zhang B, Jiang XM, et al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science. 2020. Jun 19;368(6497):1331–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jin Z, Du X, Xu Y, et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020. Jun;582(7811):289–293. [DOI] [PubMed] [Google Scholar]
- 36.Osman EEA, Toogood PL, Neamati N. COVID-19: living through another pandemic. ACS Infect Dis. 2020. Jul 10;6(7):1548–1552. [DOI] [PubMed] [Google Scholar]; • This study included a strategy to quickly discover antiviral compounds with clinical potential.
- 37.Liu D, Ahmet A, Ward L, et al. A practical guide to the monitoring and management of the complications of systemic corticosteroid therapy. Allergy Asthma Clin Immunol. 2013. Aug 15;9(1).30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hasan SS, Capstick T, Ahmed R, et al. Mortality in COVID-19 patients with acute respiratory distress syndrome and corticosteroids use. a systematic review and meta-analysis. Expert Rev Respir Med. 2020. Nov;14(11):1149–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Z, Xu X, Ni H. Small studies may overestimate the effect sizes in critical care meta-analyses: a meta-epidemiological study. Crit Care. 2013. Jan 9;17(1).R2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dechartres A, Boutron I, Trinquart L, et al. Single-center trials show larger treatment effects than multicenter trials. evidence from a meta-epidemiologic study. Ann Intern Med. 2011. Jul 5;155(1).39–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.


