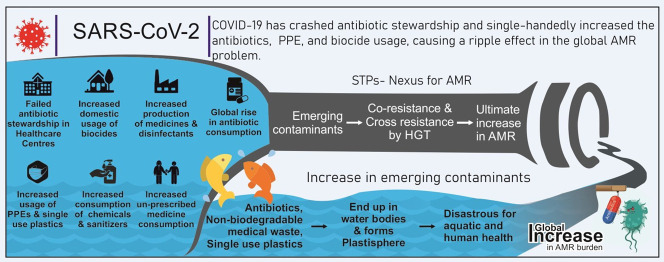
Abstract
Antimicrobial resistance (AMR) is emerging as a severe concern due to the escalating instances of resistant human pathogens encountered by health workers. Consequently, there is a shortage of antibiotics to treat Multidrug Resistance (MDR) and Extensively Drug Resistance (XDR) patients. The primary cause of AMR is the vast array of anthropogenic disturbances in natural microfauna brought about by the extensive use of antibiotics. Coronavirus Disease of 2019 (COVID-19) has crashed antibiotic stewardship and single-handedly increased the global usage of antibiotics, Personal Protective Equipment (PPE), and biocide, causing a ripple effect in the existing global AMR problem. This surge in antibiotic usage has escalated the residual antibiotics reaching Wastewater Treatment Plants (WWTPs) from pharmaceutical companies, health care centers, and domestic settings. Ultimately the natural water bodies receiving their effluents will have higher concentrations of emerging contaminants as the WWTPs cannot remove the Pharmaceuticals and Personal Care Products (PPCPs) completely. Furthermore, increased biocides usage will increase AMR by co-resistance, and increasing plastics will turn into microplastics and get converted to plastisphere, which will further enhance its propagation. Therefore, it is crucial to curb antibiotic usage, implement antibiotic stewardship dynamically; and, ameliorate the present condition of WWTPs to remove residual PPCPs efficiently. The need of the hour is to address the grave threat of AMR, which is loitering silently; if not the mankind will endure more affliction hereafter.
Keywords: Pandemic, Antibiotic resistance, Emerging contaminants, Pharmaceuticals and Personal Care Products, Health risk
Graphical abstract
1. Introduction
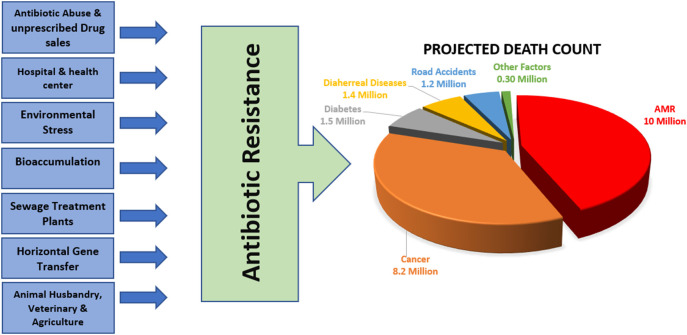
The pandemic caused by “Severe Acute Respiratory Syndrome Corona Virus 2 (SARS-CoV-2)” called Coronavirus Disease of 2019 (COVID-19) has brought a global storm. Nonetheless, the bitter truth is that COVID-19 is just the tip of the iceberg as the real danger with the potential to sink the world is “Antibiotic Resistance,” – which is looming in the darkness. On 17th July 2021, COVID-19 had a total death count of 4.08 million (“covid death worldwide,”n.d.) but, Antimicrobial Resistance (AMR) is projected to be responsible for approximately 10 Million death worldwide in the upcoming 30 years as per the pre-COVID-19 analysis; surpassing the death due to cancer (8.2 million), diabetes (1.5 million) and other factors (Fig. 1 ) (Neill, 2014). The post-COVID-19 study is expected to alter this data, and the death attributed to AMR is expected to approach a much higher number due to a global change in antibiotic consumption patterns.
Fig. 1.
Anthropogenic disturbances increase Antibiotic Resistance in the environment either directly or indirectly. Due to the pandemic, there is a surge in antibiotic usage in various sectors, especially health care centers and pharmaceutical industries which will escalate the existing Antimicrobial Resistance burden. According to Neill (2014) Antibiotic Resistance will emerge as the primary cause of death globally in the upcoming 30 years, outpassing cancer.
When SARS-CoV-2 became cosmopolitan, all the beliefs, faiths, and practices were put to the test. In that moment of global crisis, the first ray of hope that emerged was remdesivir (RDV) (Beigel et al., 2020; Diaz et al., 2021) and chloroquine (CQ)/hydroxychloroquine (HCQ)- the antiviral and anti-malarial drug, respectively (Colson et al., 2020). Baricitinib and tofacitinib- immunomodulators inhibiting Janus kinase- were also assessed for COVID-19 treatment, and both showed a significant reduction in mortality (Guimarães et al., 2021; Kalil, n.d.). Remdesivir in combination with baricitinib proved superior to remdesivir alone (Kalil et al., 2021). WHO Solidarity Trial Consortium conducted a mortality trial of four repurposed antiviral drugs- hydroxychloroquine, remdesivir, interferon beta-1a, and lopinavir result indicated little or no effect on the COVID-19 patients (Pan et al., 2021). Rivaroxaban- an anticoagulant factor X-a inhibitor- for high-risk adults patients with mild COVID-19; too did not demonstrate any significant impact (Ananworanich et al., 2020). Fostamatinib- a tyrosine kinase inhibitor, showed positive results in a small study group of 59 COVID-19 patients (Strich et al., 2021).
COVID-19 infection is a viral disease- untreatable by antibiotics, but the viral respiratory infections clinically progressed to bacterial pneumonia- the bacterial superinfection and other nosocomial infections- requiring antibiotic administration. Initial reports of Wuhan Pulmonary Hospital, China, published in ‘The Lancet,’ show an approximate 50% secondary bacterial infection occurrence rate in COVID-19 casualties (F. Zhou et al., 2020; H. Zhou et al., 2020). Thus, worldwide research accompanied by clinical trials with different antibiotics and other drugs were all over the news and research platforms. There was an immediate demand for organizing well-designed ‘randomized controlled clinical trials on a global scale’. Subsequently, scientists worldwide started formulating all sorts of permutations and combinations of antibiotics and other pre-approved drugs.
When the world stood still, the researchers and doctors across the globe worked perpetually to help mankind in its darkest days. The research from Marseille, France, showed promising results in combining HCQ with azithromycin (AZI) (Gautret et al., 2020). Even though AZI has potent antiviral activity and anti-inflammatory properties, in the case of COVID-19, it did not accord survival benefits; instead, this drug combination has arrhythmogenic potential and possesses serious safety concerns (Sultana et al., 2020). Therefore, as a better alternative, ivermectin (IVER) (anti-helminthic drug)- a familiar molecule with proven safety was explored. Various experts came forward, and their collective deliberation was published as a ‘white paper’ under the sponsorship of the Academy of Advanced Medical Education, suggesting the usage of ivermectin alone or combined as a therapeutic option for COVID-19 (Agam et al., 2020). Use of dexamethasone -a corticosteroid- decreased mortality in COVID-19 patients (Horby et al., 2021). The case study from Bangladesh Medical College by Dr. Tarek Alam on ivermectin combined with doxycycline (DOX) and azithromycin for SARS-CoV-2 treatment shows promising results (Agam et al., 2020; Hosseini et al., 2021). Slovakia became the first European Union Nation to approve this drug combination for COVID-19 treatment (“Slovakia,” n.d.).
However, the Food and Drug Administration (FDA) has not approved ivermectin usage for treating or preventing COVID-19 in humans (“FDA,” n.d.-a). Further, in June 2020 FDA revoked the usage of chloroquine and hydroxychloroquine for COVID-19 patients (“FDA,” n.d.-b). Meta-analysis of randomized controlled trials on ivermectin concludes it as a non-viable treatment option for COVID-19 and should be limited only for clinic trials (Roman et al., 2021). Similarly, there was a plea for reassessing the remdesivir treatment regime for COVID-19 (Paules et al., 2021). These expeditious trials have increased antibiotic, antiviral and antiparasitic consumption for no good, and humanity will pay its price– the inevitable global rise in AMR burden.
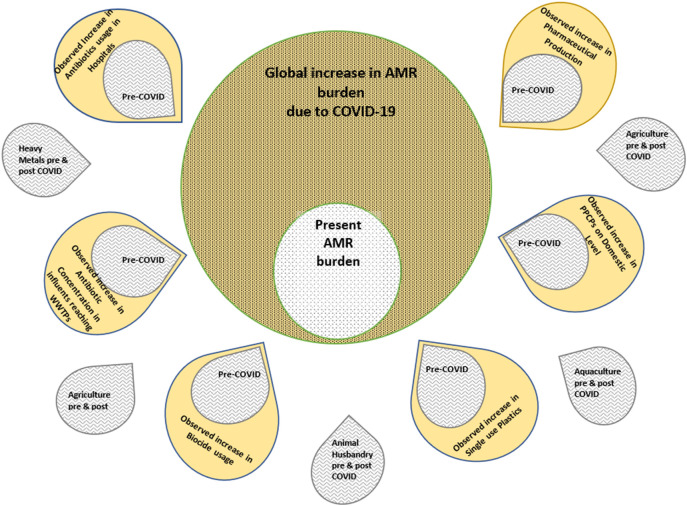
This scoping review aims to draw attention to the inevitable AMR surge expected to accompany the COVID-19 era (Fig. 2 ). The primary focus is on the overuse of Pharmaceuticals and Personal Care Products (PPCPs), their short- and long-term side effects on human health and the environment, the probable impact of increased usage of plastic on AMR, the effect of increased PPCPs in effluents, and heavy metal on AMR, problems associated with the complete removal of PPCPS from the Wastewater Treatment Plants (WWTPs), associated health risk et cetera. We have explored the cross-reactivity pattern between co-exposure of microbes to biocides and antibiotics as both increases tremendously in the environment. The severity of such interactions on AMR development is also discussed.
Fig. 2.
Some contributors of Antimicrobial Resistance have increased significantly due to the pandemic, while others remain unchanged. Antibiotics, biocides, and single-use plastics have drastically increased in the environment- predominantly in aquatic habitats. These factors act synergistically in promoting Antimicrobial Resistance. Consequently, this existing problem will possibly increase in the Post-COVID-19 Era.
2. The global crash of antimicrobial stewardship
Infectious Diseases Society of America (IDSA) states that only 8% of the COVID-19 patients acquired bacterial/fungal superinfections requiring antibiotics. However, astonishing data from another study show the administration rate of broad-spectrum antibiotics around 72% in SARS-CoV-2 patients, even in the absence of bacterial superinfection (Neto et al., 2020; Rawson et al., 2020). The study from Milan, Italy, shows an approximate 66.67% antibiotic administration rate for SARS-CoV-2 patients. Hospitals in Barcelona, Spain, have an antibiotic administration rate of 80% (Vidal et al., 2021). Data from ‘The Lancet’ show an antibiotic administration rate of 95% to patients in Jinyintan and Wuhan Pulmonary Hospital, China (F. Zhou et al., 2020; H. Zhou et al., 2020). In general, 70% to 95% of the patients are administered either DOX or amoxicillin (AMOX) (“Antibiotics used for treating COVID-19 patients may result in increased resistance to drugs' benefits,” n.d.). Neil Powell from Royal Cornwall Hospitals Trust states that “it was difficult for the doctors to know whether the patients admitted with COVID-19 symptoms had an overlying bacterial infection or not.” This issue is more challenging in the low and middle-income class (LMIC) due to the inadequacy of health care services.
Further, the standard guidelines include antibiotics like DOX, AMOX, benzylpenicillin (BENPEN), clarithromycin (CLR), co-AMOX, metronidazole (MTZ), moxifloxacin (MOXI), ciprofloxacin (CIPRO), et cetera as the first line of treatment for COVID-19 (Buckinghamshire Healthcare NHS, n.d.). Another concern is SARS-CoV-2 infection in people already carrying the AMR burden and have developed resistance. According to the Center for Disease Control and Prevention (CDC), about two in every hundred people carry methicillin-resistant Streptococcus aureus (“Healthcare Settings | MRSA | CDC,” n.d.), and infection with ESBL/AmpC producing bacteria is also persistent. CDC clearly states an increased susceptibility of hospitalized COVID-19 patients to secondary infections by resistant pathogens (approximately two hundred cases) (“CDC,” n.d.-a; “CDC,” n.d.-b; “CDC,” n.d.-c). There is an increase in reported cases of aspergillosis, antibiotic-resistant Acinetobacter, Candida MRSA bacteremia outbreak during the pandemic (“CDC,” n.d.-a; “CDC,” n.d.-b; “CDC,” n.d.-c; Srinivasan, 2021). The most commonly prescribed drugs for these infections are vancomycin (VAN) or teicoplanin (TEC) (Peng et al., 2013) and meropenem (MERO), respectively (all three are broad-spectrum antibiotics). There is a rapid increase in antibiotic consumption globally; AMOX, DOX -especially ceftriaxone and azithromycin has doubled; (Giacomelli et al., 2021; Nestler et al., 2020; Srinivasan, 2021), and the usage of broad-spectrum agents “did not escalate in few countries” (“CDC,” n.d.-a; “CDC,” n.d.-b; “CDC,” n.d.-c; Srinivasan, 2021); but simultaneously we have evidence of an increase in the prescription of broad-spectrum antibiotics (Alonso et al., 2020). It is essential to mention that antibiotic usage varies geographically- the health care facilities with higher COVID-19 inpatients consumed higher quantities of certain antibiotics and vice versa. In nursing homes, the consumption of azithromycin, ceftriaxone, and doxycycline increased but levofloxacin and amoxicillin decreased. The usage trend of antibiotics in hospitals is highly variable; CDC consolidated data from National Healthcare Safety Network and Premier Healthcare Database comprising 710 and 716 hospitals, respectively. For azithromycin, in April 2020, there is an increase of 65% and 44.6%, respectively, from both the data sources. (“CDC,” n.d.-a; “CDC,” n.d.-b; “CDC,” n.d.-c; Srinivasan, 2021). However, there is a marked laxity in implementing antimicrobial stewardship and a dearth of evidence to associate respiratory bacterial/fungal coinfections (Rawson et al., 2020).
The ‘black fungus’ or mucormycosis is emerging as one of the early side effects of the current COVID-19 treatment regime. The most probable trigger for this aggressive fungal infection is the unfortunate crossover of steroids therapy, reduced immunity, and dysglycemia (BBC News, 2021; Narayanan et al., 2021). This infection is life-threatening in immunocompromised individuals and diabetic patients. Mucormycosis has been declared an epidemic in Rajasthan, India (The Economic Times, 2021). Also, cases of white and yellow fungal infections are being reported. SARS-CoV-2 also affects audio-vestibular systems (Bhattacharjee, n.d.), causing the multisystem inflammatory syndrome, autoimmune conditions, severe weakness, et cetera. (“CDC,” n.d.-a; “CDC,” n.d.-b; “CDC,” n.d.-c). These may be the first of many other severe complications that the world will witness post-COVID-19 apart from the AMR crisis. As quoted by prof. Mathew Upton from the University of Plymouth; “Mass prescribing of antibiotics will lead to increased levels in the environment, and we know that this can select for resistant bacteria.” Due to the ongoing pandemic, it is challenging to establish controlled human infection models and is still under consideration (Rapeport et al., 2021). Hence, due to the absence of any study model or methodology, it is not easy to understand the holistic effect of COVID-19 treatment regimes.
3. Overuse of biocides and its side effects
Globally there is an increase in hygiene sensitivity which has spiked the usage of different disinfectants and a broad spectrum of pharmaceutical products to curb the spread of the virus. These pharmaceutical products, including antibiotics, can be collectively referred to as Pharmaceutical and Personal Care Products (PPCPs). The guidelines issued by CDC for disinfecting and cleaning the facilities include a list of 573 products (“CDC,” n.d.-a; “CDC,” n.d.-b; “CDC,” n.d.-c) with these active ingredients (isolated or combined form) hydrogen peroxide, quaternary ammonium compounds, isopropyl alcohol, dodecylbenzene sulfonic acid, Et cetera; and they can disinfect in contact time of 1 min (EPA, n.d.). Hypochlorous acid is 80 to 100 times more efficacious than sodium hypochlorite (bleach) (“The science of chlorine-based disinfectant,” n.d.). The spraying of disinfectants in hospitals, residential and commercial buildings, open areas, public transports, Et cetera., is done regularly as a precautionary measure. Grignard Pure™ has emerged as an efficient air treatment solution with 98% efficiency at a concentration of 0.5 to 3 mg/m3 (“GrignardPure,” n.d.) is used by airlines and other indoor spaces (Us-Epa, 2000). The sales data from Reckitt Benckiser show a 50% increase in the sales of disinfectants in the first quarter of 2020 and 20 times more production of hand sanitizer (“CEO of Lysol maker: Sales are up as a result of coronavirus pandemic,” n.d.) even though the Chinese Center for Disease Control and Prevention, United States Environmental Protection Agency and World Health Organization have successively released guidelines for the PPCP usage; the lack of knowledge combined with panic can lead to its adverse effect on health. A survey conducted by the School of Public Health, Iran, reveals that 87% of the population uses the wrong ratios of water and alcohol in homemade disinfectants, and 74.2% use the wrong proportion of sodium hypochlorite (Dindarloo et al., 2020). Data published in April 2020 show an approximate usage of 2000 tons of disinfectant (chlorine-based) in Wuhan, China alone (Zhang et al., 2020). This increase in consumption of biocides or disinfectants is a concern as they can pollute drinking water or can combine with sewage system- either direct runoff or indirect sewage effluent- eventually ending up in the environment as most of the Wastewater treatment plants are not equipped with appropriate pre-treatment techniques to completely remove PPCPs and can remove only up to 20% to 80% of it (Hassan et al., 2020). Eventually, all the PPCPs will end up in natural water bodies or soil, creating toxicity and stress on the macrofauna and microfauna.
Overuse of disinfectants has associated side effects, both acute and chronic. Few products contain non-volatile ‘active ingredients’ like chlorhexidine digluconate, triclosan (TCS), mecetronium ethyl sulfate, et cetera along with alcohol. These additional biocides fail to provide superior bactericidal efficacy but are a potential risk, and its dermal exposure must be avoided (Kampf et al., 2017) as it can cause itching, redness, dryness, and sores. These volatile chemicals cause lung irritation, shortness of breath, cough, sneezing, et cetera, and if ingested, cause abdominal pain, diarrhea, vomiting, and other gastrointestinal issues; the most frequently reported side effects are throat irritation, short attention span, headache, fatigue, Et cetera. (Dindarloo et al., 2020). The overuse of sodium hypochlorite is a significant concern due to its high oxidizing nature. It has a severe side effect spectrum based upon the route and duration of exposure- the topical application results in inflammation, inhalation in high dose leads to respiratory failure et cetera (Peck et al., 2011), the vapors cause a burning sensation in the esophagus, and edema of the mucosa. In very high concentration, it has the potential of causing tissue necrosis, rhabdomyolysis, the superoxide and hydroxide radicals formed can damage renal epithelium et cetera (Chatterjee, 2020). Triclosan causes oxidative stress, mitochondrial toxicity, affects a wide array of signal cascades, Et cetera that ultimately leads to cholestatic liver injury (Boas et al., 2019). The enrichment, bioaccumulation, and biomagnification of PPCPs need immediate focus as humans, the top predators, are at extreme risk.
4. Toxicity and cross-resistance between biocides and antibiotics
There is a lack of awareness that some biocides may have more risk of promoting AMR than other anthropogenic factors. Biocides promote antibiotic tolerance, decreased antibiotic sensitivity (Bataillon et al., 2011), cross-resistance (González et al., 2014) and co-resistance in pathogenic bacterial strains by increasing HGT (Jutkina et al., 2018), overexpression of efflux pumps (Jutkina et al., 2018; Sonbol et al., 2019), et cetera (Table 1 ). Pathogens acclimatized to BZK develop cross-resistance to ampicillin (AMP), AMOX, and erythromycin (ERY) (Bataillon et al., 2011; Lerma et al., 2015). Similarly, chlorohexidine acclimatized pathogens develop co-resistances to major antibiotics like ceftazidime, sulfamethoxazole (SMX), imipenem, CTX, tetracycline (TET), CIPRO, gentamycin, ERY (Cuenca et al., 2015; Gajadhar et al., 2003; Kampf, 2018; Morita et al., 2003). Exposure of pathogens to triclosan reduced susceptibility to chloramphenicol, TET, AMP, et cetera (Karatzas et al., 2007). Exposure to sodium hypochlorite assists in the development of cross-resistance in pathogens against AMP, chloramphenicol, and TET (González et al., 2014).
Table 1.
The cross-resistance/cross-tolerance pattern observed on co-exposure of antibiotics and biocides.
| Biocides | Microorganisms under experiment | Cross-resistance/cross tolerance pattern | References |
|---|---|---|---|
| Benzalkonium chloride | E. coli | Decreased susceptibility to cotrimoxazole. Expression of MdtM multi-drug efflux pump. |
(Holdsworth and Law, 2013) |
| Pseudomonas spp. | Resistance to ampicillin, amoxicillin, erythromycin, and trimethoprim. Induction of MexCD-OprJ multi-drug efflux pump. |
(Bataillon et al., 2011; Lerma et al., 2015; Morita et al., 2003) | |
| Salmonella typhimurium | High tolerance to chloramphenicol, tetracycline, ampicillin. Overexpression of AcrAB or AcrEF acriflavine efflux pump. | (Guo et al., 2014; Karatzas et al., 2007) | |
| Chlorhexidine digluconate | E. coli, S. aureus, S. haemolyticus | Increase in HGT of MGE carrying ARGs. | (Jutkina et al., 2018) |
| Pseudomonas spp. | Co-resistance to ciprofloxacin, norfloxacin, tobramycin, gentamicin, ampicillin, polymyxin, and erythromycin. Reduced adsorption of biocide to the cell surface and induction of MexCD-OprJ multi-drug efflux pump. |
(Gajadhar et al., 2003; Morita et al., 2003; Nagai et al., 2003; Tattawasart et al., 1999) | |
| A. baumannii | Co-resistance to carbapenem, aminoglycoside, tetracycline, and ciprofloxacin. Increased expression of multiple efflux pump genes. |
(Cuenca et al., 2015) | |
| Triclosan | E. coli | Enhance of efflux pump system. Increase in HGT of MGE carrying ARGs. |
(Jutkina et al., 2018; Sonbol et al., 2019) |
| Acinetobacter johnsonii | Decreased susceptibility with chloramphenicol. | (Lear et al., 2002) | |
| Pseudomonas spp. | Cross-tolerance with ampicillin, amoxicillin, erythromycin, imipenem, and trimethoprim. | (Lerma et al., 2015) | |
| Salmonella spp. | Reduced susceptibility to chloramphenicol, tetracycline, ampicillin et cetera. Overexpression of AcrAB acriflavine efflux pump. |
(Karatzas et al., 2007) | |
| Didecyldimethylammonium chloride | E. coli | Decreased sensitivity to cotrimoxazole and amoxicillin. | (Bataillon et al., 2011) |
| Sodium hypochlorite | Salmonella spp. | Cross-resistance with class aminoglycosides and cephalosporins, amikacin, ampicillin, chloramphenicol, tetracycline, and teicoplanin. | (González et al., 2014) |
The antibiotic resistance due to commonly used biocides is studied in a controlled environment and has minor relevance if used in small quantities. However, lifestyle modifications due to pandemics are changing the usage pattern of these disinfectants and have a realistic potential to enhance AMR development. Thus, these findings need immediate reviewing as MDR human pathogens like Salmonella spp., E. coli, Acinetobacter spp., Staphylococcus spp. have developed cross-resistance (in laboratory settings) and can evolve and become XDR which will be impossible to treat with the currently available antibiotics.
5. Heavy metals and antibiotics - a synergetic AMR surge
Studies prove the synergistic effect of heavy metals and antibiotics on AMR development (Table 2 ). The nexus of effluents from different domestic and healthcare sectors poses a considerable risk as higher concentrations of PPCPs are reaching STPs along with the residual metal-laden industrial effluents. Sludge dumping from WWTPs laden with residual PPCPs and heavy metals in agricultural fields as manure needs risk assessment. It has the potential of disseminating Antibiotic-Resistant Gene (ARG) between soil and crops and can ultimately result in bioaccumulation and human health hazard. The soil contaminated with oxytetracycline (OTC) and cadmium enhance the transfer of human pathogens into leaf tissues (Guo et al., 2021). Pathogenic bacteria like Mycobacterium and Legionella harbor a high copy number of ARG tnpA in biofilm formed on polyvinyl chloride microplastic in the presence of copper, TET, and AMP (Zhao et al., 2021). Selective pressure of heavy metals like copper and zinc increases ampicillin-resistant opportunistic pathogens like Pseudomonas, Aeromonas, Acinetobacter, Staphylococcus (Wang et al., 2021a). P. putida harboring RP4 plasmid has the potential risk of disseminating ARGs to a broad host range in soil (Fan et al., 2019). Divalent cadmium at sub-inhibitory concentration shows a significant increase in conjugation frequency of RP4 plasmid, which contains ARGs between P. putida KT2442 and much freshwater microbial community, thereby facilitating the ARG transfer to a broader taxon (Pu et al., 2021). The linkage between antibiotic resistance and metal tolerance and very high minimum inhibitory concentration values for antibiotics like AMP >10,000 μg/ml, kanamycin 4800 μg/ml, chloramphenicol 800 μg/ml is found in heterotrophic cultivable bacteria isolated from Rozalia Gold mines, Slovakia (Timková et al., 2020). In the metal mining region of Xiangjiang River, China, heavy metals (Cu+2 and Zn+2) have aggravated horizontal gene transfer of plasmids – especially conjugative plasmids- in pathogenic bacteria like P. monteilli, Aeromonas hydrophila, A. baumani, and Staphylococcus epidermis pertaining to increased MDR bacteria in that river (Wang et al., 2021a, Wang et al., 2021b). E. coli isolates from dairy wastewater in China with high iron, nickel, and arsenic concentrations show a multidrug-resistance pattern (C. Liu et al., 2021; Y. Liu et al., 2021). Thus, the increase in the consumption of antibiotics will create a ripple effect, and an increase in the synergistic effect of co-resistance of antibiotics and heavy metals will be inevitable in all the possible nexuses.
Table 2.
Co-Resistance pattern on simultaneous exposure to heavy metals and antibiotics.
| Heavy metals | Antibiotics | Source | Microorganism Under Study | Mechanism of co-resistance | Reference |
|---|---|---|---|---|---|
| Cadmium | NA. | Water (Xinlin Bay, China) | Pseudomonas putida | Increase in frequency of HGT -conjugation- by increased membrane permeability, increased catalase and superoxide dismutase activity, and overexpression of trbBpa and trfApb genes. | (Pu et al., 2021) |
| Oxytetracycline | Soil and lettuce tissue (China) | Clostridium, Burkholderia, Salmonella | Increased expression of tetracycline resistance protein (tetG, tetC, tetW), sulphonamide resistance gene sul2, erythromycin resistance gene ermX, intl1c | (Guo et al., 2021) | |
| Copper | N.A. | Swine manure (Anaerobic Digester) | Firmicutes spp., Bacteroidetes spp., Actinobacteria spp. | Cu influenced the co-occurrence of MGEs and co-selection of ARGs; affected the microbial community. High abundance of tetG, sul1, sul2, ermQ, blaCTX-Md |
(Zhang et al., 2020) |
| Copper and Zinc | Ampicillin | Water (Xiangjiang River, China) | Pseudomonas monteilii, Aeromonas hydrophilla, Acinetobacter baumannii, Staphylococcus epidermidis | Increase in HGT by conjugation and co-selection of ARGs and HRG. | (Wang et al., 2021a, Wang et al., 2021b) |
| Ampicillin Tetracycline |
Domestic sewage (China) |
Mycobacterium Legionella |
Overexpression of transposase tnpA | (Zhao et al., 2021) | |
| Zinc Nickel Lead Copper |
Ampicillin Chloramphenicol |
Soil (Rozalia Gold Mine, Slovakia) | NA. | Co-selection, co-resistance and cross-resistance mechanism. | (Timková et al., 2020) |
| Zinc Nickel Lead Copper Cobalt Chromium |
Tetracycline β-Lactams Erythromycin |
Soil (Scotland) | NA. | Abundance of ARGs tetM, tetW, blaOXA, blaCTX-M, ermB, ermF | (Knapp et al., 2011) |
| Zinc Nickel Arsenic Iron Copper Strontium Cobalt |
Vancomycin | Soil (Savannah river, USA) |
Acidobacteriaceae, Bradyrhizobium Mycobacterium Streptomyces Verrumicrobium Actinomadura |
Abundance of MDR genes mdtB, vancomycin resistance genes vanR, emrB and cusS. Overexpression of efflux pumps. |
(Thomas 4th et al., 2020) |
| Iron Copper Zinc Nickel Arsenic Selenium Lead Mercury |
β-Lactams Tetracyclines Quinolones |
Water (Dawen river, China) | E. coli | Overexpression of aminoglycoside resistance genes (aadA and aacC2), blaCTX-M, tetA and tetB, and sul2 | (C. Liu et al., 2021; Y. Liu et al., 2021) |
| Chromium Nickle Manganese Copper Lead Cobalt Arsenic |
Kanamycin Fluoroquinolone |
Water (Sabarmati river, and STP India) | E. coli | Similar cross-resistance pattern observed. | (Ram and Kumar, 2020) |
Mating pair formation gene.
DNA transfer and replication gene.
Class 1 Integrase is a site-specific recombination enzyme with recognition site of attI1 where gene cassettes get inserted.
Extended spectrum β-lactamases encoding gene.
6. Impact of the pandemic on plastisphere and AMR
The contagion of the virus requires collaborative protective measures like an increase in the use of disposable cutleries and packaging materials in markets, restaurants, take-outs, et cetera (major contributors of Food-related Marine Macroplastic Litter) and plastic-based PPE to an estimated 129 billion face masks and 65 billion gloves per month (Prata et al., 2020). According to Thailand Environment Institute, plastic waste has increased from 1500 tons to 6300 tons per day (“World Economic Forum,” n.d.). The PPE market in 2016 valued 40.06 billion and is estimated to reach 58.34 billion by 2022; further, estimated annual growth of 20% in facial and surgical mask supply by 2025 is expected (“Personal Protective Equipment Market,” n.d.). A single-use surgical mask is made of non-woven non-biodegradable polymers like polystyrene, polycarbonate, polyethylene (PE), polypropylene, and polyurethane, which impart hydrophobicity and prolongs the afterlife. Plastics never fully degrade and somewhat shrink into smaller pieces of less than five millimeters (<5 mm) called microplastics (MP). Synthetic industries are the primary source of airborne MP. Polyamide and polyvinylchloride (PVC) are the most abundant polymers in the Rhine river (Gras et al., 2021). Detailed review by Kumar et al. (2021) on the occurrence of MP in rivers across the globe concludes a disturbing concentration of microplastics. 13.7 particles/L of polystyrene in Los Angeles River, USA; 25–340 particles/kg of MP in Yangtze River, China; Wuhan rivers has 1660–8925 particles/m−3; 99.27–409.86 particles/kg in Ganga River, India et cetera. The increase in PPE production is expected to increase microplastic concentration and its ultimate return to terrestrial or aquatic ecosystems as fallout (Chen et al., 2020). Due to the physicochemical properties of microplastics like hydrophobic surface, buoyancy, et cetera, it is a stable substrate and pristine ecological niche for microbes and enables ARG exchange in any habitat- “the plastisphere.” The microbes hitching ride on microplastics can travel across the globe, converging with other species and giving impetus to AMR dissemination. The biofilm study on the riverine microplastics from the Rhine river, Netherlands, shows pathogenic bacteria like Pseudomonas spp., Acinetobacter spp., Arcobacter spp. harboring ARGs like sul1 (sulfonamide resistance gene) and erm (erythromycin resistance methylase gene) (Gras et al., 2021). The sediments from the Sabarmati River in India show a high concentration of microplastics, and at that site, the highest antibiotic resistance is present (Ram and Kumar, 2020). Bacterial species isolated from the plastic surface from the coastline of Northern Ireland show high resistance of 98.1% to beta-lactams and other critically essential antibiotics (Moore et al., 2020). (C. Liu et al., 2021; Y. Liu et al., 2021) reports distinct ARG composition on biodegradable and non-biodegradable microplastics due to selective enrichment. Profiling of bacterial isolates from microplastics in the coastal lagoon in the northern Gulf of Mexico show a similar abundance of ARGs, but different resistome pattern on polyhydroxyalkanoate (PHA) (biodegradable) with MDR gene abundance of 2.05 copies per 16S rRNA and polyethylene terephthalate (PET) (non-biodegradable) with 3.05copies per 16S rRNA and most of the ARG hosts were human pathogens; thereby posing potential human health risk (Sun et al., 2021).
Apart from harboring microbes in aquatic habitats, microplastic is the preferred substrate for growth than other organic surroundings. Studies on microplastics in landfill leachate show a higher abundance of Mobile Genetic Elements (MGEs) associated with ARGs. Microplastics harbor distinct bacterial communities than the surrounding leachate, and selective enrichment of ARGs and pathogens is on it than the surrounding leachates (Shi et al., 2020). The emerging pollutants -MP and PPCPs- are ubiquitous; and, the concentration of both is increasing tremendously due to the pandemic. The triad formation of antibiotics, bacteria, and microplastic will become much more facile, and the plastisphere will emerge as a potential AMR depot between different ecosystems. A detailed study by Wang et al., 2021a, Wang et al., 2021b compares the adsorption capacity of three major antibiotics on different MPs and their associated microbial community. Ampicillin has maximum adsorption capacity followed by tetracycline and triclosan on PVC compared to polyethylene, but microbes easily attach to the latter. Further, the ARGs were higher in the system having MPs as compared to control. Human pathogens like Legionella, Mycobacterium, Neisseria, and Arcobacter concentrated on the MP compared to sewage. PPEs, disposable cutleries, empty sanitizer pouches, soiled tissue papers, et cetera together are creating a massive trail of clinical waste in the environment. The environmental impact of such clinical debris during this pandemic era is far-reaching.
7. Pre-COVID-19 levels of PPCPs in natural water bodies
The increase in antibiotic and disinfectant usage has put the WWTPs under pressure to deal with this sudden surge in effluents, ultimately increasing the residual PPCPs-particularly antibiotics in the potential water bodies (Anh et al., 2021; Russell and Yost, 2021; Wang et al., 2020). The pre-COVID-19 studies on the Asian river systems give disturbing results. Hai river system in northern China has exceptionally high levels of thirteen antibiotics and is declared as priority contaminants, primarily oxytetracycline (361,000 ng/L) and chlortetracycline (CTC) (68,900 ng/L) (Chen et al., 2018). The Han water system in South Korea has a significant concentration of various antibiotics like CLR 72 ng/L, carbamazepine (CBZ) 29 ng/L, and sulfamethoxazole (SMX) 3 ng/L (Im et al., 2020). The studies on the urban canals of Hanoi city, Vietnam, show a high concentration of SMX, AMOX, and erythromycin (>1000 ng/L) (Tran et al., 2019). The tropical rivers of the southwestern Indian river have a significant concentration of SMX (up to 2 ng/L) and trimethoprim (TMP) (up to 8 ng/L) in the river and sources of drinking water (Joshua et al., 2020). These antibiotics will undoubtedly rise further because the effluents from hospitals will indirectly reach water bodies. After all, COVID-19 patients receive cotrimoxazole (a combination of TMP and SMX in a 1:5 ratio) (Arafat, 2020). The stress induced by the high concentration of antibiotics causes altered gene expression in aquatic microbiota and assists in resistant species' evolution. Thus, in this present scenario, the over-prescription of antibiotics is emerging as a significant environmental concern.
8. Fate of PPCPs
PPCPs have complex persistence patterns in nature due to their physicochemical properties and environmental factors. All PPCPs are not persistent, but their prolonged usage and release in the environment make them “pseudo-persistent” with a more significant environmental threat. Most antibiotics get metabolized only up to 30% to 80% in the body, and the rest gets excreted. The over-prescription of AMOX has a severe threat as the pharmacokinetics data reveal that approximately 60% of the orally administered dose gets excreted in the urine in unchanged form (“Amoxil (Amoxicillin): Uses, Dosage, Side Effects, Interactions, Warning,” n.d.). Conclusive studies show higher antibiotic residues in urban rivers, contributed by antibiotics from the domestic sewage effluent than veterinary medicine in livestock wastewater (Im et al., 2020; Murata et al., 2011); and the former has a very high potential of AMR selection. After coming into the environment, antibiotics undergo various degradation pathways to form products with lower antibacterial properties. However, the latter has a higher toxicity than parent compounds and can persist in either aqueous medium or solid medium, depending on its solubility. Carbamazepine and diazepam are highly persistent drugs with a dissipation time of around 300 days, while ivermectin- one of the most commonly used drugs for COVID-19 treatment – is moderately persistent -approximately up to 50 days (Löffler et al., 2005). Tetracycline has low solubility in aqueous media, but it is predominant in soil and sediments. The swine manure has a very high concentration of TET, and if mixed with soil, its concentration reaches several nanograms (Lehmann and Bloem, 2021). The conventional methods of WWTPs are not efficient enough to completely remove PPCPs, and as a result, the residual or transformed PPCP products end up in environmental settings like aquatic environment receiving treated water from STPs, on soil via the organic manures from farms, leach deep inside soil from the landfills and sludge.
9. Challenges to curb medical waste
Pandemic is affecting the normal functioning of solid-waste management and disposal. Ministry of Ecology and Environment, China, estimates 240 tons of waste generated daily during the pandemic compared to 40 tons per day before the pandemic in Wuhan. Similarly, United States is generating a year's medical waste in just two months due to COVID-19 (“World Economic Forum,” n.d.). Therefore, improper bio-medical waste handling is a serious concern.
Moreover, contagious diseases like hepatitis and HIV have a high transmission rate from patients to health care workers due to improper biowaste handling (“Health-care waste,” n.d.). A similar pattern of contagion holds true for novel coronavirus. In addition, the traditional waste handling methods like open burning or incineration release toxic dioxins, furans, and particulate matter in the atmosphere - some of them being potent carcinogens pose a significant concern.
Liquid waste management is dealing with a similar obstacle. The increase in antibiotic consumption leads to an increase in antibiotic residue in the wastewater reaching the WWTP. As a result, more residual PPCPs are reaching the river bodies, especially from the WWTPs receiving effluents from big cities with major hospitals and other health centers. At a suboptimal concentration of antibiotics, the microbiota of the waterbody comes under stress, affecting the non-target organisms too. The increasing PPCP concentrations in the rivers or coastal waters is deleterious for the microbiota, and they are already present in surface waters, groundwaters, sediments, and wetlands up to high concentrations like 1 mg/L (Chen et al., 2021).
Removing antibiotics from wastewater treatment plants is always challenging because the traditional secondary biological treatment methods like activated sludge treatment, anoxic/oxic oxidation ditch, and trickling filters involving hydrolysis, biodegradation, and adsorption have low removal efficiency of antibiotics. The different classes of antibiotics have different removal efficiency attributed to their physiochemical properties, chemical lattice, treatment process, and operational conditions (pH, temperature, SRT, HRT). According to the study by Wang et al. (2019) on the prevalence of antibiotics in WWTPs for 19 years, the removal efficiency of the various secondary biological treatment process for major antibiotics is as follows-azithromycin 3%–90%, ciprofloxacin 26%–100%, sulfamethoxazole 20%–100%, and tetracycline 69.1%. Antibiotics of class quinolones, lincomycins, and lactams have 73% to 77% removal efficiency. On the other hand, the antibiotics with a high potential to resist biodegradation belonging to class macrolides, sulfonamides, and TMP have a poor removal efficiency of 59.3%, 52.6%, and 55%, respectively. Another set of data of antibiotic removal by WWTP show these removal rates- tetracycline-100%, chlortetracycline-85.9%, oxytetracycline-87.9%, sulfadiazine-76.2%, sulfamethoxazole-75%, sulfamethazine-71.7%, sulfamerazine-54.7%, and trimethoprime-58% (Li et al., 2016). It concludes that antibiotics cannot be removed entirely by the conventional wastewater treatment processes, and the recent increase in PPCP in wastewater increases the load on already compromised WWTPs. Hence, there is an immediate need to address this issue of the antibiotic surge; and to minimize its potential risk to the environment, novel technologies need to be implemented to remove residual PPCPs in WWTP before releasing its effluents in water bodies.
10. Novel remediation techniques to reduce PPCP load in WWTP
The contemporary techniques used in WWTPs are insufficient to eradicate the residual PPCPs; consequently, their concentration is ever-increasing in the natural water bodies. Conventional biogas digester used in WWTPs cannot reduce PPCP concentration below harmful limits at a higher influx rate (HongMei et al., 2016); hence, it is coupled with different processes to enhance performance. Anaerobic digestion has a removal efficiency of 73%, manure composting 84.7%, and constructed wetland 90% for veterinary antibiotics (Gaballah et al., 2021). Aerobic/anoxic and sequential anaerobic + aerobic/anoxic process successfully reduces triclosan and its metabolites from sludge (Abbott and Eskicioglu, 2020). In-situ advanced anaerobic digestion enhanced with zero-valent iron powder has a removal efficiency of 97.39% for sulfamethoxazole (F. Zhou et al., 2020; H. Zhou et al., 2020). The combined anaerobic and aerobic biological processes give removal efficiency of 92% for antibiotics (Han et al., 2019). The anaerobic/anoxic/oxic process combined with membrane bioreactor has a removal efficiency of 87% for a wide range of PPCPs (Meng et al., 2021). 100% removal efficiency is necessary as residual antibiotics pose a higher hazard to the biome. Also, these techniques impart contamination to the surrounding environment. The antibiotics can leach into the soil, and if it comes in contact with the groundwater reservoir, it can pose a severe health hazard. Hence novel hybrid techniques with better removal efficiency are the need of the hour.
The tertiary treatment like the advanced oxidation process (AOPs), is efficient to treat PPCPs and other toxic products like dyes, pesticides, explosives, and many more. The significant advantage of this method is that it has a high reaction rate and a nonselective mechanism that treats a broad range of organics simultaneously, thereby reducing the toxicity and completely mineralizes organic contaminants (Ameta et al., n.d.). Hybrid technology using metal-organic frameworks as an adsorbent for PPCPs in wastewater is promising as it degraded oxytetracycline into harmless end products (Wang et al., 2016). The photo-Fenton process has 50% removal efficiency in 90 min for ciprofloxacin and fluoxetine in anaerobic pre-treated hospital effluent (Perini et al., 2017); and ofloxacin up to 98.1% in just 2 h in the presence of a catalyst (Du et al., 2020). The ozonation process can remove the mixture of eight antibiotics below their detection limit (Iakovides et al., 2019). Electrochemical advanced oxidation process alone or coupled with membrane filtration has a removal rate of 98% for carbamazepine (Ganzenko et al., 2021). The catalytic oxidation process using sludge-derived carbon (a potential low-cost catalyst) has a removal rate of 92.6% in just 260 min for oxytetracycline (Liu et al., 2019). These methods show promising results and can be used to pre-treat sanitary waste with high PPCP load like health care facilities and pharmaceutical industries before they reach WWTP.
11. Risk assessment studies
More than four thousand PPCPs are available on the market for various purposes; they can get dissipated in the environment by transforming into non-extractable residues that can be bioaccumulated and enter the food web. The impact of PPCPs on non-targeted species poses an ecological threat. Bioaccumulation factor in algal samples for triclosan (TCS) and its metabolites triclocarban and methyl triclosan is in the range of 700 to 2700 (Ebele et al., 2017). The expected global medicine usage in 2020 was 4.5 trillion doses, increasing 24% from 2015 (Pennente et al., 2015). However, this was the conjecture before COVID-19. The five-year compound annual growth rate (CGAR) to 2025 is projected to change from 4.5% to 4.6% due to pandemic (“IQVIA,” n.d.). The current PPCP usage data is challenging to obtain due to insufficient resources, self-medication, and non-prescription drug sales.
Risk assessment studies of 66 PPCPs in Tejo estuary, Portugal, show Risk Quotient (RQ) for the accumulated mixture of 16 pharmaceutical residues over 380 folds higher than the no-risk threshold driven mainly by antibiotics (Fonseca et al., 2021). The risk assessment studies on the Han water system in South Korea show RQ of more than one for aquatic organisms; clarithromycin (CLR) and sulfamethoxazole (SMX) are the potential threats (Im et al., 2020). The risk assessment studies on the Tama river system in Japan have Hazard Quotient (HQ) for five PPCPs more than one, and three of those PPCPs (AZI, CLR, and TCS) pose a potential ecological risk (Mano and Okamoto, 2016). Another study shows a high concentration of SMX and other PPCPs in the Cau river, Vietnam, and the risk assessment studies conclude moderate risk on the aquatic organism (Ngo et al., 2021). Finally, risk assessment studies on the overuse of amoxicillin (AMOX) and doxycycline (DOX)- (most prescribed antibiotics for COVID-19) the PEC: PNEC ratio of <1 for DOX and up to 5.70 for AMOX in two different settings in the United Kingdom; and the data for AMOX indicates a potential environmental concern for selection for AMR (Comber et al., 2020). However, such risk-assessment modeling is complicated in LMIC due to the unavailability of data. Nevertheless, such studies can assist in making new policies for antibiotic stewardship and help select the location of emergency hospitals in the future to minimize the damage to the environment.
12. COVID-19 and human behavior
As quoted by Desmond Tutu- “A time of crisis is not just a time of anxiety and worry. It gives a chance, an opportunity, to choose well or to choose badly”. COVID-19 shows various colors of humanity and has affected all the echelons in psychological and emotional ways. There is an increase in medicine hoarding, counter drug selling, black marketeering of drugs, et cetera. There is an immediate need to increase awareness among the masses regarding the judicious use of antibiotics and other biocides. The increase in unprescribed drug sales, over-prescription of antibiotics by health care workers, increase in self-medication rather than facing the isolation of COVID ward, google doctors and WhatsApp clinics, fake news, circulation of unresearched treatment methods, superstitions et cetera are playing an active role in the global AMR surge.
13. Conclusion
Drug resistance can develop in almost any type of microbe- viruses, fungi, bacteria, or protozoa- and the ability to treat such infections gets hampered to an immense extent. AMR recommendations from Food and Agriculture Organization, World Organization for Animal Health, and the World Health Organization state that the SUPERBUG PROBLEM needs to be solved by prudent antibiotic usage combined with improving the living standards and raising awareness among the masses. If not taken care of – the next pandemic might be worse than COVID-19. The increase in AMR post-COVID-19 is undeniable, and it is surmised that there will be an acute inadequacy of antibiotics available as the frequency of multidrug-resistant and extensively drug-resistant cases will escalate (“Scientists around the world are already fighting the next pandemic,” n.d.). The worldwide disruption in health services during this pandemic interferes with the treatments for chronic diseases like tuberculosis and hepatitis C (Barocas et al., 2021) which has the potential of causing novel resistance patterns and require aggressive efforts for treatment. Similarly, the vaccination services hampered due to the pandemic can increase the risk of other diseases, and ultimately overuse of antibiotics is speculated. The increase in AMR in environmental settings bears the pragmatic potential to introduce novel resistance patterns in human pathogens against the “now effective” antibiotics, entering the food web, and ultimately emerging as “superbug.”
Immediate attention is needed to evaluate the worldwide evolution of AMR in the COVID-19 era with a particular focus on low to middle-income countries where data on resistance patterns are scarce, the transmission of infectious pathogens is optimal, and treatment resources are suboptimal (Lucien et al., 2021). The potential impact of COVID-19 related prescription of antibiotics on the global AMR burden and its associated toxicity on the environment needs to be addressed. There should be social awareness camps to educate the masses over the use and misuse of PPCPs. There is an immediate need to formulate new and dynamic policies on water quality parameters entering the environment, focusing on emerging contaminants like antibiotics. The wastewater with a very high concentration of antibiotics emerging from healthcare settings and pharmaceutical companies needs to be monitored and pre-treated to lower the contamination level before reaching the WWTPs. Further, the WWTPs need to be upgraded by novel techniques that thoroughly remove residual antibiotics before releasing their effluents in rivers. This pandemic is creating a domino effect – the unforeseen overload on front line health workers leading to the over-prescription of antibiotics as the antimicrobial stewardship crashed on a global level, and last but not at all the least- if not immediately addressed, the global AMR surge will be inevitable, and the world will not escape from its aftermath.
Funding resources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Sidra Ghazali Rizvi: Conceptualization, Writing - Original draft, Reviewing and editing.
Dr. Shaikh Ziauddin Ahammad: Conceptualization, Reviewing and editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Editor: Frederic Coulon
References
- Abbott T., Eskicioglu C. 2020. Comparison of anaerobic, cycling aerobic/anoxic, and sequential anaerobic/aerobic/anoxic digestion to remove triclosan and triclosan metabolites from municipal biosolids. [DOI] [PubMed] [Google Scholar]
- Agam V., Arora V.K., Behera D. White paper on ivermectin as a potential therapy for COVID-19 keywords. Indian J. Tuberc. 2020:448–451. doi: 10.1016/j.ijtb.2020.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso G.A., Padullés A., Rombauts A., Gudiol C., Pujol M., Alvarez-Pouso C., Jodar R., Carratalà J. Antibiotic prescription during the COVID-19 pandemic: a biphasic pattern. Infect. Control Hosp. Epidemiol. 2020;41:1371–1372. doi: 10.1017/ICE.2020.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameta et al., n.d.Ameta C. Suresh, Ameta Rakshit, n.d. Advanced Oxidation Processes for Wastewater Treatment [WWW Document]. URL https://books.google.co.in/books?hl=en&lr=&id=hyk0DwAAQBAJ&oi=fnd&pg=PP1&ots=1PWWBc4IoW&sig=aaQOwX2Gpw4EDPQ1qgXDVFp8AaY&redir_esc=y#v=onepage&q&f=false (accessed 5.8.21).
- Amoxil (Amoxicillin): Uses, Dosage, Side Effects, Interactions, Warning [WWW Document], n.d.Amoxil (Amoxicillin): Uses, Dosage, Side Effects, Interactions, Warning [WWW Document], n.d. URL https://www.rxlist.com/amoxicillin-drug.htm#clinpharm (accessed 2.15.21).
- Ananworanich J., Mogg R., Dunne M.W., Bassyouni M. Randomized study of rivaroxaban vs. placebo on disease progression and symptoms resolution in high-risk adults with mild COVID-19. Infect. Dis. Soc. Am. 2020;1–35 doi: 10.1093/cid/ciab813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anh H.Q., Le T.P.Q., Da Le N., Lu X.X., Duong T.T., Garnier J., Rochelle-Newall E., Zhang S., Oh N.H., Oeurng C., Ekkawatpanit C., Nguyen Tien Dat, Nguyen Q.T., Nguyen Tran Dung, Nguyen T.N., Tran T.L., Kunisue T., Tanoue R., Takahashi S., Minh T.B., Le H.T., Pham T.N.M., Nguyen T.A.H. Antibiotics in surface water of east and southeast asian countries: a focused review on contamination status, pollution sources, potential risks, and future perspectives. Sci. Total Environ. 2021;764 doi: 10.1016/j.scitotenv.2020.142865. [DOI] [PubMed] [Google Scholar]
- Antibiotics used for treating COVID-19 patients may result in increased resistance to drugs’ benefits [WWW Document], n.d.Antibiotics used for treating COVID-19 patients may result in increased resistance to drugs’ benefits [WWW Document], n.d. URL https://www.news-medical.net/news/20200825/Antibiotics-used-for-treating-COVID-19-patients-may-result-in-increased-resistance-to-drugs-benefits.aspx (accessed 2.15.21).
- Arafat S.M. 2020. Role of Co-trimoxazole in Severe COVID-19. [Google Scholar]
- Bataillon B.S., Branger B., Cormier M., Bonnaure-Mallet M., Jolivet-Gougeon A. Effect of higher minimum inhibitory concentrations of quaternary ammonium compounds in clinical E. coli isolates on antibiotic susceptibilities and clinical outcomes. J. Hosp. Infect. 2011;79:141–146. doi: 10.1016/j.jhin.2011.06.008. [DOI] [PubMed] [Google Scholar]
- Barocas, J.A., Savinkina, A., Lodi, S., Epstein, R.L., Bouton, T.C.. Projected long-term impact of the COVID-19 pandemic on hepatitis C outcomes in the United States: a modelling study. Clin. Infect. Dis. 10.1093/cid/ciab779. [DOI] [PMC free article] [PubMed]
- BBC News, 2021 BBC News 2021
- Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., Lopez de Castilla D., Finberg R.W., Dierberg K., Tapson V., Hsieh L., Patterson T.F., Paredes R., Sweeney D.A., Short W.R., Touloumi G., Lye D.C., Ohmagari N., Oh M., Ruiz-Palacios G.M., Benfield T., Fätkenheuer G., Kortepeter M.G., Atmar R.L., Creech C.B., Lundgren J., Babiker A.G., Pett S., Neaton J.D., Burgess T.H., Bonnett T., Green M., Makowski M., Osinusi A., Nayak S., Lane H.C. Remdesivir for the treatment of Covid-19 — final report. N. Engl. J. Med. 2020;383:1813–1826. doi: 10.1056/nejmoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharjee n.d.Bhattacharjee, S., n.d. Have Hearing Loss After Covid-19 [WWW Document]. URL https://www.ndtv.com/opinion/opinion-millions-of-people-have-hearing-loss-after-covid-19-2453615 (accessed 6.7.21).
- Boas V.V., Gijbels E., Cooreman A., Van Campenhout R., Gustafson E., Leroy K., Vinken M. Industrial, biocide, and cosmetic chemical inducers of cholestasis. Chem. Res. Toxicol. 2019;32:1327–1334. doi: 10.1021/acs.chemrestox.9b00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckinghamshire Healthcare NHS, n.d.Buckinghamshire Healthcare NHS, n.d. Antibiotic therapy for pneumonia where covid-19 infection is suspected 1–5.
- CDC [WWW Document], n.d.CDC [WWW Document], n.d. URL https://www.cdc.gov/coronavirus/2019-ncov/community/disinfecting-building-facility.html (accessed 9.10.21).
- CDC [WWW Document], n.d.CDC [WWW Document], n.d. URL https://www.cdc.gov/drugresistance/covid19.html (accessed 9.15.21a).
- CDC [WWW Document], n.d.CDC [WWW Document], n.d. URL https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects.html (accessed 6.7.21b).
- CEO of Lysol maker: Sales are up as a result of coronavirus pandemic [WWW Document], n.d.CEO of Lysol maker: Sales are up as a result of coronavirus pandemic [WWW Document], n.d. URL https://www.cnbc.com/2020/07/16/ceo-of-durex-condom-maker-intimate-occasions-down-during-pandemic.html (accessed 2.16.21).
- Chatterjee A. Use of hypochlorite solution as disinfectant during COVID-19 outbreak in India: from the perspective of human health and atmospheric chemistry. Aerosol Air Qual. Res. 2020;20:1516–1519. doi: 10.4209/aaqr.2020.05.0253. [DOI] [Google Scholar]
- Chen H., Jing L., Teng Y., Wang J. Characterization of antibiotics in a large-scale river system of China: occurrence pattern, spatiotemporal distribution and environmental risks. Sci. Total Environ. 2018;618:409–418. doi: 10.1016/j.scitotenv.2017.11.054. [DOI] [PubMed] [Google Scholar]
- Chen G., Feng Q., Wang J. Mini-review of microplastics in the atmosphere and their risks to humans. Sci. Total Environ. 2020;703 doi: 10.1016/j.scitotenv.2019.135504. [DOI] [PubMed] [Google Scholar]
- Chen Z., Guo J., Jiang Y., Shao Y. High concentration and high dose of disinfectants and antibiotics used during the COVID-19 pandemic threaten human health. Environ. Sci. Eur. 2021;33 doi: 10.1186/s12302-021-00456-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colson P., Rolain J.M., Raoult D. Chloroquine for the 2019 novel coronavirus SARS-CoV-2. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comber S.D.W., Upton M., Lewin S., Powell N., Hutchinson T.H. COVID-19, antibiotics and one health: a UK environmental risk assessment. J. Antimicrob. Chemother. 2020;75:3411–3412. doi: 10.1093/jac/dkaa338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- covid death worldwide [WWW Document], n.d.covid death worldwide [WWW Document], n.d. URL https://www.google.com/search?q=covid death worldwide&oq=covid death worldwide&aqs=chrome..69i57j0l9.8400j0j7&sourceid=chrome&ie=UTF-8 (accessed 4.22.21).
- Cuenca F.-F., Tomá M., Caballero-Moyano F.-J., Bou G., Martínez-Martínez L., Vila J., Nimo Pachó J., Miguel Cisneros J., Rodríguez-Bañ J., Pascual L. Reduced susceptibility to biocides in Acinetobacter baumannii: association with resistance to antimicrobials, epidemiological behaviour, biological cost and effect on the expression of genes encoding porins and efflux pumps on behalf of the Spanish Group. J. Antimicrob. Chemother. 2015;70:3222–3229. doi: 10.1093/jac/dkv262. [DOI] [PubMed] [Google Scholar]
- Diaz George A., Christensen Alyssa B., Pusch Tobias, Goulet Delaney, Chang Shu-Ching, Grunkemeier Gary L., McKelvey Paul A., Robicsek Ari, French Tom, Parsons Guilford T., Doherty Glenn, Laurenson Charles, Roper Ryan, Hadlock Jennifer, Cover Cameron J., Footer Brent, Robinson Philip, Micikas Mary, Marfori Jennifer E., Cronenweth Charlotte, Mukkamala Yogavedya, Mackiewicz Jamie, Rai Ekra, Matson Martha Dickinson, Davila Jodie, Rueda Justin, Tipton Reda, Algren Heather, Ward Brittney C., Malkoski Stephen, Gluckman Tyler, Tallman Gregory B., Arguinchona Henry, Hammond Terese C., Standaert Steven, Christensen Joshua, Echaiz Jose F., Choi Robert, McClung Daniel, Pacifico Albert, Fee Martin, Sarafian Farjad, Berrington William R., Goldman Jason D. Remdesivir and mortality in patients with COVID-19. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dindarloo K., Aghamolaei T., Ghanbarnejad A., Turki H., Hoseinvandtabar S., Pasalari H., Hamid, Ghaffari R. 2020. Pattern of disinfectants use and their adverse effects on the consumers after COVID-19 outbreak. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z., Li K., Zhou S., Liu X., Yu Y., Zhang Yunhai, Zhang Yongjun. 2020. Degradation of ofloxacin with heterogeneous photo-Fenton catalyzed by biogenic Fe-Mn oxides. [DOI] [Google Scholar]
- Ebele A.J., Abou-Elwafa Abdallah M., Harrad S. Pharmaceuticals and personal care products (PPCPs) in the freshwater aquatic environment. Emerg. Contam. 2017;3:1–16. doi: 10.1016/j.emcon.2016.12.004. [DOI] [Google Scholar]
- EPA, n.d.EPA, n.d. Disinfectants Pesticides.
- Fan X.-T., Li H., Chen Q.-L., Zhang Y.-S., Ye J., Zhu Y.-G. Fate of antibiotic resistant pseudomonas putida and broad host range plasmid in natural soil microcosms. Front. Microbiol. 2019 doi: 10.3389/fmicb.2019.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA [WWW Document], n.d.FDA [WWW Document], n.d. URL https://www.fda.gov/consumers/consumer-updates/why-you-should-not-use-ivermectin-treat-or-prevent-covid-19 (accessed 5.19.21a).
- FDA [WWW Document], n.d.FDA [WWW Document], n.d. URL https://www.fda.gov/drugs/drug-safety-and-availability/fda-cautions-against-use-hydroxychloroquine-or-chloroquine-covid-19-outside-hospital-setting-or (accessed 6.10.21b).
- Fonseca V.F., Duarte I.A., Duarte B., Freitas A., Sofia A., Pouca V., Barbosa J., Gillanders B.M., Reis-Santos P. 2021. Environmental risk assessment and bioaccumulation of pharmaceuticals in a large urbanized estuary. [DOI] [PubMed] [Google Scholar]
- Gaballah M.S., Guo J., Sun H., Aboagye D., Sobhi M., Muhmood A., Dong R. A review targeting veterinary antibiotics removal from livestock manure management systems and future outlook. Bioresour. Technol. 2021;333 doi: 10.1016/j.biortech.2021.125069. [DOI] [PubMed] [Google Scholar]
- Gajadhar Tswana, Lara Alicia, Sealy Patricia, Adesiyun Abiodun A. Microbial contamination of disinfectants and antiseptics in four major hospitals in Trinidad. Rev. Panam. Salud Publica. 2003;14(3):193–199. doi: 10.1590/s1020-49892003000800006. http://www.scielosp.org/scielo.php?script=sci_arttext&pid=S1020-49892003000800006&lng=pt&nrm=iso&tlng=en Sept. 2003. Retrieved from. [DOI] [PubMed] [Google Scholar]
- Ganzenko O., Sistat P., Trellu C., Bonniol V., Rivallin M., Cretin M. Reactive electrochemical membrane for the elimination of carbamazepine in secondary effluent from wastewater treatment plant. Chem. Eng. J. 2021;419 doi: 10.1016/j.cej.2021.129467. [DOI] [Google Scholar]
- Gautret P., Lagier J.C., Parola P., Hoang V.T., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Tissot Dupont H., Honoré S., Colson P., Chabrière E., La Scola B., Rolain J.M., Brouqui P., Raoult D. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Giacomelli A., Ridolfo A.L., Oreni L., Vimercati S., Albrecht M., Cattaneo D., Rimoldi S.G., Rizzardini G., Galli M., Antinori S. Consumption of antibiotics at an italian university hospital during the early months of the COVID-19 pandemic: were all antibiotic prescriptions appropriate? Pharmacol. Res. 2021;164 doi: 10.1016/j.phrs.2020.105403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González M.D., Calleja A.C., Hernando A.-A., Capita R. Effect of sub-lethal concentrations of biocides on the susceptibility to antibiotics of multi-drug resistant salmonella enterica strains. Food Control. 2014;40:329–334. doi: 10.1016/j.foodcont.2013.11.046. [DOI] [Google Scholar]
- Gras L.M., van der Plaats R.Q.J., van der Wielen P.W.J.J., Bauerlein P.S., de Roda Husman A.M. Riverine microplastic and microbial community compositions: a field study in the Netherlands. Water Res. 2021;192 doi: 10.1016/j.watres.2021.116852. [DOI] [PubMed] [Google Scholar]
- GrignardPure [WWW Document], n.d.GrignardPure [WWW Document], n.d. URL https://grignardpure.com/frequently-asked-questions/ (accessed 9.10.21).
- Guimarães P.O., Quirk D., Furtado R.H., Maia L.N., Saraiva J.F., Antunes M.O., Kalil Filho R., Junior V.M., Soeiro A.M., Tognon A.P., Veiga V.C., Martins P.A., Moia D.D.F., Sampaio B.S., Assis S.R.L., Soares R.V.P., Piano L.P.A., Castilho K., Momesso R.G.R.A.P., Monfardini F., Guimarães H.P., Ponce de Leon D., Dulcine M., Pinheiro M.R.T., Gunay L.M., Deuring J.J., Rizzo L.V., Koncz T., Berwanger O. Tofacitinib in patients hospitalized with Covid-19 pneumonia. N. Engl. J. Med. 2021;385:406–415. doi: 10.1056/nejmoa2101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo X., Liu S., Wang Z., Zhang X.X., Li M., Wu B. Metagenomic profiles and antibiotic resistance genes in gut microbiota of mice exposed to arsenic and iron. Chemosphere. 2014;112:1–8. doi: 10.1016/j.chemosphere.2014.03.068. [DOI] [PubMed] [Google Scholar]
- Guo H., Xue S., Nasir M., Gu J., Lv J. Impacts of cadmium addition on the alteration of microbial community and transport of antibiotic resistance genes in oxytetracycline contaminated soil. J. Environ. Sci. (China) 2021;99:51–58. doi: 10.1016/j.jes.2020.04.015. [DOI] [PubMed] [Google Scholar]
- Han Y., Yang L., Chen Xueming, Cai Y., Zhang X., Qian M., Chen Xingkui, Zhao H., Sheng M., Cao G., Shen G. 2019. Removal of veterinary antibiotics from swine wastewater using anaerobic and aerobic biodegradation. [DOI] [PubMed] [Google Scholar]
- Hassan M., Zhu G., Lu Yong-ze, AL-Falahi A.H., Lu Yuan, Huang S., Wan Z. Removal of antibiotics from wastewater and its problematic effects on microbial communities by bioelectrochemical technology: current knowledge and future perspectives. Environ. Eng. Res. 2020;26 doi: 10.4491/eer.2019.405. [DOI] [Google Scholar]
- Healthcare Settings | MRSA | CDC [WWW Document], n.d.Healthcare Settings | MRSA | CDC [WWW Document], n.d. URL https://www.cdc.gov/mrsa/healthcare/index.html (accessed 2.13.21).
- Health-care waste [WWW Document], n.d.Health-care waste [WWW Document], n.d. URL https://www.who.int/news-room/fact-sheets/detail/health-care-waste (accessed 3.11.21).
- Holdsworth S.R., Law C.J. The major facilitator superfamily transporter MdtM contributes to the intrinsic resistance of Escherichia coli to quaternary ammonium compounds. J. Antimicrob. Chemother. 2013;68(4):831–839. doi: 10.1093/jac/dks491. [DOI] [PubMed] [Google Scholar]
- HongMei J., HongYing H., YongXiang G., CaiYun X., ZhiZhou C., YuTing Q. Characteristics of degradation tetracyclines and sulfonamides during wastewater treating processes in an intensive swine farm. J. Ecol. Rural Environ. 2016;32 doi: 10.11934/j.issn.1673-4831.2016.06.017. [DOI] [Google Scholar]
- Horby P., Lim S.W., Emberson J.R. Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med. 2021;384:693–704. doi: 10.1056/nejmoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini F.S., Malektojari A., Ghazizadeh S., Hassaniazad M., Davoodian P., Dadvand H., Nikpoor A.R., Nikoofal-Sahlabadi S., Kahoori S., Sepandi M., Hassanipour S., Fathalipour M. The efficacy and safety of ivermectin in patients with mild and moderate COVID-19: a structured summary of a study protocol for a randomized controlled trial. Trials. 2021;22:20–22. doi: 10.1186/s13063-020-04988-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iakovides I.C., Michael-Kordatou I., Moreira N.F.F., Ribeiro A.R., Fernandes T., Pereira M.F.R., Nunes O.C., Manaia C.M., Silva A.M.T., Fatta-Kassinos D. Continuous ozonation of urban wastewater: removal of antibiotics, antibiotic-resistant Escherichia coli and antibiotic resistance genes and phytotoxicity. Water Res. 2019;159:333–347. doi: 10.1016/j.watres.2019.05.025. [DOI] [PubMed] [Google Scholar]
- Im J.K., Kim S.H., Noh H.R., Yu S.J. Temporal-spatial variation and environmental risk assessment of pharmaceuticals in tributaries of the Han River watershed, South Korea. 2020;741 doi: 10.1016/j.scitotenv.2020.140486. [DOI] [PubMed] [Google Scholar]
- IQVIA [WWW Document], n.d.IQVIA [WWW Document], n.d. URL https://www.iqvia.com/insights/the-iqvia-institute/reports/global-medicine-spending-and-usage-trends-outlook-to-2025 (accessed 6.10.21).
- Joshua D.I., Praveenkumarreddy Y., Prabhakaranunni Prabhasankar V., Petula A.D., Yamashita N., Balakrishna K. First report of pharmaceuticals and personal care products in two tropical rivers of southwestern India. Environ. Monit. Assess. 2020 doi: 10.1007/s10661-020-08480-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutkina J., Marathe N.P., Flach C.F., Larsson D.G.J. Antibiotics and common antibacterial biocides stimulate horizontal transfer of resistance at low concentrations. Sci. Total Environ. 2018;616–617:172–178. doi: 10.1016/j.scitotenv.2017.10.312. [DOI] [PubMed] [Google Scholar]
- Kalil, A.C., n.d.Kalil, A.C., n.d. Baricitinib_ the first immunomodulatory treatment to reduce COVID-19 mortality in a placebo-controlled trial _ Enhanced Reader.pdf. lancet Respir. Med. [DOI] [PMC free article] [PubMed]
- Kalil A.C., Patterson T.F., Mehta A.K., Tomashek K.M., Wolfe C.R., Ghazaryan V., Marconi V.C., Ruiz-Palacios G.M., Hsieh L., Kline S., Tapson V., Iovine N.M., Jain M.K., Sweeney D.A., El Sahly H.M., Branche A.R., Regalado Pineda J., Lye D.C., Sandkovsky U., Luetkemeyer A.F., Cohen S.H., Finberg R.W., Jackson P.E.H., Taiwo B., Paules C.I., Arguinchona H., Erdmann N., Ahuja N., Frank M., Oh M., Kim E.-S., Tan S.Y., Mularski R.A., Nielsen H., Ponce P.O., Taylor B.S., Larson L., Rouphael N.G., Saklawi Y., Cantos V.D., Ko E.R., Engemann J.J., Amin A.N., Watanabe M., Billings J., Elie M.-C., Davey R.T., Burgess T.H., Ferreira J., Green M., Makowski M., Cardoso A., de Bono S., Bonnett T., Proschan M., Deye G.A., Dempsey W., Nayak S.U., Dodd L.E., Beigel J.H. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N. Engl. J. Med. 2021;384:795–807. doi: 10.1056/nejmoa2031994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampf G. Biocidal agents used for disinfection can enhance antibiotic resistance in gram-negative species. Antibiotics. 2018;7 doi: 10.3390/antibiotics7040110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampf G., Kramer A., Suchomel M. Lack of sustained efficacy for alcohol-based surgical hand rubs containing ‘residual active ingredients’ according to EN 12791. J. Hosp. Infect. 2017 doi: 10.1016/j.jhin.2016.11.001. [DOI] [PubMed] [Google Scholar]
- Karatzas K.A.G., Webber M.A., Jorgensen F., Woodward M.J., Piddock L.J.V., Humphrey T.J. Prolonged treatment of salmonella enterica serovar typhimurium with commercial disinfectants selects for multiple antibiotic resistance, increased efflux and reduced invasiveness. J. Antimicrob. Chemother. 2007;60:947–955. doi: 10.1093/jac/dkm314. [DOI] [PubMed] [Google Scholar]
- Knapp C.W., McCluskey S.M., Singh B.K., Campbell C.D., Hudson G., et al. Antibiotic resistance gene abundances correlate with metal and geochemical conditions in archived Scottish soils. PLoS ONE. 2011;6(11) doi: 10.1371/journal.pone.0027300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Sharma P., Manna C., Jain M. Abundance, interaction, ingestion, ecological concerns, and mitigation policies of microplastic pollution in riverine ecosystem: a review. Sci. Total Environ. 2021;782 doi: 10.1016/j.scitotenv.2021.146695. [DOI] [Google Scholar]
- Lear J., Maillard J.Y., Dettmar P., et al. Chloroxylenol- and triclosan-tolerant bacteria from industrial sources. J. Ind. Microbiol. Biotechnol. 2002;29:238–242. doi: 10.1038/sj.jim.7000320. [DOI] [PubMed] [Google Scholar]
- Lehmann L., Bloem E. 2021. Antibiotic residues in substrates and output materials from biogas plants e Implications for agriculture. [DOI] [PubMed] [Google Scholar]
- Lerma L.L., Benomar N., Casado Muñoz M.del C., Gálvez A., Abriouel H. Correlation between antibiotic and biocide resistance in mesophilic and psychrotrophic Pseudomonas spp. isolated from slaughterhouse surfaces throughout meat chain production. Food Microbiol. 2015 doi: 10.1016/j.fm.2015.04.010. [DOI] [PubMed] [Google Scholar]
- Li J., Cheng W., Xu L., Jiao Y., Baig S.A., Chen H. Occurrence and removal of antibiotics and the corresponding resistance genes in wastewater treatment plants: effluents’ influence to downstream water environment. Environ. Sci. Pollut. Res. 2016;23:6826–6835. doi: 10.1007/s11356-015-5916-2. [DOI] [PubMed] [Google Scholar]
- Liu X., Huang F., He Y., Yu Y., Lv Y., Xu Y., Zhang Y. Oxytetracycline degradation and toxicity evolution by catalytic oxidation process over sludge derived carbon. 2019 doi: 10.1016/j.jece.2019.102889. [DOI] [Google Scholar]
- Liu C., Liu Y., Feng C., Wang P., Yu L., Liu D., Sun S., Wang F. Distribution characteristics and potential risks of heavy metals and antimicrobial resistant Escherichia coli in dairy farm wastewater in Tai’an, China. 2021;262 doi: 10.1016/j.chemosphere.2020.127768. [DOI] [PubMed] [Google Scholar]
- Liu Y., Liu W., Yang X., Wang J., Lin H., Yang Y. Microplastics are a hotspot for antibiotic resistance genes: Progress and perspective. Sci. Total Environ. 2021;773 doi: 10.1016/j.scitotenv.2021.145643. [DOI] [PubMed] [Google Scholar]
- Löffler D., Römbke J., Meller M., Ternes T.A. Environmental fate of pharmaceuticals in water/sediment systems. Environ. Sci. Technol. 2005;39:5209–5218. doi: 10.1021/es0484146. [DOI] [PubMed] [Google Scholar]
- Lucien M.A.B., Canarie M.F., Kilgore P.E., Jean-Denis G., Fénélon N., Pierre M., Cerpa M., Joseph G.A., Maki G., Zervos M.J., Dely P., Boncy J., Sati H., del Rio A., Ramon-Pardo P. Antibiotics and antimicrobial resistance in the COVID-19 era. Int. J. Infect. Dis. 2021;104:250–254. doi: 10.1016/j.ijid.2020.12.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mano H., Okamoto S. Preliminary ecological risk assessment of 10 PPCPs and their contributions to the toxicity of concentrated surface water on an algal species in the Middle Basin of Tama River. J. Water Environ. Technol. 2016;14:423–436. doi: 10.2965/jwet.15-045. [DOI] [Google Scholar]
- Meng Y., Liu W., Fiedler H., Zhang J., Wei X., Liu X., Peng M., Zhang T. Fate and risk assessment of emerging contaminants in reclaimed water production processes. 2021 doi: 10.1007/s11783-021-1392-8. [DOI] [Google Scholar]
- Moore R.E., Millar B.C., Moore J.E. Antimicrobial resistance (AMR) and marine plastics: can food packaging litter act as a dispersal mechanism for AMR in oceanic environments? Mar. Pollut. Bull. 2020 doi: 10.1016/j.marpolbul.2019.110702. [DOI] [PubMed] [Google Scholar]
- Morita Y., Murata T., Mima T., Shiota S., Kuroda T., Mizushima T., Gotoh N., Nishino T., Tsuchiya T. Induction of mexCD-oprJ operon for a multidrug efflux pump by disinfectants in wild-type Pseudomonas aeruginosa PAO1. J. Antimicrob. Chemother. 2003;51:991–994. doi: 10.1093/jac/dkg173. [DOI] [PubMed] [Google Scholar]
- Murata A., Takada H., Mutoh K., Hosoda H., Harada A., Nakada N. Nationwide monitoring of selected antibiotics: distribution and sources of sulfonamides, trimethoprim, and macrolides in japanese rivers. Sci. Total Environ. 2011;409:5305–5312. doi: 10.1016/j.scitotenv.2011.09.014. [DOI] [PubMed] [Google Scholar]
- Nagai K., Murata T., Ohta S., Zenda H., Ohnishi M., Hayashi T. Two different mechanisms are involved in the extremely high-level benzalkonium chloride resistance of a Pseudomonas fluorescens strain. Microbiol. Immunol. 2003;47(10):709–715. doi: 10.1111/j.1348-0421.2003.tb03440.x. [DOI] [PubMed] [Google Scholar]
- Narayanan Shivakumar, Chua Joel V., Baddley John W. COVID-19 associated Mucormycosis (CAM): risk factors and mechanisms of disease. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neill J.O. The Review on Antimicrobial Resistance Chaired. 2014. Antimicrobial resistance: tackling a crisis for the health and wealth of nations. [Google Scholar]
- Nestler M., Godbout E., Lee K., Kim J., Noda A.J., Taylor P., Pryor R., Markley J.D., Doll M., Bearman G., Stevens M.P. Impact of COVID-19 on pneumonia-focused antibiotic use at an academic medical center. Infect. Control Hosp. Epidemiol. 2020;1–3 doi: 10.1017/ice.2020.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neto A.G., Lo K.B., Wattoo A., Salacup G., Pelayo J., DeJoy R., Bhargav R., Gul F., Peterson E., Albano J., Patarroyo-Aponte G., Rangaswami J., Azmaiparashvili Z. Bacterial infections and patterns of antibiotic use in patients with COVID-19. J. Med. Virol. 2020;2019:1489–1495. doi: 10.1002/jmv.26441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo T.H., Van D.A., Tran H.Le, Nakada N., Tanaka H., Huynh T.H. Occurrence of pharmaceutical and personal care products in Cau River, Vietnam. 2021;28:12082–12091. doi: 10.1007/s11356-020-09195-0. [DOI] [PubMed] [Google Scholar]
- Pan H., Peto R., Henao A.M. Repurposed antiviral drugs for Covid-19 — interim WHO solidarity trial results. N. Engl. J. Med. 2021;384:497–511. doi: 10.1056/nejmoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paules C.I., Gallagher S.K., Rapaka R.R. Remdesivir for the prevention of invasive mechanical ventilation or death in COVID-19 - a posthoc analysis of the adaptive COVID-19 treatment Trial-1 cohort data. Clin. Infect. Dis. 2021:1–35. doi: 10.1093/cid/ciab695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck B., Workeneh B., Kadikoy H., Patel S.J., Abdellatif A. Spectrum of sodium hypochlorite toxicity in man - also a concern for nephrologists. NDT Plus. 2011;4:231–235. doi: 10.1093/ndtplus/sfr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y., Ye X., Li Y., Bu T., Chen X., Bi J., Zhou J., Yao Z. Teicoplanin as an effective alternative to vancomycin for treatment of MRSA infection in Chinese population: a meta-analysis of randomized controlled trials. PLoS One. 2013;8:1–8. doi: 10.1371/journal.pone.0079782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennente K., Lyle J., Gardocki B. IMS Institute for Healthcare Informatics; 2015. [Google Scholar]
- Perini J.A.d.L., Silva B.C.e., Tonetti A.L., Nogueira R.F.P. Photo-Fenton degradation of the pharmaceuticals ciprofloxacin and fluoxetine after anaerobic pre-treatment of hospital effluent. Environ. Sci. Pollut. Res. 2017;24:6233–6240. doi: 10.1007/s11356-016-7416-4. [DOI] [PubMed] [Google Scholar]
- Personal Protective Equipment Market [WWW Document], n.d.Personal Protective Equipment Market [WWW Document], n.d. URL https://www.marketsandmarkets.com/Market-Reports/personal-protective-equipment-market-132681971.html (accessed 3.11.21).
- Prata J.C., Silva A.L.P., Walker T.R., Duarte A.C., Rocha-Santos T. COVID-19 pandemic repercussions on the use and management of plastics. Environ. Sci. Technol. 2020;54:7760–7765. doi: 10.1021/acs.est.0c02178. [DOI] [PubMed] [Google Scholar]
- Pu Q., Fan X.T., Li H., An X.L., Lassen S.B., Su J.Q. Cadmium enhances conjugative plasmid transfer to a fresh water microbial community. Environ. Pollut. 2021;268 doi: 10.1016/j.envpol.2020.115903. [DOI] [PubMed] [Google Scholar]
- Ram B., Kumar M. Correlation appraisal of antibiotic resistance with fecal, metal and microplastic contamination in a tropical Indian River, lakes and sewage. npj clean. Water. 2020;3:1–12. doi: 10.1038/s41545-020-0050-1. [DOI] [Google Scholar]
- Rapeport G., Ch B., Smith E., Ph D., Gilbert A., Ch B., Catchpole A., Phil D., Mcshane H., Sci F.M., Chiu C., Ch B., Ph D. The New England journal of medicine. N. Engl. J. Med. 2021:961–964. doi: 10.1056/NEJMp2106970. [DOI] [PubMed] [Google Scholar]
- Rawson T.M., Moore L.S.P., Zhu N., Ranganathan N., Skolimowska K., Gilchrist M., Satta G., Cooke G., Holmes A. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin. Infect. Dis. 2020;71:2459–2468. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman Y.M., Burela P.A., Pasupuleti V. Remdesivir for the treatment of COVID-19: a systematic review and meta-analysis of randomized controlled trials. Clin. Infect. Dis. 2021 doi: 10.1016/j.cct.2021.106272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J.N., Yost C.K. Alternative, environmentally conscious approaches for removing antibiotics from wastewater treatment systems. Chemosphere. 2021;263 doi: 10.1016/j.chemosphere.2020.128177. [DOI] [PubMed] [Google Scholar]
- Scientists around the world are already fighting the next pandemic [WWW Document], n.d.Scientists around the world are already fighting the next pandemic [WWW Document], n.d. URL https://theconversation.com/scientists-around-the-world-are-already-fighting-the-next-pandemic-115246 (accessed 2.6.21).
- Shi J., Wu D., Su Y., Xie B. 2020. Selective enrichment of antibiotic resistance genes and pathogens on polystyrene microplastics in landfill leachate. [DOI] [PubMed] [Google Scholar]
- Slovakia [WWW Document], n.d.Slovakia [WWW Document], n.d. URL https://trialsitenews.com/slovakia-becomes-the-first-eu-nation-to-formally-approve-ivermectin-for-both-prophylaxis-and-treatment-for-covid-19-patients/ (accessed 2.15.21).
- Sonbol F.I., El-Banna T.E., Abd El-Aziz A.A., El-Ekhnawy E. Impact of triclosan adaptation on membrane properties, efflux and antimicrobial resistance of Escherichia coli clinical isolates. J. Appl. Microbiol. 2019;126:730–739. doi: 10.1111/jam.14158. [DOI] [PubMed] [Google Scholar]
- Srinivasan A. 2021. Antibiotic Resistance (AR), Antibiotic Use (AU), and COVID-19. [Google Scholar]
- Strich Jeffrey R., Tian Xin, Samour Mohamed, King Christopher S., Shlobin Oksana, Reger Robert, Cohen Jonathan, Ahmad Kareem, Brown A. Whitney, Khangoora Vikramjit, Aryal Shambhu, Migdady Yazan, Kyte Jennifer Jo, Joo Jungnam, Hays Rebecca, Collins A. Claire, Battle Edwinia, Valdez Janet, Rivero Josef, Kim Ick-ko, Erb-Alvarez Julie, Shalhoub Ruba, Chakraborty Mala, Wong Susan, Colton Benjamin, Ramos-Benitez Marcos J., Warner Seth, Chertow Daniel S., Olivier Kenneth N., Aue Georg, Davey Richard T., Suffredini Anthony F., Childs Richard W., Nathan Steven D. Fostamatinib for the treatment of hospitalized adults with COVID-19. A randomized trial. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana J., Cutroneo P.M., Crisafulli S., Puglisi G., Caramori G., Trifirò G. Azithromycin in COVID-19 patients: pharmacological mechanism, clinical evidence and prescribing guidelines. Drug Saf. 2020;43:691–698. doi: 10.1007/s40264-020-00976-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Cao N., Duan C., Wang Q., Ding C., Wang J. Selection of antibiotic resistance genes on biodegradable and non-biodegradable microplastics. J. Hazard. Mater. 2021;409 doi: 10.1016/j.jhazmat.2020.124979. [DOI] [PubMed] [Google Scholar]
- Tattawasart U., Maillard J.Y., Furr J.R., Russell A.D. Development of resistance to chlorhexidine diacetate and cetylpyridinium chloride in Pseudomonas stutzeri and changes in antibiotic susceptibility. J. Hosp. Infect. 1999;42(3):219–229. doi: 10.1053/jhin.1999.0591. [DOI] [PubMed] [Google Scholar]
- The science of chlorine-based disinfectant [WWW Document], n.d.The science of chlorine-based disinfectant [WWW Document], n.d. URL https://www.cleanroomtechnology.com/news/article_page/The_science_of_chlorine-based_disinfectant/93824 (accessed 9.1.21).
- The Economic Times, 2021 The Economic Times 2021
- Thomas J.C., 4th, Oladeinde A., Kieran T.J., Finger J.W., Jr., Bayona-Vásquez N.J., Cartee J.C., Beasley J.C., Seaman J.C., McArthur J.V., Rhodes O.E., Jr., Glenn T.C. Co-occurrence of antibiotic, biocide, and heavy metal resistance genes in bacteria from metal and radionuclide contaminated soils at the Savannah River Site. Microb. Biotechnol. 2020;13(4):1179–1200. doi: 10.1111/1751-7915.13578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timková I., Lachká M., Kisková J., Maliničová L., Nosáľová L., Pristaš P., Sedláková-Kaduková J. High frequency of antibiotic tolerance in deep subsurface heterotrophic cultivable bacteria from the Rozália gold mine, Slovakia. 2020;27:44036–44044. doi: 10.1007/s11356-020-10347-5. [DOI] [PubMed] [Google Scholar]
- Tran N.H., Hoang L., Nghiem L.D., Nguyen N.M.H., Ngo H.H., Guo W., Trinh Q.T., Mai N.H., Chen H., Nguyen D.D., Ta T.T., Gin K.Y.H. Occurrence and risk assessment of multiple classes of antibiotics in urban canals and lakes in Hanoi, Vietnam. 2019;692:157–174. doi: 10.1016/j.scitotenv.2019.07.092. [DOI] [PubMed] [Google Scholar]
- Us-Epa . 3rd edition. United States Environ. Prot. Agency; Washington, DC 1, 823-B-00–008: 2000. Guidance for assessing chemical contaminant data for use in fish advisories, volume 2: Risk assessment and fish consumption limits. [Google Scholar]
- Vidal C.G., Sanjuan G., Moreno-García E., Puerta-Alcalde P., Garcia-Pouton N., Chumbita M., Fernandez-Pittol M., Pitart C., Inciarte A., Bodro M., Morata L., Ambrosioni J., Grafia I., Meira F., Macaya I., Cardozo C., Casals C., Tellez A., Castro P., Marco F., García F., Mensa J., Martínez J.A., Soriano A., Rico V., Hernández-Meneses M., Agüero D., Torres B., González A., de la Mora L., Rojas J., Linares L., Fidalgo B., Rodriguez N., Nicolas D., Albiach L., Muñoz J., Almuedo A., Camprubí D., Angeles Marcos M., Cilloniz C., Fernández S., Nicolas J.M., Torres A. Incidence of co-infections and superinfections in hospitalized patients with COVID-19: a retrospective cohort study. Clin. Microbiol. Infect. 2021;27:83–88. doi: 10.1016/j.cmi.2020.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Yuan X., Wu Y., Zeng G., Dong H., Chen X., Leng L., Wu Z., Peng L. In situ synthesis of in 2 S 3 @MIL-125(Ti) core-shell microparticle for the removal of tetracycline from wastewater by integrated adsorption and visible-light-driven photocatalysis. Appl. Catal. B Environ. 2016;186:19–29. doi: 10.1016/j.apcatb.2015.12.041. [DOI] [Google Scholar]
- Wang J., Zhuan R., Chu L. The occurrence, distribution and degradation of antibiotics by ionizing radiation: an overview. Sci. Total Environ. 2019;646:1385–1397. doi: 10.1016/j.scitotenv.2018.07.415. [DOI] [PubMed] [Google Scholar]
- Wang J., Chu L., Wojnárovits L., Takács E. Occurrence and fate of antibiotics, antibiotic resistant genes (ARGs) and antibiotic resistant bacteria (ARB) in municipal wastewater treatment plant: an overview. Sci. Total Environ. 2020;744 doi: 10.1016/j.scitotenv.2020.140997. [DOI] [PubMed] [Google Scholar]
- Wang Q., Xu Y., Liu L., Li L.Y., Lin H., Wu X.Y., Bi W.J., Wang L.T., Mao D.Q., Luo Y. The prevalence of ampicillin-resistant opportunistic pathogenic bacteria undergoing selective stress of heavy metal pollutants in the Xiangjiang River, China. 2021;268 doi: 10.1016/j.chemosphere.2020.127768. [DOI] [PubMed] [Google Scholar]
- Wang Q., Xu Y., Liu L., Li L.Y., Lin H., Wu X.Y., Bi W.J., Wang L.T., Mao D.Q., Luo Y. The prevalence of ampicillin-resistant opportunistic pathogenic bacteria undergoing selective stress of heavy metal pollutants in the Xiangjiang River, China. 2021;268 doi: 10.1016/j.envpol.2020.115362. [DOI] [PubMed] [Google Scholar]
- World Economic Forum [WWW Document], n.d.World Economic Forum [WWW Document], n.d. URL https://www.weforum.org/agenda/2020/07/plastic-waste-management-covid19-ppe/ (accessed 3.15.21).
- Zhang H., Tang W., Chen Y., Yin W. Disinfection threatens aquatic ecosystems. Science (80-. ) 2020 doi: 10.1126/science.abb8905. [DOI] [PubMed] [Google Scholar]
- Zhao Y., Gao J., Wang Z., Dai H., Wang Y. Responses of bacterial communities and resistance genes on microplastics to antibiotics and heavy metals in sewage environment. J. Hazard. Mater. 2021;402 doi: 10.1016/j.jhazmat.2020.123550. [DOI] [PubMed] [Google Scholar]
- Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou H., Cao Z., Zhang M., Ying Z., Ma L. 2020. Zero-valent iron enhanced in-situ advanced anaerobic digestion for the removal of antibiotics and antibiotic resistance genes in sewage sludge. [DOI] [PubMed] [Google Scholar]