Abstract
Objectives
During the COVID-19 pandemic, maintenance of safe and timely oncologic care has been challenging. The goal of this study is to compare presenting symptoms, staging, and treatment of head and neck mucosal squamous cell carcinoma during the pandemic with an analogous timeframe one year prior.
Materials and methods
Retrospective cohort study at a single tertiary academic center of new adult patients evaluated in a head and neck surgical oncology clinic from March -July 2019 (pre-pandemic control) and March - July 2020 (COVID-19 pandemic).
Results
During the pandemic, the proportion of patients with newly diagnosed malignancies increased by 5%, while the overall number of new patients decreased (n = 575) compared to the control year (n = 776). For patients with mucosal squamous cell carcinoma (SCC), median time from referral to initial clinic visit decreased from 11 days (2019) to 8 days (2020) (p = 0.0031). There was no significant difference in total number (p = 0.914) or duration (p = 0.872) of symptoms. During the pandemic, patients were more likely to present with regional nodal metastases (adjusted odds ratio (OR) 2.846, 95% CI 1.072-3.219, p = 0.028) and more advanced clinical nodal (N) staging (p = 0.011). No significant difference was seen for clinical tumor (T) (p = 0.502) or metastasis (M) staging (p = 0.278). No significant difference in pathologic T (p = 0.665), or N staging (p = 0.907) was found between the two periods.
Conclusion
Head and neck mucosal SCC patients presented with more advanced clinical nodal disease during the early months of the COVID-19 pandemic despite no change in presenting symptoms.
Keywords: Mucosal squamous cell carcinoma, Head and neck cancer, Covid-19
1. Introduction
The COVID-19 pandemic has created an unprecedented challenge for the United States healthcare system. While the pandemic has focused medical attention on treating COVID-19 patients, caring for patients with non-COVID-related diseases has also been impacted. The field of head and neck oncology, in particular, has faced unique challenges due to the SARS-CoV-2 virus’ transmission through the nasal and respiratory tracts in many patients [1].
Oncologic treatment continued at many tertiary hospital systems across the country during the COVID-19 pandemic [2], [3]. For many solid malignancies, treatment delays have been reported and patient encounters for cancer treatment have decreased during the pandemic [4], [5], [6]. There have been reports of delays associated with head and neck cancer care [7], [8], although there is minimal evidence around how delays impact oncologic care. Patients themselves acknowledge delaying cancer care due to pandemic-related concerns [9]. Fear of seeking medical attention as well as decreased access to medical care at entry points such as primary care providers and referring specialists may impact patients’ ability to be evaluated in a timely manner, prompting shifts in paradigms for balancing risk and benefit of oncologic treatment [10]. This is particularly important for patients with head and neck cancer, as it is well-established that delays in treatment or screening result in tumor progression, advanced stage of disease and decreased survival [11], [12], [13], [14], [15], [16], [17].
While existing literature has discussed guidelines for the care of head and neck cancer patients during the COVID-19 pandemic [18], [19], [20], [21], [22], [23], [24], the impact of potential pandemic-related changes in presentation and definitive management of head and neck cancer patients has yet to be fully explored. The purpose of the present study is to determine the impact of the COVID-19 pandemic on presentation and clinical and pathologic staging in head and neck cancer patients. We hypothesize that patients presenting during the COVID-19 will present with longer duration of symptoms, higher number of presenting symptoms, and more advanced clinicopathologic staging.
2. Materials and methods
This was a retrospective cohort study via chart review at a single tertiary academic center. We aimed to capture clinical practice at a head and neck surgical oncology clinic impacted by the COVID-19 pandemic. Data was collected for patients presenting from 1) March – July 2020 during the pandemic period and from 2) March – July 2019 as a pre-pandemic control period. Of note, the head and neck oncology team did not have any major changes in clinical staff during the entire study period. This study received institutional review board approval (IRB #201879).
2.1. Inclusion and exclusion criteria
All new patients who presented to the head and neck surgical oncology clinic were identified. Patients were excluded if they were already established with the clinic, less than 18 years of age, or had incomplete data available in the electronic medical record for clinical staging. Patients with mucosal SCC were included for further analyses, and patients with benign or systemic disease (such as lymphoma) were excluded.
2.2. Data collection
Patient demographics (age, gender, race/ethnicity, insurance status) and reason for the visit (benign vs. malignant pathology) were identified. Additional data for patients with newly diagnosed malignancy was gathered, including referral pattern, symptoms and duration at presentation, histology and location of malignancy, p-16 status, and oncologic treatment. Referrals are provider mediated and patient self-referrals. Symptoms included dysphagia, dyspnea, pain, bleeding, neck mass, otalgia and weight loss. Duration of symptoms was calculated in days based on patient report as documented in the medical record. Treatment modality was identified for each patient as 1) primary surgical intervention, 2) primary radiotherapy (RT), 3) primary chemoradiotherapy (CRT), 4) palliative RT, 5) palliative chemotherapy, 6) palliative chemoradiotherapy 7) lost to follow-up. These were further grouped into curative intent (groups 1-3), palliative intent (groups 4-6) and lost to follow-up.
In addition, clinical and pathologic staging data was collected for patients with mucosal squamous cell carcinoma (SCC) of the head and neck. These were staged by the authors (MS, AP and MT) in accordance with the latest AJCC 8th Edition Cancer Staging Guidelines [25]. Tumor (T) stage was evaluated using ordinal scales and then categorized into two subgroups of early (T1, T2) and advanced (T3, T4). Nodal (N) disease was evaluated using ordinal scales and grouped into three ordinal disease categories: early (N 0, N1, N2a), middle (N2, N2b, N2c) or advanced (N3, N3a, N3b). Nodal staging was further grouped into binary categories: no clinical nodal disease (N0) and any nodal disease present. For patients that underwent surgical resection, pathologic TNM staging and whether cancers were pathologically upstaged was also recorded. Upstaging was derived from reported pathologic and clinical staging and defined as pathologic staging more advanced than clinical staging. The following upstaging outcomes were evaluated: 1) clinical T stage to pathologic T stage, 2) clinical N stage to pathologic N stage, and 3) overall upstaging. All data was collected and managed using secure REDCap electronic data capture tools [26].
2.3. Statistical analysis
Patient characteristics including demographics, referral pattern, presenting symptoms, oncologic treatment, clinical staging, and pathological staging and upstaging were summarized for the pandemic and non-pandemic time frame using mean, standard deviation and quartiles for continuous variables and frequency and proportions for categorical variables. Comparisons on patient characteristics between two periods were conducted using Wilcoxon Rank-sum test for continuous variables and Chi-square test for categorical variables. Regression models were conducted for outcomes of clinical TNM staging at presentation and pathologic TNM staging and upstaging, using logistic regression for binary outcomes and proportional odds model ordinal outcomes. Adjusting variables included potential confounding factors such as age, gender, insurance status and location of malignancy. All analyses were conducted using R (version 4.0.4).
3. Results
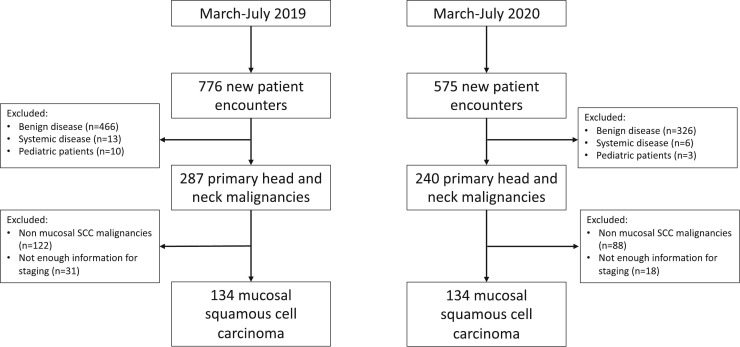
The total number of new patient encounters decreased from March - July during the pandemic year (n = 575) compared to the pre-pandemic year (n = 776). A decrease was seen for patients with benign conditions (60% to 56%), while the overall proportion of patients with newly identified malignancies increased from 2019 to 2020 (37% to 42%). Two hundred and sixty-eight patients with mucosal SCC were evaluated during the study period and included in further analysis (n = 134 from 2019, n = 134 from 2020) (Fig. 1 ).
Fig. 1.
Consort diagram for patients included in the study during the pre-pandemic and pandemic time periods.
3.1. Demographics
No significant differences were found between age, gender, race, insurance status or primary site of malignancy. There was no significant difference in referral patterns during the pandemic compared to the control year, with the majority of patients referred from ENT providers outside the hospital system (53.7% in 2019 v 56.7% in 2020, p = 0.505). The median time from receipt of referral to initial clinic visit decreased from 11 days during the control year to 8 days during the pandemic (p = 0.003). While a lower percentage of patients reported a history of prior cancer in 2020, this was not significant compared to 2019 (26.9% in 2020 v 35.1% in 2019, p = 0.186) (Table 1 ).
Table 1.
Demographic and clinical characteristics of study participants for years 2019 and 2020. Bolded p-value indicates statistical significance (p < 0.05). OMFS = oromaxillofacial surgeon.
| Year 2019 |
Year 2020 |
p-Value | |
|---|---|---|---|
| (n = 134) | (n = 134) | ||
| Age (years) | |||
| Mean (standard deviation) | 62.9 (11.9) | 64.5 (10.5) | 0.26 |
| Gender | n (%) | n (%) | |
| Female | 33 (24.6%) | 36 (26.9%) | 0.78 |
| Male | 101 (75.4%) | 98 (73.1%) | |
| Race | |||
| White/Caucasian | 120 (89.6%) | 117 (87.3%) | 0.833 |
| African-American | 4 (3.0%) | 6 (4.5%) | |
| Other | 10 (7.5%) | 10 (7.5%) | |
| Insurance status | |||
| Medicare/Medicaid | 60 (44.8%) | 72 (53.7%) | 0.281 |
| Private Insurance | 67 (50.0%) | 54 (40.3%) | |
| Self-pay/Uninsured | 7 (5.2%) | 8 (6.0%) | |
| Malignancy | |||
| Hypopharyngeal | 2 (1.5%) | 5 (3.7%) | 0.561 |
| Laryngeal | 25 (18.7%) | 25 (18.7%) | |
| Sinonasal/Nasopharyngeal | 9 (6.7%) | 8 (6.0%) | |
| Oral cavity | 49 (36.6%) | 56 (41.8%) | |
| Oropharyngeal p16 positive | 38 (28.4%) | 34 (25.4%) | |
| Oropharyngeal p16 negative | 5 (3.7%) | 3 (2.2%) | |
| p16 negative unknown primary | 6 (4.5%) | 2 (1.5%) | |
| Specialty of referring provider | |||
| Dentist/OMFS | 20 (14.9%) | 17 (12.7%) | 0.505 |
| Oncologist | 13 (9.7%) | 12 (9.0%) | |
| Community otolaryngologist | 72 (53.7%) | 76 (56.7%) | |
| Primary care provider | 10 (7.5%) | 8 (6.0%) | |
| Emergency department | 1 (0.7%) | 5 (3.7%) | |
| Vanderbilt-affiliated otolaryngologist | 10 (7.5%) | 9 (6.7%) | |
| Other | 8 (6.0%) | 3 (2.2%) | |
| History of prior cancer | |||
| Yes | 47 (35.1%) | 36 (26.9%) | 0.186 |
| No | 87 (64.9%) | 98 (73.1%) | |
| Primary treatment | |||
| Chemoradiation | 33 (24.6%) | 37 (27.6%) | 0.705 |
| Surgery | 79 (59.0%) | 69 (51.5%) | |
| Radiation | 3 (2.2%) | 4 (3.0%) | |
| Palliative chemotherapy | 5 (3.7%) | 6 (4.5%) | |
| Palliative radiation | 0 (0%) | 1 (0.7%) | |
| Palliative chemoradiation | 3 (2.2%) | 1 (0.7%) | |
| Lost to follow up | 11 (8.2%) | 16 (11.9%) | |
| Patient-reported symptoms: | |||
| Dysphagia | |||
| Yes | 39 (29.1%) | 42 (31.3%) | 0.79 |
| No | 95 (70.9%) | 92 (68.7%) | |
| Dyspnea | |||
| Yes | 10 (7.5%) | 10 (7.5%) | 1 |
| No | 124 (92.5%) | 124 (92.5%) | |
| Pain | |||
| Yes | 83 (61.9%) | 88 (65.7%) | 0.611 |
| No | 51 (38.1%) | 46 (34.3%) | |
| Otalgia | |||
| Yes | 29 (21.6%) | 15 (11.2%) | 0.031 |
| No | 105 (78.4%) | 119 (88.8%) | |
| Bleeding | |||
| Yes | 8 (6.0%) | 12 (9.0%) | 0.487 |
| No | 126 (94.0%) | 122 (91.0%) | |
| Neck mass | |||
| Yes | 36 (26.9%) | 39 (29.1%) | 0.786 |
| No | 98 (73.1%) | 95 (70.9%) | |
| Weight loss | |||
| Yes | 23 (17.2%) | 20 (14.9%) | 0.74 |
| No | 111 (82.8%) | 114 (85.1%) | |
| Number of symptoms | |||
| Mean (standard deviation) | 1.70 (1.16) | 1.69 (1.09) | 0.914 |
| Duration of symptoms (weeks) | |||
| Mean (standard deviation) | 6.82 (14.3) | 6.54 (13.0) | 0.872 |
3.2. Presenting symptoms and treatment patterns
There was a significant decrease in otalgia between 2019 and 2020 (21.6% v 11.2%, p = 0.031). Otherwise, there was no difference in presence of any of the recorded presenting symptoms between the pandemic and non-pandemic year. Similarly, there was no difference in total number of presenting symptoms or duration of presenting symptoms. There was no significant difference in treatment choice between 2019 and 2020 (p = 0.705), although there was a trend to fewer patients treated with surgery in 2020 (n = 79 in 2019, n = 69 in 2020) and more patients treated with chemoradiation (n- = 33 in 2019 and n = 37 in 2020). More patients were lost to follow up during the pandemic (n = 11 in 2019 and n = 16 in 2020).
3.3. Clinical staging, pathological staging and upstaging
During the COVID-19 pandemic, a significantly higher proportion of patients presented with more advanced clinical N staging compared to a pre-pandemic period (Table 2A). Patients during the COVID-19 pandemic were significantly more likely to present with nodal metastases (≥ N1) compared to the pre-pandemic period with adjusted odds ratio (aOR) of 1.846 (95% CI 1.072-3.219, p = 0.028) (Table 3A). Similar results were observed for ordinal outcome of clinical N staging with proportional odds model (Table 3B). Patients during the COVID-19 pandemic were more likely to have advanced clinical N staging (when comparing early, middle, and advanced levels) (aOR 2.141, 95% CI 1.195-3.836, p = 0.011) (Fig. 2 ). Patients with hypopharyngeal (aOR 4.482, 95% CI 1.030-19.500, p = 0.047), laryngeal (aOR 2.195, 95% CI 1.024-4.706, p = 0.044), and p16 negative unknown primary disease (aOR 12.915, 95% CI 2.876-57.996, p = 0.001) were more likely to present with advanced nodal disease compared to the reference level (oral cavity). Patients who were male gender were less likely to present with advanced nodal disease (aOR 0.511, 95% CI 0.271-0.966, p = 0.040). No significant difference was seen for clinical T staging (aOR 1.190, 95% CI 0.715-1.984, p = 0.502) or M staging (aOR 2.307, 95% CI 0.543-12.479, p = 0.278) between the pandemic and pre-pandemic periods (Supplemental Table 1 and Supplemental Table 2).
Table 2.
(A): Clinical staging of study participants for years 2019 and 2020. (B): Pathologic staging of study participants for years 2019 and 2020. Upstaging calculated as described in the methods section. T = Tumor, N=Nodal, M = Metastases. Bolded p-value indicates statistical significance (p < 0.05).
| A. Clinical staging | Year 2019 |
Year 2020 |
p-Value |
|---|---|---|---|
| (n = 134) |
(n = 134) |
||
| n (%) | n (%) | ||
| Clinical T stage | |||
| 0 | 13 (9.7%) | 6 (4.5%) | 0.488 |
| 1 | 22 (16.4%) | 25 (18.7%) | |
| 2 | 39 (29.1%) | 34 (25.4%) | |
| 3 | 25 (18.7%) | 34 (25.4%) | |
| 4 | 14 (10.4%) | 18 (13.4%) | |
| 4a | 18 (13.4%) | 15 (11.2%) | |
| 4b | 3 (2.2%) | 2 (1.5%) | |
| Clinical N stage | |||
| 0 | 69 (51.5%) | 57 (42.5%) | 0.028 |
| 1 | 33 (24.6%) | 32 (23.9%) | |
| 2 | 7 (5.2%) | 9 (6.7%) | |
| 2a | 5 (3.7%) | 0 (0%) | |
| 2b | 10 (7.5%) | 19 (14.2%) | |
| 2c | 4 (3.0%) | 12 (9.0%) | |
| 3 | 3 (2.2%) | 1 (0.7%) | |
| 3a | 0 (0%) | 2 (1.5%) | |
| 3b | 3 (2.2%) | 2 (1.5) | |
| Clinical M stage | |||
| 0 | 131 (97.8%) | 128 (95.5%) | 0.5 |
| 1 | 3 (2.2%) | 6 (4.5%) | |
| B. Pathologic staging & upstaging | Year 2019 |
Year 2020 |
p-Value |
|---|---|---|---|
| (n = 77) |
(n = 69) |
||
| n (%) | N (%) | ||
| Pathologic T stage | |||
| 0 | 10 (13.0%) | 4 (5.8%) | 0.368 |
| 1 | 8 (10.4%) | 15 (21.7%) | |
| 2 | 18 (23.4%) | 13 (18.8%) | |
| 3 | 16 (20.8%) | 14 (20.3%) | |
| 4 | 1 (1.3%) | 2 (2.9%) | |
| 4a | 21 (27.3%) | 20 (29.0%) | |
| 4b | 3 (3.9%) | 1 (1.4%) | |
| Pathologic N stage | |||
| 0 | 40 (51.9%) | 39 (56.5%) | 0.993 |
| 1 | 13 (16.9%) | 11 (15.9%) | |
| 2 | 2 (2.6%) | 1 (1.4%) | |
| 2a | 3 (3.9%) | 2 (2.9%) | |
| 2b | 7 (9.1%) | 5 (7.2%) | |
| 2c | 0 (0%) | 0 (0%) | |
| 3 | 0 (0%) | 0 (0%) | |
| 3a | 0 (0%) | 0 (0%) | |
| 3b | 12 (15.6%) | 11 (15.9%) | |
| Pathologic M stage | |||
| 0 | 77 (100%) | 69 (100%) | 1 |
| 1 | 0 (0%) | 0 (0%) | |
| Presence of T upstaging | |||
| Yes | 20 (26.0%) | 18 (26.1%) | 1 |
| No | 57 (74.0%) | 51 (73.9%) | |
| Presence of N Upstaging | |||
| Yes | 21 (27.3%) | 14 (20.3%) | 0.34 |
| No | 56 (72.7%) | 55 (79.7%) | |
| Presence of T or N Upstaging | |||
| Yes | 34 (44.2%) | 28 (40.6%) | 0.738 |
| No | 43 (55.8%) | 41 (59.4%) | |
Table 3.
(A) Binary logistic modeling for clinical staging for regional nodal metastases (≥N1 disease) and (B) Ordinal logistic modeling for clinical staging for advanced regional nodal metastases (grouping as described in methods). Bolded p-value indicates statistical significance (p < 0.05).
| (A) | ||||
|---|---|---|---|---|
| Variable | OddsRatio | LowerBound | UpperBound | p-Value |
| Intercept | 0.930 | 0.104 | 8.250 | 0.948 |
| Age (Years) | 0.987 | 0.959 | 1.016 | 0.383 |
| Male | 0.788 | 0.422 | 1.461 | 0.451 |
| Private insurance | 1.191 | 0.607 | 2.333 | 0.609 |
| Self-pay/uninsured | 1.879 | 0.557 | 6.671 | 0.313 |
| Hypopharyngeal | 4.769 | 0.936 | 35.348 | 0.076 |
| Laryngeal | 1.524 | 0.747 | 3.111 | 0.246 |
| Sinonasal/Nasopharyngeal | 0.735 | 0.215 | 2.210 | 0.599 |
| Oropharyngeal | 7.766 | 3.932 | 16.062 | 0.000 |
| p16 negative unknown primary | 37,492,219.710 | 0.000 | NA | 0.983 |
| Year 2020 | 1.846 | 1.072 | 3.219 | 0.028 |
| (B) | ||||
|---|---|---|---|---|
| Variable | OddsRatio | LowerBound | UpperBound | p-Value |
| Age (Years) | 0.988 | 0.959 | 1.019 | 0.451 |
| Male | 0.511 | 0.271 | 0.966 | 0.040 |
| Private insurance | 0.973 | 0.486 | 1.948 | 0.938 |
| Self-pay/uninsured | 1.896 | 0.574 | 6.260 | 0.295 |
| Hypopharyngeal | 4.482 | 1.030 | 19.500 | 0.047 |
| Laryngeal | 2.195 | 1.024 | 4.706 | 0.044 |
| Sinonasal/Nasopharyngeal | 0.495 | 0.102 | 2.412 | 0.385 |
| Oropharyngeal | 1.415 | 0.686 | 2.916 | 0.348 |
| p16 negative unknown primary | 12.915 | 2.876 | 57.996 | 0.001 |
| Year 2020 | 2.141 | 1.195 | 3.836 | 0.011 |
Fig. 2.
Forest plot of adjusted regression analysis for clinical T staging, N staging in binary (≥N1 disease) and ordinal grouping (as described in methods section), and M staging (top). Forest plot of adjusted regression analysis for pathologic N staging in binary and ordinal grouping and T staging (bottom). OR = odds ratio with confidence intervals in parenthesis. T = Tumor, N=Nodes, M = Metastases.
No significant difference in pathologic tumor (T) (p = 0.368), nodal (N) (p = 0.993) staging or pathologic upstaging (p = 0.738) was found between the two periods (Table 2B). Adjusted logistic analyses revealed no significant difference in pathologic T (aOR 0.847, 95% CI 0.398-1.796, p = 0.665), or N staging (a OR 0.959, 95% CI 0.472-1.943, p = 0.907) (Supplemental Table 3 and Supplemental Table 4A/B).
4. Discussion
To our knowledge, this is the largest comprehensive report of the impact of the COVID-19 pandemic on clinical presentation, clinical and pathologic staging, and treatment choice for any solid malignancy over a multi-month timeframe. Consistent with previous reports [3], overall number of patient encounters decreased during the initial stages of the pandemic. Contrary to what has been previously reported [7], we report stability in number of patients presenting with new head and neck malignancies. The proportional increase in patients with malignant diseases, decrease in benign patient visits, and decrease in time from referral to initial clinic visit for mucosal SCC patients suggests that there were more available head and neck surgical oncology clinic appointments, likely due to less patients with benign pathology seeking medical care during the early months of the pandemic.
This study reports the effects of the COVID-19 pandemic on TNM staging at presentation and treatment choices for patients with newly diagnosed head and neck cancer. There was an odds ratio of 2.141 for having more advanced regional nodal metastatic disease at initial presentation during the pandemic, confirming what has been previously reported in a smaller population [8]. This finding was in the absence of any change in presenting symptoms. Given that there was a shorter time from date of referral to initial clinic visit, this suggests delays elsewhere in the process of diagnosis and treatment of mucosal SCC. Patients may have chosen to delay initial evaluation by referring providers (PCPs, dentists, oral surgeons, otolaryngologists, dermatologists, etc.) due to pandemic-related fears [9]. In addition, while our head and neck surgical oncology clinic never closed, many outside clinics did temporarily close during the early months of the pandemic which would delay initial evaluation or result in bottlenecks with access at the patient's local providers. Of note, a prior study evaluating nodal progression between diagnostic and radiation treatment-planning scans of head and neck cancer patients showed that with a median interval of just 28 days, 20% of patients developed new nodal metastasis [9].
Despite more advanced clinical nodal staging for our cohort, T staging was not significantly more advanced during the pandemic. This is in contrast to a prior study by Kiong et al. which used similar methods over only a six-week period during the pandemic and found that in a smaller cohort, mucosal SCC patient's median primary tumor size was greater and T stage was more advanced during the pandemic [7]. It is important to note that our cohort consists of a higher proportion of oral cavity and larynx subsite patients compared to the paper by Kiong et al. of which nearly half the mucosal SCC patients were oropharynx. We hypothesize that the lack of more advanced T staging is due in part to a relatively large percentage of patients with advanced (T3 or T4) clinical T stage at initial presentation (44% in 2019, increased to 51.5% in 2020) creating a ceiling effect within our cohort.
Pathologic staging was also not significantly different between the two time periods. We hypothesize that this is due in part to patients with more advanced disease more likely to be treated with a primary non-surgical approach during the pandemic. This is illustrated by the fact that there was a trend towards fewer patients undergoing surgery in 2020 compared to 2019. In addition, 28% of patients in 2019 and 25% of patients in 2020 had p16 positive disease which would not be expected to have an increase in pathologic nodal staging unless contralateral nodes were identified. While others have reported a bias towards considering nonsurgical treatment of early glottic or HPV-related oropharyngeal cancers during the pandemic [18], [27], in our cohort we did not find an association between treatment choice and a particular malignancy type. We hypothesize that the non-significant increase in patients lost to follow up during 2020 was potentially a direct consequence of pandemic-related clinical care interruptions.
There was a trend towards fewer patients reporting a history of malignancy in 2020. We hypothesize that this is attributable to patients with a history of cancer being less likely to pursue medical care due to concerns for immunocompromised status that could be negatively impacted by COVID-19 exposure. Of note, while much has been written about safely caring for head and neck cancer patients during the COVID era [18], [19], [20], [21], [22], [23], [24], no departmental changes were made at our institution to specifically delay or change oncologic care other than hospital-wide COVID-19 screening protocols.
There are several expected limitations of this study. The timeframe for analysis was chosen to capture both the onset of the pandemic and ongoing effects as the pandemic continued. As a rapidly changing phenomenon, there are certainly effects that may not be captured during the 5-month timeframe. However, a multi-month timeframe was chosen in an attempt to mitigate this limitation. In addition, there was no significant change to our head and neck oncology clinical team during the entire timeframe of the study, which mitigates this limitation. There are also limitations associated with evaluating patients at a single academic tertiary care center that may not be extrapolated to other locations. Generalizability is further limited to regional prevalence of COVID-19 cases as well as regional attitudes towards the COVID-19 pandemic. Nearly 90% of our cohort was Caucasian compared to 60% white ethnicity within the entire United States [28], which also limits generalizability. Finally, long-term follow-up data for new consultations seen in our cohort would allow for better characterization of post-treatment outcomes, disease recurrence, and patient survival which may be influenced by this period of disruption from COVID-19.
5. Conclusion
Using the largest cohort to date, this study comprehensively reports the profound impact of the COVID-19 on presenting symptoms, TNM staging and treatment for patients presenting with any solid malignancy over a multi-month timeframe. We found a significantly increased risk for more advanced clinical nodal disease in patients with mucosal SCC of the head and neck during the pandemic timeframe despite no change in presenting symptoms. These findings are important as we continue to identify and treat patients with malignancies during the pandemic and future periods of oncologic care disruption.
Funding
This work was supported in part by the Vanderbilt CTSA grant from NCATS/NIH [UL1TR002243].
Declaration of competing interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amjoto.2021.103263.
Appendix A. Supplementary data
Supplementary tables
References
- 1.Vukkadala N., Rosenthal E., Patel Z.M. In response to COVID-19 and the otolaryngologist:preliminaryevidence-basedreview. Laryngoscope. 2020 Oct;130(10):E526. doi: 10.1002/lary.28770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brody R.M., Albergotti W.G., Shimunov D., Nicolli E., Patel U.A., Harris B.N., et al. Changes in head and neck oncologic practice during the COVID-19 pandemic. Head Neck. 2020 Jul;42(7):1448–1453. doi: 10.1002/hed.26233. Epub 2020 Jun 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CovidSurg Collaborative Head and neck cancer surgery during the COVID-19 pandemic: an international, multicenter, observational cohort study. Cancer. 2020 Dec;127(14):2476–2488. doi: 10.1002/cncr.33320. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 4.London J.W., Fazio-Eynullayeva E., Palchuk M.B., Sankey P., McNair C. Effects of the COVID-19 pandemic on cancerrelatedpatientencounters. JCO Clin Cancer Inform. 2020;4:657–665. doi: 10.1200/CCI.20.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maringe C., Spicer J., Morris M., et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. Lancet Oncol. 2020;21(8):1023–1034. doi: 10.1016/S1470-2045(20)30388-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sud A., Jones M.E., Broggio J., et al. Collateral damage: the impact on outcomes from cancer surgery of the COVID-19 pandemic. Ann Oncol. 2020;31(8):1065–1074. doi: 10.1016/j.annonc.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kiong K.L., Diaz E.M., Gross N.D., Hanna E.Y. The impact of COVID-19 on head and neck cancer diagnosis and disease extent. Head Neck. 2021;1–8 doi: 10.1002/hed.26665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tevetoğlu F., Kara S., Aliyeva C., Yıldırım R., Yener H.M. Delayed presentation of head and neck cancer patients during COVID-19 pandemic. Eur Arch Otorhinolaryngol. 2021 Mar;6:1–5. doi: 10.1007/s00405-021-06728-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.EL Papautsky T Hamlish Patient-reported treatment delays in breast cancer care during the COVID-19 pandemic [published online ahead of print, 2020 Aug 9]. Breast Cancer Res Treat. 2020;1–6. [DOI] [PMC free article] [PubMed]
- 10.E Garrett-Mayer BI Rini To treat or not to treat—balancing benefits and risks of treatment delay among patients with cancer during the COVID-19 pandemic. JAMA Oncol. Published online October 29, 2020. [DOI] [PubMed]
- 11.Jensen A.R., Nellemann H.M., Overgaard J. Tumor progression in waiting time for radiotherapy in head and neck cancer. Radiother Oncol. 2007;84(1):5–10. doi: 10.1016/j.radonc.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 12.Xiao R., Ward M.C., Yang K., Adelstein D.J., Koyfman S.A., Prendes B.L., et al. Increased pathologic upstaging with rising time to treatment initiation for head and neck cancer: a mechanism for increased mortality. Cancer. 2018 Apr 1;124(7):1400–1414. doi: 10.1002/cncr.31213. Epub 2018 Jan 9. [DOI] [PubMed] [Google Scholar]
- 13.Chen M.M., Harris J.P., Orosco R.K., Sirjani D., Hara W., Divi V. Association of time between surgery and adjuvanttherapy with survival in oral cavitycancer. Otolaryngol Head Neck Surg. 2018;158(6) doi: 10.1177/0194599817751679. pp. 1051–1056.1. [DOI] [PubMed] [Google Scholar]
- 14.Graboyes E.M., Kompelli A.R., Neskey D.M., et al. Association of Treatment Delays with Survival for patientswithhead and neckcancer:asystematicreview. JAMA Otolaryngol Head Neck Surg. 2019;145(2):166–177. doi: 10.1001/jamaoto.2018.2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morse E., Judson B., Husain Z., et al. Treatment delays in primarilyresectedoropharyngealsquamouscellcarcinoma: National Benchmarks and SurvivalAssociations. Otolaryngol Head Neck Surg. 2018;194599818779052 [Google Scholar]
- 16.Goel A.N., Frangos M., Raghavan G., et al. Survival impact of treatment delays in surgically managed oropharyngeal cancer and the role of human papillomavirus status. Head Neck. 2019;41(6):1756–1769. doi: 10.1002/hed.25643. [DOI] [PubMed] [Google Scholar]
- 17.Fujiwara R.J., Judson B.L., Yarbrough W.G., Husain Z., Mehra S. Treatment delays in oral cavity squamous cell carcinoma and association with survival. Head Neck. 2017;39(4):639–646. doi: 10.1002/hed.24608. [DOI] [PubMed] [Google Scholar]
- 18.Topf M.C., Shenson J.A., Holsinger F.C., et al. Framework for prioritizing head and neck surgery during the COVID-19 pandemic. Head Neck. 2020;42(6):1159–1167. doi: 10.1002/hed.26184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mehanna H., Hardman J.C., Shenson J.A., Abou-Foul A.K., Topf M.C., AlFalasi M., et al. Recommendations for head and neck surgical oncology practice in a setting of acute severe resource constraint during the COVID-19 pandemic: an international consensus. Lancet Oncol. 2020 Jul;21(7):e350–e359. doi: 10.1016/S1470-2045(20)30334-X. Epub 2020 Jun 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brody R.M., Albergotti W.G., Shimunov D., et al. Changes in head and neck oncologic practice during the COVID-19 pandemic. Head Neck. 2020;42:1448–1453. doi: 10.1002/hed.26233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Day A.T., Sher D.J., Lee R.C., et al. Head and neck oncology during the COVID-19 pandemic: reconsidering traditional treatment paradigms in light of new surgical and other multilevel risks. Oral Oncol. 2020;105 doi: 10.1016/j.oraloncology.2020.104684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Givi B., Schiff B.A., Chinn S.B., et al. Safte recommendations for evaluation and surgery of the head and neck during the COVID-19 pandemic. JAMA Otolaryngol Head Neck Surg. 2020;146:579–584. doi: 10.1001/jamaoto.2020.0780. [DOI] [PubMed] [Google Scholar]
- 23.Maniakas A., Jozaghi Y., Zafereo M.E., et al. Head and neck surgical oncology in the time of a pandemic: subsite specific triage guidelines during the COVID-19 pandemic. Head Neck. 2020;42:1194–1201. doi: 10.1002/hed.26206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kowalski L.P., Sanabria A., Ridge J.A., et al. COVID-19 pandemic: effects and evidence-based recommendations for otolaryngology and head and neck surgery practice. Head Neck. 2020;42:1259–1267. doi: 10.1002/hed.26164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Amin M.B., Edge S., Greene F., (Eds)., et al. Springer International Publishing; 2017. [Google Scholar]
- 26.Harris P.A., Taylor R., Minor B.L., Elliott V., Fernandez M., O’Neal L., et al. REDCap consortium,the REDCap consortium: building an international community of software partners. J Biomed Inform. 2019 Jul;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.SH Huang B O'Sullivan J Su J Ringash SV Bratman J Kim A Hosni A Bayley J Cho M Giuliani A Hope A Spreafico AR Hansen LL Siu R Gilbert JC Irish D Goldstein J de Almeida L Tong W Xu J Waldron Hypofractionated radiotherapy alone with 2.4 Gy per fraction for head and neck cancer during the COVID-19 pandemic: The Princess Margaret experience and proposal. Cancer. 2020 Aug 1;126(15):3426–3437. doi: 10.1002/cncr.32968. Epub 2020 Jun 1. PMID: 32478895; PMCID: PMC7300809. [DOI] [PMC free article] [PubMed]
- 28.US Census Bureau https://www.census.gov/quickfacts/fact/table/US/PST045219. Accessed April 15, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables