Objective:
We conducted the updated systematic review and meta-analysis of the best available quantitative and qualitative evidence to evaluate the effects and safety of duloxetine for the treatment of knee osteoarthritis (OA) pain.
Methods:
A comprehensive literature search used 3 English and 4 Chinese biomedical databases from inception through July 10, 2020. We included randomized controlled trials of duloxetine with intervention duration of 2 weeks or longer for knee OA. The primary outcome was pain intensity measured by Brief Pain Inventory and the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain subscale. Secondary outcome measurements included 36-Item Short Form Health Survey, Patient’s Global Impression of Improvement, Clinical Global Impressions of Severity, and adverse events (AEs). The quality of all included studies was evaluated using the Cochrane risk-of-bias criteria. The review was registered in the PROSPERO (CRD 42020194072).
Results:
Six studies totaling 2059 patients met the eligibility criteria. Duloxetine had significant reductions in Brief Pain Inventory 24 hours average pain (mean difference [MD]=−0.74; 95% confidence interval [CI], −0.92 to −0.57; P<0.00001; I 2=13%; 5 trials; 1695 patients); patient general activity (MD=−0.76; 95% CI, −0.96 to −0.56; P<0.00001; I 2=0%; 5 trials; 1694 patients) WOMAC physical function subscale (MD=−4.22; 95% CI, −5.14 to −3.30; P<0.00001; I 2=26%; 5 trials; 1986 patients); Patient’s Global Impression of Improvement (MD=−0.48; 95% CI, −0.58 to −0.37; P<0.00001; I 2=29%; 5 trials; 1741 patients); and Clinical Global Impressions of Severity (MD=−0.34; 95% CI, −0.44 to −0.24; P<0.00001; I 2=0%; 4 trials; 1178 patients) compared with placebo control. However, no difference on WOMAC pain subscale (standard mean difference=−1.68; 95% CI, −3.45 to 0.08; P=0.06; I 2=100%; 3 trials; 1104 patients) and in serious AEs (risk ratio=0.92; 95% CI, 0.40-2.11; P=0.84; I 2=0%; 5 trials; 1762 patients) between duloxetine and placebo. Furthermore, duloxetine failed to show superior effects for improving the life quality and demonstrated more treatment-emergent AEs.
Conclusion:
Duloxetine may be an effective treatment option for knee OA patients but further rigorously designed and well-controlled randomized trials are warranted.
Key Words: duloxetine, knee osteoarthritis, pain, treatment
Knee pain is a common symptom in patients with knee osteoarthritis (OA),1,2 which is a major age-related public health problem and a leading cause of long-term disability and reduced quality of life.3–5 There are no effective disease-modifying remedies available to treat knee OA6 and the underlying mechanisms of knee OA still remain unknown.7 Current guidelines of knee OA management recommend a comprehensive combination of educational, physical, behavioral, psychosocial, mind-body, and pharmacologic interventions, but the availability, accessibility, and affordability vary of these interventions.8 Nonsteroidal anti-inflammatory drugs and acetaminophen are used to treat the OA but could increase the risk of side effects after the long-term utilization.9,10 In addition, depressed symptoms are reportedly associated with knee OA, especially among the geriatric community11,12 and in Asian Americans.13 Some studies revealed depression is prevalent among patients with chronic pain due to OA.14,15 However, the latest guidelines indicated that no interventions were strongly recommended for use in patients who have concomitant OA and depression.16 Therefore, other new safe and efficient therapeutic approaches are required that have been validated by clinical experience. As understanding of the pathophysiology of OA pain has progressed, it has become apparent that much of the refractory pain associated with OA may be of neurogenic origin or may be responsive to neutralizing specific neurotransmitters.17,18 Consequently, the centrally acting serotonin and norepinephrine reuptake inhibitor, duloxetine, was approved for the treatment of musculoskeletal pain, including OA.19,20
Duloxetine is a selective, relatively balanced serotonin and norepinephrine reuptake inhibitor with antidepressant, central pain inhibitory, and anxiolytic activities that has shown efficacy in the treatment of chronic pain conditions such as peripheral neuropathic pain, fibromyalgia, low back pain, and knee OA pain.21–24 Research on duloxetine for knee OA has been growing, some systematic reviews were conducted to establish the association of duloxetine with knee OA pain,25–27 but none arrived at a definitive conclusion. In addition, more clinical trials of duloxetine and related therapies published in recent years were not included in previous systematic reviews.
In light of the growing number of clinical researches of duloxetine use for knee OA pain and the ensuing need for critical evaluation, we conducted the updated meta-analysis of all available data to determine the efficacy of duloxetine for pain relief in patients with knee OA to better inform future research and clinical practice.
METHODS
This meta-analysis is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement guidelines.28 The review was registered in the PROSPERO 2020 (registration number: CRD 42020194072).29
Search Strategy
We conducted a comprehensive literature search on 3 English and 4 Chinese biomedical databases from inception through July 10, 2020. These databases included PubMed, the Cochrane Library, Springer, the Chinese National Knowledge Infrastructure, Chongqing VIP information, Wanfang, and the Chinese Biomedical Databases. In addition, ClinicalTrials.gov and the reference lists of previously published reviews related to duloxetine and knee OA were also screened for eligible clinical trials. The search terms used duloxetine, cymbalta, knee pain, knee osteoarthritis, randomized controlled trial (RCT), and clinical trial.
Eligibility Criteria and Study Selection
We included RCTs that compared duloxetine with nonduloxetine intervention, usual care, or placebo in adults with knee pain. To be eligible for this study, each trial was required to have at least 2 weeks of duloxetine interventions with >10 patients in each group, and report original data. There was no language restriction in the literature search. We excluded review articles and case reports.
Two authors independently screened all potential eligible studies. Titles and abstracts were first screened to exclude irrelevant citations. Full texts of all articles of potentially relevant abstracts were retrieved and screened according to the study eligibility criteria. Disagreements were resolved by consensus or discussion with a third author. The diagnostic criterion derived from the American College of Rheumatology (Table 1).36
TABLE 1.
Characteristics of the Included Randomized Controlled Trials
| References | Location | Age (y) | Diagnostic Criteria | Sample Size (Male/Female); Duration of OA (y) | Treatment Group | Control Group | Main Outcome |
|---|---|---|---|---|---|---|---|
| Chappell et al30 | USA | T: 62.1±9.5 C: 62.5±9.3 | ACR OA criteria | T: 111 (41/70), 6.9±8.4 C: 120 (39/81), 7.1±7.2 | Duloxetine 60/120 mg/d for 13 wk | Placebo | BPI-S; BPI-I; WOMAC; CGI-S; SF-36; EQ-5D; TEAEs; SAEs |
| Chappell et al31 | USA | T: 63.2±8.8 C: 61.9±9.2 | ACR OA criteria | T: 128 (39/89), 6.2±5.9 C: 128 (21/107), 5.6±6.2 | Duloxetine 60/120 mg/d for 13 wk | Placebo | BPI-S; BPI-I; WOMAC; CGI-S; SF-36; TEAEs; SAEs |
| Frakes et al32 | USA | T: 61.6±9.2 C: 60.3±9.2 | ACR OA criteria | T: 264 (112/152), 9.8±8.9 C: 260 (113/147), 9.2±8.9 | Duloxetine 60/120 mg/d +NSAID+PPI for 8 wk | Placebo+NSAID+PPI | BPI-S; BPI-I; WOMAC; TEAEs; SAEs |
| Abou-Raya et al33 | Egypt | T: 68.9±6.2 C: 68.5±5.8 | ACR OA criteria | T: 144 (23/121), 5.7±4.9 C: 144 (24/120), 5.6±4.5 | Duloxetine 60 mg/d for 16 wk | Placebo | WOMAC |
| Wang et al34 | China | T: 61.2±8.2 C: 59.8±8.4 | ACR OA criteria | T: 205 (45/160), 2.9±4.4 C: 202 (51/151), 2.7±4.2 | Duloxetine 60 mg/d for 13 wk | Placebo | BPI-S; BPI-I; WOMAC; CGI-S; PGI-I; TEAEs; SAEs |
| Uchio et al35 | Japan | T: 65.5±8.0 C: 66.4±8.4 | ACR OA criteria | T: 177 (35/142), 4.0±4.2 C: 176 (44/132), 4.5±4.3 | Duloxetine 20-60 mg/d for 14 wk | Placebo | BPI-S; BPI-I; CGI-S; PGI-I; EQ-5D; SF-36; TEAEs; SAEs |
ACR indicates American College of Rheumatology; BPI, Brief Pain Inventory; BPI-I, Brief Pain Inventory-Interference; BPI-S, Brief Pain Inventory-Severity; C, control group; CGI-S, Clinical Global Impressions of Severity; EQ-5D, European Quality of Life Questionnairee5 Dimension; NSAID, nonsteroidal anti-inflammatory drug; OA, osteoarthritis; PGI-I, Patient’s Global Impression of Improvement; PPI, proton pump inhibitor; SAEs, serious adverse events; SF-36, 36-Item Short-Form Health Status Survey; T, treatment group; TEAEs, treatment-emergent adverse events; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Primary outcomes concerned the pain intensity of knee joint as measured by validated instruments including Brief Pain Inventory Severity (BPI-S)37 and the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC)38 pain subscale in this study. The interference of pain was also evaluated by Brief Pain Inventory-Interference (BPI-I).37 Secondary outcomes were quality of life, measured on the 36-Item Short Form Health Survey (SF-36)39 scale, illness severity and patient’s global impression measured by Patient’s Global Impression of Improvement (PGI-I)40 and Clinical Global Impressions of Severity (CGI-S),40 and adverse events (AEs).
Data Collection and Quality Assessment
We extracted the data from included studies using a predesigned data extraction table, including publication information, origin of study, study setting, time frame of study, age, sex, definition of knee OA, detailed information of interventions and controls, outcome measures, and main conclusion. The accuracy of the data extraction was verified by another author (Table 1).
We assessed the risk of bias for each study using the items in Cochrane Collaboration’s tool for assessing quality in randomized trials,41 which covered the following items: selection bias included random sequence generation and allocation concealment, blinding of participants, personnel, and outcome assessment, incomplete outcome data, selective reporting, and other potential bias. Two authors independently evaluated the methodological quality of the included studies using the risk-of-bias tools. Disagreements were resolved by the third author through discussion.
Data Synthesis and Statistical Analysis
We qualitatively synthesized all included studies in summary Table 1. Included studies on pain were synthesized based on the BPI-S and the WOMAC pain subscale separately. The BPI37 is a self-reported scale that measures the severity of pain and the interference of pain on function. Severity of pain is assessed with 4 questions: patients assign scores to characterize their worst pain, least pain, and average pain in the previous 24 hours and pain right now. Pain ratings range from 0 (no pain) to 10 (pain as severe as you can imagine). There are 7 questions assessing the interference of pain in the past 24 hours on patient general activity, mood, walking ability, normal work, relations with other people, sleep, and enjoyment of life. The interference ratings range from 0 (does not interfere) to 10 (completely interferes), and a mean across the interference items is derived as a summary interference measure. The WOMAC38 scale is designed to assess pain, stiffness, and physical function in patients with OA of the knee or hip. It consists of 24 questions: 5 on pain, 2 on stiffness, and 17 on physical function. Higher scores on the WOMAC indicate worst pain, stiffness, and functional limitations.
Other measures included the PGI-I and CGI-S40 to assess the patient’s global impression and the SF-36 to observe the life quality of patients. The safety of duloxetine versus placebo were assessed during the treatment phase of the study and were based on the incidence rate of treatment-emergent adverse events (TEAEs) and serious adverse events (SAEs).
We used RevMan V.5.3 software (The Cochrane Collaboration, Oxford, England, available online at www.cochrane.com) to perform the meta-analysis of the outcome data.42 Statistical heterogeneity across included studies was estimated using the Cochran Q statistic (considered significant when the P<0.10) and quantified the extent of heterogeneity with the I 2 index.43 Continuous outcomes, such as pain (measured by BPI or WOMAC pain subscale) and quality of life (measured by SF-36 scale), were expressed as mean difference (MD) with a 95% confidence interval (CI). Other forms of continuous data were converted into MD values. Dichotomous data, such as AEs, were expressed as risk ratio (RR) with a 95% CI. Other binary data were converted into an RR value. The fixed-effect model was used if I 2 <50% and the random-effect model was used if I 2 >50%. All reported P values were 2 sided and a P value <0.05 was considered to be statistically significant.
RESULTS
Studies Selection
Figure 1 summarizes the detailed study selection process. We screened a total of 2473 abstracts identified from 3 English and 4 Chinese databases and additional records from ClinicalTrials.gov. After initially screening, we excluded 2341 abstracts which did not meet the inclusion criteria (ie, participants did not have knee OA, reviews, case reports or duplicate publications). We retrieved and reviewed 132 full articles. A total of 125 articles were excluded due to lack of randomization or absence of a control group, and insufficient data for meta-analysis. Finally, 6 RCTs30–35 involving 2059 patients met our inclusion criteria.
FIGURE 1.
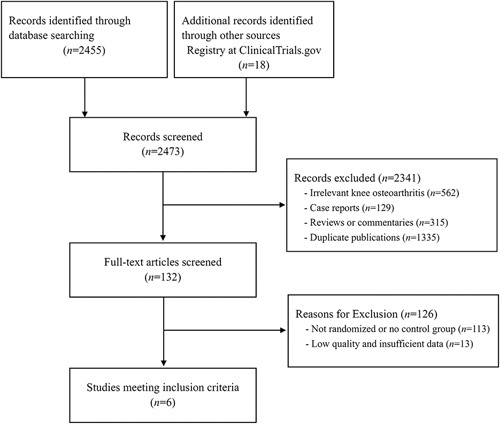
Study selection flow chart.
Study Characteristics
Table 1 summarizes the characteristics of the 6 trials.30–35 These studies were published from 2009 to 2018. Three studies30–32 were conducted in the United States, and 1 each in Egypt,33 China,34 and Japan.35 The number of participants in the studies varied from 231 to 524. The mean age was 63 years and 71.49% were women. The mean disease duration was 5.85 years, and the treatment duration ranged from 8 to 16 weeks with 20 to 120 mg/d of duloxetine in these included trials. Study participants were diagnosed with knee OA by the American College of Rheumatology criteria.36
Quality Assessment
The quality assessment of the trials was performed using the Cochrane Collaboration’s risk-of-bias tool. The detailed results are presented in Figure 2. Randomization sequence generation was adequate in all 6 trials (100%). Three studies30,33,34 declared appropriate allocation concealment (50.0%) but other 3 trials31,32,35 were unclear (50.0%). Blinding of participants and personnel occurred in all 6 trial (100%), but blinding of outcome assessment was unclear in 5 trials (83.3%).30–33,35 One study33 had a high risk of bias due to incomplete outcome data and only reported the WOMAC score data with high risk of bias of selective reporting (16.7%). In addition, regarding other potential sources of bias, no studies reported intention-to-treat items and 1 study33 with high risk of bias due to insufficient information about the treatment.
FIGURE 2.
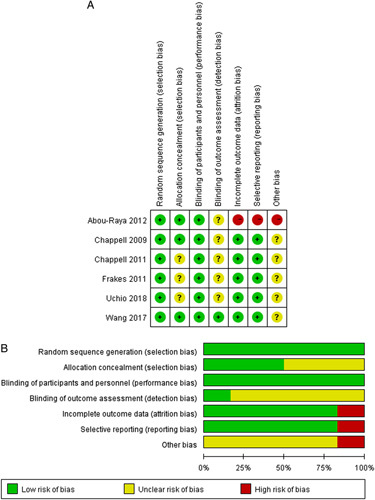
Risk of bias for randomized, controlled trials (n=6). A, Risk of bias summary. B, Risk of bias distribution.
Meta-analysis
In the 6 eligible RCTs, 5 trials30–32,34,35 measured pain level using the BPI and 3 trials30,32,34 assessed by the WOMAC pain subscale. Six trials30–35 evaluated the physical function using WOMAC physical function and stiffness subscale, 3 trials30,31,35 assessed the quality of life by SF-36, and some trials compared the patient’s global impression measured by PGI-I30–32,34,35 and CGI-S.30,31,34,35 Six trials30–35 mentioned the AEs and 5 trials30–32,34,35 of them reported the numbers of TEAEs and SAEs.
Pain Reductions
Five trials30–32,34,35 contributed to the meta-analysis of pain outcomes based on the BPI-S. The fixed-effects meta-analysis results indicated that patients in the duloxetine groups had significant reductions in the previous 24 hours on average pain (1695 patients; MD=−0.74; 95% CI, −0.92 to −0.57; P<0.00001; I 2=13%) (Fig. 3A); worst pain (1696 patients; MD=−0.87; 95% CI, −1.07 to −0.66; P<0.00001; I 2=0%) (Fig. 3B); least pain (1696 patients; MD=−0.54; 95% CI, −0.71 to −0.37; P<0.00001; I 2=0%) (Fig. 3C); and pain right now (1696 patients; MD=−0.68; 95% CI, −0.87 to −0.48; P<0.00001; I 2=0%) (Fig. 3D) than those in the placebo control groups after 8 to 14 weeks of 60/120 mg duloxetine treatment. These studies suggest that duloxetine was associated with significant pain reduction in patients with knee OA.
FIGURE 3.
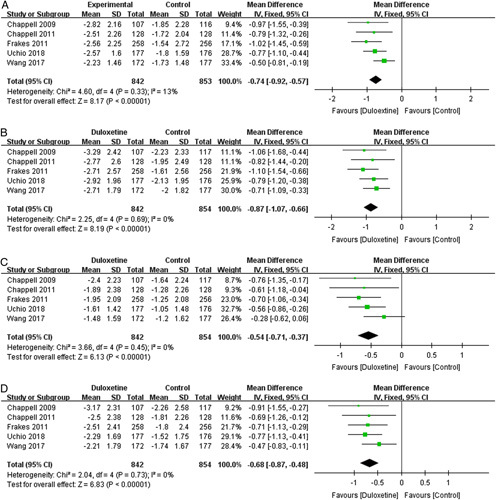
Effects of duloxetine on pain measured by Brief Pain Inventory-Severity: (A) average pain; (B) worst pain; (C) least pain; and (D) pain right now. CI indicates confidence interval.
However, meta-analysis of 3 trials30,32,34 involving 1104 patients failed to show superior analgesic effects of duloxetine on WOMAC pain subscale (standard MD=−2.11; 95% CI, −4.93 to 0.72; P=0.14) with a high heterogeneity score (I 2=100%) (Fig. 4).
FIGURE 4.
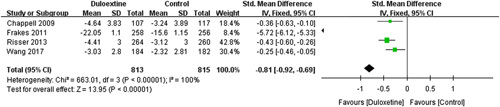
Effects of duloxetine on pain measured by Western Ontario and McMaster Universities Osteoarthritis Index pain subscale. CI indicates confidence interval.
The Interference of Pain
Five trials30–32,34,35 contributed to the meta-analysis of the interference of pain based on the BPI-I. Compared with the placebo control group, the duloxetine group showed significant improvement in the interference of pain on patient general activity (5 trials; 1694 patients, MD=−0.76; 95% CI, −0.96 to −0.56; P<0.00001; I 2=0%) (Fig. 5A); mood (4 trials; 1438 patients, MD=−0.55; 95% CI, −0.75 to −0.35; P<0.00001; I 2=9%) (Fig. 5B); walking ability (4 trials; 1438 patients, MD=−0.71; 95% CI, −0.93 to −0.50; P<0.00001; I 2=4%) (Fig. 5C); normal work (5 trials; 1694 patients, MD=−0.70; 95% CI, −0.90 to −0.50; P<0.00001; I 2=49%) (Fig. 5D); relations with other people (4 trials; 1437 patients, MD=−0.40; 95% CI, −0.75 to −0.06; P=0.02; I 2=70%) (Fig. 5E); sleep (4 trials; 1438 patients, MD=−0.53; 95% CI, −0.81 to −0.25; P=0.0002; I 2=52%) (Fig. 5F); enjoyment of life (4 trials; 1438 patients, MD=−0.60; 95% CI, −1.03 to −0.17; P=0.006; I 2=76%) (Fig. 5G); and average interference (3 trials; 924 patients, MD=−0.46; 95% CI, −0.66 to −0.27; P<0.00001; I 2=45%) (Fig. 5H) in the past 24 hours, which indicated that duloxetine can improve the interference of pain significantly.
FIGURE 5.
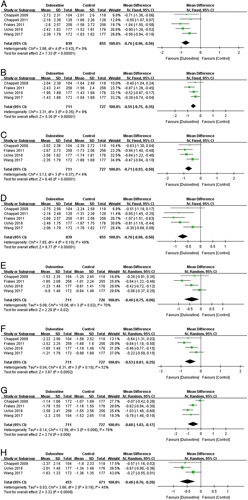
Effects of duloxetine in the interference of pain measured by Brief Pain Inventory-Interference: (A) patient general activity; (B) mood; (C) walking ability; (D) normal work; (E) relations with other people; (F) sleep; (G) enjoyment of life; and (H) average interference. CI indicates confidence interval.
Physical Function and Quality of Life
Six trials30–35 reported specific relevant data for the meta-analysis showed that duloxetine had a significant improvement on WOMAC physical function subscale (1986 patients; MD=−4.22; 95% CI, −5.14 to −3.30; P<0.00001; I 2=26%) (Fig. 6A), and on WOMAC stiffness subscale (2002 patients; MD=−0.47; 95% CI, −0.60 to −0.34; P<0.00001; I 2=21%) (Fig. 6B). These evidences showed that duloxetine can improve the physical function for the knee OA patients.
FIGURE 6.
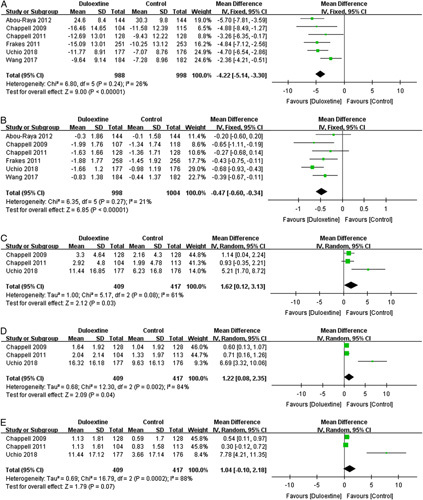
Effects of duloxetine on the physical function measured by (A) Western Ontario and McMaster Universities Osteoarthritis Index physical function subscale and (B) Western Ontario and McMaster Universities Osteoarthritis Index stiffness subscale. The quality of life measured by (C) 36-Item Short Form Health Survey (SF-36) physical functional subscale, (D) SF-36 bodily pain subscale, and (E) SF-36 role physical subscale. CI indicates confidence interval.
However, the results of the random-effects meta-analysis of 3 trials30,31,35 involving 826 patients reported the negative effects of duloxetine for improving the life quality measured by SF-36 physical functional subscale (MD=1.62; 95% CI, 0.12-3.13; P=0.03; I 2=61%) (Fig. 6C) and by SF-36 bodily pain subscale (MD=1.22; 95% CI, 0.08-2.35; P=0.04; I 2=84%) (Fig. 6D). There was also no significant difference in SF-36 role physical subscale (MD=1.04; 95% CI, −0.10 to 2.18; P=0.07; I 2=88%) (Fig. 6E).
Patient’s Global Impression
Meta-analysis showed that duloxetine had significantly improvement of patient’s global impression measured by PGI-I (1741 patients; MD=−0.48; 95% CI, −0.58 to −0.37; P<0.00001; I 2=29%) (Fig. 7A) in 5 trials30–32,34,35 and by CGI-S (1178 patients; MD=−0.34; 95% CI, −0.44 to −0.24; P<0.00001; I 2=0%) (Fig. 7B) in 4 trials30,31,34,35 compared with placebo control.
FIGURE 7.
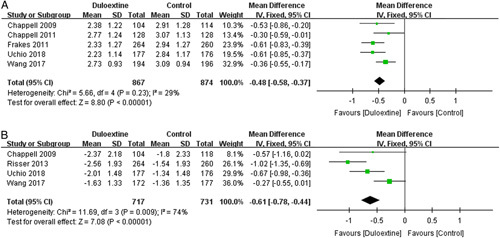
Effects of duloxetine in patient’s global impression measured by: (A) Patient’s Global Impression of Improvement; (B) Clinical Global Impression of Severity. CI indicates confidence interval.
Safety
Six trials30–35 described the reasons of main TEAEs in the duloxetine group were constipation, nausea, hyperhidrosis, cough, myalgia, arthralgia, palpitations, dry mouth, and so on. No deaths or suicide-related events were reported. Five trials30–32,34,35 involving 1762 patients compared the safety of duloxetine with placebo control interventions. The results of our meta-analysis showed that duloxetine had higher incidence of TEAEs (RR=1.31; 95% CI, 1.20-1.43; P<0.00001; I 2=0%) (Fig. 8A), but there was no significant difference in the rate of SAEs between duloxetine and control groups (RR=0.92; 95% CI, 0.40-2.11; P=0.84; I 2=0%) (Fig. 8B).
FIGURE 8.
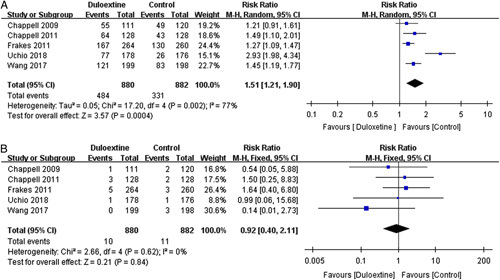
Effects of duloxetine in patient’s safety: (A) incidence rate of treatment-emergent adverse events, and (B) serious adverse events. CI indicates confidence interval.
DISCUSSION
This updated systemic review and meta-analysis of 6 RCTs in 2059 individuals indicate that duloxetine appears to be effective on pain reduction on BPI-S and benefit for improving of the physical function and patient’s global impression compared with placebo for people who with knee OA. However, duloxetine failed to demonstrate superior analgesic effects on the WOMAC pain subscale and beneficial effects on quality of life improvement. In addition, there was no substantial difference between duloxetine and the control groups in the incidence of SAEs.
Three systematic reviews have been published.25–27 In a system review and meta-analysis of 3 RCTs enrolled 1011 patients published in 2015 revealed that duloxetine 60/120 mg/d resulted in a greater reduction in pain improved function and patient-rated impression of improvement, and acceptable AEs for treating knee OA pain after ~10 to 13 weeks of treatment.25 Another system review and meta-analysis conducted in 2018 indicated that duloxetine has statistically significant, moderate benefits on pain, function, and quality of life in knee OA patients, but use of this drug is associated with a significantly higher risk of AEs.26 The third system review and meta-analysis published in 2019 is effective in the management of chronic pain and loss of physical function but having no advantage in treating joint stiffness.27 The consistent findings from above system reviews and meta-analyses were duloxetine had a beneficial impact on pain relief, function improvement across all studies. In addition, our evidence showed that duloxetine can also ameliorate the knee stiffness, although the significant change in the quality of life cannot be verified. In terms of safety, all the studies demonstrated that duloxetine is at higher risk for TEAEs, but that there is no substantial SAEs between duloxetine and the control group. This research suggests that the side effects of duloxetine are mild to moderate in severity and can be appropriate. Previous study had found that the most TEAEs occur early in therapy, and have been steadily tolerated by extending the length of therapy.44
There is increasing evidence indicated that a correlation between pain and depression can be promoted by norepinephrine systems and 5-hydroxytryptamine.45,46 Serotonin and noradrenaline can dampen peripheral pain signals by mediating a bidirectional feedback between a central pain modulation system and a peripheral nociceptive stimulus such as OA pain.47,48 Duloxetine can be effective in alleviating pain by modulating the descending brain and spinal cord pressure pathways as a selective, relatively controlled serotonin and norepinephrine reuptake inhibitor.
Our study also has limitations. First, in this meta-analysis, we included only 6 trials although we screened all the available studies using the comprehensive literature search strategy, and most of the included trials had a high quality and lower risk bias. Second, this meta-analysis lacked long-term follow-up studies as the qualifying criteria for inclusion, but the potential value of duloxetine during long-term therapy has been shown by a study undertaken in China to be effective and tolerable.49 Third, the trials compared treatment of knee OA pain between duloxetine and other therapies are still lacking. There is a study have demonstrated that both duloxetine and gabapentin have similar and acceptable effects on pain reduction and improvement of functional status in patients with knee OA,50 but no methodologically rigorous studies directly compared duloxetine with other first-line therapies, including nonsteroidal anti-inflammatory drugs, in the treatment of OA.51 Fourth, in this meta-analysis, we found the included trials concentrated on the knee joint and synthesized evidence from studies of patients affected by knee OA. Four studies30,31,34,35 explicitly excluded the patients who had the depressive disorders, and 1 study33 excluded the participants if they were taking any other antidepressants, while duloxetine has been reported to be effective for other chronic pain conditions with depression or anxiety symptoms.52,53 Even though 4 trials30,32,34,35 involving 1438 patients showed significant improvement in mood and relations with other people based on the BPI-I (Figs. 5B, E), future studies should indicate the real benefits to support the clinical application of duloxetine for patients suffering from knee OA and depression simultaneously.
CONCLUSIONS
In summary, our study reveals that duloxetine may be an effective treatment option for knee OA patients. However, owing to the presence of some contradictory pain relief evidence and the higher risk of AEs, duloxetine still requires further additional large-scale, high-quality, rigorously designed, and well-controlled RCTs to evaluate the long-term safety and determine the advantage for patients with knee OA and depressive conditions at the same time.
Footnotes
Supported by the National Natural Science Foundation of China (no. 81774340, 81973875) and Shanghai Medical Innovation Research Project (no. 20MC1920600, 21Y11921600).
B.C. and J.D. contributed equally.
Y.Z. and B.C.: designed the review protocol. S.W. and J.D.: carried out the literature search. B.C. and M.Z.: contributed to data extraction. B.C. and J.P.: contributed to quality assessment. Y.Z. and H.Z.: provided statistical supports for meta-analysis. B.C. and J.D. performed the analyses and drafted the manuscript. Y.Z.: revised this manuscript. All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published.
The authors declare no conflict of interest.
Contributor Information
Bo Chen, Email: cbm818@126.com.
Jingrui Duan, Email: jrscofield@live.com.
Shengyue Wen, Email: freedaydayday@163.com.
Jian Pang, Email: lidazul@126.com.
Min Zhang, Email: zm_602@sina.com.
Hongsheng Zhan, Email: shgsyjs@139.com.
Yuxin Zheng, Email: sg_zyx1728@126.com.
REFERENCES
- 1.Busija L, Bridgett L, Williams SR, et al. Osteoarthritis. Best Pract Res Clin Rheumatol. 2010;24:757–768. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Q, Yue J, Golianu B, et al. Updated systematic review and meta-analysis of acupuncture for chronic knee pain. Acupunct Med. 2017;35:392–403. [DOI] [PubMed] [Google Scholar]
- 3.Wang C, Schmid CH, Iversen MD, et al. Comparative effectiveness of Tai Chi versus physical therapy for knee osteoarthritis: a randomized trial. Ann Intern Med. 2016;165:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McAlindon TE, Cooper C, Kirwan JR, et al. Determinants of disability in osteoarthritis of the knee. Ann Rheum Dis. 1993;52:258–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muraki S, Akune T, Oka H, et al. Association of occupational activity with radiographic knee osteoarthritis and lumbar spondylosis in elderly patients of population-based cohorts: a large-scale population-based study. Arthritis Rheum. 2009;61:779–786. [DOI] [PubMed] [Google Scholar]
- 6.Jotanovic Z, Mihelic R, Sestan B, et al. Emerging pathways and promising agents with possible disease modifying effect in osteoarthritis treatment. Curr Drug Targets. 2014;15:635–661. [DOI] [PubMed] [Google Scholar]
- 7.Liu R, Yuan X, Yu J, et al. An updated meta-analysis of the asporin gene D-repeat in knee osteoarthritis: effects of gender and ethnicity. J Orthop Surg. 2017;12:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res (Hoboken). 2020;72:149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smalley WE, Griffin MR. The risks and costs of upper gastrointestinal disease attributable to NSAIDs. Gastroenterol Clin North Am. 1996;25:373–396. [DOI] [PubMed] [Google Scholar]
- 10.Nagi R, Yashoda Devi BK, Rakesh N, et al. Clinical implications of prescribing nonsteroidal anti-inflammatory drugs in oral health care−a review. Oral Surg Oral Med Oral Pathol Oral Radiol. 2015;119:264–271. [DOI] [PubMed] [Google Scholar]
- 11.Sugai K, Takeda-Imai F, Michikawa T, et al. Association between knee pain, impaired function, and development of depressive symptoms. J Am Geriatr Soc. 2018;66:570–576. [DOI] [PubMed] [Google Scholar]
- 12.Chen YP, Huang YY, Wu Y, et al. Depression negatively affects patient-reported knee functional outcome after intraarticular hyaluronic acid injection among geriatric patients with knee osteoarthritis. J Orthop Surg. 2019;14:387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ahn H, Weaver M, Lyon D, et al. Depression and pain in Asian and White Americans with knee osteoarthritis. J Pain. 2017;18:1229–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Russell IJ, Mease PJ, Smith TR, et al. Efficacy and safety of duloxetine for treatment of fibromyalgia in patients with or without major depressive disorder: results from a 6-month, randomized, double-blind, placebo-controlled, fixed-dose trial. Pain. 2008;136:432–444. [DOI] [PubMed] [Google Scholar]
- 15.Mossey JM, Gallagher RM. The longitudinal occurrence and impact of comorbid chronic pain and chronic depression over two years in continuing care retirement community residents. Pain Med. 2004;5:335–348. [DOI] [PubMed] [Google Scholar]
- 16.Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27:1578–1589. [DOI] [PubMed] [Google Scholar]
- 17.Malfait AM, Schnitzer TJ. Towards a mechanism-based approach to pain management in osteoarthritis. Nat Rev Rheumatol. 2013;9:654–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller RE, Miller RJ, Malfait AM. Osteoarthritis joint pain: the cytokine connection. Cytokine. 2014;70:185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller RE, Malfait AM, Block JA. Current status of nerve growth factor antibodies for the treatment of osteoarthritis pain. Clin Exp Rheumatol. 2017;35(suppl 107):85–87. [PMC free article] [PubMed] [Google Scholar]
- 20.Smelter E, Hochberg MC. New treatments for osteoarthritis. Curr Opin Rheumatol. 2013;25:310–316. [DOI] [PubMed] [Google Scholar]
- 21.Smith EM, Pang H, Cirrincione C, et al. Effect of duloxetine on pain, function, and quality of life among patients with chemotherapy-induced painful peripheral neuropathy: a randomized clinical trial. JAMA. 2013;309:1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bidari A, Moazen-Zadeh E, Ghavidel-Parsa B, et al. Comparing duloxetine and pregabalin for treatment of pain and depression in women with fibromyalgia: an open-label randomized clinical trial. Daru. 2019;27:149–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Konno S, Oda N, Ochiai T, et al. Randomized, double-blind, placebo-controlled phase III trial of duloxetine monotherapy in Japanese patients with chronic low back pain. Spine. 2016;41:1709–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lunn MP, Hughes RA, Wiffen PJ. Duloxetine for treating painful neuropathy, chronic pain or fibromyalgia. Cochrane Database Syst Rev. 2014;1:CD007115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang ZY, Shi SY, Li SJ, et al. Efficacy and safety of duloxetine on osteoarthritis knee pain: a meta-analysis of randomized controlled trials. Pain Med. 2015;16:1373–1385. [DOI] [PubMed] [Google Scholar]
- 26.Osani MC, Bannuru RR. Efficacy and safety of duloxetine in osteoarthritis: a systematic review and meta-analysis. Korean J Intern Med. 2019;34:966–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen L, Gong M, Liu G, et al. Efficacy and tolerability of duloxetine in patients with knee osteoarthritis: a meta-analysis of randomised controlled trials. Intern Med J. 2019;49:1514–1523. [DOI] [PubMed] [Google Scholar]
- 28.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bo Chen MZ, Lin X, Pang J, et al. An updated systematic review and meta-analysis of Duloxetine for knee pain. 2020. Available at: https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=194072. [DOI] [PMC free article] [PubMed]
- 30.Chappell AS, Ossanna MJ, Liu-Seifert H, et al. Duloxetine, a centrally acting analgesic, in the treatment of patients with osteoarthritis knee pain: a 13-week, randomized, placebo-controlled trial. Pain. 2009;146:253–260. [DOI] [PubMed] [Google Scholar]
- 31.Chappell AS, Desaiah D, Liu-Seifert H, et al. A double-blind, randomized, placebo-controlled study of the efficacy and safety of duloxetine for the treatment of chronic pain due to osteoarthritis of the knee. Pain Pract. 2011;11:33–41. [DOI] [PubMed] [Google Scholar]
- 32.Frakes EP, Risser RC, Ball TD, et al. Duloxetine added to oral nonsteroidal anti-inflammatory drugs for treatment of knee pain due to osteoarthritis: results of a randomized, double-blind, placebo-controlled trial. Curr Med Res Opin. 2011;27:2361–2372. [DOI] [PubMed] [Google Scholar]
- 33.Abou-Raya S, Abou-Raya A, Helmii M. Duloxetine for the management of pain in older adults with knee osteoarthritis: randomised placebo-controlled trial. Age Ageing. 2012;41:646–652. [DOI] [PubMed] [Google Scholar]
- 34.Wang G, Bi L, Li X, et al. Efficacy and safety of duloxetine in Chinese patients with chronic pain due to osteoarthritis: a randomized, double-blind, placebo-controlled study. Osteoarthritis Cartilage. 2017;25:832–838. [DOI] [PubMed] [Google Scholar]
- 35.Uchio Y, Enomoto H, Alev L, et al. A randomized, double-blind, placebo-controlled Phase III trial of duloxetine in Japanese patients with knee pain due to osteoarthritis. J Pain Res. 2018;11:809–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29:1039–1049. [DOI] [PubMed] [Google Scholar]
- 37.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singap. 1994;23:129–138. [PubMed] [Google Scholar]
- 38.Bellamy N, Buchanan WW, Goldsmith CH, et al. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 39.Ware JE, Snow KK, Kosinski M, et al. SF-36 health survey manual and interpretation guide. Boston, MA: The Health Institute New England Medical Center; 1993. [Google Scholar]
- 40.Guy W. ECDEU Assessment Manual for Psychopharmacology. Rockville, MD: National Institute of Mental Health (U.S.). Psychopharmacology Research Branch. Division of Extramural Research Programs; 1976. [Google Scholar]
- 41.Higgins JP, Altman DG, Sterne JA. Chapter 8: assessing risk of bias in included studies. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions version 5.1.0.2011. [Google Scholar]
- 42.Review Manager (RevMan). Version 5.3 ed: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014.
- 43.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brunton S, Wang F, Edwards SB, et al. Profile of adverse events with duloxetine treatment: a pooled analysis of placebo-controlled studies. Drug Saf. 2010;33:393–407. [DOI] [PubMed] [Google Scholar]
- 45.Iyengar S, Webster AA, Hemrick-Luecke SK, et al. Efficacy of duloxetine, a potent and balanced serotonin-norepinephrine reuptake inhibitor in persistent pain models in rats. J Pharmacol Exp Ther. 2004;311:576–584. [DOI] [PubMed] [Google Scholar]
- 46.Wong DT, Bymaster FP. Dual serotonin and noradrenaline uptake inhibitor class of antidepressants potential for greater efficacy or just hype? Prog Drug Res. 2002;58:169–222. [DOI] [PubMed] [Google Scholar]
- 47.Hirakawa N, Tershner SA, Fields HL, et al. Bi-directional changes in affective state elicited by manipulation of medullary pain-modulatory circuitry. Neuroscience. 2000;100:861–871. [DOI] [PubMed] [Google Scholar]
- 48.Fields HL. Pain modulation: expectation, opioid analgesia and virtual pain. Prog Brain Res. 2000;122:245–253. [DOI] [PubMed] [Google Scholar]
- 49.Wang G, Bi L, Li X, et al. Maintenance of effect of duloxetine in Chinese patients with pain due to osteoarthritis: 13-week open-label extension data. BMC Musculoskelet Disord. 2019;20:174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Enteshari-Moghaddam A, Azami A, Isazadehfar K, et al. Efficacy of duloxetine and gabapentin in pain reduction in patients with knee osteoarthritis. Clin Rheumatol. 2019;38:2873–2880. [DOI] [PubMed] [Google Scholar]
- 51.Weng C, Xu J, Wang Q, et al. Efficacy and safety of duloxetine in osteoarthritis or chronic low back pain: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2020;28:721–734. [DOI] [PubMed] [Google Scholar]
- 52.Happich M, Schneider E, Wilhelm S, et al. Depression treatment with duloxetine and reduction of inability to work. Depress Res Treat. 2012;2012:264854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Russell JM, Weisberg R, Fava M, et al. Efficacy of duloxetine in the treatment of generalized anxiety disorder in patients with clinically significant pain symptoms. Depress Anxiety. 2008;25:E1–E11. [DOI] [PubMed] [Google Scholar]


