Abstract
Background:
Human alcohol self-administration studies employing oral intake are subject to high variability of the resulting blood alcohol concentrations due to idiosyncrasies of gastrointestinal absorption kinetics among subjects. We sought to improve the subjects’ opportunity to control their brain alcohol exposure by computer-assisted i.v. self-administration.
Methods:
Instead of drinking, subjects could request increments of their arterial blood alcohol concentration (aBAC) of precisely 7.5 mg% at any time they wanted by pressing a button, provided their aBAC would not exceed 100 mg%. The latency between pushing the button and reaching the new aBAC peak was preset to be 2.5 minutes on the first day and was randomly changed to 1.5 or 3.5 minutes on days two and three in a crossover design. The necessary rate and amount of alcohol infusion was calculated by the software about once every second. Nine healthy social drinkers (4 females/5 males; mean age 25.0 ± 4.0 yr) participated in 3 sessions each. Outcome measures were mean and maximum observed aBAC, and the number of alcohol requests.
Results:
Maximum aBAC was 76.5 ± 26.3 mg% on average over all experiments. When grouping days two and three according to latency (1.5 vs. 3.5 minutes), maximum aBAC and the number of requests in the session were significantly higher with the faster rise and all three outcome measures were significantly correlated between days. No such correlations were found between the first and either of the following days.
Conclusion:
These data suggest that CASE is practical and safe, and results in considerable alcohol exposure that can be manipulated with parameters chosen for the incremental exposure. Following one practice day, test-retest stability was good, suggesting a potential for use in scientific studies.
Keywords: Ethanol, self administration, infusion
Introduction
Self-administration of ethanol is a useful tool to study human drinking behavior in the laboratory. In the standard design, subjects are presented with a tray of alcoholic drinks and are invited to consume as many of them as they like, or to receive monetary compensation for each drink they reject. Outcome measures are the number of drinks consumed or the achieved blood alcohol concentration. This type of experiments is presumably influenced by several distinct factors, including motivation to drink, sensitivity and tolerance to alcohol, maintenance or loss of control, taste preferences, personality traits such as impulsivity, and the kinetics of gastrointestinal absorption. Alcohol self-administration therefore is an integrative measure which implies the drawback that the impact of the many influential factors cannot be easily dissected. On the other hand, it is a highly valid measure with respect to alcohol use disorders, since the dependent variables comprise the behavior under question.
Prior research using self-administration experiments produced some important positive results showing how alcohol drinking can be modulated in humans. For example, nicotine was shown to increase self-administration in male and to decrease it in female social drinkers, both effects being dose-dependent (Acheson et al., 2006). Naltrexone pretreatment with a fixed dose of 50 mg/day for 6 days significantly decreased alcohol self-administration in non-treatment-seeking alcoholics, resulting in maximum venous blood alcohol concentrations (vBAC) of 20mg%, compared to 60 mg% after pretreatment with placebo (O’Malley et al., 2002). In a later study, this could only be replicated in family history positive (FHP) alcoholics after 6 days on 100 mg/day naltrexone compared to placebo (maximum vBAC 25 vs. 46 mg%, respectively), while the same naltrexone dose in family history negative (FHN) alcoholics raised maximum vBAC from 28 to 43 mg% (Krishnan-Sarin et al., 2007). The 50 mg/day naltrexone dosage had no significant effect in this study. Naltrexone and nalmefene were both found to reduce the number of drinks consumed in a bar lab environment in non-treatment-seeking alcoholics but not in social drinkers (Drobes et al., 2003). vBAC during self-administration, however, was not reported in this paper.
In other studies employing oral alcohol self-administration, the starting hypotheses could not be substantiated. For example, no difference in laboratory alcohol drinking could be observed when comparing FHN and FHP male social drinkers (de Wit and McCracken, 1990), after pretreatment with the nicotinic acetylcholine receptor antagonist mecamylamine vs. placebo (Young et al., 2005), after tryptophan depletion vs. sham depletion (Petrakis et al., 2002), or after exposure to a psychosocial stress test vs. a control condition (de Wit et al., 2003). These negative findings could imply that the hypotheses were incorrect or, alternatively, that the method of oral self-administration was inadequate to detect subtle effects. Indeed, in those studies which measured BAC the average peak level was surprisingly low, ranging from 46 (Krishnan-Sarin et al., 2007) to 60 mg% (O’Malley et al., 2002) in venous blood of non-treatment-seeking alcoholics. These low peak levels imply that subjects might be rather reluctant to drink according to their habits in a laboratory setting. In any case, the range of the outcome measure is small, constricting the ability to document effects of experimental manipulations.
Another problem with oral alcohol administration is that even after adjusting dosages for total body water (minimizing the effects of sex and body morphology) and performing the ingestion with identical experimental procedures, the maximum observed BAC and the time of its occurrence after oral ingestion vary about threefold between subjects (Ramchandani et al., 2006). This variability complicates the interpretation of self-administration experiments, since subjects ingesting the same sequence of drinks will differ substantially in their brain alcohol exposure.
The latter problem can be overcome by infusing ethanol intravenously. Over the last years, a physiologically based pharmacokinetic (PBPK) model of alcohol administration and elimination was under continuing development (Plawecki et al., 2007;Plawecki et al., 2008). Specifying the parameters of the model for each individual allows the achievement of the same, prescribed time course of arterial BAC (aBAC) in every given subject. This type of experiment controls arterial rather than venous BAC, since aBAC is a much better representation of brain alcohol exposure and can be reliably measured using breath samples (Lindberg et al., 2007). When used for clamping the aBAC, the PBPK model calculates an individualized infusion protocol maintaining aBAC within ± 5mg% of the target concentration (O’Connor et al., 1998). More recently, the same principle helped to achieve rapid linear changes of aBAC with minimal experimental variability across subjects (O’Connor et al., 2007). CASE employs the PBPK model to achieve an identical increment in aBAC each time any subject chooses to self-infuse rather than administering a fixed dose as with drinking.
We employed the capabilities of CASE to prescribe the characteristics of that controlled incremental exposure to see if healthy subjects would reliably achieve a substantial level of inebriation and to learn if varying the parameters of the incremental exposure would influence that level. All subjects participated in 3 experimental days separated by at least one week.
Materials and methods
Subjects
All subjects were recruited by a local newspaper advertisement. The 77 respondents were screened via telephone in order to pre-check inclusion and exclusion criteria. Inclusion criteria were: Healthy male and female volunteers aged 21 to 30 years; social drinking (i.e., consuming 4 drinks or less per occasion at least twice a week throughout the preceding two months); being able to abstain from tobacco smoking for four hours without developing nicotine withdrawal; agreeing to abstain from any illegal drugs starting three weeks before the first experiment; agreeing to abstain from alcohol for two days before each experiment; effective contraception in women, and a body mass index (BMI) between 20 and 27 kg/m2. Exclusion criteria were: Any physical or mental disorder requiring current medical treatment or psychotherapy; current or prior alcohol or illegal substance dependence; premenstrual dysphoric disorder; teetotalers; a history of epileptic seizures; liver or pancreatic disorders, or laboratory signs indicating such disorders; known intolerance against ethanol; known pregnancy, breast-feeding, or positive urine pregnancy testing on any of the test days, and any prior alcohol intake on the test day or the day before. During the telephone interview, subjects were informed about the experimental procedures of the project to gauge the subject’s willingness to undertake the intravenous infusion.
Twenty-five of the respondents fulfilled all criteria. One of them had a history of alcohol abuse, the others had no alcohol or illegal substance abuse. From those, we selected the first 6 qualified males and first 6 qualified females, based on the chronological order of response to the original advertisement. Subsequently, 1 female was excluded based on a face-to-face interview conducted on the first day of testing, since we could not confidently rule out alcohol dependence. Another female dropped out after the second day of testing without providing a reason, and the data from one male could not be evaluated due to equipment malfunction in one session.
Thus, the final sample comprised 9 subjects (5 males), with a mean ± SD age of 25.0 ± 4.0, weight 83.8 ± 11.8 kg, Fagerstrom test of nicotine dependence (FTND) score of 1.0 ± 2.0, alcohol use disorders identification test (AUDIT) score of 7.8 ± 2.6, and Beck depressive inventory (BDI) score of 1.3 ± 1.0. There were 3 regular smokers in our sample. Two participants thought one parent was alcohol-dependent, and two others thought so of one second-degree relative, while the others denied alcoholism in their families.
Experimental design and procedures of the “Freibier” paradigm
On the first of 3 testing days, subjects reported to the laboratory at 1300h. Written informed consent was obtained after a full explanation of all study procedures. This study protocol complied with the Declaration of Helsinki and was approved by the University of Heidelberg ethical committee. A medical and psychiatric history was taken to confirm inclusion and exclusion criteria. For any substance which subjects reported having used more often than 5 times in their life, the composite diagnostic interview (CIDI) (Lachner et al., 1998) substance abuse section questions were asked to rule out dependence from the respective substance. Subjects completed a time-line follow-back (TLFB) interview to specify the number of drinks consumed on each day during the preceding four weeks. A urine sample was obtained to screen for illegal substances and to perform a pregnancy test in women, all of which were negative in all instances. Next, an 18G i.v. line was established in the antecubital fossa of the nondominant arm, followed by a 20-minute break to give subjects a chance to relax and get acquainted with the laboratory environment. At 1450h the subject was shown how the software would lead them through the experiment and how to use a button connected to the CASE computer to request “drinks”. S/he was told that pressing the button increased the infusion rate for several minutes, during which time the button would be inactive to prevent them from requesting more alcohol before the requested “drink” was fully delivered. Subjects were instructed to make use of the infusion in order to produce pleasant alcohol effects of the same extent as they usually would when drinking at a week-end party, but to avoid untoward alcohol effects. To give them a rationale for doing so, they were misinformed that the goal of the study was to vary the alcohol concentration in the infusate between days and to test how that affects the subjective alcohol effects.
At 1455h the experiment started with a priming period during which subjects were prompted to request a “drink” four times in a row by the software. Each request increased aBAC by 7.5 mg% in precisely 2.5 min, defining a linear ascending limb slope (ALS) of +3 mg%/min. The four requests served to produce a priming alcohol exposure of 30 mg% after 10 minutes and to demonstrate to the subjects that each request produced only a rather small increase of alcohol effects. For the next 15 minutes, no “drinks” could be requested while the subjects were asked to find out how much they felt the effect of the four “drinks” they had requested so far. During this waiting period, aBAC fell by a descending limb slope (DLS) of −1.0 mg%/min, resulting in a aBAC of 15 mg% at 25 minutes in all participants. At that time, subjects took a mandatory bathroom break, after which the self-administration period was started by reactivating the “drink” button. Subjects were informed that during the next two hours they were free to request more alcohol or to refrain from doing so, in order to achieve their preferred level of alcohol effect when drinking at a party. We called this the “Freibier” (German for “free beers”) paradigm, since the general idea was that no work or pay was required to obtain alcohol. During the experiment, subjects could listen to a broad selection of music files presented on the laboratory laptop. Reading was discouraged in order to prevent distraction from the messages on the CASE computer screen.
Subjects completed the biphasic alcohol effects scale (BAES) (Martin et al., 1993) as translated by the authors and reported alcohol craving by rating agreement with the German statements corresponding to “I would like to have an alcoholic drink now” and “I feel an urge to have an alcoholic drink now” on an 11-point Likert scale. These subjective responses were obtained at baseline and at 15, 35, 70, 96 and 125 minutes after starting the infusion. At 2:25 h, the experiment was terminated, the i.v. line was removed and subjects were offered a full meal. They were free to leave the lab as soon as aBAC had fallen to 20 mg%, or to take a taxi cab or be collected by a friend at a aBAC of 45 mg%, provided they were not visibly impaired by alcohol.
On the second and third days, subjects arrived at the lab at 1400 h. A brief history regarding the time since the last experiment was taken, including questions for major life events, for side effects after the last experiment, when their most recent drinking day occurred, and how many drinks they had consumed on that occasion. The incremental increase after each request remained at 7.5mg%. In a randomized crossover design, the ALS was increased to +5 mg%/min (“quick day”) in half of the subjects and slowed to +2.14mg%/min (“Slow day” in the other half, without their knowledge. The change in ALS was reversed on the third experimental day. One month after the last session, a questionnaire concerning overall adverse effects was mailed to all participants. It included a time-line follow-back diary of daily alcohol intake throughout the 4 weeks after the last session.
Methods and equipment for ethanol infusion
The infusion solutions were prepared by mixing standard Ringer’s solution with 95% ethanol (Alkohol Konzentrat 95% Braun, Melsungen, Germany) to give a final concentration of 6% (v/v). The infusion was warmed to body temperature and delivered by a dual infusion pump (Gemini PC2-TX, Cardinal Health, Dublin, OH). The PBPK model was incorporated into the CASE software, which was implemented on a specifically configured personal computer equipped with two screens. One screen displayed all information needed for the technician to begin the infusion and to enter occasional aBAC readings. The second screen always displayed one of 8 messages to the participant, as described in Table 1, and was located in the subject’s compartment of the laboratory, together with a push button connected to the computer. The CASE software controlled the infusion pumps via its RS 232 interface.
Table 1:
Messages displayed on the subject’s screen guiding the participant through the experiment.
| No. | Message to subject | “Drink” button status | Duration displayed | Possible next messages |
|---|---|---|---|---|
| 1 | “Priming drink: please push the drink button NOW” | Active | Until button is pressed | 2, 3 |
| 2 | “Priming drink ordered, waiting for the new peak to be reached” | Inactive | Either 1.5, 2.5, or 3.5 minutesa | 1 |
| 3 | “We’ ve closed the bar until the priming interval is over. We want you to pay attention for about 15 minutes to the effects of the four drinks you have had so far.” | Inactive | 15 minutes | 4 |
| 4 | “The bar is now open: order another drink at your discretion” | Active | Until next drink is ordered | 5, 6,7,8 |
| 5 | “Another drink ordered, waiting for the new peak to be reached” | Inactive | Either 1.5, 2.5, or 3.5 minutes | 4,6 |
| 6 | “The bar is closed. We will reopen as soon as a breath alcohol reading is entered” | Inactive | Until a breath reading is entered | 4, 5, 7,8 |
| 7 | “The bar is closed. We will reopen as soon as another drink would not expose your brain to more than the safety limit” | Inactive | Up to 7.5 minutesb | 4,8 |
| 8 | “The experiment is over. Thanks.” | Inactive | none |
Message 1 and 2 describe the priming period, 3 describes the waiting period, and 4–8 the self-administration period.
depending on the ascending limb slope selected;
defined by the increment divided by descending limb slope, here 7.5 mg% by −1 mg%/min.
Before starting a session, the subject’s age, height, weight and sex were entered into the CASE software which transformed these measurements into the 6 PBPK parameters that individualized the model of alcohol distribution and elimination (O’Connor et al., 2000). Whenever the subject pressed the button, the button was deactivated (switching to message 5 in Table 1) and the model calculated the infusion rates necessary to linearly increase BAC by 7.5 mg% within the preset time, then produce a steady decline of BAC by a DLS of −1 mg%/min. Then CASE controlled the pump to deliver this infusion profile. Upon reaching the next peak, the “drink” button would be reactivated and the aBAC declined until one of three events occurred: (i) the subject ordered a new “drink”, in which case the above described events reiterated; or (ii) the calculated infusion rate decreased to the minimal rate that kept the line open (4 ml/h), in which cased it remained at that rate until (i); or (iii) the 2.5 hour session was over, in which case the pump was turned off. An output of the simulated instantaneous aBAC values throughout the experiment was provided on the technician’s screen with a time resolution of 30 seconds (See figure 1).
Figure 1:
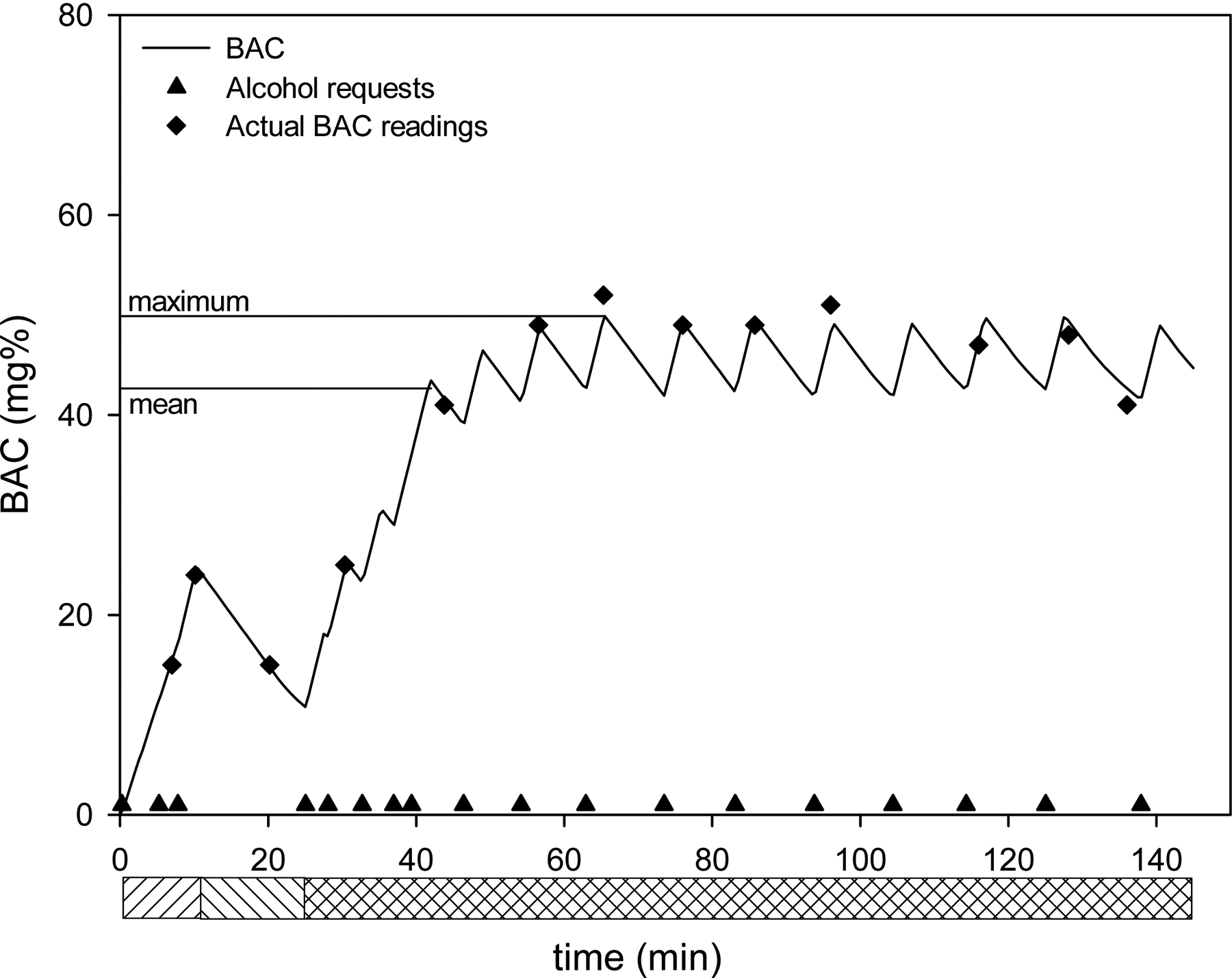
Example of a typical CASE experiment showing alcohol requests by the subject and the ensuing blood alcohol concentration (aBAC) course over time. Solid line: instantaneous aBAC as simulated by the software; upward hatched bars: priming period; downward hatched bars: waiting period; cross-hatched bars: self-administration period. Note the small differences between simulated and actual aBAC. Mean and maximum refer to simulated aBAC throughout the self-administration period.
Once each second in the background, the CASE software calculated the future time course of aBAC as if a new drink had been ordered at that moment. If that projection would raise the aBAC beyond the preset safety limit, of 100 mg%, message 7 in table 1 was displayed and the “drink” button would be inactivated. For safety reasons, the software required the experimenter to enter time-stamped aBAC readings at least once every 20 minutes; otherwise message 6 would be displayed, also inactivating the “drink” button. The software used any difference between the simulated and the actual aBAC readings for cumulative adjustments of the individual’s pharmacokinetic model and corrected future infusion rate calculations correspondingly.
aBAC was estimated from breath alcohol samples using an Alcotest 7410 med breathalyzer (Draeger Sicherheitstechnik, Lübeck, Germany) applying the factor 210 to convert breath alcohol (mg/l air) to arterial blood alcohol concentration (mg%, i.e. mg/100ml whole blood). This method was shown to give a precise estimate of aBAC even if alcohol is rapidly administered by either oral (Lindberg et al., 2007) or i.v. route (Jones et al., 1997).
Outcome measures and statistical evaluation
An example for a typical experiment is given in Figure 1, depicting the “drink” requests by the subject, the simulated instantaneous aBAC and the actual aBAC readings. Figure 1 is the first session record for a 22 year old female, who was 170 cm tall and weighed 71.7 kg. The aBAC-time profile was achieved with a total of 625 ml of infusate, corresponding to 37.5 g ethanol. The infusion rate varied between 212 and 532 ml/h to produce the ascending limbs, and between 200 and 4 ml/h during descending limbs.
Outcome variables of the self-infusion behavior were (i) the mean aBAC, defined as the arithmetic mean of all simulated aBAC values throughout the self-administration period; (ii) the maximum aBAC, defined as the maximum of all simulated aBAC readings throughout the self-administration period and (iii) the number of requests made by the subject to obtain “drinks” throughout the self-administration phase of the session. These measurements were obtained for each session day and were analyzed using bivariate correlations to assess stability over the sessions. The effects of different ALS and their sequence between days were assessed by multiple analyses of variance (MANOVA).
Results:
All subjects made use of the experimental setup to self-infuse alcohol. Unstructured interviews after each experiment revealed that all subjects understood the experiment and how to operate the self-administration. It turned out, however, that during the first day some of them had not fully complied with the instructions. Some had not understood that the purpose of the experiment was to induce the same alcohol effect as that individual sought at a party, which resulted in rather low alcohol exposure. Others, knowing that there was a aBAC safety limit of 100 mg%, deliberately tried to reach that level out of curiosity about what that feels like. In doing, so they ignored the emerging side effects which usually would have prevented them from drinking that much. Noncompliance of that kind was not reported during the second or third experimental sessions.
All subjects reported they had enjoyed the experiment and experienced a similar alcohol effect as with drinking. When asked to guess what was different between the days, the majority of participants correctly reported that the time between possible alcohol requests varied, but none realized that this implied quicker or slower administration of the alcohol. One of the participants reported he would have preferred the “slow” over the “quick” pattern, one preferred the “quick”, and the others had no preference.
No adverse effects occurred during the experiments. Specifically, none of the subjects complained about nausea or vomited, and no experiment was terminated prematurely. Two subjects reported side-effects of headache or tiredness on the evening or the day after one or more of the experiments. Seven of the nine subjects who participated in all three experiments provided the second TLFB diary. The post-testing number of drinks per month (d/m), compared to that recorded before participation, decreased by −3.4 ± 18.3 (median 0; range −37 to +17), as did the mean number of drinks per drinking day (d/dd); −1 ± 1.2 (median −0.24, range −2.8 to 0). The +17 difference in d/m occurred in a subject who increased his monthly drinks from 19 to 36, while his d/dd remained unchanged at 3.2, suggesting an increase in drinking days but arguing against loss of control due to study participation. The cumulative volume of administered infusion solution was recorded by the software and averaged 1067, 1152, and 991 ml on the first, “quick”, and “slow” day, respectively. Most subjects required one additional bathroom break during self-administration, and one subject required two.
The mean aBAC during the first day was not significantly correlated to that of the second or third experimental day, and did not correlate between second and third day, per se. Likewise, no associations between days one to three could be detected for the other two outcome measures, i.e. maximum aBAC and number of requests.
However, when accounting for the sequence of ALS variation between the second and third day, the outcome measures for the “quick” and “slow” days turned out to be significantly interrelated (Pearson’s r= 0.82, 0.79, and 0.72 for mean aBAC, maximum aBAC and number of requests; p<.05, respectively).
All three outcome measures scored higher during the “quick” compared to the “slow” days (See Figure 2). This was confirmed in a MANOVA with mean aBAC, maximum aBAC, and number of requests as dependent variables, “ALS” as a within-subjects factor (5 vs. 2.14 mg%/min, i.e., “quick” vs. “slow”), and “sequence” (second day quick/ third day slow vs. vice versa) as between-subjects factor. A significant influence was observed for “ALS” (Wilks lambda, F[3,5]= 10.5, p=.013), but not for “sequence” or the factor interaction. Univariate tests revealed that ALS exerted its influence predominantly on maximum aBAC and on number of requests (p<.05), while its effect on mean aBAC was marginal (p=.073). The ALS effect is also reflected by more cumulative ethanol mass administration during the “quick” (55.3 ± 20.8 g) compared to the “slow” days (47.6 ± 18.7 g; t(8)=14.9, p<0.05). The three CASE outcome measures of both “quick” and “slow” days were not significantly correlated with the subject’s percent drinking days, drinks per drinking day, or AUDIT scores.
Figure 2:
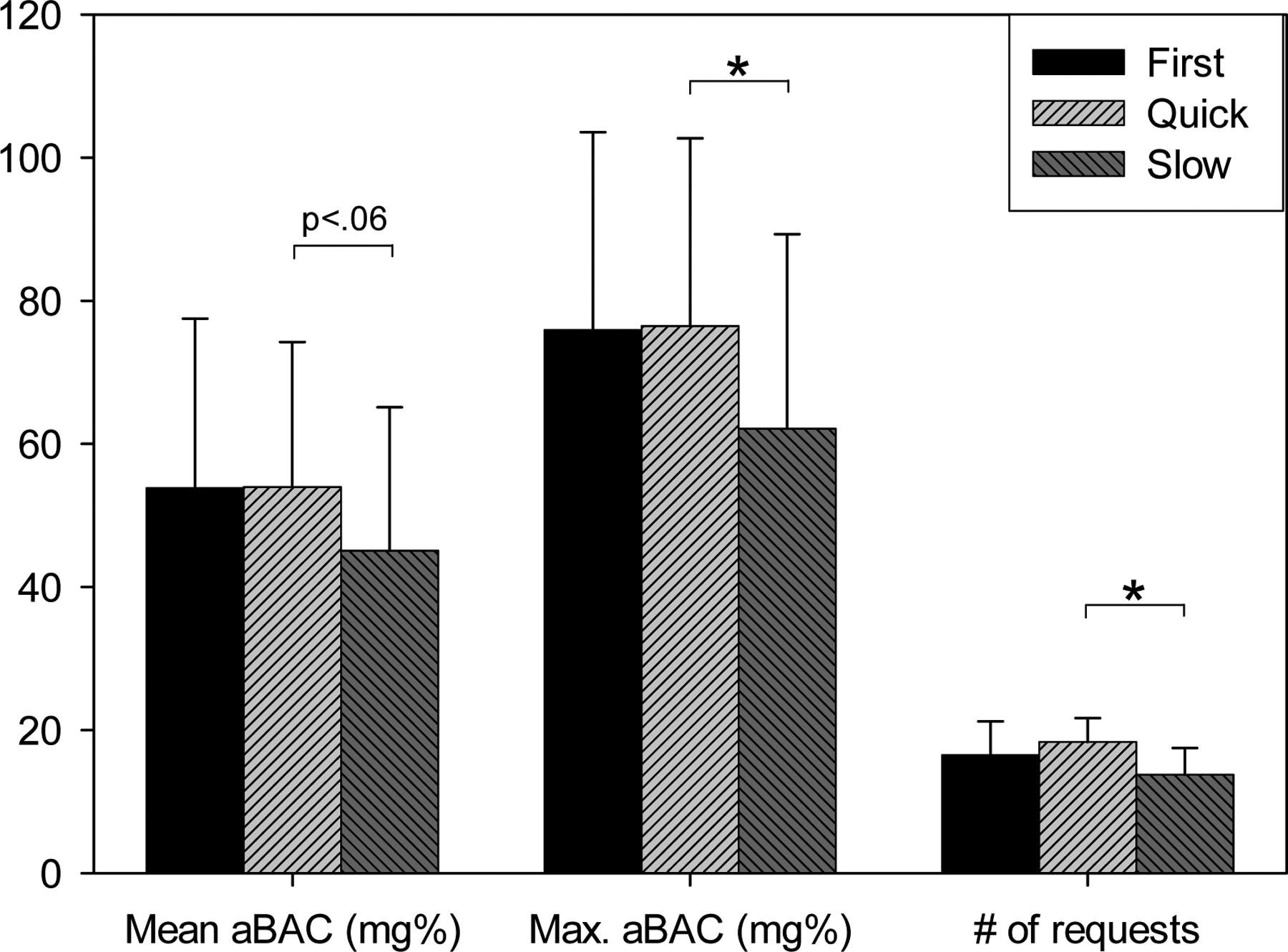
Mean and SD outcome measures by experimental day, involving different experimental parameters: Mean and maximum arterial blood alcohol concentration (aBAC) in mg%, and number of alcohol requests made by the subjects.
The BAES stimulant subscore increased by 0.5 ± 0.5 during the priming period of the “quick” and by 0.2 ± 1.2 during the priming period of the “slow” days, compared to baseline. During the same time, the BAES sedative subscale decreased by 0.1 ± 0.2 on the “quick”, and by 0.0 ± 0.9 on the “slow” days. Those differences were not statistically different between days and were not significantly correlated to any of the three outcome measures of the respective experimental day. The stimulant subscale scores obtained during the self-administration period of the experiment correlated with aBAC at the time of BAES scoring during both “quick” and “slow” experiments (Pearson’s r= 0.42 and r= 0.51; p < 0.01, respectively), while the sedative subscale scores were not significantly related to aBAC.
The change between alcohol craving scores obtained at baseline and at the end of the 25-minute priming interval was significantly correlated with all three outcome measures of the “slow” experiments (Pearson’s r= 0.94 for mean and max aBAC, and number of requests,; p<0.01, respectively). No significant correlations of that kind could be found during the “quick” days (see figure 3).
Figure 3:
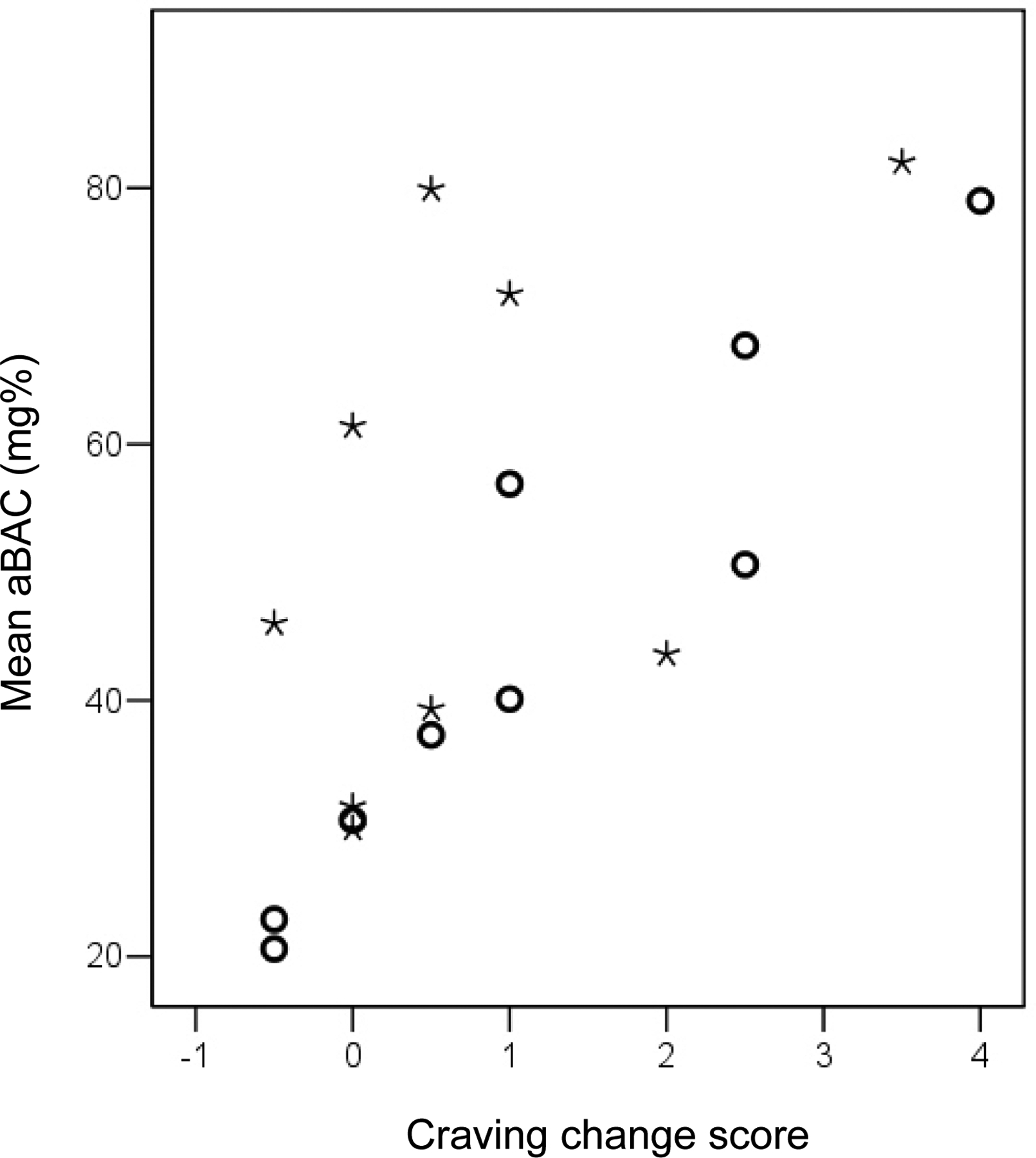
Scatterplot of change in alcohol craving during the 25 minute- priming/waiting period versus mean blood alcohol concentration (aBAC) during the self-administration period. Open circles= “slow” days; asterisks= “quick” days.
Discussion:
These results demonstrate that the CASE “Freibier” paradigm provides a practical and safe method to investigate alcohol self-administration in healthy volunteers. The low frequency of both observed immediate side effects and reported delayed adverse reactions (i.e. hangovers) is remarkable, given that the subjects achieved considerable levels of alcohol exposure.
Results of our follow-up questionnaire do not support concerns that participating in three CASE experiments might increase future drinking. This reassuring finding is in line with more thorough studies of drinking after experiments involving laboratory alcohol administration, which found that drinking measures decreased rather than increased during the first weeks after participation (Pratt and Davidson, 2005), (Drobes and Anton, 2000).
Comparing self-infusion behavior over the three test days demonstrated that test-retest stability was poor between the first and either consecutive session, but was good between the “quick” and “slow” days. A likely explanation is that on the first day, subjects might not have felt confident enough with their first-time experience of i.v. alcohol, resulting in overly cautious self-administration. After one reassuring experience, this phenomenon appears to have resolved and not have affected the following experiments. For the time being, it appears appropriate to schedule one practice session in order to acquaint subjects with the experience of i.v. alcohol, before evaluating hypotheses with subsequent CASE experiments. This observation also implies that the 25-minute priming/waiting period which precedes the self-administration might be omitted in future studies, since one of the goals it was thought to achieve was to acquaint subjects with the self-infusion procedure in order to circumvent the need of a practice session. On the other hand, another goal was to induce mild alcohol craving, increasing the probability of successive self-administration, as was done in prior studies (Drobes et al., 2003;O’Malley et al., 2002).
While all measures of alcohol self-administration were interrelated between “quick” and “slow” experiments, they were also significantly higher in the former. This result implies that subjects achieved more alcohol exposure when the same aBAC increment per request (i.e., +7.5 mg%) happened more quickly (at a rate of +5 vs. +2,14 mg%/min), consistent with the general concept that drugs of abuse are more rewarding, more addictive, and are consumed in higher amounts if their route of administration warrants quicker access to the brain. By providing control over the kinetics of aBAC change, CASE is the first method allowing study of this aspect with regard to alcohol.
The maximum aBAC was 76.5 ± 26.3 mg% (mean ± SD), averaged over all experimental sessions, which is considerably higher than in all the oral self-administration studies reviewed in the introduction. The increase is particularly remarkable since our subjects were non-dependent social drinkers, while the foregoing studies all investigated non treatment-seeking alcoholics who would be expected to drink rather more. In 34% of our sessions, the subjects actually reached the safety limit of 100 mg% and, in 25 %, this happened several times during a session, suggesting that some sessions might have been limited by a ceiling effect.
The higher ethanol levels achieved with CASE cannot be due to the methodological difference that prior studies reported vBAC, since they observed the maximum vBAC at around 180 min after starting to drink. This is the time when vBAC usually begins to exceed aBAC, as gastrointestinal absorption is complete (Lindberg et al., 2007). We rather suggest that two aspects distinguishing oral from i.v. self-administration might explain why CASE results in higher alcohol exposure: First, during oral ingestion experiments subjects remember how much they already ingested and, from the taste, they can guess how strong the drinks were. Since subjects know that their drinking behavior is being observed by the experimenter, they might be embarrassed to have a lot of drinks out of concern for what the laboratory staff might think of them. This concern might especially apply to non treatment-seeking alcoholics, who very likely have experienced criticism for their drinking in the past. A second issue possibly impeding alcohol self-administration by ingestion is that the subjects usually are offered a fixed number of prepared drinks per hour. This procedure is in the service of limiting the maximum aBAC achieved in the fastest absorber among the smallest subjects who may elect to ingest all available drinks at once. The limited number of drinks offered necessarily communicates some expectancy to the subjects as to how many drinks the experimenters think they might or should ingest at the most. Some subjects might be reluctant to take all the possible drinks because they perceive this would be an extreme behavior and they would score top in consumption.
These two issues are abolished with CASE, since subjects do not know how much they have already infused nor how often they are supposed to push the “drink” button. Therefore, they need to make their decisions for or against electing another “drink” on the sole basis of the pharmacological alcohol effects they perceive. Whatever the reason, the present CASE validation study resulted in higher levels of alcohol exposure than observed in previous experiments using oral self-administration, providing more potential for modulation of this signal by manipulation of the sample population characteristics or by pretreatment with a pharmaceutical. Utility would be enhanced if future studies show that it is safe to increase the maximum allowable aBAC.
It is intriguing that self-administration behavior changed between “quick” and “slow” days although the subjects did not recognize these differences in infusion kinetics and did not score their craving and the stimulant and sedative alcohol effects differently during the priming period of “quick” vs. “slow” days. This observation implies that CASE might be sensitive to subtle differences in the rewarding effects of alcohol, which are not picked up by these questionnaires. Two alternative explanations are that the sample size might have been too small to demonstrate such an association and that craving/ BAES questionnaires were not obtained at peak aBAC of the priming phase, rather five minutes later. In fact, neither BAES nor craving questions measure how much subjects like the alcohol effect they perceive. Such a measure should be included in future experiments. It is also noteworthy that alcohol self-administration was closely predicted by the craving change during the priming period, i.e. the first 25 minutes during the “slow” experiments. Since no such association was found during the “quick” days (due to relatively high alcohol exposure in the face of small craving changes in four of the nine participants, see figure 3), both craving and the reinforcement associated with rapid ascent may have influenced the outcome. This notion is further supported by the fact that three of these four subjects achieved considerably lower aBAC levels on the slow day. We also recognize the need for a more complete assessment of craving in future studies.
From a learning theory point of view, one advantage of the CASE/ Freibier paradigm should be that the contingency between behavior (pushing a button to receive a “drink”) and its consequence (feeling a change in alcohol effect) is closer than with oral administration for three reasons: First, each button press results in exactly the same amount of aBAC increase in every subject at any time throughout the experiment. Second, all these aBAC increments follow exactly the same kinetics, i.e. a linear increase over a preset period of time, thus, increments are achieved much more reliability than would be possible with drinking. Finally, after an increment was delivered, tissue redistribution of alcohol is used to produce a linear decline of aBAC at three times the alcohol clearance rate achieved by hepatic metabolism. The more rapid decline continues until the subject requests a new “drink”, or the distribution volume is equilibrated. These rapid changes in aBAC can be expected to result in almost equally quick changes of brain exposure since the brain is a low-volume, high-perfusion organ and has little capacity to store alcohol due to its low water content. For these reasons, CASE enables human subjects to gain more direct control over their brain alcohol exposure than with oral self-administration. Other implications are that individual preferences for specific alcoholic beverages, brands, tastes and/or smells are irrelevant.
The particular manifestation of CASE in this study was the Freibier paradigm, optimized to investigate the pure pharmacodynamic effects causing humans to use alcohol, and using the the simplest form of an operant behavioral paradigm in an effort to bridge the gap between animal and human behavioral studies. There was neither intention nor attempt to provide face validity for the procedure when benchmarked to the usual environmental and social aspects of drinking; we targeted only the pharmacological experience. Thus, finding no relation between our outcome measures and the variables describing the subjects’ usual drinking habits was of little concern and may well have been due to the small sample size. On the other hand, CASE technology in general offers a broad methodological platform which can be adopted to mimic many aspects of human alcohol intake that an interested researcher might intend to investigate. Many paradigms are possible; examples include truly blinded placebo administration, pairing alcohol administration to other behaviors such as sipping an alcoholic or nonalcoholic beverage, performing CASE experiments in an artificial bar lab environment with or without social interaction, or perform self-administration inside an MRI scanner. Other groups have recently established methods to study human i.v. self-administration of nicotine (Sofuoglu et al., 2008) and cocaine (Donny et al., 2006), which offers the future perspective to broadly validate how everyday substance use compares to laboratory i.v. self-administration.
The authors offer to provide all necessary software for any new paradigm, and equipment specifications to those colleagues who are interested in using CASE technology for intravenous alcohol administration, whether in animals or humans, and who contract to keep technical details confidential. European investigators should contact Dr. Zimmermann at Ulrich.Zimmermann@uniklinikum-dresden.de; North American investigators should contact Dr. O’Connor at oconnor1@iupui.edu.
We conclude that CASE and its “Freibier” paradigm provide a practical and safe method for alcohol self-administration and results in considerable alcohol exposure. The resulting time course of aBAC appears to be reproducible, corresponds to subjective ratings of craving and alcohol effects and is in line with general concepts about reinforcement by drugs of abuse. Together, these preliminary data suggest that CASE might be a practical and sensitive method to investigate factors influencing human self-administration of ethanol.
Acknowledgments
This project was supported by Grant No. P60 AA007611-20 from the National Institute on Alcohol Abuse and Alcoholism. The contents of this report are solely the responsibility of the authors and do not necessarily represent the official view of the National Institute on Alcohol Abuse and Alcoholism or NIH.
Reference List
- (1).Acheson A, Mahler SV, Chi H, de WH (2006) Differential effects of nicotine on alcohol consumption in men and women. Psychopharmacology (Berl) 186:54–63. [DOI] [PubMed] [Google Scholar]
- (2).de Wit H, McCracken SG (1990) Ethanol self-administration in males with and without an alcoholic first-degree relative. Alcohol Clin Exp Res 14:63–70. [DOI] [PubMed] [Google Scholar]
- (3).de Wit H, Soderpalm AH, Nikolayev L, Young E (2003) Effects of acute social stress on alcohol consumption in healthy subjects. Alcohol Clin Exp Res 27:1270–1277. [DOI] [PubMed] [Google Scholar]
- (4).Donny EC, Bigelow GE, Walsh SL (2006) Comparing the physiological and subjective effects of self-administered vs yoked cocaine in humans. Psychopharmacology (Berl) 186:544–552. [DOI] [PubMed] [Google Scholar]
- (5).Drobes DJ, Anton R (2000) Drinking in alcoholics following an alcohol challenge research protocol. J Stud Alcohol 61:220–224. [DOI] [PubMed] [Google Scholar]
- (6).Drobes DJ, Anton RF, Thomas SE, Voronin K (2003) A clinical laboratory paradigm for evaluating medication effects on alcohol consumption: naltrexone and nalmefene. Neuropsychopharmacology 28:755–764. [DOI] [PubMed] [Google Scholar]
- (7).Jones AW, Norberg A, Hahn RG (1997) Concentration-time profiles of ethanol in arterial and venous blood and end-expired breath during and after intravenous infusion. J Forensic Sci 42:1088–1094. [PubMed] [Google Scholar]
- (8).Krishnan-Sarin S, Krystal JH, Shi J, Pittman B, O’Malley SS (2007) Family History of Alcoholism Influences Naltrexone-Induced Reduction in Alcohol Drinking. Biol Psychiatry 62:694–697. [DOI] [PubMed] [Google Scholar]
- (9).Lachner G, Wittchen HU, Perkonigg A, Holly A, Schuster P, Wunderlich U, Turk D, Garczynski E, Pfister H (1998) Structure, content and reliability of the Munich-Composite International Diagnostic Interview (M-CIDI) substance use sections. European Addiction Research 4:28–41. [DOI] [PubMed] [Google Scholar]
- (10).Lindberg L, Brauer S, Wollmer P, Goldberg L, Jones AW, Olsson SG (2007) Breath alcohol concentration determined with a new analyzer using free exhalation predicts almost precisely the arterial blood alcohol concentration. Forensic Sci Int 168:200–207. [DOI] [PubMed] [Google Scholar]
- (11).Martin CS, Earleywine M, Musty RE, Perrine RE, Swift RM (1993) Development and validation of the biphasic alcohol effects scale. Alcoholism: Clinical & Experimental Research 17:140–146. [DOI] [PubMed] [Google Scholar]
- (12).O’Connor S, Morzorati S, Christian J, Li TK (1998) Clamping breath alcohol concentration reduces experimental variance: Application to the study of acute tolerance to alcohol and alcohol elimination rate. Alcohol Clin Exp Res 22:202–210. [PubMed] [Google Scholar]
- (13).O’Connor S, Morzorati S, Zimmermann US, Flury L. Ascending or descending BrAC; who knows? Alcohol Clin Exp Res. 31, 252A. 2007. [Google Scholar]
- (14).O’Connor S, Ramchandani VA, Li TK (2000) PBPK modeling as a basis for achieving a steady BrAC of 60 +/− 5 mg% within ten minutes. Alcoholism: Clinical & Experimental Research 24:426–427. [PubMed] [Google Scholar]
- (15).O’Malley SS, Krishnan-Sarin S, Farren C, Sinha R, Kreek J (2002) Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology (Berl) 160:19–29. [DOI] [PubMed] [Google Scholar]
- (16).Petrakis IL, Buonopane A, O’Malley S, Cermik O, Trevisan L, Boutros NN, Limoncelli D, Krystal JH (2002) The effect of tryptophan depletion on alcohol self-administration in non-treatment-seeking alcoholic individuals. Alcohol Clin Exp Res 26:969–975. [DOI] [PubMed] [Google Scholar]
- (17).Plawecki MH, DeCarlo RA, Ramchandani VA, O’Connor S (2007) Improved Transformation of Morphometric Measurements for A Priori Parameter Estimation in a Physiologically-Based Pharmacokinetic Model of Ethanol. Biomedical Signal Processing and Control 2:97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Plawecki MH, Han JJ, Doerschuk PC, Ramchandani VA, O’Connor S: A Physiologically-Based Pharmacokinetic (PBPK) Model for Alcohol: Mathematical Foundations. IEEE Transactions on Biomedical Engineering. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Pratt WM, Davidson D (2005) Does participation in an alcohol administration study increase risk for excessive drinking? Alcohol 37:135–141. [DOI] [PubMed] [Google Scholar]
- (20).Ramchandani VA, O’Connor S, Neumark YD, Zimmermann US, Morzorati S, de Wit H (2006) The alcohol clamp: applications, challenges and new directions- an RSA 2004 symposium summary. Alcohol Clin Exp Res 30:155–164. [DOI] [PubMed] [Google Scholar]
- (21).Sofuoglu M, Yoo S, Hill KP, Mooney M (2008) Self-administration of intravenous nicotine in male and female cigarette smokers. Neuropsychopharmacology 33:715–720. [DOI] [PubMed] [Google Scholar]
- (22).Young EM, Mahler S, Chi H, de WH (2005) Mecamylamine and ethanol preference in healthy volunteers. Alcohol Clin Exp Res 29:58–65. [DOI] [PubMed] [Google Scholar]


