Abstract
The information of plasma technologies applications for environmental clean-up on treating and degrading metals, metalloids, dyes, biomass, antibiotics, pesticides, volatile organic compounds (VOCs), bacteria, virus and fungi is compiled and organized in the review article. Different reactor configurations of plasma technology have been applied for reactive species generation, responsible for the pollutants removal, hydrogen and methane production and microorganism inactivation. Therefore, in this review article, the reactive species from discharge plasma are presented here to provide the insight into the environmental applications. The combinations of plasma technology with flux agent and photocatalytic are also given in this review paper associated with the setup of the plasma system on the removal process of metals, VOCs, and microorganisms. Furthermore, the potential of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) inactivation via plasma technology is also described in this review paper. Detailed information of plasma parameter configuration is given to support the influence of the critical process in the plasma system to deal with contaminants.
Keywords: Reactive species, Plasma technologies, Discharge plasma, Photocatalytic, Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)
Graphical Abstract
1. Introduction
Plasma is the fourth state of matter because of the high temperature, energetic molecules, and state transform of matter on the sequence: solid, liquid, gas, and plasma Fridman (2008) (Gomez et al., 2009). Molecules of the gas dissociate to another form as the atom of gas, and it moves freely to free charged particles, including electrons, reactive species (radicals, peroxides, super oxides, hydroxyl radical, singled oxygen, alpha oxygen) and positive ions (Hayyan et al., 2016). Moreover, the presence of free charge carriers confirm that plasmas are electrically conductive and can achieve ultra-high electrical conductivities, for example copper and gold. Also, plasmas generate better energetic species compared to ordinary chemical because of high temperature and energy densities. Furthermore, a chemical reaction from energetic species occurs even at the low temperatures that harder to be obtained by ordinary chemical reaction, giving excited ions, atoms and molecules related to the selective chemical transition (Fridman et al., 1999, Fridman and Kennedy, 2011). Thereby, plasma technology shows a good opportunity for environmental clean-up applications.
The collision of atoms in plasma technology plays a key role for pollutants degradation during the treatment due to high energy atoms Heberlain and Murphy (2008) . The collision of atoms is generated from a chemical reaction between the two electrodes that convert electrical energy to thermal energy. Moreover, the collision occurs from a gas phase environment under a glow discharge with electrons and ions, neutral atoms, molecules, reactive species, and photons (Chang, 2009, Chapman, 1980, Taylor and Pirzada, 1994). The main collision processes are ionization, dissociation, photo-ionization, excitation, relaxation or de-excitation, radiative recombination, ion-neutral collision, and electron attachment ( Table 1). Thereby, the degradation of pollutants during plasma treatment can be obtained during the plasma processes because of the collision of gas phase in the plasma technology. Fig. 1 shows the difference between collision processes in plasma technology.
Table 1.
Gas phase collision process in plasma technology.
| Gas phase collision process in plasma technology | Brief explanations | Electron reactions | References |
|---|---|---|---|
| Ionization | Sources are from energy input and discharge, and including a thermal and photon ionization. Electron removes electrons from atom. Thus, it generates a positive ion and two electrons. | e + Ar → 2e- + Ar+ | Chapman (1980) |
| e + He → He ++ 2e | |||
| Photoionization or photoexcitation process | When photons are absorbed by a molecule, radiation is also absorbed to the degree that molecules are ionized and occurs. | O2 + hv → O + O + e– | Mattox (2010) |
| Penning Ionization and Penning Excitation | Excitation is the ionization (or excitation) of an atom by the transfer of the excitation energy from that other atom whose energy is greater than the ionization (or excitation) energy of the other. | A* + B → A + B +,A*+ B → A + B*Ar* (metastable)Cu → Ar + Cu ++ e– | Rossnagel et al. (1991), Mattox (2010) |
| Excitation | Electrons are excited to higher orbitals, and the resulting ions are detached from the atom. During an exciting collision, the primary electron loses kinetic energy equal to the excitation energy. | e + He → He* + e | Chapman (1980) |
| Relaxation or de-excitation | Process related to excited atoms and molecules which is unstable and electron configuration return to its original state in several transition. Each transition is accompanied by photon emission and every transition is accompanied by energy between quantum level. | CF4 + O2 | Chapman (1980) |
| Dissociation | This process breaks apart of molecule. An oxygen molecule dissociates to two oxygen atoms. | E + O2 → e + O + O, e + AB → A + B + e, e + AB → A + B + 2e | Chapman (1980) |
| Recombination | Recombination is an ionization-an electron coalesces with a positive ion to form a neutral atom. | He+ + 2e- à He + e, He ++ e- à He + hvAr+ + e → surface → Ar°. | Chapman (1980), Mattox (2010) |
| Radiative Recombination | The excess energy process of recombination is conducted by radiation, and Electron–ion recombination (neutralization) occurs when ions and electrons combine to form a neutral species. | Ar+ + e– → surface → Ar0 | Mattox (2010) |
| Ion Neutral Collision | Further ionization is associated with ions and neutral collision where A and B are categorized as the same species. | A + A+ → A+ + A, A+ + B à A + B+ | Chapman (1980) |
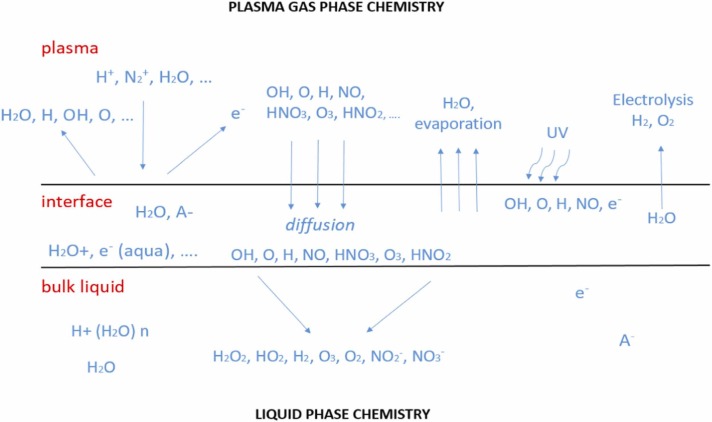
Fig. 1.
An overview of transfer process at the plasma-liquid interface
Modified from Samukawa et al. (2012).
Plasma technology shows a promising result on the environmental clean-up for dealing with contaminants, such as volatile organic compounds (Wessenback, 2016, Chung et al., 2018, Du et al., 2018, Du et al., 2019; Sanito et al., 2020a), recalcitrant organic compounds and dyes (Zhang et al., 2021, Zhou et al., 2021a, Zhou et al., 2021c, Zhou et al., 2021b), metals, metalloids (Cubas et al., 2014, Cubas et al., 2015, Sanito et al., 2020b, Sanito et al., 2020c), carbon from plastic waste (Sanito et al., 2020b) and microorganism (Santos et al., 2018), and mostly studies are based on the laboratory scale which are promising to be applied in the real application. Decomposition trichloroethylene (C2HCl3), dichloromethane (CH2Cl2), 1,1,1-Tricholorethane (C2H3Cl3), carbon tetra chloride (CCl4), chloroform (CHCl3), carbon tetraflouride (CF4), hexafluoroethane (C2F6), and Sulfur hexafluoride (SF6) showed a good progress conducted by corona discharge and dielectric barrier discharge (Du et al., 2018). An application of atmospheric-microwave plasma bubble reactor for dealing with cefixime, azophloxine, and dyes pollutants have been conducted by Zhang et al. (2021), Zhou et al. (2021a) and Zhou et al. (2021b). The standard operating condition considering discharge power (10–400 W), AC frequency (100–3000 Hz) and peak voltage (10.4–14.4 kV), in different plasma configuration, such as atmospheric – pressure plasma bubble and dielectric barrier discharge. Zhou et al. (2021a) confirmed that single pollutants, namely, methylene blue, orange II, and alizarin yellow can be degraded via the atmospheric – pressure plasma bubble. Sanito et al. (2020a) found that the treatment of epoxy resin mixed with CaCO3 via an atmospheric-pressure microwave plasma showed a good removal rate of benzene and toluene, and Chung et al. (2018) stated that active species plays an integral role in the oxidation that causes reduction of VOC concentration during plasma treatment. The addition of flux agents improves the composition of the OH radicals that responsible for degrading the nitrogen oxide (NOx) (Sanito et al., 2020b). Cubas et al. (2014) observed that the thermal plasma treatment of galvanic sludge is suitable for eliminating Cr, Fe, and Zn as the same author (Cubas et al., 2015) discovered that reduction of Mn and Zn from the moist paste batteries can be obtained. Furthermore, the addition of quartz sand is considered as the one of parameters that improves the vitrification of material. In the inactivation of microorganism, bacteria such as Escherichia coli, Pseudomonas flourescens (Santos et al., 2018; Mai-Prochnow et al., 2020), and Pseudomonas aeruginosa (Scally et al., 2018) can be reduced by cold plasma corona discharge, plasma bubble reactor, and dielectric barrier discharge, respectively. Thus, plasma technology is a promising method in many environmental clean-up applications.
Recently, plasma technology has been applied to remove pollutants from hazardous waste (Cubas et al., 2015; Sanito et al., 2020a), wastewater (Zhang et al., 2021, Zhou et al., 2021a, Zhou et al., 2021b), agriculture products, and foods and even generate syngas from the biomass (Muvhiiwa et al., 2018). The reactive species in plasma system plays an integral role in the degradation of pollutants (Locke and Shih, 2011). However, the mechanism of pollutant degradation from plasma discharged is directly associated with electricity power, gas flow rates, chemical composition of carrier gases, and electron densities. Also, the main challenge is associated with the lack of knowledge on the quantities of radicals by plasma and its mechanism for further recombination and interaction (Adamovich et al., 2017). In addition, the fuse of flux agents or catalyst also improves the role reactive species during the degradation of pollutants from the solid waste or wastewater (Wessenback, 2016, Zhang et al., 2021; Sanito et al., 2020a). Consequently, the role of reactive species must be fully understood to obtain a good performance during the treatment of contaminants based on each plasma parameters reactors via the plasma systems, for dealing with solid wastes, wastewater, biomass conversion, and inactivation of microorganisms.
This review aims to explore the mechanism of reactive species for degrading pollutants and microorganisms from solid waste, wastewater, agriculture products, aerosols, and conversion of biomass to useful gases product during the treatment in plasma technology from recent publications and literature reviews. Also, the information on plasma technology to convert biomass to syngas is also presented herein. Furthermore, the mechanism on how reactive species destroy VOCs, metals, metalloids, bacteria and virus during various plasma systems. Furthermore, the most important parameters of the plasma system and their effect on the removal of pollutants are also discussed. A detailed review of the literature and the significance of insight reactive species mechanism in plasma technology is explored, and a future outlook is given in this review paper. Information was collected from several journals via science direct and web of science.
2. Reactive species in plasma discharges
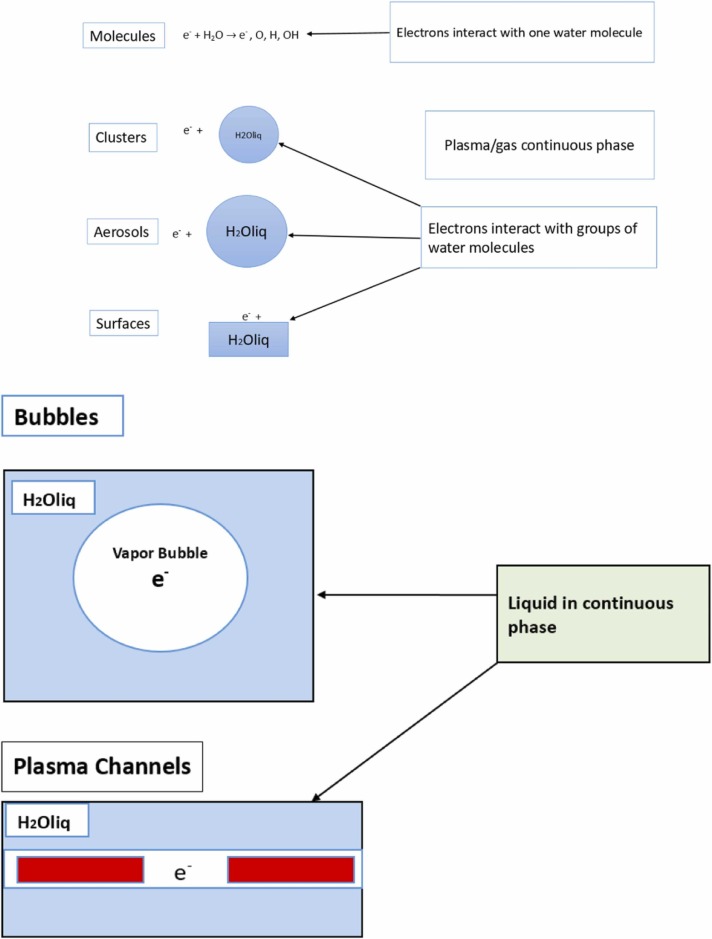
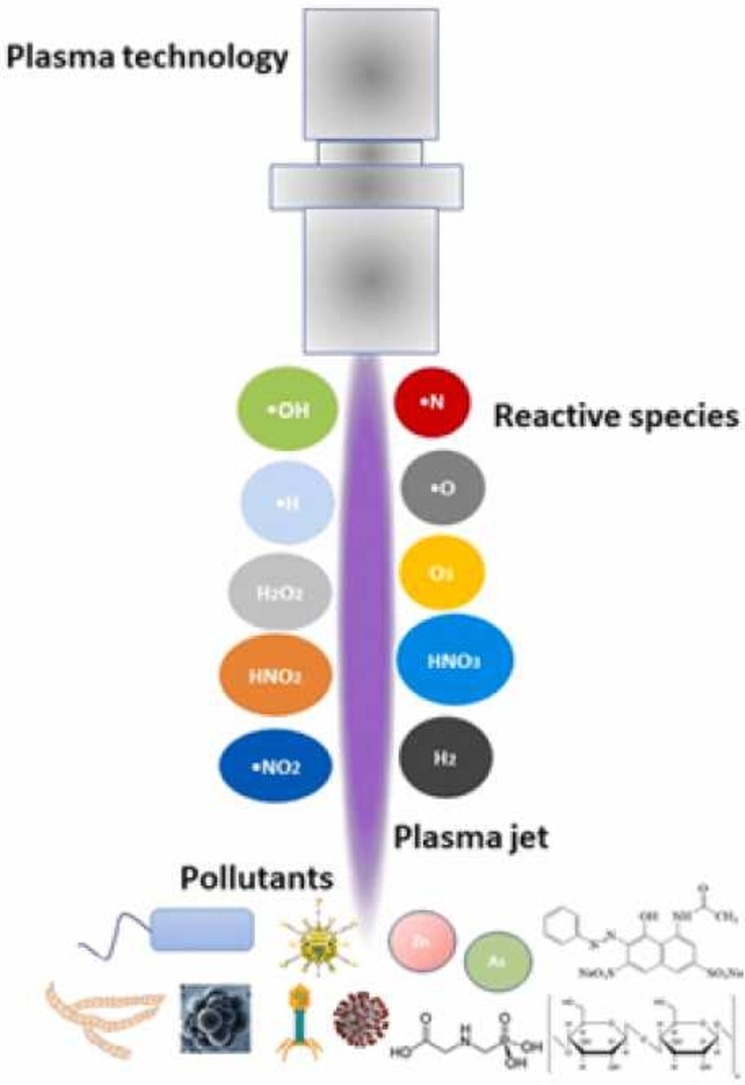
Reactive species plays a major role in the degradation of pollutants because they create some reactions, namely, acid-base reactions (H3O+, HNO2, HNO3), oxidation reactions (•H, H2O2, O3, ONOOH), reduction reactions (•H, •HO2), and photochemical reactions (UV reaction from plasma), and it can be generated from plasma discharged (Lukes et al., 2014, Iervolino et al., 2020, Zhou et al., 2021a, Zhou et al., 2021b, Zhou et al., 2021c, Zeghioud et al., 2020). Nayak et al. (2017) pointed out that the essential species, namely, O3, O2, NO, N2O, NO2, NO3, and N2O5, are usually found in the discharges zones. An overview of the transfer process is shown in Fig. 2. In more precise, based on the electrical discharges in liquid and in gas-liquid environment, reactive species are classified into three types, namely, primary species, secondary species and tertiary species (Lukes et al., 2012). Reactive species may be formed directly in liquid or bubbles in the liquid and the gas with contact with droplets, aerosols, clusters, and water surfaces. In contact with a solid surface, addition of catalyst or flux agent improves the elimination of pollutants during the process of high energy collision of the atom, such as reactive species in water is recognized as plasma activated water (PAW). Furthermore, the reactive oxygen species (ROS) and reactive nitrogen species (RNS) will be produced from water environment under plasma reaction (Hefny et al., 2016, Nayak et al., 2017). According to Snoecx et al. (2019), the trapping of oxygen (O) species and hydrogen (H) species plays an important role in suppressing the nitrogen oxide (NOx) and nitrous oxide (N2O) formation. From the study of Li et al. (2019) pointed out that reactive species also might be generated and then combined with photocatalytic reactions through some reactions ((1), (2), (3), (4)). Thus, reactive species from plasma discharged play an important role to degrade pollutants in plasma technology application.
| Photocatalyst + Plasma (UV irradiation) → e– + h+ | (1) |
| h+ + H2O → •OH + H+ | (2) |
| e– + H2O → •H + •OH + e– | (3) |
| •OH + •OH → H2O | (4) |
Fig. 2.
The schematic of plasma-water interactions. (a) Continuous gas phase, (b) continuous liquid phase (modified from Locke and Shih, 2011).
According to Locke and Shih (2011), reactions of primary and secondary species during the treatment in water can be generated from water (H2O), oxygen (O2), and nitrogen (N2). Sanito et al. (2020a) confirmed that the addition of shell powder improved elimination of VOC due to •OH radical composition contained in the shell powder. In addition, Lukes et al. (2014) found that plasma gas discharged at the gasliquid interface consists of reactive species such as hydroxyl radicals (•OH), nitrogen dioxide (•NO2), nitric oxide radicals (NO radicals) and long-lived chemical products, such as ozone (O3), hydrogen peroxide (H2O2), nitrate (NO3 –), nitric oxide (NO–) and directly depend on the gas atmosphere. Notably, the existence of reactive species is strongly generated by jet discharged. Table 2 shows the classification of reactive species from jet discharges in plasma technology.
Table 2.
The list of reactive species in plasma discharged (Lukes et al., 2012).
| Reactive species | Categories | Information |
|---|---|---|
|
Plasma | Primary species or parent species |
| •H | ||
| •O | ||
| •N | ||
| ||
| H2O2 | Plasma, gas/liquid interface, liquid | Secondary species |
| H2 | ||
| O3 | ||
| HNO2 | ||
| HNO3 | ||
| O=NOOH | ||
| O=NOOH | liquid (post-discharged) | Tertiary species |
| ||
|
3. Plasma system configuration and critical parameters
The operation of plasma to treat waste (solid waste and wastewater), biomass conversion and inactivation of microorganisms should consider several factors related for facilitating plasma discharged, such as electric power, carrier gases, gas flow rate and reaction time. The electric power, type of gases, addition of flux agents and gas flow rates under jet discharge play a dominant role in treating contaminants (Gomez et al., 2009; Cubas et al., 2014; Cubas et al., 2015; Sanito et al., 2020a, Sanito et al., 2020b). In an atmospheric-pressure microwave plasma reactor, higher microwave power with proper control of gas flow rate gives the high energy excitation that is responsible for generating reactive species (Leins et al., 2015). For creating vitrification, degrading pollutants and generating syngas via thermal plasma or atmospheric-pressure microwave plasma reactor, the addition of flux agents is suggested (Lin et al., 2014, Cubas et al., 2015, Cubas et al., 2016; Sanito et al., 2020a, Sanito et al., 2020b). Thereby, the different plasma system has different critical parameters for different application.
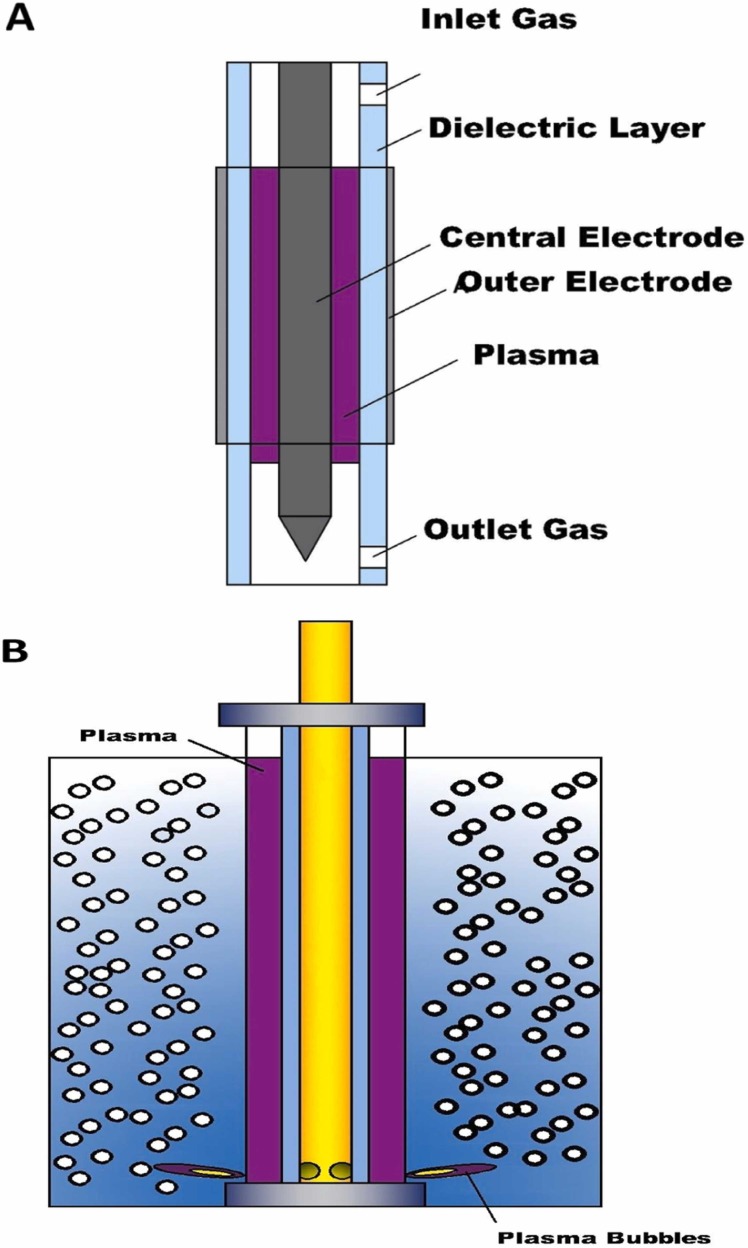
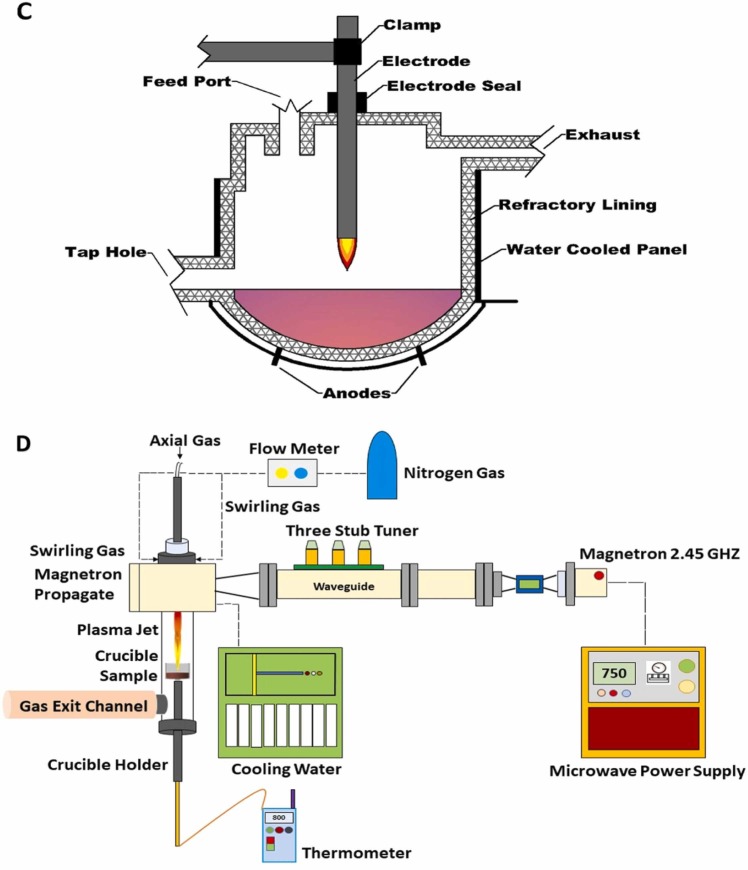
Fig. 3 shows the different configurations of plasma technology for dealing with solid waste and liquid waste (a), Dielectric barrier discharges, (b) Micro-plasma bubble reactor, (c). Thermal plasma, (d) Atmospheric-pressure microwave plasma reactor. However, different plasma systems have different support systems for eliminating different contaminants from different sources. For example, atmospheric-pressure microwave plasma configuration requires cooling water system and dielectric barrier discharge is vice versa. The system of thermal plasma is simpler compared to the atmospheric-pressure microwave plasma reactor. Thus, each plasma technology has different system. Table 3 informs a comparison of plasma systems, parameters set-ups, and supporting systems.
Fig. 3.
The schematic of plasma systems (a). Continuousgas phase dielectric barrier discharges (modified from Belov et al., 2016), (b). Micro-plasma bubblereactor (modified from Zhou et al., 2021a),(c). Thermal plasma (modified from Tetronics, 2015). (d). Atmospheric-pressure microwave plasmareactor (modified from Sanito et al., 2021a).
Table 3.
Different plasma systems on treat solid waste, wastewater, agriculture and inactivation of bacteria and virus.
| No | Plasma systems | Setting of parameters | Supporting systems | Targets of treatment | References |
|---|---|---|---|---|---|
| 1 | Atmospheric-pressure microwave plasma reactor | Microwave power supply, carrier gases, gas flow, pyrolysis duration, crucible position | Gas Exhaust (chimney), cooling water system, crucible, crucible holder, quartz tube, magnetron, waveguide, gas tube, nebulizer. | Epoxy resin | Sanito et al., (2020a); Sanito et al. (2020c);Sanito et al. (2021a). |
| 2 | Thermal Plasma | Carrier gas, pyrolysis time, gas flow rate, position of torch | Anode, tungsten inert gas, cathode (graphite), plasma torch. | Galvanic sludge, moist paste batteries | Cubas et al., 2015, Cubas et al., 2016 |
| 3 | Atmospheric-Pressure plasma bubble | AC Frequency, discharge peak voltage, gas flow rate, plasma treatment time | Plasma power supply (Plasma leap technology), bubble column reactor, inner quartz tube, high-voltage stainless-steel electrode rod, outer dielectric tube, digital oscilloscope, air compressor, dielectric tubes. | Antibiotics and dyes from aqueous solution | Zhang et al. (2021), Zhou et al. (2021a) |
| 4 | Rotating gliding arc discharge reactor | Gas flow rate and applied voltage. | Air blower, flow meter, drying column, pumps, buffer tank, heat chamber, autotransformer, high-voltage transformer, buffer tank | Toxic gas (Toluene) | Lu et al. (2012) |
| 5 | Gliding arc discharge | Power frequency, carrier gas flow rate, electrode gap, length of electrode, voltage | nozzle, flange, stainless steel pipe, ceramic pipe, knife shape electrode, thermal couple, power supply, grounding, gliding arc reactor, flow meter, control panel, air compressor. | PCDD/Fs | Yan et al. (2007) |
| 6 | DC arc discharge | Arc power and feed rate | Evaporator, anode, nozzle case, cathode rod, insulator, water tank, evaporator, negative and positive power supply. | Hydrofluoroethylene (HFC) | Watanabe and Tsuru (2008) |
| 7 | Dielectric barrier discharge (DBD) | Current, voltage, frequency, treatment times, carrier gas, feed rate, waveform, discharge gap | Oscilloscope, electrodes (outer and inner), transistor, transformer, mass flow controller (MFC), COx analyzer, peristaltic pump and magnetic stirrer. | Antimicrobial activity, dyes, antibiotics | Reddy et al. (2013),Laurita et al. (2015),Butscher et al. (2016),Markovic et al. (2015) |
| 8 | Pulsed Dielectric Barrier Discharged Reactor | Treatment time, sample flow rate | Quartz vessel, quartz tube, electrode, alternative voltage, reactor cell, waveforms, and oscilloscope. | Pesticides | Wardenier et al. (2019) |
| 8 | Plasmatron plasma reactor | Electricity power, gas flow rate | Fume exhaust-chimney, molten product collection, automatic waste package feeder, automation and data collection apparatus, scrubber. | Printed circuit board | Szalatkiewicz et al. (2013), Szalatkiewiczm (2013) |
| 9 | Cold Plasma type corona discharged gas-liquid type | Carrier gas, treatment time | Cylindrical tube body, teflon, electrodes, and colorimeter. | Inactivation of bacteria | Santos et al. (2018) |
| 10 | Atmospheric pressure plasma jet | Gas mixture, gas flow rate | Glass, electrodes, ceramics, jet nozzle, and vortexes. | Phenolic in an aqueous solution | Hefny et al. (2016) |
| 11 | Non-thermal plasma | Carrier gas (argon), air mixture, AC power supply, current, NTP treatment time | Distinct geometries, gas inlet, gas outlet, sampling facilities, ac power supply, spherical glass reactor, tubular quartz reactor, tip-plate double concentric rods electrodes, and parallel electrodes. | Inactivation of bacteria | Cubas et al. (2021) |
| 12 | Non-thermal plasma with a packed-bad dielectric barrier discharges reactor | Applied voltage or power supply, air flow rate, and flow meter | Induced draft (ID) fan, DBD reactor, aerosol generator, a digital oscilloscope, a high voltage amplifier, a digital function generator, 20 kV high voltage amplifiers, 30 kV neon transformer, high-voltage probe, alma pulse generator, monitor capacitor, low-voltage probe, and oscilloscope. | Airborne virus, SARS-CoV-2 | Xia et al. (2019);Liao et al. (2017);Bisag et al. (2020) |
4. Degradation of contaminants in plasma technology
4.1. Degradation of metals and metalloids
Some studies have been performed that plasma technology shows a promising result in the elimination of metals and metalloids using thermal plasma (Cubas et al., 2014, Cubas et al., 2015, Cubas et al., 2016) and atmospheric-pressure microwave plasma reactor (Sanito et al., 2020a, Sanito et al., 2020c, Sanito et al., 2021a). Moist paste batteries, galvanic sludge, leachate and epoxy resin, have been treated through a plasma system for eliminating Cr, Fe, Mn, and Zn with flux agents (quartz sand and shell powder) addition.
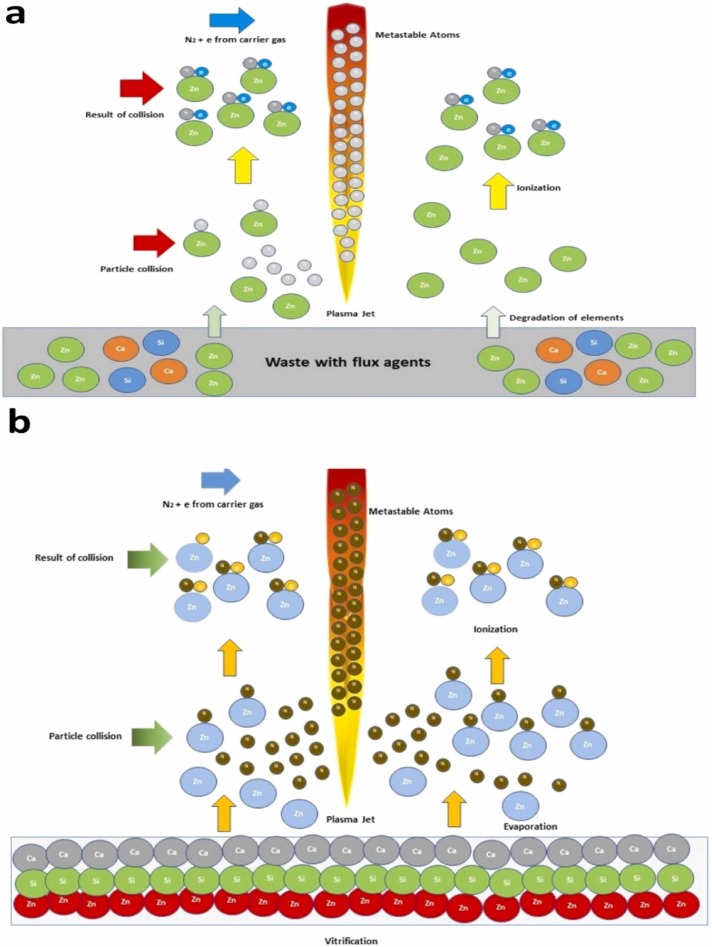
The fuse of quartz sand creates an increased reduction of Ce, Fe and Zn in the galvanic sludge and Mn and Zn in the moist paste batteries. Decreasing of metals and metalloids from galvanic sludge and moist paste batteries strongly depends on the fuse of quartz sand because it creates the silicate network from the Si structure and traps the elements. The addition of flux agent creating the fusion of hazardous waste to form a single entity (Sanito et al., 2020a). Moreover, plasma leaves through the nozzle causing recombine and gaseous state. As a result, the energy is released. In this case, the elimination of elements occurs, and quartz sand converts to a glassy formation (Cubas et al., 2014). Zn’s reduction also may be obtained at a good result with the fuse of shell powder in the resin because the presence of OH• radicals from shell powder and N metastable atoms from plasma discharges that play a significant role as the catalyst (Sanito et al., 2020a). Fig. 4a displays the mechanism of element degradation, and Fig. 4b gives the vitrification of pollutants with the addition of the flux agents.
Fig. 4.
Vitrification of waste, (a) degradation of elements, (b) vitrification of waste with addition of flux agents (modified from Sanito et al., 2020b).
According to flame atomic absorption spectroscopy (FAAS) data, Cubas et al. (2014) found that Cr, Fe and Zn eliminations were 100%, 99.6%, and 100%, respectively, in galvanic sludge fuse with the quartz sand during the treatment in the thermal plasma. The flow rate was controlled by 25 L/min – 1, and 2.4 kW of thermal plasma power, and 12 min of plasma pyrolysis. Cubas et al. (2015), on their further, research confirmed that removals of Mn and Zn from moist paste battery, respectively, can be obtained by more than 97%, while the quartz sand were added via the thermal plasma in 5 min, using argon gas with the flow rate of 5 L/min – 1, and 2.4 kW of thermal plasma power. Removal of Zn from resin can be obtained by more than 96%, while the pyrolysis duration was maintained at 5 min with the fuse of shell powder from Babylonia formosae as the flux agents. Furthermore, gas flow rate and microwave power were controlled at 6 L/min and at 900 W, respectively (Sanito et al., 2020a). Moreover, other analyses using scanning electron microscope (SEM) and X-ray fluorescence (XRF) confirmed the conversion of residue structure of the material with the increasing of Si in the final residue. It confirms that there is a transformation of material in plasma treatment.
Sanito et al. (2020a) confirmed that x-ray diffraction (XRD) analysis showed the amorphous structure of final residue. It means that Zn was not detected in the surface of the final residue and is successfully vitrified in the final residue. Thus, an amorphous element cannot be detected via XRD analysis, but it eliminates elements based on the inductively coupled plasma atomic emission spectroscopy (ICP-AES) analysis. Thereby, it can be concluded that degradation of the element occurs due to the reaction of reactive species from the plasma jet. The reaction of Cr, Fe, Mn and Zn combined with SiO2 and CaCO3 can be shown as follows:
| Zn(OH)2 (s) + SiO2 (s) → Zn2SiO4 (s) + H2O (g) | (5) |
| Zn (s) + H2O (g)⇌ ZnO (s) + H2 (g) | (6) |
| Zn (s) + CaCO3 (s) → ZnO (s) + CaO (s) + CO (g) | (7) |
Higher removal of elements is obtained because of the high energy excitation from the plasma jet. Furthermore, the collision of atoms from plasma discharged occurs and is ionized by a high energetic reaction and high temperature. Also, the metastable atom in carrier gas plays the integral role in an ionization of elements’ atoms from waste. Owing to this mechanism, harmful elements can be eliminated perfectly (Fridman, 2008, Mattox, 2010). Thereby, there is a significant decrease in elements. The reactions of elements ionization can be explained as follows:
| N (Metastable) + Cr → N + Cr+ + e– | (8) |
| N (Metastable) + Fe → N + Fe+ + e– | (9) |
| N (Metastable) + Mn → N + Mn+ + e– | (10) |
| N (Metastable) + Zn → N + Zn+ + e– | (11) |
To summarize, the removal of elements via the plasma technology can be considered in the treatment of hazardous waste, and it shows a promising result. Also, it depends on the addition of flux agents, high energies, and metastable atoms. XRD, XRF, and SEM analyses help to confirm the transformation of material due to the addition of flux agent. Vitrification of final residue also can be obtained due to the fuse SiO2 and CaCO3 that may fulfill the concept of circular economy (Sanito et al., 2020c).
4.2. Converting carbon from biomass to hydrogen
Plasma technology has been applied for converting the biomass or agriculture waste to hydrogen and methane production, and it creates an opportunity for reducing the environmental impact of petroleum-based fuel and generate a useful syngas product (Chen et al., 2008; Tsai and Chen, 2009; Wang et al., 2010; Lin et al., 2014; Huang et al., 2016; Muvhiiwa, 2018). In the plasma gasification, the oxidation reaction and conversion reaction occur during the in the plasma reactions. Oxidation reactions can be described as follows (Lemmens et al., 2007):
| C + O ↔ CO2 | (12) |
| C + ½ O2↔ CO | (13) |
| C + O2↔ CO2 | (14) |
Conversion reactions can be described as follows:
| C + H2O ↔ CO + H2 | (15) |
| CO + H2O ↔ CO2 + H2 | (16) |
| 2 CO + 4H2↔ 2CH4 + O2 | (17) |
| CO + NH3 → HCN + H2O | (18) |
The formation of gases, such as hydrogen (H2), carbon dioxide (CO2), and carbon monoxide (CO) can be formed via the devolatilization, secondary gas reaction, and char gasification. Conversion of cellulose is related to dehydration reaction to form the char where it competes with each other (Lin et al., 2014). Muvhiiwa et al. (2018) found that temperature plays an integral role in the biomass conversion with the value of 82% at 1000 °C, and may prevent the production of tar from the wood pellets. 800 – 900 °C of reaction temperature for biomass conversion was recommended by Higman and van der Burgt (2008). Additionally, the use of a water-stabilized arc has a significant effect on the composition and low flow rate plasma generated during gasification (Hrabovsky et al., 2010). As a consequence, biomass can be converted effectively to syngas.
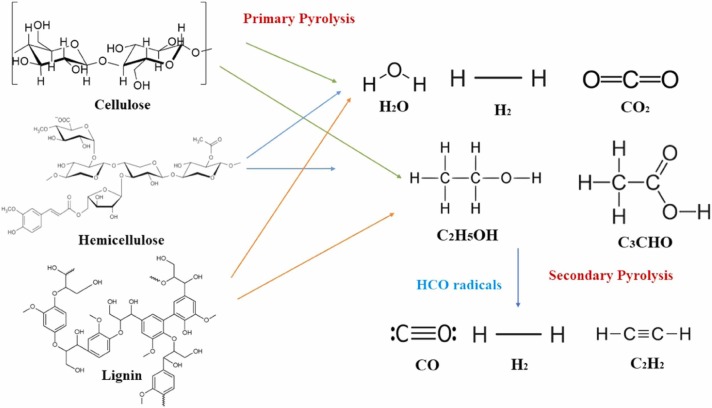
According to Lin et al., 2014, active cellulose may be decomposed due to competing reactions, generate volatile species and char. Furthermore, volatile products, namely, glycolaldehyde (C2H4O2), glyoxal (C2H2O2), acetyldehyde (CH3CHO), acetone (C3H6O), hydroxymethylfurfural (C6H6O3), carbon dioxide (CO2), carbon monoxide (CO), methane (CH4), water (H2O) and levoglucosan (C6H10O5) are generated from this process. Then, H2 gases are generated in a secondary gas-phase pyrolysis reaction through hydrogen abstraction reaction with hydrogen atoms. Formyl radicals (HCO) are formed by the active cellulose and contributed to the most reaction to the CO formation. Bio-molecular reaction plays an integral role in the degradation of formaldehyde (CH2O) assisted with HCO. The information of conversion pathway of biomass to hydrogen is shown in Fig. 5. The reaction of conversion pathway from the biomass is described as follow (Lin et al., 2014):
| Cellulose → Active Cellulose (Active Cellulose reaction) |
| Active cellulose → |
| 0·95C2H4O2 + 0·25C2H2O2 + 0·20 CH3CHO + 0·20C3H6O + 0·25C6H6O3 + 0·20 CO2 |
| + 0·15 CO + 0·1 CH4 + 0·9H2O + 0·65 Char | (19) |
| Active cellulose → C6H10O5 (Active decompose cellulose) | (20) |
| Cellulose → 5H2O + 6 char (Dehydration reaction) | (21) |
Fig. 5.
Conversion pathway of biomass to hydrogen in plasma reactor (Huang et al., 2016).
Reaction during cellulose degradation can be described as follows:
| CH2O + H → HCO + H2 | (22) |
| C2H2O2 + H → CO + HCO + H2 | (23) |
| C6H6O3 + H → C5H4O2 + CO + H + H2 | (24) |
| C5H4O2 + H → C4H3O2 + CO + H2 | (25) |
| C2H4O2 + H → CO + CH2OH + H2 | (26) |
| CH3CHO + H → CH3CO + H2 | (27) |
| C6H10O5 + H → C3H4O3 + CH2CHO + CH2O + H2 | (28) |
| C3H4O3 + H → CO + C2H2O2 + H + H2 | (29) |
H-atom reactants and the importance of HCO radicals during in the reactions.
| HCO + M → CO + H + M | (30) |
| C2H2O2 → 2HCO | (31) |
| C4H3O → CO + C3H3 | (32) |
| CH3CO + M → CH3 + CO + M | (33) |
The H-atom reactants indicate a reaction and formed a HCO radical with the reaction that is mentioned as follows:
| HCO + CH2O → CO2 + CH3 | (34) |
| HCO + CH3CHO → CO2 + C2H5 | (35) |
Total hydrogen (H2) was generated from the experiment. The settings of microwave power were 800 W, 900 W and 1000 W, resulting the total hydrogen production can be obtained by 15.56%, 21.00% and 23.73%, respectively. Higher H2 can be obtained due to higher microwave power at value of 1000 W, producing high thermal energy responsible for producing high reactive species. It can be shown on the high conversion rate with the value of 67.33%. Moreover, it generates twice as much as hydrogen gas mass compared to 800 W of microwave power. The amounts of H2, CO2, and CO volume fractions were 90.57%, 91.16%, and 91.25%, respectively.
Microwave plasma generated a high energetic electron, responsible for generating free radicals and active species that are released through the electron impact dissociation reaction, penning dissociation reaction, and molecule radical reactions. Thereby, a higher microwave power plays a significant impact in generating high hydrogen production and hydrogen conversion rate (Lin et al., 2014). An example of penning ionization can be described as follows:
| A* + B → A + B+ + e | (36) |
Basu (2010) and Sirkawar et al. (2016) stated that the generated of tar formation is a serious issue because it consists of hydrocarbon and heavy metals. Also, it causes issue related to the quality of the gas product. To solve the problems, thermal cracking, steam reforming, dry reforming, carbon formation, and partial oxidation are suggested to be conducted in the main treatment of plasma gasification.
To conclude, the conversion of biomass to methane and hydrogen can be obtained via plasma technology from the biomass. Also, it shows plasma technology as an alternative fuel source. The presence of Tar formation should be considered to maintain the quality of the gas produced in the plasma gasification.
4.3. Degradation of dye
Some scholars have performed degradation of dyes via plasma technologies and it shows a promising result (Jiang et al., 2012, Lukes et al., 2012, Reddy et al., 2013, Medel et al., 2012; Bansode et al., 2017, Chandana et al., 2015; Zhou et al., 2021a).
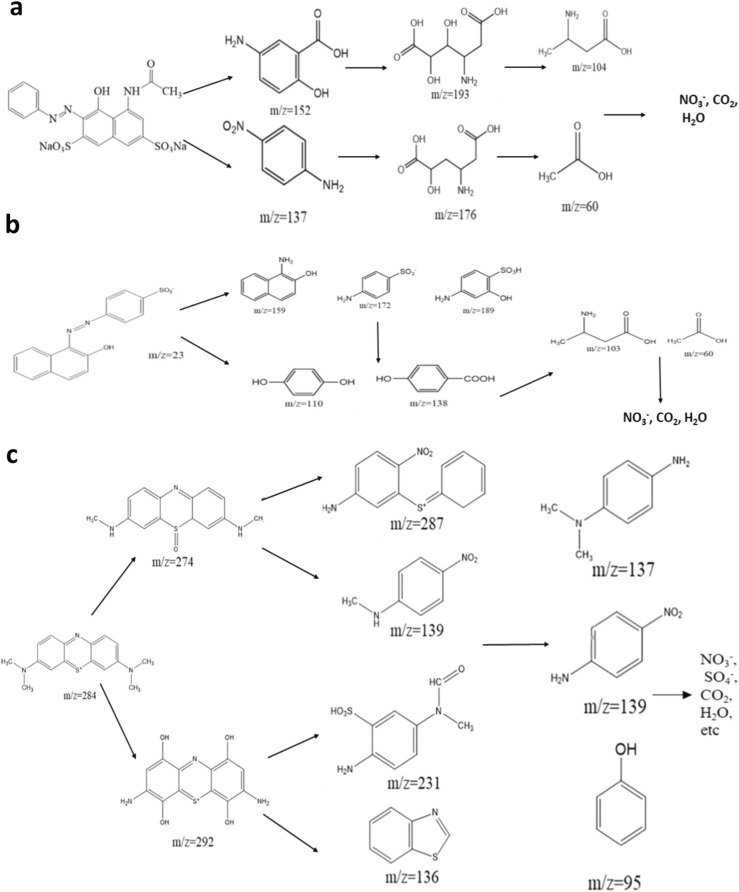
Zhou et al. (2021a) investigated the degradation of mixture organic pollutants using the micro-plasma bubble, and dye products were analyzed by High-Performance Liquid Chromatography (HPLC) coupled with a mass spectrometry analyzer. The selections of dye combinations are azo, dye mixture (AY and Orng-II), azo, and cationic dye (AY and MB), and combinations of all dyes (AY, Orng-II, and MB). A micro-plasma bubble column reactor consists of an electrical circuit measurement, optical emission spectroscopy (OES), and liquid chromatography (LC-MS system). The power supply of the system was provided from Leap 100, (PlasmaLeap Technologies, Australia). Moreover, this power supply provides the high voltage output, ranging from 0 to 80 kV with frequency 100 – 3000 Hz generate plasma discharges. Plasma bubble column reactor, the part of the system, consists of a 5 mm-thick stainless-steel rod (acting as the HV electrodes), an inner and outer diameter of 5.0 and 8.0 mm, respectively, and a 12 mm-inner-diameter outer dielectric tube. Plasma treatment was controlled from 1 to 10 min, and discharge peak voltages were controlled at 10.4 kV, 11.6 kV, 12.4 kV, 13.6 kV, 14.4 kV, respectively, and frequencies were maintained at 500 Hz, 750 Hz, 1000 Hz, 1250 Hz, and 1500 Hz, respectively. Air flow rate was controlled at 2.0 standard liters per minute (SLM) to generate the plasma discharges. The results showed that degradation of azo dye AY could be obtained by 99.99% in 10 min, at 14.4 kV of voltage. The kinetic constant was confirmed at 0.413 min– 1. Fig. 6a, Fig. 6b, and Fig. 6c give the information on the degradation pathway of azo dye, orng-II, and methylene blue (MB). Bansode et al. (2017) stated that higher discharge voltage generates high plasma densities and reactive species indicating similar degradation trends in the Orng-II and MB dyes in the Microbubble Plasma Systems. A setting of higher frequency at 1500 Hz confirmed a high kinetic constant of 1.585 min– 1 (Methylene Blue), 1.1263 min– 1 (Orng-II) and 0.548 min– 1 (Azo Dye AY). Also, alkaline conditions support the degradation of dye in the systems due to generated H2O2, •OH, and •O2 − that plays an important role as strong oxidation to degrade pollutants·H2O2 is generated from the interaction of energetic electrons and water molecules. Furthermore, O3 molecules are generated from plasma bubbles contact with water, combining with the H2O2 to generate the O2 and •OH. During the treatment of mixed dye pollutants, namely, azo dye mixed with methylene blue, azo dye mixed with Orng-II, and azo dye combined with Orng-II and methylene blue, removal of TOC can be obtained by 92.2%, 88.5%, and 81.9%, respectively, in 20 min. It confirms that reactive plasma bubbles play an integral role in dye degradation ( Fig. 7). The reaction of reactive species can be described as follow:
| H2O + *e– → H + •OH | (37) |
| •OH + •OH – → H2O2 | (38) |
| O2 + *e– → 2O + e– | (39) |
| O + O2 + M → O3 + M | (40) |
| H2O2+ UV → •OH + •OH | (41) |
| O3 + H2O2 → HO2 + •OH + O3 | (42) |
| O3 + HO2 → 2O2 + •OH | (43) |
Fig. 6.
Degradation pathway of dyes represent by (modified from: Zhou et al., 2021a).
Fig. 7.
Reactive oxygen species in the plasma bubbles (Concepts and mechanisms are modified from Zhou et al. (2021c)).
Another study by Chandana et al. (2015) confirmed that atmospheric-pressure non-thermal plasma jet indicated a promising result on the degradation of methylene blue pollutants. The establishment of H2O2 is recognized to play a major role in the elimination of dyes. Plasma jet was maintained by applying a potential difference of electrodes from 16 to 20 kV at 50 Hz, and power variation was set up between 2 W and 5 W. Excited plasma species and water molecules play a major role in establishing H2O2 formation and increase the function of time. After that, H2O2 may be converted to hydroxyl radicals due to the reaction with UV light. Formation of reaction can be seen as follow:
| *e– + H2O → •H + •OH + e– | (44) |
| •OH + •OH → H2O2 | (45) |
| H2O2 + UV → •OH + •OH | (46) |
pH plays a significant role in establishing the formation of various ionic species. In the presence of zero air and nitrogen bubbling, excited nitrogen plays a major role during the association to form NO and NO2 that convert it to NHO3 and HNO2. Furthermore, there is an excitation of oxygen and nitrogen molecules that transformed it to NO, NO2 molecules, and inorganic acid. In more precise, the concentration of excited species formed and transformation of mineral acids occurs, and there was an establishment of acid proton in an argon plasma discharge because of H2O* molecules and acidic proton may be generated from argon metastable species Ar (3p) that provide energy transfer (Medel et al., 2012). Moreover, acid–base dissociation is recognized as chemical species in water and gas– liquid environments (Lukes et al., 2012). Degradation of methylene blue can be obtained by 99% in 40 min using argon plasma jet compared to nitrogen and air. In addition, argon plasma jet indicated a better result in removing methylene blue with values of 99%, 97%, and 96% from initial concentration 30 ppm, 40 ppm, and 50 ppm, respectively. Also, higher reactive species may be generated from the process that become the main contribution of dye degradation. The reactions can be described as follows:
| *e– + O2 → •O + •O + e– | (47) |
| *e– + N2 → •N + •N + e– | (48) |
| *N + O→ NO | (49) |
| *O + NO → NO2 | (50) |
| NO2 + H2O → NO3 + 2H+ | (51) |
| •OH + NO /NO2 → HNO2 / HNO3 | (52) |
| •OH ↔ O−• + H+ (X) | (53) |
| H2O2↔ HO2−2• + H+ (X) | (54) |
| HO2↔ O2−• + H+ (X) | (55) |
| *e– + H2O → •H + •OH + e– | (56) |
| *OH + OH → H2O2 | (57) |
In addition, Garcia et al. (2017) treated methylene blue in an aqueous solution using a non-thermal microwave plasma jet at an atmospheric-pressure. Argon was used as a carrier gas, and microwave power was set up at 200 W. Quartz tube was used with value of 1.5 and 4 mm of inner and outer diameter, and the flow rate range from 350 to 1400 sccm. Concentrations of methylene blue were ranging from 5 to 250 mg/L and at the temperature 55 °C. Long–lived excited neutrals creates reactive species from the thermal energy in microwave plasma jet that containing of N2, NO, and O. Furthermore, metastable argon atoms from penning reactions generates argon atoms, argon ions (Ar+), and argon dimmer ions Ar2 + throughout Arm ionization collision, and metastable associative ionization with the recombination reactions of ions Ar+, and Ar2 + produce argon excited atoms (Ar*), and combination of H2O2 species, charged particles, ozone, hydrogen peroxide, and UV radiation, causes further dissociation and produce OH aq. Lastly, OH reactive species in water react due to the presence of a third molecule to generate hydrogen peroxide. Then, diffuse to the bulk liquid (Garcia et al., 2010). Arm ionization collisions can be described as:
| Ar m + Ar m → Ar + + Ar + e– | (58) |
| Ar m + Ar m → Ar 2+ + e– | (59) |
| Ar + e– + e– → Ar* + e– | (60) |
| Ar + e– + Ar → Ar* + Ar | (61) |
| Ar2+ + e– → Ar* + Ar | (62) |
| Ar*, m + H2O → Ar + OH* + H | (63) |
| Arm + N2 → Ar + N2* | (64) |
| N2* + H2O(gas) → N2 + OH* (gas) + H | (65) |
| OHaq + OHaq + M → H2O2aq + H | (66) |
Dojcinovic et al. (2011) investigated whether dielectric barrier discharges (DBD) combined with advanced oxidation processes (AOPs) can be used on decolorization of reactive textile dyes, namely, reactive black 5. Some catalysts such as H2O2, Fe2+ and Cu2+ were used. Energy densities were controlled at 45 – 315 kJ/L, and plasma treatment was performed at 24 h. An effective result of reactive black 5 decolorization can be obtained by 97% with the addition of 10 mM H2O2. The best performance on the degradation of reactive black 5 can be obtained with a decrease in the dosage of H2O2 that may prevent the consumption of •OH radicals and increase the decolorization. Then, degradation efficiency and decolorization reaction can be shown as follows:
| H2O2 + •O2– → •OH + OH– + O | (67) |
| HO2• + •OH → H2O + O2 | (68) |
To conclude, atmospheric-plasma bubble, non-thermal plasma jet and dielectric barrier discharges show a good result on decolorization of contaminants in the aqueous solution, confirming a promising result on the degradation of dyes.
4.4. Degradation of antibiotics, pesticides and other organic compounds
Many scholars have conducted studies looking at degradation of organic compounds and it shows a promising result (Qu et al., 2013, Markovic et al., 2015; Dobrin et al., 2013; Wardenier et al., 2019). Wardenier et al. (2019) used pulsed dielectric barrier discharge to remove pesticides atrazine (ATZ), alachlor (ALA), dichlorvos (DVOS), diuron (DIU), pentachlorophenol (PCP), and pharmaceuticals carbamazepine (CBZ), 1.7-α-ethinylestradiol (EE2) and plasticizer bisphenol A (BPA). Plasma discharged was generated by air, argon, or oxygen, and it was continuously flown through the reactor. 50 kHz of frequency was applied in the reactor cell, and discharge was produced in a pulsed mode with 15%. During the experiment, 2.5 L of synthetic water solution was fed to the reactor at flow rates of 56.3 mL min– 1, and 60 mL, respectively. Samples were collected at the regular time intervals, namely, 2.5 min, 5 min, 10 min, 15 min, 20 min, 25 min, and 30 min, respectively. The efficiency removals of ATZ, ALA, DIU, PCP, DVOS, BPA, CBZ, and EE2 were 56.9%, 57.8%, 62.4%, 69.3%, 70.2%, 76.7%, 82.4% and 87.8%, respectively. The decomposition of complex pollutants is related to the role of oxidative species such as hydroxyl radicals (•HO), nitrogen oxide (•NO), atomic oxygen (•O), ozone (O3), and hydrogen peroxide (H2O2). NO plays a major role in fast ozone quenching that leading to nitrogen oxide (NO2). The formation of reaction can be described as follows:
| N + e– → 2N* + e– | (69) |
| N + e– → 2O* + e– | (70) |
| N + O2 → NO + O* | (71) |
| O* + O2 + M → O3 + M | (72) |
| NO* + O3 → NO2 + O2 | (73) |
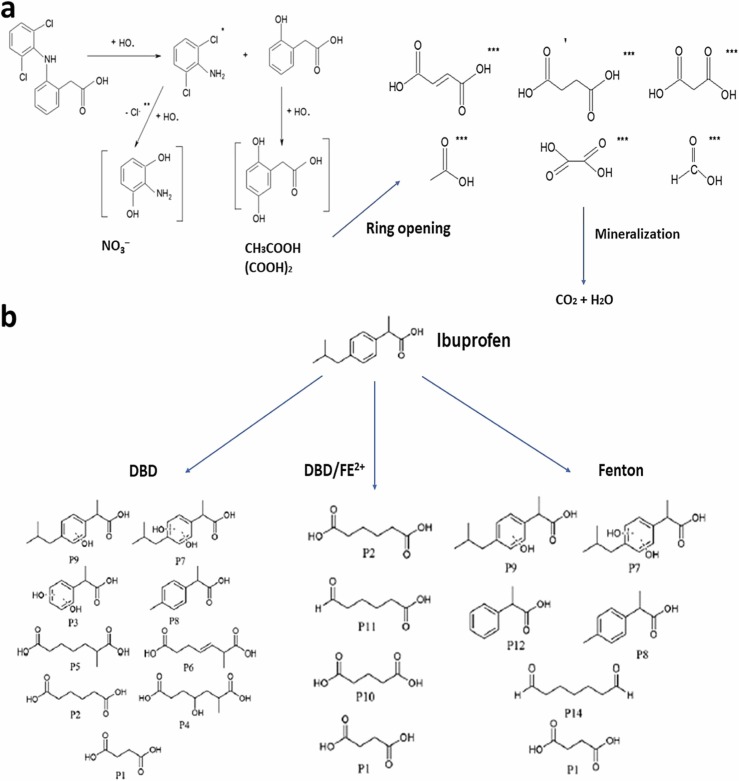
Another study by Dobrin et al., (2013) found that pulsed corona discharged showed a promising result on the degradation of pharmaceutical drug diclofenac (DCF). Oxygen was used as carrier gas with flow rate 1 L/min. Discharged of the gap was controlled at 3.5 mm. An amplitude of the voltage was controlled at 18 kV, and the voltage was maintained at 2 kV. Time of plasma was controlled under 2,5,7 and 10 min. Final concentration of DCF was obtained less than 5 mg/K after the treatment. In 5 min, approximately 70% of DCF can be degraded properly, and only 50% of chlorine remained in the residue as the organic form. pH of DCF solution decreased from 7 to 5.3 in 15 min. The most striking finding was the removal of 200 mg/L DCF in 30 min of ozonation. After that, Cl– remained in the aqueous solution and formed the by-product associated with the attack of hydroxyl radical. Fig. 8a shows the degradation of diclofenac.
Fig. 8.
Degradation pathway of organic pollutants (a) Diclofenac (modified from Dobrin et al. (2013)), and (b) Ibuprofen (modified from Markovic et al. (2013)).
Furthermore, the application of a non-thermal plasma reactor with dielectric barrier discharged and combined with Fenton reaction to destroy ibuprofen from aqueous solution was investigated by Markovic et al. (2015). Ibuprofen is categorized as a pharmaceutical compound that mostly contaminated surface and groundwater. Three experimental systems are installed for degrading the Ibuprofen, namely, dielectric barrier discharge reactor (DBD), DBD combined with Fe2+, and Fenton treatment were generated by hydrogen peroxide and iron salt. The solution of Ibuprofren was prepared via the double distillated water with the concentration 60 mg/L. The DBD/Fe2+ preparation was performed with the addition of the catalyst of Fe2+ , with the concentration at 5 mg/L. Furthermore, for preparing the Fe2+/H2O2 solution, concentrations of Ibuprofen, Fe2+, and H2O2 were 60 mg/L, 25.2 mg/L, and 306 mg/L, respectively. 17 kV of high voltage and 300 Hz of frequency were applied to the outer electrode. Moreover, filamentary barrier discharged was generated with 3 mm gap between inner metal electrode and glass tube. Degradation of ibuprofen can be obtained by 85% (DBD) and 99% (DBD/Fe2+). In the 10 min, the DBD/Fe2+ had 30% higher efficiency removal degradation than others. In 12 min, the percentage degradation between treatments decreased to 20%, and after 15 min, the treatment was less than 20%. Thus, the homogenous catalyst significantly affected the efficiency degradation of Ibuprofen in non-thermal plasma reactor. Degradation efficiency can be obtained by 78%, and it remains at 85%. Catalyst creates the reaction of •OH radicals, and it can be explained as follow:
| H2O2 + hv → 2HO• | (74) |
| H2O2 + O2– → OH• + OH– + O2 | (75) |
| H2O2 + Fe2+ → OH• + OH– + Fe3+ | (76) |
Fenton reaction plays a significant role in the degradation of products such as four aromatics products and two aliphatic products. OH• radicals play a significant role as an oxidative species for the ibuprofen oxidation and oxidation of hydrogen peroxide. In addition, the Fenton reduce the total organic carbon (TOC) value by 15%. Also, the TOC value can be reduced by 35% with the catalytic NTP (DBD/Fe2+). The measurement of TOC represents the oxidation of molecules to the smaller organic pollutants (Zhou et al., 2021a). Hydrogen peroxide inside the reactor plays a major role in the degradation of Ibuprofen, and all the catalyst may enhance the oxidizing power through the Fenton (Photo-Fenton) reaction. Unfortunately, water from the treatment should be neutralized from the post-treatment. An illustration of the degradation pathway of Ibuprofen is outlined in Fig. 8.
Qu et al. (2013) researched the removal of phenol from water solution using corona pulsed discharged combined with activated carbon (PCDP/GAC). 1.0 g GAC was filled in wastewater in the high voltage multi-need electrode and ground plate electrode. The volume of wastewater is 250 mL, and the circulating rate of wastewater was controlled at 100 mL/min. Gas flow rate and electricity power were controlled at 6 L/min and 23.6 kV, respectively. Removal efficiencies of Cd2+ and phenol were obtained at 69% and 87.3%, respectively, in 60 min during the treatment from PCDP/GAC. Furthermore, increasing the pulsed voltage and feeding gas significantly reduces phenol and Cd2+ during the treatment. OH radicals, H2O2, and O3 degrade phenol through the hydroxylated intermediates and organic acids. Reactive species react with phenol, forming decomposition products, namely, hydroxylated and nitrated aromatic ring molecules (Banaschik et al., 2017). Also, a chemical exchange between Cd2+ and surface oxygen groups that play an integral role in Cd2+ extraction responsible for generating intermediate oxygen and byproduct from a PCDP improves the adsorption of Cd2+ on GAC. Moreover, the Fourier transform infrared spectroscopy analysis indicates that OH, CH, and CC bonds are cleaved, and Ar O C and CO groups increased during the treatment. Unfortunately, the appears of peaks do not represent the original GAC. This confirms that phenol is transformed to benzoquinone or small organic compounds, for example, carboxylic acid, aldehyde, and ester.
In conclusion, plasma technology systems can be modified to degrade organic compounds combined with Fenton and activated carbon to deal with harmful pollutants. OH radicals generated from plasma play a major role to degrade the contaminants.
4.5. Removal of volatile organic compounds (VOCs) and other toxic gas
Treatment of toxic gases, namely, volatile organic compounds (VOCs) and PCDD/Fs in plasma technology shows promising results by some scholars (Vandenbroucke et al., 2011; Lu et al., 2012; Mao et al., 2018; Li et al., 2021) (Table S1). Mao et al. (2018) used dielectric barrier discharged (DBD) to eliminate benzene. The total gas flow rate was controlled at 300 mL/min, and purity of He was 99.99%. An electric furnace was used to control the temperature at 500 °C. A power supply includes a high voltage probe, a pulse power supply, and a digital phosphor oscilloscope were used in the unit. Decomposition of benzene can be obtained because of the CeO2 addition and the presence of O3. Furthermore, Ce3+ can be oxidized to the Ce4+ that generates O* species that enhance decomposition of benzene.
Lu et al. (2012) used a rotating gliding arc discharge reactor for degrading toluene. The gas flow rate was controlled at 5.4–10.8 m3 h–1. A discharged power was controlled at the range 6–10 kV with an autotransformer. The results indicated that removal efficiency can be obtained maximum of 77% from the initial concentration 200 mg m–3. In addition, the combination of water vapor and gas mixture play a major role in the decomposition of toluene because of the presence of some reactive species, such as electrons, positive and negative ions, and free radicals. Li and collaborators (Li et al., 2021) found that the combination of dielectric barrier discharges and photocatalyst (Ag-TiO2/γ-Al2O3) could remove the toluene with the percentage of 81% CO2. In fact, the radicals, namely, the O3 and OH radicals (Vandenbroucke et al., 2011), and electron hole pairs, such as H+ and e–– from the photocatalyst, create a reaction for degrading toluene and its intermediate products. Thereby, degradation of toluene can be obtained from the gliding arc discharge.
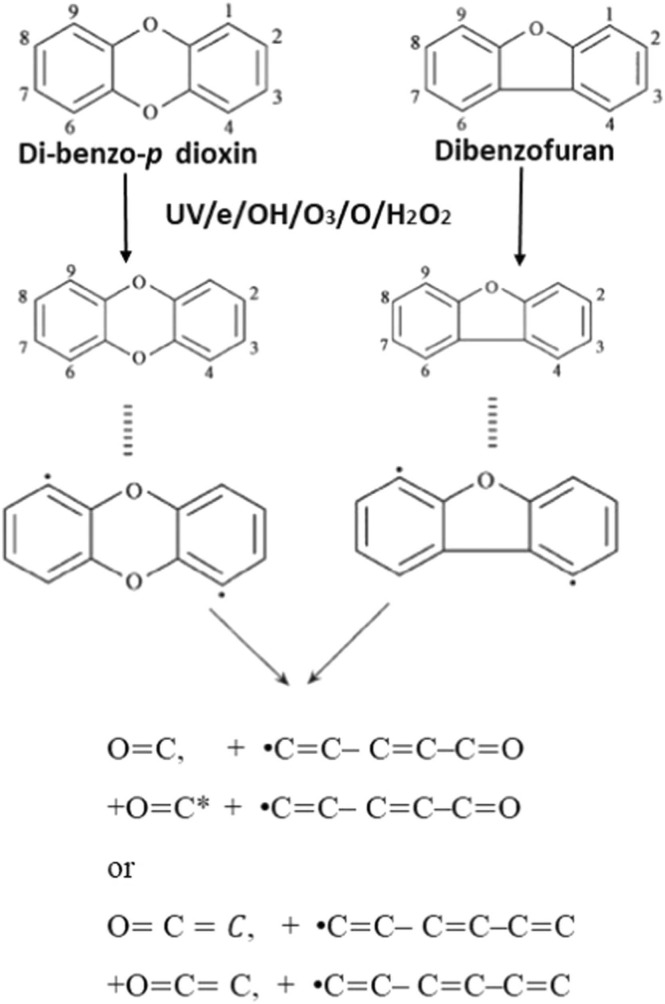
Mizeraczyk et al. (2005) investigated the using the atmospheric-pressure microwave discharge for treating the hazardous gas that consists of volatile organic compounds (VOCs) combined with nitrogen gas and the microwave power ranging from 200 to 400 watt under 10% of gaseous concentration. This result confirmed that the VOCs may be degraded fully with the relatively low-cost energy, where the energy calculation was 1000 g (kW-h)−1. This study also confirmed that volatile organic compound (VOCs) and VOC(s) reforming may be transformed to hydrogen. Also, the corresponding energy of the mass yield can be obtained from the waveguide-supplied MS. Thereby, an atmospheric-pressure microwave plasma reactor shows a promising result on the treatment of gaseous pollutants. Yan et al. (2007) used gliding arc discharges to degrade PCDD/Fs. The dry air was set up at 300 mL/min, and power frequency was controlled at 50 Hz. Then, the reactor was run with 10 kV of the voltage, and the length of electrode was 150 mm. The total concentration reduction ranged from 25% to 79%, and the TEQ value was 61%. Electron collision occurs that provides broad energy from the broad energy distribution consisting of O2, N2, and H2O ( Fig. 9). Thereby, energy transfer occurs, causes excitation, ionization and dissociation.
Fig. 9.
Degradation pathway of Dibenzodioxin by a dielectric barrier discharges (modified from Yan et al. (2007)).
To conclude, Dielectric barrier discharge gives a promising result on degrading the VOC and PCDD/Fs from air pollution. The setting of parameters helps to give the proper parameters that generate some reactive species that responsible for crushing the pollutants during the operation.
4.6. Deactivation of bacteria
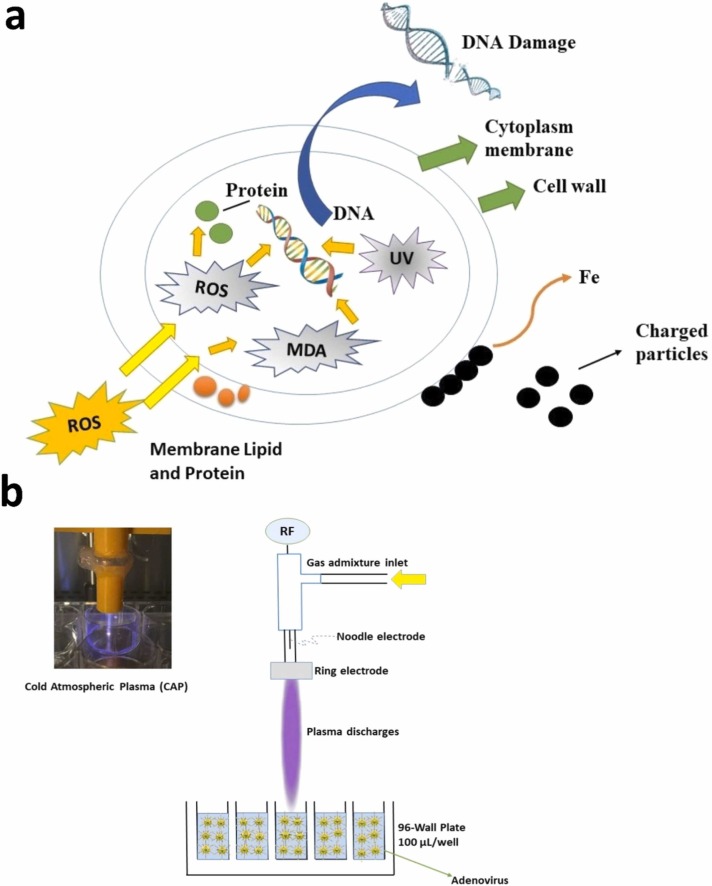
Plasma technology shows a promising result on the deactivation of bacteria from different fields of studies (Kim et al., 2013, Laurita et al., 2015, Liao et al., 2017, Cubas et al., 2019, Cubas et al., 2021). Perez et al., (2019) used cold atmospheric plasma (CAP) for killing Xanthomonas vesicatoria (XV) strain IPV-BO 2684. To generate the plasma activated water (PAW), CAP was combined with a DBD reactor consisting of a polystyrene cylindrical case. The plasma source was supported by the HV generator to generate pulses with a slow rate of kV/ns and 50 mJ energy per pulse. The system was controlled for 10 min with the source of peak voltage at 20 kV and the frequency of I kHz. Then, the air gap was maintained at 1 mm for in vitro and in planta experiments (1–3 h). As a result, the plasma activated water can be generated containing of hydrogen peroxides (H2O2), nitrates (NO3 –), and nitrites (NO2 –). These PAW were analyzed using the Amplex Hydrogen Peroxide Assay Kit. The results showed that PAW affect antimicrobial activity in in vitro experiments. In a plant experiment on the bacterial leaf spot of tomato showed a lower infection rate percentage of relative protection (RP) at values of 61% and 51%, respectively. OH radicals can be generated from the cold atmospheric pressure plasma containing heavy particles (positive and negative ions, atoms, free radicals and excited or non-excited molecules), electrons, and UV-rays. Sanito et al. (2021b) stated that the presence of OH radical shows a promising result in killing the bacteria because of this reactive species prevented the transcription of deoxyribonucleic acid (DNA) and penetrated the membrane cells. Chemical radicals play an integral role for the oxidation of the cell membrane of organism via the lipid peroxidation and reducing the ozone (Cubas et al., 2019). Thereby, microorganism can be killed during the process.
Butscher et al., (2016) investigated the plasma inactivation of microorganisms on sprout seeds of onion (Allium cepa), radis (Raphanus sativus), cress (Lepidium sativum), and alifalfa (Medicago sative) in a dielectric barrier discharge (DBD). Two parallel aluminum electrodes with the size 100 mm x 200 mm covered by dielectric barriers from polymethylmethacrylate or polycarbonate with a thickness 2 mm and a 5 mm gas gap. Argon is used as a working gas with 5.6 nlm. The pulses were controlled at 2.5–10 kHz, 6–10 kV, and 500 ns. The results show that the maximum inactivation of E. coli was 2.47 log. A reactive oxygen species from plasma contributes to the inactivation of microorganisms that related to the improvement of the plasma power density. Machala et al. (2013) stated that chemical and bactericidal effects are associated with the formation of ROS (reactive oxygen species) and RNS (reactive nitrogen species) in bio-decontamination. RNS is well known as the active agent in the virus inactivation and causes disruption of multiple cellular organelles (Auten and Davis, 2009). Moreover, plasma gas provides a strong bactericidal efficacy with O2 or air admixtures (Nayak et al., 2017). Thereby, gas has an effect for creating the reactive species. Stoffles et al., (2008) stated that energetic electrons from reactive chemistry are associated with the formation of free radicals and photon-emitting excited species. This statement is supported by the opinion of Zhu et al. (2009) that metastable He atoms play an integral role in generating N2 + through a penning ionization. Also. argon gas mix with air in non-thermal plasma indicates the oxidizing species in the gas/liquid interface, namely, RNS, ROS, H2O/H2O2, and ozone responsible for crushing the membrane and cytoplasm of bacteria ( Fig. 10a). In addition, chemical species derived from the air plasma indicates a better result compared to the chemical species from Ar plasma (Cubas et al., 2021). Then, the upper electrode of the gas phase that discharges close to the water surface indicates the high damage of bacteria, followed by reactive species’ attack (Estifaee, 2019). Thus, the inactivation of bacteria can be obtained by plasma technology.
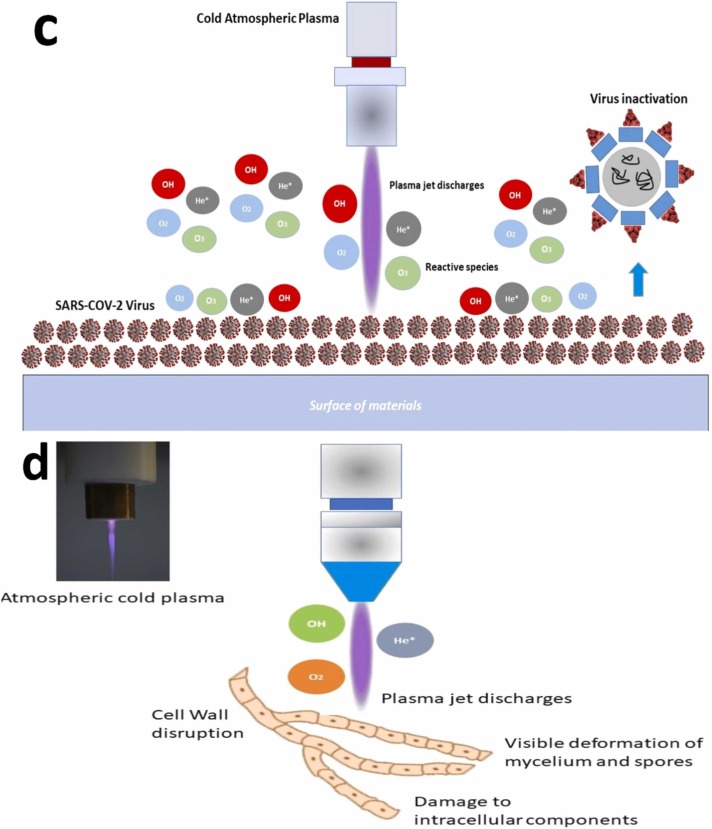
Fig. 10.
Inactivation mechanism of bacteria and virus viaatmospheric-cold plasma (a). Mechanism of ROS to kill bacteria (illustration ismodified from Liao et al. (2017)), (b) Mechanism of cold atmospheric plasma to inactivated ofadenovirus in the in-vivo experiment (modified from Aboubakr et al. (2016)), (c). Mechanism deactivation of SARS-CoV-2virus, (d). Mechanism of cold atmospheric plasma in killing fungi.
Furthermore, Lukes et al. (2014) stated that the formation of transient species of OH, NO2, NO radicals, and long-lived chemical products, for example, O3, H2O2, NO3 –, NO2 – generated by plasma discharged at the liquid interface. These species related to the chemical and biocidal effects that play a significant role in the degradation of bacteria are evaluated using E. coli. pH value of the plasma-treated solution is controlled using a phosphate buffer at pH 3.3, 6.9, or 10.1. The mixtures of oxygen with nitrogen or argon gases were used during the treatment. Experiment with E. coli shows that inactivation depends on the gas phase plasma and pH, similar to inactivation with PAW. Moreover, the concentration of NO2 – and H2O2 associated directly with peroxynitrite may be formed in the PAW, confirming the bacterial inactivation. NO from the residue is generated by different isoforms of nitric oxide synthase (NOS) and (ROS) by NADPH oxidase (NOX) that responsible for inhibition of adenoviral replication. It is clearly can be understood that reactive species from plasma treatment reliable in killing bacteria.
To summarize, the inactivation of bacteria depends on the reactive species radicals generated from the plasma system. The carrier gases (mixing and non-mixing with air), power supply set-up provide the diversity of reactive species to kill bacteria in an aqueous solution.
4.7. Deactivation of virus
Some studies have confirmed that plasma technology shows a promising result to eliminate the virus, for instance, spring viremia carp virus (SVCV) model, adenovirus and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Zimmerman et al., 2011, Chen et al., 2020, Filipic et al., 2020, Guo et al., 2021, Moldgy et al., 2020, Song et al., 2022).
Song and colleagues (Song et al., 2022) applied a pulsed corona discharged plasma (PCDCP). The voltage and frequency were controlled between 0 and 30 kV and 0–200 Hz, respectively. The gas flow rates were maintained at 6 L/min–1. Furthermore, the peristaltic pump was controlled at 100 rpm. During the study, 5 mL of virus suspension were used as the model. Each sample were collected at 15, 30, 60, 90, 120, 180, 240, and 300 s, respectively, and the control (sample with the absence of virus) were also prepared. The 4-log 10 reduction of SVCV can be obtained by 120 s of treatment. In more precise, the higher reductions of virus inactivation can be obtained with the frequencies of 70 Hz and 80 Hz. Moreover, the input power controlled at 0.024 kW (19.0 kV) reduced the titer more than 0.018 kW (16.8 kV) and 0.010 kW (14.3 kV), where virus titer was detected below the detection limit in 180 s of exposure time (4-log 10) reduction·H2O2 and O3 play an integral role on the degradation SVCV after 60 s. Furthermore, •OH radical and 1O2 increases after the treatment for 60 and 30 s, respectively. It can be assumed that reactive species reacts with the viral proteins and genome causes the virus inactivation, especially the 1O2 (Cho et al., 2010). In addition, O3 and H2O2 play an important role for the viral inactivation (Wolf et al., 2018, Su et al., 2018). This confirmed that reactive species oxidizing the protein, damages the structure and function of genome. Thereby, it causes the virus inactivation or deterioration of viral infectivity (Ersoya et al., 2019, Guo et al., 2018).
Zimmerman et al. (2011) investigated an effect of cold atmospheric plasma (CAP) in an adenovirus solution. The plasma device in this study consists of a UV emission spectrum. This UV power density was set up in length 170–340 nm wavelength. Furthermore, the measurement density was controlled by 12 mm. Ambient temperature was controlled at 20 °C and humidity was controlled at 40%. This device has one surface micro discharge (SMD) plasma electrode (10×10 cm2) with an insulator plate (10 mm thick). The diameter of the grid was 0.5 mm. Plasma is generated from the mesh grid side with a high sinusoidal ac voltage with a value of 4.7 kV pp at 10 kHz between the metal plate and mesh grid. Furthermore, the power consumption of the electrode was controlled at 0.5 W cm–2. HEK293 cells with E1 region of adenovirus were used in the experiment. The mouse fibrosarcoma cell line CMS-5 was infected with adenovirus at a density of 5 × 105 cells. The application of CAP indicates inhibition of virus with 100% of survival of cells, and it shows inhibition of virus replication because the use of CAP protects the lysis of cells from the infection of the virus. Moreover, inactivation of adenovirus ranging from 7.5 × 102 PFU mL–1. NO with its intermediates of NO radicals, NO–, NO+, N2O3, N2O4, ONOO–, and ROS (O–2, H2O2) are presumed responsible for attacking the virus, where these reactive species have an integral role as antimicrobial and antiviral. Mohamed et al. (2021) confirmed that there are specific reactive species responsible for dealing with the inactivation of virus that causes the viral pathogen, such as, O3, O2, and H2O2. The presence of O3 and NO2 usually generates the production of N2O5 that transform it to peroxynitrate (ONOO – / ONOOH) on the layer of water (Guo et al., 2021, Mohamed et al., 2021) that responsible for dealing with the virus inactivation. The presence of peroxynitrate, therefore, conclude that it is the most crucial point for the inactivation of the virus.
Another study conducting a cold atmospheric plasma reactor for inactivation of the virus has been performed by Aboubakr et al. (2016). A radio-frequency (RF) atmospheric plasma jet is applied with an ignited argon (1% admixture of molecular gas) and a 1.9 mm quartz tube during the study. The flow of gas was set at 1.5 standard liters per minute (SLM). Moreover, RF power frequency and power of jet were controlled at 20 kHz and 2.5 W, respectively. Four types of gas mixtures were used during the study, namely, Ar, Ar +1% O2, Ar +1% air, and Ar +0.27% H2O. The specific scavengers were used to evaluate plasma product activity. Feline calici virus strain 255 was chosen as a model for treatment. In the study, the combination of Ar +0.27% H2O showed a high concentration of H2O2 with a value of 4790 μM after 2 min of plasma exposure. The addition of H2O2 and •NO2 is a dominant reactive species for virus inactivation. In addition, pH reduction shows that O3 and NO2 – contribute significantly to virus inactivation related to the various gas mixtures. A scavenger experiment indicates that oxygen plays an integral role in the deactivation of the virus with Ar +1% O2 plasma. Increasing humidity in an argon plasma improves the number of H2O2 and •OH (Fig. 10b). Thereby, this confirms that plasma is a useful tool for virus inactivation.
Xia et al. (2019) studied the inactivation of airborne viruses by a packed bed non-thermal plasma reactor. For example, one or two AC voltage amplifiers power dielectric barrier discharge (DBD) reactor, variable 0–20 kV high voltage amplifier, and a 30 kV neon transformer. There is a significant increase of virus with the value of more than 2.3 log infective MS2 (2 log inactivation). 90% (1 log) inactivation of airborne MS2 can be achieved with an ozone dose with a value of 1.28 min mg m–3 from reactive oxygen species. Thus, the inactivation of the virus can be obtained via the treatment of DBD.
Since the outbreak of the SARS-CoV-2 in 2019, the infection via the aerosol and water has become a serious issue because of the transmission over 30 m in an atmospheric condition (Gorbunov 2020). Furthermore, their Virions exists on the surface of plastic, metals, and cardboard for some hours. Thus, some scholars have developed an investigation on the performance of cold plasma to deal with the SARS-CoV-2 (Bisag et al., 2020, Chen et al., 2020). Bisag et al. (2020) used a parallel-plate direct DBD configuration consisting of aluminum electrodes (5 × 150 × 2 mm) supported and covered by two dielectric supports, with the electrode gap of 2 mm. The device was set up by a micro-pulsed high voltage generator (AlmaPULSE; AlmaPlasma) for generating 56 kV of peak-to-peak voltage and the 14 kHz of frequency with the flow rate 0.6 slpm. The nebulizer was loaded with the 5 mL SARS-CoV-2 RNA, where the aerosol was collected with 1 mL distilled water. The degradation of SARS-CoV-2 RNA is shown in Fig. 10c. The result showed that CAP-treated with the RNA suspensions indicated a negative result based on the RNA suspension compared to the untreated sample with positive results.
The performance of CAP inactivation on SARS-CoV-2 was also assessed by Chen et al. (2020). Ar and He gases were controlled at 6.4 L/min and 16.5 L/min, respectively, at 16.8 kV and 16.6 kV. The reactive species was measured using optical emission spectroscopy. The SARS-CoV-2 samples were obtained from the swab test from the patient with respiratory illness. Various material surfaces were treated using the SARS-CoV-2 at the 2×105 PFU in 25 µl of volume. After that, the surface of the material was exposed with the argon and helium gases. As a result, CAP inactivated the SARS-CoV-2 less than 180 s of treatment. From the surface of metal, there was a decontamination of the 30 s from the exposure study. The plastic and leather ball surface was deactivated in 30 s and 60 s. The striking findings of this study were the surface inactivation of SARS-CoV-2 using a CAP related to composition, roughness, and absorptivity. Moreover, the higher discharge intensity generates more reactive species. As a result, the highest inactivation efficiency of the virus on the metal surface can be obtained from the study.
To conclude, CAP shows a promising result on the virus’s deactivation since reactive species generated from the plasma discharged. Furthermore, for further application to the inactivation of SARS-CoV-2 that attach to the surface of the material, such as composition, roughness, and absorptivity, the higher discharged of CAP is thus recommended in the treatment of SARS-CoV-2.
4.8. Deactivation of fungi
Some researchers have investigated the deactivation of fungi using plasma technology for different aspects, namely, health and agriculture (Zhang et al., 2014; Borges et al., 2018, Borges et al., 2017; Misra et al., 2019; Los et al., 2020). Borges et al. (2017) investigated the use of cold atmospheric pressure jet to deal with Candida albicans. 99.5% purity He was used as the carrier gas. The gas flow rate was controlled at 2 SLM. Moreover, discharge power was controlled at 1.8 W. Nozzle and sample surface were maintained at 1.5 cm in all experiments. The results indicated that fungal reduction can be obtained more than 1-log reduction during viable cell count after 90 s exposure. According to Liebmann et al. (2011), destruction of cells occurs because of the presence of reactive species, namely, nitric oxide, nitrogen oxide, hydroxyl radical, superoxide ozone, and singlet ozone (Fig. 10d).
Borges et al. (2018) confirmed an effect of cold atmospheric pressure plasma jet for treating the oral candidiasis. 99.5% purity helium was used as the carrier gas with a constant flow 2 SLM is controlled by a mass flow controller. The reactor nozzle was connected to a 1-m-long, 2.5-mm-diam polyurethane tube. The plasma jet device was controlled by a Miniplus4 AC power supply that generates an AC voltage signal with an amplitude up to 20 kV. The frequency was set up at 40 and 40 kHz. C. albicans ATCC 18804 cells were grown in a Sabouraud dextrose agar at 37 °C. After that, C albicans ATCC18804 suspension with a value of 1×108 cells mL–1 was contacted with the mice tongue for 3 min. The results showed that plasma jet may reduce the infection of C. albicans since there is a significant difference in the number of invading hyphae. Modification of plasma treatment or protocols significantly impacts the production of reactive species to fungal cell. Modifying amplitude modulation and reducing power play an integral role in generating reactive species from atmospheric-cold plasma.
In addition, Zhang et al. (2014) tested an effect of an atmospheric-pressure cold plasma on fungus-infected plant leaves (P. erubescens). Leaves of P. erubescens infected with plant pathogens was treated by needle-shaped plasma jet during different treatment time. Infection was showed with the presence of black spots on the leaves. Based on the study, deterioration of leaves can occur during the treatment on 30 s. Longer duration caused the damage of the leaf blade and leaf vein in 40 s during the treatment because of the plasma activated-species. As a consequence, the weak leaf veins were responsible for the deterioration of P. erubescens. There was a decrease of black spots on leaves during the plasma treatment time, confirming a good degradation of fungi from the atmospheric-pressure cold plasma jet. During the treatment, the number of black spots with a diameter < 2 mm rapidly decreased with the treatment time, emphasizing that small black spots were relatively easier to heal by the plasma jet. Conversely, larger diameters, which the value > 5 mm, were more difficult to be eliminated by atmospheric-pressure plasma jet. The inhibition mechanism is dominated by excited helium (He*), N2 (C → B), OH, O, and Hα (Stoffels et al., 2008).
To conclude, plasma technology shows a promising result on degrading fungi in different fields, such as health and agriculture. Thus, plasma technology is thus suggested in the elimination of fungi for the control the fungi.
5. Further research development opportunities in plasma technology
Plasma technology has the excellent opportunity to be implemented in the real application and many specific fields, not only to deal with the hazardous waste and wastewater but also for the conversion of biomass and inactivation of microorganisms (bacteria, fungi, and virus).
Some plasma technologies have been applied to treat pollutants, for instance, STARTECH (plasma electric waste converter), PACT (Plasma Arc Centrifugal Treatment), PLASCON (In Flight-Plasma Arc System) (Rahuman et al., 2000, Zinovlev et al., 2007), direct current plasma power supply and plasma furnace stations (Tetronics, 2015) and high-temperature plasma torch (APEXGREEN, 2021). The goals of the system are to treat hazardous waste and resource recovery. However, there is no information on the treatment of SARS-CoV-2.
Focuses on the virus, since the outbreak of the coronavirus 2 (SARS-CoV-2) pandemics worldwide, it has many challenges to the societies. Especially, the transmission via the aerosol should be concerned seriously (Bayarri et al., 2021). As the information of the treatment of SARS-CoV-2 is described in this paper from many literatures, plasma technology shows a promising result to deal with virus and give many strategies for preventing the virus transmission via the aerosol that causes the transmission of the virus, including SARS-CoV-2 virus (Zimmerman et al., 2011, Guo et al., 2021, Bisag et al., 2020, Chen et al., 2020, Mohamed et al., 2021). Thus, the development of plasma technology to inactivate the SARS-CoV-2 from the aerosol should be further investigated during the pandemic situation.
6. Conclusion
On the basis of the aforementioned discussion in this review paper, there are some important conclusions that can be obtained. Plasma technology with different systems can be considered to degrade pollutants and microorganisms because of the presence of reactive species generated in the plasma discharged. In this aspects, reaction oxygen species (ROS), reactive nitrogen species (RNS), and OH radicals in the plasma discharges play an important role to degrade the contaminants such as metals, metalloids, VOCs, dyes, and microorganisms (bacteria, fungi and virus), depends on the setup of parameters from the plasma systems. Combination of plasma technology with other process such as the photocatalyst, fenton and activated carbon are suggested to improve the degradation of difficult organic compounds from the aqueous solutions. Also, the formation of methane and hydrogen can be obtained from the degradation of biomass via plasma technology. The addition of flux agents and a combination of the photocatalyst in the plasma systems shows a promising result on degrading metals and VOCs. The scavenger test of electron holes combined with the photocatalyst gives the information of the electron holes. Furthermore, the plasma system also indicates a promising result on the inactivation of bacteria, fungi and virus. Further development should be focused on the economic analysis for the large-scale treatment in the various application that dealing with recent issues related to hazardous waste treatment, biomass conversion and preventing the transmission of SARS-CoV-2 virus. In addition, the research focusing on the treatment of the SARS-CoV-2 virus via plasma technology will become an interesting topic for studying the pandemic issues. Therefore, these research topics should be considered in nearby the future.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This current work is financially supported by the Chung Yuan Christian University (CYCU) with contract number 109609432. The first author would like to thank Prof. Ya-Fen Wang for supporting the work of this manuscript. We thank the Department of Environmental Engineering, Chung Yuan Christian University, where the study was undertaken.
Editor: Dr. C. Baiyang
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.jhazmat.2021.127390.
Appendix A. Supplementary material
Supplementary material
.
References
- Aboubakr H.A., Gangalk U., Youssef M.M., Goyai S.M., Bruggeman P.J. Inactivation of virus in solution by cold atmospheric pressure plasma: identification of chemical inactivation pathways. J.Phys. D: Appl. Phys. 2016;49 [Google Scholar]
- Adamovich I., Baalard S.D., Bogaerts A., Bruggeman P.J., Cappelli M., Colombo V., Czarnetzki U., Ebert U., Eden J.G., Favia P., Graves D.B., Hamaguchi S., Hieftje G., Hori M., Kaganovich I.D., Kortshagen U., Kusher M.J., Mason N.J., Mazouffre S., Thagard S.M., Metelmann H.R., Mizuno A., Moreau E., Murphy A.B., Niemira B.A., Oehrlein G.S., Petrovic Z.L., Pitchford L.C., Pu Y.K., Rauf S., Sakai O., Samukawa S., Starikovskaia S., Tennyson J., Terashima K., Turnere M.M., van de Sanden M.C.M., Verdelle A. The plasma roadmap: Low temperature plasma science and technology, topical review. J. Phys. D: Appl. Phys. 2017;50 [Google Scholar]
- APEXGREEN, 2021, Ding Jian Green Energy Technology Company Limited. 〈http://www.apexgreen.com.tw/〉.
- Auten R.L., Davis J.M. Oxygen toxicity and reactive oxygen species: the devil is in the details. Pediatr. Res. 2009;66:121–127. doi: 10.1203/PDR.0b013e3181a9eafb. [DOI] [PubMed] [Google Scholar]
- Banaschik R., Petr Lukes, Miron C., Banaschik R., Pipa A.V., Fricke K., Bednarski P.J., Kolb J.F. Fenton chemistry promoted by sub-microsecond pulsed corona plasmas for organic micropollutant degradation in water. Electrochimica Acta. 2017;245:539–548. [Google Scholar]
- Bansode A.V., More S.E., Siddiqui E.A., Satpute S., Ahmad A., Bhoraskar S.V., Mathe V.L. Effective degradation of organic water pollutants by atmospheric non-thermal plasma torch and analysis of degradation process. Chemosphere. 2017;167:396–405. doi: 10.1016/j.chemosphere.2016.09.089. [DOI] [PubMed] [Google Scholar]
- Basu P. In: Biomass gasification and Pyrolysis. Basu, editor. Academic Press; Boston: 2010. pp. 97–116. [Google Scholar]
- Bayarri B., Alcalde A.C., Lopez-Vinent N., Mico M.M., Sans C. Can ozone inactivate SARS-CoV-2? A review of mechanisms and performance on viruses. J. Hazard. Mater. 2021;415 doi: 10.1016/j.jhazmat.2021.125658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belov I., Vanneste J., Aghaee M., Paulussen S., Bogaerts A. Synthesis of micro-and nanomaterials in CO2 and CO dielectric barrier discharges. Plasma Process Polym. 2016 [Google Scholar]
- Bisag A., Isabelli P., Laurita R., Bucci C., Capelli F., Dirani G., Gherardi M., Laghi G., Paglianti A., Sambri V., Colombo V. Cold atmospheric plasma inactivation of aerosolized microdroplets containing bacteria and purified SARS-CoV-2 RNA to contrast airborne indoor transmission. Plasma Process Polym. 2020:1–8. [Google Scholar]
- Borges A.C., Lima G.D.M.G., Nishime T.M.C., Gontijo A.V.L., Kostov K.G., Ito C.Y.K. Amplitude-modulated cold atmospheric pressure plasma jet for treatment of oral candidiasis: In vivo study. PLoS One. 2018;13(6):1–19. doi: 10.1371/journal.pone.0199832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges A.C., Nishime T.M.C., Kostov K.G., Lima G.D.M.G., Gontijo A.V.L., Carvalho J.N.M.M.D., Honda R.Y., Ito C.Y.K. Cold atmospheric pressure plasma jet modulates Candida albicans virulence traits. Critical plasma medicine. 2017;7-8:9–15. [Google Scholar]
- Butscher D., Loon H.V., Waskow A., van Rohr P.R., Schuppler M. Plasma inactivation of microorganisms on sprout seeds in a dielectric barrier discharge. Int. J. Food Microbiol. 2016;238:222–232. doi: 10.1016/j.ijfoodmicro.2016.09.006. [DOI] [PubMed] [Google Scholar]
- Chandana L., Reddy P.M.K., Subrahmanyam C. Atmospheric pressure non-thermal plasma jet for the degradation of methylene blue in aqueous medium. Chemical Engineering Journal. 2015;282:116–122. [Google Scholar]
- Chang J.S. Thermal plasma solid waste and water treatments: a critical review. Int. J. Plasma. Environ. Sci. Technol. 2009;3:67–84. [Google Scholar]
- Chapman . John Wiley and Sons, Inc; 1980. Glow Discharge Processing: Sputtering and Plasma Etching. [Google Scholar]
- Chen M.Q., Wang J., Zhang M.X., Chen M.G., Zhu X.F., Min F.F., Tan Z.C. Catalytic effects of eight inorganic additives on pyrolysis of pine wood sawdust by microwave heating. J. Anal. Appl. Pyrolysis. 2008;82:145–150. [Google Scholar]
- Chen Z.T., Junior G.G., Arumugaswami V., Wirz R.E. Cold atmospheric plasma for SARS-CoV-2 inactivation. Phys. Fluids. 2020;32(111702):1–6. doi: 10.1063/5.0031332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho M., Lee J., Mackeyev Y., Wilson L.J., Alvarez P.J., Hughes J.B., Kim J.H. Visible light sensitized inactivation of MS-2 bacteriophage by a cationic amine functionalized C60 derivative. Environ. Sci. Technol. 2010;44:6685–6691. doi: 10.1021/es1014967. [DOI] [PubMed] [Google Scholar]
- Chung W.C., Mei D.H., Tu X., Chang M.B. Catalysis Reviews. Taylor & Francis; 2018. Removal of VOCs from gas streams via plasma and catalysis. Cold atmospheric plasma for SARS-CoV-2 inactivation, Physics of fluids, [Google Scholar]
- Cubas A.L.V., Ferreira M.F., Goncalves D.B., Machado M.D.M., Debacher N.A., Moecke E.H.S. Influence of non-thermal plasma reactor geometry and plasma gas on the inactivation of Eschericia coli in water. Chemosphere. 2021;271 doi: 10.1016/j.chemosphere.2021.130255. [DOI] [PubMed] [Google Scholar]
- Cubas A.L.V., Machado M.D.M., Machado M.D.M., Gross F., Magnago R.F., Moecke E.H., Souza G.D.I. Inertization of heavy metals present in galvanic sludge by DC thermal plasma. Environ. Sci. Technol. 2014;48:2853–2861. doi: 10.1021/es404296x. [DOI] [PubMed] [Google Scholar]
- Cubas A.L.V., Machado M.D.M., Machado M.D.M., Gross F., Magnago R.F., Moecke E.H., Souza G.D.I. Final treatment of spent batteries by thermal plasma. J. Environ. Manag. 2015;159:202–208. doi: 10.1016/j.jenvman.2015.05.004. [DOI] [PubMed] [Google Scholar]
- Cubas A.L.V., Machado M.D.M., Machado M.D.M., Moecke E.H.S., Dutra A.R.D.A., Fiedler H., Bueno P. Application of thermal plasma for inertization of sludge produced during treatment of landfill leacheate. Quim. Nova. 2016;Vol. 39(No. 8):906–913. [Google Scholar]
- Cubas A.L.V., Machado M.D.M., Santos J.R.D., Zanco J.J., Ribeiro D.H.B., Andre A.S., Debacher N.A., Moecke E.H.S. Effect of chemical species generated by different gemoetries of air and argon non-thermal plasma reactors on bacteria inactivation in water. Sep. Purif. Technol. 2019;222:68–74. [Google Scholar]
- Dobrin D., Bradu C., Magureanu M., Mandache N.B., Parvulescu V.I. Degradation of diclofenac in water using a pulsed corona discharged. Chem. Eng. J. 2013;234:389–396. [Google Scholar]
- Dojcinovic B.P., Roglic G.M., Obradovic B.M., Kuraica M.M., Kostic M.M., Nesic J., Manojlovic D.D. Decolorization of reactive textile dyes using water falling film dielectric barrier discharge. J. Hazard. Mater. 2011;192:763–771. doi: 10.1016/j.jhazmat.2011.05.086. [DOI] [PubMed] [Google Scholar]
- Du C.M., Gong X.J., Lin Y.C. Decomposition of volatile organic compounds using corona discharge plasma technology. J. Air Waste Manag. Assoc. 2019;Vol 69(No 8):879–899. doi: 10.1080/10962247.2019.1582441. [DOI] [PubMed] [Google Scholar]
- Du C.M., Huang Y., Gong X.J., Wei X.G. Plasma purification of halogen volatile organic compounds. IEEE Trans. Plasma Sci. 2018;Vol 46(No 4):838–858. [Google Scholar]
- Ersoya Z.G., Barisci S., Dinc O. Mechanisms of the Escherichia coli and Enterococcus faecalis inactivation by ozone. LWT-Food Sci. Technol. 2019;100:306–313. doi: 10.1016/j.lwt.2018.10.095. [DOI] [Google Scholar]
- Estifaee P., Su X., Yannam S.K., Rogers S., Thagard S.M. Mechanism of E. coli 905 inactivation by direct-in-liquid electrical discharge plasma in low conductivity 906 solutions. Sci.Rep, UK. 2019;9(1) doi: 10.1038/s41598-019-38838-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipic A., Guttierez-Aguirre I., Prmic G., Mozetic M., Dobnik D. Cold plasma, a new hope in the field of virus inactivation, Cell Press Reviews. Trends Biotechnol. 2020;Vol. 38(No. 11):1278–1291. doi: 10.1016/j.tibtech.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman A. Cambridge University Press; United Kingdom: 2008. Plasma Chemistry. [Google Scholar]
- Fridman A., Kennedy L.A. Plasma Physics and Engineering. Second edition., CRC Press, Taylor and Francis Group; 2011. [Google Scholar]
- Fridman A., Nester S., Kennedy L.A., Saveliev A., Mutaf-Yardimci O. Gliding arc gas discharge. Prog. Energy Combust. Sci. 1999;25:211–213. [Google Scholar]
- Garcia M.C., Varo M., Martinez P. Excitation of species in an expanded argon microwave plasma at atmospheric pressure. Plasma Chem. Plasma Process. 2010;30:241–255. [Google Scholar]
- Garcia M.C., Mora M., Esquivel D., Foster J.E., Rodero A., Jimenez-Sanchidiran C., Romero-Salguero F.J. Microwave atmospheric pressure plasma jets for wastewater treatment: degradation of methylene blue as a model dye. Chemosphere. 2017;180:239–246. doi: 10.1016/j.chemosphere.2017.03.126. [DOI] [PubMed] [Google Scholar]
- Gomez E., Rani D.A., Cheeseman C.R., Deegan D., Wise M., Boccaccini A.R. Thermal plasma technology for the treatment of wastes: a critical review. J. Hazard. Mater. 2009;161:614–626. doi: 10.1016/j.jhazmat.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Gorbunov B. Aerosol particles generated by coughing and sneezing of a SARS-CoV-2 (COVID-19) host travel over 30 m distance. Aerosol air Qual. Res. 2020;Vol 21(3):1–16. [Google Scholar]
- Guo L., Xu R.B., Liu Z.C., Zhao Y.M., Liu D.X., Zhang L., Chen H., Kong M.G. Mechanism of virus inactivation by cold atmospheric-pressure plasma and plasma –activated water. Appl. Environ. Microbiol., Public Environ. Health Probl. 2018;84(17):1–10. doi: 10.1128/AEM.00726-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Yao Z.Q., Yang L., Zhang H., Qi Y., Guo L., Xi W., Liu D.X., Zhang L., Cheng Y., Wang X.H., Rong M.Z., Chen H.L., Kong M.G. Plasma activated water: an alternative disinfectant for S protein inactivation to prevent SARS-CoV-2 infection. Chem. Eng. J. 2021;421 doi: 10.1016/j.cej.2020.127742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayyan M., Hashim M.A., Al Nashef I.M. Superoxide ion: generation and chemical implications. Chem., Rev. 2016;116:3029–3085. doi: 10.1021/acs.chemrev.5b00407. [DOI] [PubMed] [Google Scholar]
- Heberlein J., Murphy A.B. Thermal plasma waste treatment. J. Phys. D Appl. Phys. 2008;41:1–20. [Google Scholar]
- Hefny M.M., Pattyn C., Lukes P., Benediktm J. Atmospheric plasma generates oxygen atoms as oxidizing species in aqueous solutions. J. Phys. D: Appl. Phys. 2016;49:1–13. [Google Scholar]
- Higman C., van der Burgt M. Gasification. 2nd ed., Elsevier Science; USA: 2008. [Google Scholar]
- Hrabovsky, M., Konrad, M., Kopecky, V., Hlina, M., Kavka, T., Chumak, O., Maslani, A., Oost, G.V., 2010, Plasma aided gasification of biomass and plastics using CO2 as oxidizer. International symposium on non-thermal/thermal plasma pollution control technology & sustainable energy, ISNTP 7. St John’s, newfoundland, Canada.
- Huang X.Y., Cheng D.G., Chen F.Q., Zhan X.L. Reaction pathways of hemicellulose and mechanism of biomass pyrolysis in hydrogen plasma: a density functional theory study. Renew. Energy. 2016;96:490–497. [Google Scholar]
- Iervolino G., Vaiano V., Pepe G., Campiglia P., Palma V. Degradation of acid orange 7 azo dye in aqueous solution by a catalytic-assisted, non-thermal plasma process. Catalysis. 2020;10:888. [Google Scholar]
- Jiang B., Zheng J.T., Liu Q., Wu M.B. Degradation of azo dye using non-thermal plasma advanced oxidation process in a circulatory airtight reactor system. Chem. Eng. J. 2012;204–206:32–39. [Google Scholar]
- Kim H.J., Yong H.I., Park S.H., Choe W.H., Jo C.R. Effects of dielectric barrier discharge plasma on inactivation and the physicochemical and sensory characteristics of pork loin. Curr. Appl. Phys. 2013:1420–1425. [Google Scholar]
- Laurita R., Barbieri D., Gherardi M., Colombo V., Lukes P. Chemical analysis of reactive species and antimicrobial activity of water treated by nanosecond pulsed DBD air plasma. Clin. Plasma Med. 2015;3:53–61. [Google Scholar]
- Leins M., Gaiser S., Schulz A., Walker M., Schumacher U., Hirth T. How to ignite an atmospheric-pressure microwave plasma torch without any additional igniters. J. Vis. Exp. 2015;Vol. 98:1–12. doi: 10.3791/52816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmens B., Elslander H., Vanderreydt I., Peys K., Diels L., Oosterlinck M., Joos M. Assessment of plasma gasification of high caloric waste streams. waste management. 2007;27:1562–1569. doi: 10.1016/j.wasman.2006.07.027. [DOI] [PubMed] [Google Scholar]
- Li C.J., Rao Y.H., Zhang B.W., Huang K., Cao X., Peng D.D., Wu J.S., Xiao L.Q., Huang Y.Z. Extraordinary catalysis induced by titanium foil cathode plasma for degradation of water pollutant. Chemosphere. 2019;21:341–348. doi: 10.1016/j.chemosphere.2018.09.138. [DOI] [PubMed] [Google Scholar]
- Li M.Y., Li D.D., Zhang Z.Q., Ji C.J., Zhou S., Guo W.W., Zhao C.C., Liu F., Han F.L. Study on the performance and mechanism of degradation of toluene with non-thermal plasmas synergized supported TiO2/γ-Al2O3 catalyst. J. Environ. Chem. Eng. 2021;9 [Google Scholar]
- Li and collaborators (Li et al., 2001). -→ Li and collaborators (Li et al., 2021).
- Liao X.Y., Liu D.H., Xiang Q., Ahn J.H., Chen S., Ye X.Q., Ding T. Inactivation mechanism of non-thermal plasma on microbes: a review. Food Control. 2017;75:83–91. [Google Scholar]
- Liebmann J., Scherer J., Bibinov N., Rajasekaran P., Kovacs R., Gesche R., wakowicz P., Kolb-Bachofen V. Biological effects of nitric oxide generated by an atmospheric pressure gas-plasma on human skin cells. Nitric Oxide. 2011;24:8–16. doi: 10.1016/j.niox.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Lin C.Y., Wu T.Y., Jhang S.R., Yang P.M., Hsiao Y.H. Hydrogen production from banyan leaves using an atmospheric-pressure microwave plasma reactor. Bio-Resour. Technol. 2014;161:304–309. doi: 10.1016/j.biortech.2014.03.067. [DOI] [PubMed] [Google Scholar]
- Locke B.R., Shih K.Y. Review of the methods to form hydrogen peroxide in electrical discharge plasma with liquid water. Plasma Sources Sci. Technol. 2011;20:1–13. (Plasma Sources and Science and Technology) [Google Scholar]
- Los A., Ziuzina D., Boehm D., Cullen P.J., Bourke P. Inactivation efficacies and mechanisms of gas plasma and plasma-activated water against Aspergillus flavous spores and biofilms: a comparative study. Appl. Environ. Microbiol. Food Microbiol. 2020;86(9):1–17. doi: 10.1128/AEM.02619-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y.S., Sun X.M., Li X.D., Yan J.H., Diu C.M. Decomposition of toluene in a rotating glaidarc discharge reactor. IEEE Trans. Plasma Sci. 2012;Vol. 40(No 9):2151–2156. [Google Scholar]
- Lukes P., Locke B.R., Brisset J.L. Plasma Chemistry and Catalysis in Gases and Liquids. first edition., Wiley-VCH Verlag GmBH & Co; 2012. Aqueous-phase chemistry of electrical discharged plasma in water and in gas-liquid environments. [Google Scholar]
- Lukes P., Dolezalova E., Sisrova I., Clupek M. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: evidence for the formation of peroxynitritethrough a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Source Sci. Technol. 2014;23 [Google Scholar]
- Machala Z., Taabova B., Hensel K., Spetlikova E., Sikurova L., Lukes P. Formation of ROS and RNS in water electro-sprayed through transient spark discharge in air and their bactericidal effects. Plasma process. polym. 2013:10–649-659. [Google Scholar]
- Mai-Prochnow A., Alam D., Zhou R.W., Zhang T.Q., Ostrikov K., Cullen P.J. Microbial decontamination of chicken using atmospheric-plasma bubbles. Plasma Process Polym. 2020:1–11. [Google Scholar]
- Mao L.G., Chen Z.Z., Wu X.Y., Tang X.J., Yao S.L., Zhang X.M., Jiang B.Q., Han J.Y., Wu Z.L., Lu H., Nozaki T. Plasma-catalyst hybrid reactor with CeO2/γ-Al2O3 for benzene decomposition with synergetic effect and nano particle by-product reduction. J. Hazard. Mater. 2018;347:150–159. doi: 10.1016/j.jhazmat.2017.12.064. [DOI] [PubMed] [Google Scholar]
- Markovic M., Jovic M., Stankovic D., Kovacevic V., Roglic G., Gojgic-Cvijovic G., Manojlovic D. Application of non-thermal plasma reactor and fenton reaction for degradation of ibuprofen. Sci. Total Environ. 2015;505:1148–1155. doi: 10.1016/j.scitotenv.2014.11.017. [DOI] [PubMed] [Google Scholar]
- Mattox D.M. Elsevier Inc; 2010. The Low-pressure Plasma Processing Environment in Handbook of Physical Vapor Deposition (PVD) Processing; pp. 157–193. [Google Scholar]
- Medel A., Bustos E., Esquivel K., Godinez L.A., Meas Y. Electrochemical incineration of phenolic compounds from the hydrocarbon industry using boron-doped diamond electrodes. Int. J. Photo. 2012;681875:1–6. [Google Scholar]
- Misra, N.N., Yadav, B., Roopesh, M.S., Jo, C., 2019, Cold plasma for effective fungal mycotoxin control in foods: mechanisms, Inactivation effects and applications. [DOI] [PubMed]
- Mizeraczyk J., Jasinski M., Zakrzewski Z. Hazardous gas treatment using atmospheric pressure microwave discharges. Plasma Phys. Control. Fusion. 2005;47:B589–B602. [Google Scholar]
- Mohamed H., Nayak G., Rendine N., Wigdahl B., Krebs F.C., Bruggeman P.J., Miller V. Non-thermal plasma as a novel strategy for treating or preventing viral infection and associated disease, review. Front. Phys. 2021;9 [Google Scholar]
- Moldgy A., Nayak G., Aboubakr H.A., Goyal S.M., Bruggeman P.J. Inactivation of virus and bacteria using cold atmospheric pressure air plasmas and the role of reactive nitrogen species. J. Phys. D: Appl Phys. 2020;53(4) [Google Scholar]
- Muvhiiwa F.R., Sempuga B., Hilderbant D., van der Walt J. Study of the effects of temperature on syngas composition from pyrolysis of wood pellets using a nitrogen plasma torch. J. Anal. Appl. Pyrolysis. 2018;130:159–168. [Google Scholar]
- Nayak G., Aboubakr H.A., Goyal S.M., Bruggeman P.J. Reactive species responsible for the inactivation of feline calicivirus by a two-dimensional array of integrated coaxial microhollow dielectric barrier discharges in air. Plasma Process Polym. 2017;15:1–12. [Google Scholar]
- Perez S.M., Biondi E., Laurita R., Proto M., Sarti F., Gherardi M., Bertaccini A., Colombo V. Plasma activated water as resistance inducer against bacterial leaf spot of tomato. PLoS ONE. 2019;14(5) doi: 10.1371/journal.pone.0217788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu G.Z., Liang D.L., Qu D., Huang Y.M., Liu T., Mao H., Ji P.H., Huang D.L. Simultaneous removal of cadmium ions and phenol from water solution by pulsed corona discharged plasma combine with activated carbon. Chem. Eng. J. 2013;228:28–35. [Google Scholar]
- Rahuman, M.M.S.M., Pistone, L., Trifiro, F., Miertus, S., 2000, Destruction technologies for polychlorinated biphenyls (PCBs). In: Proceedings of Expert Group Meetings on POPs and Pesticides Contaminations: Remediation Technologies and on Clean Technologies for the Reduction and Elimination of POPs, pp. 1–55.
- Reddy P.M.K., Raju B.R., Karuppiah J., Reddy E.L., Subrahmanyam C. Degradataion and mineralization of methylene blue by dielectric barrier discharge non-thermal plasma reactor. Chem. Eng. J. 2013;217:41–47. [Google Scholar]
- Rossnagel S.M., Cuomo J.J., Westwood W.D. Material Science and Processing Technology. 1st edition., William Andrews; 1991. Handbook of plasma processing technology: fundamental, etching, deposition, and surface interactions. [Google Scholar]
- Samukawa S., Hori M., Rauf S., Tachibana K., Bruggeman P., Kroesen G., Whitehead J.C., Murphy A.B., Gutsol A.F., Starikovskaia S., Kortshagen U., Boeuf J.P., Sommerer T.J., Kushner M.J., Czanetzki U., Mason N. The 2012 plasma road map. J. Phys. D: Appl. Phys. 2012;45 1-37. [Google Scholar]
- Sanito R.C., You S.J., Wang Y.F. Application of plasma technology for treating e-waste: a review. J. Environ. Manag. 2021;288 doi: 10.1016/j.jenvman.2021.112380. [DOI] [PubMed] [Google Scholar]
- Sanito R.C., You S.J., Chang G.M., Wang Y.F. Effect of shell powder on removal of metals and volatile organic compounds (VOCs) from resin in atmospheric-pressure microwave plasma reactor. J. Hazard. Mater. 2020;394 doi: 10.1016/j.jhazmat.2020.122558. [DOI] [PubMed] [Google Scholar]
- Sanito R.C., You S.J., Chang T.J., Wang Y.F. Economic and environmental evaluation of flux agents in the vitrification of resin waste: A SWOT analysis. J. Environ. Manag. 2020;270 doi: 10.1016/j.jenvman.2020.110910. [DOI] [PubMed] [Google Scholar]
- Sanito R.C., Chen Y.W., You S.J., Yang H.S., Hsieh Y.K., Wang Y.F. Hydrogen and methane production from Styrofoam Waste using an atmospheric-pressure microwave plasma reactor, Aerosol and Air Quality. Research. 2020;20:2226–2238. [Google Scholar]
- Sanito, R.C., Yeh, T.Z., You, S.J., Wang, Y.F., 2021b, Novel TiO2/PANI composites as a disinfectant for the elimination of Escherichia coli and Staphylococcus aureus in aquaculture water.
- Santos L.C.O., Cubas A.L.V., Moecke E.H.S., Ribeiro D.H.B., Amante E.R. Use of cold plasma to inactivate Eschericia coli and physicochemical evaluation in Pumpkin Puree. J. Food Prot. 2018;Vol. 81(No. 11):1897–1905. doi: 10.4315/0362-028X.JFP-18-136. [DOI] [PubMed] [Google Scholar]
- Scally L., Gulan M., Weigang L., Cullen P.J., Milosavljevic V. Significant of a non-thermal plasma treatment on LDPE biodegradation with Pseudomonas aeruginosa. Materials. 2018;11:192. doi: 10.3390/ma11101925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirkawar V.S., Zhao M., Clough P., Yao J., Zhong X., Memon M.Z., Shah N., Anthony E.J., Fennell P.S. An overview of advances in biomass gasification. Energy Environ. Sci. R. Soc. 2016;9:2927–3304. [Google Scholar]
- Snoecx R., Wessenbeeck K.V., Lenaerts S., Cha M.S., Bogaerts A. Suppressing the formation of NOx and N2O in CO2 / N2 dielectric barrier discharge plasma by adding CH4: scavenger chemistry at work. Sustain. Energy Fuels. 2019;3:1388. [Google Scholar]
- Song K., Wang H., Jiao Z., Qu G.Z., Chen W.C., Wang G.X., Wang T.C., Zhang Z.Q., Ling F. Inactivation efficacy and mechanism of pulsed corona discharge plasma on virus in water. J. Hazard. Mater. 2022;422 doi: 10.1016/j.jhazmat.2021.126906. [DOI] [PubMed] [Google Scholar]
- Stoffels E., Sakiyama Y., Graves D.B. Cold atmospheric plasma: charged species and their interactions with cells and tissues. IEEE Trans. Plasma Sci. 2008;36:1441–1457. [Google Scholar]
- Su X., Tian Y., Zhou H.Z., Li Y., Zhang Z.H., Jiang B.Y., Yang B., Zhang J., Fang J. Inactivation efficacy of nonthermal plasma-activated solutions against Newcastle disease virus. Appl. Environ. Microbiol. 2018;84 doi: 10.1128/AEM.02836-17,02836-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalatkiewicz J., Szewczyk R., Budny E., Missala T., Winiarski W. Construction aspects of plasma based technology for waste of electrical and electronic equipment (WEEE) management in urban areas, 11 th international conference on modern building materials, structures and technique, MBMST 2013. Procedia Eng. 2013;57:1100–1108. [Google Scholar]
- Szalatkiewiczm J., 2013, Metals recovery from waste of printed circuit boards processed in plasmatron plasma reactor, 16th IFAC symposium in automation in mining, mineral and metal processing, 478–483.
- Taylor R.P., Pirzada S.A. Thermal plasma processing of materials: a review. Adv. Perform. Mater. 1994;1:35–50. [Google Scholar]
- Tetronics . Tetronic International Limited; Marston Gate, Swindon, United Kingdom: 2015. Tetronics International: About Tetronics. [Google Scholar]
- Tsai C.H., Chen K.T. Production of hydrogen and nano carbon powders from direct plasmalysis of methane. International journal of hydrogen energy. 2009;34:833–838. [Google Scholar]
- Vandenbroucke A.M., Morent R., De Geyter N., Leys C. Non-thermal plasmas for non-catalytic and catalytic VOC abatement. J. Hazard. Mater. 2011;195:30–54. doi: 10.1016/j.jhazmat.2011.08.060. [DOI] [PubMed] [Google Scholar]
- Wang Y.F., You Y.S., Tsai C.H., Wang L.C. Production of hydrogen by plasma-reforming of methanol. Int. J. Hydrog. Energy. 2010;35:9637–9640. [Google Scholar]
- Wardenier N., Vanraes P., Nikiforov A., van Hulle S.W.H., Leys C. Removal micro-pollutants from water in a continuous-flo electrical discharge reactor. J. Hazard. Mater. 2019 doi: 10.1016/j.jhazmat.2018.08.095. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Tsuru T. Water plasma generation under atmospheric pressure for HFC destruction. Thin Solid Films. 2008;516:4391–4396. [Google Scholar]
- Wessenback K.V. Universiteit Antwerpen; Antwerpen: 2016. Plasma Catalysis as an Efficient and Sustainable Air Purification; pp. 1–193. [Google Scholar]
- Wolf C., von Gunten U., Kohn T. Kinetics of inactivation of waterborne enteric viruses by Ozone. Environ. Sci. Technol. 2018;52:2170–2177. doi: 10.1021/acs.est.7b05111. [DOI] [PubMed] [Google Scholar]
- Xia, T., Kleinheksel, A., Lee, E.M., Qiao, Z., Wigginton, K.R., Clack, H.L., 2019, Inactivation of airborne viruses using a packed bed non-thermal plasma reactor. J. Phys. D: Appl. Phys. 52, 255201. Journal of Applied Physics. [DOI] [PMC free article] [PubMed]
- Yan J.H., Peng Z., Lu S.Y., Du C.M., Li X.D., Chen T., Ni M.J., Cen K.F. Destruction of PCDD/Fs by gliding arc discharges. J. Environ. Sci. 2007;19:1404–1408. doi: 10.1016/s1001-0742(07)60229-0. [DOI] [PubMed] [Google Scholar]
- Zeghioud H., Nguyen-Tri P., Khezami L., Amrane A., Assadi A.A. Review on discharge plasma for water treatment: mechanism, reactor geometris, active species and combined process. J. Water Process Eng. 2020;387 [Google Scholar]
- Zhang T.Q., Zhou R.W., Wang P.Y., Mai-Prochnow A., McConchie R., Li W.S., Zhou R., Thompson E.W., Ostrikov K., Cullen P.J. Degradation of cefexime antibiotic in water by atmospheric plasma bubbles: performance, degradation pathways and toxicity evaluation. Chem. Eng. J. 2021 [Google Scholar]
- Zhang T.Q., Zhou R.W., Wang P.Y., Mai-Prochnow A., McConchie R., Li W.S, Zhou R., Thompson E.W., Ostrikov K., Cullen P.J. Degradation of cefexime antibiotic in water by atmospheric plasma bubbles: performance, degradation pathways and toxicity evaluation. Chemical Engineering Journal. 2021 [Google Scholar]
- Zhang X.H., Liu D.P., Zhou R.W., Song Y., Sun Y., Sun Y., Zhang Q., Niu J.H., Fan H.Y., Yang S.Z. Atmospheric cold plasma jet for plant disease treatment. Appl. Phys. Lett. 2014;104 [Google Scholar]
- Zhou R.W., Zhang T.Q., Zhou R.S., Mai-Prochnow A., Ponraj S.B., Fang Z., Masood H., Kanangh J., McClure D., Alam D., Ostrikov K., Cullen P.J. Underwater microplasma bubbles for efficient and simultaneous degradation of mixed dye pollutants. Sci. Total Environ. 2021;750 doi: 10.1016/j.scitotenv.2020.142295. [DOI] [PubMed] [Google Scholar]
- Zhou R.W., Zhang T.Q., Zhou R.S., Mai-Prochnow A., Ponraj S.B., Fang Z., Masood H., Kanangh J., McClure D., Alam D., Ostrikov K., Cullen P.J. Underwater microplasma bubbles for efficient and simultaneous degradation of mixed dye pollutants. Sci. Total Environ. 2021;750 doi: 10.1016/j.scitotenv.2020.142295. [DOI] [PubMed] [Google Scholar]
- Zhou R.W., Zhou R.S., Alam D., Zhang T.Q., Li W.S., Xia Y., Mai-Prochnow A., An H.J., Lovell E.C., Masood H., Amal R., Ostrikov K., Cullen P.J. Plasmalytic bubbles using CeO2 for organic pollutant degradation. Chem. Eng. J. 2021;403 3. [Google Scholar]
- Zhu W.C., Li Q., Zhu X.M., Pu Y.K. Characteristics of atmospheric pressure plasma jets emerging into ambient air and helium. J. Phys. D: Appl. Phys. 2009;42 [Google Scholar]
- Zimmerman, J.L., Dumler, K., Shimizu, T., Morfill, G.E., Wolf, A., Boxhammer, V., Schlegel, J., Gansbacher, B., Anton, M. 2011, Effectof cold atmospheric plasmas on adenoviruses in solution.
- Zinovlev S., Fornasiero P., Lodolo A., Miertus S. Non-combustion technologies for POPs destruction. Rev. Eval. 2007:1–182. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material