Abstract
Disruptions in STI testing infrastructure during the COVID-19 pandemic threaten to impact STI service delivery for adolescents. Within a large pediatric primary care network, we compared STI testing encounters between the pandemic period and an analogous pre-pandemic period. STI test counts decreased and test positivity increased during the pandemic period.
Keywords: STI testing, adolescents, COVID-19
Summary:
In this retrospective study, gonorrhea and chlamydia test volumes decreased sharply and test positivity increased during the pandemic period within a large pediatric primary care network.
Introduction
Adolescents and young adults, ages 15–24, are at increased risk for HIV and other sexually transmitted infections (STIs), accounting for one in five new HIV diagnoses and nearly half of new STI diagnoses each year.1,2 The Centers for Disease Control and Prevention (CDC) recommends routine STI and HIV screening for sexually active adolescents and young adults.3 In the months following the COVID-19 pandemic, youth faced new obstacles to accessing sexual health services, including interruptions to public transportation, clinic closures, and barriers related to telemedicine (i.e. privacy concerns, limited access to broadband internet, inability to collect laboratory assays during telemedicine visits).4 Health systems were forced to reduce clinic hours and close some walk-in services, including STI and HIV testing. Additionally, the demand for COVID-19 testing both impacted the supply chain for Neisseria gonorrhoeae and Chlamydia trachomatis nucleic acid amplification test (NAAT) kits, causing a national stock-out, and delays in laboratory result reporting as some hospital-based lab systems were forced to send STI testing to reference laboratories to accommodate in-house COVID-testing needs. In September 2020, the CDC issued a “Dear colleagues” letter announcing the national supply shortages and calling for conservation strategies to preserve the limited testing supplies.5
While it is presumed that these changes affected the availability of STI testing and treatment services for young people, the extent to which these major changes in STI testing infrastructure and service availability have impacted STI service delivery during the on-going pandemic is currently unknown. Our research objective was to examine changes to STI testing counts, case counts, and positivity rates within a large, academic primary care network in the United States from the pre-COVID-19 pandemic period to the ongoing pandemic period.
Materials and Methods
This study is a retrospective cross-sectional analysis of electronic health record (EHR) data from a 31-clinic, hospital-affiliated, pediatric primary care network. The health system serves over 250,000 youth annually. All clinics provide comprehensive health services, and two of the clinics have embedded Title X-funded family planning clinics offering free STI testing and treatment. We utilized data from a commercial business intelligence platform (Qlikview-Qlik, Radnor, PA) which automatically extracts STI testing data from the EHR for continuous quality improvement. We included all STI testing encounters from patients aged 15–21 during the eight months following the beginning of the COVID-19 pandemic in the United States (March 1st, 2020 - October 31st, 2020) and during the corresponding eight month period in the previous year (March 1st, 2019 - October 31st, 2019) to account for seasonal variation in testing rates. An STI testing encounter was defined as a visit recorded in the EHR that included at least one order for an STI or HIV diagnostic test. Some patients attended multiple STI testing encounters during the study period for risk or symptom-based screening or test for reinfection after STI treatment. A syphilis test was considered positive if the patient had a newly positive rapid plasma reagin test or a four-fold rise in RPR titer, and a corresponding positive fluorescent treponemal antibody absorption test. All positive syphilis and HIV cases underwent a manual chart review to confirm diagnosis and to identify the stage of syphilis disease.
We measured outcomes related to STI testing (test counts, case counts, and test positivity rates). Test positivity rates were compared from the pre-pandemic period to the pandemic period using chi-square tests for differences in proportions. Analyses were performed using R 3.5.1.4 This research was deemed exempt by the Children’s Hospital of Philadelphia Institutional Review Board.
Results
During the pre-pandemic period, there were 4699 STI testing encounters among 3723 unique patients. During the pandemic period there were 3476 STI testing encounters from 2770 unique patients. The median age of patients was 17 years, 71.9% of patients were Black or African American, and 54.6% used public insurance.
The largest decreases in the number of chlamydia and gonorrhea tests performed occurred in the three months following the onset of the COVID-19 pandemic. After some stabilization, a decrease of similar magnitude occurred in September and October, when supplies and laboratory resources for STI screening were interrupted by the national NAAT shortage and conservative strategies for testing were recommended by the CDC. In both instances, the decrease in chlamydia and gonorrhea test volume was accompanied by an increase in the test positivity rate (see Figure 1).
Figure 1. Total test counts, positive and negative test counts, and test positivity rates from March 2019 - October 2020 for chlamydia and gonorrhea.
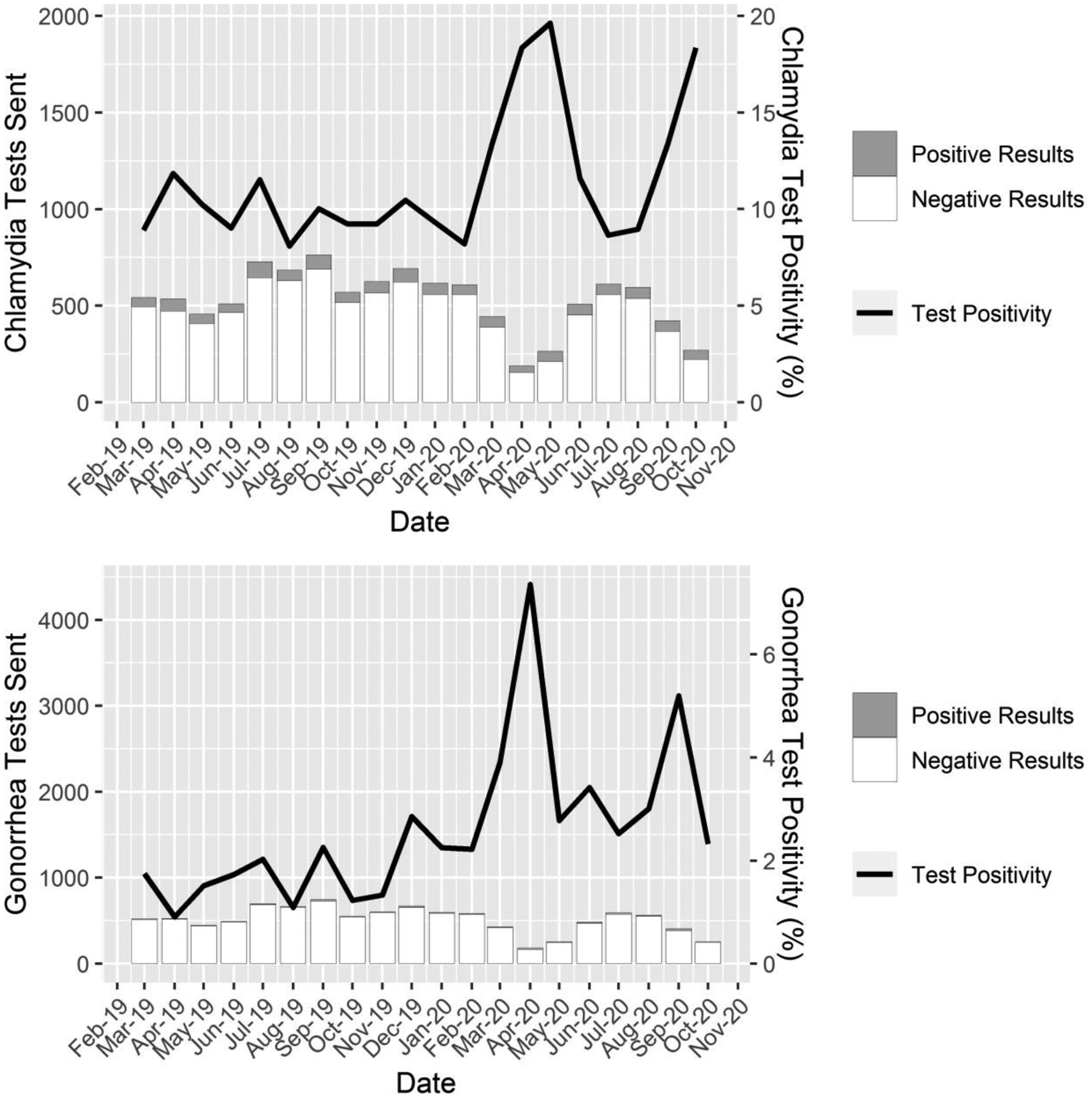
Note: Bars represent the total number tests sent for each STI each month, the shaded region of the bars represents positive results from these tests, the unshaded region represents negative results from these tests, and the lines represent test positivity rate. Test counts are shown on the left axis, test positivity rates are shown on the right axis.
Comparing the entire pre-pandemic period to the entire pandemic period, we found a sharp decline in the number of STI tests that were sent (chlamydia: 4,368 vs. 3,145, a 28% decrease; gonorrhea: 4,229 vs. 3,011, a 29% decrease; syphilis: 1,604 vs. 1,296, a 19% decrease; HIV: 1,717 vs. 1,384, a 19% decrease), as well as a significant increase in test positivity rate for chlamydia (10.4% vs. 12.7%, p= 0.003) and gonorrhea (1.7% vs. 3.4%, p< 0.001). We found no significant changes in test positivity rates for syphilis or HIV (0.1% vs 0.2%, p=.81; 0.1% vs 0.1%, p=1), however case counts for both were low and therefore we caution against making wider inference based on these positivity rates. All syphilis cases were determined to be either primary or early latent stage. Despite these reductions in testing volume, the raw case count of infections identified was relatively stable from the pre-pandemic period to the pandemic period (chlamydia: 453 vs. 398; gonorrhea: 72 vs. 103; syphilis: 2 vs. 3; HIV: 2 vs. 1).
Discussion
During the first eight months of the COVID-19 pandemic, fewer adolescents and young adults received STI testing within a large primary care network in a high STI prevalence region. However, the positivity rate of these tests was markedly higher for both chlamydia and gonorrhea. These higher test positivity rates suggest that during the COVID-19 pandemic symptomatic patients are likely being prioritized for testing, and that asymptomatic cases may be going undetected and untreated as asymptomatic youth may be less likely to present for in-office primary care visits. Given that an estimated 77% of chlamydia infections and 45% of gonorrhea infections among young people present without symptoms, routine asymptomatic screening is a critical tool for STI diagnosis and treatment.6 During the pandemic, prioritizing symptomatic patients and deferring preventive STI screening is recommended by several published guidelines7,8 as the benefits of avoiding exposure to COVID-19 in clinical settings may outweigh the negative sequelae of untreated STIs.7,9 However, for adolescents and young adults who carry the vast majority of the STI burden in the U.S., this risk calculus may differ in a patient population at relatively low risk of severe disease from SARS CoV-2 infection and at high risk of severe sequelae from untreated STIs (i.e., pelvic inflammatory disease, infertility, susceptibility to HIV infection, and increased community STI transmission).10–12 It should be noted that although adolescents are at decreased risk of severe disease from COVID-19, there is growing evidence that this population could still contribute to community spread of the virus through pre-symptomatic and asymptomatic transmission, a fact which should be considered when developing best practices for conducting routine STI screening.13
Importantly, although the total volume of testing decreased at the study sites, over 500 episodes of chlamydia, gonorrhea, HIV, and syphilis were identified during the pandemic period, and the raw case count for gonorrhea increased by 31 cases. These data are sobering for a number of reasons. First, these data highlight that many young people have remained sexually active throughout the pandemic, despite recommendations for social distancing, and continue to be highly vulnerable to STIs and their sequelae. While it would be easy to assume that social distancing recommendations would lead to decreased STI risk, system-wide school shutdowns have been associated with stable or increasing rates of STIs.14 Secondly, by remaining open and continuing to provide STI services throughout the pandemic (when many community STI testing sites closed), the primary care network in this study served as a critical sexual health safety net for youth. Although the outpatient network had limited capacity for in-person visits for a period of several weeks immediately following the onset of the pandemic, by maintaining key parts of their sexual health infrastructure, it was possible to detect and treat a large number of STIs. Importantly, the raw chlamydia case counts during the pandemic period were only slightly lower than before the pandemic, and a higher raw number of gonorrhea cases were detected during the pandemic period. These data underscore the importance of keeping STI testing services available during this present surge of the COVID-19 pandemic, and the need for systems to scale up testing as the pandemic resolves to account for likely missed cases during the disruption in services.
While decreased testing may be partially attributed to COVID-19 risk mitigation, other factors related to the pandemic may have also functioned to limit STI screening. An interruption in the supply chain for urine STI screening within our health system resulted in significant challenges to providing STI testing during September and October. This interruption likely caused the visible decrease in the number of tests sent by providers, and a corresponding increase in test positivity rate. Similar to the immediate effects of the pandemic, this shock to the system likely forced prioritization of symptomatic cases and deferral of preventive screening, leading to the potential for missed detection and treatment of STIs in the community. Given the present COVID-19 surge in testing, national advocacy efforts are needed to assure that there is consistency in the required supply chains for these critical sexual health services during pandemics and other events that could cause limitations to resources and infrastructure.
Our study has limitations as well as strengths. First, given the cross sectional nature of our study design, we are unable to ascribe causality for the reduction in STI test volume and increased test positivity during the pandemic period. However, using near-real time EHR data visualizations has allowed us to rapidly identify a critical need for ongoing STI services, irrespective of causality. Second, we were unable to determine the anatomic site of collection or whether multiple anatomic sites were sampled for each patient, limiting our ability to detect changes in provider ordering behavior regarding multi-site specimen collection. Third, although we suspect that the decline in total test count can be partially attributed to a decrease in asymptomatic screening, we were unable to ascertain which tests were ordered in response to presenting symptoms and which were ordered as routine screening. Finally, our study was limited by an inability to account for cases where patients were treated syndromically without having a test sent—another practice that is likely to have increased during the pandemic and in response to limited STI testing supplies during the national NAAT stock-out.
STI/HIV testing services remain a critical piece of health infrastructure in the setting of a global pandemic, especially for adolescents. The effects of this unprecedented shock to the health system are evident in the patterns of STI testing data seen in this study. Efforts should be focused on maintaining continuous access to STI services, reinforcing supply chains and mitigating interruptions, and establishing infrastructure to provide routine preventive screening while minimizing potential exposures to COVID-19.
Conflicts of Interest and Source of Funding:
This work was supported by grants K23MH119976 (Wood) and K23MH102128 (Dowshen) from the National Institute of Mental Health; and by a Children’s Hospital of Philadelphia Center for Pediatric Clinical Effectiveness and PolicyLab pilot grant. The authors have no conflicts of interest to disclose.
References
- 1.Kreisel KM, Spicknall IH, Gargano JW, et al. Sexually Transmitted Infections Among US Women and Men: Prevalence and Incidence Estimates, 2018. Sex Transm Dis. Published online 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention HIV Surveillance Report, 2018 (Preliminary). Vol 30.; 2019. http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html [Google Scholar]
- 3.Workowski KA, Bolan GA. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep Morb Mortal Wkly Rep Recomm Rep. 2015;64(RR-03):1. [PMC free article] [PubMed] [Google Scholar]
- 4.Thomson-Glover R, Hamlett H, Weston D, Ashby J. Coronavirus (COVID-19) and young people’s sexual health. Sex Transm Infect. 2020;96(7):473–474. [DOI] [PubMed] [Google Scholar]
- 5.Bachmann LH, Bolan G. Dear Colleague Letter: Shortage of STI Diagnotic Test Kits and Laboratory Supplies. Published online September 8, 2020. https://www.cdc.gov/std/general/DCL-Diagnostic-Test-Shortage.pdf
- 6.Farley TA, Cohen DA, Elkins W. Asymptomatic sexually transmitted diseases: the case for screening. Prev Med. 2003;36(4):502–509. [DOI] [PubMed] [Google Scholar]
- 7.Barbee LA, Dombrowski JC, Hermann S, et al. “Sex in the time of covid”: clinical guidelines for sexually transmitted disease management in an era of social distancing. Sex Transm Dis. 2020;47(7):427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.DiMarco D Guidance: Strategies for STI Screening and Treatment During COVID-19. The Clinical Guidelines Program. Published online 2020. https://www.hivguidelines.org/sti-care/sti-covid-19-guidance/ [Google Scholar]
- 9.Mmeje OO, Coleman JS, Chang T. Unintended Consequences of the COVID-19 Pandemic on the Sexual and Reproductive Health of Youth. J Adolesc Health. 2020;67(3):326–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bernstein KT, Marcus JL, Nieri G, Philip SS, Klausner JD. Rectal gonorrhea and chlamydia reinfection is associated with increased risk of HIV seroconversion. JAIDS J Acquir Immune Defic Syndr. 2010;53(4):537–543. [DOI] [PubMed] [Google Scholar]
- 11.Haggerty CL, Gottlieb SL, Taylor BD, Low N, Xu F, Ness RB. Risk of sequelae after Chlamydia trachomatis genital infection in women. J Infect Dis. 2010;201(Supplement_2):S134–S155. [DOI] [PubMed] [Google Scholar]
- 12.Robertson J, Ward M, Conway D, Caul E. Chlamydial and gonococcal antibodies in sera of infertile women with tubal obstruction. J Clin Pathol. 1987;40(4):377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guilamo-Ramos V, Benzekri A, Thimm-Kaiser M, Hidalgo A, Perlman DC. Reconsidering Assumptions of Adolescent and Young Adult Severe Acute Respiratory Syndrome Coronavirus 2 Transmission Dynamics. Clin Infect Dis. Published online 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McBride JA, Eickhoff J, Wald ER. Impact of COVID-19 Quarantine and School Cancelation on Other Common Infectious Diseases. Pediatr Infect Dis J. 2020;39(12):e449–e452. [DOI] [PubMed] [Google Scholar]


