Abstract
Neural tube defects (NTDs) are one of the greatest causes of childhood mortality and disability-adjusted life years worldwide. Global prevalence at birth is approximately 18.6 per 10,000 live births, with more than 300,000 infants with NTDs born every year. Substantial strides have been made in understanding the genetics, pathophysiology, and surgical treatment of NTDs, yet the natural history remains one of high morbidity and profound impairment of quality of life. Direct and indirect costs of care are enormous, which ensures profound inequities and disparities in the burden of disease in countries of low and moderate resources. All indices of disease burden are higher for NTDs in developing countries. The great tragedy is that the majority of NTDs can be prevented with folate fortification of commercially produced food. Unequivocal evidence of the effectiveness of folate to reduce the incidence of NTDs has existed for more than 25 years. Yet, the most comprehensive surveys of effectiveness of implementation strategies show that more than 100 countries fail to fortify, and consequently only 13% of folate-preventable spina bifida is actually prevented. Neurosurgeons harbor a disproportionate, central, and fundamental role in the management of NTDs and enjoy high standing in society. No organized group in medicine can speak as authoritatively or convincingly. As a result, neurosurgeons and organized neurosurgery harbor disproportionate potential to advocate for more comprehensive folate fortification, and thereby prevent the most common and severe birth defect to impact the human nervous system. Assertive, proactive, informed advocacy for folate fortification should be a central and integral part of the neurosurgical approach to NTDs. Only by making the prevention of dysraphism a priority can we best address the inequities often observed worldwide.
Keywords: pediatric neurosurgery, mandatory folic acid fortification, neural tube defect, spina bifida, myelomeningocele, anencephaly, infant mortality, congenital malformations
…folic acid fortification may be the most important science-driven intervention in nutrition and public health in decades.
— I. H. Rosenberg41
It is estimated that there are 300,000 new live births affected by neural tube defects (NTDs) worldwide each year. In the US there are approximately 1500 children born with NTDs each year.58 The indices of Global Burden of Disease indicate that NTDs are among the greatest causes of childhood mortality and disability-adjusted life years worldwide.26,56 The true mortality is likely underrepresented, as stillbirths and pregnancy terminations are usually not included.5 Mortality in children affected by NTDs is 10-fold higher than in nonaffected children, and is highest in countries of modest resources due to limitations in specialized medical and surgical care.2,58 The economic and social costs are also staggering due to a combination of lost productivity and direct and indirect medical care costs.2 These costs ensure profound inequities of disease burden and pronounced disparities in care between countries of abundant versus modest resources. However, up to 92% of NTDs can be prevented with mandatory folic acid fortification.37 As a result, it is highly likely that the strategy most likely to ameliorate inequities of disease burden in NTDs is prevention rather than surgical refinement.
The most common NTD forms are myelomeningocele (MMC) and anencephaly, which represent 90% of all NTDs.10 Open MMC is classically and widely referred to as spina bifida (SB), but the spectrum of dysraphism also encompasses other variants such as meningoceles, encephaloceles, craniorachischisis, lipomyelomeningoceles, and occult spinal defects.7,10,16,31,49 Occult defects frequently go underdetected or undetected, particularly in low-resource settings, and are therefore likely underreported in the literature.49
The term “spina bifida” was first coined in 1641 by Nicholas Tulp, while the first isolated reports of surgical repair of open NTDs were recorded in the late 19th and early 20th centuries.18,19,53 These were largely proofs of a technical capability, and widespread surgical treatment of open NTDs was not embraced until the 1970s. Prior to this, most cases of open NTDs were either not treated or highly selectively treated, with only the mildest forms being considered suitable candidates for closure and subsequent care.44 It was not until the late 20th century that substantial strides were made in understanding the pathophysiology, epidemiology, genetics, and central role of dietary folic acid fortification as an effective means of primary prevention.7,15,27
The key to many clinical advances came from epidemiological observations.37 Many of the earliest of these arose from the United Kingdom because of the prevalence and severity of NTDs in this region. Observational epidemiology studies from the middle part of the 20th century provided the key insight that the incidence of NTDs varied with multiple family variables including social class, ethnic group, maternal age, parity (number of prior pregnancies), and geographical region.37 One of the key observations was made by Smithells, who noted that poor women had babies with NTDs at a significantly higher rate, which led to the hypothesis that dietary quality was contributory.21,46
Initial studies with multivitamin supplementation were promising and set the stage for the British Medical Research Council study that found folic acid to be central to the genesis of NTDs. In this randomized trial, high-dose (5000 μg/day) oral folic acid was compared alone and in combination with other vitamin supplements.34 Only the treatment arms in which folic acid was provided realized significant reductions in NTD incidence, with a reduction of 72%.37
A multitude of subsequent studies have confirmed these findings and provided unequivocal evidence that folate fortification reliably and predictably reduces the incidence of MMC and anencephaly.15,27 These cases are referred to as folic acid preventable SB (FAP-SB) and have been subsequently shown to represent up to 92% of all clinical NTDs. This realization led to mandated fortification of wheat and corn products in the US and 80 other countries, which resulted in pronounced reductions in rates of newborn NTDs over the ensuring decades. It has been well established for at least two decades that folic acid fortification is a safe, highly effective, and inexpensive prevention strategy for NTDs, and yet rates of fortification vary widely. A 2015 survey of the extent of worldwide effectiveness of dietary folate fortification strategies found that most countries in Europe, Africa, and Asia were not fortifying grains or rice with folate, and that consequently the estimated rate of actual prevention of FAP-SB was only 13%.2 The lack of prevention occurs because there is insufficient political will in many countries to either pass regulations that require mandatory folic acid fortification, or to provide sufficient oversight to ensure industry actually follows the regulations for mandatory folic acid fortification.2,37
Incidence and Global Disparities
Determining the true incidence of NTDs is difficult due to the high rate of fetal mortality and spontaneous abortions, especially for those NTDs not compatible with life.25 This is particularly challenging in low- and middle-income countries (LMICs), where prenatal ascertainment is very low. Therefore, we rely chiefly on prevalence at birth as a surrogate epidemiological measure. Globally, more than 300,000 infants with NTDs are born every year.36 Worldwide prevalence at birth is approximately 18.6 cases per 10,000 live births.6,30 There is, however, significant regional, ethnic, and global variation. In the US, for example, the average birth prevalence is 7 cases per 10,000 live births.55 However, birth prevalence is higher in Hispanics compared to White Americans, but lower in African Americans.55 In certain areas of Africa, birth prevalence of NTDs can reach 75 cases per 10,000 live births, while in areas of Latin America up to 96 cases per 10,000 live births have been reported.42,58 However, estimating birth prevalence LMICs has proven to be very challenging. In Latin America, for example, only 2 countries have documented population-based monitoring, while most rely only on sporadic hospital-based reports, contributing to the wide range in prevalence reported in the literature.58 While some differences in prevalence may be due to genetic variations and clustering, most of this disparity is attributable to substantial gaps in prevention efforts. Mortality among infants with open defects can also vary significantly, with rural areas having rates approaching 100% and developed nations as low as 10%.33,44 Benefits in treatment and prevention have thus disproportionately bypassed LMICs, with many of these cases representing preventable deaths and disabilities.5,22,56,57 Adding to these challenges, most epidemiological studies in LMIC settings have significant limitations, and there is a lack of continued surveillance necessary to assess the efficacy of preventive efforts.
Treatment Versus Prevention
Neurosurgical treatment of NTDs is limited to prenatal or postnatal repair of MMC, treatment of associated hydrocephalus, posterior fossa decompression of the Chiari type II malformation, encephalocele or lipomyelomeningocele repair, and cord untethering.10 More recently, the MOMs (Management of Myelomeningocele Study) trial reported randomized evidence regarding the efficacy of prenatal repair, opening the possibility for improved outcomes and a potential reduction in the need for CSF shunting, but at the risk of an increase in prenatal complications and preterm birth.1 While improved technique and better patient selection can reduce these risks,3,32 adoption of in-utero repair has been modest within the US, and still remains unavailable to most individuals in developing countries. The exceptional cost and limited availability of intrauterine MMC closure make it unlikely to exert a significant impact on the broad public health perspective of global NTD management for the foreseeable future. Furthermore, prenatal screening and ultrasound is uncommon in many low-resource areas, with nearly all NTD cases discovered at birth. Because neurosurgical intervention is unable to restore function lost due to failure of proper neural tube closure,7 all neurosurgical treatment of open NTDs remains fundamentally palliative. Therefore, primary prevention remains our most important and effective tool.
Folic Acid Supplementation
Folate is a water-soluble vitamin that may be obtained naturally as endogenous food folate or in its synthetic form (folic acid) from supplements and fortified foods. Folate is an unstable molecule, while folic acid is stable and can be incorporated into food. The earliest evidence of primary prevention of NTDs with multivitamin supplementation was provided by Smithells et al. in the early 1980s,21,45–48 while later studies isolated folic acid as the major contributor to this observation.4,8,20,29,35,43,52,54 Several clinical trials and meta-analyses have since provided conclusive evidence of NTD prevention with folic acid alone, with an estimated number needed to treat of 847.5,14,23,24,27,34 A more recent meta-analysis also showed that there was no effect of folic acid supplementation on cleft palate, cleft lip, congenital cardiovascular defects, miscarriages, or other birth defects.15
A daily folic acid dose of 400 μg can prevent the great majority of NTDs.17,28 Berry et al. showed effectiveness with a daily dose of 400 μg of folic acid in the community setting in China in both high and low prevalence regions.4 The effect of prevention policies has varied substantially around the world. In the US, policies were first implemented in 1992 with a recommendation by the US Public Health Service that all women of childbearing age should consume 400 μg of folic acid daily.40 In 1998, mandatory fortification of enriched cereal grain products (140 μg/100 g) was instituted.9,11 Adoption of these policies within the US has resulted in a 28% reduction in NTD birth prevalence, from 10.7 cases per 10,000 live births in 1992 to 7 cases per 10,000 live births in 1998, preventing approximately 1326 NTDs annually.55 Fortification has also lead to a decrease in serum folate deficiency levels from 30% to < 1%.38 However, after the initial reduction these policies achieved, birth prevalence of NTDs has remained stable in the US since 1999, and there are still significant gaps and opportunities for greater impact. Currently, nearly a quarter (21.6%) of childbearing age women in the US still have red blood cell (RBC) folate concentrations levels associated with a higher risk for NTDs.50 Only a small percentage of women (17% among Hispanic women and 30% among White women) report consuming the recommended ≥ 400 μg of folic acid per day.51 In the US, NTD prevalence among Hispanics is higher than other groups, which may be due to a combination of factors such as the reported difference in consumption of the daily recommended allowance of folic acid by women of childbearing age, as well as genetic factors. Of key importance is that the FDA did not require fortification of corn masa flour, a staple food eaten by many Hispanic Americans. There could be other factors as well; for example, the methylenetetrahydrofolate reductase T allele, a common genetic polymorphism in Hispanics, has been associated with relatively lower plasma folate and RBC folate concentrations.13
Globally, fortification mandates are still not universally adopted (https://fortificationdata.org/#data). For example, although maize and corn play a significant role in diet for Latin American countries, only a few have adopted fortification mandates (Fig. 1). Wheat flour fortification mandates are more widely adopted, but there are still countries that could benefit significantly from adopting these policies (Fig. 2). Neurosurgeons should take a leading role in national and international policy as well as research aimed at promoting the implementation of folic acid fortification in all countries.
FIG. 1.
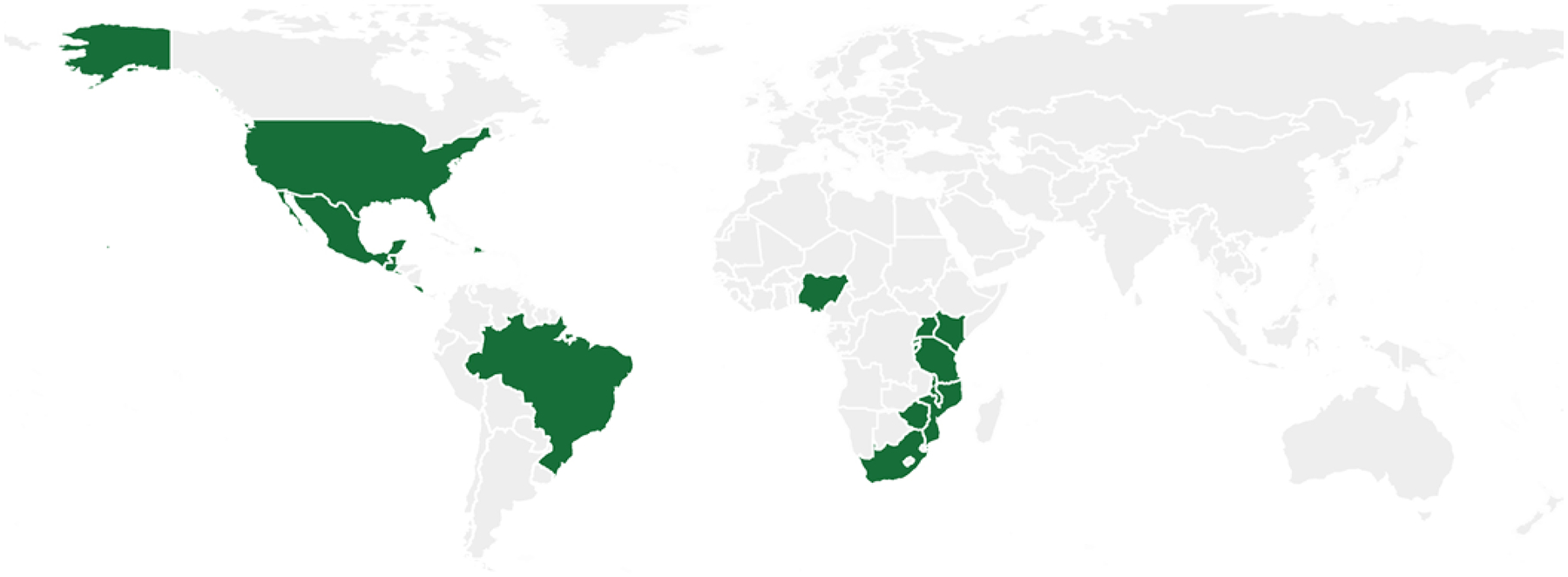
Maize flour folic acid supplementation. The map displays (in green) countries that have legal documentation to either standardize (“voluntary fortification”) or mandate (“mandatory fortification”) food fortification with maize flour folic acid, or countries that have written standards for folic acid (“fortification standards”). Source: Global Fortification Data Exchange (https://fortificationdata.org/#data).
FIG. 2.
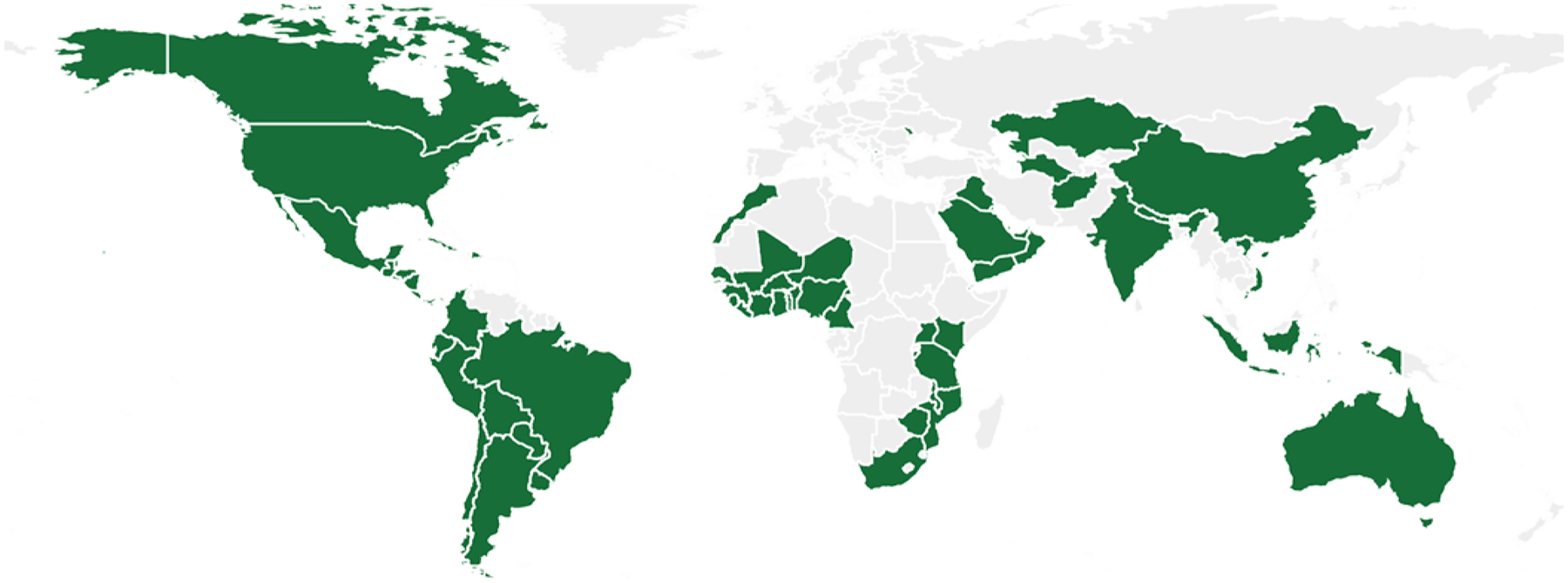
Wheat flour folic acid supplementation. The map displays (in green) countries that have legal documentation to either standardize (“voluntary fortification”) or mandate (“mandatory fortification”) food fortification with wheat flour folic acid, or countries that have written standards for folic acid (“fortification standards”). Source: Global Fortification Data Exchange (https://fortificationdata.org/#data).
Mobilizing Neurosurgeons: A Call to Action
From the first day of life, open NTDs are the purview of neurosurgeons. Through primary closure, management of hydrocephalus, posterior fossa decompression, and tethered cord release, these patients with NTDs are more closely connected to neurosurgery than virtually any other specialty. All other morbidities are secondary to disorders in neuronal innervation of end organs. Because neurosurgeons take the primary role in management of these conditions, it is incumbent on neurosurgeons to embrace a strong leadership role in prevention efforts. With increasing interest in global neurosurgery, and recognition of the vital role for neurosurgeons in global health, neurosurgeons are perfectly positioned to take a leading role in advocating for folic acid fortification efforts.
Neurosurgery is a small field that exerts a profoundly disproportionate impact. This is in large part due to the critical importance of the human central nervous system and the unparalleled ability of neurosurgeons to favorably impact the lives of patients with neurological illness. As a result, neurosurgery holds a position of societal privilege and prominence. This provides a unique and powerful position to advocate for the prevention of a profoundly severe neurological illness. Despite decades of diligent work, surgical advances remain largely palliative and the vast majority of patients with NTDs have significant lifelong morbidity. In LMICs, mortality rates remain high and the burdens of disease are profound. Associated emotional, psychological, and financial costs remain extremely high, and suffering persists at an extraordinary level. Yet, the fundamental disease process is largely preventable with a safe, cost effective, and simple dietary vitamin fortification. Brazil showed a 10-fold reduction in prevalence of NTDs following fortification, while the US observed a 20% reduction in SB and a 34% reduction in anencephaly.12,37,58 There is, to date, no significant evidence of adverse public health consequences of folic acid fortification and the cost-effectiveness ratio is a staggering 150:1.2,12
Implementation Steps
Reducing inequities in neurosurgical care will require more than simply increasing capacity and advancing technical expertise. Fundamentally, neurosurgeons seek to reduce the burden of neurological diseases across the world. Prevention of FAP-SB represents the single greatest opportunity for neurosurgeons to contribute to this goal. As the primary stewards of SB care, neurosurgeons must divorce themselves from the idea of prevention as belonging solely to the realm of public health policy. Comprehensive neurosurgical care delivery ought to incorporate prevention efforts as a central aim and inherent part of an overall strategy that improves care and eliminates inequities and disparities in the burden of dysraphic disease.
Steps to Improve SB Care
We propose the following concrete steps for both individuals and organized neurosurgery to work together to enhance SB care.
First, neurosurgeons must serve as powerful advocates for mandatory folic acid fortification of centrally processed foods. Our professional organizations should forward evidence-based, scientifically valid position statements that support fortification and call upon Ministers of Health and elected political officials to support public health through proven prevention strategies. Neurosurgeons and neurosurgical organizations can collaborate with experts in genetics, epidemiology, and public health to call for a resurgence of commitment to programs of fortification and associated surveillance and enforcement efforts, which ensure that the vast majority of women of reproductive age consume products that are appropriately fortified with folic acid and other micronutrients recommended by the WHO. The potential power of neurosurgery in contributing to this cause is substantial. Success in a single country can prevent more disease than any neurosurgeon or neurosurgical organization can treat (palliatively) in a lifetime.
Second, neurosurgeons must form partnerships with local and international colleagues to advance basic and clinical research. This can be accomplished by provision of tissue for large-scale genomic studies of human patients with NTDs and by advocating for patient registries. Mosaic and differential tissue expression ensures the value of tissue obtained directly from regions of NTDs. Only neurosurgeons can provide the key tissue available in abundance at the time of surgical treatment.
Third, neurosurgeons must support improved registry and surveillance efforts in North America and beyond. An example of this is the National Spina Bifida Patient Registry, which is the largest currently available registry of patients with SB. This registry is sponsored and maintained by the US Centers for Disease Control and Prevention and currently has almost 9000 patients enrolled from 17 centers in North America.
Fourth, neurosurgeons should also advocate for greater prenatal screening, including ultrasound, by which open NTDs can be diagnosed.
Fifth, initiatives such as the Food Fortification Initiative, Push! Global Alliance, and the Center for Spina Bifida Prevention at Emory University can provide country-level information on NTD prevalence and local prevention, and can serve as partners to effect significant change (Fig. 3).39
FIG. 3.
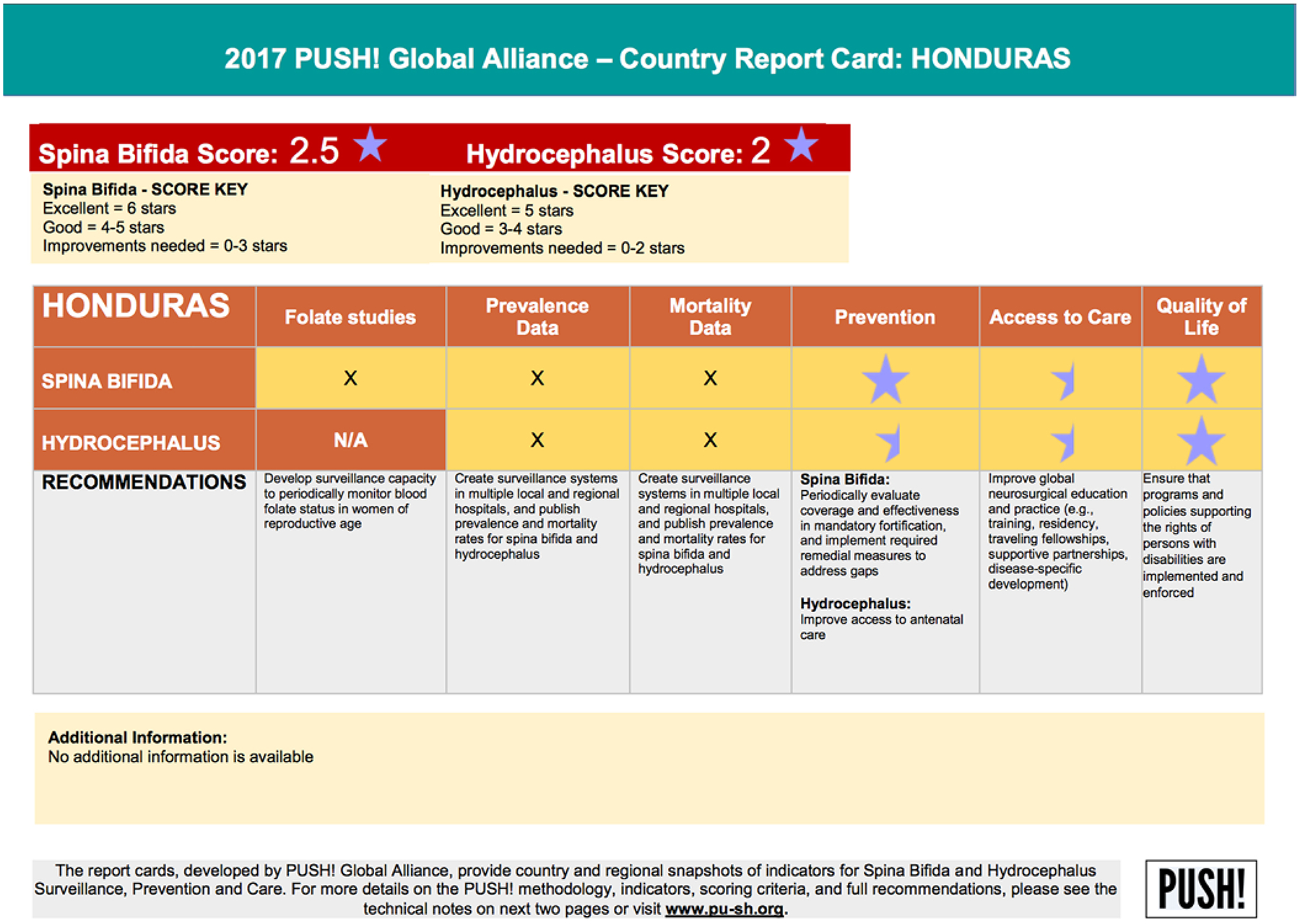
Sample Global Push report card. The report cards, developed by the PUSH! Global Alliance To Improve Nutrition and the Center for Spina Bifida Prevention/Emory University, provide country and regional snapshots of indicators for SB and hydrocephalus surveillance, prevention, and care. Reproduced with permission from the PUSH! Global Health Alliance (http://pu-sh.org/global-report-cards). For more details on the PUSH! methodology, indicators, scoring criteria, and full recommendations, visit www.pu-sh.org.39
Sixth, neurosurgeons should support the establishment of comprehensive countrywide MMC centers of excellence through a combination of advocacy, international collaboration, and funding.
Seventh, SB advocacy organizations and organized neurosurgical groups such as the World Federation of Neurological Societies, the Foundation for International Education in Neurological Surgery, and the International Society for Pediatric Neurosurgery must expand availability of multidisciplinary conferences on SB prevention and multidisciplinary management across the world. Such conferences must be interdisciplinary and include urology, orthopedics, and physical medicine and rehabilitation.
And lastly, neurosurgeons should work to establish and expand collaborations between their own institution and existing SB centers in developing countries.
Conclusions
Neurosurgeons will continue to enthusiastically debate and disseminate new surgical techniques and management strategies for patients with NTD. However, unlike the vast majority of neurosurgical diseases, in NTDs we have a condition that is eminently preventable. We have had the scientific evidence for at least 25 years, yet it is only being partially utilized and embraced. We must have science-based advocacy to impress upon public policy decision makers the critical role that folic acid fortification can play in the prevention of an otherwise devastating birth defect. Neurosurgeons must take an active leadership role in primary prevention, which is—and should be—our most powerful tool.
ABBREVIATIONS
- FAP-SB
folic acid preventable SB
- LMICs
low- and middle-income countries
- MMC
myelomeningocele
- NTD
neural tube defect
- RBC
red blood cell
- SB
spina bifida
Footnotes
Disclosures
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1.Adzick NS, Thom EA, Spong CY, Brock JW III, Burrows PK, Johnson MP, et al. : A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med 364:993–1004, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arth A, Kancherla V, Pachón H, Zimmerman S, Johnson Q, Oakley GPA Jr: A 2015 global update on folic acid-preventable spina bifida and anencephaly. Birth Defects Res A Clin Mol Teratol 106:520–529, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Bennett KA, Carroll MA, Shannon CN, Braun SA, Dabrowiak ME, Crum AK, et al. : Reducing perinatal complications and preterm delivery for patients undergoing in utero closure of fetal myelomeningocele: further modifications to the multidisciplinary surgical technique. J Neurosurg Pediatr 14:108–114, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Berry RJ, Li Z, Erickson JD, Li S, Moore CA, Wang H, et al. : Prevention of neural-tube defects with folic acid in China. N Engl J Med 341:1485–1490, 1999 [DOI] [PubMed] [Google Scholar]
- 5.Blencowe H, Cousens S, Modell B, Lawn J: Folic acid to reduce neonatal mortality from neural tube disorders. Int J Epidemiol 39 (Suppl 1):i110–i121, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blencowe H, Kancherla V, Moorthie S, Darlison MW, Modell B: Estimates of global and regional prevalence of neural tube defects for 2015: a systematic analysis. Ann N Y Acad Sci 1414:31–46, 2018 [DOI] [PubMed] [Google Scholar]
- 7.Botto LD, Moore CA, Khoury MJ, Erickson JD: Neural-tube defects. N Engl J Med 341:1509–1519, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Bower C, Stanley FJ: Dietary folate as a risk factor for neural-tube defects: evidence from a case-control study in Western Australia. Med J Aust 150:613–619, 1989 [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention (CDC): Spina bifida and anencephaly before and after folic acid mandate—United States, 1995–1996 and 1999–2000. MMWR Morb Mortal Wkly Rep 53:362–365, 2004 [PubMed] [Google Scholar]
- 10.Copp AJ, Stanier P, Greene ND: Neural tube defects: recent advances, unsolved questions, and controversies. Lancet Neurol 12:799–810, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cordero A, Mulinare J, Berry R, Boyle C, Dietz W, Johnston R Jr, et al. : CDC grand rounds: additional opportunities to prevent neural tube defects with folic acid fortification. MMWR Morb Mortal Wkly Rep 59:980–984, 2010 [PubMed] [Google Scholar]
- 12.Crider KS, Bailey LB, Berry RJ: Folic acid food fortification—its history, effect, concerns, and future directions. Nutrients 3:370–384, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crider KS, Zhu JH, Hao L, Yang QH, Yang TP, Gindler J, et al. : MTHFR 677C->T genotype is associated with folate and homocysteine concentrations in a large, population-based, double-blind trial of folic acid supplementation. Am J Clin Nutr 93:1365–1372, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Czeizel AE, Dudás I, Métneki J: Pregnancy outcomes in a randomised controlled trial of periconceptional multivitamin supplementation. Final report. Arch Gynecol Obstet 255:131–139, 1994 [DOI] [PubMed] [Google Scholar]
- 15.De-Regil LM, Peña-Rosas JP, Fernández-Gaxiola AC, Rayco-Solon P: Effects and safety of periconceptional oral folate supplementation for preventing birth defects. Cochrane Database Syst Rev (12):CD007950, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Detrait ER, George TM, Etchevers HC, Gilbert JR, Veke-mans M, Speer MC: Human neural tube defects: developmental biology, epidemiology, and genetics. Neurotoxicol Teratol 27:515–524, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duffy ME, Hoey L, Hughes CF, Strain JJ, Rankin A, Souverein OW, et al. : Biomarker responses to folic acid intervention in healthy adults: a meta-analysis of randomized controlled trials. Am J Clin Nutr 99:96–106, 2014 [DOI] [PubMed] [Google Scholar]
- 18.Forestus P: De capitis et cerebre morbis ac symptomatic, in: Observationum et curationum medicinalium, libri III. Leiden: Officina Platiniana, 1587 [Google Scholar]
- 19.Fraser J: Spina bifida. Edinburgh Med J 36:284, 1929 [Google Scholar]
- 20.Friel JK, Frecker M, Fraser FC: Nutritional patterns of mothers of children with neural tube defects in Newfoundland. Am J Med Genet 55:195–199, 1995 [DOI] [PubMed] [Google Scholar]
- 21.Hibbard ED, Smithells R: Folio acid metabolism and human embryopathy. Lancet 1:1254, 1965 [Google Scholar]
- 22.Higashi H, Barendregt JJ, Kassebaum NJ, Weiser TG, Bickler SW, Vos T: The burden of selected congenital anomalies amenable to surgery in low and middle-income regions: cleft lip and palate, congenital heart anomalies and neural tube defects. Arch Dis Child 100:233–238, 2015 [DOI] [PubMed] [Google Scholar]
- 23.Kirke PN, Daly LE, Elwood JH: A randomised trial of low dose folic acid to prevent neural tube defects. Arch Dis Child 67:1442–1446, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laurence KM, James N, Miller MH, Tennant GB, Campbell H: Double-blind randomised controlled trial of folate treatment before conception to prevent recurrence of neural-tube defects. Br Med J (Clin Res Ed) 282:1509–1511, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Little J: Fetal loss, in Elwood J, Little J, Elwood H (eds): Epidemiology and Control of Neural Tube Defects. Oxford: Oxford University Press, 1992 [Google Scholar]
- 26.Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. : Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 379:2151–2161, 2012 [DOI] [PubMed] [Google Scholar]
- 27.Lumley J, Watson L, Watson M, Bower C: Periconceptional supplementation with folate and/or multivitamins for preventing neural tube defects. Cochrane Database Syst Rev (3):CD001056, 2001 [DOI] [PubMed] [Google Scholar]
- 28.Marchetta CM, Devine OJ, Crider KS, Tsang BL, Cordero AM, Qi YP, et al. : Assessing the association between natural food folate intake and blood folate concentrations: a systematic review and Bayesian meta-analysis of trials and observational studies. Nutrients 7:2663–2686, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Milunsky A, Jick H, Jick SS, Bruell CL, MacLaughlin DS, Rothman KJ, et al. : Multivitamin/folic acid supplementation in early pregnancy reduces the prevalence of neural tube defects. JAMA 262:2847–2852, 1989 [DOI] [PubMed] [Google Scholar]
- 30.Mitchell LE: Epidemiology of neural tube defects. Am J Med Genet C Semin Med Genet 135:88–94, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Mitchell LE, Adzick NS, Melchionne J, Pasquariello PS, Sutton LN, Whitehead AS: Spina bifida. Lancet 364:1885–1895, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Moldenhauer JS, Adzick NS: Fetal surgery for myelomeningocele: after the Management of Myelomeningocele Study (MOMS). Semin Fetal Neonatal Med 22:360–366, 2017 [DOI] [PubMed] [Google Scholar]
- 33.Moore CA, Li S, Li Z, Hong SX, Gu HQ, Berry RJ, et al. : Elevated rates of severe neural tube defects in a high-prevalence area in northern China. Am J Med Genet 73:113–118, 1997 [PubMed] [Google Scholar]
- 34.MRC Vitamin Study Research Group: Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. Lancet 338:131–137, 1991 [PubMed] [Google Scholar]
- 35.Mulinare J, Cordero JF, Erickson JD, Berry RJ: Periconceptional use of multivitamins and the occurrence of neural tube defects. JAMA 260:3141–3145, 1988 [PubMed] [Google Scholar]
- 36.Murray CJ, Lopez AD: The Global Burden of Disease: A Comprehensive Assessment of Mortality and Disability from Diseases, Injuries, and Risk Factors in 1990 and Projected to 2020. Cambridge, MA: Harvard School of Public Health, 1996, Vol 1 [Google Scholar]
- 37.Oakley GP Jr: The scientific basis for eliminating folic acid-preventable spina bifida: a modern miracle from epidemiology. Ann Epidemiol 19:226–230, 2009 [DOI] [PubMed] [Google Scholar]
- 38.Pfeiffer CM, Hughes JP, Lacher DA, Bailey RL, Berry RJ, Zhang M, et al. : Estimation of trends in serum and RBC folate in the U.S. population from pre- to postfortification using assay-adjusted data from the NHANES 1988–2010. J Nutr 142:886–893, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Push! Global Alliance: Global report card for spina bifida and hydrocephlaus prevention and care. PU-SH.org (http://www.pu-sh.org/global-report-cards) [Accessed August 14, 2018]
- 40.Recommendations for the use of folic acid to reduce the number of cases of spina bifida and other neural tube defects. MMWR Recomm Rep 41 (RR-14):1–7, 1992 [PubMed] [Google Scholar]
- 41.Rosenberg IH: Science-based micronutrient fortification: which nutrients, how much, and how to know? Am J Clin Nutr 82:279–280, 2005 [DOI] [PubMed] [Google Scholar]
- 42.Rosenthal J, Casas J, Taren D, Alverson CJ, Flores A, Frias J: Neural tube defects in Latin America and the impact of fortification: a literature review. Public Health Nutr 17:537–550, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shaw GM, Schaffer D, Velie EM, Morland K, Harris JA: Periconceptional vitamin use, dietary folate, and the occurrence of neural tube defects. Epidemiology 6:219–226, 1995 [DOI] [PubMed] [Google Scholar]
- 44.Shurtleff DB, Luthy DA, Nyberg DA, Benedetti TJ, Mack LA: Meningomyelocele: management in utero and post natum. Ciba Found Symp 181:270–286, 1994 [DOI] [PubMed] [Google Scholar]
- 45.Smithells RW, Nevin NC, Seller MJ, Sheppard S, Harris R, Read AP, et al. : Further experience of vitamin supplementation for prevention of neural tube defect recurrences. Lancet 1:1027–1031, 1983 [DOI] [PubMed] [Google Scholar]
- 46.Smithells RW, Sheppard S, Schorah CJ: Vitamin deficiencies and neural tube defects. Arch Dis Child 51:944–950, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smithells RW, Sheppard S, Schorah CJ, Seller MJ, Nevin NC, Harris R, et al. : Apparent prevention of neural tube defects by periconceptional vitamin supplementation. Arch Dis Child 56:911–918, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smithells RW, Sheppard S, Schorah CJ, Seller MJ, Nevin NC, Harris R, et al. : Possible prevention of neural-tube defects by periconceptional vitamin supplementation. Lancet 1:339–340, 1980 [DOI] [PubMed] [Google Scholar]
- 49.Stevenson RE, Hall JG: Human Malformations and Related Anomalies. Oxford: Oxford University Press, 2006 [Google Scholar]
- 50.Tinker S, Hamner H, Crider K: Red blood cell folate concentrations among non-pregnant United States women of childbearing age, National Health and Nutrition Examination Survey, 2007–2010. Presented at the 47th Annual SER Meeting, Seattle, 2014. (Abstract) (https://epiresearch.org/wp-content/uploads/2014/08/abstract-book-printed.pdf) [Accessed August 14, 2018] [Google Scholar]
- 51.Tinker SC, Cogswell ME, Devine O, Berry RJ: Folic acid intake among U.S. women aged 15–44 years, National Health and Nutrition Examination Survey, 2003–2006. Am J Prev Med 38:534–542, 2010 [DOI] [PubMed] [Google Scholar]
- 52.Vergel RG, Sanchez LR, Heredero BL, Rodriguez PL, Martinez AJ: Primary prevention of neural tube defects with folic acid supplementation: Cuban experience. Prenat Diagn 10:149–152, 1990 [DOI] [PubMed] [Google Scholar]
- 53.Von Recklinghausen F: Untersuchungen über die Spina bifida. Virchows Arch Pathol Anat Physiol Klin Med 105:373–455, 1886 [Google Scholar]
- 54.Werler MM, Shapiro S, Mitchell AA: Periconceptional folic acid exposure and risk of occurrent neural tube defects. JAMA 269:1257–1261, 1993 [PubMed] [Google Scholar]
- 55.Williams J, Mai CT, Mulinare J, Isenburg J, Flood TJ, Ethen M, et al. : Updated estimates of neural tube defects prevented by mandatory folic acid fortification—United States, 1995–2011. MMWR Morb Mortal Wkly Rep 64:1–5, 2015 [PMC free article] [PubMed] [Google Scholar]
- 56.World Health Organization: Mortality and burden of disease estimates for WHO member states, in: WHO Health Statistics and Information Systems. Geneva: World Health Organization, 2018 [Google Scholar]
- 57.Wu VK, Poenaru D, Poley MJ: Burden of surgical congenital anomalies in Kenya: a population-based study. J Trop Pediatr 59:195–202, 2013 [DOI] [PubMed] [Google Scholar]
- 58.Zaganjor I, Sekkarie A, Tsang BL, Williams J, Razzaghi H, Mulinare J, et al. : Describing the prevalence of neural tube defects worldwide: a systematic literature review. PLoS One 11:e0151586, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]


