ABSTRACT
Primary leiomyosarcomas of the colon (PLC) are rare tumors, representing 0.12% of all colon malignancies. We report a 59-year-old man with weight loss, mild anemia, and rectal bleeding. Colonoscopy revealed a 3.2 × 2.6-cm mass at the ileocecal valve. Histopathological examination of the biopsy showed a spindle cell neoplasm that stained positive for smooth muscle actin, caldesmon, and desmin. A diagnosis of PLC was made. Subsequently, a laparoscopic right hemicolectomy was performed, and no local recurrence was noted 6 months after the resection. Given the rarity of PLC, more studies on the clinical features and treatments of this tumor are warranted.
INTRODUCTION
Primary leiomyosarcomas of the colon (PLC) are rare malignant tumors that are derived from smooth muscle cells of the colonic muscularis propria or/and muscularis mucosa.1–3 Although leiomyosarcomas are common in the uterus, stomach, and retroperitoneum, they account for only 0.12% of colonic malignancies.1,2 These colonic leiomyosarcomas have been reported primarily in Japan.1,2 Only a few articles have come out about PLC in the United States.1,4 PLC are aggressive tumors with a 5-year survival of 45.6%.5 Because of the rarity and aggressiveness of PLC, studies on clinical features and treatments of this tumor are needed. Here, we present a case of PLC located at the ileocecal valve that was diagnosed by immunohistochemistry and curatively resected through laparoscopic surgery.
CASE REPORT
A 59-year-old man with a medical history of hyperlipidemia and hypertension presented with weight loss, mild anemia, and painless intermittent rectal bleeding. The patient denied abdominal pain or a change in bowel habits. The patient had a family history of liver cancer, uterine cancer and glioblastoma, and melanoma. Colonoscopy was performed with a finding of a 2.5 to 3-cm ulcerated and round mass at the level of the ileocecal valve (Figure 1). Biopsy of the mass showed a spindle cell neoplasm raising the differential diagnosis of gastrointestinal stromal tumor (GIST) and PLC. The subsequent computerized tomography (CT) study with contrast revealed a hyperdense 3.2 × 2.6-cm mass seen in the ileocecal valve and multiple hypodense areas in the liver, raising the suspicion of metastasis (Figure 2A and Figure 3). The magnetic resonance imaging study of the liver suggested hemangioma with no sign of enhancing hepatic mass. A decision was made to proceed with diagnostic laparoscopy as a part of the metastatic workup. Laparoscopy revealed the presence of adhesions to the right lower quadrant abdominal wall with an unremarkable appearance of the cecum and appendix.
Figure 1.
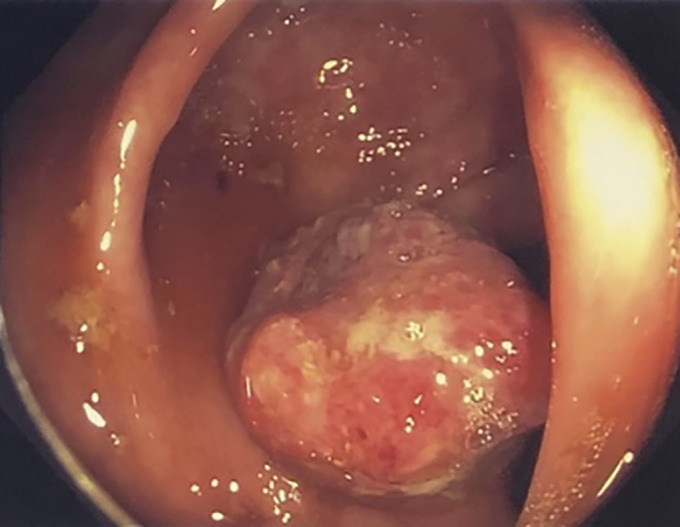
Gross image of the cecum obtained from colonoscopy showing a 2.5 to 3-cm ulcerated and round mass at the level of the ileocecal valve.
Figure 2.
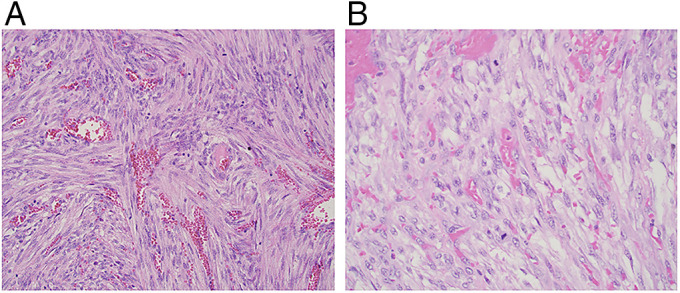
Hematoxylin and eosin stain of the leiomyosarcoma of the colon showing a spindle cell tumor arranged in perpendicular fascicles with prominent blood vessels and mitotic figures at (A) 20× magnification and (B) 40× magnification.
Figure 3.
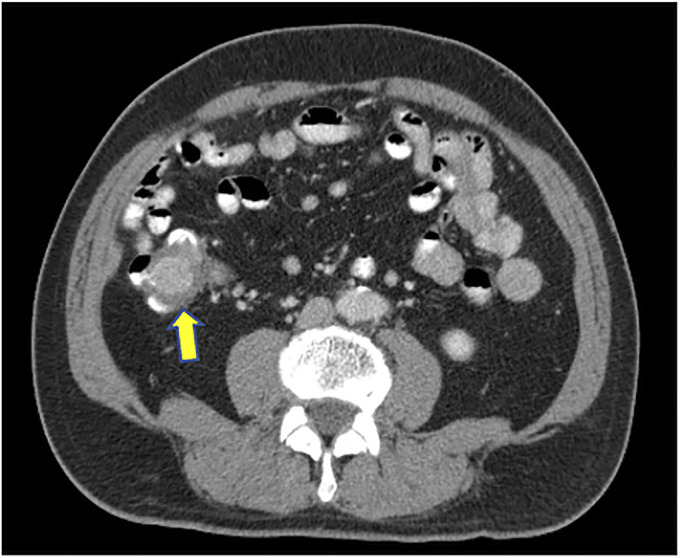
Abdominal computed tomography showing a hyperdense mass seen in the cecum measuring 3.2 × 2.6 cm (arrow).
This case was discussed with a multidisciplinary tumor board who decided that endoscopic resection of the tumor was not amendable, given the ulcerated nature and the large size of this tumor. Subsequently, the patient underwent a laparoscopic right hemicolectomy. Gross pathologic analysis showed a 2 × 2 × 0.7-cm tan-red, indurated sessile polyp on the ileocecal valve. It grossly appeared to extend into the submucosa but did not seem to involve the muscularis propria or extend into the surrounding pericolic fat. Histologically, the lesion was a spindle cell neoplasm with intersecting fascicles and prominent mitotic figures (Figure 2). Immunohistochemistry revealed that the spindle cells stained positive for smooth muscle actin, caldesmon, and desmin (focal) but negative for CK AE 1/3, CD34, CD117, DOG-1, S100, HHV8, EBV-encoded small noncoding RNAs (in-situ hybridization), and erythroblast transformation-specific related gene (Figure 4). The Ki-67 proliferation index was approximately 10%. All surgical margins were negative. Forty-six regional lymph nodes were harvested, and no metastasis was found. Final pathology revealed an intermediate grade, pT1N0 pathologic stage PLC at the ileocecal valve.
Figure 4.
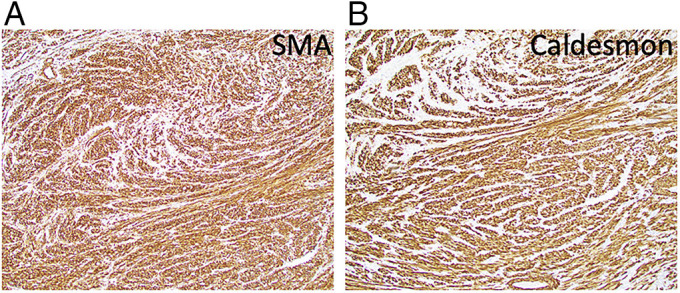
Immunohistochemistry study of leiomyosarcoma of the colon using the (A) SMA stain positive (10× magnification) and (B) caldesmon stain positive (10× magnification). SME, smooth muscle actin.
The patient did not receive any adjuvant chemotherapy because the risks associated with intensive adjuvant chemotherapy outweighed the potential benefit. By extrapolating from the retroperitoneal sarculator nomogram, our patient would be predicted to be disease-free at 7 years with approximately 40% risk of relapse, and adjuvant chemotherapy would only lower the risk by 10% to 30%. Therefore, the patient decided to be followed up by a long-term surveillance plan: abdomen and pelvic computed tomography (every 3 months for 2 years, then every 6 months for 2 years, and then annually), lungs imaging with thoracic CT (annually and alternate every 6 months with a thoracic x-ray), and colonoscopy (1 year and then 3 years). Six months after the resection, abdominal CT and thoracic x-rays showed the absence of local recurrence and metastatic disease.
DISCUSSION
PLC are rare and aggressive malignant tumors that are derived from smooth muscle cells of the colonic muscularis propria and/or muscularis mucosa.1–3 The first case of PLC was reported by Scott6 in 1923. As of 2019, 40 additional cases have been reported.5 PLC represent only 0.12% of all colon malignancies.2,3 PLC are most typically diagnosed during the fifth or sixth decades of life and slightly more frequent in men (53.7%) than in women (46.3%).5,7 Clinical presentation is often nonspecific, including abdominal pain, abdominal mass, and rectal bleeding,5 so clinical diagnosis of PLC is a challenge. The clinical differential diagnoses for PLC include submucosal tumors, such as adenocarcinoma, lymphoma, GIST, kaposi sarcoma, and schwannoma. Adenocarcinoma and lymphoma can readily be differentiated from PLC on microscopic examination.8 PLC tumor cells are usually spindle-shaped and arranged in perpendicular fascicles. They have eosinophilic cytoplasm and cigar-shaped nuclei with blunted ends. Tumor necrosis and pleomorphism can also be present.9 However, PLC, GIST, kaposi sarcoma, and schwannoma share resemblance in histological appearance, so immunohistochemistry is needed to distinguish them apart.8 PLC express smooth muscle actin, desmin, and caldesmon but do not express GIST markers (CD34, CD117, and DOG-1), S100 (differentiating it from schwannoma), or HHV8 (differentiating it from Kaposi sarcoma).9–11
PLC are known to be more aggressive with poorer prognoses than other tumors of the colon.1 The 5-year overall survival is 45.6% with a mean overall survival of 95.5 ± 18.6 months.5 Recurrence rates of colorectal sarcomas ranging from 20 to 85% have been reported.4 It was a challenge in determining the recurrence rates of our patient because there are relatively limited data to guide the medical team. However, we extrapolated the risk of recurrence by the retroperitoneal sarculator nomogram. The limitation of this prediction is that it is not directly applicable to our patient's type or location of the sarcoma, but it was the best approximation that the medical team can make. Typically, PLC metastasize to distance sites, mainly in the liver and lungs.5 Multiple studies have shown that longer survival of patients with PLC was correlated with low tumor mitotic activity, smaller tumor size < 8 cm, and age < 60 years.5,12
Currently, the most widely performed treatment of PLC is surgical resection, whereas the use of systemic adjuvant chemotherapy is still controversial and has not yet been established.13 For example, Yaren et al3 reported that a 66-year-old woman diagnosed with high-grade PLC remained 33 months symptom-free after right hemicolectomy and adjuvant ifosfamide plus doxorubicin chemotherapy. On the other hand, Kiran et al14 reported that a 54-year-old man diagnosed with grade III PLC underwent laparotomy and 6 cycles of ifosfamide and doxorubicin and remained only 6 months event-free before the local recurrence of tumor occurred at the anastomotic site. Furthermore, in the review of the national cancer database, no difference was noted in survival among patients with colorectal sarcoma who received radiation compared with those who did not.4 Given the conflicting data regarding the use of adjuvant chemotherapy and radiation, active surveillance through imaging studies of the abdomen and lung along with colonoscopy, similar to the guideline for resected colon cancer by the National Comprehensive Cancer Network,15 should be recommended to patients with PLC after tumor resection. More studies, such as a multicenter randomized control trial, are warranted to help us better understand the pathological characteristics of PLC and evaluate the efficacy of different treatments on patients with PLC.
DISCLOSURES
Author contributions: GS Wong reviewed the literature, wrote the article, and approved the final article and is the article guarantor. SV Yudina edited and reviewed the article. MCD Reyes edited and reviewed the article.
Financial disclosure: None to report.
Informed consent was obtained for this case report.
Contributor Information
Svetlana V. Yudina, Email: Svetlana_Yudina@URMC.Rochester.edu.
Maria Cecilia D. Reyes, Email: Mariacecilia_Reyes@URMC.Rochester.edu.
REFERENCES
- 1.Crystal JS, Korderas K, Schwartzberg D, Tizio SC, Zheng M, Parker G. Primary leiomyosarcoma of the colon: A report of two cases, review of the literature, and association with immunosuppression for ibd and rheumatoid arthritis. Case Rep Surg. 2018;2018:6824643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iwasa K, Taniguchi K, Noguchi M, Yamashita H, Kitagawa M. Leiomyosarcoma of the colon presenting as acute suppurative peritonitis. Surg Today. 1997;27(4):337–44. [DOI] [PubMed] [Google Scholar]
- 3.Yaren A, Değirmencioğlu S, Callı Demirkan N, Gökçen Demiray A, Taşköylü B, Doğu GG. Primary mesenchymal tumors of the colon: A report of three cases. Turk J Gastroenterol. 2014;25(3):314–8. [DOI] [PubMed] [Google Scholar]
- 4.Thiels CA, Bergquist JR, Krajewski AC, et al. Outcomes of primary colorectal sarcoma: A national cancer data base (NCDB) review. J Gastrointest Surg. 2017;21(3):560–8. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Wang H, Yuan ZL, Zhao JF, Dong DB, Gao Q. A pooled analysis of risk factors of surgically treated leiomyosarcoma of the colon in adults. World J Surg Oncol. 2020;18(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scott CR. Myoma malignum particularly other than uterine. Northwest Med. 1923;23:436–9. [Google Scholar]
- 7.Resch T, Oberhuber R, Zitt M, et al. Leiomyosarcoma of the colon: Unresolved issues of a rare but highly aggressive malignancy. Am Surg. 2011;77(4):E62–4. [PubMed] [Google Scholar]
- 8.Wong YC, Chan SY, Yuen KY, Chong LC. Locally invasive and obstructive colonic leiomyosarcoma: A diagnostic and therapeutic challenge. Hong Kong Med J. 2020;26(1):73–5. [DOI] [PubMed] [Google Scholar]
- 9.Beauchamp A, Hajjar R, Khullar S, Latour M, Schwenter F, Sebajang H. Mesenteric lymph node recurrence of a primary colorectal leiomyosarcoma. Case Rep Surg. 2020;2020:6935834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miettinen M, Sarlomo-Rikala M, Lasota J. Gastrointestinal stromal tumors: Recent advances in understanding of their biology. Hum Pathol. 1999;30(10):1213–20. [DOI] [PubMed] [Google Scholar]
- 11.Nagata N, Igari T, Shimbo T, et al. Diagnostic value of endothelial markers and HHV-8 staining in gastrointestinal Kaposi sarcoma and its difference in endoscopic tumor staging. World J Gastroenterol. 2013;19(23):3608–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warkel RL, Stewart JB, Temple AJ. Leiomyosarcoma of the colon: Report of a case and analysis of the relationship of histology to prognosis. Dis Colon Rectum. 1975;18(6):501–6. [DOI] [PubMed] [Google Scholar]
- 13.Yahagi M, Ishii Y, Hara A, Watanabe M. Laparoscopic surgery to treat leiomyosarcomas of the sigmoid colon: A case report and literature review. Surg Case Rep. 2019;5(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kiran P, Shiny PM, Dhanya KS, Aravindan KP. Diagnosis of leiomyosarcoma of colon. J Cancer Res Ther. 2015;11(4):1035. [DOI] [PubMed] [Google Scholar]
- 15.National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology. https://www.nccn.org/professionals/physician_gls. Accessed October 14, 2020. [Google Scholar]


