Abstract
Background:
Biological therapy is effective for the treatment of psoriasis and psoriatic arthritis; however, adverse effects related to immunosuppression, such as viral infections, have been reported. Amongst these infections, herpes zoster (HZ) is common.
Objective:
To evaluate the risk of HZ in psoriasis and psoriatic arthritis patients treated with biological therapy.
Data sources:
A comprehensive literature search of PubMed, Embase, and Web of Science was performed using certain keywords until October 9, 2020. Nine studies were included after a detailed assessment.
Study eligibility criteria:
The eligibility criteria included randomized controlled trials (RCTs) and observational studies of patients with psoriasis or psoriatic arthritis treated with biological therapies; compared with non-biological therapies, non-biological systemic therapies, or controls; with the incidence of HZ reported in case and control groups. The Cochrane risk of bias tool and Newcastle-Ottawa scale were used to assess the quality of the RCTs and observational studies, respectively. Data were extracted from 9 eligible studies and then analyzed using Stata software (Version 12.0).
Results:
The risk of HZ in biological therapies was higher than that in non-biological (odds ratios [OR]: 1.48; 95% confidence interval [CI]: 1.18–1.86; I2 = 0%) and non-biological systemic (OR: 1.32; 95% CI: 1.02–1.71; I2 = 0%) therapies. Furthermore, the risk of HZ associated with tumor necrosis factor-α inhibitors increased significantly (OR: 1.50; 95% CI: 1.11–2.02; I2 = 0%). Notably, infliximab (OR: 2.43; 95% CI: 1.31–4.50; I2 = 0%) and etanercept (OR: 1.65; 95% CI: 1.07–2.56; I2 = 0%) increased the risk of HZ, while adalimumab (OR: 1.21; 95% CI: 0.64–2.30; I2 = 0%), ustekinumab (OR: 2.20; 95% CI: 0.89–5.44; I2 = 0%), alefacept (OR: 1.46; 95% CI: 0.20–10.47; I2 = 0%), and efalizumab (OR: 1.58; 95% CI: 0.22–11.34; I2 = 0%) did not.
Limitations:
Few RCTs have reported HZ incidents; thus, our results require confirmation via large-scale RCTs.
Conclusions and implications of key findings:
Biological therapies, especially tumor necrosis factor-α inhibitors, may lead to the risk of HZ in psoriasis and psoriatic arthritis patients. Amongst these agents, infliximab and etanercept have been shown to significantly increase the risk of HZ. Additionally, younger age and female sex may be risk factors.
Systematic review registration number:
INPLASY202110027.
Keywords: biological therapy, herpes zoster, psoriasis, psoriatic arthritis, risk
1. Introduction
Psoriasis is a common chronic skin disease affecting many people worldwide, while psoriatic arthritis is traditionally associated with it as a comorbidity.[1] The overall prevalence of psoriasis is 2% to 3% of the world's population and it is difficult to cure, which has greatly increased its burden on a country's health care economy.[2] Fortunately, the development of biological drugs has brought new hope for psoriasis patients. In recent years, many studies have reported that biological therapies greatly improved clinical responses in psoriasis and psoriatic arthritis patients[3]; adalimumab, etanercept, infliximab, alefacept, efalizumab, and ustekinumab are representative drugs.[3] “Non-biological systemic therapies,” such as cyclosporine, methotrexate (MTX), and conventional disease-modifying antirheumatic drugs (c-DMARDs), are the most commonly used systemic drugs worldwide.[4] The efficacy of biological drugs for patients has indeed improved, but their safety is always a concern. While some scholars have claimed that biological drugs could offer the same safety as non-biological systemic therapies when administered long term,[5–7] others have shown that they increased the risk of infections.[4,8] Therefore, the safety profile of biological therapy requires further investigation.
Herpes zoster (HZ) is one of the viral infections potentially caused by biological therapy.[9] Compared with bacterial infections, little is known about the risk of HZ in psoriasis and psoriatic arthritis patients treated with biological drugs; however, the lifetime risk of HZ is estimated at 10% to 20% in the general population.[10] The overall incidence rates for HZ in psoriasis and non-psoriasis patients are 4.50 and 3.44 per 1000 person-years, respectively.[11,12] Moreover, postherpetic neuralgia is a complication of HZ that usually occurs in immunocompromised patients, which seriously affects their quality of life.[13] With the wide application of biological therapy, the risk of HZ infection in psoriasis and psoriatic arthritis patients has received more attention.
Several studies have reported that the risk of HZ was associated with biologic drugs in psoriasis and psoriatic arthritis patients[9,10,14–20]; however, the results are contradictory, and statistical significance was often not determined due to the low incidence of HZ. We therefore conducted a systematic review and meta-analysis of published studies to evaluate the association between biologic drugs and HZ in psoriasis and psoriatic arthritis patients.
2. Methods
This meta-analysis was reported according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines,[21] and this study did not involve the patients’ private information, so this meta-analysis did not require ethical approval. The systematic review registration number is INPLASY202110027.
2.1. Literature search strategy
We searched for articles published before October 9, 2020, in the following electronic databases: PubMed, Embase, and Web of Science. Searches were limited to human studies and English-language publications. The search terms included (“adalimumab,” “etanercept,” “infliximab,” “golimumab,” “alefacept,” “efalizumab,” “rituximab,” “ustekinumab,” “ixekizumab,” “secukinumab,” “brodalumab,” “guselkumab,” “biological therapy”) and (“HZ,” “herpes zoster,” “shingles”) and (“psoriasis”) or (“psoriatic arthritis”). Additionally, we performed a manual search by reviewing the reference lists of all included studies.
2.2. Inclusion criteria
Studies were included if they met the following criteria: subjects, including patients with psoriasis or psoriatic arthritis; interventions, including biological therapies (adalimumab, etanercept, infliximab, alefacept, efalizumab, ustekinumab, etc); comparators, including non-biological therapies, non-biological systemic therapies, or controls; outcomes, including studies reporting the incidence of HZ in case and control groups; and study designs, including case-control, cohort, or cross-sectional studies.
2.3. Exclusion criteria
Studies were excluded if they were classified as follows: in vitro or animal studies; non-English articles; studies that did not report cases of HZ infection; and reviews, case reports, theses, or conference abstracts.
2.4. Data extraction
The data were extracted from the included studies as follows: first author's name, year of publication, study design, total number of subjects, general characteristics of patients (disease, age, and sex), treatment duration, follow-up duration, number of patients in each treatment group or per drug, and number of HZ cases in each treatment group or per drug. Data were extracted independently by 2 authors (ZAL and CYJ); any discrepancy was resolved by the other authors (SN and YY).
2.5. Quality assessment
We independently assessed the quality of randomized controlled trials (RCTs) and non-RCTs (retrospective studies and prospective cohort studies) using the Cochrane risk of bias tool[22] and Newcastle-Ottawa scale,[23] respectively. A score of 0 to 9 (marked as stars) was allocated to each study, except RCTs. The studies, which are RCTs and non-RCTs acquiring ≥5 stars, were regarded as high quality.
2.6. Subgroup analysis, sensitivity analysis, and publication bias
Subgroup analyses were performed by study design, mean age, and sex, while sensitivity analyses were performed for heterogeneity. Begg funnel plots and the Egger test were used to determine publication bias.[24]
2.7. Statistical analysis
Data analysis was performed using Stata version 12.0 (StataCorp, College Station, TX). The odds ratio (OR) was utilized for dichotomous variables, and all results are shown with 95% confidence intervals (CIs). Heterogeneity was quantified using the I2 statistic; I2 > 50% indicated heterogeneity. If there was heterogeneity between 2 groups, the random-effects model was used; otherwise, the fixed-effects model was used.[25]
3. Results
3.1. Study selection
The literature search process is shown in Fig. 1. The literature and manual search initially identified 2170 studies; most were excluded after reviewing the title and/or abstract. Nine studies were finally included after a detailed assessment; specifically, there were 2 RCTs,[15,18] 2 cohort studies,[9,10] and 5 retrospective studies.[14,16,17,19,20] Reasons for exclusion included duplication, irrelevance, or lack of quantitative data regarding the incidence of HZ associated with the case and control groups. The general characteristics of the included studies were shown in Table 1. The numbers of HZ infection in biologics and non-biologics group were shown in Table 2.
Figure 1.
PRISMA flow diagram. Finally, 9 studies were included after a detailed assessment. PRISMA = preferred reporting items for systematic reviews and meta-analyses.
Table 1.
The general characteristics of included studies.
| Study | Study design | Total subjects | Disease | Mean age (years ± SD) | Female n (%) | Treatment time | Follow-up time | Quality score |
| Dreiher et al 2012 | RS | 22,330 | Psoriasis | 48.6 ± 20.1 | 11,723 (52.5) | NA | 9 years | ∗∗∗∗∗ |
| Kalb et al 2015 | PC | 11,466 | Psoriasis | 58.4 ± 13.8 | 5145 (44.9) | Median 2.26 years | 8 years | ∗∗∗∗∗∗∗ |
| Leonardi et al 2008 | RCT | 766 | Moderateto severe psoriasis | 44.3 ± 11.7 | 235 (30.7) | 76 weeks | Median 36weeks | RCT |
| Levandoski et al 2018 | RS | 5889 | Psoriasis | 51.0 ± 15 | 2914 (49) | NA | 1 years | ∗∗∗ |
| Megna et al 2016 | RS | 502 | Psoriasis | 51.7 (16–88) | 180 (35.9) | NA | NA | ∗∗∗ |
| Papp et al 2017 | RCT | 6501 | Moderateto severe psoriasis | 45.4 ± 13 | 2074 (31.9) | 1–2 years | 3 years | RCT |
| Shalom et al 2015 | RS | 95,941 | Psoriasis | 45.8 ± 20 | 48,872 (51) | At least 3 months | 11 years 7 months | ∗∗∗∗∗∗∗ |
| Shalom et al 2019 | PC | 10,469 | Psoriasis | 48.5 ± 13.8 | 4667 (44.6) | 6 months | Median 3.2 years | ∗∗∗∗∗∗∗ |
| Zisman et al 2016 | RS | 3128 | Psoriatic arthritis | 50.3 ± 14.5 | 1683 (53.8) | At least 6 months | 12 years | ∗∗∗∗∗∗∗ |
Total subjects, total number of patients included in the study, regardless of abandoning or receiving multiple treatments for each patient.
NA = data not available, PC = prospective cohort, RCT = randomized controlled trail, RS = retrospective study.
The significant value of asterisks is that the more asterisks, the higher the quality of the articles.
Table 2.
Numbers of HZ infection in biologics group and non-biologics group.
| Study | Biologic treatment group (patients no.) | Numbers of HZ infection in biologic treatments | Non-biologic treatment group (patients no.) | Numbers of HZ infection in nonbiologic treatments |
| Dreiher et al 2012 | Adalimumab (129) Etanercept (271) Infliximab (112) Alefacept (71) Efalizumab (41) | 0 4 2 0 0 | UVB (1074) PUVA (1074) Acitretin (2497) Cyclosporine (94) MTX (1382) Corticosteroids (839) Control (14,746) | 6 11 13 4 24 22 68 |
| Kalb et al 2015 | Adalimumab (2675) Etanercept (1854) Infliximab (1151) Ustekinumab (3474) | 1 2 0 1 | MTX (490) NonMTX/Nonbiologics∗ (1610) | 0 0 |
| Leonardi et al 2008 | Ustekinumab (511) | 1 | Placebo (255) | 0 |
| Levandoski et al 2018 | Biologics† (2258) | 23 | Nonbiological systemic therapies‡ (3631) | 33 |
| Megna et al 2016 | Adalimumab (102) Etanercept (82) Infliximab (12) Golimumab (3) Ustekinumab (67) | 2 0 0 0 0 | Topical treatments (52) Phototherapy (23) Acitretin (29) Cyclosporine (65) MTX (67) | 0 0 0 1 0 |
| Papp et al 2017 | Ixekizumab (4209) Etanercept (739) | 5 4 | Placebo (1553) | 4 |
| Shalom et al 2015 | Adalimumab (719) Etanercept (1030) Infliximab (392) Efalizumab (38) Alefacept (21) Ustekinumab (63) Methotrexate and biologics (739) | 7 13 8 0 0 2 16 | UVB (2895) PUVA (1063) Acitretin (4094) Cyclosporine (148) MTX (4320) Control (94073) | 36 11 27 3 54 826 |
| Shalom et al 2019 | TNF-α inhibitors§ (5076) Ustekinumab (2704) | 13 8 | MTX (1201) NonMTX∗ /Nonbiologics (1488) | 2 2 |
| Zisman et al 2016 | TNF-α inhibitors§ (587) TNF-α inhibitors + c-DMARD (427) | 5 8 | No DMARDs (1066) c-DMARDs (2156) | 8 20 |
c-DMARDs = conventional disease-modifying antirheumatic drugs, HZ = Herpes Zoster, MTX = methotrexate, TNF-α inhibitors = tumor necrosis factor-α inhibitors.
NonMTX/Nonbiologics included topical therapy, phototherapy, systemic steroids, acitretin, and cyclosporine.
Biologics included adalimumab, etanercept, infliximab, ustekinumab, golimumab, certolizumab, tocilizumab, abatacept, anakinra, and rituximab.
Nonbiological systemic therapies included methotrexate, retinoids, cyclosporine, hydroxyurea, mycophenolate mofetil, sulfasalazine, and thioguanine.
TNF-α inhibitors included adalimumab, etanercept, infliximab, and golimumab.
3.2. Risk of HZ associated with use of biologics compared with non-biological and non-biological systemic therapies
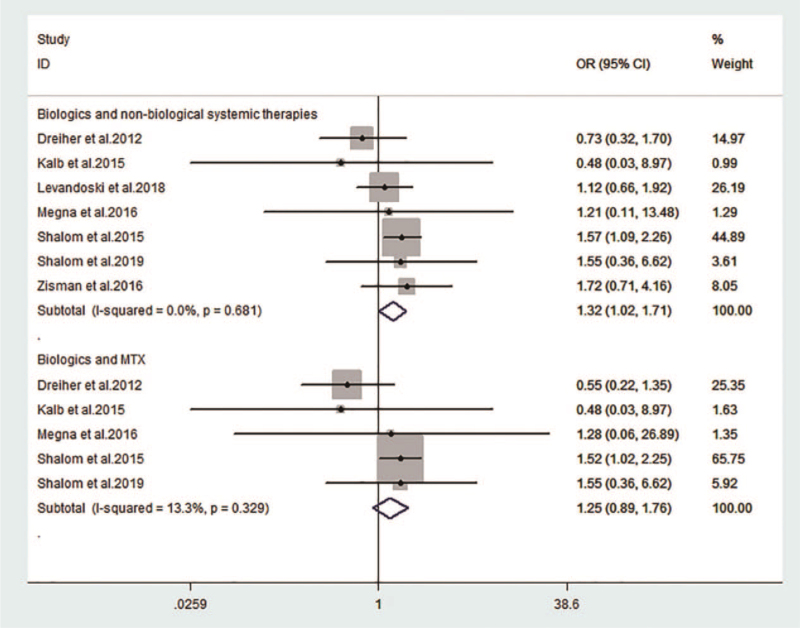
Non-biological therapies included topical therapy, phototherapy, MTX, acitretin, cyclosporine, corticosteroids, c-DMARDs, control, and placebo. Non-biological systemic therapies included MTX, acitretin, cyclosporine, corticosteroids, and c-DMARDs. Compared with non-biological (OR: 1.48; 95% CI: 1.18–1.86; I2 = 0%; Fig. 2) and non-biological systemic (OR: 1.32; 95% CI: 1.02–1.71; I2 = 0%; Fig. 3) therapies, the risks of HZ increased with the use of biologics; however, such a risk was not observed when compared with MTX (OR: 1.25; 95% CI: 0.89–1.76; I2 = 0%; Fig. 3).
Figure 2.
Risk of herpes zoster with biologics compared with non-biological therapies. Compared with non-biological therapies, the risks of HZ increased with the use of biologics (OR: 1.48; 95% CI: 1.18–1.86; I2 = 0%). CI = confidence interval, HZ = herpes zoster, OR = odds ratio.
Figure 3.
Risk of herpes zoster with biologics compared with non-biological systemic therapies and MTX. Compared with non-biological systemic therapies, the risks of HZ increased with the use of biologics (OR: 1.32; 95% CI: 1.02–1.71; I2 = 0%); when compared with MTX, such a risk was not observed. CI = confidence interval, MTX = methotrexate, OR = odds ratio.
3.3. Risk of HZ associated with use of TNF-α inhibitors and non-TNF-α inhibitors, compared with non-biological therapies
Tumor necrosis factor alpha (TNF-α) inhibitors included adalimumab, etanercept, infliximab, and golimumab, while other biological drugs were called non-TNF-α inhibitors. Compared with non-biological therapies, the risk of HZ increased significantly with use of TNF-α inhibitors (OR: 1.50; 95% CI: 1.11–2.02; I2 = 0%; Fig. 4). However, no significant differences were observed with use of non-TNF-α inhibitors (OR: 1.20; 95% CI: 0.61–2.34; I2 = 0%; Fig. 4).
Figure 4.
Risk of herpes zoster with TNF-α inhibitors and non-TNF-α inhibitors compared with non-biological therapies. Compared with non-biological therapies, the risk of HZ increased significantly with use of TNF-α inhibitors (OR: 1.50; 95% CI: 1.11–2.02; I2 = 0%); no significant differences were observed with use of non-TNF-α inhibitors (OR: 1.20; 95% CI: 0.61–2.34; I2 = 0%). CI = confidence interval, HZ = herpes zoster, OR = odds ratio, TNF-α = tumor necrosis factor alpha.
3.4. Risk of HZ associated with each biological drug compared with non-biological therapies
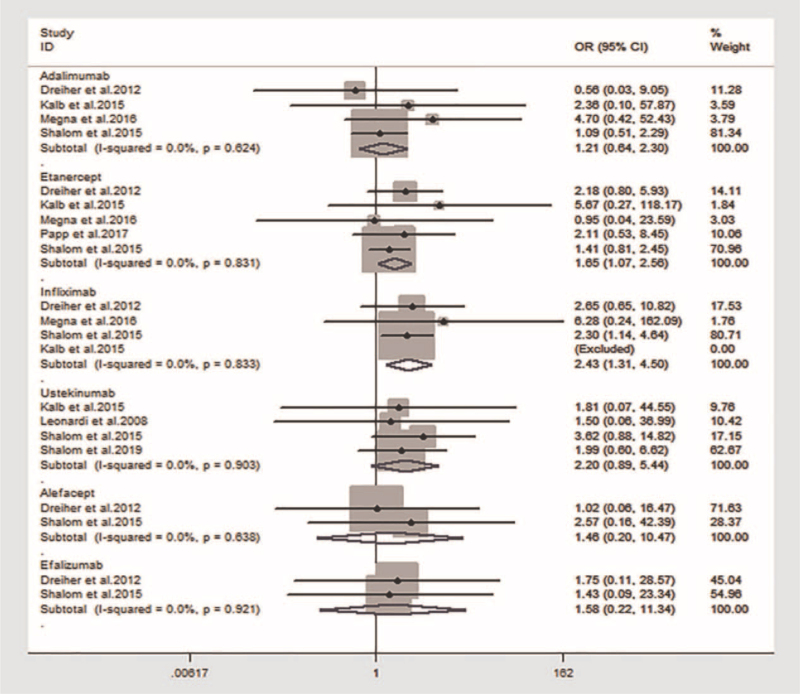
The risk of HZ associated with infliximab (OR: 2.43; 95% CI: 1.31–4.50; I2 = 0%) and etanercept (OR: 1.65; 95% CI: 1.07–2.56; I2 = 0%) increased significantly when compared with that of non-biological therapies; however, adalimumab (OR: 1.21; 95% CI: 0.64–2.30; I2 = 0%), ustekinumab (OR: 2.20; 95% CI: 0.89–5.44; I2 = 0%), alefacept (OR: 1.46; 95% CI: 0.20–10.47; I2 = 0%), and efalizumab (OR: 1.58; 95% CI: 0.22–11.34; I2 = 0%) did not show increased risk (Fig. 5). Due to the limited data regarding other biological drugs, we could not conduct statistical analyses.
Figure 5.
Risk of herpes zoster with each biological drug compared with non-biological therapies. Compared with non-biological therapies, the risk of HZ associated with infliximab (OR: 2.43; 95% CI: 1.31–4.50; I2 = 0%) and etanercept (OR: 1.65; 95% CI: 1.07–2.56; I2 = 0%) increased significantly; however, adalimumab (OR: 1.21; 95% CI: 0.64–2.30; I2 = 0%), ustekinumab (OR: 2.20; 95% CI: 0.89–5.44; I2 = 0%), alefacept (OR: 1.46; 95% CI: 0.20–10.47; I2 = 0%), and efalizumab (OR: 1.58; 95% CI: 0.22–11.34; I2 = 0%) did not show increased risk. CI = confidence interval, OR = odds ratio.
3.5. Subgroup analysis, sensitivity analysis, and publication bias
Subgroup analyses were performed by study design, mean age, and sex. The results indicated that a high risk of HZ was associated with a mean age ≤50 years (OR: 1.60; 95% CI: 1.22–2.09; I2 = 0%), rate of being women >50% (OR: 1.64; 95% CI: 1.27–2.13; I2 = 0%), and non-RCTs (OR: 1.53; 95% CI: 1.27–2.13; I2 = 0%). Given that I2 = 0%, a sensitivity analysis was not required. The Egger tests showed that there was no statistically significant publication bias (Fig. 6).
Figure 6.
Evaluation of publication bias. The Egger tests showed that there was no statistically significant publication bias.
4. Discussion
Indeed, biological therapies are known to improve the efficacy of treatment for psoriasis and psoriatic arthritis. However, although adverse effects related to immunosuppression (such as HZ) have gained much research attention in recent years, the risk of HZ associated with biologics remains controversial. This meta-analysis compared the risk of HZ between biological and non-biological therapies. The results showed an elevated risk of HZ associated with psoriasis and psoriatic arthritis patients when treated via biological therapy, compared with non-biological or non-biological systemic therapies. An elevated risk was also observed with use of TNF-α inhibitors, but not with non-TNF-α inhibitors. Similarly, the risk of HZ did not increase with the use of biologics when compared with MTX. Specifically, individual drug comparison results showed that the risk of HZ increased in patients treated with etanercept and infliximab, while no increased risk was observed with use of adalimumab, ustekinumab, and alefacept.
Subgroup analyses revealed that younger and female patients were at a higher risk of HZ infection when treated with biological therapies; this indicates younger age and female sex as potential risk factors. Furthermore, our results are consistent with findings reported in previous studies. For example, El Hayderi et al[26] reported that the administration of biologics in psoriasis patients increased the risk of HZ by 2- to 3-fold; infliximab conferred an increased risk for HZ, whereas adalimumab, etanercept, and ustekinumab did not. Moreover, younger age groups were expected to contain more cases of HZ.[26,27] Although the risk of HZ associated with the use of biologics in our meta-analysis was lower than that in previously reported studies, our results correspond with these reports. Similarly, Dreiher et al[14] reported an association between HZ and infliximab that approached statistical significance; however, there was no significance observed with respect to the other biological agents: adalimumab, etanercept, alefacept, and efalizumab.
There were also many studies that conflict with our results; for instance, Shalom et al[10] demonstrated that TNF-α inhibitors and ustekinumab did not significantly increase the risk of HZ in psoriasis patients, while Levandoski et al[16] found no significant differences regarding the risk of HZ amongst psoriasis patients treated with biological and non-biological therapies. The data presented by Zisman et al[20] showed that the risk of HZ in patients with psoriatic arthritis increased with age, but not with TNF-α inhibitors; however, Galloway et al[28] illustrated an increased risk of HZ associated with biologics versus c-DMARDs in those with rheumatoid arthritis (RA). Although many studies have presented findings contradictory to our results, this higher risk of HZ seemed reasonable in psoriasis and psoriatic arthritis patients treated with biological therapies.
HZ risk has been shown to be higher in individuals treated with immunosuppressive medications, including biological drugs.[29] As TNF-α is a major factor in the immune defence against HZ, we observe an increased risk in patients undergoing treatment with TNF-α inhibitors.[26,30] Among the TNF-α inhibitors, infliximab and etanercept were associated with high risks of HZ,[31] while ustekinumab was associated with a comparable risk of HZ in patients undergoing non-biological therapies, possibly due to its relatively short application and different targeted cytokine pathways.[26] The other second-generation biologics did not yet have sufficient data that would allow for further analysis.
A surprising finding was that younger patients were more susceptible to HZ when treated with biological therapies, contrary to the results of some studies.[20] Amongst the 9 studies, 6 involved patients with a mean age <50 years while only 3 involved patients with a mean age >50 years. Overall, the mean age of patients treated with biological therapies was younger than 50 years, which may explain the increased risk of HZ linked to younger patients. As El Hayderi et al[26] reported, HZ might undergo an age-shift toward younger patients. In addition, female sex may be another risk factor for HZ incidents when patients are treated with biological therapies.[14]
Our results indicate that biological therapies, especially infliximab and etanercept, may correlate with a higher risk of HZ, while younger age and female sex may be risk factors. Dermatologists and their patients treated with biological drugs should therefore be alerted to the possible risk of HZ associated with these medications. The live attenuated viral vaccine is recommended for administration before starting some biological drugs in these patients,[26] while the intravenous administration of acyclovir (10 mg/kg/8 h for at least 7 days) is additionally advised for patients who are at a higher risk of developing HZ.[26,32]
The current study has some limitations. Given that few RCTs have exclusively reported HZ incidents, especially those regarding second-generation biologics, most of the included studies were observational; some studies that did not provide integrated data on HZ incidents were excluded. We may have also missed smaller studies; therefore, our results require confirmation via large-scale RCTs.
5. Conclusion
Biological therapies, especially TNF-α inhibitors, may contribute to the risk of HZ in psoriasis and psoriatic arthritis patients. Amongst these agents, infliximab and etanercept have been shown to significantly increase the risk of HZ. Younger age and female sex may also be risk factors.
Acknowledgments
The authors would like to thank Editage (www.editage.cn) for English language editing.
Author contributions
Conceptualization: Ailing Zou.
Datacuration: Nian Shi, Yu Ye.
Investigation: Ailing Zou, Yongjun Chen, Nian Shi.
Software: Yongjun Chen, Yu Ye.
Supervision: Ailing Zou, Yongjun Chen.
Writing – original draft: Ailing Zou.
Writing–review & editing: Nian Shi, Yu Ye.
Footnotes
Abbreviations: c-DMARDs = conventional disease-modifying antirheumatic drugs, CI = confidence interval, HZ = herpes zoster, MTX = methotrexate, OR = odds ratios, RCT = randomized controlled trial, TNF-α = tumor necrosis factor-α.
How to cite this article: Zou A, Chen Y, Shi N, Ye Y. Risk of herpes zoster associated with biological therapies for psoriasis and psoriatic arthritis: a systematic review and meta-analysis. Medicine. 2021;100:40(e27368).
Availability of data and materials: Materials used in this review were obtained from published research and papers.
Competing interests: The authors declare that they have no competing interests.
The authors have no funding and conflicts of interest to disclose.
The datasets generated during and/or analyzed during the current study are publicly available.
References
- [1].Staubach P, Fau - Zimmer S, Zimmer S. Plaque psoriasis – more than a skin disorder. Med Monatsschr Pharm 2017;40:231–3. [PubMed] [Google Scholar]
- [2].Perera GK, Di Meglio P, Nestle FO. Psoriasis. Annu Rev Pathol 2012;7:385–422. [DOI] [PubMed] [Google Scholar]
- [3].Ronholt K, Iversen L. Old and new biological therapies for psoriasis. Int J Mol Sci 2017;18:2297–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Siegel SAR, Winthrop KL. In the real world: infections associated with biologic and small molecule therapies in psoriatic arthritis and psoriasis. Curr Rheumatol Rep 2019;21:36–42. [DOI] [PubMed] [Google Scholar]
- [5].Papp K, Thaçi D, Marcoux D, et al. Efficacy and safety of adalimumab every other week versus methotrexate once weekly in children and adolescents with severe chronic plaque psoriasis: a randomised, double-blind, phase 3 trial. Lancet 2017;390:40–9. [DOI] [PubMed] [Google Scholar]
- [6].van de Kerkhof PC, Griffiths CE, Reich K, et al. Secukinumab long-term safety experience: A pooled analysis of 10 phase II and III clinical studies in patients with moderate to severe plaque psoriasis. J Am Acad Dermatol 2016;75:83.e84–98.e84. [DOI] [PubMed] [Google Scholar]
- [7].Gordon KB, Papp KA, Langley RG, et al. Long-term safety experience of ustekinumab in patients with moderate to severe psoriasis (Part II of II): results from analyses of infections and malignancy from pooled phase II and III clinical trials. J Am Acad Dermatol 2012;66:742–51. [DOI] [PubMed] [Google Scholar]
- [8].Ritchlin CT, Stahle M, Poulin Y, et al. Serious infections in patients with self-reported psoriatic arthritis from the Psoriasis Longitudinal Assessment and Registry (PSOLAR) treated with biologics. BMC Rheumatol 2019;3:52–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Kalb RE, Fiorentino DF, Lebwohl MG, et al. Risk of serious infection with biologic and systemic treatment of psoriasis: results from the psoriasis longitudinal assessment and registry (PSOLAR). JAMA Dermatol 2015;151:961–9. [DOI] [PubMed] [Google Scholar]
- [10].Shalom G, Naldi L, Lebwohl M, et al. Biological treatment for psoriasis and the risk of herpes zoster: results from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). J Dermatolog Treat 2019;30:534–9. [DOI] [PubMed] [Google Scholar]
- [11].Tsai SY, Chen HJ, Lio CF, et al. Increased risk of herpes zoster in patients with psoriasis: a population-based retrospective cohort study. PLoS One 2017;12:e0179447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Takeshita J, Shin DB, Ogdie A, et al. Risk of serious infection, opportunistic infection, and herpes zoster among patients with psoriasis in the United Kingdom. J Invest Dermatol 2018;138:1726–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cohen E. Herpes zoster and postherpetic neuralgia. Clin Infect Dis 2020;2020:ciaa1192. [DOI] [PubMed] [Google Scholar]
- [14].Dreiher J, Kresch FS, Comaneshter D, et al. Risk of Herpes zoster in patients with psoriasis treated with biologic drugs. J Eur Acad Dermatol Venereol 2012;26:1127–32. [DOI] [PubMed] [Google Scholar]
- [15].Leonardi CL, Kimball AB, Papp KA, et al. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1). Lancet 2008;371:1665–74. [DOI] [PubMed] [Google Scholar]
- [16].Levandoski KA, Quesenberry CP, Tsai AL, et al. Herpes zoster rates in a large cohort of patients with systemically treated psoriasis. JAMA Dermatol 2018;154:218–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Megna M, Napolitano M, Ayala F, et al. The risk of herpes zoster in patients with psoriasis: a retrospective records-based observational study. Indian J Dermatol Venereol Leprol 2016;82:744. [DOI] [PubMed] [Google Scholar]
- [18].Papp KA, Bachelez H, Blauvelt A, et al. Infections from seven clinical trials of ixekizumab, an anti-interleukin-17A monoclonal antibody, in patients with moderate-to-severe psoriasis. Br J Dermatol 2017;177:1537–51. [DOI] [PubMed] [Google Scholar]
- [19].Shalom G, Zisman D, Bitterman H, et al. Systemic therapy for psoriasis and the risk of herpes zoster: a 500,000 person-year study. JAMA Dermatol 2015;151:533–8. [DOI] [PubMed] [Google Scholar]
- [20].Zisman D, Bitterman H, Shalom G, et al. Psoriatic arthritis treatment and the risk of herpes zoster. Ann Rheum Dis 2016;75:131–5. [DOI] [PubMed] [Google Scholar]
- [21].McInnes MDF, Moher D, Thombs BD, et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA Statement. JAMA 2018;319:388–96. [DOI] [PubMed] [Google Scholar]
- [22].Higgins JP, Altman Dg Fau - Gøtzsche PC, Gøtzsche Pc Fau - Jüni P, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Ottawa Hospital Research Institute Web site. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed March 1, 2020. [Google Scholar]
- [24].Egger M, Davey Smith G, Fau - Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cumpston M, Li T, Fau - Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev 2019;10:ED000142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].El Hayderi L, Colson F, Dezfoulian B, et al. Herpes zoster in psoriasis patients undergoing treatment with biological agents: prevalence, impact, and management challenges. Psoriasis (Auckl) 2016;6:145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Adelzadeh L, Jourabchi N, Wu JJ. The risk of herpes zoster during biological therapy for psoriasis and other inflammatory conditions. J Eur Acad Dermatol Venereol 2014;28:846–52. [DOI] [PubMed] [Google Scholar]
- [28].Galloway JB, Mercer LK, Moseley A, et al. Risk of skin and soft tissue infections (including shingles) in patients exposed to anti-tumour necrosis factor therapy: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis 2013;72:229–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Marra F, Lo E, Kalashnikov V, et al. Risk of herpes zoster in individuals on biologics, disease-modifying antirheumatic drugs, and/or corticosteroids for autoimmune diseases: a systematic review and meta-analysis. Open Forum Infect Dis 2016;3: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Arvin AM, Moffat JF, Sommer M, et al. Varicella-zoster virus T cell tropism and the pathogenesis of skin infection. Curr Top Microbiol Immunol 2010;342:189–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Serac G, Tubach F, Mariette X, et al. Risk of herpes zoster in patients receiving anti-TNF-alpha in the prospective French RATIO registry. J Invest Dermatol 2012;132(3 pt 1):726–9. [DOI] [PubMed] [Google Scholar]
- [32].Werner RN, Nikkels AF, Marinovic B, et al. European consensus-based (S2k) guideline on the Management of Herpes Zoster - guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV), Part 2: treatment. J Eur Acad Dermatol Venereol 2017;31:20–9. [DOI] [PubMed] [Google Scholar]