Abstract
Background:
Incidence, risk factors, and perinatal morbidity and mortality rates related to amniotic fluid embolism remain a challenge to evaluate, given the presence of differing international diagnostic criteria, the lack of a gold standard diagnostic test, and a significant overlap with other causes of obstetric morbidity and mortality.
Objective:
The aims of this study were (1) to analyze the clinical features and outcomes of women, using the largest United States-based contemporary international amniotic fluid embolism registry, and (2) to investigate differences in demographic and obstetric variables, clinical presentation, and outcomes between women with typical versus atypical amniotic fluid embolism, using previously published and validated criteria for the research reporting of amniotic fluid embolism.
Materials and Methods:
The AFE Registry is an international database established at Baylor College of Medicine (Houston, TX) in partnership with the Amniotic Fluid Embolism Foundation (Vista, CA) and the Perinatology Research Branch of the Division of Intramural Research of the NICHD/NIH/DHHS (Detroit, MI). Charts submitted to the registry between August 2013 and September 2017 were reviewed, and cases were categorized into typical, atypical, non-amniotic fluid embolism, and indeterminate, using the previously published and validated criteria for the research reporting of AFE. Demographic and clinical variables, as well as outcomes for patients with typical and atypical AFE, were recorded and compared. Student t tests, X2 tests, and analysis of variance tables were used to compare the groups, as appropriate, using SAS/STAT software, version 9.4.
Results:
A total of 129 charts were available for review. Of these, 46% (59/129) represented typical amniotic fluid embolism and 12% (15/129) atypical amniotic fluid embolism, 21% (27/129) were non-amniotic fluid embolism cases with a clear alternative diagnosis, and 22% (28/129) had an uncertain diagnosis. Of the 27 women misclassified as an amniotic fluid embolism with an alternative diagnosis, the most common actual diagnosis was hypovolemic shock secondary to postpartum hemorrhage. Ten percent (6/59) of the women with typical amniotic fluid embolism had a pregnancy complicated by placenta previa, and 8% (5/61) had undergone in vitro fertilization to achieve pregnancy. In all, 66% (49/74) of the women with amniotic fluid embolism reported a history of atopy or latex, medication, or food allergy, compared to 34% of the obstetric population delivered at our hospital over the study period (P < .05).
Conclusion:
Our data represent a series of women with amniotic fluid embolism whose diagnosis has been validated by detailed chart review, using recently published and validated criteria for research reporting of amniotic fluid embolism. Although no definitive risk factors were identified, a high rate of placenta previa, reported allergy, and conceptions achieved through in vitro fertilization was observed.
Keywords: anaphylactoid syndrome of pregnancy, cardiovascular collapse, disseminated intravascular coagulopathy, history of atopy, hypotension, hypertension, neurologic injury, registry
INTRODUCTION
Amniotic fluid embolism (AFE) is a catastrophic obstetric complication typically presenting as a complex sequence of peripartum events that result in cardiovascular collapse and disseminated intravascular coagulation (DIC).1–9 The reported incidence of AFE ranges from 1 in 15,200 to 1 in 53,800 deliveries worldwide.9–22
Recognition of the true incidence and mortality rates associated with AFE are confounded by several factors – most importantly, the absence of a definitive “gold standard” diagnostic test. In addition, many of the signs and symptoms of AFE overlap with those seen in other obstetric complications.10,13–15,23–27 The existence of several differing international criteria for the diagnosis of AFE further compound the challenges of defining risk factors, diagnosis, prognosis, and understanding of the underlying pathophysiology of this disease.10–12,21–23 Studies based on administrative data sets consistently demonstrate both a higher incidence and a lower mortality rate than those based on data from individual chart review, suggesting that former studies likely contain significant numbers of patients with conditions other than AFE.10,13–24, 28
Diagnostic criteria for the research reporting of AFE have been published to promote uniformity in data collection, validate prior studies, refine the identification of clinical risk factors, and improve treatment strategies for AFE.24 When applied to the largest United States-based contemporary international registry for AFE, the diagnostic performance of this criteria set demonstrated a sensitivity of 79.4% and a specificity of 100% for the diagnosis of AFE in 115 enrolled women.25 The aims of the present study were to analyze the clinical features and outcomes of women in this registry, and to investigate differences in clinical presentation and outcomes between women with typical vs atypical AFE.
MATERIALS and METHODS
Following Institutional Review Board approval in 2012, an international AFE registry was established at Baylor College of Medicine, (Houston, TX) in collaboration with the Amniotic Fluid Embolism Foundation, (Foundation; Vista, CA) and the Perinatology Research Branch of the Division of Intramural Research of the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health/US Department of Health and Human Services (Detroit, MI). Charts submitted to the registry between August 2013 and September 2017 were reviewed for this descriptive study. Cases identified prior to 1995 and included in the original registry for AFE were excluded.2 All cases were initially identified as survivors or family members of decedents who contacted the Foundation’s website and were invited to submit the medical records through the Foundation to the investigators. All consented individuals were informed that the result of case categorization would not be disclosed to them. No cases originated as medico-legal consultations to authors of the study. Charts, analyzed by the four authors with expertise in the field of AFE and critical care obstetrics, were categorized as one of the following:
Typical amniotic fluid embolism. These charts met current published and validated diagnostic criteria recommended for the research reporting of AFE.14,24,25
Atypical amniotic fluid embolism. This group included women who, while not strictly meeting each of the above criteria, were felt by the reviewing experts to have a clinical course that could not be explained by any recognized pathophysiologic process other than a forme fruste of AFE.24,25
Non-amniotic fluid embolism. A critical review of the medical records for these women suggested an alternative medical diagnosis much more likely than AFE.
Indeterminate. For this group, medical records were not consistent with typical or atypical AFE, but a definitive alternative diagnosis could not be reached. In most cases, the medical records submitted were insufficiently detailed to allow a definitive alternative diagnosis.
Inter-reviewer agreement was determined by independent review of each case by three different authors reviewing cases and were categorized only with agreement, using the published and validated criteria for the research reporting of AFE as well as predetermined categories of indeterminate and non-AFE. Demographic and clinical variables, as well as outcomes for patients with typical and atypical AFE, were recorded and compared. Data were reported as means and percentages. Student’s t tests, X2 tests, and ANOVA tables were used to compare the groups, as appropriate, using SAS/STAT® software, version 9.4 (SAS Institute, Cary, NC).
RESULTS
Of the 136 cases submitted to the registry, 129 had charts available for review. Sixteen international cases were included. Of these, 46% (59/129) met the criteria for typical AFE, 12% (15/129) were considered atypical AFE, 21% (27/129) appeared to represent a different condition, and 22% (28/129) were considered to have an uncertain diagnosis (Figure 1). Available demographic and pre-event medical and obstetric features of patients with typical and atypical AFE are described in Tables 1 and 2. Of all women with AFE, only 2 (3%) had twins. Racial/ethnic makeup was self-reported as 70% white, 7% Hispanic, 3% Asian, 1% black, and 19% mixed or unspecified. In all, 66% (49/74) of women with AFE reported a history of atopy, or latex, medication, or food allergy, compared to 31% (4739/15091) of the obstetric population delivered at our hospital over the study period (P < .05), and 10% (6/59) of women with AFE had placenta previa at term. Clinical features and maternal outcomes associated with the occurrence of AFE are presented in Table 3. Maternal death occurred in 10% of cases of typical AFE. Of all women with AFE, 17.6% (13/74) delivered vaginally and 79.7% (59/74) underwent cesarean delivery, of which 32.2% (19/59) were emergent, in response to clinical AFE. Fewer maternal deaths were seen among women with atypical AFE; however, given the small number of deaths in the overall population, this difference did not reach statistical significance. Both pulmonary edema and maternal neurologic injury were significantly more frequent among survivors of typical AFE (P = .04, P = .02, respectively).
Figure 1:
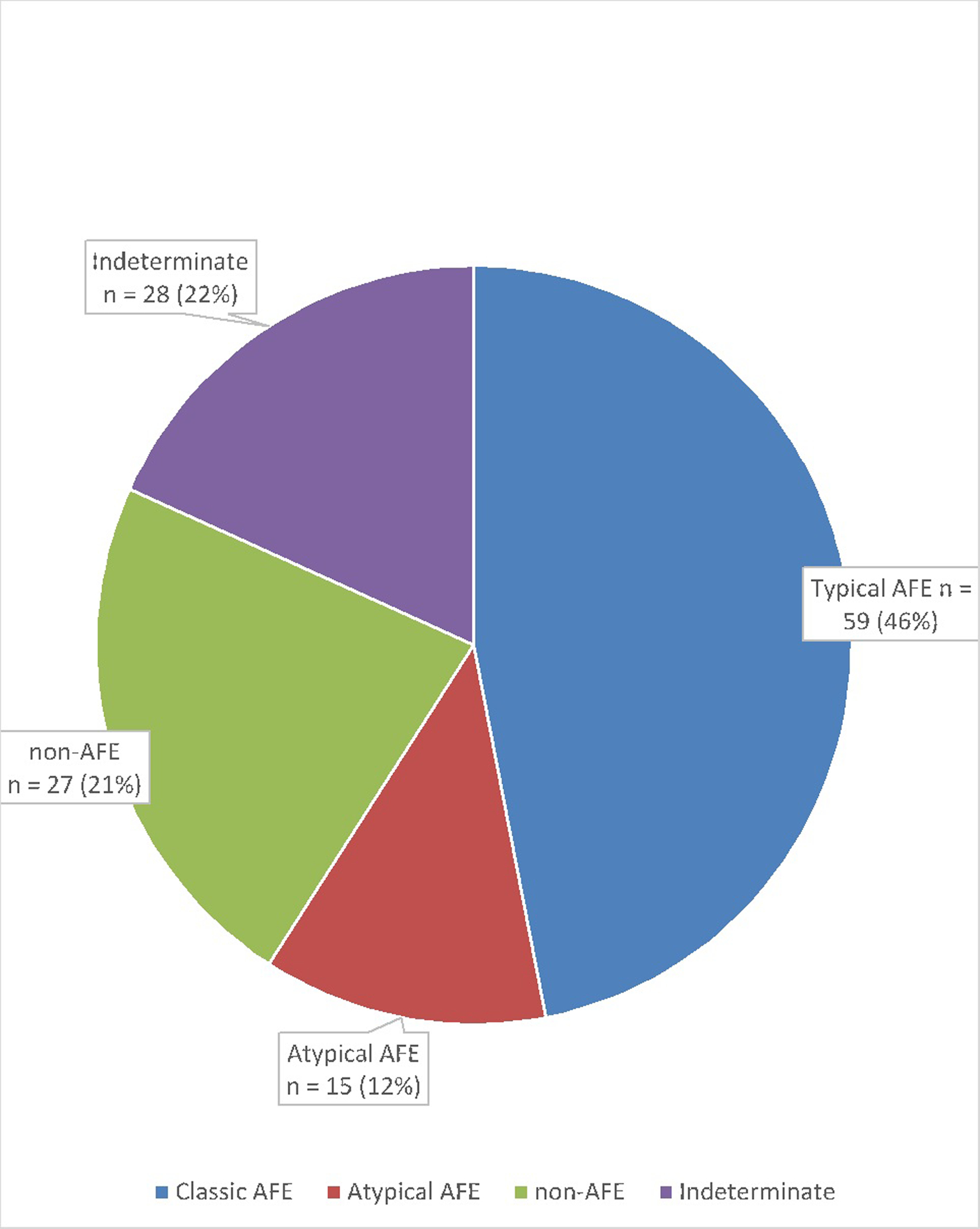
AFE Classification using Diagnostic Criteria Recommended for the Research Reporting of AFE.14,24
Table 1:
Demographic Data for Patients with Typical and Atypical Amniotic Fluid Embolism
| Variable | Typical AFE | Atypical AFE | P-Value |
|---|---|---|---|
| Maternal Age (years) | 33.2 (±4.7) | 33.3 (±4.5) | 0.9 |
| Parous | 4(3,5) | 4(3,5) | |
| Maternal BMI (kg/m2) on admission | 31.4 (±7.2) | 30.9 (±3.9) | 0.8 |
| Maternal Weight Gain (lbs) | 21.4 (±15.1) | 37.4 (±15.2) | 0.002 |
| Gestational age at delivery (weeks) | 37.9 (±4.1) | 38.4 (±3.4) | 0.6 |
| Birth Weight (gm) | 3539 (±507.1) | 3277 (±855.3) | 0.2 |
| Living Children | 1.1 (±1.7) | 0.9 (±1.2) | 0.6 |
| Number of Prenatal Visits | 12.8 (±4.2) | 12.8 (±3.8) | 0.9 |
Data expressed as mean and SD or median (1st, 3rd quartile).
BMI, body mass index.
Table 2:
Obstetrical and Medical characteristics for Patients with Typical (N=59) and Atypical Amniotic Fluid Embolism (N=15)
| Variable | Typical AFE | Atypical AFE: | P-value |
|---|---|---|---|
| Atopy | 8/47 (17.02) | 0/15 (0) | 0.1 |
| Asthma | 4/54 (7.41) | 2/14 (14.29) | 0.4 |
| Thyroid | 4/53 (7.55) | 0/15 (0) | 0.3 |
| Latex Allergy | 0/59 (0) | 1/15 (6.67) | 0.1 |
| Food Allergy | 8/59 (13.56) | 2/15 (13.33) | 0.9 |
| Medication Allergy | 23/59 (38.98) | 7/15 (46.67) | 0.3 |
| Gestational Diabetes | 6/49 (12.24) | 0/15 (0) | 0.2 |
| Pre-gestational Diabetes | 1/54 (1.85) | 0/15 (0) | 0.6 |
| Gestational Hypertension | 5/49 (10.20) | 0/15 (0) | 0.2 |
| Cancer | 1/51 (1.96) | 1/14 (7.14) | 0.3 |
| Thrombophilia | 0/59 (0) | 1/13 (7.69) | 0.1 |
| Cerclage | 1/50 (2.00) | 0/15 (0) | 0.6 |
| Tobacco | 2/53 (3.77) | 1/15 (6.67) | 0.6 |
| Prior ethanol use | 5/52 (9.62) | 4/15 (26.67) | 0.1 |
| In vitro-fertilization | 4/48 (8.83) | 1/13 (7.69) | 0.8 |
| Amniotic Fluid: | |||
| Oligohydramnios | 1/57 (1.75) | 0/15 (0) | 0.8 |
| Polyhydramnios | 4/57 (7.02) | 1/15 (6.67) | |
| Normal Fluid | 44/57 (77.19) | 13/15 (86.67) | |
| Placenta Previa | 6/59 (10.17) | 0/15 (0) | 0.4 |
| Induction: | |||
| Oxytocin | 31/40 (77.50) | 8/9 (88.89) | 0.4 |
| Prostaglandin | 11/46 (23.91) | 4/13 (30.77) | 0.6 |
| Induction Agents: Gel | 6/11 (54.55) | 0/15 (0) | 0.1 |
| Induction Agents: Tablets | 5/11 (45.45) | 3/3 (100) | |
| Anesthesia: | |||
| Epidural/spinal | 42/50 (84.00) | 13/15 (86.67) | 0.7 |
| General/combined | 8/50 (16.00) | 2/15 (13.33) |
Data expressed as N (%)
Table 3:
Clinical Signs and Symptoms of patients with Typical and Atypical AFE
| Variable | Typical AFE | Atypical AFE | P-value |
|---|---|---|---|
| Bronchospasm | 5/34 (14.7) | 2/10 (20.0) | 0.7 |
| Chest Pain | 6/48 (12.5) | 3/13 (23.1) | 0.3 |
| Cough | 2/48 (4.1) | 2/13 (15.4) | 0.1 |
| Cyanosis | 11/41 (26.8) | 3/11 (27.3) | 0.9 |
| Dyspnea | 29/45 (64.4) | 8/13 (61.5) | 0.8 |
| Headache | 3/50 (6.0) | 4/13 (30.8) | 0.01 |
| Hypotension | 54/54 (100.0) | 8/10 (80.0) | 0.001 |
| Pulmonary Edema | 9/46 (19.6) | 5/10 (50.0) | 0.04 |
| Seizure | 11/53 (20.8) | 2/13 (15.4) | 0.7 |
| Transient Hypertension | 7/48 (14.6) | 1/11 (9.1) | 0.6 |
| Uterine Atony | 28/51 (54.9) | 3/11 (27.2) | 0.1 |
| Uterine Tachysystole | 4/41 (9.8) | 2/10 (20.0) | 0.4 |
| Maternal Death | 6/59 (10.2) | 1/15 (6.7) | 0.7 |
| Neurological Injury | 32/59 (54.3) | 3/10 (20.0) | 0.01 |
Data expressed as N (%)
Neonatal outcomes are listed in Table 4. A total of 30 newborns (51%) born to women with typical AFE had Apgar scores < 7 at 1 minute and were acidemic (mean arterial pH was 6.95). Neonatal mortality was 6%; however, no neonatal deaths were reported by women with atypical AFE. Table 5 outlines the frequency with which each of the 5 criteria necessary for the diagnosis of AFE was met by both typical and atypical cases. Maternal hypotension was the only criterion that was significantly less common among atypical AFE cases (P = .001).
Table 4:
Neonatal Outcomes in cases of Typical and Atypical AFE
| Variable | Typical AFE (N=59) |
Atypical AFE (N=15) |
P-value |
|---|---|---|---|
| Birth Weight (gm) | (48/59) 3539.1 (±507.06) | (11/15) 3277.2 (±855.28) | 0.2 |
| Umbilical artery pH | (18/59) 6.95 (±0.20) | (6/15) 7.01 (±0.24) | 0.6 |
| Female | 29/59 (49) | 5/15 (33) | 0.3 |
| Male | 21/59 (36) | 7/15 (47) | |
| 5 min APGAR: 1–3 | 11/59 (18.6) | 6/15 (40.0) | 0.2 |
| 5 min APGAR: 4–6 | 19/59 (32.2) | 4/15 (26.7) | |
| 5 min APGAR: 7–10 | 29/59 (49.2) | 5/15 (33.3) | |
| Neonatal Death | 3/59 (5) | 0 | 0.4 |
Data expressed as mean (SD) or N (%)
Table 5:
Clinical Criteria for Patients with Typical (N=59) and Atypical Amniotic Fluid Embolism (N=15)
| Criterion Number | Typical AFE | Atypical AFE |
|---|---|---|
| 1 | 100% | 66.7% |
| 2 | 100% | 78.6% |
| 3 | 100% | 78.6% |
| 4 | 100% | 100% |
| 5 | 100% | 92.9% |
Data expressed as (%)
Criterion 1: Sudden onset of cardiorespiratory arrest or hypotension (systolic blood pressure <90 mm Hg)
Criterion 2: respiratory compromise (dyspnea, cyanosis, or peripheral capillary oxygen saturation (SpO2) <90% or respiratory arrest.
Criterion 3: Documentation of overt disseminated intravascular coagulation (DIC) using the scoring system of the Scientific and Standardization Committee on Disseminated Intravascular Coagulation of the International Society on Thrombosis and Hemostasis (ISTH), modified for pregnancy.
Criterion 4: Clinical onset during labor, cesarean delivery or dilation and evacuation or within 30 minutes of delivery of the placenta.
Criterion 5: No fever (38.0°C) and absence of any other significant confounding condition or explanation for the signs and symptoms observed.
Amniotic fluid embolism outcomes
Of the 27 women misclassified as having AFE with a definitive alternative diagnosis, the most common actual diagnosis was hypovolemic shock secondary to postpartum hemorrhage (n=21, 77.7%), followed by anesthetic complications (n = 4, 14.8%) and sepsis-related cardiovascular collapse (n = 2, 7.4%).
Among women who survived either atypical or typical AFE, long-term complications that include medical and psychological manifestations involving depression and post-traumatic stress disorder (PTSD) symptoms, were recorded from those contacted and available for interview at least 1 year post-event. Of 29 survivors available for interview, 2 reported thyroid abnormalities, 1 reported persistent liver dysfunction, and 16 (55.1%) endorsed symptoms consistent with PTSD, including anger outbursts, self-destructive behavior, and flashbacks. Only 1 case (3%) of PTSD was associated with fetal/neonatal demise. All of the patients who reported post-traumatic stress symptoms were surveyed about their mental health resources and were referred to local facilities for mental health care.
Seven women who experienced either typical or atypical AFE had a subsequent pregnancy: 1 miscarried, and the other 6 had an uncomplicated pregnancy and delivered at term with no recurrence of AFE.
COMMENT
Principal findings
Strict diagnostic criteria for the research reporting of AFE were developed to reduce or eliminate the likelihood of including non-AFE cases in published data, thus providing valid data sets for improved analysis.23–25 These criteria have been applied and validated, using the largest contemporary United States-based international AFE registry.25
In this current report of the registry, these criteria have been applied to identify risk factors, determine outcomes, and distinguish typical from clinically atypical cases of AFE. This analysis more accurately describes the current clinical spectrum of this disease than do older studies, especially those based on administrative and coding data. With the exception of hypotension at presentation, criteria for typical and atypical cases of AFE did not differ.
Although no definitive risk factors were identified, in vitro fertilization, reported history of allergy, and placenta previa were notably increased among women with AFE, and both maternal and neonatal outcomes were comparably improved in relation to other research studies and the original national registry.2,28–39 Our data represent the largest available series of women with AFE whose diagnosis has been validated by chart review, according to recently published criteria, and support the following findings.24,25
Results in the Context of What is Known
First, the maternal mortality rate found in this analysis was 10% with a neurologically intact survival of 46%, compared to other studies, including the original registry, where maternal mortality was 61% with a neurologically intact survival rate of 15%.2 While recognizing the limitations, including reporting bias, of any registry-derived outcome data, these data suggest that maternal outcomes may be improving due to increased recognition and evolving critical care medicine, likely linked to our enhanced understanding of the pathophysiology of this condition.2,8,9,23,29–32,40–43 The fact that neurologic injury in survivors was also less frequent in atypical cases would be expected, given the less extensive nature of their disease process.
Despite a high percentage (36 %) of fetuses delivered by emergency cesarean or operative vaginal delivery due to maternal cardiovascular instability or collapse, there were only 3 neonatal deaths in women with AFE; in the report of an earlier national registry 3 decades ago, fetal or neonatal death rates approached 60% with one-half of the newborns experiencing neurologic sequelae.2 As with the apparently improved maternal outcome statistics, improved neonatal outcomes may reflect an increased appreciation for the importance of prompt delivery in the face of preventing maternal cardiovascular collapse and improving neonatal care.23,44 International registries and case series have also demonstrated improved perinatal outcomes secondary to early recognition and prompt resuscitation efforts for suspected cases.17–22,28–32,38,40,43 Recommendations for the management of AFE have been published to assist providers with key lifesaving maneuvers, e.g., early involvement of advanced cardiac life support, management of cardiovascular changes, and transfusion recommendations, all of which have contributed to provider education and training.10,23,49
Second, our data confirm the frequent misdiagnosis of AFE; almost one-half of patients whose diagnosis was believed to be sufficiently secure to prompt submission to the national registry did not, upon careful review of medical records, have confirmed AFE. The implications for the accuracy of general, administrative data-derived diagnoses of AFE are clear.2–4,9,10,14–22,24–26,35–39,45–47
Third, as demonstrated in Table 5, patients with typical vs atypical AFE were differentiated by the absence of 1 or more criteria needed to confirm the diagnosis for research reporting purposes, rather than demonstrating different timing of the onset of signs or symptoms of this condition. This is consistent with the recognized pathophysiology of AFE.
Finally, while our data suggest few demographic or obstetric differences between women with AFE and those in the general population, 3 observations were noteworthy. Ten percent of women with typical AFE had placenta previa complicating their pregnancy, compared to a rate at term of < 1% in the general population, suggesting that abnormal placentation may increase the risk of activation of pathogenic pro-inflammatory or reactive mediators during the delivery process, or may enhance the opportunity for large volumes of amniotic fluid to enter maternal circulation. Other studies have found similar rates of placenta previa in women who have suffered AFE.30,42 Additionally, 66% of women with AFE reported a history of atopy, or latex, medication, or food allergy, compared to 34% of the obstetric population delivered at our hospital over the study period (p <.05). This doubling of reported atopy among cases suggests that an anaphylactoid reaction may be the inciting event for the cascade of adverse events that characterize AFE.2,33 Lastly, we also noted that 8% of women with typical AFE conceived through in vitro fertilization. This compares to 1–2% of women in the general US population.26 While valid statistical conclusions regarding the significance of such data are not possible, the recognized involvement of an abnormal immune response in the pathogenesis of AFE makes these data intriguing.2,3,8,48
Clinical implications
The classic clinical signs of AFE, including peripartum respiratory distress, hypoxia, profound hypotension, cardiovascular collapse, and disseminated intravascular coagulopathy, have been consistently reported in the literature as commonalities in AFE, regardless of varying global case definitions.8,15–22,24,30–32,40–43,46–49 When applying these diagnostic criteria for the research reporting of AFE to our registry, 100% of typical and 67% of atypical AFE cases met the definition.24
Clinical features of AFE also include profound cardiovascular changes. Available evidence suggests that the hemodynamic response to AFE initially presents with increased pulmonary vascular resistance, and right ventricular failure, followed by left ventricular dysfunction.9,14–22,25,35–39 In all registries and research reports, 83–100% of patients demonstrated laboratory abnormalities or clinical findings consistent with DIC, regardless of mode of delivery.6,14–22,24,25,46,47 Although debated, the reporting criteria used for the diagnosis of DIC in AFE-related coagulopathy has mainly relied on the scoring system of Scientific and Standardization Committee on DIC of the ISTH (International Society on Thrombosis and Haemostasis), modified for pregnancy, which, in one study, showed low sensitivity for critical care parturients. This limitation should be interpreted with caution as AFE cases were not included in the study.24,44
Research implications
Since AFE was first being recognized as a distinct clinical syndrome, over 1300 case reports and series have been published in efforts to clarify the etiology, risk factors, and pathogenesis.1–8,10–12,15–22,27,34–39,44–47,50 Historically, such analyses have often been challenged by inconsistent reporting criteria.35 Series relying mainly on hospital diagnostic codes for case identification are often inaccurate, as coding may report the cause of maternal death as AFE with inclusion of patients who, in fact, did not have AFE, in the absence of diagnostic validation.3,9,13,14,24 Such errors may be related to the nature of the coding process, in which any diagnosis considered and ultimately discarded, regardless of how temporary or speculative, often finds its way into a list of diagnostic codes at the time of discharge, and will be identified as a case in any electronic data extraction process. Such errors complicate calculations of frequency, risk factors, and morbidity and mortality associated with this uncommon condition.9–11,14–25
Continued erroneous reliance on the detection of squamous cells in the pulmonary circulation as a pathognomonic criterion for AFE diagnosis regardless of clinical presentation also plays a role in erroneous diagnoses.28 Romero et al hypothesize subclinical infection as an etiology of AFE based on 2 cases of proposed AFE where supralethal maternal plasma TNF-α levels were present prior to the clinical AFE event.48 Laboratory analyses of various substances found in amniotic fluid or thought to be involved in the pathophysiologic response (zinc coproporphyrin, Sialyl Tn antigen, serum tryptase, complement C3 and C4, intrinsic and extrinsic immunologic pathways) have been studied and reported, but, to date, none appear reliable in predicting or diagnosing AFE.11–13,20,24
As a result of these inconsistencies, the recognition of disease, pathophysiology, and treatment strategies have remained elusive, and further studies that will involve a more comprehensive analysis of maternal and fetal biomarkers collected from women who have had typical and unequivocal AFE are necessary and a priority for the authors.9,14,24,25
Strengths and Limitations
Strengths of this study include the extensive experience of the authors in clinical AFE research and publication as well as the nature of the data origin; the investigators who analyzed the data had no part in determining submission of cases through the Foundation website for analysis. In addition, this represents the largest reported series of patients with AFE that has been diagnosed and analyzed by expert chart review. Limitations of the study include those of any investigation based on voluntary self-reporting of data. Rational, but speculative, arguments exist supporting the concept that such a database may be biased to include either more, or fewer, patients with adverse outcomes than actually exist in the general population. For example, it seems likely that atypical and, hence, less severe cases of AFE are underrepresented in the reported registry data and that fatal cases may not have been entered due to family or provider enrollment apprehension. There is also a potential of over-reporting according to socioeconomic status. Thus, extrapolations of data describing specific mortality rates to the general population of women experiencing AFE would be invalid. However, we believe that a comparison of these data to those in the original registry report of 1995 and to other research studies and registries remains instructive and supports an improvement in maternal and neonatal outcome since that time. While this large, contemporary series represents a useful picture of the clinical details of women with AFE in contemporary practice, the nature of registry data precludes a direct statistical comparison of demographic variables and medical/obstetric risk factors between women with and without AFE. Such comparisons have been attempted using large populations derived from administrative data sets; however, the contamination of such data with significant numbers of women who did not have AFE makes such data equally problematic. Although our data suggest a possible relationship between in vitro fertilization, maternal atopic history, and placenta previa, the absolute risk of AFE for women with any of these conditions is so small as to preclude their use in clinical management; at the present time, no risk factors exist that would alter standard obstetric care. AFE remains both unpredictable and unpreventable.
Conclusion
Future collaborations and ongoing collection of cases and lab specimens in this international registry will help clarify various aspects of this critical condition and will allow for improved diagnosis and management.
AJOG MFM at a Glance.
Why was this study conducted?
To analyze the largest United States-based international amniotic fluid embolism (AFE) registry, using the published and validated criteria for the research reporting of AFE, and to determine whether there were differences in clinical characteristics and outcomes between patients with typical vs atypical AFE.
What are the key findings?
There were no consistent differences in demographic or obstetric variables in women with typical vs atypical AFE. Women with AFE were twice as likely to report a history of atopy, latex, medication, or food allergy than the obstetric population delivered at our hospital during the same time period, several times more likely to have conceived through in vitro fertilization, and more than 10 times more likely to have placental previa than the general US population. Overall maternal and neonatal morbidity and mortality were improved compared to those in previously published reports.
Of women misclassified as having an AFE with an alternative diagnosis, the most common diagnosis was hypovolemic shock secondary to postpartum hemorrhage, followed by anesthetic complications and sepsis-related cardiovascular collapse.
There were no instances of recurrence of AFE among the women with either typical or atypical AFE in subsequent pregnancies.
What does this study add to what is already known?
Contemporary clinical characteristics and perinatal outcomes of women with AFE are defined through an analysis of the largest available medical records-based data set, using the criteria published for the research reporting of AFE. Although no definitive risk factors were identified, an unexpected observation was the high rate of reported allergy, consistent with a putative mechanism of disease – an anaphylactoid reaction.
Funding Statement:
This research was supported, in part, by the Perinatology Research Branch, Division of Obstetrics and Maternal-Fetal Medicine, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, US Department of Health and Human Services (NICHD/NIH/DHHS); and, in part, with Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C. Dr. Romero has contributed to this work as part of his official duties as an employee of the United States Federal Government.
Footnotes
Disclosure: The authors declare no conflicts of interest.
Abstracts were presented at The Society for Maternal Fetal Medicine 38th and 39th Annual Pregnancy Meeting, Jan. 29–Feb. 3, 2018, Las Vegas, NV, and will be presented Feb. 11–16, 2020, in Dallas, TX.
REFERENCES
- 1).Steiner PE, Lusbaugh C. Maternal pulmonary embolism by amniotic fluid as a cause of obstetric shock and unexplained death in obstetrics. JAMA. 1941;117:1245–1254. [DOI] [PubMed] [Google Scholar]
- 2).Clark SL, Hankins GD, Dudley DA, Dildy GA, Porter TF. Amniotic fluid embolism: analysis of the national registry. Am J Obstet Gynecol. 1995;172:1158. [DOI] [PubMed] [Google Scholar]
- 3).Clark SL, Christmas JT, Frye DR et al. Maternal Mortality in the U.S. – Predictability and the Impact of protocols on fatal post-cesarean pulmonary embolism and hypertension-related intracranial hemorrhage. Am J Obstet Gynecol 2014;211:32.e1–9 [DOI] [PubMed] [Google Scholar]
- 4).Moore J, Baldisseri MR. Amniotic fluid embolism. Crit Care Med. 2005;33:S279–S285. [DOI] [PubMed] [Google Scholar]
- 5).Drukker L, Sela HY, Ioscovich A, Samueloff A, Grisaru-Granovsky S Amniotic Fluid Embolism: A Rare Complication of Second-Trimester Amniocentesis. Fetal Diagn Ther. 2017;42(1):77–80. [DOI] [PubMed] [Google Scholar]
- 6).Conde-Agudelo A, Romero R. Amniotic fluid embolism: an evidence-based review. Am J Obstet Gynecol. 2009;201(5):445.e1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7).Abenhaim HA, Azoulay L, Kramer MS, Leduc L. Incidence and risk factors of amniotic fluid embolisms: a population based study on 3 million births in the United States. Am J Obstet Gynecol. 2008;199:49e1–e8. [DOI] [PubMed] [Google Scholar]
- 8).Thongrong C, Kasemsiri P, Hofmann JP, Bergese SD, Papadimos TJ, Gracias VH, et al. Amniotic fluid embolism. Int J Crit Illn Inj Sci. 2013;3:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Conde-Agudelo A, Romero R. “Amniotic fluid embolism: an evidence-based review,” Am J Obstet Gynecol, 2009; 201:445:e1–.e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Pacheco LD, Saade G, Hankins GD, Clark SL. Amniotic fluid embolism: diagnosis and management. Society for Maternal-Fetal Medicine (SMFM). Electronic address: pubs@smfm.org. Am J Obstet Gynecol. 2016;215:B16–24. [DOI] [PubMed] [Google Scholar]
- 11).Royal College of Obstetricians and Gynecologists, UK Obstetric Surveillance System. Available at: https://www.rcog.org.uk/. Accessed August 30, 2019
- 12).Nuffield Department of Human Health, University of Oxford. Amniotic fluid embolism. Available at: https://www.npeu.ox.ac.uk/ukoss/current-surveillance/amf. Accessed June 9, 2016.
- 13).Australasian Maternal Outcomes Surveillance System. Amniotic fluid embolism. Available at: http://www.amoss.com.au/?q=content/amniotic-fluid-embolism-afe. Accessed June 2016.
- 14).Hasegawa J, Sekizawa A, Tonaka H. et al. Current status of pregnancy-related maternal mortality in Japan: a report from the Maternal Death Exploratory Committee in Japan. BMJ Open. 2016;6:e010304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15).Clark SL. Amniotic fluid embolism. Obstet Gynecol 2014;123:337–48. [DOI] [PubMed] [Google Scholar]
- 16).Clark SL, Romero R, Dildy GA, Callaghan WM, et al. Proposed diagnostic criteria for the case definition of amniotic fluid embolism in research studies. Am J Obstet Gynecol. 2016;215:408–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Stafford I, Moaddab A, Klassen M, Belfort MA, Dildy GA, Clark SL. Diagnostic precision of proposed criteria for research reporting of amniotic fluid embolism. American Journal of Obstetrics and Gynecology 2019. 220(3):285–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Moaddab A, Klassen M, Preister CDE, Munoz EH, Belfort MA, Clark SL, Dildy GA. Reproductive decisions after the diagnosis of amniotic fluid embolism Eur J Obstet Gynecol Repro Biol 2017;211: 33–36 [DOI] [PubMed] [Google Scholar]
- 19).Sunderam S, Kissin DM, Crawford SB, Folger SG, Boulet SL, Warner L, Barfield WD. Assisted Reproductive Technology Surveillance – United States 2015. MMWR Surveill Summ 2018:16;67(3): 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20).Hogberg U, Joelsson I. Amniotic fluid embolism in Sweden, 1951–1980. Gynecol Obstet Invest. 1985;20:130–137. [DOI] [PubMed] [Google Scholar]
- 21).Gilbert WM, Danielsen B. Amniotic fluid embolism: decreased mortality in a population-based study. Obstet Gynecol. 1999;93:973–977. [DOI] [PubMed] [Google Scholar]
- 22).Tuffnell DJ. United kingdom amniotic fluid embolism register. BJOG. 2005;112:1625–1629. [DOI] [PubMed] [Google Scholar]
- 23).Kramer MS, Rouleau J, Baskett TF, Joseph KS. Maternal Health Study Group of the Canadian Perinatal Surveillance System. Amniotic-fluid embolism and medical induction of labour: a retrospective, population-based cohort study. Lancet. 2006;368:1444–1448. [DOI] [PubMed] [Google Scholar]
- 24).Roberts CL, Algert CS, Knight M, Morris JM. Amniotic fluid embolism in an Australian population-based cohort. BJOG. 2010;117:1417–1421. [DOI] [PubMed] [Google Scholar]
- 25).Stolk KH, Zwart JJ, Schutte J, van Roosmalen J. Severe maternal morbidity and mortality from amniotic fluid embolism in the Netherlands. Acta Obstet Gynecol Scand. 2012;91:991–995. [DOI] [PubMed] [Google Scholar]
- 26).Hasegawa J, Sekizawa A, Tanaka H, Katsuragi S, Osato K, Murakoshi T, et al. Current status of pregnancy-related maternal mortality in Japan: a report from the Maternal Death Exploratory Committee in Japan. BMJ Open 2016. 6(3), e010304. doi: 10.1136/bmjopen-2015-010304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27).Morau E, Proust A, & Ducloy JC [Maternal deaths due to amniotic fluid embolism. Results from the French confidential enquiry into maternal deaths, 2010–2012]. Gynecol Obstet Fertil Senol 2017. 45(12S), S43–S47. doi: 10.1016/j.gofs.2017.10.014 [DOI] [PubMed] [Google Scholar]
- 28).Girard P, Mal H, Laine JF, Petitpretz P, Rain B, Duroux P. Left heart failure in amniotic fluid embolism. Anesthesiology. 1986;64:262–265. [DOI] [PubMed] [Google Scholar]
- 29).Funk M, Damron A, Bandi V, Aagaard K, Szigeti R, Clark SL. Pulmonary vascular obstruction by squamous cells is not involved in amniotic fluid embolism Am J Obstet Gynecol. 2018; 218, 460 – 461. [DOI] [PubMed] [Google Scholar]
- 30).McDonnell N, Knight M, Peek MJ, Ellwood D, Homer CS, McLintock C, Vaughan G, Pollock W, Li Z, Javid N, Sullivan E, the Australasian Maternity Outcomes Surveillance System (AMOSS). Amniotic fluid embolism: an Australian-New Zealand population-based study. BMC Pregnancy Childbirth. 2015. December 24;15:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31).Spiliopoulos M, Puri I, Jain NJ, Kruse L, Mastrogiannis D, Dandolu V. Amniotic fluid embolism-risk factors, maternal and neonatal outcomes. J Matern Fetal Neonatal Med. 2009;22(5):439–44. [DOI] [PubMed] [Google Scholar]
- 32).Indraccolo U, Battistoni C, Mastrantonio I, Di Iorio R, Greco P, & Indraccolo SR Risk factors for fatality in amniotic fluid embolism: a systematic review and analysis of a data pool. J Matern Fetal Neonatal Med 2018. 31(5), 661–665. [DOI] [PubMed] [Google Scholar]
- 33.Barnhart ML, Rosenbaum K. Anaphylactoid syndrome of pregnancy. Nurs Womens Health 2019;23:38–48. [DOI] [PubMed] [Google Scholar]
- 34.Oda T, Tamura N, Kanayama N. Japanese viewpoint on amniotic fluid embolism. Am J Obstet Gynecol 2017;217:91. [DOI] [PubMed] [Google Scholar]
- 35.Hasaart TH, Essed GG. Amniotic fluid embolism after transabdominal amniocentesis. Eur J Obstet Gynecol Reprod Biol 1983;16:25–30. [DOI] [PubMed] [Google Scholar]
- 36.Judich A, Kuriansky J, Engelberg I, Haik J, Shabtai M, Czerniak A. Amniotic fluid embolism following blunt abdominal trauma in pregnancy. Injury 1998;29:475–7. [DOI] [PubMed] [Google Scholar]
- 37.Maher JE, Wenstrom KD, Hauth JC, Meis PJ. Amniotic fluid embolism after saline amnioinfusion: two cases and review of the literature. Obstet Gynecol 1994;83:851–4. [PubMed] [Google Scholar]
- 38.Rainio J, Penttila A. Amniotic fluid embolism as cause of death in a car accident—a case report. Forensic Sci Int 2003;137:231–4. [DOI] [PubMed] [Google Scholar]
- 39.Ray BK, Vallejo MC, Creinin MD, et al. Amniotic fluid embolism with second trimester pregnancy termination: a case report. Can J Anaesth 2004;51:139–44. [DOI] [PubMed] [Google Scholar]
- 40.Fitzpatrick KE, Tuffnell D, Kurinczuk JJ, Knight M. Incidence, risk factors, management and outcomes of amniotic-fluid embolism: a population-based cohort and nested case-control study. BJOG 2016;123:100–9. [DOI] [PubMed] [Google Scholar]
- 41.Kanayama N, Tamura N. Amniotic fluid embolism: pathophysiology and new strategies for management. J Obstet Gynaecol Res 2014;40:1507–7. [DOI] [PubMed] [Google Scholar]
- 42.Skolnik S, Ioscovich A, Eidelman LA, et al. Anesthetic management of amniotic fluid embolismea multi-center, retrospective, cohort study. J Matern Fetal Neonatal Med 2019;32:1262–6. [DOI] [PubMed] [Google Scholar]
- 43.Fong A, Chau CT, Pan D, Ogunyemi DA. Amniotic fluid embolism: antepartum, intrapartum and demographic factors. J Matern Fetal Neonatal Med 2015;28:793–8. [DOI] [PubMed] [Google Scholar]
- 44.Pacheco PD, Clark SL, Klassen M. Amniotic fluid embolism: principles of early clinical management. Am J Obstet Gynecol 2020;222: 48–52. [DOI] [PubMed] [Google Scholar]
- 45.Estelles A, Gilabert J, Andres C, Espana F, Aznar J. Plasminogen activator inhibitors type 1 and type 2 and plasminogen activators in amniotic fluid during pregnancy. Thromb Haemost 1990;64:281–5. [PubMed] [Google Scholar]
- 46.Clark SL. New concepts of amniotic fluid embolism: a review. Obstet Gynecol Surv 1990;45:360–8. [DOI] [PubMed] [Google Scholar]
- 47.Clark SL, Montz FJ, Phelan JP. Hemodynamic alterations associated with amniotic fluid embolism: a reappraisal. Am J Obstet Gynecol 1985;151:617–21. [DOI] [PubMed] [Google Scholar]
- 48.Romero R, Kadar N, Vaisbuch E, Hassan SS. Maternal death following cardiopulmonary collapse after delivery: amniotic fluid embolism or septic shock due to intrauterine infection? Am J Reprod Immunol 2010;64:113–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.West M Amniotic fluid embolism: a historical perspective in diagnosis and management. BJOG 2016;123:110. [DOI] [PubMed] [Google Scholar]
- 50.Erez O Proposed diagnostic criteria for the case definition of amniotic fluid embolism in research studies. Am J Obstet Gynecol 2017;217:228–9. [DOI] [PubMed] [Google Scholar]


