Abstract
Background
Patients with central nervous system (CNS) tumors may be at risk of dying from cardiovascular disease (CVD). We examined CVD mortality risk in patients with different histological subtypes of CNS tumors.
Methods
We analyzed UK(Wales)-based Secure Anonymized Information Linkage (SAIL) for 8743 CNS tumors patients diagnosed in 2000–2015, and US-based National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) for 163,183 patients in 2005–2015. We calculated age-, sex-, and calendar-year-adjusted standardized mortality ratios (SMRs) for CVD comparing CNS tumor patients to Wales and US residents. We used Cox regression models to examine factors associated with CVD mortality among CNS tumor patients.
Results
CVD was the second leading cause of death for CNS tumor patients in SAIL (UK) and SEER (US). Patients with CNS tumors had higher CVD mortality than the general population (SAIL SMR = 2.64, 95% CI = 2.39–2.90, SEER SMR = 1.38, 95% CI = 1.35–1.42). Malignant CNS tumor patients had over 2-fold higher mortality risk in US and UK cohorts. SMRs for nonmalignant tumors were almost 2-fold higher in SAIL than in SEER. CVD mortality risk particularly cerebrovascular disease was substantially greater in patients diagnosed at age younger than 50 years, and within the first year after their cancer diagnosis (SAIL SMR = 2.98, 95% CI = 2.39–3.66, SEER SMR = 2.14, 95% CI = 2.03–2.25). Age, sex, race/ethnicity in USA, deprivation in UK and no surgery were associated with CVD mortality.
Conclusions
Patients with CNS tumors had higher risk for CVD mortality, particularly from cerebrovascular disease compared to the general population, supporting further research to improve mortality outcomes.
Keywords: brain and CNS tumors, cardiovascular disease, cross-national data analysis, epidemiology, mortality
Key Points.
CVD mortality as the leading cause of noncancer death in brain tumor patients.
Raised CVD mortality risk observed in young adults and within the first year after tumor diagnosis.
More work required to delineate factors associated with CVD death.
Importance of the Study.
Incidence rates are increasing for CNS tumors and based on limited data, these patients may be at risk for cardiovascular disease (CVD) mortality. Our analysis of over 170,000 patients with CNS tumors in two large UK and US population-based cohorts shows CVD mortality is the greatest single noncancer cause of death among malignant and nonmalignant CNS tumors, and contributes to significantly worse CNS cancer survival. CVD mortality risk, particularly from cerebrovascular disease, were elevated among patients diagnosed under the age of 50 years and within the first year after CNS tumor diagnosis. Future work needs to delineate factors including whether treatment and/or localized or systemic effects of tumors are associated with CVD death among CNS tumor patients. Our analysis demonstrated the most comprehensive quantitative estimates of CVD mortality burden among malignant and nonmalignant CNS tumors, which provides important implications in clinical practice and can shed a light for future study.
Central nervous system (CNS) tumors, both malignant and nonmalignant, are associated with significant morbidity and mortality.1 The incidence of CNS tumors is increasing globally.2 Therapy options and patient survival for glioblastoma, the most common malignant CNS tumor, have changed little in almost 20 years, with median survival less than 15 months.3–5 Meningiomas, the most common nonmalignant CNS tumors, is accounting for 33–50% newly diagnosed intracranial tumors, with 5-year survival of 85%.3,6,7 There are substantial disabling effects associated with CNS tumors even if nonmalignant, with a significant burden of disease for patients and their families, healthcare systems and societies worldwide.1
An in-depth understanding of cause of death in CNS tumor patients may generate opportunities for prevention and minimization of avoidable morbidity and mortality. In a study of 906 CNS tumor patients that survived 1 year post diagnosis, and over 4365 matched controls from the UK Clinical Practice Research Datalink (CPRD), CNS tumor patients had a higher frequency of previously diagnosed CVD (21.4% vs 16.3%) and hypertension (17.2% vs 13.4%); lower frequency of CVD risk factors of current smoking (1.5% vs 5.3%) or heavy drinking (19.5% vs 23.8%), but no difference in diabetes, obesity or chronic kidney disease.8 In addition, compared with other cancer types, CNS tumor patients had markedly elevated mortality from cerebrovascular disease indicating possible different biological mechanisms related to the brain structure and functions.14 Cumulatively, data suggest CVD may be an important cause of mortality among CNS tumor patients.
Primary CNS tumors are heterogenous with different clinical presentations, treatments, and outcomes.3 Previous studies of CVD risk or mortality reported aggregated data for malignant CNS tumors without distinguishing histological subtypes,15–17 which may mask the tumor heterogeneity and discount their different effects on CVD mortality. Some studies only examined CVD mortality risk limited to childhood and younger age onset (<40 years) patients.12,13,18,19
Our study aimed to provide comprehensive quantitative estimates on risk of CVD mortality in primary CNS tumors using population-based data from the UK and US. We aimed to determine whether CVD mortality is raised among patients with primary CNS tumors and in different subtypes compared to the general population, and to assess the factors associated with increased CVD mortality among CNS tumor patients.
Methods
Overall Design
We conducted a retrospective, observational cohort study according to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines.23 First, we estimated the relative risk of CVD death for patients with CNS tumors compared to the general population, using data from UK (Wales)-based Secure Anonymized Information Linkage (SAIL) Databank24 and from US-based National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) programme. Second, we examined the factors associated with CVD mortality in CNS tumor patients using the SAIL and SEER datasets (see Supplementary Methods).
Data Sources
Both SAIL and SEER have been previously described.2,9 Summary characteristics of the SAIL and SEER database are described in Supplementary Table 1. Briefly, the SAIL Databank is a data platform holding de-identified and linkable datasets, including the Welsh Cancer Intelligence and Surveillance Unit (WCISU) data, for the population of Wales (approximately three million people), United Kingdom (UK). SEER is a registry of population-based incident tumor registries from geographically distinct regions in the USA, covering 28% of the US population, including incidence, survival, and surgical treatment data.25 SEER covers diverse population in USA with a greater proportion of cases of primary CNS tumors in non-White persons, while in SAIL 95% of the population was white. Due to the different socio-demographic compositions and healthcare system in the two cohorts, we reported results separately for SAIL UK and SEER US.
Study Population
We identified adult patients (aged ≥18 years) with CNS tumors in 2000–2015 from SAIL and SEER in 2005–2015. We classified CNS tumors using the following International Classification of Diseases 10th revision (ICD-10) codes: C70–72, C75.1–75.3, D18.0, D32–D33, D35.2–D35.4, D42–D43, D44.3–D44.5.2 We also used the International Classification of Diseases for Oncology third edition (ICD-O-3) codes to group tumors into malignancy type (malignant or nonmalignant type) and three major histologic subgroups (meningiomas, gliomas, and all others), following the definitions from the Central Brain Tumour Registry of the United States (CBTRUS) (Supplementary Appendix 1).3
Outcome Variables
We ascertained deaths by linking to mortality data and categorized causes of death using ICD-9 and ICD-10 codes for the main underlying cause: CNS tumors, cardiovascular disease (CVD) and other causes (Supplementary Appendices 2 and 3). The primary outcome of interest was death from CVD, including diseases of the heart, cerebrovascular disease, atherosclerosis, and aortic aneurysm or dissection.9
Statistical Analysis
Descriptive analyses of baseline characteristics by main causes of death in CNS tumors and subgroups were performed using the Pearson χ2 test for categorical variables (summarized as frequencies/percentages). Continuous variables were compared across subgroups using analysis of variance for normal distribution data presented as mean, 95% confidence interval (CI), or Kruskal–Wallis (summarized as medians and interquartile range) for nonnormal distribution.
We compared CVD mortality among patients with CNS tumors to the general population using standardized mortality ratios (SMRs) to calculated as the ratio of the observed to expected numbers of deaths. We calculated these for the time period 2000–2015 for SAIL and 2005–2015 for SEER, accounting for the fact that nonmalignant CNS tumors were only registered in SEER from 2004 onwards.26 We adjusted SMRs by age, sex, and calendar year to the Welsh and US general populations respectively during the study period. For both datasets, we categorized age groups as 18–49 years (due to low frequency of cases in younger people) and into 10-year age groups for ages ≥50 years. We estimated SMRs for all CVD deaths and for two major CVD subgroups (deaths due to heart disease and those due to cerebrovascular disease) as a function of calendar year, age at cancer diagnosis, and follow-up time after cancer diagnosis, respectively.
To determine factors associated with elevated CVD mortality in CNS tumor patients, we used cause-specific multivariable adjusted Cox proportional hazards regression models to calculate hazard ratios (HRs) and 95% confidence intervals (CIs) for CVD deaths as the primary outcome among different CNS tumor patient subgroups. The variables included in the models were malignancy, histological types (gliomas, meningioma, and other histological types), age at diagnosis, sex, race/ethnicity status (SEER only), marital status (SEER only), area deprivation measured by Welsh Index of Multiple Deprivation (WIMD) (SAIL only) and surgery status (surgery versus no surgery or no information). We computed survival time from the date of CNS cancer diagnosis until date of death or last contact (December 31, 2015). We calculated cumulative incidence function curves to describe the incidence of deaths from CVD, and CNS death over time. We also conducted analyses stratifying CNS tumor patients by malignant status and major histological subtypes. To reduce the chance of reverse causality, all analyses were landmark analyses, with follow-up commencing 2 months after cancer diagnoses,27 thereby excluding patients with an event of death from cancer or CVD within 2 months. To test the robustness of our results, we performed sensitivity analyses in those diagnosed from 2005 onwards when incidence and diagnosis of CNS tumors (overall and histologically confirmed) in SAIL stabilised.2
We defined statistical significance by a two-tailed P-value <.05. We prepared and analyzed data using R (Version 4, https://www.R-project.org/) for SAIL UK and SEER*Stat 8.3.8 for SEER. We conducted analyses of SAIL data within SAIL’s remotely accessible trusted research environment, having obtained approval for use of the data (Project No. 0918). No additional informed consent was required as there was no individual patient involvement.
Results
Cohort Characteristics
In SAIL, there were 8743 patients diagnosed with CNS tumors between 2000 and 2015 (Table 1). Nearly 60% (n = 5119) of patients had nonmalignant tumors, which were more frequent in women. The most common histological subtypes were gliomas (36.1%), which are mostly malignant, and meningiomas (24.3%), the majority of which are nonmalignant. There were 4804 (54.9%) deaths during 30,134 person-years at risk. The leading cause of death was CNS tumor (69.2%) followed by CVD (9.5%). The primary cause of CVD death was heart disease (52.7%), followed by cerebrovascular disease (41.8%).
Table 1.
Cohort characteristics in patients with central nervous system tumors
| SAIL 2000–2015 | Overall CNS tumors | Nonmalignant CNS tumors | Malignant CNS tumors | P-value |
|---|---|---|---|---|
| N = 8743 | N = 5119 | N = 3624 | ||
| Sex | ||||
| Female | 4736 (54.2%) | 3190 (62.3%) | 1546 (42.7%) | |
| Male | 4007 (45.8%) | 1929 (37.7%) | 2078 (57.3%) | |
| Age at diagnosis (years, mean/SD) | 61.3 (17.7) | 60.6 (18.6) | 62.2 (16.4) | <.001 |
| Age at diagnosis | <0.001 | |||
| 18–49 years | 2207 (25.2%) | 1466 (28.6%) | 741 (20.4%) | |
| Over 50 years | 6536 (74.8%) | 3653 (71.4%) | 2883 (79.6%) | |
| Area deprivation WIMD | <.006 | |||
| 1 Most deprived | 1672 (19.3%) | 1003 (19.8%) | 669 (18.7%) | |
| 2 | 1688 (19.5%) | 1002 (19.8%) | 686 (19.2%) | |
| 3 | 1821 (21.1%) | 1113 (22.0%) | 708 (19.8%) | |
| 4 | 1787 (20.7%) | 1000 (19.7%) | 787 (22.0%) | |
| 5: Least deprived | 1677 (19.4%) | 950 (18.7%) | 727 (20.3%) | |
| Histologic subtypes | <.001 | |||
| Glioma | 3156 (36.1%) | 83 (1.6%) | 3073 (84.8%) | |
| Meningioma | 2128 (24.3%) | 2036 (39.8%) | 92 (2.5%) | |
| Other | 3459 (39.6%) | 3000 (58.6%) | 459 (12.7%) | |
| Surgery | .0998 | |||
| Having surgery | 5597 (64.0%) | 3240 (63.3%) | 2357 (65.0%) | |
| Not having surgery | 176 (2.0%) | 25 (0.5) | 151 (4.2%) | |
| No information | 2970 (34.0%) | 1854 (36.2%) | 1116 (30.8%) | |
| Follow up time (months)* | 18.0 [4.0; 68.4] | 42.6 [11.0;92.6] | 6.0 [2.0;18.0] | <.001 |
| Follow up period | <.001 | |||
| <1 year | 3691 (42.2%) | 1322 (25.8%) | 2369 (65.4%) | |
| 1–4 years | 2582 (29.5%) | 1702 (33.2%) | 880 (24.3%) | |
| 5–9 years | 1554 (17.8%) | 1318 (25.7%) | 236 (6.5%) | |
| ≥10 years | 916 (10.5%) | 777 (15.2%) | 139 (3.8%) | |
| Total death | Total death | Total death | ||
| Main cause of death | N = 4804 | N = 1738 | N = 3066 | <.001 |
| CNS death | 3325 (69.2%) | 549 (31.6%) | 2776 (90.5%) | |
| CVD death | 455 (9.5%) | 398 (22.9%) | 57 (1.9%) | |
| Total CVD death | Total CVD death | Total CVD death | ||
| Main types of CVD death | N = 455 | N = 398 | N = 57 | <.001 |
| Heart disease | 240 (52.7%) | 208 (52.3%) | 32 (56.1%) | |
| Cerebrovascular disease | 190 (41.8%) | 167 (42.0%) | 23 (40.3%) | |
| SEER 2005-2015 | Overall CNS tumors | Nonmalignant CNS tumors | Malignant CNS tumors | P-value |
| N = 163,183 | N = 110,076 | N = 53,107 | ||
| Sex | <.001 | |||
| Female | 97,944 (60.0%) | 74,720 (67.9%) | 23,224 (43.7%) | |
| Male | 65,239 (40.0%) | 35,356 (32.1%) | 29,883 (56.3%) | |
| Age at diagnosis (years, mean/SD) | 60.7 (16.9) | 61.7 (16.6) | 58.8 (17.3) | <.001 |
| Age at diagnosis | <.001 | |||
| 18–49 years | 40,812 (25.0%) | 25,943 (23.6%) | 14,869 (28.0%) | |
| Over 50 years | 12,2371 (75.0%) | 84,133 (76.4%) | 38,238 (72.0%) | |
| Race/Ethnicity | <.001 | |||
| SEER 2005-2015 | Overall CNS tumors | Nonmalignant CNS tumors | Malignant CNS tumors | P-value |
| N = 163,183 | N = 110,076 | N = 53,107 | ||
| Non-Hispanic White | 116,509 (71.4%) | 76,008 (69.1%) | 40,501 (76.3%) | |
| Hispanic (all races) | 18,522 (11.4%) | 12,380 (11.2%) | 6142 (11.6%) | |
| Non-Hispanic Black | 14421 (8.84%) | 11,163 (10.1%) | 3258 (6.13%) | |
| Non-Hispanic Asian or Pacific Islander | 11,364 (6.96%) | 8599 (7.81%) | 2765 (5.21%) | |
| Non-Hispanic American Indian/Alaska Native | 955 (0.59%) | 703 (0.64%) | 252 (0.47%) | |
| Non-Hispanic Unknown Race | 1412 (0.87%) | 1223 (1.11%) | 189 (0.36%) | |
| Marital status | <.001 | |||
| Married/Partner | 88,560 (54.3%) | 57,539 (52.3%) | 31,021 (58.4%) | |
| Single/Separated/Divorced | 64,138 (39.3%) | 44,380 (40.3%) | 19,758 (37.2%) | |
| Unknown | 10,485 (6.43%) | 8157 (7.41%) | 2328 (4.38%) | |
| Histologic subtypes | <.001 | |||
| Glioma | 50,534 (31.0%) | 1705 (1.6%) | 48,829 (91.9%) | |
| Meningioma | 81431 (49.9%) | 80,535 (73.2%) | 896 (1.7 %) | <.001 |
| Other | 32,128 (19.11%) | 27,836 (22.3%) | 3382 (6.4%) | |
| Surgery | <.001 | |||
| Having surgery | 82681 (50.7%) | 45641 (41.5%) | 37,040 (69.7%) | |
| Not having surgery | 79,332 (48.6%) | 63,655 (57.8%) | 15,677 (29.5%) | |
| No information | 1170 (0.72%) | 780 (0.71%) | 390 (0.73%) | |
| Follow up time (months)a | 38.0 [14.0; 78.0] | 54.0 [25.0; 90.0] | 13.0 [4.00;33.0] | <.001 |
| Follow up period | <.001 | |||
| <1 year | 7224 (32.8%) | 3289 (21.5%) | 3935 (58.4%) | |
| 1–4 years | 2469 (11.2%) | 1220 (7.97%) | 1249 (18.5%) | |
| 5–9 years | 903 (4.09%) | 778 (5.08%) | 125 (1.85%) | |
| ≥10 years | 11458 (52.0%) | 10026 (65.5%) | 1432 (21.2%) | |
| Total death | Total death | Total death | ||
| Main cause of death | N = 64676 | N = 25,042 | N = 39,634 | <.001 |
| CNS death | 33,348 (51.6%) | 1081 (4.32%) | 32,267 (81.4%) | |
| CVD death | 7583 (11.7%) | 6525 (26.1%) | 1058 (2.67%) | |
| Total CVD death | Total CVD death | Total CVD death | ||
| Main types of CVD death | N = 7583 | N = 6525 | N = 1058 | <.001 |
| Heart disease | 5039 (66.4%) | 4354 (66.7%) | 685 (64.7%) | |
| Cerebrovascular disease | 1990 (36.2%) | 1682 (25.8%) | 308 (29.1%) |
CNS, central nervous system; CVD, cardiovascular disease includes the disease of heart, cerebrovascular disease, hypertension, atherosclerosis, aortic aneurysm/dissection, and other diseases of arteries, arterioles, or capillaries.
WIMD, Welsh Index of Multiple Deprivation is the measurement of area deprivation in Wales, UK, ranking from 1 (most deprived) to 5 (least deprived).
aNumber presented in median/Interquartile range (IQR).
In SEER, there were 163,183 patients with CNS tumors diagnosed between 2005 and 2015 (Table 1). Over 67% (n = 110,076) of patients had nonmalignant tumors, which were more frequent in women. The most common histological subtypes were meningioma (49.9%) and glioma (31.0%). Among 64,676 deaths during 684,437 person-years at risk, CNS tumor was the leading cause of death (51.6%) followed by CVD (11.7%). The primary cause of CVD death was heart disease (66.4%), followed by cerebrovascular disease (36.2%) The subtypes of other histological groups are described in Supplementary Table 2.
Cardiovascular Mortality Risk
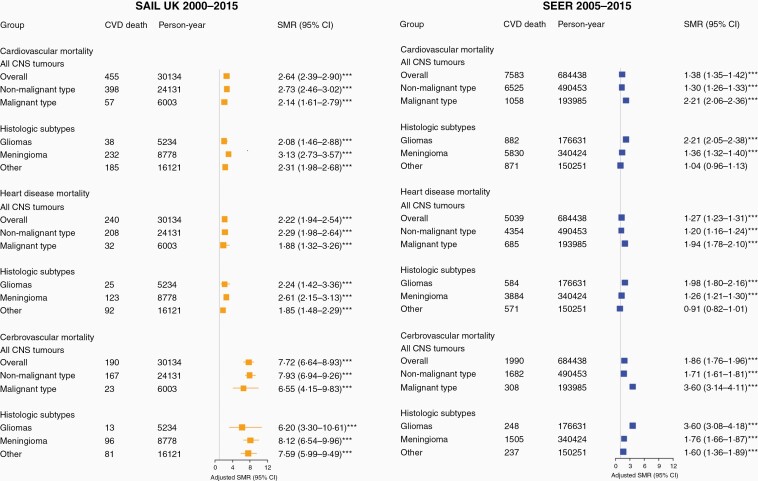
Overall, patients with CNS tumors had higher CVD mortality compared with the general population (SAIL SMR = 2.64, 95% CI = 2.39–2.90, SEER SMR = 1.38, 95% CI = 1.35–1.42, Figure 1). Malignant CNS tumor patients had more than twice the CVD mortality risk of the general population in SAIL and SEER. SMR for nonmalignant tumors was almost twice as high in SAIL as in SEER (SAIL SMR = 2.73, 95% CI = 2.46–3.02, SEER 1.30, 95% CI = 1.26–1.33). Among histological subtypes, patients with meningiomas and other types had excess CVD mortality risk (SMR 3.13 and 2.31, respectively) in SAIL than those in SEER (SMR 1.36 and 1.04, respectively). SMRs for cerebrovascular mortality were higher than for heart disease mortality, with relative risk considerably higher in SAIL than in SEER (Figure 1). Sex stratification showed similar patterns of risk with variations among subgroups. Male patients with malignant gliomas had over 2-fold higher CVD mortality risk in SAIL (SMR = 2.66, 95% CI = 1.77–3.82) and SEER (SMR = 2.09, 95% CI = 1.89–2.31); whereas female patients with nonmalignant meningiomas in SAIL had higher CVD mortality risk (SMR = 3.39, 95% CI = 2.90–3.94) than those in SEER (SMR = 1.31, 95% CI = 1.37–1.36, data not shown). Analysis of mortality trends by calendar year showed elevated CVD mortality risks across the entire period of study, albeit with some variation by subgroups, in both SAIL and SEER (Supplementary Table 3).
Figure 1.
Cardiovascular mortality in CNS tumors and stratified by subgroups. Standardized mortality ratios (SMRs) for cardiovascular disease (CVD) adjusted by age, sex and calendar-year in CNS tumor and by malignancy and main histological subtypes. SMRs for subtypes within cause of CVD (disease of heart and cerebrovascular disease) were presented. A SMR above 1 represents a higher relative risk of death for a type of cardiovascular cause compared with the general population.
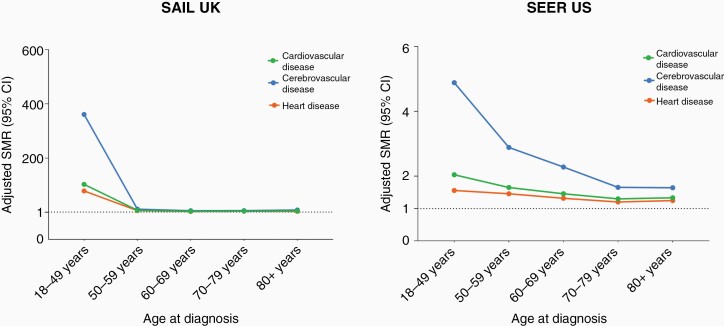
Cardiovascular Mortality Risk by Age at Cancer Diagnosis
For all CNS tumors combined, SMRs for CVD mortality were elevated for all age groups in SAIL and SEER, particularly for patients diagnosed below the age of 50 years (SAIL SMR = 96.09, 95% CI = 48.89–164.55, SEER SMR 2.03, 95% CI = 1.79–2.30; Figure 2 and Supplementary Table 4). SMRs for cerebrovascular disease were substantially elevated in SAIL (SMR 321.97, 95% CI = 138.34–622.23), less so for SEER (SMR 4.82, 95% CI = 3.84–5.99) (Supplementary Table 4). These patterns of higher relative CVD mortality risk for those with younger age at diagnosis were consistent across CNS tumor subtypes of malignancy and main histological types (Supplementary Tables 4 and 5).
Figure 2.
Cardiovascular mortality in CNS tumors by age at cancer diagnosis and stratified by subtypes. SMRs for CVD adjusted by age and sex in CNS tumors. SMRs for subtypes within cause of CVD (disease of heart and cerebrovascular disease) were presented. A SMR above 1 represents a higher relative risk of death for a type of cardiovascular cause compared with the general population.
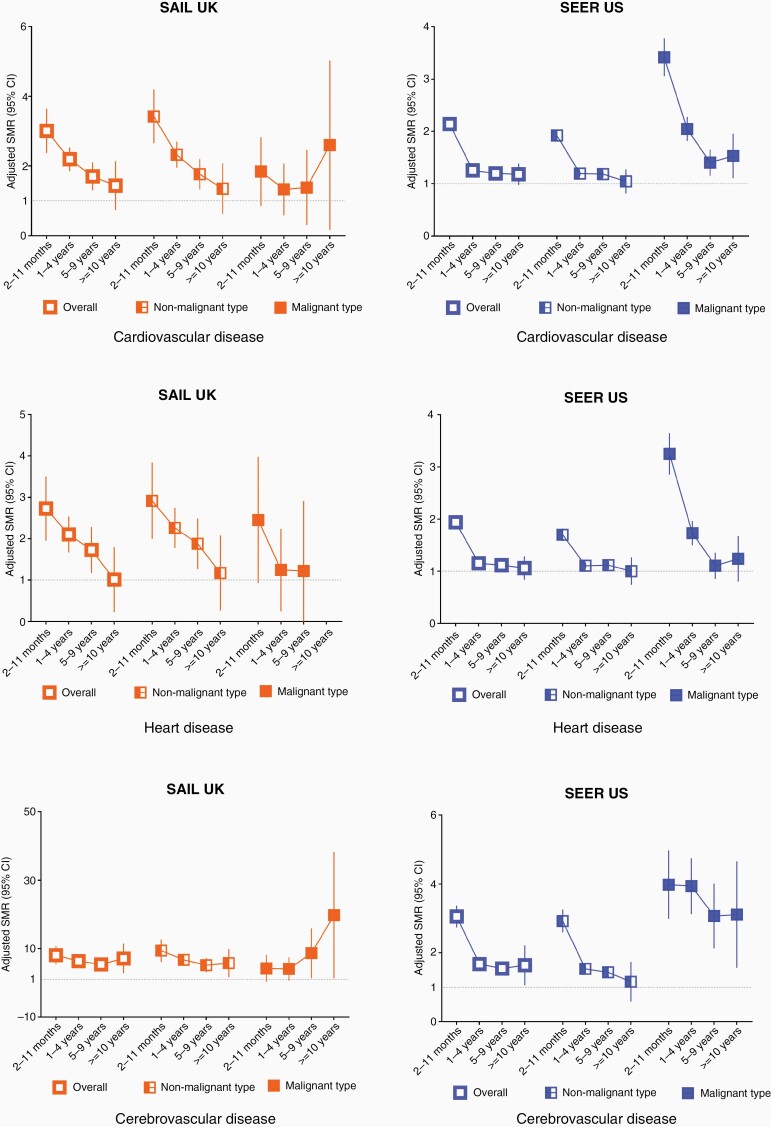
Cardiovascular Mortality Risk During Follow-Up
The highest SMRs for CVD mortality were observed in the first year after cancer diagnosis (SAIL SMR = 2.98, 2.39–3.66, SEER SMR = 2.14, 2.03–2.25, Supplementary Table 6). CVD mortality declined from 1 year post diagnosis, but remained elevated for up to 10 years (Figure 3). These patterns of higher CVD mortality risk were consistent across CNS tumor subtypes of malignancy and main histological types (Supplementary Tables 6 and 7).
Figure 3.
Cardiovascular mortality in CNS tumors by follow up year and stratified by subgroups. SMRs for CVD adjusted by age, sex and calendar year in overall CNS tumors and by malignancy. SMRs for subtypes within cause of CVD (disease of heart and cerebrovascular disease) were presented. A SMR above 1 represents a higher relative risk of death for a type of cardiovascular cause compared with the general population.
Cumulative Incidence of Death
The cumulative incidences of death from CVD and from CNS tumor by main histological group in SAIL and SEER are shown in Supplementary Figure 1. The risk from CVD death was negligible in gliomas in both datasets but constituted the leading cause of death in meningioma patients in SEER.
Factors Associated with CVD Mortality in SAIL and SEER
Multivariable Cox proportional hazards models to examine associations of factors with CVD mortality in nonmalignant meningioma and malignant gliomas are shown in Table 2. For meningiomas, strong predictors of CVD mortality were older age and area deprivation (SAIL), non-Hispanic Black ethnicity (SEER) and not being married or having a partner (SEER). Being male and not having surgery were associated with higher CVD mortality in both cohorts but nonsignificantly (P > .05) in SAIL (Table 2). For gliomas, being male, older age, and not having surgery significantly increased risk of CVD mortality in both cohorts. In the SEER cohort, being non-Hispanic Black or not being married or having a partner were also associated with higher CVD mortality. Not having surgery was associated with increase in risk of CVD death in SAIL (HR = 2.22, 1.03–4.76) and SEER (HR = 1.56, 1.35–1.56), respectively, compared with those having surgery (Table 2).
Table 2.
Cox regression multivariable HRs and 95% CIs for association between patient characteristics and CVD mortality
| SAIL UK 2000–2015 | Nonmalignant meningioma | Malignant glioma |
|---|---|---|
| Characteristics | HR (95% CI) | HR (95% CI) |
| Gender | ||
| Female | 1 | |
| Male | 1.16 (0.85–1.57) | 2.11 (1.03–4.31)a |
| Age | 1.11 (1.09–1.12) | 1.08 (1.05–1.11)b |
| Area deprivation | ||
| WIMD 5 (least deprived) | 1 | |
| WIMD 4 | 1.10 (0.70–1.71) | 0.74 (0.28–1.94) |
| WIMD 3 | 1.32 (0.84–2.05) | 0.81 (0.30–2.19) |
| WIMD 2 | 1.73 (1.12–2.66)a | 0.63 (0.21–1.9) |
| WIMD 1 (most deprived) | 2.00 (1.30–3.06)c | 1.37 (0.54–3.48) |
| Year of diagnosis | 0.98 (0.86–1.11) | 0.91 (0.83–1.00) |
| Treatment | ||
| Having surgery | 1 | 1 |
| Not having surgery | 6.23 (1.52–25.62) | Nil |
| No information | 1.35 (1.01–1.83) | 1.82 (0.81–4.09) |
| SEER 2005–2015 | Nonmalignant Meningioma | Malignant Glioma |
| Characteristics | HR (95% CI) | HR (95% CI) |
| Gender | ||
| Female | 1.00 | 1.00 |
| Male | 1.64 (1.55–0.74)b | 1.36 (1.18–1.56)b |
| Age | 1.10 (1.10–1.10)b | 1.07 (1.07–1.08)b |
| Ethnicity | ||
| Non-Hispanic White | 1.00 | 1.00 |
| Hispanic (all races) | 0.91 (0.83–1.01) | 1.10 (0.87–1.39) |
| Non-Hispanic Black | 1.48 (1.37–1.60)b | 2.01 (1.61–2.53)b |
| Non-Hispanic Asian or Pacific Islander | 0.83 (0.75–0.94)c | 0.98 (0.71–1.35) |
| Non-Hispanic American Indian/Alaska Native | 0.84 (0.54–1.30) | 1.72 (0.77–3.86) |
| Non-Hispanic Unknow Race | 0.20 (0.08–0.48)b | 0.43(0.06–3.04) |
| Marital status | ||
| Married/Having Partner | 1.00 | 1.00 |
| Single/Separate/Divorced | 1.38 (1.30–1.47)b | 1.43 (1.23–1.65)b |
| Unknown | 1.26 (1.14–1.40)b | 1.22 (0.87–1.71) |
| Year of diagnosis | 0.99 (0.98–1.00)a | 0.96 (0.94–0.99)c |
| Treatment | ||
| Having surgery | 1.00 | 1.00 |
| Not having surgery | 1.34 (1.25–1.43)b | 1.58 (1.36–1.82)b |
| No information | 1.39 (1.05–1.84)a | 0.33 (0.05–2.36) |
HR, hazard ratio; CI, confidence interval; CVD, cardiovascular disease; WIMD, Welsh Index Multiple Deprivation.
a<.05,
b<.001,
c<.01.
Model adjusted for gender, age, WIMD (SIAL UK), ethnicity (SEER US), marital status (SEER US), year of diagnosis, treatment information for surgery.
Broadly similar SMRs and HRs to those noted above were observed in analyses that were restricted to CNS tumors (overall and subtypes of malignancy and main histological groups) between 2005 and 2015 in SAIL UK (data not shown).
Discussion
Our analysis of over 170,000 patients with CNS tumors in population-based cancer registries from UK and US demonstrated CVD was the second commonest cause of death in both malignant and nonmalignant types. There was a significantly increased risk of CVD mortality after CNS tumor diagnosis compared to the general population. CVD mortality, particularly from cerebrovascular disease, was substantially greater in patients diagnosed at a younger age (<50 years) and within the first year after their cancer diagnosis. Age, sex, race/ethnicity in USA, deprivation in UK and not having surgery were associated with CVD mortality in CNS tumor patients. Targeted interventions and risk stratification tools might improve survival.
Our inclusion of all CNS tumor subtypes by malignancy and main histological groups extends previous studies of CVD mortality among patients of childhood and younger age onset. Increased CVD mortality in CNS tumor patients may reflect cardiotoxic cancer treatment. Current treatment for malignant CNS tumors includes surgery, cranial radiotherapy, and chemotherapy.4,28 Such cancer treatment is associated with adverse cardiovascular complications including cardiomyopathy, cardiac arrhythmias and stroke.29 Older age also contributes to increased risk of cardiotoxicity from cancer treatment,9 and the mean age at cancer diagnosis was 60 years in our cohorts. Increased CVD mortality risk in nonmalignant CNS tumor patients, for example, asymptomatic patients with benign meningiomas, who may not require surgical management or radiation therapy, could relate to existing CVD risk or other comorbidities, such as advancing age, or underutilized existing preventive therapies.30 This requires further study.
Patients who are younger at cancer diagnosis, and in the first year after diagnosis, were at higher risk of CVD mortality is consistent with previous studies of other cancers.9,15,17 In one study following over 200,000 cancer patients diagnosed at 15–39 years for 25 years, risk of cardiac mortality was 1.4 times the general population with decreasing SMRs for cardiac mortality at increasing ages (SMR 4.2 at 15–19 years to SMR 1.2 at 35–39 years).15 The immediate negative effects of surgery (malignant and nonmalignant tumors), and concomitant radiotherapy and chemotherapy (malignant tumors) may explain the highest risk in first-year post diagnosis. Our studies also showed CVD mortality remained elevated even 5 years after diagnosis, suggesting long-term effects from anti-cancer treatment and individual comorbidity.
Across all CNS tumors, cerebrovascular mortality risk was higher than heart disease. Greater cerebrovascular mortality risk was reported in previous studies of childhood and young adult-onset CNS tumors.13,16,31 A recent US study found malignant CNS tumors had the highest risk of stroke mortality compared to other cancer sites in the first 5 years following cancer diagnosis.17 Possible explanations included hypoxic condition leading to cerebral ischemic injuries from cranial surgery and radiation.19 A cancer-associated hypercoagulatory state could also contribute to ischemia.8,11,19 The higher cerebrovascular mortality may also be partly explained by direct effects of CNS tumors on cerebral vasculature, systemic tumor-related inflammatory and pro-coagulant factors, or in some cases, diagnostic misclassification (e.g., focal epilepsy, “tumor attacks,” or intra-tumoral hemorrhage being labeled diagnostically as stroke).
The findings of not having surgery associated with increased CVD mortality may implicate a tumor-related factor in CVD risk but may alternatively indicate that more proactive follow up of patients after surgery is of benefit for earlier identification and treatment of CVD risk factors. Another explanation is “reverse causality”, that those who did not receive surgery had worse performance status or poor neurological function at the time surgery was considered, particularly in elderly patients.32 The cohorts we examined lacked the granular clinical detail to enable further exploration of these potential explanations.
CVD mortality risk varied between the UK and US cohorts studied, especially for benign meningiomas (Supplementary Table 8). SMR estimates for CVD mortality by race/ethnicity in SEER showed the risk was significantly higher in people with CNS tumors regardless of ethnicity (Supplementary Table 9). However, meningioma patients in SAIL UK remained higher CVD mortality risk compared to their general population (SMR 3.13) than those in non-Hispanic White and Black (SMR 1.31 and 1.64) in US SEER. These may suggest the differences in risk could be related to the healthcare factors, for example, treatment patterns related to the type of facility, access to specialized medical and surgical care for early diagnosis and long-term management across countries.15,33 Highly specialized neuro-oncology care including surgical and radiation services for diagnosis and long-term management are important for improving survival outcome CNS tumors.1 Incidence rates of meningiomas in the US are higher than in other countries, including the UK.2,33 This likely reflects increasing diagnosis of meningiomas as incidental findings, partly because of growing MRI availability, more marked in the USA.6 More meningiomas are diagnosed at earlier stage.6 The rate of growth of benign meningioma is typically slow and most meningiomas found incidentally on brain imaging undertaken for unrelated symptoms remain asymptomatic.6 A larger proportion of meningiomas detected in the US when they are small, not growing, and not requiring treatment, may at least in part explain the difference in excess CVD mortality between the UK and USA. Less well-resourced healthcare and higher prevalence of CVD risk factors can compound CVD mortality risk,34 and may also contribute to the differences observed between the US and the UK.
CVD mortality was significant high in the other histological subtype group in SAIL (SMR 2.31, P < .001) but not in SEER (SMR 1.04, P > .05, Figure 1). This is consistent with the observation that the SMR for nonmalignant tumors is over twice as high in SAIL (SMR 2.27, P < .001) but not significantly high in SEER (SMR 0.94, P > .05), while malignant CNS tumor patients had similar high risk of CVD mortality in SAIL and SEER (SMR 2.68 and 2.88, respectively, P < .001, Supplementary Table 10). Further analysis showed patients with tumors of the sellar region experienced an excess CVD mortality risk in both cohorts (SMR 3.12 and 4.20, P < .001, respectively), and that patient with tumors of cranial and spinal nerve had substantially high risk in SAIL (SMR = 3.17, 95% CI = 1.96–4.84) but significantly low in SEER (SMR = 0.72, 95% CI = 0.64–0.81, Supplementary Table 10).
The findings of consistently higher CVD risk in malignant CNS tumors, particularly gliomas, but with variations in CVD risk in nonmalignant CNS tumors, especially meningiomas, between the UK and USA, may reflect the respective variations in standard approaches and guidelines on management of tumors across countries. Information on clinical characteristics and therapeutic management on meningiomas remains scarce compared to that of malignant tumors,33 because benign CNS tumors has not been included in cancer registration in many countries. A better understanding of the factors contributing to CVD risk following a nonmalignant CNS tumor diagnosis is needed to interrogate the differences further.
Our study has important implications. Our analysis shows CVD mortality is the largest single noncancer cause of death among patients with CNS tumors. Over 25% of CNS tumor patients were diagnosed aged 18–49 years, among which over 60% had a nonmalignant tumor. This is important because these patients have better survival prognosis overall and so may have most to benefit from early CVD prevention. Malignant CNS tumor patients have, on average, a much shorter-life expectancy, but there still may be advantages to CVD risk reduction, especially in the absence of other emerging therapeutic strategies. Early prevention of CVD should be a priority in cancer patients. In our study, advanced age, being male, lower SES and non-Hispanic Black ethnicity were strong predictors of CVD mortality in CNS tumors patients. These are well established CVD mortality risk factors and could inform screening to identify patients who may benefit from CVD preventive interventions. Active monitoring and treatment of CVD complications during and after cancer treatment could also help reduce CVD mortality.
Strengths and Limitations
Our results provide the most comprehensive analysis of malignant and nonmalignant CVD mortality in CNS tumor patients in two independent populations, enhancing the generalizability of our findings. Our inclusion of all CNS tumor subtypes extends previous studies of CVD mortality among patients of younger age at onset (<40 years).12,13,15,16 A key strength is stratification of nonmalignant CNS tumors, particularly meningiomas, which account for 30–50% newly diagnosed intracranial tumors. Our analysis suggest that meningioma’s might benefit from risk stratification and early prevention given elevated risk of death from CVD not previously assessed in other CNS mortality studies. Using cancer registry data from two countries allowed us to examine the CVD mortality patterns across relatively rare CNS tumor subtypes using consistent methodology. Population-based cancer registry data has strengths of capturing and identifying risk in those who may be under-represented in clinical trials, from which treatment guidelines are often developed.
Our study had several limitations. Firstly, SMRs cannot be compared to each other since they compare the relative risk with the standard population, which may vary among groups and geographical location.17 SMRs and their confidence intervals depend on person-years at risk, and if the incidence of a cancer is low and the survival is limited (e.g., certain brain cancers in the Wales, UK), the CI may be wide. Second, we could not calculate risk adjusted mortality (e.g., risk adjusted mortality index) by taking into account of health care factors such as admission types, specific treatment modalities including type of surgery, chemotherapy and radiotherapy, limiting further exploration and interpretation of our results. This may be possible in future by linking routinely collected data from different sources we can provide a better measurement to monitor survival outcomes in CNS tumors. Third, we did not have information on CVD risk factors such as obesity and smoking. However, a recent large study examining CVD risk for multiple cancer sites showed adjustment for shared risk factors had little effect on CVD risk compared with the general population.8 Fourth, the lack of individual level of sociodemographic information such as marital status and ethnicity in SAIL and deprivation in SEER made it difficult to directly compare CVD risk for these two cohorts. Another limitation is the use of death certificate information to classify cause of death, which may have limited accuracy. However, international studies have reported acceptable validity (83-98%) of case of death using such data.35,36 Lastly, we did not have detailed data in SEER to classify all stroke outcomes as ischemic or hemorrhagic.
Conclusions
Our results provide important evidence on CVD risk in CNS tumors by subtype that could inform risk stratification and prevention efforts in clinical practice, particularly in the first year and among those diagnosed at younger ages. Given the effectiveness of both primary and secondary prevention interventions for CVD, risk screening prior to, during and after cancer treatment should be emphasized and communicated within multidisciplinary teams including neuro-oncologists, general practitioners, and cardiologists.
Supplementary Material
Acknowledgments
This study makes use of anonymized data held in the Secure Anonymized Information Linkage (SAIL) Databank. We would like to acknowledge all the data providers who make anonymized data available for research.
Funding
K.J. and M.T.C.P. are funded by Cancer Research UK Brain Cancer Centre of Excellence Award (C157/A27589). Cancer Research UK did not play a role in study design, analysis, interpretation, or submission of this article.
Authorship Statement
K.J. conceived and designed the study, analyzed data, developed figures, interpreted data, and developed the first draft of the manuscript. P.M.B. conceived and designed the study, interpreted data, contributed to the writing, reviewing and editing of the manuscript. M.T.C.P. contributed to data interpretation and reviewing the manuscript. C.L.M.S. conceived and designed the study, interpreted and verified data, contributed to the writing, reviewing and editing of the manuscript. J.D.F. conceived and designed the study, interpreted and verified data, contributed to the writing, reviewing and editing of the manuscript. All authors critically revised the manuscript for important intellectual content. All authors approved the manuscript to submit for publication.
Conflict of interest statement. All authors have no conflict of interest to declare.
References
- 1. Brain GBD, Other CNSCC . Global, regional, and national burden of brain and other CNS cancer, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(4):376–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Poon MTC, Brennan PM, Jin K, Sudlow CLM, Figueroa JD. Might changes in diagnostic practice explain increasing incidence of brain and central nervous system tumors? A population-based study in Wales (United Kingdom) and the United States. Neuro oncol. 2021;23(6):979–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ostrom QT, Patil N, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2013–2017. Neuro Oncol. 2020;22(12 Suppl 2): iv1–iv96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 5. Poon MTC, Sudlow CLM, Figueroa JD, Brennan PM. Longer-term (≥ 2 years) survival in patients with glioblastoma in population-based studies pre- and post-2005: a systematic review and meta-analysis. Sci Rep. 2020;10(1):11622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Vernooij MW, Ikram MA, Tanghe HL, et al. Incidental findings on brain MRI in the general population. N Engl J Med. 2007;357(18):1821–1828. [DOI] [PubMed] [Google Scholar]
- 7. van Alkemade H, de Leau M, Dieleman EM, et al. Impaired survival and long-term neurological problems in benign meningioma. Neuro oncol. 2012;14(5):658–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Strongman H, Gadd S, Matthews A, et al. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: a population-based cohort study using multiple linked UK electronic health records databases. Lancet. 2019;394(10203):1041–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sturgeon KM, Deng L, Bluethmann SM, et al. A population-based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J. 2019;40(48):3889–3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Narayan V, Thompson EW, Demissei B, Ho JE, Januzzi JL Jr, Ky B. Mechanistic biomarkers informative of both cancer and cardiovascular disease: JACC state-of-the-art review. J Am Coll Cardiol. 2020;75(21):2726–2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ghosh MK, Chakraborty D, Sarkar S, Bhowmik A, Basu M. The interrelationship between cerebral ischemic stroke and glioma: a comprehensive study of recent reports. Signal Transduct Target Ther. 2019;4:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Olsson DS, Andersson E, Bryngelsson IL, Nilsson AG, Johannsson G. Excess mortality and morbidity in patients with craniopharyngioma, especially in patients with childhood onset: a population-based study in Sweden. J Clin Endocrinol Metab. 2015;100(2):467–474. [DOI] [PubMed] [Google Scholar]
- 13. Wijnen M, Olsson DS, van den Heuvel-Eibrink MM, et al. Excess morbidity and mortality in patients with craniopharyngioma: a hospital-based retrospective cohort study. Eur J Endocrinol. 2018;178(1):93–102. [DOI] [PubMed] [Google Scholar]
- 14. Zaorsky NG, Zhang Y, Tchelebi LT, Mackley HB, Chinchilli VM, Zacharia BE. Stroke among cancer patients. Nat Commun. 2019;10(1):5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Henson KE, Reulen RC, Winter DL, et al. Cardiac mortality among 200 000 five-year survivors of cancer diagnosed at 15 to 39 years of age: the teenage and young adult cancer survivor study. Circulation. 2016;134(20):1519–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Prasad PK, Signorello LB, Friedman DL, Boice JD Jr, Pukkala E. Long-term non-cancer mortality in pediatric and young adult cancer survivors in Finland. Pediatr Blood Cancer. 2012;58(3):421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zaorsky NG, Zhang Y, Tchelebi LT, Mackley HB, Chinchilli VM, Zacharia BE. Stroke among cancer patients. Nat commun. 2019;10(1):5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yuen KCJ, Mattsson AF, Burman P, et al. Relative risks of contributing factors to morbidity and mortality in adults with craniopharyngioma on growth hormone replacement. J Clin Endocrinol Metab. 2018;103(2):768–777. [DOI] [PubMed] [Google Scholar]
- 19. Brada M, Ashley S, Ford D, Traish D, Burchell L, Rajan B. Cerebrovascular mortality in patients with pituitary adenoma. Clin Endocrinol (Oxf). 2002;57(6):713–717. [DOI] [PubMed] [Google Scholar]
- 20. Erfurth EM, Hagmar L. Cerebrovascular disease in patients with pituitary tumors. Trends Endocrinol Metab. 2005;16(7):334–342. [DOI] [PubMed] [Google Scholar]
- 21. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;140(11):e596–e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lyon AR, Dent S, Stanway S, et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: a position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur J Heart Fail. 2020;22(11):1945–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495–1499. [DOI] [PubMed] [Google Scholar]
- 24. Lyons RA, Hutchings H, Rodgers SE, et al. Development and use of a privacy-protecting total population record linkage system to support observational, interventional, and policy relevant research. The Lancet. 2012;380:S6. [Google Scholar]
- 25. Warren JL, Klabunde CN, Schrag D, Bach PB, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care, 2002;40(8 Suppl): Iv-3-18. [DOI] [PubMed] [Google Scholar]
- 26. Forjaz G, Barnholtz-Sloan JS, Kruchko C, et al. An updated histology recode for the analysis of primary malignant and nonmalignant brain and other central nervous system tumors in the Surveillance, Epidemiology, and End Results Program. Neurooncol Adv. 2021;3(1):vdaa175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1(11):710–719. [DOI] [PubMed] [Google Scholar]
- 28. Goldbrunner R, Minniti G, Preusser M, et al. EANO guidelines for the diagnosis and treatment of meningiomas. Lancet Oncol. 2016;17(9):e383–e391. [DOI] [PubMed] [Google Scholar]
- 29. Anker MS, Hadzibegovic S, Lena A, et al. ; Heart Failure Association Cardio-Oncology Study Group of the European Society of Cardiology . Recent advances in cardio-oncology: a report from the ‘Heart Failure Association 2019 and World Congress on Acute Heart Failure 2019’. ESC Heart Fail. 2019;6(6):1140–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wang K, Eblan MJ, Deal AM, et al. Cardiac toxicity after radiotherapy for stage III non-small-cell lung cancer: pooled analysis of dose-escalation trials delivering 70 to 90 Gy. J Clin Oncol. 2017;35(13):1387–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bright CJ, Hawkins MM, Guha J, et al. Risk of cerebrovascular events in 178 962 five-year survivors of cancer diagnosed at 15 to 39 years of age: the TYACSS (Teenage and Young Adult Cancer Survivor Study). Circulation. 2017;135(13):1194–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Weller M, van den Bent M, Preusser M, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021;18(3):170–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Baldi I, Engelhardt J, Bonnet C, et al. Epidemiology of meningiomas. Neurochirurgie. 2018;64(1):5–14. [DOI] [PubMed] [Google Scholar]
- 34. Schröder SL, Richter M, Schröder J, Frantz S, Fink A. Socioeconomic inequalities in access to treatment for coronary heart disease: a systematic review. Int J Cardiol. 2016;219:70–78. [DOI] [PubMed] [Google Scholar]
- 35. Stoltzfus KC, Zhang Y, Sturgeon K, et al. Fatal heart disease among cancer patients. Nat Commun. 2020;11(1):2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zellweger U, Junker C, Bopp M; Swiss National Cohort Study Group . Cause of death coding in Switzerland: evaluation based on a nationwide individual linkage of mortality and hospital in-patient records. Popul Health Metr. 2019;17(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.