Supplemental Digital Content is available in the text.
Keywords: antiplatelet therapy, dual antiplatelet therapy, percutaneous coronary intervention
Background:
The optimal duration of antiplatelet therapy (APT) in patients at high bleeding risk with or without oral anticoagulation (OAC) after coronary stenting remains unclear.
Methods:
In the investigator-initiated, randomize, open-label MASTER DAPT trial (Management of High Bleeding Risk Patients Post Bioresorbable Polymer Coated Stent Implantation With an Abbreviated Versus Standard DAPT Regimen), 4579 patients at high bleeding risk were randomized after 1-month dual APT to abbreviated or nonabbreviated APT strategies. Randomization was stratified by concomitant OAC indication. In this subgroup analysis, we report outcomes of populations with or without an OAC indication. In the population with an OAC indication, patients changed immediately to single APT for 5 months (abbreviated regimen) or continued ≥2 months of dual APT and single APT thereafter (nonabbreviated regimen). Patients without an OAC indication changed to single APT for 11 months (abbreviated regimen) or continued ≥5 months of dual APT and single APT thereafter (nonabbreviated regimen). Coprimary outcomes at 335 days after randomization were net adverse clinical outcomes (composite of all-cause death, myocardial infarction, stroke, and Bleeding Academic Research Consortium 3 or 5 bleeding events); major adverse cardiac and cerebral events (all-cause death, myocardial infarction, and stroke); and type 2, 3, or 5 Bleeding Academic Research Consortium bleeding.
Results:
Net adverse clinical outcomes or major adverse cardiac and cerebral events did not differ with abbreviated versus nonabbreviated APT regimens in patients with OAC indication (n=1666; hazard ratio [HR], 0.83 [95% CI, 0.60–1.15]; and HR, 0.88 [95% CI, 0.60–1.30], respectively) or without OAC indication (n=2913; HR, 1.01 [95% CI, 0.77–1.33]; or HR, 1.06 [95% CI, 0.79–1.44]; Pinteraction=0.35 and 0.45, respectively). Bleeding Academic Research Consortium 2, 3, or 5 bleeding did not significantly differ in patients with OAC indication (HR, 0.83 [95% CI, 0.62–1.12]) but was lower with abbreviated APT in patients without OAC indication (HR, 0.55 [95% CI, 0.41–0.74]; Pinteraction=0.057). The difference in bleeding in patients without OAC indication was driven mainly by a reduction in Bleeding Academic Research Consortium 2 bleedings (HR, 0.48 [95% CI, 0.33–0.69]; Pinteraction=0.021).
Conclusions:
Rates of net adverse clinical outcomes and major adverse cardiac and cerebral events did not differ with abbreviated APT in patients with high bleeding risk with or without an OAC indication and resulted in lower bleeding rates in patients without an OAC indication.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT03023020.
Clinical Perspective.
What Is New?
The MASTER DAPT trial (Management of High Bleeding Risk Patients Post Bioresorbable Polymer Coated Stent Implantation With an Abbreviated Versus Standard DAPT Regimen) investigated an abbreviated (1 month) versus nonabbreviated (3−12 months) dual antiplatelet therapy and a stopping of antiplatelet therapy at 6 months strategy after coronary stenting in an all-comer population at high bleeding risk.
This prespecified subgroup analysis reports on the outcome of patients with or without an oral anticoagulation (OAC) indication.
At 12-month follow-up, ischemic and net risk did not differ with abbreviated versus nonabbreviated antiplatelet therapy regimens in both subgroups, although significantly fewer clinically relevant bleeding events occurred in the group without an OAC indication, whereas only numerically fewer bleeding events occurred in the group with an OAC indication.
What Are the Clinical Implications?
This subgroup analysis from the MASTER DAPT trial adds additional evidence that dual antiplatelet therapy beyond 1 month in patients with or without an indication for OAC has no benefit and only increases bleeding risk.
The strategy of stopping antiplatelet therapy after 6 months in patients with an OAC indication needs to be explored further.
Patients undergoing coronary stenting for obstructive coronary artery disease require dual antiplatelet therapy (DAPT), composed of aspirin and a P2Y12 receptor blocker, for a certain period to reduce the risk of ischemic events such as stent thrombosis. The optimal duration of antiplatelet therapy (APT) after implantation of drug-eluting coronary stents remains a matter of debate, especially in patients at high risk for bleeding, which is associated with a 3- to 5-fold increased risk of death.1,2 Approximately 10% of patients undergoing coronary stent implantation have an indication for oral anticoagulation (OAC), mainly because of concomitant atrial fibrillation.3 These patients present with a clinical dilemma because of the need to combine APT with OAC therapy. OAC therapy per se is associated with increased risk of bleeding, and adding APT further amplifies that risk.4 Patients without OAC therapy but ≥75 years of age or with renal insufficiency, active cancer, blood disorders, bleeding history, need for surgery, chronic use of nonsteroidal anti-inflammatory drugs or steroids, and cerebral infarcts constitute another large group at high risk of bleeding after coronary stenting.5
Little evidence exists on the optimal combination and duration of APT in patients at high bleeding risk with or without OAC therapy after coronary artery stenting. Only 2 relatively small-sized, randomized, controlled trials have investigated the combination of a vitamin K antagonist (VKA) and APT, with discordant results.6,7 With the advent of more potent P2Y12 inhibitors and nonvitamin K antagonist oral anticoagulants (NOACs), finding the optimal antithrombotic therapy after coronary stenting has become more complex. Four randomized trials with different NOACs in patients with atrial fibrillation undergoing coronary stenting or treatment for acute coronary syndrome focused primarily on dual therapy with a NOAC and a P2Y12 inhibitor (mainly clopidogrel) versus triple therapy with VKA and DAPT.8–11 Meta-analyses of these 4 trials show that dual therapy, after up to 1 week of triple therapy, significantly reduced the risk of bleeding compared with prolonged (ie, mainly 6 months or more) triple therapy, at the cost of a significant increase in stent thrombosis and a borderline higher risk of myocardial infarction.12–14 Therefore, the optimal duration of APT remains to be determined.
The MASTER DAPT trial (Management of High Bleeding Risk Patients Post Bioresorbable Polymer Coated Stent Implantation With an Abbreviated Versus Standard DAPT Regimen) was designed to investigate the safety of abbreviated versus nonabbreviated APT in patients with high bleeding risk undergoing coronary stenting. The trial protocol provided differential APT recommendations for patients at high bleeding risk with or without an indication for OAC therapy and stratified randomization accordingly. In this prespecified analysis, we assessed the treatment effects of abbreviated versus nonabbreviated APT regimens in patients with or without a concomitant indication for OAC.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for the purposes of reproducing the results or replicating the procedure.
Study Design
The MASTER DAPT trial (URL: https://www.clinicaltrials.gov; Unique identifier: NCT03023020) was an investigator-initiated, randomized, open-label, noninferiority trial with sequential superiority testing in largely unselected patients at high bleeding risk who underwent implantation with a biodegradable polymer-coated Ultimaster (Terumo Corporation, Tokyo, Japan) sirolimus-eluting stent.15 The trial was performed at 140 sites in 30 countries across Europe, South America, the Middle East, Asia, and Australia. The study protocol was approved in each country and center. All patients gave written informed consent. An independent data safety monitoring board regularly reviewed the conduct of the trial and the safety of the patients. Trial organization and participating sites are mentioned in the Data Supplement.
Patients
Patients at high risk for bleeding who underwent treatment of all planned coronary artery stenoses with Ultimaster stent implantation for acute or chronic coronary syndromes were eligible if they remained event-free until the time of randomization at 1 month after the index procedure. Patients were considered at high bleeding risk if at least 1 of the following criteria applied: OAC (VKA or NOAC) therapy for at least 12 months, recent (<12 months) nonaccess site bleeding episode(s) that required medical attention, previous bleeding episode(s) that required hospitalization if the underlying cause had not been definitively treated, ≥75 years of age, systemic conditions associated with an increased bleeding risk (eg, hematologic disorders or any known coagulation disorder associated with increased bleeding risk), documented anemia (defined as repeated hemoglobin levels <11 g/dL or transfusion within 4 weeks before randomization), need for chronic treatment with steroids or nonsteroidal anti-inflammatory drugs, diagnosed malignancy (other than skin), stroke at any time or transient ischemic attack in the previous 6 months, or predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score ≥25.16 Exclusion criteria were minimal and limited to implantation of a nonstudy stent within the previous 6 months or a bioresorbable scaffold at any time before the index procedure, or undergoing treatment because of an in-stent restenosis or stent thrombosis. Detailed inclusion and exclusion criteria are provided in the Data Supplement.
Randomization, Masking, and Procedures
Patients were centrally randomized (1:1 ratio) to an open-label abbreviated or nonabbreviated APT regimen 30 to 44 days after the index procedure. Randomization was concealed using a web-based system; randomization sequences were computer generated; blocked with randomly selected block sizes of 2, 4, or 6; and stratified by site, history of acute myocardial infarction within 12 months, and indication for at least 12 months of OAC therapy. In this subgroup analysis, we report the outcomes of the populations with or without an OAC indication.
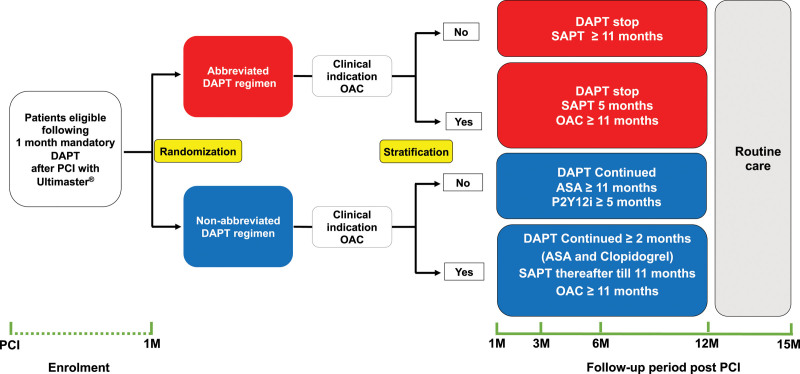
Figure 1 shows the trial design and the specific subgroups of this analysis. Patients with an indication for OAC therapy who were randomly allocated to receive abbreviated treatment immediately discontinued DAPT and continued single APT (SAPT) for 6 months after the index procedure, and continued thereafter with OAC monotherapy only. Patients on OAC therapy who were randomly allocated to receive nonabbreviated treatment continued DAPT until at least 3 months after the index stent procedure (ie, at least 2 months after randomization) and continued thereafter with SAPT until 12 months after the index procedure. Patients without an indication for OAC who were randomly allocated to the abbreviated group immediately discontinued DAPT and continued with SAPT until 12 months after the index procedure. Patients without an indication for OAC who were randomly allocated to the nonabbreviated group continued DAPT for at least 6 months after the index procedure (ie, ≥5 months after randomization), after which SAPT with aspirin was continued. All antiplatelet and OAC treatment options were dosed according to the corresponding authorization for use and locally approved regimens. Table I in the Data Supplement shows the APT regimen(s) allowed after each type of event according to the presence or absence of a clinical indication for OAC.
Figure 1.
Schematic trial design. ASA indicates acetylsalicyclic acid; DAPT, dual antiplatelet therapy; M, month; OAC, oral anticoagulation; PCI, percutaneous coronary intervention; and SAPT, single antiplatelet therapy.
Follow-up visits occurred at 60±14 and 150±14 days after randomization, preferably as on-site visits, and at 335±14 days after randomization, exclusively as an on-site visit. Two independent clinical research organizations (Cardiovascular European Research Center, Massy, France; and Cardialysis, Rotterdam, the Netherlands) performed on-site and remote monitoring visits, verified the source documents, and collected source material for event adjudication. All events were adjudicated by an independent adjudication committee that was unaware of the treatment allocations. All data were stored at a central database (Clinical Trial Unit, Bern, Switzerland). Nonadherence to the allocated treatment regimen was evaluated according to the Nonadherence Academic Research Consortium classification (Table II in the Data Supplement).17
Outcomes
Coprimary outcomes were net adverse clinical outcomes, defined as the composite of all-cause death, myocardial infarction, stroke, and Bleeding Academic Research Consortium (BARC) 3 or 5 bleeding events18; major adverse cardiac and cerebral events, expressed as a composite of all-cause death, myocardial infarction, and stroke; and major or clinically relevant nonmajor bleeding, defined as a composite of type 2, 3, or 5 BARC bleeding events.
The secondary outcomes include the individual components of the 3 coprimary outcomes; the composite of cardiovascular death, myocardial infarction, and stroke; the composite of cardiovascular death, myocardial infarction, definite or probable stent thrombosis, and any revascularization; the composite of stroke and transient ischemic attack; and all bleeding events, adjudicated according to the BARC classifications.18 All outcomes were prespecified.15 All analyses evaluated the occurrence of the adjudicated outcomes between randomization and 335 days.
Statistical Analysis
The data were analyzed according to the intention-to-treat principle. Outcomes were assessed separately for patients with and without indication for OAC therapy by calculating hazard ratios (HRs) with 95% confidence intervals. The Com-Nougue method19 was used to analyze time-to-event and calculate event rates and P values. We report cause-specific estimates throughout the article. For patients with a primary outcome, time-to-event was calculated as the difference between the date of occurrence of the outcome event and the date of randomization plus 1. For patients with an outcome event and complete follow-up until the end of day 335, time to censoring was calculated as 335 days. For patients with incomplete clinical follow-up, time to censoring was defined as the difference between the dates of last known clinical status and randomization plus 1. For the third coprimary end point, the occurrence of death was defined as a competing risk event, and follow-up was censored at the time of the occurrence of death. Kaplan-Meier curves were created for the first 2 (time-to-event) coprimary outcomes, and cause-specific Kaplan-Meier curves for the third coprimary end point (with censoring at the time of the competing risk event of unrelated death). Kaplan-Meier calculations included all (first) adjudicated outcome events that occurred between randomization and 335 days thereafter according to the randomized treatment assignment, irrespective of the dual antiplatelet regimen received at the time of the outcome event. Cause-specific HRs and 95% CIs were generated for primary and secondary end points with the use of Cox proportional hazards regression analysis with censoring at end of study and at the time of the competing risk event of unrelated death as defined above.
P values for testing homogeneity of the hazard ratio in subgroups of patients were derived in Cox proportional hazards models with interaction terms for treatment group and subgroup membership. The 95% CIs and P values for interaction were not adjusted for multiplicity and should not be used to infer definitive treatment effects.
Role of the Funding Source
The study was sponsored and coordinated by the European Cardiovascular Research Institute, a nonprofit organization, and received grant support from Terumo. The sponsor and funder had no role in the study design, data collection, data monitoring, analysis, interpretation, or writing of the report.
Results
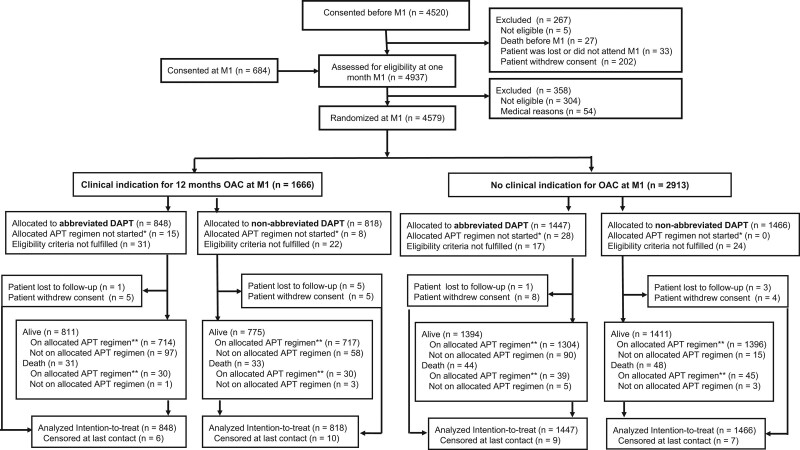
From February 28, 2017, to December 5, 2019, 5204 patients were screened, and 4579 (88.1%) were randomized a median of 34 days (interquartile range [IQR], 32–39) after stenting, of which 1666 (36.4%) patients had an indication for OAC and 2913 (63.6%) had no indication for OAC. Of the 1666 patients with OAC therapy, 848 were assigned to the abbreviated APT group and 818 to the nonabbreviated APT group (Figure 2). Of the 2913 patients without OAC, 1447 were assigned to the abbreviated APT group and 1466 to the nonabbreviated APT group. Complete follow-up in the OAC subgroup was 99.3% for the abbreviated APT arm and 98.8% for the nonabbreviated APT arm, and 99.4% and 99.5%, respectively, in the subgroup without OAC.
Figure 2.
Patient flow. M1=1 month after index coronary stent procedure, meaning the last intended coronary stent implantation. APT indicates antiplatelet therapy; DAPT, dual antiplatelet therapy; and OAC, oral anticoagulation. *Did not start within 14 days of randomization, or nonpermitted alternative regimen because of event within 14 days from randomization. †At day 335 or on allowed alternative regimen because of, for example, previous events; if not recorded on exactly day 335, the last information on adherence is used.
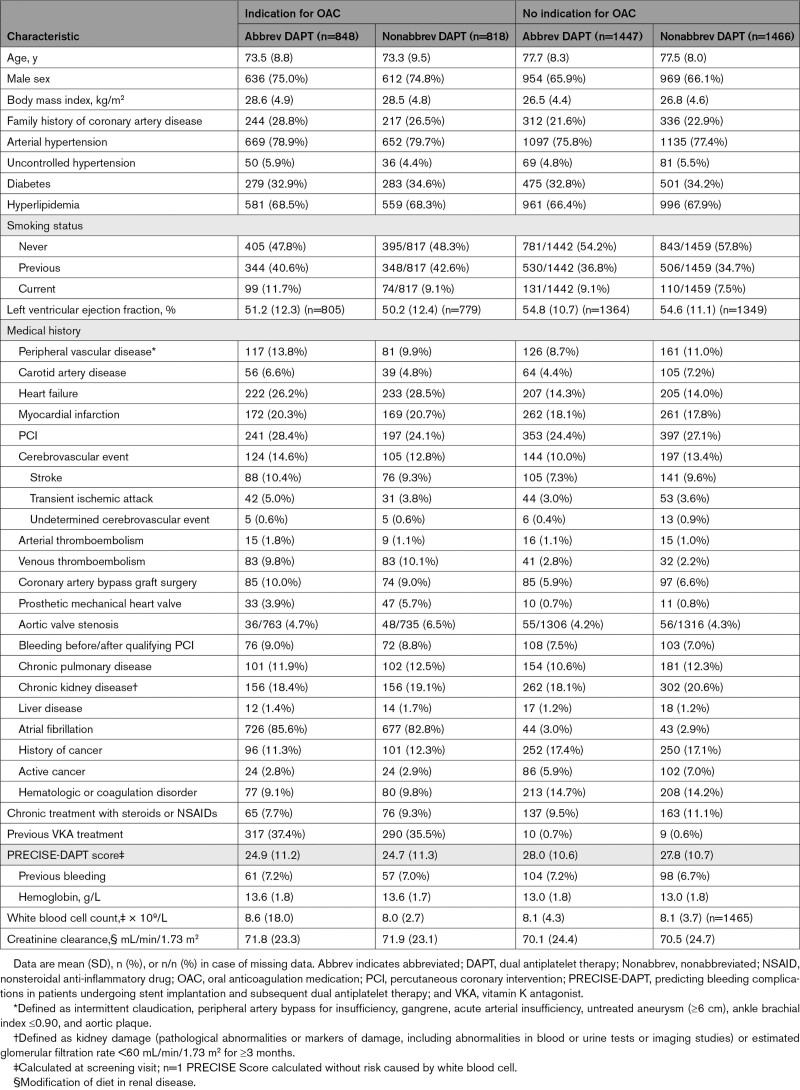
Baseline and procedural characteristics are described in Tables 1 and 2 and Table III in the Data Supplement. The overall mean±SD age was 76.0±8.7 years. On average, 33.6% of patients received treatment for diabetes. The indication for coronary stenting was acute coronary syndrome for 48.3% of patients. Atrial fibrillation was present in 84.2% of patients in the OAC group.
Table 1.
Baseline Characteristics According to Presence or Absence of Clinical Indication for OAC
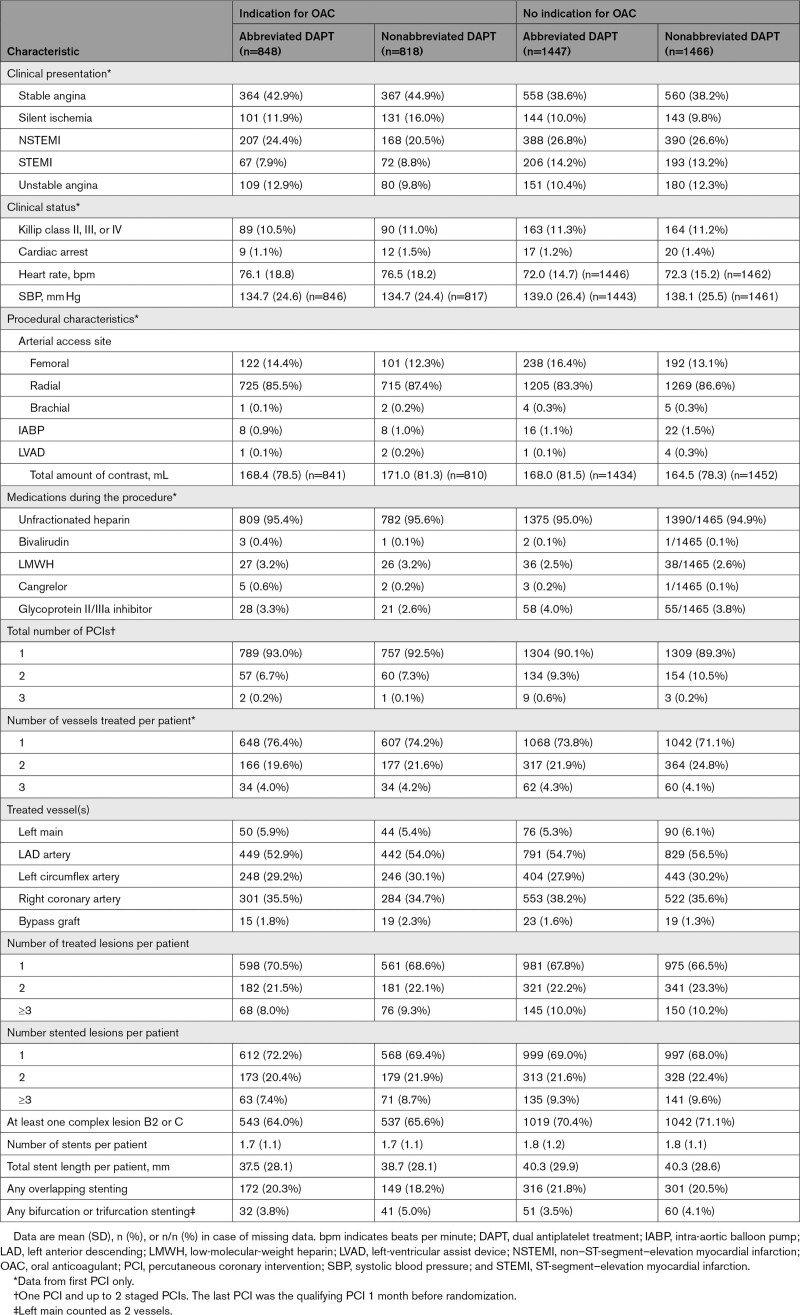
Table 2.
Procedural Characteristics According to Presence or Absence of Clinical Indication for OAC
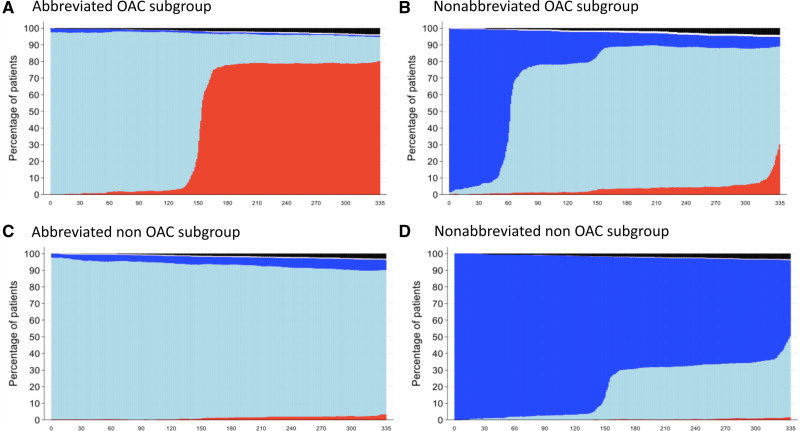
In the OAC group, 64.9% of the patients received a NOAC and 33.5% a VKA (Table IV in the Data Supplement), largely in combination with clopidogrel (98.8%) in the abbreviated group or aspirin plus clopidogrel (97.4%) in the nonabbreviated group. In the non-OAC group, aspirin plus clopidogrel (67.8%) was the most frequently implemented regimen, followed by aspirin and ticagrelor (28.4%; Table V in the Data Supplement). Median durations of DAPT since coronary stenting were 33 days (IQR, 30–39) in patients with OAC and 34 days (IQR, 31–40) in patients without OAC in the abbreviated arm; and 96 days (IQR, 90–114) and 364 days (IQR, 190–369), respectively, in the nonabbreviated group. Detailed information on antiplatelet use is shown in Figure 3.
Figure 3.
Antiplatelet use per day since randomization for patients with and without oral anticoagulation therapy. A, Patients with an OAC indication on abbreviated APT. B, Patients with an OAC indication on nonabbreviated APT. C, Patients without an OAC indication on abbreviated APT. (D) Patients without an OAC indication on nonabbreviated APT.
Dark blue = dual APT, light blue = single APT, red = no APT, black = deceased, and white = no information. APT indicates antiplatelet therapy; and OAC, oral anticoagulation.
Adherence to the allocated antiplatelet regimen decreased over time and was lower in the abbreviated versus nonabbreviated group at 12 months in the OAC subgroup (82.7% versus 95.8%; P<0.001, respectively). Detailed information on adherence is depicted in Figure I and Tables V and VI in the Data Supplement, with 16.1% of the patients with an OAC indication who still used APT at the 11 months visit after randomization in the abbreviated arm.
Outcomes Among Patients With OAC Indication
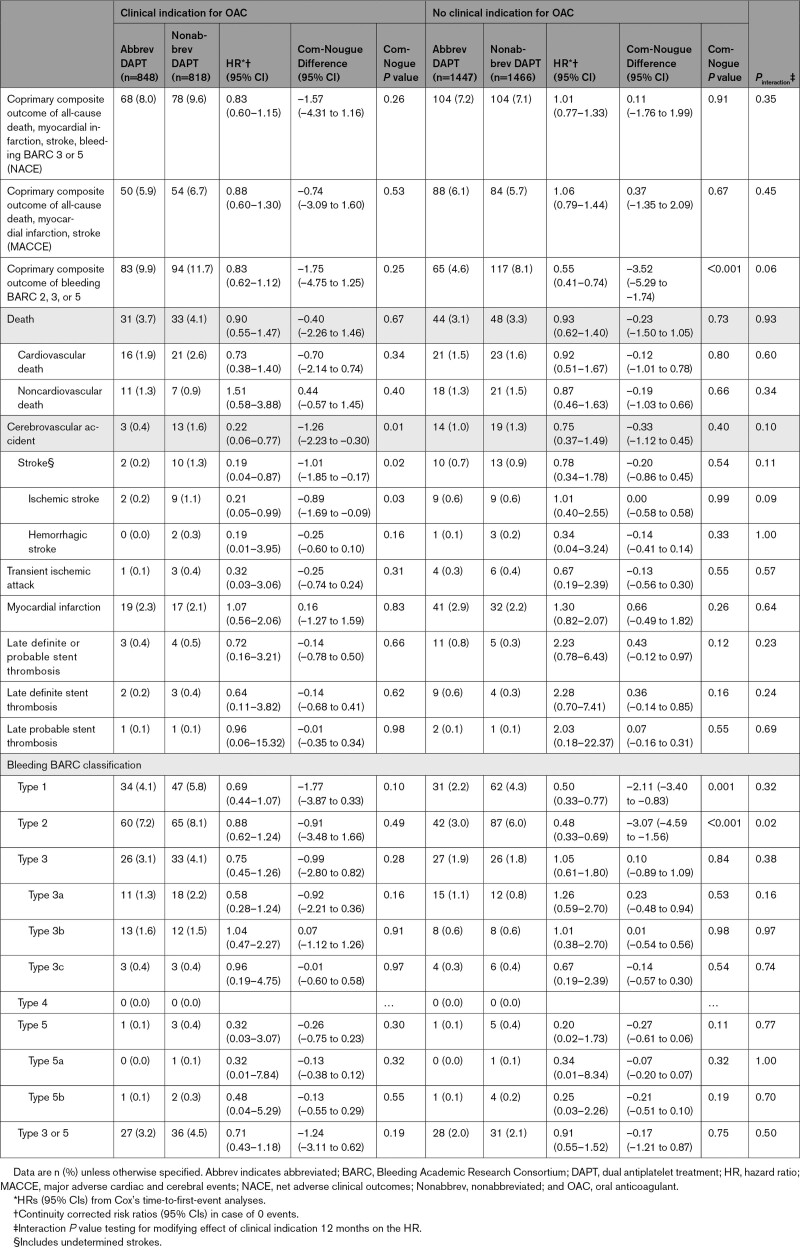
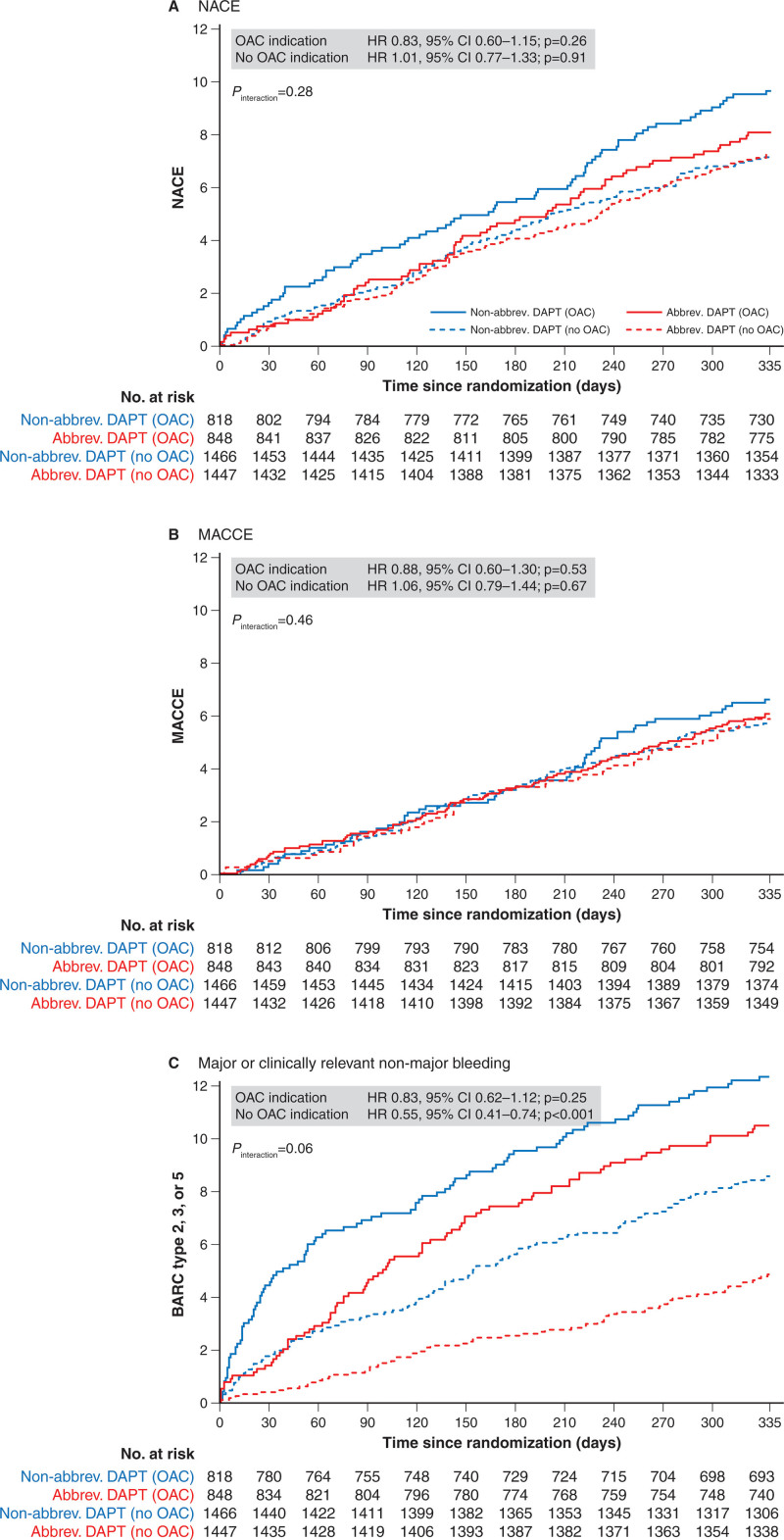
Clinical outcomes at 12 months in patients with OAC are shown in Table 3. Net adverse clinical outcomes occurred in 68 (8.0%) patients in the abbreviated arm versus 78 (9.6%) patients in the nonabbreviated arm (HR, 0.83 [95% CI, 0.60–1.15]; P=0.26; Figure 4A). Major adverse cardiac and cerebral events did not differ, occurring in 50 (5.9%) patients in the abbreviated arm versus 54 (6.7%) patients in the nonabbreviated arm (HR, 0.88 [95% CI, 0.60–1.30]; P=0.53; Figure 4B). BARC 2, 3, or 5 bleeding occurred in 83 (9.9%) patients in the abbreviated arm versus 94 (11.7%) patients in the nonabbreviated arm (HR, 0.83 [95% CI, 0.62–1.12]; P=0.25; Figure 4C). Fewer cerebrovascular events occurred in the abbreviated arm (HR, 0.22 [95% CI, 0.06–0.77]; P=0.01). Other outcomes did not differ between groups.
Table 3.
Clinical Outcomes at 11 Months After Randomization (12-Month Follow-Up) (Intention-To-Treat Population)
Figure 4.
Kaplan-Meier curves of the 3 coprimary outcomes at 11 months after randomization (12-month follow-up). Net adverse clinical events (A), major cardiovascular events (B), and major or clinically relevant nonmajor bleeding (C). abbrev. indicates abbreviated; BARC, Bleeding Academic Research Consortium; DAPT, dual antiplatelet therapy; HR, hazard ratio; MACCE, major adverse cardiac and cerebral events; NACE, net adverse clinical outcomes; and OAC, oral anticoagulation.
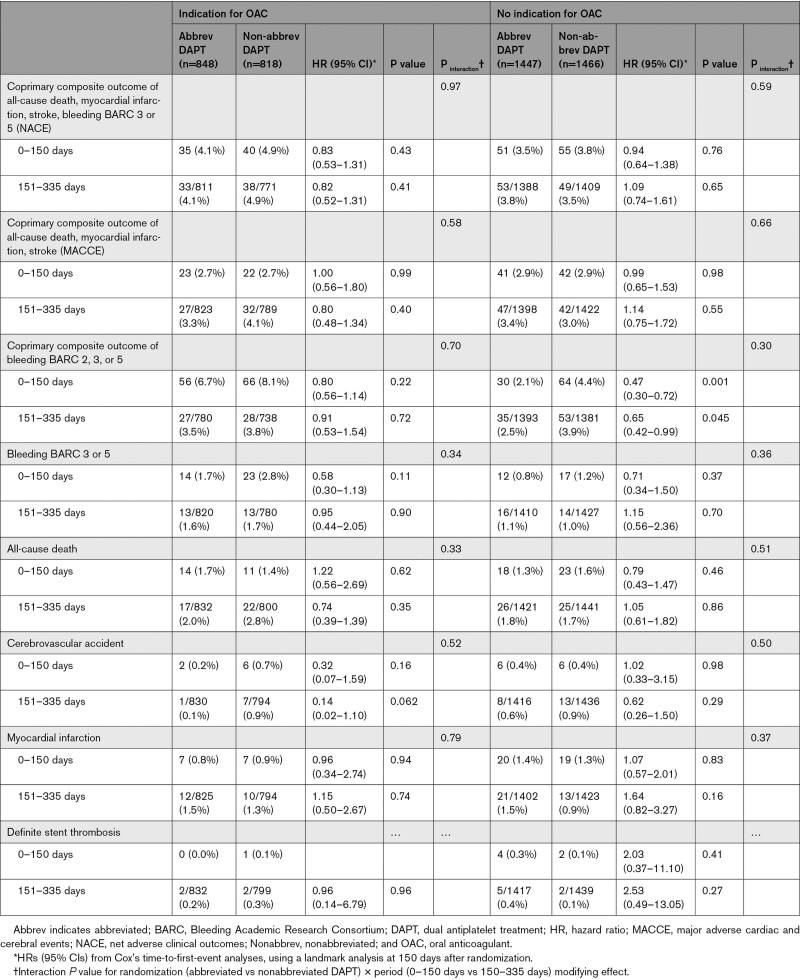
Landmark analyses at 150 days after randomization showed consistent treatment effects for the coprimary and secondary end points with respect to time among patients with an indication for OAC (Table 4). Numerically, fewer BARC 3 or 5 bleeding events occurred in the first 150 days in the abbreviated arm compared with the nonabbreviated arm. No differences in ischemic and bleeding events were noted between 150 and 335 days when APT was stopped in the abbreviated arm.
Table 4.
Clinical Outcomes Using a Landmark Analysis at 150 Days (6-Month Visit) According to Presence or Absence of Clinical Indication for OAC (Intention-To-Treat)
Outcomes Among Patients Without an OAC Indication
Clinical outcomes at 12 months in patients without an OAC indication are shown in Table 3. Net adverse clinical outcomes did not differ between the abbreviated and nonabbreviated APT groups (104 [7.2%] versus 104 [7.1%], respectively; HR, 1.01 [95% CI, 0.77–1.33]; P=0.91; Figure 4A). Major adverse cardiac and cerebral events also did not differ between the treatment groups (88 [6.1%] versus 84 [5.7%]; HR, 1.06 [95% CI, 0.79–1.44]; P=0.67; Figure 4B). BARC 2, 3, and 5 bleeding occurred less frequently in the abbreviated arm (65 [4.6%] versus 117 [8.1%]; HR, 0.55 [95% CI, 0.41–0.74]; P<0.001; Figure 4C). Fewer BARC 1 and BARC 2 bleedings occurred in the abbreviated APT arm (P=0.001 and P<0.001, respectively).
Landmark analyses at 150 days after randomization showed consistent treatment effects with respect to time for the coprimary and secondary end points among patients without an indication for OAC (Table 4).
Consistency of Treatment Effects Between Patients With and Without OAC
The treatment effects between abbreviated and nonabbreviated APT were consistent in patients with or without an OAC indication, except for the coprimary end point of BARC 2, 3, or 5 bleeding and the secondary end point of BARC 2 bleeding. Both HRs were lower in patients without an OAC indication, with a borderline (Pinteraction=0.057) and significant (Pinteraction=0.021) interaction test, respectively.
The coprimary end point of BARC 2, 3, or 5 bleedings was 2-fold higher in the OAC versus non-OAC therapy subgroup, whereas rates of first ischemic events did not differ in both subgroups (Figure 4B and 4C).
Per-Protocol Population
The coprimary findings remained unchanged in the per-protocol population (Tables VII and VIII in the Data Supplement). An overview of protocol violations used to define the per-protocol population is summarized in Table IX in the Data Supplement.
Discussion
The main findings of this subgroup analysis from the MASTER DAPT trial are 3-fold. First, in an abbreviated APT strategy, stopping DAPT at 1 month after coronary stenting with a biodegradable polymer coated sirolimus-eluting stent, the occurrence of ischemic and net events did not differ in patients with or without OAC therapy. Second, stopping DAPT at 1 month and continuing with SAPT reduces clinically relevant bleeding risk significantly in patients with high bleeding risk without an indication for OAC and numerically in patients with an indication for OAC. Third, stopping SAPT 6 months after coronary stenting demonstrated no effect on ischemic events, or in prevention of bleeding events in patients with an indication for OAC, although a significant proportion of patients were nonadherent to the allocated abbreviated APT regimen and did not stop APT 6 months after coronary stenting. These findings have the following implications: our results show that it is safe and beneficial to stop DAPT after 1 month in patients at high bleeding risk with or without an indication for OAC, and that the effect of stopping all APT after 6 months in patients on OAC needs to be explored further.
Patients at high bleeding risk constitute a considerable proportion of those undergoing coronary stenting.20 This high bleeding risk population is composed of a heterogenous group in which 2 large subgroups can be identified on the basis of the need, or not, for concomitant OAC therapy. The need for APT in combination with OAC therapy increases bleeding risk,3,21 whereas OAC therapy alone after stenting is insufficient in preventing ischemic complications such as stent thrombosis.22,23 Therefore, guidelines24–27 recommend different APT strategies for patients at high bleeding risk after coronary stenting depending on whether they receive APT or OAC therapy. On the basis of these recommendations, the MASTER DAPT trial was designed as an all-comer high bleeding risk trial with 2 APT strategies for each stratified subgroup. Patients without an indication for OAC who were allocated to the nonabbreviated APT regimen received DAPT for a minimum of 6 months, consistent with the guidelines. Patients allocated to the experimental abbreviated APT therapy arm received 1 month of DAPT, similar to other recent high bleeding risk trials investigating an abbreviated DAPT regimen.20,28,29 However, in the OAC subgroup, the duration of therapy in the nonabbreviated APT arm (with a minimum of 3 months of DAPT) is longer than recommended in the current North American guidelines24,27 and European non−ST-segment−elevation myocardial infarction25 and atrial fibrillation guidelines,26 which recommend DAPT for 1 week to 1 month in patients on OAC. No previous trial has investigated the value of 1 month versus 3 months of DAPT in the OAC population. The ISAR-TRIPLE trial (Intracoronary Stenting and Antithrombotic Regimen–Testing of a 6-Week Versus a 6-Month Clopidogrel Treatment Regimen in Patients With Concomitant Aspirin and Oral Anticoagulant Therapy Following Drug-Eluting Stenting) compared 6 weeks of clopidogrel with 6 months of clopidogrel in 614 patients receiving aspirin and OAC after drug-eluting stent implantation; the authors found no difference between treatment strategies for the composite of death, myocardial infarction, stroke, stent thrombosis, or major bleeding.6 Therefore, the recommendation of a short (ie, 1 week) duration of DAPT in patients with OAC after percutaneous coronary intervention or an acute coronary syndrome in the current NOAC era is supported by the findings of 4 studies.8–11 Three of these studies8,9,11 tested a short duration of triple therapy consisting of aspirin, NOAC, and a P2Y12 inhibitor, followed by continuation of the latter 2 drugs, versus a long duration of triple therapy composed of aspirin, VKA, and a P2Y12 inhibitor for mainly 6 months or longer. The AUGUSTUS trial is the largest among the 4 and the only study that disentangled the effect of OAC type from duration of aspirin treatment.8 When data from these studies are pooled, they show a major reduction in bleeding events with a short duration of triple therapy. However, they also show higher rates of stent thrombosis, likely clustered in the first few weeks after aspirin discontinuation,30 which are consistently observed in patients with or without an acute coronary syndrome.12 Our findings are in concert with observational data,31 showing no rebound of ischemic events after 1 month of DAPT in patients with OAC.
Although North American and European guidelines25–27 recommend stopping APT after 6 months in an OAC population (level of evidence C), no previous trial has actually investigated the value of no APT versus SAPT after 6 months. Our current study failed to show a clear benefit of stopping APT after 6 months, highly likely because of the lack of power in this subgroup analysis and the fact that a considerable proportion of patients were nonadherent to the abbreviated allocated treatment regimen and continued using SAPT after 6 months. Whether the absence of an increase in ischemic events and a decrease in bleeding events relate to this nonadherence remains to be explored.
In our trial of an all-comer population at high bleeding risk, we observed a 2-fold higher bleeding rate in the OAC subgroup compared with the non-OAC subgroup. This can be explained in part by the relative long DAPT regimens in the nonabbreviated APT arm of this subgroup but not for the abbreviated APT arm. Furthermore, most patients on OAC were treated with a NOAC (64.9%), and no outcome differences were noted between patients on VKA or NOAC medications (data not shown). The increased bleeding risk of patients on OAC versus not on OAC emphasizes the importance of defining the optimal APT duration and combination in this subgroup who are already categorized as at high bleeding risk. A numerically lower rate of BARC 3 or 5 bleedings was noted in the first 150 days after randomization in the abbreviated arm of patients on OAC, whereas this was less apparent in the abbreviated APT arm of the population without an OAC indication. In contrast, patients at high bleeding risk without an OAC indication in the abbreviated arm had significantly fewer BARC 2 bleedings, whereas this was less apparent in the OAC population. This different signal of bleeding severity between both subgroups is likely related to the higher bleeding risk of patients with OAC and differences in baseline characteristics between subgroups.
Another interesting finding is the reduced risk of ischemic stroke in the abbreviated APT arm of the OAC subgroup (P=0.03). A similar observation was noted in the WOEST trial (What is the Optimal Antiplatelet & Anticoagulant Therapy in Patients With Oral Anticoagulation and Coronary Stenting) (P=0.056).7 Whether this paradoxical finding relates to lower dosing of VKA or NOAC in the nonabbreviated DAPT arm or is a result of chance of this low-frequency event remains to be determined.
Every randomized trial has its strengths and weaknesses. The fact that randomization was done at 1 month could indicate that patients at lower ischemic risk were selected, because patients with an ischemic event before 1 month were excluded from randomization. However, to allow patients entering the trial only after 30 days of uneventful follow-up mimics clinical practice and prevents inclusion of patients in whom the clinical equipoise between DAPT continuation or discontinuation is questionable and perhaps unethical. On the other hand, patients experiencing a manageable bleeding event after percutaneous coronary intervention but who were stable 1 week before randomization could be included because they were de facto high bleeding risk. In our trial, randomization at 1 month is the preferred strategy, because at that time point, the comparison of the allocated treatment strategies started, and events that happened before randomization while both subgroups were on DAPT did not interfere with the outcome of this trial.
Nonadherence often occurs in randomized trials and can have important implications for the results. In general, nonadherence of <20% is considerate acceptable; however, the cut-off of 20% is arbitrary and encompasses several reasons that nonadherence occurred. In the MASTER DAPT trial, nonadherence was carefully monitored and classified.17 In our trial, we observed a significant drop in adherence in the abbreviated APT arm of the OAC subgroup. A considerable number of patients continued APT after 6 months, whereas the protocol stipulated stopping at 6 months. During the trial monitoring process, we noticed this phenomenon and tried to make all investigators aware of this protocol violation. Unfortunately, despite many communications during the trial to the principal investigators in each country and to local investigators, the Nonadherence Academic Research Consortium 2 (temporary) and Nonadherence Academic Research Consortium 3 (permanent discontinuation) nonadherence patterns remained prevalent at around 30% in the abbreviated APT arm of the OAC subgroup. Local physicians and patients were reluctant to stop APT at 6 months.
Limitations
Several limitations must be acknowledged. This was a subgroup analysis (albeit the investigated subgroups were prespecified and stratified at randomization). The subgroups were not powered for the coprimary outcomes and individual end points, such as myocardial infarction and stent thrombosis; therefore, the results should be interpreted with caution. Treatment allocation was open-label, which reflects a treatment-strategy trial and the impossibility of masking treatment for 3 oral P2Y12 inhibitors and aspirin. Nonadherence to the allocated APT regimen occurred more often in the abbreviated APT arm of the OAC subgroup. Our trial included patients at high risk for bleeding who underwent biodegradable polymer sirolimus-eluting stent implantation; consequently, our results may not extend to patients who are not at high bleeding risk or received other stent types.
Conclusions
In an all-comer high bleeding risk population with minimal angiographic restrictions, stopping DAPT 1 month after coronary stenting was associated with lower bleeding risk without additional ischemic risk in patients with or without OAC. Stopping APT after 6 months in patients on OAC needs to be explored further.
Acknowledgments
Editorial support was provided by Sophie Rushton-Smith, PhD (MedLink Healthcare Communications, London, United Kingdom) and was funded by the European Cardiovascular Research Institute. The help and support provided by Dragica Paunovic, MD, for the funding of this trial is greatly appreciated.
Sources of Funding
The study was sponsored by the European Cardiovascular Research Institute, a nonprofit organization, and received grant support from Terumo.
Disclosures
Dr Smits reports personal fees from Terumo and Opsense; grants and personal fees from Abbott Vascular, Microport, and Daichy Sankyo; and grants from SMT and Microport. Dr Heg reports that Clinical Trial Unit Bern, University of Bern, has a staff policy of not accepting honoraria or consultancy fees. However, Clinical Trial Unit Bern is involved in design, conduct, or analysis of clinical studies funded by not-for-profit and for-profit organizations. In particular, pharmaceutical and medical device companies provide direct funding to some of these studies. Dr Jüni reports honoraria (to the institution) from Amgen, Ava, and Fresenius; and grants from Appili Therapeutics. Dr Jüni serves as unpaid member of the steering group of trials funded by Appili Therapeutics, Abbott Vascular (EXCEL trial [Evaluation of XIENCE Versus Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularization]: https://clinicaltrials.gov/ct2/show/NCT01205776; comparing XIENCE Stent in subjects with unprotected left main coronary artery disease with coronary artery bypass graft surgery; no active involvement for over 3 years, no coauthored publication, but still listed as original member of statistical executive committee) and Terumo (MASTER DAPT trial: https://www.clinicaltrials.gov/ct2/show/NCT03023020; comparing abbreviated DAPT with prolonged DAPT in patients with a drug-eluting stent; ongoing active involvement as member of steering group), and has participated in advisory boards or consulting for Amgen, Ava, and Fresenius. Dr Vranckx reports personal fees from Daiichi Sankyo, Bayer AG, BLS Bering, and Astra Zeneca. Dr Ozaki reports grants from Takeda Pharmaceutical Company Ltd, Daiichi Sankyo Company Ltd, Otsuka Pharmaceutical Company Ltd, and Sanofi. Dr Morice is a shareholder and CEO of Cardiovascular European Research Center and a minor shareholder of Electroducer (a start-up not involved in the MASTER DAPT trial). Dr Chevalier reports personal fees from Terumo and the Cardiovascular European Research Center and holds stock options in Colibri. Dr Windecker reports grants from Abbott, Amgen, Astra Zeneca, BMS, Bayer, Biotronik, Boston Scientific, Cardinal Health, CadioValve, CSL Behring, Daiichi Sankyo, Edwards Lifesciences, Guerbet, InfraRedx, Johnson&Johnson, Medicure, Medtronic, Novartis, Polares, OrPha Suisse, Pfizer, Regeneron Pharmaceuticals, Sanofi-Aventis, Sinomed, Terumo, and V-Wave. Dr Windecker serves as unpaid advisory board member or unpaid member of the steering/executive group of trials funded by Abbott, Abiomed, Amgen, Astra Zeneca, BMS, Boston Scientific, Biotronik, Cardiovalve, Edwards Lifesciences, MedAlliance, Medtronic, Novartis, Polares, Sinomed, V-Wave, and Xeltis, but has not received personal payments by pharmaceutical companies or device manufacturers. He is also member of the steering/executive committee group of several investigator-initiated trials that receive funding by industry without effect on his personal remuneration. Dr Windecker is an unpaid member of the Pfizer Research Award selection committee in Switzerland and of the Women as One Awards Committee. He is member of the Clinical Study Group of the Deutsches Zentrum für Herz Kreislauf-Forschung and of the Advisory Board of the Australian Victorian Heart Institute. He is chairperson of the ESC Congress Program Committee, former chairperson of the ESC Clinical Practice Guidelines Committee, and Deputy Editor of JACC CV Interventions.
Dr Roffi reports institutional research grants from Biotronik, Medtronic, Boston Scientific, GE Healthcare, and Terumo. Dr Mahfoud reports grants from Terumo, Deutsche Forschungsgemeinschaft (TRR 219), and Deutsche Gesellschaft für Kardiologie; grants and personal fees from Medtronic and Recor; and personal fees from Bayer and Boehringer Ingelheim. Dr Hildick-Smith reports personal fees from Terumo. Dr Schultz reports grants and personal fees from Abbott Vascular. Dr Valgimigli reports grants and personal fees from Terumo; and personal fees from Astra Zeneca, Alvimedica/CID, Abbott Vascular, Daiichi Sankyo, Bayer, CoreFlow, Idorsia Pharmaceuticals LTD, Universität Basel, Dept Klinische Forschung, Vifor, Bristol Myers Squib SA, Biotronik, Boston Scientific, Medtronic, Vesalio, Novartis, Chiesi, and PhaseBio. The other authors report no conflicts.
Supplemental Materials
MASTER DAPT Trial Investigators
Expanded Methods
Data Supplement Tables I–IX
Data Supplement Figure I
Supplementary Material
Nonstandard Abbreviations and Acronyms
- APT
- antiplatelet therapy
- BARC
- Bleeding Academic Research Consortium
- DAPT
- dual antiplatelet therapy
- HR
- hazard ratio
- MACCE
- major adverse cardiac and cerebral events
- MASTER DAPT
- Management of High Bleeding Risk Patients Post Bioresorbable Polymer Coated Stent Implantation With an Abbreviated Versus Standard DAPT Regimen
- NACE
- net adverse clinical outcomes
- NARC
- nonadherence Academic Research Consortium
- NOAC
- nonvitamin K antagonist oral anticoagulant
- NSAID
- nonsteroidal anti-inflammatory drug
- OAC
- oral anticoagulation
- PRECISE-DAPT
- predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy
- SAPT
- single antiplatelet therapy
- VKA
- vitamin K antagonist
A complete list of the investigators in the Management of High Bleeding Risk Patients Post Bioresorbable Polymer Coated Stent Implantation With an Abbreviated Versus Standard DAPT Regimen Trial is provided in the Data Supplement.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.121.056680.
For Sources of Funding and Disclosures, see page 1209.
Contributor Information
Enrico Frigoli, Email: enricofrigoli@gmail.com.
Jan Tijssen, Email: tijssenj@outlook.com.
Peter Jüni, Email: peter.juni@utoronto.ca.
Pascal Vranckx, Email: pascal.vranckx@jessazh.be.
Yukio Ozaki, Email: yukio.ozaki7@gmail.com.
Marie-Claude Morice, Email: mc.morice@icps.com.fr.
Bernard Chevalier, Email: b.chevalier@icps.com.fr.
Yoshinobu Onuma, Email: yoshinobuonuma@gmail.com.
Stephan Windecker, Email: Stephan.Windecker@insel.ch.
Pim A.L. Tonino, Email: pim.tonino@catharinaziekenhuis.nl.
Marco Roffi, Email: vlgmrc@unife.it.
Maciej Lesiak, Email: maciej.lesiak@skpp.edu.pl.
Felix Mahfoud, Email: felix.mahfoud@uks.eu.
Jozef Bartunek, Email: Jozef.Bartunek@olvz-aalst.be.
David Hildick-Smith, Email: David.Hildick-Smith@bsuh.nhs.uk.
Antonio Colombo, Email: ac84344@gmail.com.
Goran Stankovic, Email: gorastan@gmail.com.
Andrés Iñiguez, Email: andres.iniguez.romo@sergas.es.
Carl Schultz, Email: Carl.schultz@uwa.edu.au.
Ran Kornowski, Email: ran.kornowski@gmail.com.
Paul J.L. Ong, Email: paul2ong@gmail.com.
Mirvat Alasnag, Email: mirvat@jeddacath.com.
Alfredo E. Rodriguez, Email: arodriguez@centroceci.com.ar.
Aris Moschovitis, Email: moschovitis@herzzentrum.ch.
Peep Laanmets, Email: Peep.Laanmets@regionaalhaigla.ee.
Dik Heg, Email: dierik.heg@ctu.unibe.ch.
Marco Valgimigli, Email: vlgmrc@unife.it.
References
- 1.Généreux P, Giustino G, Witzenbichler B, Weisz G, Stuckey TD, Rinaldi MJ, Neumann FJ, Metzger DC, Henry TD, Cox DA, et al. Incidence, predictors, and impact of post-discharge bleeding after percutaneous coronary intervention. J Am Coll Cardiol. 2015;66:1036–1045. doi: 10.1016/j.jacc.2015.06.1323 [DOI] [PubMed] [Google Scholar]
- 2.Valle JA, Shetterly S, Maddox TM, Ho PM, Bradley SM, Sandhu A, Magid D, Tsai TT. Postdischarge bleeding after percutaneous coronary intervention and subsequent mortality and myocardial infarction: insights from the HMO Research Network-Stent Registry. Circ Cardiovasc Interv. 2016;9:e003519.doi: 10.1161/CIRCINTERVENTIONS.115.003519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karjalainen PP, Porela P, Ylitalo A, Vikman S, Nyman K, Vaittinen MA, Airaksinen TJ, Niemelä M, Vahlberg T, Airaksinen KE. Safety and efficacy of combined antiplatelet-warfarin therapy after coronary stenting. Eur Heart J. 2007;28:726–732. doi: 10.1093/eurheartj/ehl488 [DOI] [PubMed] [Google Scholar]
- 4.Mega JL, Simon T. Pharmacology of antithrombotic drugs: an assessment of oral antiplatelet and anticoagulant treatments. Lancet. 2015;386:281–291. doi: 10.1016/S0140-6736(15)60243-4 [DOI] [PubMed] [Google Scholar]
- 5.Urban P, Mehran R, Colleran R, Angiolillo DJ, Byrne RA, Capodanno D, Cuisset T, Cutlip D, Eerdmans P, Eikelboom J, et al. Defining high bleeding risk in patients undergoing percutaneous coronary intervention. Circulation. 2019;140:240–261. doi: 10.1161/CIRCULATIONAHA.119.040167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fiedler KA, Maeng M, Mehilli J, Schulz-Schüpke S, Byrne RA, Sibbing D, Hoppmann P, Schneider S, Fusaro M, Ott I, et al. Duration of triple therapy in patients requiring oral anticoagulation after drug-eluting stent implantation: the ISAR-TRIPLE Trial. J Am Coll Cardiol. 2015;65:1619–1629. doi: 10.1016/j.jacc.2015.02.050 [DOI] [PubMed] [Google Scholar]
- 7.Dewilde WJ, Oirbans T, Verheugt FW, Kelder JC, De Smet BJ, Herrman JP, Adriaenssens T, Vrolix M, Heestermans AA, Vis MM, et al. ; WOEST study investigators. Use of clopidogrel with or without aspirin in patients taking oral anticoagulant therapy and undergoing percutaneous coronary intervention: an open-label, randomised, controlled trial. Lancet. 2013;381:1107–1115. doi: 10.1016/S0140-6736(12)62177-1 [DOI] [PubMed] [Google Scholar]
- 8.Lopes RD, Heizer G, Aronson R, Vora AN, Massaro T, Mehran R, Goodman SG, Windecker S, Darius H, Li J, et al. ; AUGUSTUS Investigators. Antithrombotic therapy after acute coronary syndrome or PCI in atrial fibrillation. N Engl J Med. 2019;380:1509–1524. doi: 10.1056/NEJMoa1817083 [DOI] [PubMed] [Google Scholar]
- 9.Vranckx P, Valgimigli M, Eckardt L, Tijssen J, Lewalter T, Gargiulo G, Batushkin V, Campo G, Lysak Z, Vakaliuk I, et al. Edoxaban-based versus vitamin K antagonist-based antithrombotic regimen after successful coronary stenting in patients with atrial fibrillation (ENTRUST-AF PCI): a randomised, open-label, phase 3b trial. Lancet. 2019;394:1335–1343. doi: 10.1016/S0140-6736(19)31872-0 [DOI] [PubMed] [Google Scholar]
- 10.Cannon CP, Lip GYH, Oldgren J. Dual antithrombotic therapy with dabigatran after PCI in atrial fibrillation. N Engl J Med. 2018;378:485–486. doi: 10.1056/NEJMc1715183 [DOI] [PubMed] [Google Scholar]
- 11.Gibson CM, Mehran R, Bode C, Halperin J, Verheugt FW, Wildgoose P, Birmingham M, Ianus J, Burton P, van Eickels M, et al. Prevention of bleeding in patients with atrial fibrillation undergoing PCI. N Engl J Med. 2016;375:2423–2434. doi: 10.1056/NEJMoa1611594 [DOI] [PubMed] [Google Scholar]
- 12.Gargiulo G, Goette A, Tijssen J, Eckardt L, Lewalter T, Vranckx P, Valgimigli M. Safety and efficacy outcomes of double vs. triple antithrombotic therapy in patients with atrial fibrillation following percutaneous coronary intervention: a systematic review and meta-analysis of non-vitamin K antagonist oral anticoagulant-based randomized clinical trials. Eur Heart J. 2019;40:3757–3767. doi: 10.1093/eurheartj/ehz732 [DOI] [PubMed] [Google Scholar]
- 13.Capodanno D, Di Maio M, Greco A, Bhatt DL, Gibson CM, Goette A, Lopes RD, Mehran R, Vranckx P, Angiolillo DJ. Safety and efficacy of double antithrombotic therapy with non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation undergoing percutaneous coronary intervention: a systematic review and meta-analysis. J Am Heart Assoc. 2020;9:e017212.doi: 10.1161/JAHA.120.017212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galli M, Andreotti F, D’Amario D, Vergallo R, Montone RA, Porto I, Crea F. Dual therapy with direct oral anticoagulants significantly increases the risk of stent thrombosis compared to triple therapy. Eur Heart J Cardiovasc Pharmacother. 2020;6:128–129. doi: 10.1093/ehjcvp/pvz030 [DOI] [PubMed] [Google Scholar]
- 15.Frigoli E, Smits P, Vranckx P, Ozaki Y, Tijssen J, Jüni P, Morice MC, Onuma Y, Windecker S, Frenk A, et al. Design and rationale of the Management of High Bleeding Risk Patients Post Bioresorbable Polymer Coated Stent Implantation With an Abbreviated Versus Standard DAPT Regimen (MASTER DAPT) Study. Am Heart J. 2019;209:97–105. doi: 10.1016/j.ahj.2018.10.009 [DOI] [PubMed] [Google Scholar]
- 16.Costa F, van Klaveren D, James S, Heg D, Räber L, Feres F, Pilgrim T, Hong MK, Kim HS, Colombo A, et al. ; PRECISE-DAPT Study Investigators. Derivation and validation of the predicting bleeding complications in patients undergoing stent implantation and subsequent dual antiplatelet therapy (PRECISE-DAPT) score: a pooled analysis of individual-patient datasets from clinical trials. Lancet. 2017;389:1025–1034. doi: 10.1016/S0140-6736(17)30397-5 [DOI] [PubMed] [Google Scholar]
- 17.Valgimigli M, Garcia-Garcia HM, Vrijens B, Vranckx P, McFadden EP, Costa F, Pieper K, Vock DM, Zhang M, Van Es GA, et al. Standardized classification and framework for reporting, interpreting, and analysing medication non-adherence in cardiovascular clinical trials: a consensus report from the Non-adherence Academic Research Consortium (NARC). Eur Heart J. 2019;40:2070–2085. doi: 10.1093/eurheartj/ehy377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449 [DOI] [PubMed] [Google Scholar]
- 19.Com-Nougue C, Rodary C, Patte C. How to establish equivalence when data are censored: a randomized trial of treatments for B non-Hodgkin lymphoma. Stat Med. 1993;12:1353–1364. doi: 10.1002/sim.4780121407 [DOI] [PubMed] [Google Scholar]
- 20.Urban P, Meredith IT, Abizaid A, Pocock SJ, Carrié D, Naber C, Lipiecki J, Richardt G, Iñiguez A, Brunel P, et al. ; LEADERS FREE Investigators. Polymer-free drug-coated coronary stents in patients at high bleeding risk. N Engl J Med. 2015;373:2038–2047. doi: 10.1056/NEJMoa1503943 [DOI] [PubMed] [Google Scholar]
- 21.Paikin JS, Wright DS, Crowther MA, Mehta SR, Eikelboom JW. Triple antithrombotic therapy in patients with atrial fibrillation and coronary artery stents. Circulation. 2010;121:2067–2070. doi: 10.1161/CIRCULATIONAHA.109.924944 [DOI] [PubMed] [Google Scholar]
- 22.Bertrand ME, Legrand V, Boland J, Fleck E, Bonnier J, Emmanuelson H, Vrolix M, Missault L, Chierchia S, Casaccia M, et al. Randomized multicenter comparison of conventional anticoagulation versus antiplatelet therapy in unplanned and elective coronary stenting. The full anticoagulation versus aspirin and ticlopidine (fantastic) study. Circulation. 1998;98:1597–1603. doi: 10.1161/01.cir.98.16.1597 [DOI] [PubMed] [Google Scholar]
- 23.Leon MB, Baim DS, Popma JJ, Gordon PC, Cutlip DE, Ho KK, Giambartolomei A, Diver DJ, Lasorda DM, Williams DO, et al. A clinical trial comparing three antithrombotic-drug regimens after coronary-artery stenting. Stent Anticoagulation Restenosis Study Investigators. N Engl J Med. 1998;339:1665–1671. doi: 10.1056/NEJM199812033392303 [DOI] [PubMed] [Google Scholar]
- 24.Kumbhani DJ, Cannon CP, Beavers CJ, Bhatt DL, Cuker A, Gluckman TJ, Marine JE, Mehran R, Messe SR, Patel NS, et al. 2020 ACC expert consensus decision pathway for anticoagulant and antiplatelet therapy in patients with atrial fibrillation or venous thromboembolism undergoing percutaneous coronary intervention or with atherosclerotic cardiovascular disease: a report of the American College of Cardiology Solution Set Oversight Committee. J Am Coll Cardiol. 2021;77:629–658. doi: 10.1016/j.jacc.2020.09.011 [DOI] [PubMed] [Google Scholar]
- 25.Collet JP, Thiele H, Barbato E, Barthélémy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, et al. ; ESC Scientific Document Group. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42:1289–1367. doi: 10.1093/eurheartj/ehaa575 [DOI] [PubMed] [Google Scholar]
- 26.Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, et al. ; ESC Scientific Document Group. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 27.Angiolillo DJ, Bhatt DL, Cannon CP, Eikelboom JW, Gibson CM, Goodman SG, Granger CB, Holmes DR, Lopes RD, Mehran R, et al. Antithrombotic therapy in patients with atrial fibrillation treated with oral anticoagulation undergoing percutaneous coronary intervention: a North American perspective: 2021 update. Circulation. 2021;143:583–596. doi: 10.1161/CIRCULATIONAHA.120.050438 [DOI] [PubMed] [Google Scholar]
- 28.Valgimigli M, Patialiakas A, Thury A, McFadden E, Colangelo S, Campo G, Tebaldi M, Ungi I, Tondi S, Roffi M, et al. ; ZEUS Investigators. Zotarolimus-eluting versus bare-metal stents in uncertain drug-eluting stent candidates. J Am Coll Cardiol. 2015;65:805–815. doi: 10.1016/j.jacc.2014.11.053 [DOI] [PubMed] [Google Scholar]
- 29.Windecker S, Latib A, Kedhi E, Kirtane AJ, Kandzari DE, Mehran R, Price MJ, Abizaid A, Simon DI, Worthley SG, et al. ; ONYX ONE Investigators. Polymer-based or polymer-free stents in patients at high bleeding risk. N Engl J Med. 2020;382:1208–1218. doi: 10.1056/NEJMoa1910021 [DOI] [PubMed] [Google Scholar]
- 30.Lopes RD, Leonardi S, Wojdyla DM, Vora AN, Thomas L, Storey RF, Vinereanu D, Granger CB, Goodman SG, Aronson R, et al. Stent thrombosis in patients with atrial fibrillation undergoing coronary stenting in the AUGUSTUS Trial. Circulation. 2020;141:781–783. doi: 10.1161/CIRCULATIONAHA.119.044584 [DOI] [PubMed] [Google Scholar]
- 31.Alexander JH, Wojdyla D, Vora AN, Thomas L, Granger CB, Goodman SG, Aronson R, Windecker S, Mehran R, Lopes RD. Risk/benefit tradeoff of antithrombotic therapy in patients with atrial fibrillation early and late after an acute coronary syndrome or percutaneous coronary intervention: insights from AUGUSTUS. Circulation. 2020;141:1618–1627. doi: 10.1161/CIRCULATIONAHA.120.046534 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.