Abstract
Glyphosate (GLY) usage for weed control is extensive. To investigate ovarian impacts of chronic GLY exposure, female C57BL6 mice were orally administered saline as vehicle control (CT) or GLY at 0.25 (G0.25), 0.5 (G0.5), 1.0 (G1.0), 1.5 (G1.5), or 2 (G2.0) mg/kg for five days per wk. for 20 wks. Feed intake increased (P < .05) in G1.5 and G2.0 mice and body weight increased (P < .05) in G1.0 mice. There was no impact of GLY on estrous cyclicity, nor did GLY affect circulating levels of 17β-estradiol or progesterone. Exposure to GLY did not impact heart, liver, spleen, kidney or uterus weight. Both ovarian weight and follicle number were increased (P < .05) by G2.0 but not affected at lower GLY concentrations. There were no detectable effects of GLY on ovarian protein abundance of pAKT, AKT, pAKT:AKT, γH2AX, STAR, CYP11A1, HSD3B, CYP19A, ERA or ERB. Increased (P < .05) abundance of ATM protein was observed at G0.25 but not higher GLY doses. A dose-dependent effect (P < .10) of GLY exposure on ovarian protein abundance as quantified by LC-MS/MS was observed (G0.25–4 increased, 19 decreased; G0.5–5 increased, 25 decreased; G1.0–65 increased, 7 decreased; G1.5–145 increased, 2 decreased; G2.0–159 increased, 4 decreased). Pathway analysis was performed using DAVID and identified glutathione metabolism, metabolic and proteasome pathways as GLY exposure targets. These data indicate that chronic low-level exposure to GLY alters the ovarian proteome and may ultimately impact ovarian function.
Keywords: Glyphosate, Ovary, Proteome, Mouse
1. Introduction
Proper ovarian function is important for reproductive and overall female health (Hoyer, 2005; Hoyer, 2002; Hoyer and Keating, 2014). Impacts of chemical exposures on ovarian function range from temporary (altered cyclicity and ovulation) to permanent (complete depletion of ovarian follicular structures; (Hoyer and Keating, 2014; Keating, and C JM, Sen N, Sipes IG, Hoyer PB., 2009)). In addition, endocrine disrupting chemicals can target the ovary, which can interfere with steroid hormone production; a scenario which can negatively impact female fertility (Gore et al., 2015; Patel et al., 2015; Rattan et al., 2017). Loss of ovarian function and cessation of associated uterine functionality result in menopause, a timeframe at which women are at heightened risk for development of a number of diseases and health disorders (De Vos et al., 2010; Hoyer and Sipes, 1996; Senapati, 2018). Thus, premature complete or partial loss of ovarian function is detrimental for female health.
Glyphosate (GLY) is a widely applied non-selective herbicide and has been used for approximately 3.5 decades (Williams et al., 2000). With the introduction of GLY-resistant crops, GLY became one of the most predominant utilized herbicides in the U.S. (Benbrook, 2016). Detection of GLY residues in food stuffs (Zoller et al., 2018) and in human urine (Curwin et al., 2007a, 2007b; Knudsen et al., 2017; Mills et al., 2017; Soukup et al., 2020) has placed potential health effects of GLY exposure under scrutiny. Urinary presence of GLY does not automatically indicate a health risk – in fact, it has been recognized that GLY is readily excreted, through feces and urine (Williams et al., 2000). Furthermore, although urinary exposure has been detected, there is no difference in urine levels in rural agricultural (presumably a higher exposure demographic) versus non-agricultural individuals (Curwin et al., 2007a, 2007b).
There are conflicting reports as to whether GLY should be considered a risk factor for female reproductive health. The Ontario Farm Family Health study correlated GLY exposure during late pregnancy with spontaneous abortion (Arbuckle et al., 2001), and association between GLY exposure and a shortened gestational length was reported in a birth cohort from Indiana (Parvez et al., 2018). Exposure in vivo for 60 days to GLY (126 or 315 mg/Kg/d) increased atretic follicle number, reduced antral follicle surface area in vivo in rats and decreased 17β-estradiol (E2) production (Hamdaoui et al., 2019). Another in vitro study determined no impact of GLY (1–300 μg/ml) on cell number, E2 or progesterone (P4) production in granulosa cells, but did note impacts of a GLY-based herbicide (GBH) exposure on steroid hormone concentration in media (Perego et al., 2017). Postnatal exposure of lambs to a GBH; 2 mg/Kg/day) did not alter ovarian weight but increased proliferation of granulosa and theca cells as evidenced by increased abundance of a proliferation marker and decreased mRNA encoding follicle stimulating hormone receptor and growth and differentiation factor 9 (Alarcon et al., 2019). GLY-based herbicide exposure (2 mg/Kg/day) in neonatal rats increased uterine luminal hyperplasia, estrogen receptor alpha, and P4 receptor (Ingaramo et al., 2019). In contrast, no effect of GLY on E2 production using a standardized H295R steroidogenic assay was observed (Hecker et al., 2011), nor was there any impact of GLY on gross phenotypic reproductive measures in acute and sub-chronic exposure studies (Williams et al., 2000), illustrating conflicting data in the literature.
Additional reasons for inconsistency between the aforementioned studies is the formulation used: GLY vs. a GBH mixture. Additionally, as alluded to above the doses used in vivo are often extremely high and at non-relevant human exposure levels. The purpose of the current study was to explore the hypothesis that chronic GLY exposure at levels relevant to human exposure would impact ovarian function in ways that could contribute to female infertility.
2. Materials and methods
2.1. Materials
Glyphosate (CAS # 1071-;83-;6), 2-β-mercaptoethanol, Tris base, Tris HCL, Sodium chloride, Sucrose, EDTA, Paraformaldehyde and Tween-20 were purchased from Sigma-Aldrich Inc. (St Louis, MO). Glycerol, Sodium citrate, Citric acid and Pierce BCA protein assay kit were from Thermo Fisher Scientific. 4–15% mini-PROTEAN TGX™ precast protein gels were obtained from BioRad, USA. 4–20% TGX stain free precast protein gels were purchased from Criterion. iBlot 2NC regular stacks were from Invitrogen. Anti-AKT (ab8805), anti-phosphorylated AKT T308 (ab38449), anti-ERα (ab32063), anti-ERβ (ab288), and anti-phosphorylated ATM S1981 (ab81292) were from Abcam (Cambridge, MA). Anti-STAR (NBP1–33485), HSD3β (NBP1–32353) and CYP19A1 (NB100-1596) were from Novus biologicals (Centennial, CO). Anti-CYP11A1 (12491), anti-rabbit IgG HRP linked antibody (7074), anti-mouse IgG HRP linked antibody (7076), anti-phosphorylated Histone H2AX (2577S), and SignalFire™ ECL Reagent (6883) from Cell Signaling (Danvers, MA). The source of E2 (EIA-2693) and P4 (EIA-1561) ELISA kits was DRG International, Inc. (Springfield, NJ).
2.2. Animals
C57BL6 mice were exposed to vehicle control (CT; saline; n = 10) or GLY at doses of 0.25, 0.5, 1.0, 1.5, or 2 mg/kg orally (n = 10 per group) from a pipette tip five days per week for 20 weeks beginning at 6 weeks of age. Body weight was measured weekly and feed intake was measured over the last 10 wks. Vaginal cytology was monitored daily for the last 21 d of the treatment period by lavaging the vagina with 15 μl saline, smearing a microscope slide with this solution and visualizing the cellular composition present. Mice were euthanized at the pro-estrus phase of the estrous cycle. Blood was collected post-euthanasia by cardiac puncture. Organs were collected and weights recorded. Organ weight was normalized to body weight.
2.3. Histology
Ovaries (n = 10 per treatment group) were fixed in 4% paraformaldehyde for 24 h, transferred to 10–30% sucrose, and embedded using OCT. Ovaries were sectioned (10 μM thickness) and every 6th section was mounted and stained by hematoxylin and eosin. Healthy oocyte-containing follicles were identified and counted in every 12th section. Unhealthy follicles were distinguished from healthy follicles by the appearance of pyknotic bodies and intense eosinophilic staining of oocytes. Healthy follicles were classified and enumerated as previously described (Flaws et al., 1994).
2.4. Western blot analysis
The ovaries (n = 5 per treatment group) were homogenized in tissue lysis buffer (containing 50 mM Tris HCL, 1 mM EDTA, pH 8.5). Samples were centrifuged at 10,000 rpm for 15 min, twice and supernatant collected. The protein concentration was measured by bicinchoninic acid assay and with Laemmli buffer onto 4–20% precast gels. Proteins were separated by SDS-PAGE and transferred to a nitrocellulose membrane. Membranes were stained with Ponceau S to visualize and quantify protein loading and then blocked for 1 h in 5% milk in 1× Tris-buffered saline containing tween 20 (TTBS) and incubated with primary antibodies against anti-AKT (primary: 1:500, secondary: 1:500), anti-γH2AX (1:750; 1:500); anti-phosphorylated AKT T308 (primary: 1:250, secondary 1:200); anti-ERα (primary: 1:500, secondary:1:500); anti-ERβ (primary:1:500, secondary: 1:250); anti-STAR (primary: 1:500, secondary: 1:100); anti-HSD3β (primary: 1:500, secondary: 1:1000); anti-CYP11A1 (primary: 1:500, secondary: 1:100) and anti-CYP19A1 (primary: 1:500, secondary: 1:200) overnight at 4 °C. Following three washes in TTBS (1×) membranes were incubated with the species-specific secondary antibody for 1 h at room temperature. Western blots were detected using chemiluminescence and exposed to X-ray film. Individual protein values were normalized to Ponceau S stained total protein. Densitometry of the appropriately-sized protein band was performed using ImageJ software (Abramoff et al., 2004). To ensure antibody specificity, negative control blots for each antibody used were performed in which the membranes were incubated with primary antibody only, secondary antibody only, or normal IgG in place of primary antibody with the inclusion of the appropriate secondary antibody. No protein bands were observed on these control blots indicating the specificity of the protein bands detected and analyzed.
2.5. Steroid hormone quantification
Serum (25 μl; n = 10 per treatment group) was added in duplicate per sample to an enzyme-linked immunosorbent assay plate to measure E2 or P4. Plates were incubated for 60–90 min after adding the enzyme conjugate (100–200 μl). The wells were rinsed with wash solution three times, substrate solution added (100–200 μl) and incubated for 15–30 min. The enzymatic reaction was stopped by adding stop solution (50–100 μl) and the signal determined within 10 min using a plate reader at 450 nm absorbance.
2.6. Ovarian proteome analysis
Protein was isolated from ovary (n = 5 per treatment group) samples using lysis buffer (50 mM Tris HCL and 1 mM EDTA, pH 8.5) and digested (50 μg) with trypsin/Lys-C, dried down and reconstituted in 5% Buffer B (0.1% formic acid/acetonitrile), 95% Buffer A (0.1% formic acid/water) to a concentration of 1 μg/μL. Peptide Retention Time Calibration mixture (PRTC, Pierce part #88320) was added into the sample (25 fmol/μL) to serve as an internal control. Peptides (10 μg initial protein) and PRTC (250 fmol) were injected onto a liquid chromatography column (Agilent Zorbax SB-C18, 0.5 mm × 150 mm, 5 μm) and separated using an Agilent 1260 Infinity Capillary Pump. The separated peptides were analyzed using a Q ExactiveTM Hybrid Quadrupole-Obrbitrap Mass Spectrometer with an HCD fragmentation cell. The resulting intact and fragmentation pattern was compared to a theoretical fragmentation pattern (MASCOT for the sample peptides Sequest HT for the PRTC peptides) to identify peptides. The relative abundance of the identified proteins was based on the areas of the top three unique peptides for each sample and the areas were normalized using the PRTC peptides areas.
The arithmetic mean of the PRTC was used as normalization factor. For each peptide, the signal intensity was divided by the arithmetic mean of the PRTC before further analysis. Metaboanalyst 3.0 (Xia et al., 2015; Xia and Wishart, 2016) was used for data analysis. Upon finding data integrity to be satisfactory (no peptide with more than 50% missing replicates, positive values for the area), missing value imputation was done using Singular Value Decomposition (SVD) method. Filtering, based on interquartile range, was then performed to remove values that are unlikely to be of use when modeling the data, followed by generalized log transformation (glog 2) before data analysis. The control and treatment samples were compared by the Student’s t-test. Differences between groups were assessed by the Mann-Whitney rank sum test. All p values were two sided. To adjust for multiple comparisons, Bonferroni correction was applied and only p values less than 0.05 were considered significant. The PCA analysis was performed using the prcomp package and pairwise score plots providing an overview of the various separation patterns among the most significant components were accessed. The PLS regression was then performed using the plsr function provided by R pls package. The classification and cross-validation were also performed using the caret package. The uniport protein ids that were up/down regulated were used to retrieve the corresponding KEGG ids using the “Retrieve/ID mapping” tool of UniProt (accessible at http://www.uniprot.org/uploadlists/). KEGG ids were then used to retrieve biological pathway association of the proteins using David 6.8.
2.7. Sample identity blinding
The identity of samples used for ELISA and follicle counting were not known to the investigator performing the analysis. Samples analyzed by LC-MS/MS and subsequent bioinformatics analysis were designated lettering as identification so that the sample identity was unknown for those analyses. In addition, protein samples used for western blotting were double blinded – the identity of the samples was unknown to the investigator and after western blots were completed, they were then identified by group for the purpose of statistical analysis. Only after the statistical analysis was completed was group identity revealed.
2.8. Statistical analysis
All data were analyzed by either unpaired t-test or one-way ANOVA. All statistical analysis was performed using Prism 5.04 software (GraphPad Software). For the estrous cycle data, statistical analysis was performed on raw values and they were expressed as a percentage time spent at each cycle stage for graphical purposes. Statistical significance was defined as P < .05 for all endpoints except the LC-MS/MS analysis which was considered biologically meaningful if P < .10.
3. Results
3.1. Effect of glyphosate on body weight and feed intake
As anticipated, body weight increased over the duration of the dosing period (Fig. 1A). There was increased (P < .05) body weight observed in the mice that received G1.0, but no other dosage groups differed from CT. Relative to CT, G2.0 and G1.5 treated mice had increased (P < .05) feed intake (Fig. 1B) but there were no other GLY-induced impacts on feed intake.
Fig. 1.
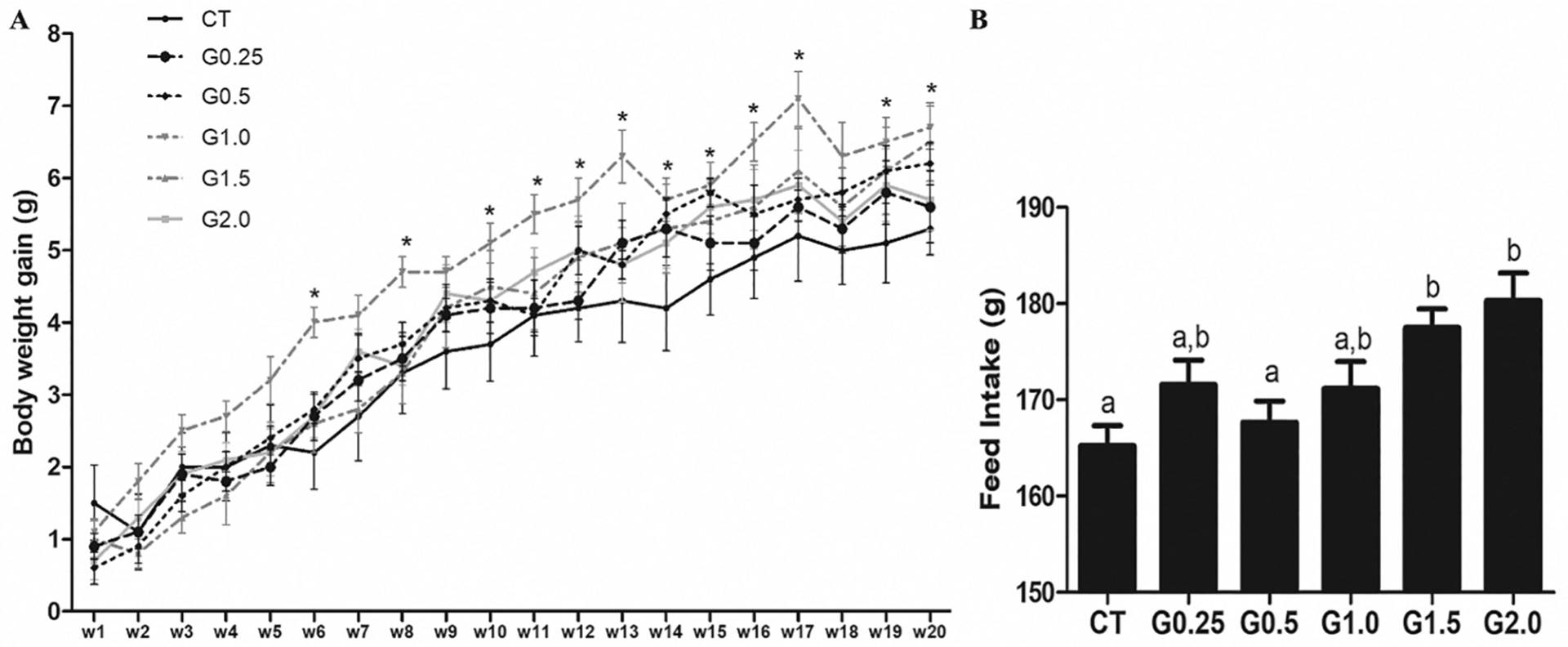
Impact of GLY exposure on body weight and feed intake. Female C57Bl/6 mice were exposed to saline vehicle control (CT) or increasing doses of GLY (0.25 mg/kg (G0.25), 0.5 mg/kg (G0.5), 1 mg/kg (G1.0), 1.5 mg/kg (G1.5) or 2 mg/kg (G2.0)) per os from a pipette tip five days per week for 20 weeks. (A) weekly body weight and (B) food intake were calculated. Data points represent mean +/− SEM. Asterix or different letters represent difference from CT; P < .05.
3.2. Impact of GLY exposure on the estrous cycle
Daily vaginal cytology was performed over 21 weeks and the percentage time spent at stages of the estrous cycle determined as a percentage for graphical purposes. There was no impact of any GLY dose exposure on the length of time spent in proestrus (PE), estrus (E) or metestrus + diestrus (MDE) combined (Fig. 2).
Fig. 2.
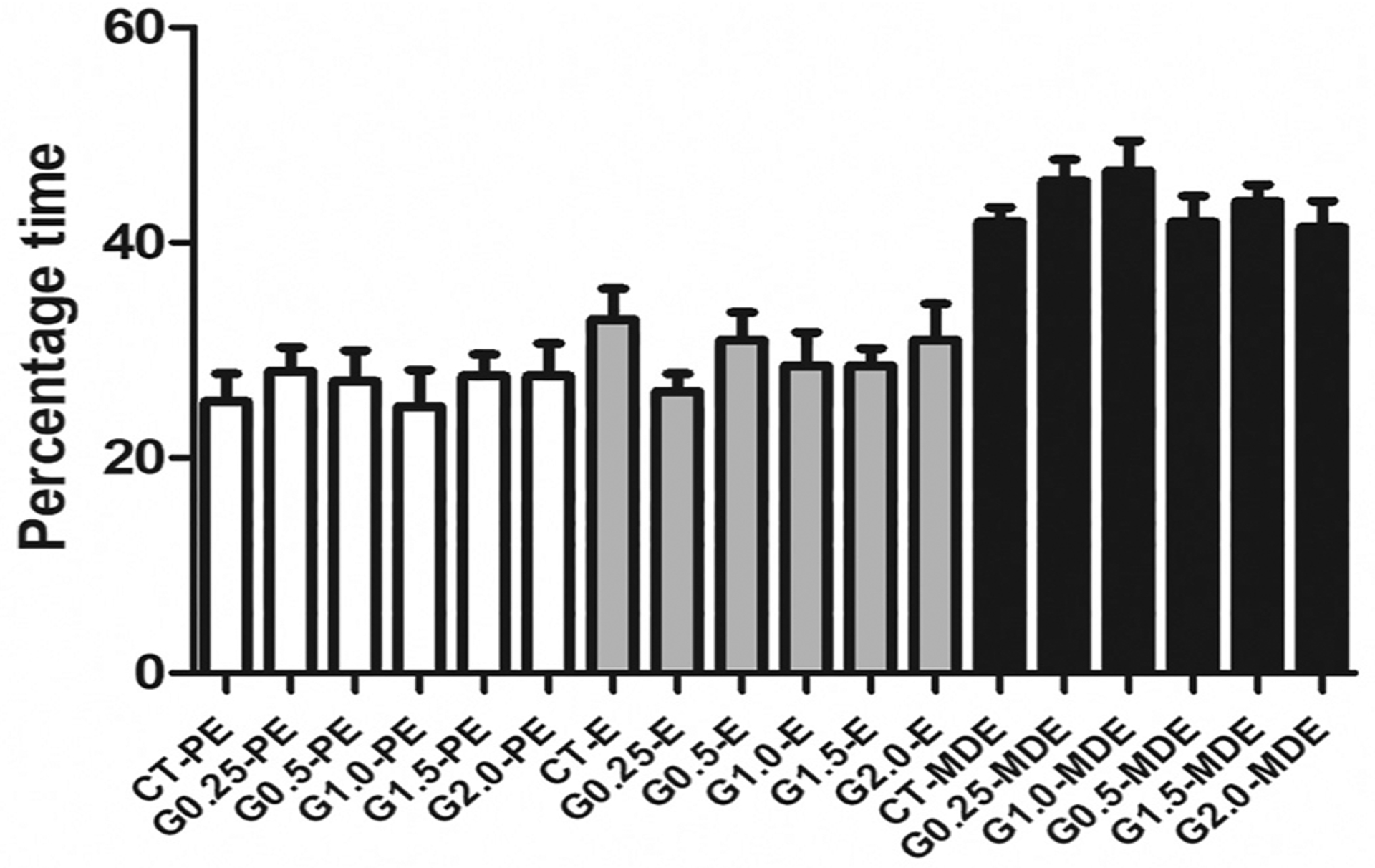
Effect of GLY exposure on percentage time spent at stages of estrous cycle. Female C57Bl/6 mice were exposed to saline vehicle control (CT) or increasing doses of GLY (0.25 mg/kg (G0.25), 0.5 mg/kg (G0.5), 1 mg/kg (G1.0), 1.5 mg/kg (G1.5) or 2 mg/kg (G2.0)) per os from a pipette tip five days per week for 20 weeks. The percentage time spent at stages of estrous cycle were calculated: proestrus = PE; estrus = E; metestrus and diestrus = MDE. Data points represent mean +/− SEM. Statistical analysis performed on raw data.
3.3. Ovarian steroid hormone effect of GLY exposure
The range of E2 (Fig. 3A) and P4 (Fig. 3B) were as expected for the pro-estrus stage of the estrous cycle in mice (Zenclussen et al., 2014). There was no observable effect of GLY exposure on E2 or P4 in circulation when compared with CT-treated mice at any dose (Fig. 3).
Fig. 3.
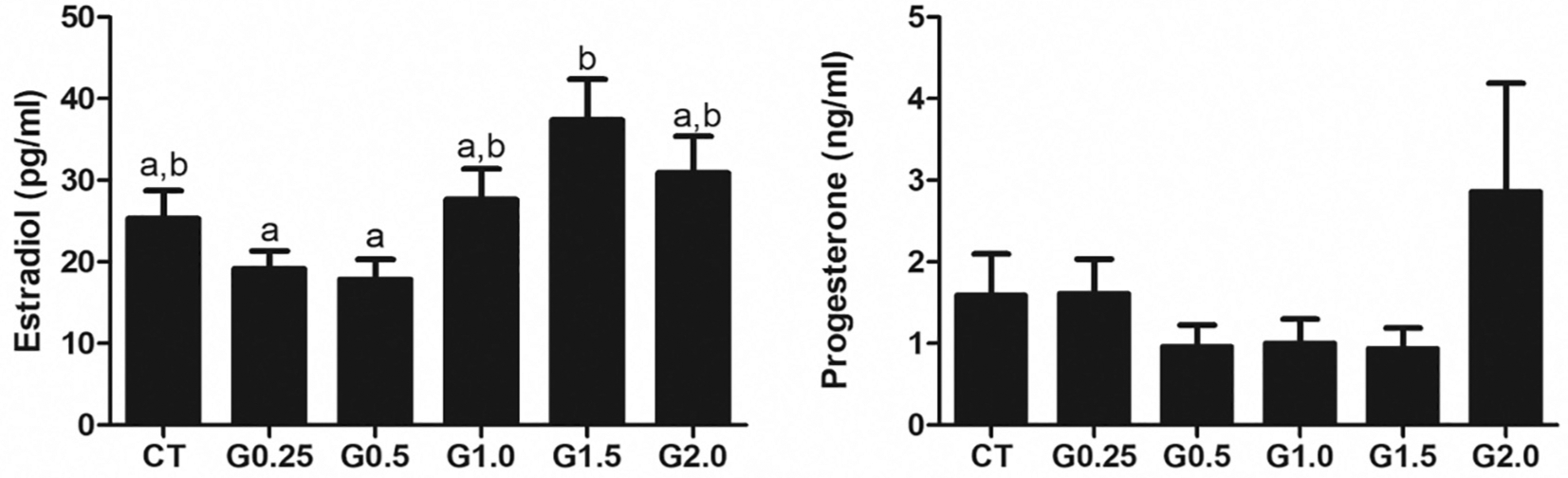
GLY exposure effect on circulating E2 and P4. Female C57Bl/6 mice were exposed to saline vehicle control (CT) or increasing doses of GLY (0.25 mg/kg (G0.25), 0.5 mg/kg (G0.5), 1 mg/kg (G1.0), 1.5 mg/kg (G1.5) or 2 mg/kg (G2.0)) per os from a pipette tip five days per week for 20 weeks. Circulating E2 and P4 were measured by ELISA. Data points represent mean +/− SEM. Different letters represent differences between treatments; P < .05.
3.4. Impact of GLY exposure on organ weight
There was no impact of GLY exposure on relative weight of heart (Fig. 4A), liver (Fig. 4B), spleen (Fig. 4C), kidney (Fig. 4D), or uterus (Fig. 4E). There was also no impact of doses lower than G2.0 on ovarian weight, relative to CT (Fig. 4F). Mice who received G2.0 had increased (P < .05) ovarian weight compared to both CT and all other GLY doses (Fig. 4F).
Fig. 4.
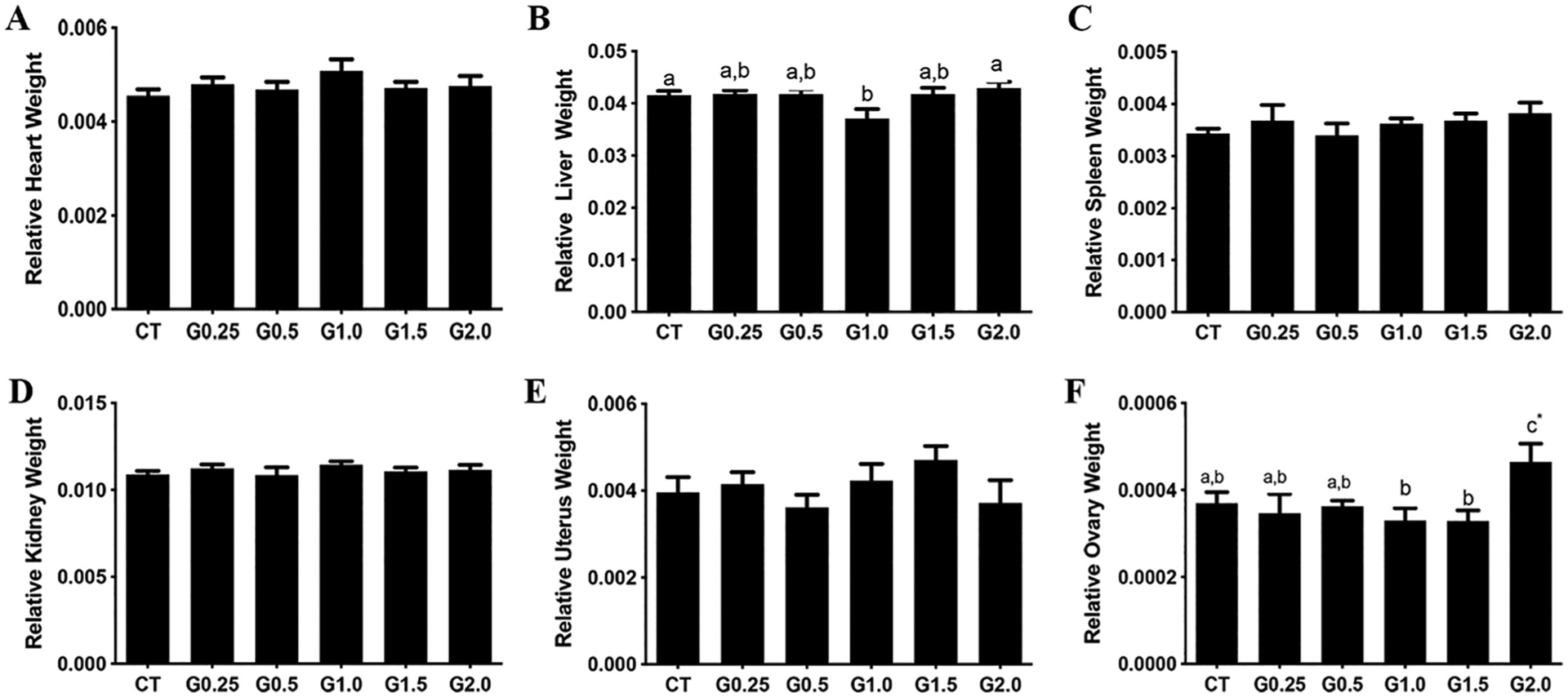
Relative organ weight impacts of GLY exposure. Female C57Bl/6 mice were exposed to saline vehicle control (CT) or increasing doses of GLY (0.25 mg/kg (G0.25), 0.5 mg/kg (G0.5), 1 mg/kg (G1.0), 1.5 mg/kg (G1.5) or 2 mg/kg (G2.0)) per os from a pipette tip five days per week for 20 weeks. Organ weights were collected post-euthanasia and normalized to body weight: (A) Heart, (B) Liver, (C) Spleen, (D) Kidney, (E) Uterus and (F) Ovary weight. Data points represent mean +/− SEM. Different letters represent differences between treatments; P < .05.
3.5. Follicular composition impact of GLY exposure
There was no effect (P > .05) of GLY exposure on the number of primordial (Fig. 5A), primary (Fig. 5B), secondary (Fig. 5C) or antral follicles (Fig. 5D). Mice exposed to G2.0 had greater (P < .05) numbers of all size follicles, relative to controls (Fig. 5A–D).
Fig. 5.
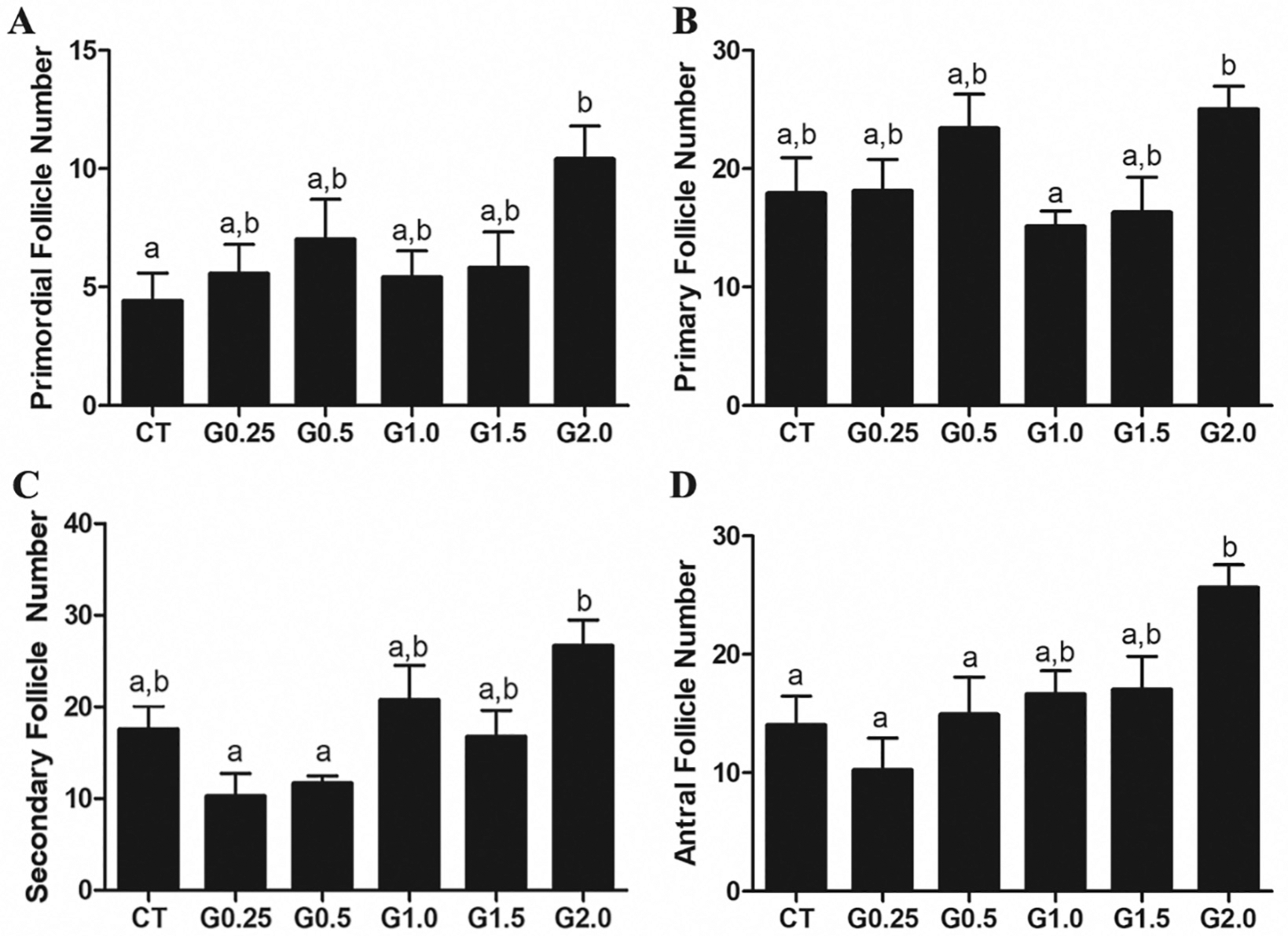
Effect of GLY exposure on ovarian follicle number. Female C57Bl/6 mice were exposed to saline vehicle control (CT) or increasing doses of GLY (0.25 mg/kg (G0.25), 0.5 mg/kg (G0.5), 1 mg/kg (G1.0), 1.5 mg/kg (G1.5) or 2 mg/kg (G2.0)) per os from a pipette tip five days per week for 20 weeks. Ovaries were sectioned, stained and follicular stages classified and counted. (A) primordial, (B) primary, (C) secondary, and (D) Antral follicle number are presented. Data points represent mean +/− SEM. Different letters represent differences between treatments; P < .05.
3.6. Phosphatidylinositol-3 kinase, DNA repair or steroidogenesis pathway impacts of GLY exposure
There was no effect of any GLY exposure level on abundance of ovarian total AKT (Fig. 6A), phosphorylated AKT T308 (pAKT) (Fig. 6B) or the ratio of pAKT:AKT protein. Exposure to G0.25 increased (P < .05) phosphorylate ATM protein abundance relative to CT-treated mice but there was no effect at any other dosage level (Fig. 7A). There was no effect of GLY exposure on the level of ovarian γH2AX (Fig. 7B). GLY exposure did not alter STAR (Fig. 8A), HSD3β (Fig. 8B), CYP11A1 (Fig. 8C), CYP19A1 (Fig. 8D) or ERA (Fig. 8E) protein abundance. While there were some dose-dependent differences in ERB within the GLY exposures (Fig. 8F), no GLY exposure treatments differed from CT-treated mice in ovarian ERB level (Fig. 8).
Fig. 6.
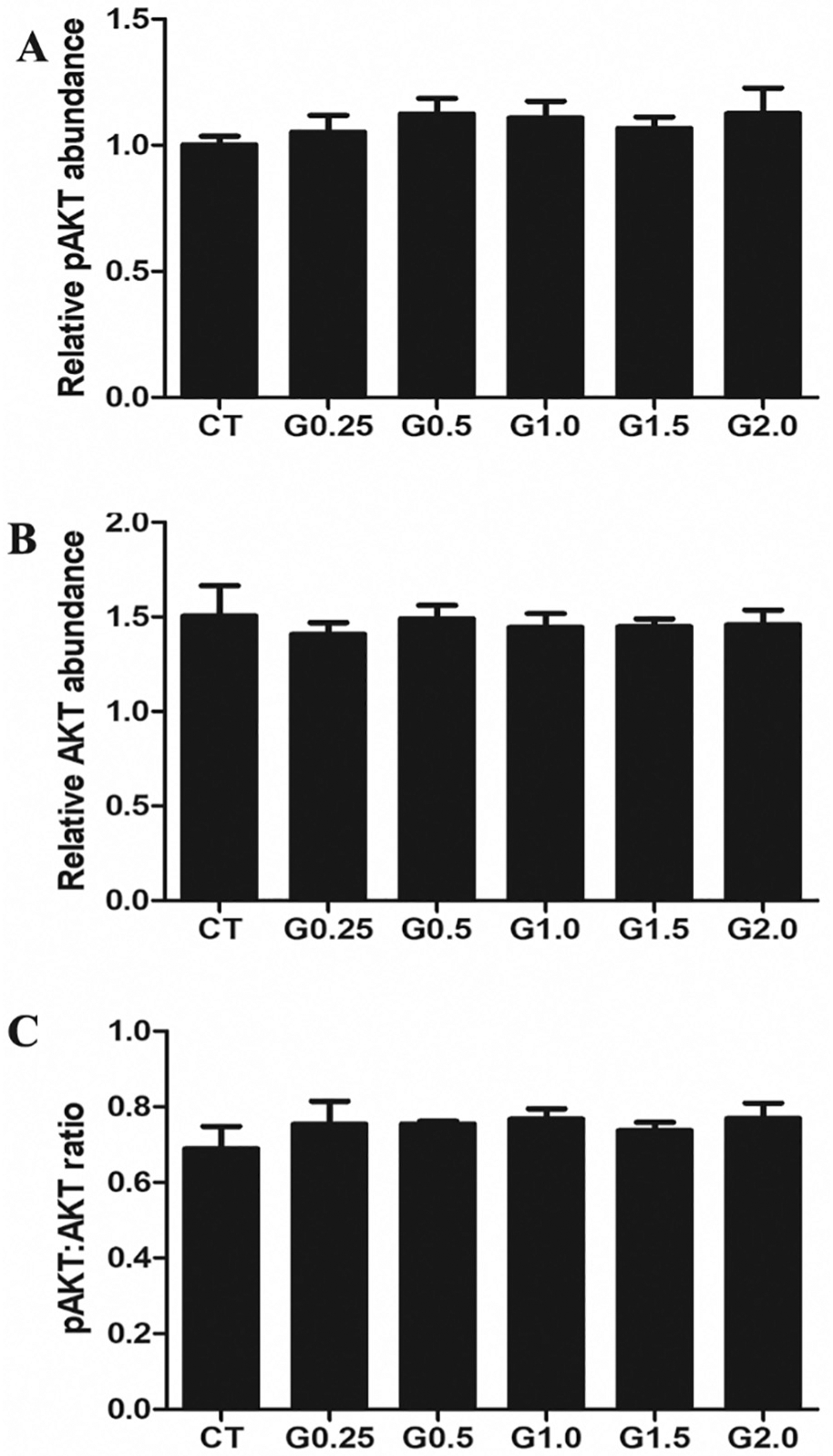
Impact of GLY exposure on ovarian pAKT and AKT protein abundance. Female C57Bl/6 mice were exposed to saline vehicle control (CT) or increasing doses of GLY (0.25 mg/kg (G0.25), 0.5 mg/kg (G0.5), 1 mg/kg (G1.0), 1.5 mg/kg (G1.5) or 2 mg/kg (G2.0)) per os from a pipette tip five days per week for 20 weeks. Ovaries were homogenized and western blotting performed to quantify protein abundance of (A) AKT, (B) pAKT and (C) pAKT:AKT ratio was calculated. Data points represent mean +/− SEM.
Fig. 7.
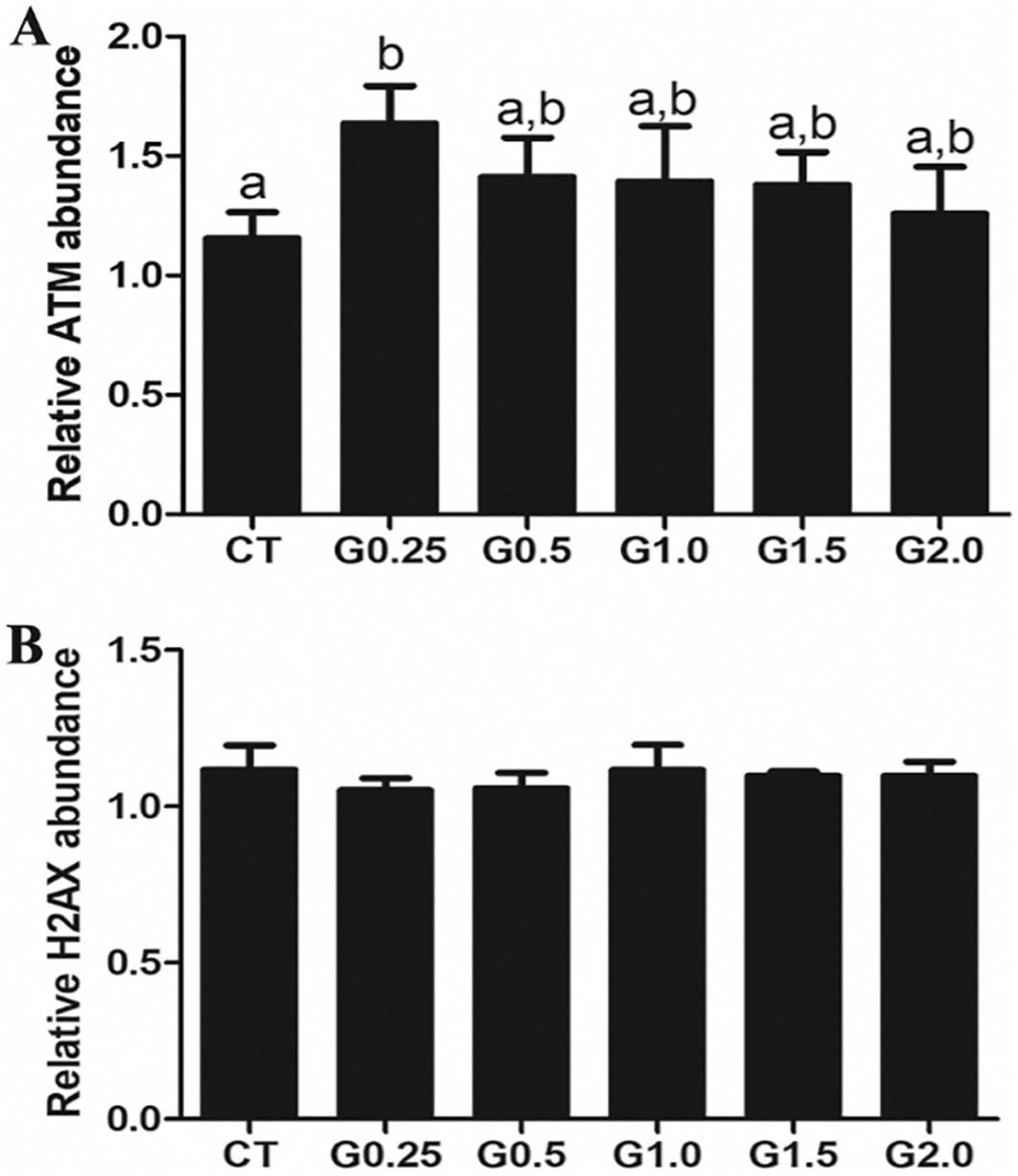
Consequence of GLY exposure on ovarian ATM and abundance. Female C57Bl/6 mice were exposed to saline vehicle control (CT) or increasing doses of GLY (0.25 mg/kg (G0.25), 0.5 mg/kg (G0.5), 1 mg/kg (G1.0), 1.5 mg/kg (G1.5) or 2 mg/kg (G2.0)) per os from a pipette tip five days per week for 20 weeks. Ovaries were homogenized and western blotting performed to quantify protein abundance of (A) ATM and (B) γH2AX. Data points represent mean +/− SEM. Different letters represent differences between treatments; P < .05.
Fig. 8.
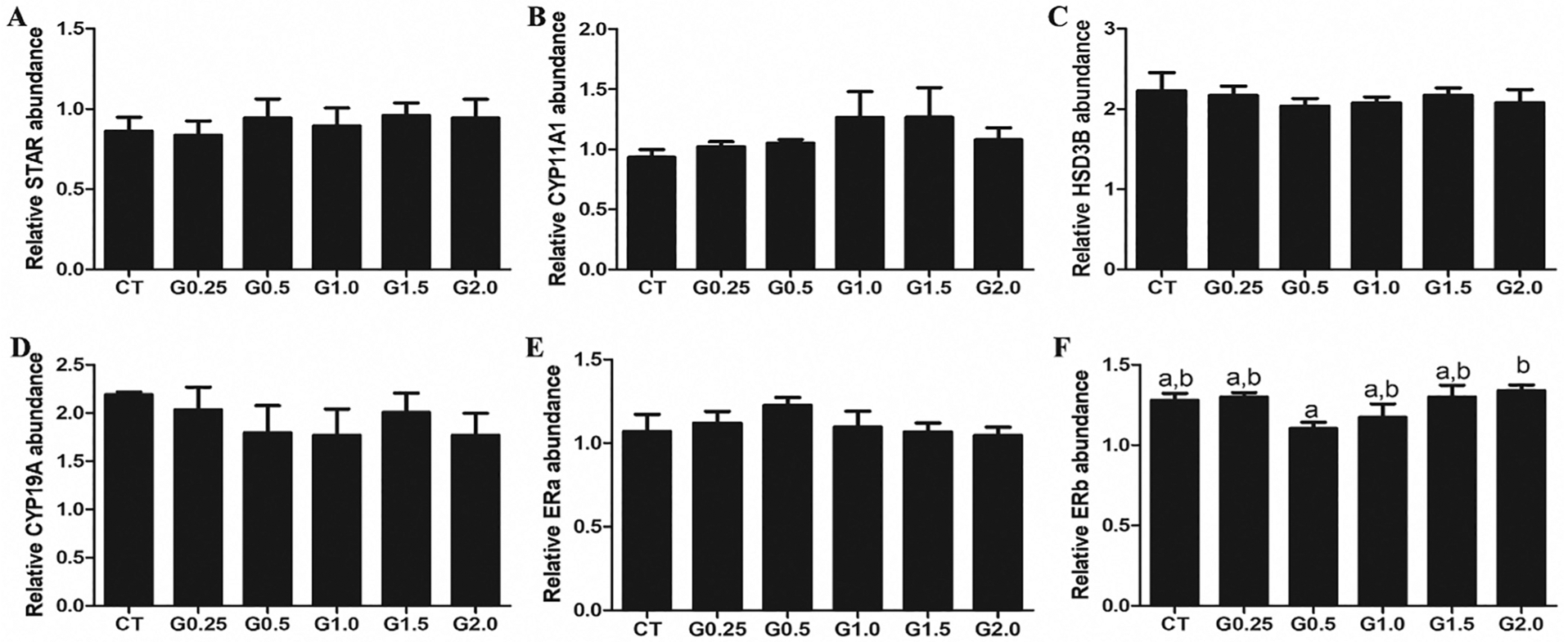
Effect of GLY exposure on ovarian steroidogenic proteins. Female C57Bl/6 mice were exposed to saline vehicle control (CT) or increasing doses of GLY (0.25 mg/kg (G0.25), 0.5 mg/kg (G0.5), 1 mg/kg (G1.0), 1.5 mg/kg (G1.5) or 2 mg/kg (G2.0)) per os from a pipette tip five days per week for 20 weeks. Ovaries were homogenized and western blotting performed to quantify protein abundance of (A) STAR, (B) CYP11A1, (C) HSD3B, (D) CYP19A, (E) ERa and (F) ERb. Data points represent mean +/− SEM. Different letters represent differences between treatments; P < .05.
4. Ovarian global proteome impacts of GLY exposure
LC-MS/MS was performed on ovarian protein homogenates and bioinformatic comparison between each dose with CT-treated mouse ovaries performed. There was a dose-dependent effect (P < .1) of GLY exposure on ovarian protein abundance. (See Fig. 9.)
Fig. 9.
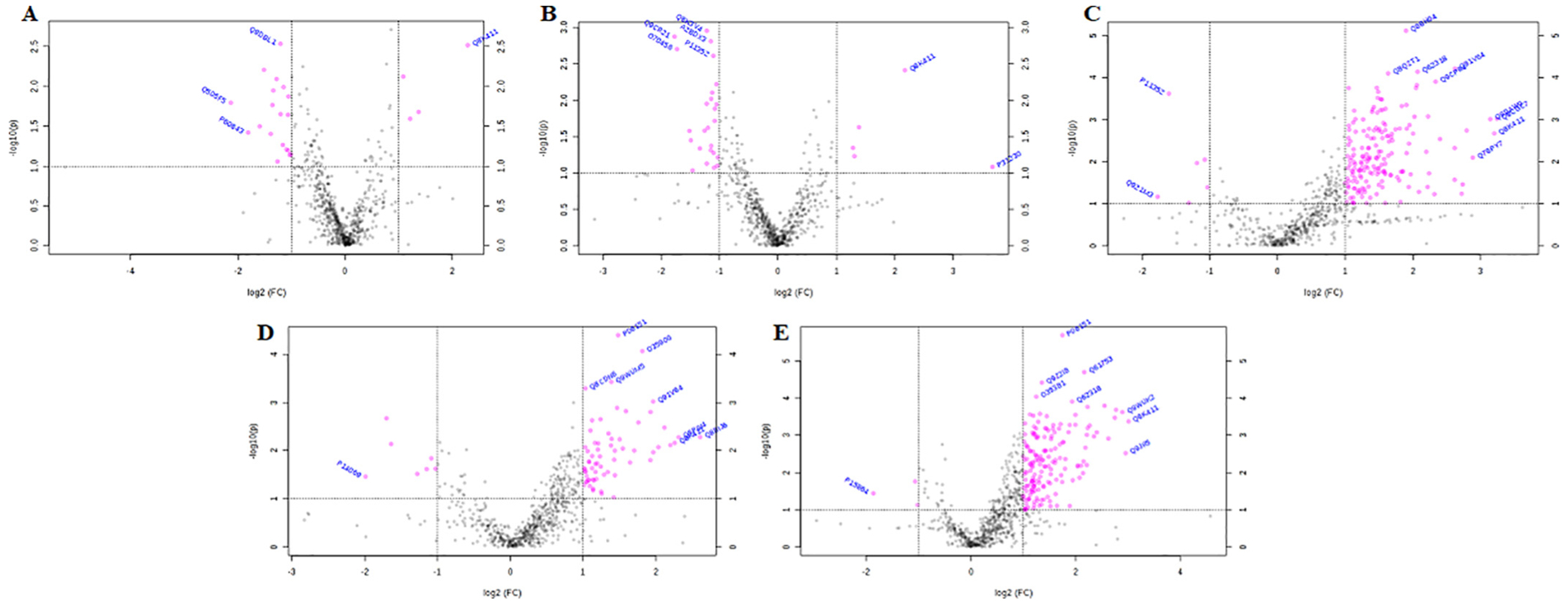
Ovarian proteomic impact of GLY exposure. Female C57Bl/6 mice were exposed to saline vehicle control (CT) or increasing doses of GLY (0.25 mg/kg (G0.25), 0.5 mg/kg (G0.5), 1 mg/kg (G1.0), 1.5 mg/kg (G1.5) or 2 mg/kg (G2.0)) per os from a pipette tip five days per week for 20 weeks. Ovaries were homogenized and LC-MS/MS was performed to detect peptides. Bioinformatic analyses were performed to determine differences in protein abundance due to (A) G0.25, (B) G0.5, (C) G1.0, (D) G1.5, and (E) G2.0 relative to CT. Pink dots in volcano plots above the horizontal dotted line indicate proteins that differed in abundance when compared to CT. Dots to the right of the right dotted vertical line are proteins that were increased (P < .05) in abundance and dots to the left of the most left vertical dotted line indicate proteins that were reduced (P < .05) in abundance (n = 10 ovaries per treatment).
Exposure to G0.25 increased four and decreased 19 proteins relative to CT (Supplemental Table 1). Ovaries from mice that received G0.5 had five increased and 25 decreased proteins. (Supplemental Table 5). G1.0 exposure increased abundance of 65 and decreased seven ovarian proteins (Supplemental Table 5). There were 145 increased and 2 decreased proteins in the G1.5, relative to CT, group (Supplemental Table 7). Finally, exposure to G2.0 increased 159 and decreased four ovarian proteins Supplemental Table 9). Interestingly, there were two proteins that was increased in all GLY dosages - bifunctional glutamate/proline-tRNA ligase synthetase and presequence protease, mitochondrial. Also, protein S100-A10 was increased (P < .05) in all exposure groups except G0.5.
Pathway analysis of ovarian protein changes was performed using DAVID. Exposure to G0.25 altered “proteasome”, “fatty acid degradation”, “fatty acid metabolism” and “valine, leucine and isoleucine degradation” pathways (Supplemental Table 2). “Glutathione metabolism” was altered by G0.5 exposure (Supplemental Table 4). The pathways altered by G1.0 were “propanoate metabolism”, “TCA cycle”, “metabolic pathways”, carbon metabolism”, “spliceosome” “oxidative phosphorylation” and “ribosome” (Supplemental Table 6). Exposure to G1.5 affected pathways involved in “carbon metabolism”, “propanoate metabolism”, “metabolic pathways”, “TCA cycle”, “ribosome”, “biosynthesis of amino acids” and “valine, leucine and isoleucine degradation” (Supplemental Table 8). The highest G2.0 dose had more pathways identified: “ribosome”, “carbon metabolism”, pyruvate metabolism”, glycolysis/gluconeogenesis”, “fatty acid degradation”, “valine, leucine and isoleucine degradation”, “metabolic pathways”, “propanoate metabolism”, “spliceosome”, “TCA cycle”, “proteasome” and “glutathione metabolism” were all identified as GLY targets (Supplemental Table 10).
5. Discussion
Glyphosate is an herbicide used for weed control since 1974 and introduction of GLY resistant crops in 1996 has dramatically increased usage (Duke and Powles, 2008). A dose of GLY of 1 mg/kg/day from chronic dietary exposure has been deemed safe (Agency, 2017) and a NOAEL for reproductive toxicity has been identified as 2132 mg/kg/day (Williams et al., 2000). While many other studies have investigated dramatically higher GLY exposure levels, the current study sought to determine ovarian impacts of GLY at an exposure level considered to elicit no reproductive or developmental effects of 2 mg/kg/day (Agency, 1993) and lower. Additionally, mice drank a GLY solution from a pipette tip to simulate oral exposure and the study lasted twenty weeks to evaluate chronic exposure. Thus, the dose, duration and route of exposure were low level and chronic. Further, we chose the reductionist approach and evaluated the effects of GLY and not additional adjuvants that are used in herbicide formulations.
Lack of overt toxicity and general malaise in the exposed mice was demonstrated as none of the GLY exposures reduced body weight relative to controls, however, the intermediary exposure of G1.0 increased body weight. In addition, feed intake was monitored for the last ten weeks of the study, and while none of the GLY exposures reduced feed intake, the higher two exposures of G1.5 and G2.0 increased feed intake. These findings raise the potential that increased body weight at G1.0, despite no overt increase in feed intake, could represent a decrease in metabolic rate. Increased feed intake at G1.5 and G2.0 could also indicate altered metabolic rate since despite increased feed intake, it did not translate to increased body weight. Regardless of the apparent dichotomy of how GLY affects gross system energetics, the potential for xenobiotic exposures to alter metabolism has previously been documented (Heindel et al., 2017), presenting an interesting observation that warrants future investigation.
The potential impact of GLY exposure on the percentage time spent in stages of the estrous cycle was investigated. Metestrus and diestrus were combined due to the similarity in vaginal cytology between these two stages as previously performed in other studies (Hannon et al., 2016). At completion of the study, the mice were 26 weeks of age, and it was notable that the length of proestrus and estrus were longer than would be observed in younger aged mice. This observation in reproductively aged mice have been previously noted (Hannon et al., 2016). Exposure to GLY did not alter the time spent at any stage of the estrous cycle, correlating with findings from safety studies on GLY (Williams et al., 2000), supporting that exposure at this level would not translate to an apparent change to the menstrual cycle.
Since GLY has been implicated as an endocrine disruptor in both in vitro or in vivo studies (De Almeida et al., 2018; Mesnage et al., 2017; Nardi et al., 2017; Thongprakaisang et al., 2013; Walsh et al., 2000), circulating E2 and P4 levels were measured. Similar to our findings with estrous cyclicity, there was no impact of GLY exposure on the level of E2 or P4 in circulation. These findings are in agreement with the safety evaluation for GLY in which standard endocrinological assessments determined that GLY should not be considered a disruptor of ovarian-produced hormones (Williams et al., 2000). Additionally, there was no impact of GLY exposure at any dosage on abundance of proteins involved in ovarian steroidogenesis (STAR, CYP11A1, HSD3B or CYP19A). Further, there was no effect of GLY exposure on the estrogen receptor, ERA and while there were some observable differences between doses of GLY on ERB, there was no difference from control-treated mice at any dose of GLY. Taken together, our data do not support GLY as an ovarian endocrine disruptor.
In agreement with lack of overt toxicity in terms of body weight loss or reduced feed intake, there was no effect of chronic GLY exposure on the weight of the heart, liver, spleen, kidney or uterus. There was a small reduction in relative liver weight due to G1.0, attributable to the mice in the G1.0 group having increased body weight. Interestingly, the weight of the ovary was increased in the group exposed to G2.0, thought these mice were not heavier than the control-treated counter-parts. Alterations to organ weights in adult rats have been reported however the GLY dose at which these effects were observed was 500 mg/kg (Tang et al., 2017). Consequently, at human relevant GLY exposures, we detected little to no overt issues on key organ weights.
Despite lack of an observable effect of GLY exposure on ovarian cyclicity or hormone production but considering the increased ovarian weight of the G2.0-exposed mice, the potential for GLY to alter ovarian follicular composition was of interest. It is known that chemical exposures can alter numbers of follicles within the ovary (Borgeest et al., 2002; Flaws et al., 1994; Ganesan et al., 2014; Hannon et al., 2015; Keating, and C JM, Sen N, Sipes IG, Hoyer PB., 2009; Lim et al., 2013; Madden et al., 2014) and that fertility outcomes post-xenobiotic exposure are dependent on the follicle pool targeted (Hoyer and Keating, 2014). The number of follicles were classified as primordial, primary, secondary or antral to determine any effects on specific follicular stages of development. Interestingly, numbers of all follicle stages were low, likely reflective of the age of the mice. There was no effect of G0.25-G1.5, however, the number of all follicle types was increased in the G2.0 group, which was in line with the increased ovarian weight observed also at this dosage. While difficult to appreciate the reason for this, it may be that follicular recruitment towards ovulation had stalled. A study that exposed adult rats to GLY (126 or 315 mg/kg) for 60 days documented increased follicular atresia and reduced antral follicle surface area (Hamdaoui et al., 2018), however, this exposure was up to 150-fold higher than is deemed low risk (Agency, 2017). A reduction in transition of primordial to the primary stage due to exposure to a GBH formulation has been reported in rats, though it was not clear if this was due to the gestational or postnatal exposure (Türkmen and Türkmen, 2019).
The phosphatidylinositol-3 kinase (PI3K) pathway plays a key role in follicular recruitment (Keating, and C JM, Sen N, Sipes IG, Hoyer PB., 2009; Liu et al., 2006; Reddy et al., 2009; Reddy et al., 2005), oocyte viability (Brown et al., 2010) and ovarian steroidogenesis (Chen et al., 2007; Fukuda et al., 2009; Zeleznik et al., 2003). Also, chemical exposures can target PI3K signaling to impart ovarian toxicity (Fernandez et al., 2008; Hannon et al., 2014; Keating, and C JM, Sen N, Sipes IG, Hoyer PB., 2009; Sobinoff et al., 2011; Sobinoff et al., 2012). The proxy for PI3K activation is AKT (Datta et al., 1999), thus, the abundance total and phosphorylated AKT were assessed in ovarian homogenates. There was no impact of GLY exposure at any dosage on total or phosphorylated AKT, nor was the ratio of pAKT:AKT altered in any way. Thus, despite higher follicle numbers at G2.0, a mechanistic role for PI3K signaling is not supported.
A genotoxic potential for GLY has been recently suggested by observations of DNA damage in other tissue (Santovito et al., 2018; Suarez-Larios et al., 2017; De Almeida et al., 2018; Milic et al., 2018; Wozniak et al., 2018; Hong et al., 2018; Milic et al., 2018; Avdatek et al., 2018; Anifandis et al., 2018). Ataxia mutated telangiectasia (ATM) is a serine/threonine kinase that phosphorylates histone 2AX (γH2AX) within seconds in a 1:1 ratio with the DNA break (Svetlova et al., 2010) and this event is critical for DNA double strand break repair (Burma et al., 2001; Paull et al., 2000; Rogakou et al., 1998). Exposure of mice to G0.25 increased ATM abundance but there was no impact of any higher GLY doses. There was also no effect of GLY exposure at any dose on ovarian abundance of γH2AX, indicating lack of an ovarian response to double-stranded DNA breaks, discounting GLY as a genotoxicant at the investigated GLY level of exposure.
Finally, LC-MS/MS was used as an unbiased non-targeted approach to determine if GLY affected ovarian protein abundance. Interestingly, there was a dose-dependent impact of GLY exposure on the ovarian proteome. At G0.25 and G0.5, most proteins affected were reduced in abundance, relative to the control-treated mouse ovaries. In the G1.0, G1.5 and G2.0, the majority of proteins that differed from control-treated shifted towards being increased in abundance. Three proteins of interest emerged from this analysis: bifunctional glutamate/proline-tRNA ligase synthetase and presequence protease, mitochondrial were increased at all GLY dosages. In addition, protein S100-A10 was increased in all exposure groups except G0.5. Some deficiencies with this approach are use of total ovarian homogenates and that post-translational modification are not identified, however, protein that are altered in the ovary due to GLY exposure were identified.
Bifunctional glutamate/proline-tRNA ligase synthetase (EPRS) has a role in translational silencing (Sampath et al., 2004) but little to no information on an ovarian role is available. Interestingly, EPRS is associated with tumorigenesis (Fahrmann et al., 2016) and is increased in estrogen-responsive breast cancer cells (Katsyv et al., 2016), thus there could be a function for EPRS in modulating the ovarian response to mutagenic compounds. Ovarian EPRS was increased in a dose-dependent manner relative to control-treated mice at all GLY doses tested.
The annexin A2 protein binding partner S100A10 (Grindheim et al., 2017) is implicated in cancer progression (Huang et al., 2017) including serous ovarian cancer (Lokman et al., 2019). Interestingly, increased S100a10 transcript abundance is associated with oocyte growth in a bovine model (Ghanem et al., 2007). The S100A10 protein was increased relative to control-treated mice at all doses of GLY in a dose-dependent manner, and potentially could contribute to altered folliculogenesis observed at the G2.0 exposure.
Presequence protease, mitochondrial (PITRM1) was increased in a dose-dependent manner due to GLY exposure at all five dosages. Located in the mitochondrial matrix, PITRM1 is involved in limb development (Town et al., 2009). Loss of PITRM1 function is associated with a number of disease states for which mitochondrial insufficiency is involved (Brunetti et al., 2016; Langer et al., 2018; Yoshida et al., 2009). There is no information on the role of ovarian PITRM1, however, a potential role in ovarian development and an importance in the female germ cell cannot be discounted.
Some commonalities in pathways affected emerged in the GLY-exposed groups. There were effects on nutrient metabolism and degradation as indicated by changes in proteins involved in fatty acid degradation, fatty acid metabolism, valine, leucine and isoleucine degradation, propanoate metabolism, TCA cycle, metabolic pathways, carbon metabolism, biosynthesis of amino acids, and glycolysis/gluconeogenesis. Alterations to ovarian energetics have been described to occur during follicular growth (Cinco et al., 2016). In addition, proteins involved in mitochondrial function and glutathione (GSH) metabolism were identified to be altered in abundance by GLY exposure.
GLY has been suggested to cause mitochondrial dysfunction in other models and non-ovarian tissues (Bailey et al., 2018; Burchfield et al., 2018; Lopes et al., 2018; Pereira et al., 2018; Bonvallot et al., 2018). In the male reproductive tract, GBH formulations caused mitochondrial dysfunction in a mouse Sertoli cell line where reduced SDH activity was demonstrated and reduced total GST activity was demonstrated (Vanlaeys et al., 2018). In human sperm, GLY exposure ex vivo caused a reduction in mitochondrial viability in the midpiece of spermatozoa which was associated with reduced motility (Anifandis et al., 2017). Mitochondria from the oocyte are critically important organelles, not only because of their function but also due to their uniparental transmission from the maternal lineage. Declining mitochondrial number and function are associated with oocyte aging (May-Panloup et al., 2016). Since ovarian aging is not concurrent with systemic aging, any factor that hastens ovarian aging can have long-term detrimental effects on women’s health.
Ovarian GSH metabolism was identified as being affected by GLY exposure. It is recognized that GSH has many cellular roles, but of particular interest is that GSH conjugation to chemicals represents predominantly a detoxification reaction (Awasthi et al., 2009; Bhattacharya and Keating, 2012; Cole and Deeley, 2006; Cortes-Wanstreet et al., 2009; Hayes and Pulford, 1995). Further, the enzymes that catalyze conjugation of GSH to chemicals, glutathione-S transferases, play additional roles in modulating apoptosis (Adler et al., 1999; Bhattacharya and Keating, 2012; Bhattacharya et al., 2013; Cho et al., 2001; Keating et al., 2010). Increases in GSTA4 and GSTK1 were identified at higher GLY exposures, while GSTP and GSTM were decreased by lower GLY exposures. Glutathione peroxidase 1 (GPX1) was decreased in abundance by G2.0, G1.0 and G0.5, respectively. Also, superoxide dismutase (SOD1) was decreased by G0.5. The antioxidant peredoxin 6 (PRDX6) was increased by G1.5, and interestingly has been demonstrated to be important in protecting sperm from DNA damage and oxidative stress (Fernandez and O’Flaherty, 2018; O’Flaherty, 2018) supporting a role for PRDX8 in germ cells. Oxidative stress contributes to ovarian aging (Lim and Luderer, 2010) and reduces fecundity (Ozer et al., 2016; Papalou et al., 2016). Chemical exposures including chromium (Banu et al., 2016), cigarette smoke (Camlin et al., 2016; Siddique et al., 2014; Tsai-Turton and Luderer, 2006), phthalates (Wang et al., 2012a; Wang et al., 2012b), bisphenol A (Berger et al., 2016), and methoxychlor (Gupta et al., 2006) induce ovarian ROS generation as a mode of toxicity.
Taken together, this study has determined lack of any effect of chronic GLY exposure at dosages relevant to human exposure (Agency, 2017) on liver, heart, spleen, kidney or uterine weight, AKT or γH2AX protein abundance, or the ovarian level of steroidogenic proteins. Nor was there any impact of GLY on estrous cyclicity and circulating E2 or P4. There were dose specific effects on feed intake (G1.5 and G2.0 increased feed intake) and body weight (increased by G1.0). There was also an increase in follicle number and ovarian weight by G2.0 but no effects at lower exposures. A moderate reduction in ATM protein was observed at the G0.25 dose but this was not sustained into higher dosages. Use of a global non-targeted proteomics technique revealed alterations to proteins involved in energetics, oxidative stress and mitochondrial function. Some of these observations may be primary targets of GLY and others could be at least partly attributable to altered feed intake (G1.5 and G2.0), altered body weight (G1.0) and altered ovarian composition (G2.0). Whether these protein changes translate to alterations in protein function within the ovary remains unclear and the findings reflect a single snap-shot in time within the ovary. Examination of timepoints post-exposure would be valuable in understanding long-term outcomes of GLY exposure. These findings pave the way for future studies understanding how low-level chronic chemical exposures can alter ovarian protein abundance.
Supplementary Material
Funding
Supported by R21ES026282 from the National Institute of Environmental Health Sciences to AFK.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.taap.2020.115116.
References
- Abramoff MD, Magalhaes PJ, Ram SJ, 2004. Image processing with imagej. Biophoton. Int 11 (7), 36–42. [Google Scholar]
- Adler V, Yin Z, Fuchs SY, Benezra M, Rosario L, Tew KD, Pincus MR, Sardana M, Henderson CJ, Wolf CR, et al. , 1999. Regulation of jnk signaling by gstp. EMBO J. 18 (5), 1321–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agency, U.S.E.P., 1993. Glyphosate. Prevention PaTS. [Google Scholar]
- Agency, U.S.E.P., 2017. Glyphosate. Dietary exposure analysis in support of registration review In: Prevention OoCSaP, (Washington D.C.). [Google Scholar]
- Alarcon R, Ingaramo PI, Rivera OE, Dioguardi GH, Repetti MR, Demonte LD, Milesi MM, Varayoud J, Munoz-de-Toro M, Luque EH, 2019. Neonatal exposure to a glyphosate-based herbicide alters the histofunctional differentiation of the ovaries and uterus in lambs. Mol. Cell. Endocrinol 482, 45–56. [DOI] [PubMed] [Google Scholar]
- Anifandis G, Amiridis G, Dafopoulos K, Daponte A, Dovolou E, Gavriil E, Gorgogietas V, Kachpani E, Mamuris Z, Messini CI, et al. , 2017. The in vitro impact of the herbicide roundup on human sperm motility and sperm mitochondria. Toxics. 6 (1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anifandis G, Katsanaki K, Lagodonti G, Messini C, Simopoulou M, Dafopoulos K, Daponte A, 2018. The effect of glyphosate on human sperm motility and sperm DNA fragmentation. Int. J. Environ. Res. Public Health 15 (6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuckle TE, Lin Z, Mery LS, 2001. An exploratory analysis of the effect of pesticide exposure on the risk of spontaneous abortion in an Ontario farm population. Environ. Health Perspect 109 (8), 851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avdatek F, Birdane YO, Turkmen R, Demirel HH, 2018. Ameliorative effect of resveratrol on testicular oxidative stress, spermatological parameters and DNA damage in glyphosate-based herbicide-exposed rats. Andrologia 109 (8), 851–857 e13036. [DOI] [PubMed] [Google Scholar]
- Awasthi YC, Chaudhary P, Vatsyayan R, Sharma A, Awasthi S, Sharma R, 2009. Physiological and pharmacological significance of glutathione-conjugate transport. J Toxicol Environ Health B Crit Rev. 12 (7), 540–551. [DOI] [PubMed] [Google Scholar]
- Bailey DC, Todt CE, Burchfield SL, Pressley AS, Denney RD, Snapp IB, Negga R, Traynor WL, Fitsanakis VA, 2018. Chronic exposure to a glyphosate-containing pesticide leads to mitochondrial dysfunction and increased reactive oxygen species production in caenorhabditis elegans. Environ. Toxicol. Pharmacol 57, 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banu SK, Stanley JA, Sivakumar KK, Arosh JA, Burghardt RC, 2016. Resveratrol protects the ovary against chromium-toxicity by enhancing endogenous antioxidant enzymes and inhibiting metabolic clearance of estradiol. Toxicol. Appl. Pharmacol 303, 65–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benbrook CM, 2016. Trends in glyphosate herbicide use in the United States and globally. Environ. Sci. Eur 28 (1), 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A, Ziv-Gal A, Cudiamat J, Wang W, Zhou C, Flaws JA, 2016. The effects of in utero bisphenol a exposure on the ovaries in multiple generations of mice. Reproductive Toxicol. (Elmsford, NY) 60, 39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya P, Keating AF, 2012. Protective role for ovarian glutathione s-transferase isoform pi during 7,12-dimethylbenz[a]anthracene-induced ovotoxicity. Toxicol. Appl. Pharmacol 260 (2), 201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharya P, Madden JA, Sen N, Hoyer PB, Keating AF, 2013. Glutathione s-transferase class mu regulation of apoptosis signal-regulating kinase 1 protein during vcd-induced ovotoxicity in neonatal rat ovaries. Toxicol. Appl. Pharmacol 267 (1), 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonvallot N, Canlet C, Blas YEF, Gautier R, Tremblay-Franco M, Chevolleau S, Cordier S, Cravedi JP, 2018. Metabolome disruption of pregnant rats and their offspring resulting from repeated exposure to a pesticide mixture representative of environmental contamination in brittany. PLoS One 13 (6), e0198448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgeest C, Symonds D, Mayer LP, Hoyer PB, Flaws JA, 2002. Methoxychlor may cause ovarian follicular atresia and proliferation of the ovarian epithelium in the mouse. Toxicol. Sci 68 (2), 473–478. [DOI] [PubMed] [Google Scholar]
- Brown C, LaRocca J, Pietruska J, Ota M, Anderson L, Smith SD, Weston P, Rasoulpour T, Hixon ML, 2010. Subfertility caused by altered follicular development and oocyte growth in female mice lacking pkb alpha/akt1. Biol. Reprod 82 (2), 246–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetti D, Torsvik J, Dallabona C, Teixeira P, Sztromwasser P, Fernandez-Vizarra E, Cerutti R, Reyes A, Preziuso C, D’Amati G, et al. , 2016. Defective pitrm1 mitochondrial peptidase is associated with abeta amyloidotic neurodegeneration. EMBO molecular medicine. 8 (3), 176–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchfield SL, Bailey DC, Todt CE, Denney RD, Negga R, Fitsanakis VA, 2018. Acute exposure to a glyphosate-containing herbicide formulation inhibits complex ii and increases hydrogen peroxide in the model organism caenorhabditis elegans. Environ. Toxicol. Pharmacol 66, 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burma S, Chen BP, Murphy M, Kurimasa A, Chen DJ, 2001. Atm phosphorylates histone h2ax in response to DNA double-strand breaks. J. Biol. Chem 276 (45), 42462–42467. [DOI] [PubMed] [Google Scholar]
- Camlin NJ, Sobinoff AP, Sutherland JM, Beckett EL, Jarnicki AG, Vanders RL, Hansbro PM, McLaughlin EA, Holt JE, 2016. Maternal smoke exposure impairs the long-term fertility of female offspring in a murine model. Biol. Reprod 94 (2), 39. [DOI] [PubMed] [Google Scholar]
- Chen YJ, Hsiao PW, Lee MT, Mason JI, Ke FC, Hwang JJ, 2007. Interplay of pi3k and camp/pka signaling, and rapamycin-hypersensitivity in tgfbeta1 enhancement of fsh-stimulated steroidogenesis in rat ovarian granulosa cells. J. Endocrinol 192 (2), 405–419. [DOI] [PubMed] [Google Scholar]
- Cho SG, Lee YH, Park HS, Ryoo K, Kang KW, Park J, Eom SJ, Kim MJ, Chang TS, Choi SY, et al. , 2001. Glutathione s-transferase mu modulates the stress-activated signals by suppressing apoptosis signal-regulating kinase 1. J. Biol. Chem 276 (16), 12749–12755. [DOI] [PubMed] [Google Scholar]
- Cinco R, Digman MA, Gratton E, Luderer U, 2016. Spatial characterization of bioenergetics and metabolism of primordial to preovulatory follicles in whole ex vivo murine ovary. Biol. Reprod 95 (6), 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SP, Deeley RG, 2006. Transport of glutathione and glutathione conjugates by mrp1. Trends Pharmacol. Sci 27 (8), 438–446. [DOI] [PubMed] [Google Scholar]
- Cortes-Wanstreet MM, Giedzinski E, Limoli CL, Luderer U, 2009. Overexpression of glutamate-cysteine ligase protects human cov434 granulosa tumour cells against oxidative and gamma-radiation-induced cell death. Mutagenesis. 24 (3), 211–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curwin BD, Hein MJ, Sanderson WT, Striley C, Heederik D, Kromhout H, Reynolds SJ, Alavanja MC, 2007a. Pesticide dose estimates for children of Iowa farmers and non-farmers. Environ. Res 105 (3), 307–315. [DOI] [PubMed] [Google Scholar]
- Curwin BD, Hein MJ, Sanderson WT, Striley C, Heederik D, Kromhout H, Reynolds SJ, Alavanja MC, 2007b. Urinary pesticide concentrations among children, mothers and fathers living in farm and non-farm households in Iowa. Ann. Occup. Hyg 51 (1), 53–65. [DOI] [PubMed] [Google Scholar]
- Datta SR, Brunet A, Greenberg ME, 1999. Cellular survival: a play in three akts. Genes Dev. 13 (22), 2905–2927. [DOI] [PubMed] [Google Scholar]
- De Almeida LKS, Pletschke BI, Frost CL, 2018. Moderate levels of glyphosate and its formulations vary in their cytotoxicity and genotoxicity in a whole blood model and in human cell lines with different estrogen receptor status. 3. Biotech 8 (10), 438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos M, Devroey P, Fauser BC, 2010. Primary ovarian insufficiency. Lancet. 376 (9744), 911–921. [DOI] [PubMed] [Google Scholar]
- Duke SO, Powles SB, 2008. Glyphosate: a once-in-a-century herbicide. Pest Manag. Sci 64 (4), 319–325. [DOI] [PubMed] [Google Scholar]
- Fahrmann JF, Grapov D, Phinney BS, Stroble C, DeFelice BC, Rom W, Gandara DR, Zhang Y, Fiehn O, Pass H, et al. , 2016. Proteomic profiling of lung adenocarcinoma indicates heightened DNA repair, antioxidant mechanisms and identifies lasp1 as a potential negative predictor of survival. Clin. Proteomics 13, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez MC, O’Flaherty C, 2018. Peroxiredoxin 6 Is the Primary Antioxidant Enzyme for the Maintenance of Viability and DNA Integrity in Human Spermatozoa. Human Reproduction. Oxford, England [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez SM, Keating AF, Christian PJ, Sen N, Hoying JB, Brooks HL, Hoyer PB, 2008. Involvement of the kit/kitl signaling pathway in 4-vinylcyclohexene diepoxide-induced ovarian follicle loss in rats. Biol. Reprod 79 (2), 318–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flaws JA, Doerr JK, Sipes IG, Hoyer PB, 1994. Destruction of preantral follicles in adult rats by 4-vinyl-1-cyclohexene diepoxide. Reproductive toxicology (Elmsford, NY). 8 (6), 509–514. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Orisaka M, Tajima K, Hattori K, Kotsuji F, 2009. Luteinizing hormone-induced akt phosphorylation and androgen production are modulated by map kinase in bovine theca cells. J Ovarian Res. 2 (1), 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesan S, Nteeba J, Keating AF, 2014. Enhanced susceptibility of ovaries from obese mice to 7,12-dimethylbenz[a]anthracene-induced DNA damage. Toxicol. Appl. Pharmacol 281 (2), 203–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanem N, Holker M, Rings F, Jennen D, Tholen E, Sirard MA, Torner H, Kanitz W, Schellander K, Tesfaye D, 2007. Alterations in transcript abundance of bovine oocytes recovered at growth and dominance phases of the first follicular wave. BMC Dev. Biol 7, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT, 2015. Edc-2: the endocrine society’s second scientific statement on endocrine-disrupting chemicals. Endocr. Rev 36 (6), E1–e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grindheim AK, Saraste J, Vedeler A, 2017. Protein phosphorylation and its role in the regulation of annexin a2 function. Biochim. Biophys. Acta, Gen. Subj 1861 (11 Pt A), 2515–2529. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Schuh RA, Fiskum G, Flaws JA, 2006. Methoxychlor causes mitochondrial dysfunction and oxidative damage in the mouse ovary. Toxicol. Appl. Pharmacol 216 (3), 436–445. [DOI] [PubMed] [Google Scholar]
- Hamdaoui L, Naifar M, Rahmouni F, Harrabi B, Ayadi F, Sahnoun Z, Rebai T, 2018. Subchronic exposure to Kalach 360 sl-induced endocrine disruption and ovary damage in female rats. Arch. Physiol. Biochem 124 (1), 27–34. [DOI] [PubMed] [Google Scholar]
- Hamdaoui L, Naifar M, Rahmouni F, Ayadi F, Rebai T, 2019. Sub-chronic exposure to Kalach 360 sl-induced damage in rats’ liver and hematological system. Environ. Sci. Pollut. Res. Int 26 (36), 36634–36646. [DOI] [PubMed] [Google Scholar]
- Hannon PR, Peretz J, Flaws JA, 2014. Daily exposure to di(2-ethylhexyl) phthalate alters estrous cyclicity and accelerates primordial follicle recruitment potentially via dysregulation of the phosphatidylinositol 3-kinase signaling pathway in adult mice. Biol. Reprod 90 (6), 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon PR, Brannick KE, Wang W, Gupta RK, Flaws JA, 2015. Di(2-ethylhexyl) phthalate inhibits antral follicle growth, induces atresia, and inhibits steroid hormone production in cultured mouse antral follicles. Toxicol. Appl. Pharmacol 284 (1), 42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannon PR, Niermann S, Flaws JA, 2016. Acute exposure to di(2-ethylhexyl) phthalate in adulthood causes adverse reproductive outcomes later in life and accelerates reproductive aging in female mice. Toxicol. Sci 150 (1), 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JD, Pulford DJ, 1995. The glutathione s-transferase supergene family: regulation of gst and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit. Rev. Biochem. Mol. Biol 30 (6), 445–600. [DOI] [PubMed] [Google Scholar]
- Hecker M, Hollert H, Cooper R, Vinggaard AM, Akahori Y, Murphy M, Nellemann C, Higley E, Newsted J, Laskey J, et al. , 2011. The oecd validation program of the h295r steroidogenesis assay: phase 3. Final inter-laboratory validation study. Environ. Sci. Pollut. Res. Int 18 (3), 503–515. [DOI] [PubMed] [Google Scholar]
- Heindel JJ, Blumberg B, Cave M, Machtinger R, Mantovani A, Mendez MA, Nadal A, Palanza P, Panzica G, Sargis R, et al. , 2017. Metabolism disrupting chemicals and metabolic disorders. Reproductive toxicology (Elmsford, NY) 68, 3–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y, Yang X, Huang Y, Yan G, Cheng Y, 2018. Assessment of the oxidative and genotoxic effects of the glyphosate-based herbicide roundup on the freshwater shrimp, macrobrachium nipponensis. Chemosphere. 210, 896–906. [DOI] [PubMed] [Google Scholar]
- Hoyer PB, 2005. Damage to ovarian development and function. Cell Tissue Res. 322 (1), 99–106. [DOI] [PubMed] [Google Scholar]
- Hoyer PB, Keating AF, 2014. Xenobiotic effects in the ovary: temporary versus permanent infertility. Expert Opin. Drug Metab. Toxicol 10 (4), 511–523. [DOI] [PubMed] [Google Scholar]
- Hoyer PB, Sipes IG, 1996. Assessment of follicle destruction in chemical-induced ovarian toxicity. Annu. Rev. Pharmacol. Toxicol 36, 307–331. [DOI] [PubMed] [Google Scholar]
- Hoyer PBDP, 2002. Endocrinology and toxicology: The female reproductive system. In: Derelanko MJ, Hollinger MA (Eds.), Handbook of toxicology. CRC Press, pp. 573–596. [Google Scholar]
- Huang D, Yang Y, Sun J, Dong X, Wang J, Liu H, Lu C, Chen X, Shao J, Yan J, 2017. Annexin a2-s100a10 heterotetramer is upregulated by pml/raralpha fusion protein and promotes plasminogen-dependent fibrinolysis and matrix invasion in acute promyelocytic leukemia. Front. Med 11 (3), 410–422. [DOI] [PubMed] [Google Scholar]
- Ingaramo PI, Guerrero Schimpf M, Milesi MM, Luque EH, Varayoud J, 2019. Acute uterine effects and long-term reproductive alterations in postnatally exposed female rats to a mixture of commercial formulations of endosulfan and glyphosate. Food Chem. Toxicol 134, 110832. [DOI] [PubMed] [Google Scholar]
- Katsyv I, Wang M, Song WM, Zhou X, Zhao Y, Park S, Zhu J, Zhang B, Irie HY, 2016. Eprs is a critical regulator of cell proliferation and estrogen signaling in er + breast cancer. Oncotarget. 7 (43), 69592–69605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating AF, C JM, Sen N, Sipes IG, Hoyer PB., 2009. Effect of phosphatidylinositol-3 kinase inhibition on ovotoxicity caused by 4-vinylcyclohexene diepoxide and 7, 12-dimethylbenz[a]anthracene in neonatal rat ovaries. Toxicol. Appl. Pharmacol 241 (2), 127–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keating AF, Sen N, Sipes IG, Hoyer PB, 2010. Dual protective role for glutathione s-transferase class pi against vcd-induced ovotoxicity in the rat ovary. Toxicol. Appl. Pharmacol 247 (2), 71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudsen LE, Hansen PW, Mizrak S, Hansen HK, Morck TA, Nielsen F, Siersma V, Mathiesen L, 2017. Biomonitoring of danish school children and mothers including biomarkers of pbde and glyphosate. Rev. Environ. Health 32 (3), 279–290. [DOI] [PubMed] [Google Scholar]
- Langer Y, Aran A, Gulsuner S, Abu Libdeh B, Renbaum P, Brunetti D, Teixeira PF, Walsh T, Zeligson S, Ruotolo R, et al. , 2018. Mitochondrial pitrm1 peptidase loss-of-function in childhood cerebellar atrophy. J. Med. Genet 55 (9), 599–606. [DOI] [PubMed] [Google Scholar]
- Lim J, Luderer U, 2010. Oxidative damage increases and antioxidant gene expression decreases with aging in the mouse ovary. Biol. Reprod 84 (4), 775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J, Lawson GW, Nakamura BN, Ortiz L, Hur JA, Kavanagh TJ, Luderer U, 2013. Glutathione-deficient mice have increased sensitivity to transplacental benzo [a]pyrene-induced premature ovarian failure and ovarian tumorigenesis. Cancer Res. 73 (2), 908–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu K, Rajareddy S, Liu L, Jagarlamudi K, Boman K, Selstam G, Reddy P, 2006. Control of mammalian oocyte growth and early follicular development by the oocyte pi3 kinase pathway: new roles for an old timer. Dev. Biol 299 (1), 1–11. [DOI] [PubMed] [Google Scholar]
- Lokman NA, Ho R, Gunasegaran K, Bonner WM, Oehler MK, Ricciardelli C, 2019. Anti-tumour effects of all-trans retinoid acid on serous ovarian cancer. J.Exp. & Clin. Cancer Res. CR 38 (1), 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes FM, Sandrini JZ, Souza MM, 2018. Toxicity induced by glyphosate and glyphosate-based herbicides in the zebrafish hepatocyte cell line (zf-l). Ecotoxicol. Environ. Saf 162, 201–207. [DOI] [PubMed] [Google Scholar]
- Madden JA, Hoyer PB, Devine PJ, Keating AF, 2014. Involvement of a volatile metabolite during phosphoramide mustard-induced ovotoxicity. Toxicol. Appl. Pharmacol 277 (1), 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May-Panloup P, Boucret L, Chao de la Barca JM, Desquiret-Dumas V, Ferre-L’Hotellier V, Moriniere C, Descamps P, Procaccio V, Reynier P, 2016. Ovarian ageing: the role of mitochondria in oocytes and follicles. Hum. Reprod. Update 22 (6), 725–743. [DOI] [PubMed] [Google Scholar]
- Mesnage R, Phedonos A, Biserni M, Arno M, Balu S, Corton JC, Ugarte R, Antoniou MN, 2017. Evaluation of estrogen receptor alpha activation by glyphosate-based herbicide constituents. Food Chem. Toxicol 108 (Pt A), 30–42. [DOI] [PubMed] [Google Scholar]
- Milic M, Zunec S, Micek V, Kasuba V, Mikolic A, Lovakovic BT, Semren TZ, Pavicic I, Cermak AMM, Pizent A, et al. , 2018. Oxidative stress, cholinesterase activity, and DNA damage in the liver, whole blood, and plasma of wistar rats following a 28-day exposure to glyphosate. Arhiv za higijenu rada i toksikologiju. 69 (2), 154–168. [DOI] [PubMed] [Google Scholar]
- Mills PJ, Kania-Korwel I, Fagan J, McEvoy LK, Laughlin GA, Barrett-Connor E, 2017. Excretion of the herbicide glyphosate in older adults between 1993 and 2016. Jama. 318 (16), 1610–1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nardi J, Moras PB, Koeppe C, Dallegrave E, Leal MB, Rossato-Grando LG, 2017. Prepubertal subchronic exposure to soy milk and glyphosate leads to endocrine disruption. Food Chem. Toxicol 100, 247–252. [DOI] [PubMed] [Google Scholar]
- O’Flaherty C, 2018. Peroxiredoxin 6: the protector of male fertility. Antioxidants (Basel, Switzerland) 7 (12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer A, Bakacak M, Kiran H, Ercan O, Kostu B, Kanat-Pektas M, Kilinc M, Aslan F, 2016. Increased oxidative stress is associated with insulin resistance and infertility in polycystic ovary syndrome. Ginekol. Pol 87 (11), 733–738. [DOI] [PubMed] [Google Scholar]
- Papalou O, Victor VM, Diamanti-Kandarakis E, 2016. Oxidative stress in polycystic ovary syndrome. Curr. Pharm. Des 22 (18), 2709–2722. [DOI] [PubMed] [Google Scholar]
- Parvez S, Gerona RR, Proctor C, Friesen M, Ashby JL, Reiter JL, Lui Z, Winchester PD, 2018. Glyphosate exposure in pregnancy and shortened gestational length: a prospective Indiana birth cohort study. Environ. health : a global access science source 17 (1), 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S, Zhou C, Rattan S, Flaws JA, 2015. Effects of endocrine-disrupting chemicals on the ovary. Biol. Reprod 93 (1), 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull TT, Rogakou EP, Yamazaki V, Kirchgessner CU, Gellert M, Bonner WM, 2000. A critical role for histone h2ax in recruitment of repair factors to nuclear foci after DNA damage. Curr. Biol. : CB 10 (15), 886–895. [DOI] [PubMed] [Google Scholar]
- Perego MC, Caloni F, Cortinovis C, Schutz LF, Albonico M, Tsuzukibashi D, Spicer LJ, 2017. Influence of a roundup formulation on glyphosate effects on steroidogenesis and proliferation of bovine granulosa cells in vitro. Chemosphere. 188, 274–279. [DOI] [PubMed] [Google Scholar]
- Pereira AG, Jaramillo ML, Remor AP, Latini A, Davico CE, da Silva ML, Muller YMR, Ammar D, Nazari EM, 2018. Low-concentration exposure to glyphosate-based herbicide modulates the complexes of the mitochondrial respiratory chain and induces mitochondrial hyperpolarization in the danio rerio brain. Chemosphere. 209, 353–362. [DOI] [PubMed] [Google Scholar]
- Rattan S, Zhou C, Chiang C, Mahalingam S, Brehm E, Flaws JA, 2017. Exposure to endocrine disruptors during adulthood: consequences for female fertility. J. Endocrinol 233 (3), R109–r129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy P, Shen L, Ren C, Boman K, Lundin E, Ottander U, Lindgren P, Liu YX, Sun QY, Liu K, 2005. Activation of akt (pkb) and suppression of fkhrl1 in mouse and rat oocytes by stem cell factor during follicular activation and development. Dev. Biol 281 (2), 160–170. [DOI] [PubMed] [Google Scholar]
- Reddy P, Adhikari D, Zheng W, Liang S, Hamalainen T, Tohonen V, Ogawa W, Noda T, Volarevic S, Huhtaniemi I, et al. , 2009. Pdk1 signaling in oocytes controls reproductive aging and lifespan by manipulating the survival of primordial follicles. Hum. Mol. Genet 18 (15), 2813–2824. [DOI] [PubMed] [Google Scholar]
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM, 1998. DNA double-stranded breaks induce histone h2ax phosphorylation on serine 139. J. Biol. Chem 273 (10), 5858–5868. [DOI] [PubMed] [Google Scholar]
- Sampath P, Mazumder B, Seshadri V, Gerber CA, Chavatte L, Kinter M, Ting SM, Dignam JD, Kim S, Driscoll DM, et al. , 2004. Noncanonical function of glutamyl-prolyl-trna synthetase: gene-specific silencing of translation. Cell. 119 (2), 195–208. [DOI] [PubMed] [Google Scholar]
- Santovito A, Ruberto S, Gendusa C, Cervella P, 2018. In vitro evaluation of genomic damage induced by glyphosate on human lymphocytes. Environ. Sci. Pollut. Res. Int 25 (34), 34693–34700. [DOI] [PubMed] [Google Scholar]
- Senapati S, 2018. Infertility: a marker of future health risk in women? Fertil. Steril 110 (5), 783–789. [DOI] [PubMed] [Google Scholar]
- Siddique S, Sadeu JC, Foster WG, Feng YL, Zhu J, 2014. In vitro exposure to cigarette smoke induces oxidative stress in follicular cells of f(1) hybrid mice. Journal of applied toxicology : JAT 34 (2), 224–226. [DOI] [PubMed] [Google Scholar]
- Sobinoff AP, Mahony M, Nixon B, Roman SD, McLaughlin EA, 2011. Understanding the villain: Dmba-induced preantral ovotoxicity involves selective follicular destruction and primordial follicle activation through pi3k/akt and mtor signaling. Toxicol. Sci 123 (2), 563–575. [DOI] [PubMed] [Google Scholar]
- Sobinoff AP, Nixon B, Roman SD, McLaughlin EA, 2012. Staying alive: Pi3k pathway promotes primordial follicle activation and survival in response to 3-mc induced ovotoxicity. Toxicol. Sci 128 (1), 258–271. [DOI] [PubMed] [Google Scholar]
- Soukup ST, Merz B, Bub A, Hoffmann I, Watzl B, Steinberg P, Kulling SE, 2020. Glyphosate and ampa levels in human urine samples and their correlation with food consumption: results of the cross-sectional karmen study in Germany. Arch. Toxicol 94 (5), 1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suarez-Larios K, Salazar-Martinez AM, Montero-Montoya R, 2017. Screening of pesticides with the potential of inducing dsb and successive recombinational repair. J. Toxicol 2017, 3574840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svetlova MP, Solovjeva LV, Tomilin NV, 2010. Mechanism of elimination of phosphorylated histone h2ax from chromatin after repair of DNA double-strand breaks. Mutat. Res 685 (1–2), 54–60. [DOI] [PubMed] [Google Scholar]
- Tang J, Hu P, Li Y, Win-Shwe T-T, Li C, 2017. Ion imbalance is involved in the mechanisms of liver oxidative damage in rats exposed to glyphosate. Front. Physiol 8, 1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongprakaisang S, Thiantanawat A, Rangkadilok N, Suriyo T, Satayavivad J, 2013. Glyphosate induces human breast cancer cells growth via estrogen receptors. Food Chem. Toxicol 59, 129–136. [DOI] [PubMed] [Google Scholar]
- Town L, McGlinn E, Fiorenza S, Metzis V, Butterfield NC, Richman JM, Wicking C, 2009. The metalloendopeptidase gene pitrm1 is regulated by hedgehog signaling in the developing mouse limb and is expressed in muscle progenitors. Dev. Dyn 238 (12), 3175–3184. [DOI] [PubMed] [Google Scholar]
- Tsai-Turton M, Luderer U, 2006. Opposing effects of glutathione depletion and follicle-stimulating hormone on reactive oxygen species and apoptosis in cultured preovulatory rat follicles. Endocrinology. 147 (3), 1224–1236. [DOI] [PubMed] [Google Scholar]
- Türkmen R, Türkmen T, 2019. Prenatal and neonatal exposure to glyphosate-based herbicide reduces the primordial to primary transition in the newborn rat ovary: a preliminary study. Kocatepe Veterinary J 12 (2), 168–177. [Google Scholar]
- Vanlaeys A, Dubuisson F, Seralini GE, Travert C, 2018. Formulants of glyphosate-based herbicides have more deleterious impact than glyphosate on tm4 sertoli cells. Toxicol. in Vitro 52, 14–22. [DOI] [PubMed] [Google Scholar]
- Walsh LP, McCormick C, Martin C, Stocco DM, 2000. Roundup inhibits steroidogenesis by disrupting steroidogenic acute regulatory (star) protein expression. Environ. Health Perspect 108 (8), 769–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Craig ZR, Basavarajappa MS, Gupta RK, Flaws JA, 2012a. Di (2-ethylhexyl) phthalate inhibits growth of mouse ovarian antral follicles through an oxidative stress pathway. Toxicol. Appl. Pharmacol 258 (2), 288–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Craig ZR, Basavarajappa MS, Hafner KS, Flaws JA, 2012b. Mono-(2-ethylhexyl) phthalate induces oxidative stress and inhibits growth of mouse ovarian antral follicles. Biol. Reprod 87 (6), 152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GM, Kroes R, Munro IC, 2000. Safety evaluation and risk assessment of the herbicide roundup and its active ingredient, glyphosate, for humans. Regul. Toxicol. Pharmacol 31 (2 Pt 1), 117–165. [DOI] [PubMed] [Google Scholar]
- Wozniak E, Sicinska P, Michalowicz J, Wozniak K, Reszka E, Huras B, Zakrzewski J, Bukowska B, 2018. The mechanism of DNA damage induced by roundup 360 plus, glyphosate and ampa in human peripheral blood mononuclear cells - genotoxic risk assessement. Food Chem. Toxicol 120, 510–522. [DOI] [PubMed] [Google Scholar]
- Xia J, Wishart DS, 2016. Using metaboanalyst 3.0 for comprehensive metabolomics data analysis. Curr. Protoc. Bioinformatics 55 14 10 11–14 10 91. [DOI] [PubMed] [Google Scholar]
- Xia J, Sinelnikov IV, Han B, Wishart DS, 2015. Metaboanalyst 3.0–making metabolomics more meaningful. Nucleic Acids Res. 43 (W1), W251–W257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Kato K, Yokoi K, Oguri M, Watanabe S, Metoki N, Yoshida H, Satoh K, Aoyagi Y, Nozawa Y, et al. , 2009. Association of gene polymorphisms with chronic kidney disease in japanese individuals. Int. J. Mol. Med 24 (4), 539–547. [DOI] [PubMed] [Google Scholar]
- Zeleznik AJ, Saxena D, Little-Ihrig L, 2003. Protein kinase b is obligatory for follicle-stimulating hormone-induced granulosa cell differentiation. Endocrinology. 144 (9), 3985–3994. [DOI] [PubMed] [Google Scholar]
- Zenclussen ML, Casalis PA, Jensen F, Woidacki K, Zenclussen AC, 2014. Hormonal fluctuations during the estrous cycle modulate heme oxygenase-1 expression in the uterus. Front Endocrinol (Lausanne) 5, 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller O, Rhyn P, Rupp H, Zarn JA, Geiser C, 2018. Glyphosate residues in swiss market foods: monitoring and risk evaluation. Food Addit Contam Part B Surveill. 11 (2), 83–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


