PURPOSE
Physical activity (PA) is a promising intervention for cancer-related cognitive decline, yet research assessing its use during chemotherapy is limited. This study evaluated patterns of PA before, during, and after chemotherapy in patients with breast cancer and the association between PA and cognitive function.
METHODS
In a nationwide, prospective cohort study, we assessed PA (Aerobics Center Longitudinal Study PA measure) and perceived and objectively measured cognitive functioning (Functional Assessment of Cancer Therapy–Cognitive, Delayed Match to Sample, and Rapid Visual Processing measures) at prechemotherapy (T1), postchemotherapy (T2), and 6 months postchemotherapy (T3) in patients with breast cancer and cancer-free, age-matched controls at equivalent time points. Longitudinal linear mixed-effects models (LMMs) characterized PA changes over time between patients and controls, adjusting for demographic and clinical factors. LMMs further estimated the role of prechemotherapy PA and changes in PA during chemotherapy on cognitive changes over time.
RESULTS
Patients with stage I-IIIC breast cancer (n = 580; age M [standard deviation] = 53.4 [10.6] years) and controls (n = 363; age M [standard deviation] = 52.6 [10.3] years) were included. One third of patients met national PA guidelines at T1, dropping to 21% at T2 before rising to 37% at T3. LMMs revealed declines in PA from T1 to T2 in patients compared with controls (all P < .001). Patients meeting guidelines at T1 demonstrated better cognitive scores over time on the Functional Assessment of Cancer Therapy–Cognitive and Rapid Visual Processing (all P < .05), with similar patterns of objectively-measured cognitive function as controls. In patients, greater moderate-to-vigorous PA at the previous time point was significantly associated with better cognitive trajectories (all P < .05), and adherence to PA guidelines throughout chemotherapy was associated with better self-reported cognition (P < .01).
CONCLUSION
This nationwide study demonstrates that PA maintenance before and during chemotherapy is associated with better cognitive function immediately and 6 months after chemotherapy completion.
INTRODUCTION
Cancer-related cognitive decline (CRCD) is an important clinical problem. Upward of 75% of patients with breast cancer report cognitive problems during chemotherapy,1-5 and evidence suggests that CRCD may persist years after chemotherapy completion.6 The onset of CRCD contributes to an accelerated aging phenotype, increasing morbidity and, subsequently, mortality.7,8
CONTEXT
Key Objective
Cancer-related cognitive decline is an important clinical problem for which interventions are needed. Physical activity (PA) is a promising intervention; however, its use in patients undergoing active treatment is limited. This study sought to investigate timing and dose of PA in a large nationwide, prospective cohort study in patients with breast cancer as well as its relationship to cognitive function. To our knowledge, this is one of the largest analyses of PA and cognitive function before and after chemotherapy in patients with breast cancer.
Knowledge Generated
PA in patients declined during chemotherapy before recovering to pretreatment levels 6 months after chemotherapy completion; however, most patients remained insufficiently active. More PA prechemotherapy, and adhering to national PA guidelines during chemotherapy, were associated with better cognition over time.
Relevance
This study supports the use of PA before and during chemotherapy for maintaining cognitive function.
We previously reported on the impact of chemotherapy on cognition in patients with breast cancer compared with individuals without a cancer diagnosis serving as controls.1,9 This large, nationwide study examined trajectories of cognitive change from prechemotherapy (T1) to postchemotherapy (T2) and 6 months postchemotherapy completion (T3) in a cohort of 943 women with breast cancer and age- and sex-matched cancer-free controls. We showed that cognitive function declined in patients compared with controls from T1 to T31,9; domains of sustained attention (ie, Rapid Visual Processing [RVP]) and visual memory (ie, Delayed Match-to-Sample [DMS]) were among those revealing significant performance reductions in patients relative to controls, where patients either declined or improved less over time. Cognitive complaints (ie, Functional Assessment of Cancer Therapy–Cognitive [FACT-Cog]) among patients increased significantly compared with controls.1
Physical activity (PA) in cancer survivors has been consistently associated with improved health outcomes, including better physical function and quality of life, and improved survival.10,11 Although PA following primary treatments has shown the greatest evidence for benefits on CRCD,6,10,12-18 the American College of Sports Medicine and others have recommended more research on the timing and dose of PA10; studies focusing on PA before and during chemotherapy are lacking.12 It remains unclear whether PA before (ie, prehabilitation) or during chemotherapy may provide lasting cognitive benefits.19
To address these gaps, we conducted an analysis of PA and cognition in a cohort of patients with breast cancer and controls.1,9 We investigated the patterns and trajectories of PA in patients with breast cancer undergoing chemotherapy compared with age-matched, cancer-free controls. We then examined how prechemotherapy PA was associated with changes in cognitive functioning in both patients and controls, as well as how change in PA from prechemotherapy to postchemotherapy was associated with cognitive trajectories in patients only. We hypothesized that (1) patients would engage in lower levels of PA compared with controls, (2) PA levels in patients would decline from prechemotherapy to postchemotherapy, and (3) patients with high levels of prechemotherapy PA, and those who maintained high levels postchemotherapy, would demonstrate better cognitive functioning, comparable to controls, compared with those who were less active.
METHODS
Participants and Study Design
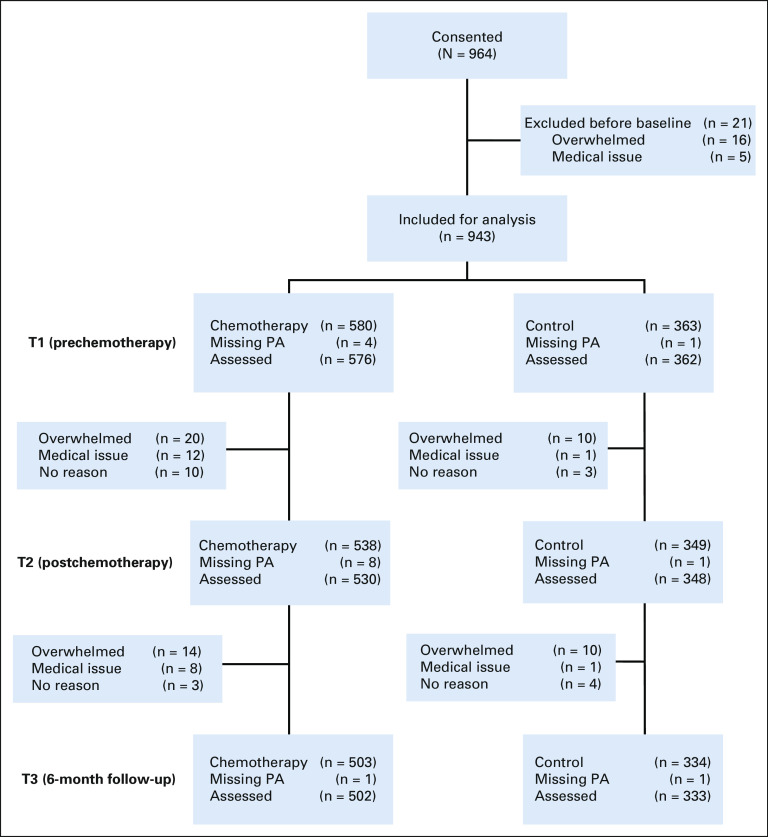
Details of the study design, sample size determination, and enrollment procedures have been previously reported.1,9 All available data were used in the current analysis. Herein, cancer survivors are women diagnosed with breast cancer, and are referred to as patients throughout, given their entry into the study began before receiving curative intent treatment. Participants were recruited from 22 NCI Community Oncology Research Program (NCORP) sites nationwide to participate in a longitudinal study assessing the impact of chemotherapy on cognitive function in female patients with breast cancer. Eligibility criteria for patients included female with stage I-IIIC disease, at least 21 years of age, ability to write and speak English, not pregnant, chemotherapy-naïve, scheduled for chemotherapy with no plan to receive concurrent radiation therapy during chemotherapy, free from central nervous system disease, no neurodegenerative disease, and no hospitalization because of major psychiatric illness within the last year. Controls were eligible if they were cancer-free, within 5 years of age of the matched patients, and met non–cancer-specific eligibility criteria. The study was approved by all institutional review boards at each NCORP site and the University of Rochester Cancer Center NCORP Research Base (ClinicalTrials.gov identifier: NCT01382082). All participants provided written informed consent.
Measurements were assessed prechemotherapy—within 7 days before the first chemotherapy administration (T1); postchemotherapy—within 1 month of the last chemotherapy administration (T2); and 6 months postchemotherapy—6 months after the final chemotherapy administration (T3) —and at equivalent time points for controls. Covariates were assessed once at T1.
Measures
Physical activity.
PA was assessed using the validated leisure time PA questionnaire from the Aerobics Center Longitudinal Study.20 Participants indicated the frequency of varying activities over the past 3 months, ranging from household activities to vigorous sports. When applicable, a statistician (E.C.) calculated intensity using reported frequency and distance. Two researchers (E.A.S. and A.S.K.) independently assigned metabolic equivalent of task (MET) hours for each activity classification based on the updated Physical Activity Compendium.21 Disagreements were discussed among all three researchers until consensus was achieved based on existing literature, erring on the side of conservative MET assignment. MET assignments then estimated the energy cost of individual activities: light-intensity (1.6-2.9 METs),22 moderate-intensity (3.0-5.9 METs), and vigorous-intensity (≥ 6.0 METs).22,23 Moderate-to-vigorous-intensity physical activity (MVPA) included all activities ≥ 3.0 METs. In line with the current Physical Activity Guidelines for Americans,23 MVPA was categorized to either meeting PA guidelines (≥ 150 min/wk MVPA) or not (< 150 min/wk MVPA). Analytic variables included index-specific minutes and MET h/wk, index-specific intensities (eg, exercise MVPA), and dichotomous meeting guidelines (yes or no).
Cognitive measures.
For this analysis, we selected a combination of validated objective and self-reported cognitive measures administered in person and previously identified as being significantly reduced in patients with breast cancer compared with controls across T1-T3 in this cohort.1,9 The FACT-Cog (version 2.0) Perceived Cognitive Impairment (PCI; assessing perceived impairments) and FACT-Cog Total Score (assessing all four domains) were studied.24 The RVP assessed sustained attention25,26 and the DMS assessed visual memory after a 12-second delay26—both from the CANTAB eclipse software (Cambridge Cognition, Cambridge, United Kingdom). Changes over time of more than 1/2 standard deviation of the FACT-Cog measured at baseline were considered to be clinically meaningful.1,27
Covariates.
Age, race, and menopausal status were self-reported by participants. Body mass index (BMI) was calculated using the standard kg/m2 calculation. Reading ability, a proxy for cognitive reserve, was assessed by the Wide Range Achievement Test, 4th Edition, reading subscale.28 Anxiety was assessed by the Spielberger State-Trait Anxiety Inventory State score.29 Depressive symptoms were assessed by a single item on the Multidimensional Fatigue Symptom Inventory.30 Chemotherapy regimens were categorized as anthracycline-containing or non–anthracycline-containing from clinic notes, based on previous anthracycline-cognition literature.31,32
Statistical Analysis
Physical activity patterns.
To investigate PA patterns and trajectories in patients with cancer compared with controls, we conducted t and χ2 tests. To account for the full trajectory of PA in patients and controls, we used longitudinal linear mixed-effects models (LMMs) with PA at T1, T2, and T3 as the outcome. Fixed effects were assessment (T1, T2, and T3 treated nominally), group (patient or control), and group × time interaction. The random effect (independent of residual error) was participant-specific PA at the three time points, represented as an unstructured covariance matrix. Estimation was performed using the restricted maximum likelihood method. Marginal adjusted means quantified the between-group difference (patients v control) at T1, T2, and T3, changes from T1 to T2 and T1 to T3, and between-group differences in these changes. Models were adjusted for age, race, BMI, menopausal status, cognitive reserve, anxiety, and depressive symptoms.
Physical activity and cognitive functioning in patients and controls over time.
We first explored how the PA-cognition association differed between patients and controls. Longitudinal LMMs estimated the association between prechemotherapy PA and change in cognitive functioning over time using three-way interactions between time (T1, T2, and T3), group (patients and controls), and PA (meeting guidelines at T1 and not meeting guidelines at T1), with cognition at T2 and T3 as outcome variables. Between-group differences in trajectories of cognitive tests were evaluated using linear contrasts (eg, patients meeting guidelines compared with controls not meeting guidelines). Analyses were adjusted for age, race, BMI, menopausal status, cognitive reserve, anxiety, and depressive symptoms.
Physical activity and cognitive functioning in patients only.
For patient-only analyses, we conducted repeated-measures longitudinal LMMs with minutes of MVPA activities at T1 and T2 as lagged time-varying covariates and cognition at T2 and T3 as outcomes. Then, we categorized patients into four groups based on change in meeting PA guidelines from T1 to T2 (never met guidelines, went from meeting to not meeting guidelines, went from not meeting to meeting guidelines, and always met guidelines). This four-level group variable, time (nominal T1, T2, T3), and group × time interaction were fixed effects in longitudinal LMMs with cognition at T1, T2, and T3 as the outcome. Between-group differences in trajectories of cognitive tests were evaluated using linear contrasts. Estimation used restricted maximum likelihood and assumed unstructured covariance matrices for the random effects. Analyses were adjusted for all previous covariates plus treatment type (anthracycline yes or no). The significance level for all models was set to 0.05, and analyses were conducted using SAS (SAS Institute, Cary, NC) and SPSS (IBM Corp, Chicago, IL).
RESULTS
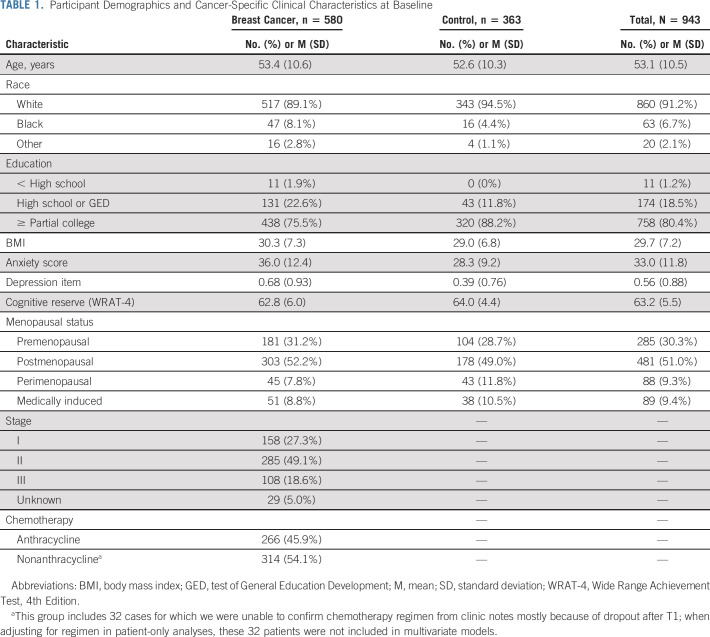
Participant characteristics are detailed in Table 1. Briefly, participants (N = 943) included 580 patients with breast cancer (53.4 ± 10.6 years) and 363 controls (52.6 ± 10.3 years) who provided complete cognitive data at each time point with minimal missing data (< 5%) on the PA questionnaire at each time point (Fig 1). Participants were mostly White (91.2%), completed at least some college training (80.4%), and postmenopausal (51.0%) at T1. Patients had mostly stage II disease (49.1%) and almost half had chemotherapy regimens containing anthracycline (45.9%).
TABLE 1.
Participant Demographics and Cancer-Specific Clinical Characteristics at Baseline
FIG 1.
CONSORT diagram. PA, physical activity.
PA Patterns
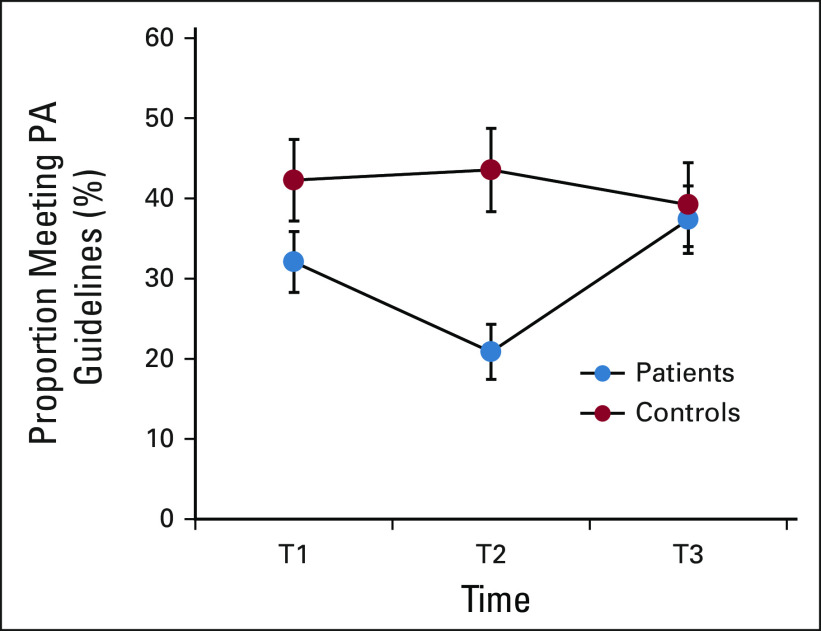
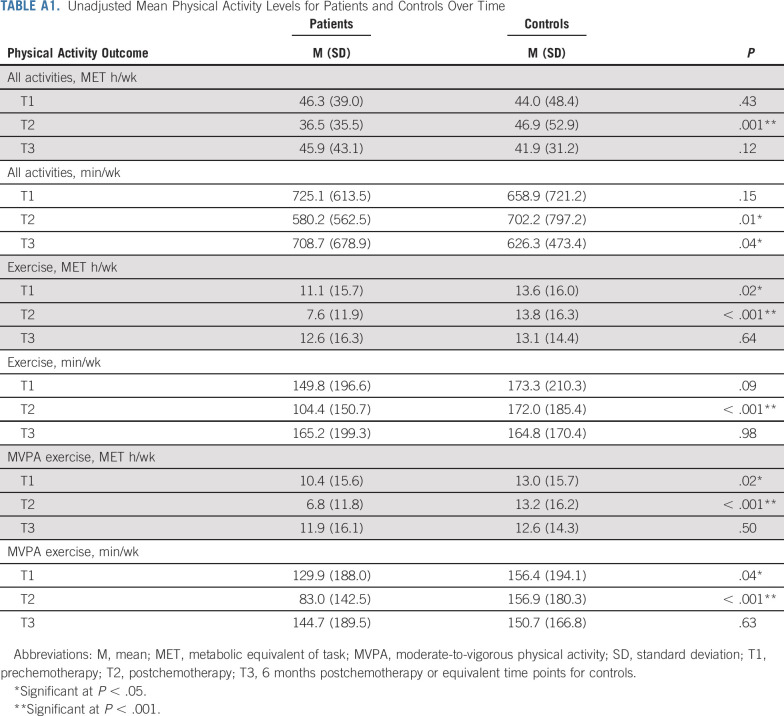
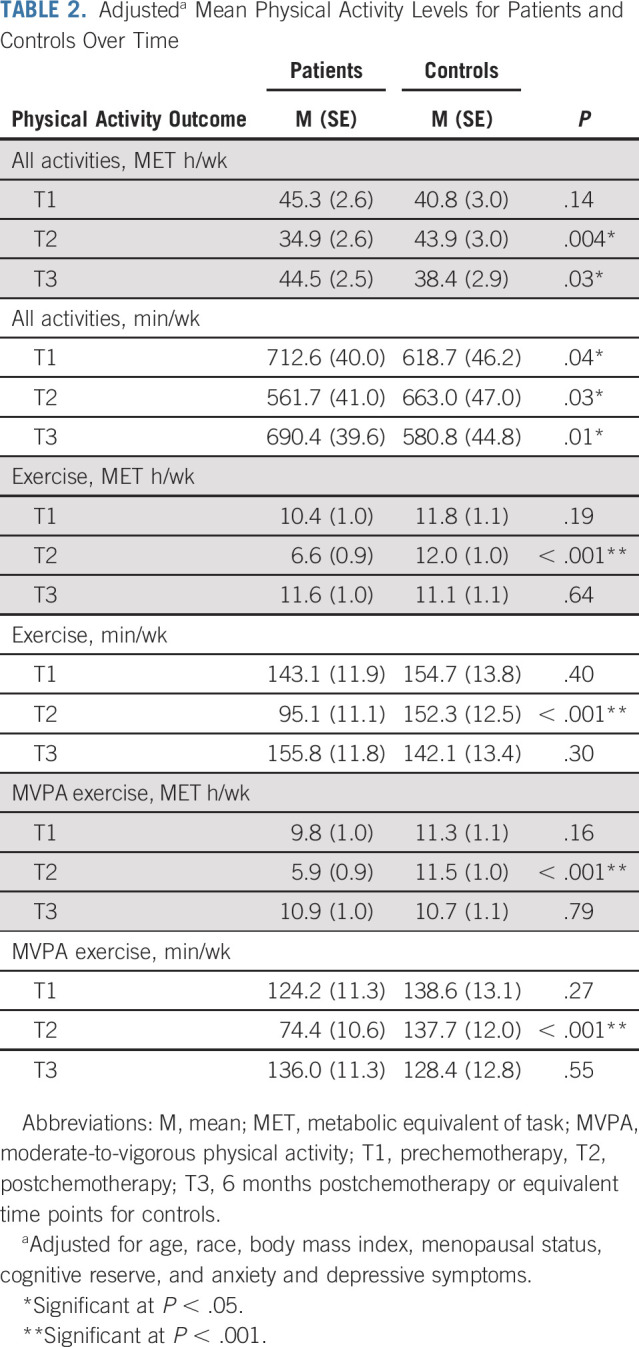
One third of patients with breast cancer met PA guidelines at T1, dropping to 21% at T2 before returning to 37% at T3. Figure 2 details the proportion of patients and controls meeting guidelines over time. At T1, patients reported an average of 11.1 ± 15.7 exercise MET h/wk and 10.4 ± 15.6 MVPA exercise MET h/wk, significantly lower than controls (both Ps = .02; Appendix Table A1 [online only, unadjusted]). These levels declined significantly in patients from T1 to T2 (both Ps < .001), remaining substantially lower than those in controls. Patients also reported significantly lower levels of total minutes and MET h/wk for all activities combined at T2 (all P < .01). Mean levels and group differences for all other PA outcomes are depicted in Table 2 (adjusted), Appendix Table A1, and Appendix Figure A1 (online only). Appendix Table A2 (online only) details the most common activities in patients and controls at T1. Longitudinal LMMs indicated several significant group × time interactions, confirming a decline in patients with breast cancer from T1 to T2 for all PA outcomes compared with controls (all P < .001). From T2 to T3, patients reported significantly increased PA across all outcomes compared with controls (all P < .03), with no significant difference in exercise levels between groups at T3 (all P > .64).
FIG 2.
Proportion of patients with cancer and controls meeting PA guidelines over time. Error bars represent 95% CIs. PA, physical activity; T1, prechemotherapy; T2, postchemotherapy; T3, 6 months postchemotherapy or equivalent time points for controls.
TABLE 2.
Adjusteda Mean Physical Activity Levels for Patients and Controls Over Time
PA and Cognitive Functioning in Patients and Controls Over Time
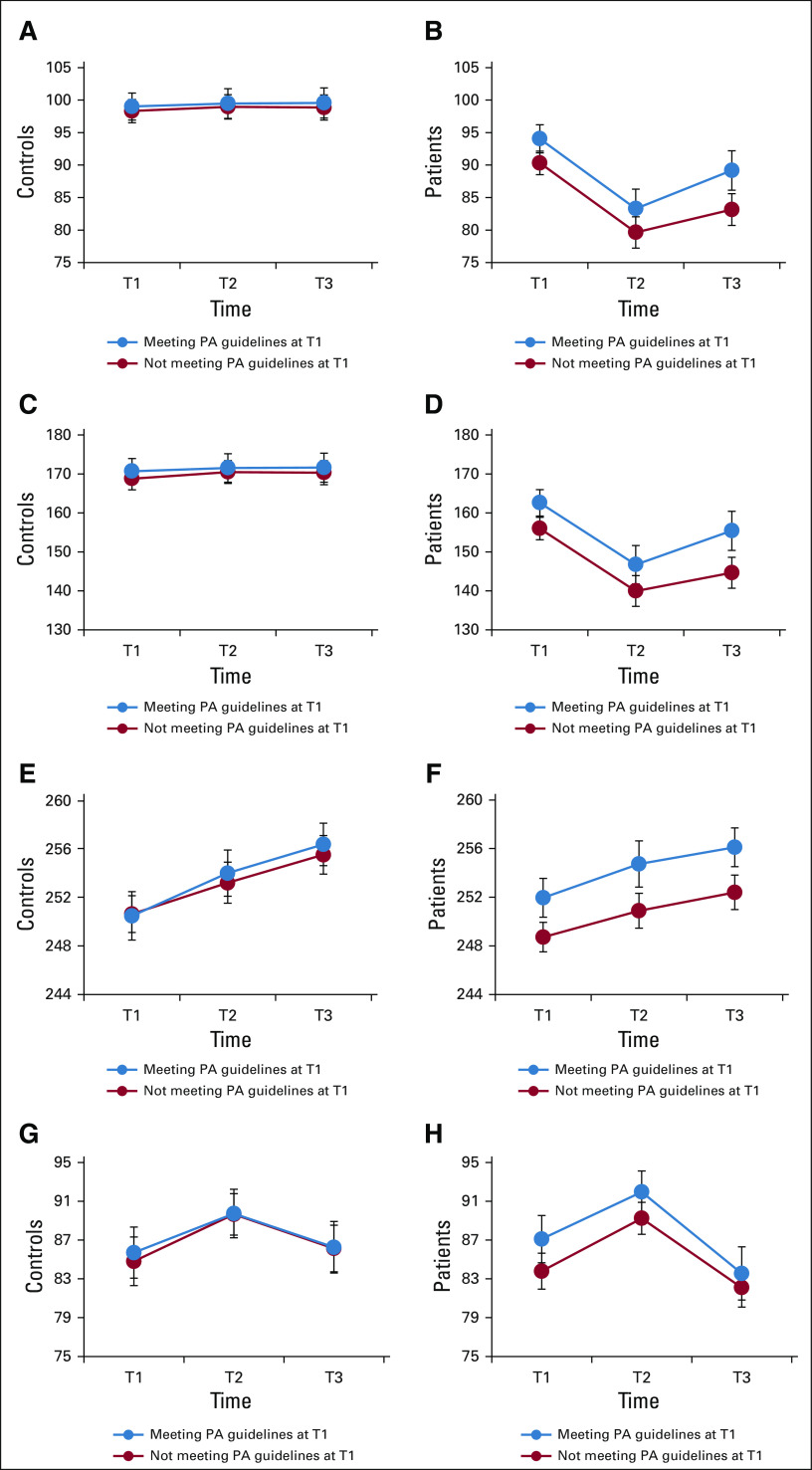
Unadjusted changes in cognitive functioning over time in patients and controls by meeting PA guidelines at baseline are detailed in Appendix Figure A2 (online only). Longitudinal LMM linear contrasts indicated that patients meeting national PA guidelines at baseline demonstrated comparable cognitive scores as controls who did not meet guidelines for RVP (β = −1.5, P = .14) and DMS (β = −1.2, P = .33). For the FACT-Cog PCI and Total scores, patients meeting PA guidelines at baseline scored lower (ie, worse function; than controls who did not meet guidelines, (β = 7.2, P < .001) and (β = 9.6, P < .001). Comparing patients and controls who both met PA guidelines at baseline, patients demonstrated comparable scores as controls on RVP and DMS, (β = −2.0, P = .07) and (β = −0.7, P = .57), respectively, but still reported more problems than controls on the FACT-Cog (Ps < .001). Similar findings were evident when comparing patients and controls who both did not meet PA guidelines at baseline (Appendix Fig A2).
PA and Cognitive Functioning in Patients Only
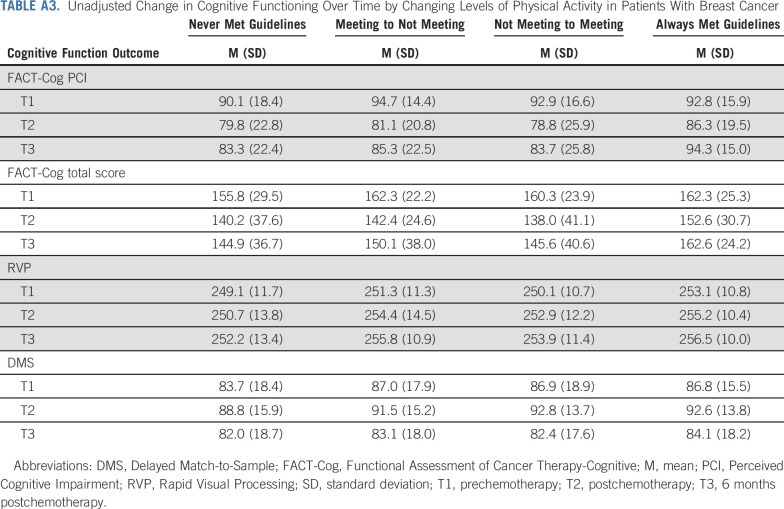
In patients only, meeting PA guidelines at baseline was associated with significantly higher scores (ie, better function or perceived function) on the FACT-Cog PCI (β = 2.7, P = .05), FACT-Cog Total (β = 4.9, P = .02), and RVP (β = 2.2, P = .01) over time, compared with patients not meeting PA guidelines at baseline. A similar pattern emerged for the DMS (β = 1.9, P = .07), albeit not statistically significant. Longitudinal LMMs with PA as a lagged time-varying covariate indicated that more minutes of MVPA measured at the previous time point was significantly associated with better cognitive function trajectories on the FACT-Cog PCI (P = .01), FACT-Cog Total (P = .04), and RVP (P = .05). No significant associations emerged between all activities and cognition (all P > .23). Assessing the association between change in meeting PA guidelines from T1 to T2, 316 patients never met guidelines, 102 patients went from meeting to not meeting guidelines, 36 patients went from not meeting to meeting guidelines, and 76 patients always met guidelines (Fig 3 and Appendix Table A3, online only). On the FACT-Cog, patients who maintained guidelines demonstrated better cognitive function scores at T2 (P = .01), better cognitive recovery at T3 (T1 v T3 scores, P = .97), and a higher mean score at T3 compared with the other three groups (P < .01). Importantly, this group did not experience a clinically meaningful decline on the FACT-Cog from T1 to T3, as assessed by effect size (ES; difference in scores standardized to the baseline score standard deviation; PCI ES = 0.08, P = .57; Total ES = −0.06, P = .97). On the RVP, patients who met guidelines at T1 scored higher at all three time points regardless of meeting guidelines at T2 (P = .05). On the DMS, patients who never met guidelines performed worse than the other groups (P = .12). Indeed, patients who never met guidelines experienced clinically meaningful declines on the FACT-Cog (PCI ES = −0.45, P < .001; Total ES = −0.46, P < .001).
FIG 3.
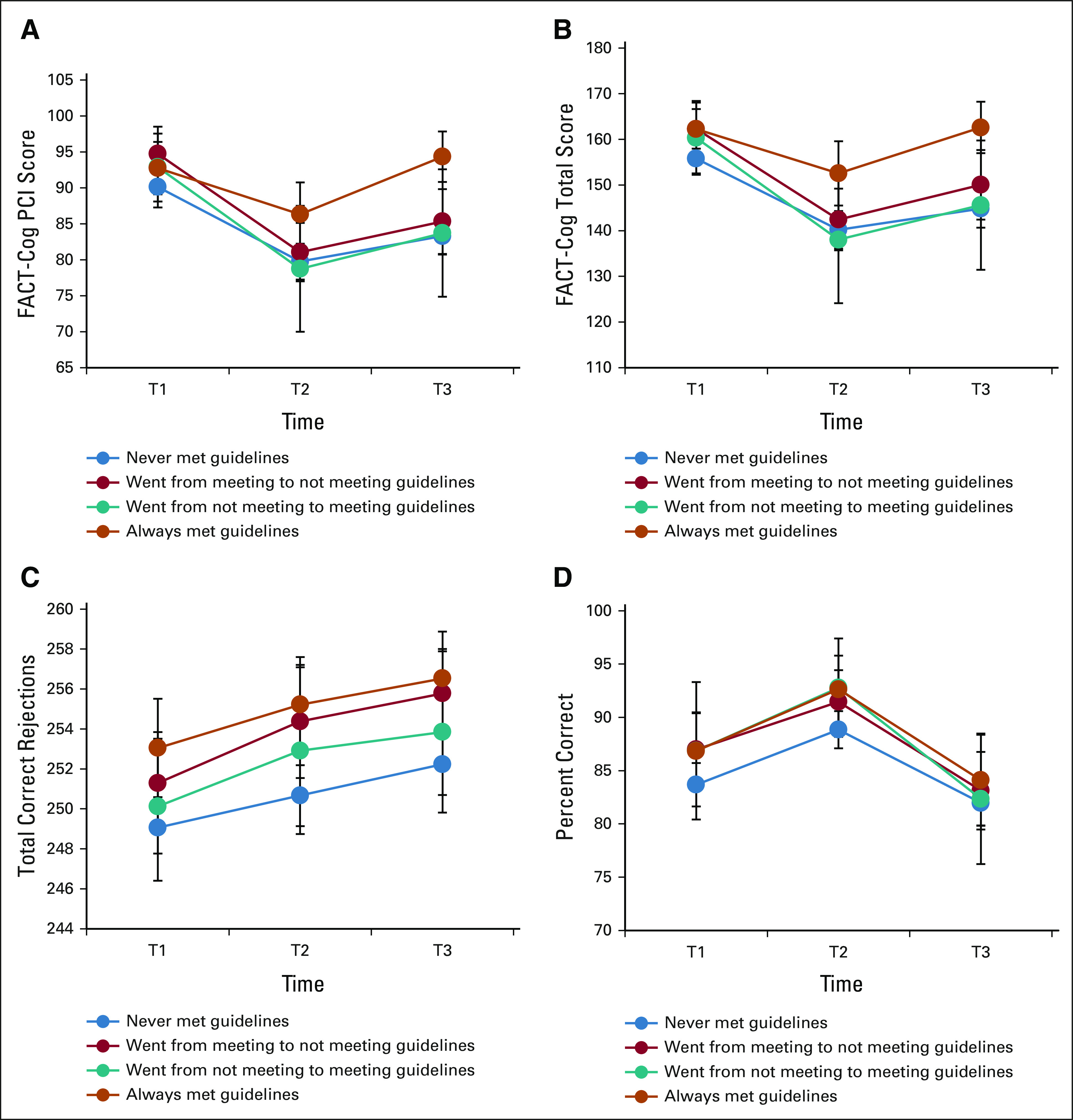
Change in cognitive functioning over time by changing levels of PA in patients with breast cancer. (A) FACT-Cog PCI, (B) FACT-Cog total score, (C) RVP, and (D) DMS. Error bars represent 95% CIs, unadjusted data, higher scores = better function. DMS, Delayed Match-to-Sample; FACT-Cog, Functional Assessment of Cancer Therapy–Cognitive; PCI, Perceived Cognitive Impairment; RVP, Rapid Visual Processing; T1, prechemotherapy; T2, postchemotherapy; T3, 6 months postchemotherapy.
DISCUSSION
To our knowledge, this study provides one of the largest assessments of PA and cognitive function before and after chemotherapy in patients with breast cancer. Our results suggest that chemotherapy negatively affects the ability to maintain optimal PA levels, and higher levels of PA before initiating chemotherapy are associated with better perceived and objectively assessed cognitive functioning after chemotherapy completion. Although patients in the current sample returned to prechemotherapy PA levels 6 months postchemotherapy, more than two thirds of patients still did not meet PA guidelines. Importantly, patients who met PA guidelines pretreatment had comparable cognitive scores, at least for certain domains, as their cancer-free peers. Our results suggest that maintaining higher levels of MVPA from prechemotherapy to postchemotherapy, or at least meeting national PA guidelines before treatment, may assist in alleviating CRCD.
Our finding that PA significantly declined during chemotherapy is consistent with the documented detrimental effects of chemotherapy on lifestyle behaviors after cancer.33-36 Most of this work has been conducted cross-sectionally,35,36 with time frames centered around diagnosis rather than treatment,33,34 and without comparison to appropriate controls. This large, longitudinal cohort allowed us to determine how PA levels changed before, during, and acutely postchemotherapy. Despite a recovery to baseline activity 6 months after chemotherapy completion, 63% of patients were insufficiently active, emphasizing the need to encourage activity regardless of time on or off chemotherapy. Further understanding of behavioral factors underlying this PA recovery (eg, renewed motivation or postchemotherapy improved health status) will be important to shape behavioral interventions in this population.
Despite the low proportion of patients meeting PA guidelines and exercising prechemotherapy, those who did had substantially better cognitive outcomes after chemotherapy. Few observational studies have examined the association between PA and cognitive functioning in patients with cancer,12 and most were conducted in small samples37 or after chemotherapy completion.38-40 Our findings reveal the importance of prechemotherapy PA on changes in cognitive function after chemotherapy in more than 500 patients with breast cancer. Future work should explore whether this association is the result of an epidemiologic relationship between precancer PA accumulated across the lifespan and cognition, or rather if increasing PA within a discrete time window before treatment (eg, prehabilitation) can effectively reduce CRCD. Further exploration of prechemotherapy PA change is warranted.12,19,41,42
The importance of prechemotherapy PA is underscored by our finding of comparable scores on objective measures of cognition (eg, RVP and DMS) over time between patients who met PA guidelines at baseline and controls. These associations did not emerge for self-reported cognition, where active patients still scored lower than controls on the FACT-Cog. Many self-reported cognitive measures, such as the FACT-Cog, are multidimensional and capture additional psychosocial and emotional symptoms,43,44 which may explain this discrepancy. The fact that active patients scored similarly to both inactive and active controls on objective measures of cognitive functioning suggests that PA may help attenuate accelerated cognitive aging. These results point to potential aging-specific mechanisms that warrant further exploration.
The robust PA-cognition relationship persisted in our analysis of patients only. Lagged analyses indicated that higher levels of recent PA were associated with better cognition for most cognitive domains assessed. This finding was consistent over time, emphasizing the importance of PA across the entire chemotherapy window. Patients who adhered to PA guidelines from prechemotherapy to postchemotherapy had the highest cognitive functioning across domains with no clinically meaningful declines in self-reported cognitive functioning. Regardless of PA change during treatment, those who met guidelines prechemotherapy had the highest scores on attention. Those who never met guidelines scored worse on visual memory than all other groups. These findings demonstrate the importance of PA both before and during chemotherapy for preventing meaningful declines in self-reported cognition. However, it remains unclear as to whether increasing PA during chemotherapy can improve cognition; only 36 patients increased their PA levels during chemotherapy. Further randomized controlled trials in this space are warranted. MVPA demonstrated the most robust associations with cognition, confirming the need to focus on PA dosing when designing such trials in cancer.10
Our findings should be interpreted in the context of their strengths and limitations. This study was conducted in a large, nationwide longitudinal sample within the NCORP network. We used multiple well-validated measures of cognitive function that have previously been identified as impaired by chemotherapy,1,9 across the treatment window. The use of age-matched, cancer-free controls allowed for comparisons to the general population. We acknowledge that PA was self-reported, which may introduce recall or social desirability biases45; however, this cost-effective method surveyed a large cohort over time during a challenging life phase (cancer treatment) and captured contextual PA information. Both PA and cognitive function were assessed simultaneously in this sample. Although our statistical analyses accounted for and maintained temporality, it is possible that patients with better executive functioning and self-regulation were more likely to engage in PA behavior.46
In summary, patients with breast cancer resumed their pretreatment PA levels 6 months postchemotherapy; however, a majority of patients remained insufficiently active. Importantly, PA before and during chemotherapy was associated with better cognitive outcomes. Promoting PA across the treatment continuum should continue, and further research should focus on how PA both before and during chemotherapy may minimize CRCD.
ACKNOWLEDGMENT
The authors thank the participants of this study and all staff at the University of Rochester Cancer Center NCORP Research Base and NCORP affiliate sites that recruited and observed participants. The authors thank the National Cancer Institute Clinical Community Oncology Program (CCOP) and NCORP programs for their funding and support of this project. The authors thank Dr Sue Rosenthal for providing feedback on this manuscript.
Appendix
FIG A1.
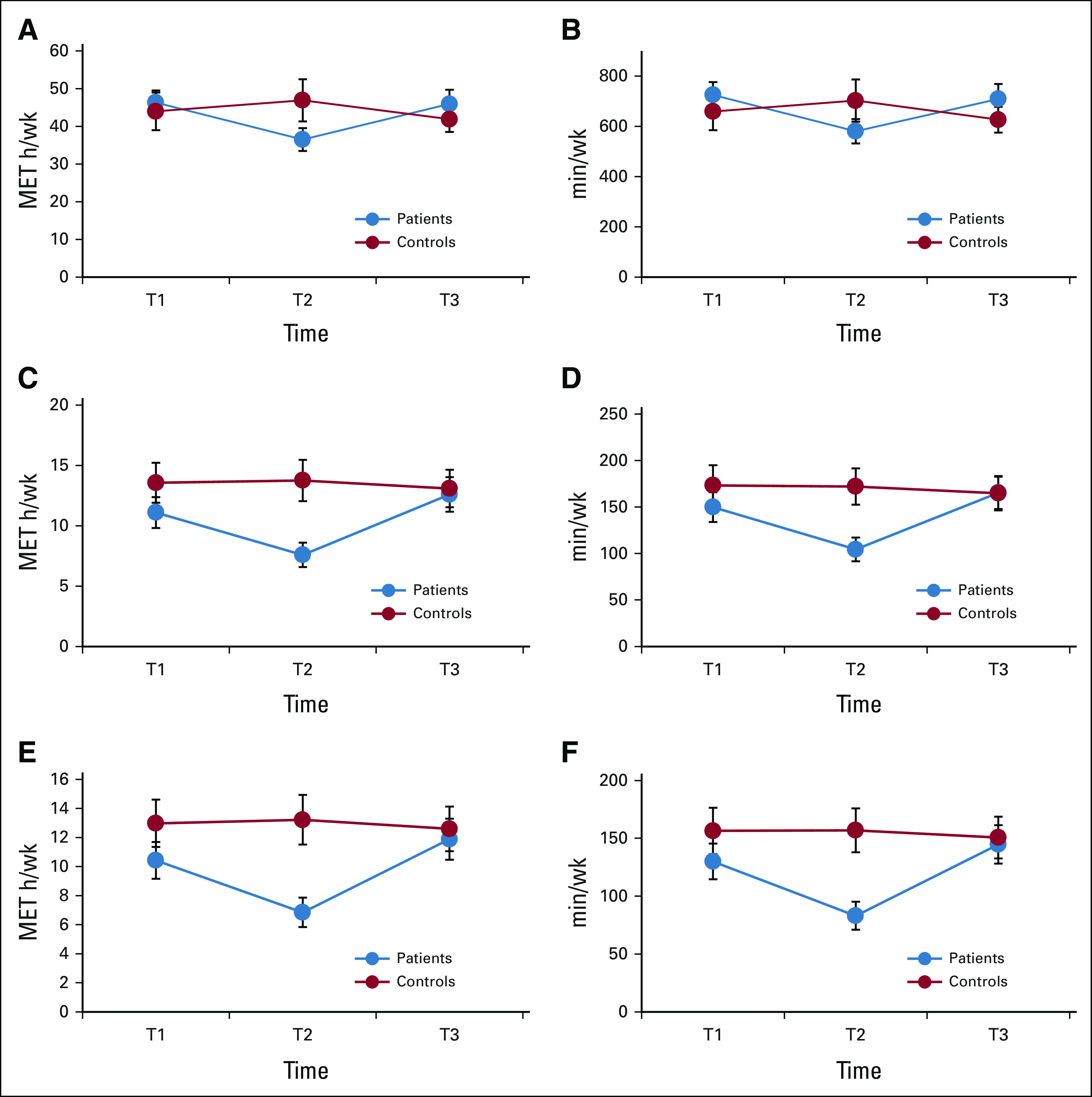
PA levels for patients and controls over time: (A and B) all activities, (C and D) exercise, and (E and F) moderate-to-vigorous physical activity. Error bars represent 95% CIs. MET, metabolic equivalent of task; T1, prechemotherapy; T2, postchemotherapy; T3, 6 months postchemotherapy or equivalent time points for controls.
FIG A2.
Change in cognitive functioning over time for patients and controls, by meeting PA guidelines at baseline: (A and B) FACT-Cog: perceived cognitive impairment (PCI), (C and D) FACT-Cog: total score, (E and F) rapid visual processing (RVP), and (G and H) delayed match to sample (DMS). Error bars represent 95% CIs, unadjusted data, higher scores = better function. DMS, Delayed Match-to-Sample; FACT-Cog, Functional Assessment of Cancer Therapy–Cognitive; PA, physical activity; PCI, Perceived Cognitive Impairment; RVP, Rapid Visual Processing; T1, prechemotherapy; T2, postchemotherapy; T3, 6 months postchemotherapy or equivalent time points for controls.
TABLE A1.
Unadjusted Mean Physical Activity Levels for Patients and Controls Over Time
TABLE A2.
Most Common Activities at T1 in Patients and Cancer-Free Controls
TABLE A3.
Unadjusted Change in Cognitive Functioning Over Time by Changing Levels of Physical Activity in Patients With Breast Cancer
Alison Conlin
Consulting or Advisory Role: Pfizer, Novartis, Seattle Genetics/Astellas, BioTheranostics
Speakers' Bureau: Seagen
Jerry Mitchell
Employment: Immunomedics, Amgen, Seattle Genetics, Foundation Medicine
Stock and Other Ownership Interests: Seattle Genetics
Consulting or Advisory Role: Regeneron, BioTheranostics, Rigel
Speakers' Bureau: Sun Pharma, Regeneron
Michelle C. Janelsins
Consulting or Advisory Role: Charles River Analytics
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented in part as a poster at the International Cognition and Cancer Task Force (ICCTF) meeting, February 4, 2020, Denver, CO.
SUPPORT
Supported by National Cancer Institute Grants No. U10CA037420 Supplement, UG1CA189961, DP2195765, K07CA168886, T32CA102618, and R01CA231014. E.A.S. was supported by the Cancer Prevention Fellowship Program (National Cancer Institute, National Institutes of Health) from 2017-2020.
AUTHOR CONTRIBUTIONS
Conception and design: Elizabeth A. Salerno, Eva Culakova, Michelle C. Janelsins
Financial support: Karen M. Mustian, Michelle C. Janelsins
Administrative support: Charles E. Matthews, Michelle C. Janelsins
Provision of study materials or patients: Alison Conlin, Lora Weiselberg, Jerry Mitchell, Michelle C. Janelsins
Collection and assembly of data: Eva Culakova, Amber S. Kleckner, Charles E. Heckler, Alison Conlin, Lora Weiselberg, Jerry Mitchell, Karen M. Mustian, Michelle C. Janelsins
Data analysis and interpretation: Elizabeth A. Salerno, Eva Culakova, Amber S. Kleckner, Charles E. Heckler, Po-Ju Lin, Charles E. Matthews, Karen M. Mustian, Michelle C. Janelsins
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Physical Activity Patterns and Relationships With Cognitive Function in Patients With Breast Cancer Before, During, and After Chemotherapy in a Prospective, Nationwide Study
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Alison Conlin
Consulting or Advisory Role: Pfizer, Novartis, Seattle Genetics/Astellas, BioTheranostics
Speakers' Bureau: Seagen
Jerry Mitchell
Employment: Immunomedics, Amgen, Seattle Genetics, Foundation Medicine
Stock and Other Ownership Interests: Seattle Genetics
Consulting or Advisory Role: Regeneron, BioTheranostics, Rigel
Speakers' Bureau: Sun Pharma, Regeneron
Michelle C. Janelsins
Consulting or Advisory Role: Charles River Analytics
No other potential conflicts of interest were reported.
REFERENCES
- 1.Janelsins MC, Heckler CE, Peppone LJ, et al. : Cognitive complaints in survivors of breast cancer after chemotherapy compared with age-matched controls: An analysis from a nationwide, multicenter, prospective longitudinal study. J Clin Oncol 35:506-514, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brezden CB, Phillips K-A, Abdolell M, et al. : Cognitive function in breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol 18:2695-2701, 2000 [DOI] [PubMed] [Google Scholar]
- 3.Wefel JS, Lenzi R, Theriault RL, et al. : The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: Results of a prospective, randomized, longitudinal trial. Cancer 100:2292-2299, 2004 [DOI] [PubMed] [Google Scholar]
- 4.van Dam FS, Schagen SB, Muller MJ, et al. : Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: High-dose versus standard-dose chemotherapy. J Natl Cancer Inst 90:210-218, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Pendergrass JC, Targum SD, Harrison JE: Cognitive impairment associated with cancer: A brief review. Innov Clin Neurosci 15:36-44, 2018 [PMC free article] [PubMed] [Google Scholar]
- 6.Janelsins MC, Kesler SR, Ahles TA, et al. : Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry 26:102-113, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mandelblatt JS, Hurria A, McDonald BC, et al. : Cognitive effects of cancer and its treatments at the intersection of aging: What do we know; what do we need to know? Semin Oncol 40:709-725, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahles TA, Root JC, Ryan EL: Cancer- and cancer treatment-associated cognitive change: An update on the state of the science. J Clin Oncol 30:3675-3686, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Janelsins MC, Heckler CE, Peppone LJ, et al. : Longitudinal trajectory and characterization of cancer-related cognitive impairment in a nationwide cohort study. J Clin Oncol 36:3231-3239, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell KL, Winters-stone KM, Wiskemann J, et al. : Exercise guidelines for cancer survivors: Consensus statement from international multidisciplinary roundtable exercise guidelines for cancer survivors: Consensus statement from international multidisciplinary roundtable SPECIAL COMMUNICATIONS. Med Sci Sport Exerc 51:2375-2390, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel AV, Friedenreich CM, Moore SC, et al. : Introduction: The American College of Sports Medicine convened an inter-national multidisciplinary roundtable on exercise. Med Roundtable Rep Phys Act 51:2391-2402, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zimmer P, Baumann FT, Oberste M, et al. : Effects of exercise interventions and physical activity behavior on cancer related cognitive impairments: A systematic review. Biomed Res Int 2016:1820954, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salerno EA, Rowland K, Kramer AF, et al. : Acute aerobic exercise effects on cognitive function in breast cancer survivors: A randomized crossover trial. BMC Cancer 19:371, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Von Ah D, Jansen CE, Allen DH: Evidence-based interventions for cancer- and treatment-related cognitive impairment. Clin J Oncol Nurs 18:17-25, 2014 [DOI] [PubMed] [Google Scholar]
- 15.Mustian KM, Sprod LK, Janelsins M, et al. : Exercise recommendations for cancer-related fatigue, cognitive impairment, sleep problems, depression, pain, anxiety, and physical dysfunction: A review. Oncol Hematol Rev 8:81-88, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pergolotti M, Battisti NML, Padgett L, et al. : Embracing the complexity: Older adults with cancer-related cognitive impairment—A Young International Society of Geriatric Oncology position paper. J Geriatr Oncol 11:237-243, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mustian KM, Janelsins MC, Peppone LJ, et al. : EXCAP exercise effects on cognitive impairment and inflammation: A URCC NCORP RCT in 479 cancer patients. J Clin Oncol 33, 2015. (suppl; abstr 9504) [Google Scholar]
- 18.Northey JM, Cherbuin N, Pumpa KL, et al. : Exercise interventions for cognitive function in adults older than 50: A systematic review with meta-analysis. Br J Sports Med 52:154-160, 2018 [DOI] [PubMed] [Google Scholar]
- 19.Silver JK, Baima J: Cancer prehabilitation. Am J Phys Med Rehabil 92:715-727, 2013 [DOI] [PubMed] [Google Scholar]
- 20.Kohl HW, Blair SN, Paffenbarger RS, et al. : The aerobics center longitudinal study physical activity questionnaire. Med Sci Sport Exerc 29:S10-S14, 1997. (6 suppl) [Google Scholar]
- 21.Ainsworth BE, Haskell WL, Herrmann SD, et al. : Compendium of physical activities: A second update of codes and MET values. Med Sci Sport Exerc 43:1575-1581, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Tudor-Locke C, Washington TL, Ainsworth BE, et al. : Linking the American Time Use Survey (ATUS) and the compendium of physical activities: Methods and rationale. J Phys Act Heal 6:347-353, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Piercy KL, Troiano RP, Ballard RM, et al. : The physical activity guidelines for Americans. JAMA 320:2020-2028, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner LI, Sweet J, Butt Z, et al. : Measuring patient self-reported cognitive function: Development of the functional assessment of cancer therapy—Cognitive function instrument. J Support Oncol 7:W32-W39, 2009 [Google Scholar]
- 25.Fray PJ, Robbins TW: CANTAB battery: Proposed utility in neurotoxicology. Neurotoxicol Teratol 18:499-504, 1996 [DOI] [PubMed] [Google Scholar]
- 26.Robbins TW, James M, Owen AM, et al. : Cambridge Neuropsychological Test Automated Battery (CANTAB): A factor analytic study of a large sample of normal elderly volunteers. Dement Geriatr Cogn Disord 5:266-281, 1994 [DOI] [PubMed] [Google Scholar]
- 27.Cheung YT, Foo YL, Shwe M, et al. : Minimal clinically important difference (MCID) for the functional assessment of cancer therapy: Cognitive function (FACT-Cog) in breast cancer patients. J Clin Epidemiol 67:811-820, 2014 [DOI] [PubMed] [Google Scholar]
- 28.Wilkinson GS, Robertson GJ: WRAT 4: Wide Range Achievement Test. Lutz, FL, Psychological Assessment Resources, 2006 [Google Scholar]
- 29.Spielberger C, Sydeman S, Owen A, et al. : Measuring anxiety and anger with the state-trait anxiety inventory (STAI) and the state-trait anger expression inventory (STAXI). Mahwah, NJ, Lawrence Erlbaum Associates Publishers, 1999 [Google Scholar]
- 30.Mendoza TR, Wang XS, Cleeland CS, et al. : The rapid assessment of fatigue severity in cancer patients. Cancer 85:1186-1196, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Janelsins MC, Mustian KM, Palesh OG, et al. : Differential expression of cytokines in breast cancer patients receiving different chemotherapies: Implications for cognitive impairment research. Support Care Cancer 20:831-839, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kesler SR, Blayney DW: Neurotoxic effects of anthracycline- vs nonanthracycline-based chemotherapy on cognition in breast cancer survivors. JAMA Oncol 2:185-192, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kwan ML, Sternfeld B, Ergas IJ, et al. : Change in physical activity during active treatment in a prospective study of breast cancer survivors. Breast Cancer Res Treat 131:679-690, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnsson A, Johnsson A, Johansson K: Physical activity during and after adjuvant chemotherapy in patients with breast cancer. Physiotherapy 99:221-227, 2013 [DOI] [PubMed] [Google Scholar]
- 35.Lahart I, Metsios G, Nevill A, et al. : Physical activity levels in women attending breast screening, receiving chemotherapy and post-breast cancer treatment: A cross-sectional study. Int J Environ Res Public Health 11:5487-5496, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Midtgaard J, Baadsgaard MT, Møller T, et al. : Self-reported physical activity behaviour; exercise motivation and information among Danish adult cancer patients undergoing chemotherapy. Eur J Oncol Nurs 13:116-121, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Fitzpatrick TR, Edgar L, Holcroft C: Assessing the relationship between physical fitness activities, cognitive health, and quality of life among older cancer survivors. J Psychosoc Oncol 30:556-572, 2012 [DOI] [PubMed] [Google Scholar]
- 38.Crowgey T, Peters KB, Hornsby WE, et al. : Relationship between exercise behavior, cardiorespiratory fitness, and cognitive function in early breast cancer patients treated with doxorubicin-containing chemotherapy: A pilot study. Appl Physiol Nutr Metab 39:724-729, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hartman SJ, Marinac CR, Natarajan L, et al. : Lifestyle factors associated with cognitive functioning in breast cancer survivors. Psychooncology 24:669-675, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marinac CR, Godbole S, Kerr J, et al. : Objectively measured physical activity and cognitive functioning in breast cancer survivors. J Cancer Surviv 9:230-238, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stout NL, Silver JK, Baima J, et al. : Prehabilitation: An emerging standard in exercise Oncology, in Exercise Oncology. Switzerland AG, Springer International Publishing, 2020, pp 111-143 [Google Scholar]
- 42.Campbell KL, Zadravec K, Bland KA, et al. : The effect of exercise on cancer-related cognitive impairment and applications for physical therapy: Systematic review of randomized controlled trials. Phys Ther 100:523-542, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hutchinson AD, Hosking JR, Kichenadasse G, et al. : Objective and subjective cognitive impairment following chemotherapy for cancer: A systematic review. Cancer Treat Rev 38:926-934, 2012 [DOI] [PubMed] [Google Scholar]
- 44.Costa DSJ, Fardell JE: Why are objective and perceived cognitive function weakly correlated in patients with cancer? J Clin Oncol 37:1154-1158, 2019 [DOI] [PubMed] [Google Scholar]
- 45.Sallis JF, Saelens BE: Assessment of physical activity by self-report: Status, limitations, and future directions. Res Q Exerc Sport 71:1-14, 2000. (suppl 2) [DOI] [PubMed] [Google Scholar]
- 46.Daly M, McMinn D, Allan JL: A bidirectional relationship between physical activity and executive function in older adults. Front Hum Neurosci 8:1044, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]