Abstract
In vitro antimicrobial activity and susceptibility testing interpretation criteria and quality control were studied for gatifloxacin, a new 8-methoxy fluoroquinolone, tested against Haemophilus influenzae. Moraxella catarrhalis (600 strains) and H. influenzae (1,400 strains) from the SENTRY Antimicrobial Surveillance Program in North America (Canada and the United States) were also tested against gatifloxacin and 12 other antimicrobial agents. Gatifloxacin (MIC at which 90% of the isolates are inhibited [MIC90], ≤0.03 μg/ml; 100.0% of strains inhibited at ≤2 μg/ml) was the most active agent tested against H. influenzae and was similar to four comparison fluoroquinolones (MICs, ≤0.03 to 2 μg/ml) against M. catarrhalis. A subset of 300 recent clinical isolates of H. influenzae were tested by using media (Haemophilus Test Medium agar and broth) and procedures recommended by the National Committee for Clinical Laboratory Standards (NCCLS) and with the E-test (AB BIODISK, Solna, Sweden). Gatifloxacin (MIC50, 0.008 μg/ml) was slightly more active than levofloxacin, and E-test results were generally elevated by 0.5 log2 dilution step compared to reference MICs. The gatifloxacin 5-μg disk test produced zone diameters that were routinely above 30 mm for H. influenzae strains, corresponding to gatifloxacin MICs of 0.008 or 0.016 μg/ml. The gatifloxacin susceptibility breakpoint proposed for nonfastidious species (≤2 μg/ml; ≥18 mm) was also suggested for H. influenzae testing. No interpretive errors were observed. Quality control guidelines for H. influenzae ATCC 49247 were determined by using the NCCLS M23-T3 (1998) study design. The results from the nine-laboratory protocol suggested the following control ranges: for broth microdilution tests, 0.004 to 0.03 μg/ml; for disk diffusion testing, 33 to 41 mm. Gatifloxacin appears to be a potent anti-Haemophilus fluoroquinolone compound with in vitro testing interpretive criteria that will produce accurate results (disk diffusion, broth microdilution, and E-test).
Gatifloxacin, formerly AM-1155 or CG5501, is a newer 8-methoxy fluoroquinolone with expanded activity against aerobic gram-positive cocci and some anaerobes (3, 15). Activity against important gram-negative bacilli and the commonly isolated pathogens in community-acquired respiratory tract infections (Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, Mycoplasma, Chlamydia, and Legionella) has been maintained (1, 3, 4, 11–13). For the H. influenzae strains, results for gatifloxacin MICs at which 50% of the isolates are inhibited (MIC50s) have been remarkably low (range, ≤0.006 to 0.008 μg/ml), and the highest reported MIC was 0.03 μg/ml (13). This degree of activity of gatifloxacin was comparable to those of trovafloxacin (1) and ciprofloxacin (1, 13), although there have been some variations from this conclusion, possibly produced by differences in testing methods or media (3, 12, 13).
In this report, we summarize several studies of gatifloxacin to clarify its potency against H. influenzae and M. catarrhalis by using methods of the National Committee for Clinical Laboratory Standards (NCCLS). Furthermore, three methods (E-test [AB BIODISK, Solna, Sweden], disk diffusion, and broth microdilution) (7, 8) that represent the most commonly used methods in clinical practice were compared. Quality control guidelines were also established for H. influenzae ATCC 49247 by applying a nine-laboratory study design (10).
MATERIALS AND METHODS
Strains.
The H. influenzae and M. catarrhalis strains were isolates from the 1997 SENTRY Antimicrobial Surveillance Program (North America). Among the 300-strain subset of H. influenzae used for further studies, 110 strains (36.7%) produced a β-lactamase as demonstrated by a positive chromogenic cephalosporin test (Cephinase-2; BDMS, Cockeysville, Md.). All isolates were derived from clinical infections of the respiratory tract or bloodstream. SENTRY participant identifications of H. influenzae were confirmed by the monitor (in Iowa City, Iowa) by standard laboratory methods, which included growth requirements for X and V factors and a negative porphyrin test.
Antimicrobial agents.
Gatifloxacin and levofloxacin (the control fluoroquinolone) laboratory standard powders were obtained from Bristol-Myers Squibb (Princeton, N.J.) and Ortho-McNeil Pharmaceuticals (Raritan, N.J.), respectively. Other tested drugs were provided by their manufacturers in the United States. These compounds were dispersed into Mueller-Hinton broth (one lot for regression and five lots for quality control evaluations) conforming to the HTM formulation recommended by the NCCLS (8). Disk reagents, both 5 μg in content (gatifloxacin and ciprofloxacin), were produced by Difco (Detroit, Mich.). All quality assurance test results with the ciprofloxacin disks were within guidelines listed in NCCLS tables (9).
Susceptibility testing.
All MIC and disk diffusion tests were processed by the methods recommended for H. influenzae in the NCCLS documents (7, 8). The HTM broth microdilution trays were manufactured by Dade MicroScan (Sacramento, Calif.) and were rehydrated and inoculated with a single lot of broth (PML Microbiologics, Wilsonville, Oreg.). The final inoculum concentration was approximately 5 × 105 CFU/ml and was controlled by regularly performed colony counts. Disk diffusion zone diameters were measured on HTM agar plates (Remel, Lenexa, Kans.) with a digital caliper.
Quality control study.
Nine laboratories were recruited to perform replicate testing of the H. influenzae ATCC 49247 quality control strain (7–10). The MIC study design followed the NCCLS M23-T3 guideline (10) by using five lots of HTM from at least three commercial manufacturers (Accumedia lot 9704131; BBL lots ARDFKF, I9DGXC, and GODIGL; and Difco lot 116440JD), and 10 replicates over as many days. Fifty gatifloxacin MIC results were generated in each laboratory (i.e., a total of 450 MIC determinations). Levofloxacin (two broth lots produced by BBL and Difco) was used as a control, with all 180 MIC results within NCCLS control limits (9).
Disk diffusion quality control for H. influenzae ATCC 49247 also employed a nine-laboratory protocol with three HTM agar lots (60 replicates per participant [each using two disk lots] × 9 laboratories = 540 zone diameters). Control fluoroquinolone zone diameters were acceptable at all sites except laboratory B, where larger zones were reported (see Results and Discussion). The results of these disk diffusion quality control investigations were analyzed by using methods noted in NCCLS guideline M23-T3 (10) and described earlier by Gavan et al. (2).
RESULTS AND DISCUSSION
In Table 1, the activity of gatifloxacin is compared to those of 12 other antimicrobial agents. Gatifloxacin (MIC50 and MIC90, ≤0.03 μg/ml) was very active against H. influenzae and M. catarrhalis. The highest MICs recorded for gatifloxacin were 0.25 and 1 μg/ml. A total of 36.2% of the H. influenzae strains were resistant to amoxicillin-ampicillin, and 20.3% were refractory to cefaclor (MIC90, 32 μg/ml). Like gatifloxacin, the other tested fluoroquinolones demonstrated potent activity against the H. influenzae isolates from the United States and Canada. The M. catarrhalis strains were also quite susceptible to the fluoroquinolones, orally administered cephalosporins (cefaclor, cefuroxime, and cefixime), macrolides, tetracycline, trimethoprim-sulfamethoxazole, and the β-lactamase inhibitor combination. Only amoxicillin tested alone was less active, due to destruction by β-lactamase (resistance) in nearly 91% of the isolates.
TABLE 1.
Activity of gatifloxacin compared to those of 12 other orally administered antimicrobial agents against 1,400 strains of H. influenzae and 600 strains of M. catarrhalis (SENTRY Antimicrobial Surveillance Program, North America, 1997)
| Organism (no. of strains tested) | Antimicrobial agent | MIC (μg/ml)
|
% of strains inhibited (breakpoint [μg/ml]a) | ||
|---|---|---|---|---|---|
| MIC50 | MIC90 | Range | |||
| H. influenzae (1,400) | Amoxicillin | 1 | >8 | ≤0.06–>8 | 63.8 (≤2) |
| Amoxicillin-clavulanate (2:1) | 0.5 | 2 | ≤0.06–>8 | 99.4 (≤4) | |
| Cefaclor | 4 | 32 | ≤0.25–>32 | 79.7 (≤8) | |
| Cefuroxime | 0.5 | 4 | ≤0.06–>8 | 95.9 (≤4) | |
| Cefixime | ≤0.03 | 0.12 | ≤0.03–>4 | 99.9 (≤1) | |
| Clarithromycin | 8 | 16 | ≤0.25–>32 | 62.2 (≤8) | |
| Tetracycline | ≤2 | ≤2 | ≤2–>16 | 98.6 (≤2) | |
| Trimethoprim-sulfamethoxazole (1:19) | ≤0.25 | 8 | ≤0.25–>8 | 76.8 (≤0.5) | |
| Gatifloxacin | ≤0.03 | ≤0.03 | ≤0.03–0.25 | 100.0 (≤2) | |
| Ciprofloxacin | ≤0.016 | ≤0.016 | ≤0.016–0.25 | 100.0 (≤1) | |
| Levofloxacin | ≤0.5 | ≤0.5 | ≤0.5–1 | 100.0 (≤2) | |
| Sparfloxacin | ≤0.12 | ≤0.12 | ≤0.12–0.25 | 100.0 (≤1) | |
| Trovafloxacin | ≤0.03 | ≤0.03 | ≤0.03–0.25 | 100.0 (≤1) | |
| M. catarrhalis (600) | Amoxicillin | 4 | 8 | ≤0.06–>8 | 8.9 (≤0.12) |
| Amoxicillin-clavulanate (2:1) | 0.25 | 0.25 | ≤0.06–8 | 99.8 (≤4) | |
| Cefaclor | 1 | 2 | ≤0.25–32 | 99.7 (≤8) | |
| Cefuroxime | 1 | 2 | 0.12–8 | 99.0 (≤4) | |
| Cefixime | 0.25 | 0.5 | ≤0.03–2 | 99.5 (≤1) | |
| Clarithromycin | ≤0.25 | ≤0.25 | ≤0.25–16 | 99.5 (≤2) | |
| Tetracycline | ≤2 | ≤2 | ≤2 | 100.0 (≤4) | |
| Trimethoprim-sulfamethoxazole (1:19) | ≤0.25 | 0.5 | ≤0.25–>8 | 98.3 (≤2) | |
| Gatifloxacin | ≤0.03 | ≤0.03 | ≤0.03–1 | 100.0 (≤2) | |
| Ciprofloxacin | 0.03 | 0.03 | ≤0.016–1 | 100.0 (≤1) | |
| Levofloxacin | ≤0.5 | ≤0.5 | ≤0.5–2 | 100.0 (≤2) | |
| Sparfloxacin | ≤0.12 | ≤0.12 | ≤0.12–0.5 | 100.0 (≤1) | |
| Trovafloxacin | ≤0.03 | ≤0.03 | ≤0.03–0.5 | 100.0 (≤1) | |
MIC breakpoint for susceptibility; NCCLS criteria when available (9).
Table 2 summarizes the activity of gatifloxacin compared to that of levofloxacin against 300 recent clinical isolates of H. influenzae selected for in vitro test development. Gatifloxacin broth microdilution and E-test MICs are compared to those of levofloxacin (broth microdilution test only), subcategorized by organism β-lactamase production. No relevant difference in activity between the β-lactamase-producing and nonproducing isolates was observed for either fluoroquinolone (identical MIC50 and MIC90 results). Gatifloxacin (MIC50, 0.008 μg/ml) was slightly more active than levofloxacin (MIC50, 0.016 μg/ml); however, the MIC90s were identical (0.016 μg/ml).
TABLE 2.
Activities of gatifloxacin and a control fluoroquinolone (levofloxacin) tested against 300 recent clinical isolates of H. influenzae
| β-Lactamase activity (no. of isolates tested) | Antimicrobial agent | Method used | MIC (μg/ml)
|
||
|---|---|---|---|---|---|
| Range | MIC50 | MIC90 | |||
| Negative (190) | Gatifloxacin | BMDa | 0.004–0.06 | 0.008 | 0.016 |
| E-test | 0.004–0.125 | 0.016 | 0.023 | ||
| Levofloxacin | BMD | 0.008–0.125 | 0.016 | 0.016 | |
| Positive (110) | Gatifloxacin | BMD | 0.004–0.03 | 0.008 | 0.016 |
| E-test | 0.006–0.125 | 0.016 | 0.023 | ||
| Levofloxacin | BMD | 0.008–0.125 | 0.016 | 0.016 | |
BMD, broth microdilution reference test (8).
The comparison of the gatifloxacin E-test and broth microdilution MIC results is more clearly visualized in Table 3, which lists E-test variations by log2 dilution steps. There was a tendency toward higher E-test values when gatifloxacin was tested against H. influenzae. Only 1.0% of E-test MICs were lower (0.5 log2 dilution) than the broth microdilution results. However, the E-test MICs tended to be only slightly higher, by 0.5 to 1 log2 dilution, and the essential agreement was acceptable at 92.7%.
TABLE 3.
Comparison of E-test and broth microdilution results for gatifloxacin tested against 300 recent isolates of H. influenzaea
| Variation in log2 dilution steps | No. of isolates |
|---|---|
| −0.5 | 3 |
| 0b | 61 |
| 0.5 | 133 |
| 1.0 | 81 |
| 1.5 | 19 |
| 2.0 | 3 |
The correlation coefficient (r) for these tests was 0.71.
Ideal correlation of no variation.
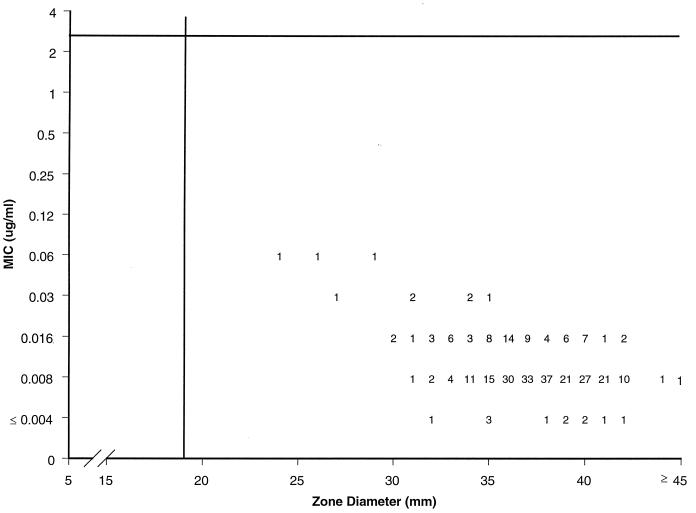
All 300 H. influenzae isolates for which the results are summarized in Tables 2 and 3 were also tested by using 5-μg gatifloxacin disks. The resulting zone diameters were then compared to broth microdilution MICs by using regression analysis and scattergram plots (Fig. 1). The single-cluster character of these organisms resulted in a low correlation coefficient (r = 0.44), and a proposed susceptible-only breakpoint appears to be appropriate. A gatifloxacin-susceptible MIC breakpoint of ≤2 μg/ml and a zone diameter of ≥18 mm, proposed previously for other species, would correctly categorize all tested H. influenzae strains as susceptible to this new fluoroquinolone (5). No interpretive error was observed.
FIG. 1.
Scattergram comparing broth microdilution MICs (HTM broth) and 5-μg disk zone diameters for gatifloxacin tested against 300 recent clinical isolates of H. influenzae (SENTRY Antimicrobial Surveillance Program, North America, 1997). Regression equation, y = −0.09x + 5.6 (r = 0.44).
Gatifloxacin broth microdilution MIC quality control results for H. influenzae ATCC 49247 are shown in Table 4. Nine laboratories each contributed 50 replicate MIC results for a combined total of 450 MIC data points for this control strain. All but two laboratories produced MICs that were either 0.008 or 0.016 μg/ml. Only five results (1.1%) were outside this range. The proposed MIC range of 0.004 to 0.03 μg/ml (4 log2 dilutions) for gatifloxacin and H. influenzae ATCC 49247 would contain all of the results generated in this trial and was suggested because of the approximately equal number of MIC occurrences found at 0.008 and 0.016 μg/ml.
TABLE 4.
Gatifloxacin MIC quality control results for H. influenzae ATCC 49247 from a nine-laboratory trial (10)a
| Gatiflox-acin MIC (μg/ml) | No. of occurrences for laboratory:
|
Total no. of occur-rences | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | ||
| 0.004 | 0 | 3 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 5b |
| 0.008 | 8 | 47 | 45 | 0 | 40 | 44 | 23 | 24 | 13 | 244b |
| 0.016 | 42 | 0 | 3 | 50 | 10 | 6 | 27 | 26 | 37 | 201b |
| 0.03 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0b |
| Total | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 450 |
Each laboratory used five HTM medium lots over 10 trial days; the total number of MICs determined was 50.
The proposed MIC range (0.004 to 0.03 μg/ml) contained all results generated in the trial.
The disk diffusion quality control results for gatifloxacin and H. influenzae ATCC 49247 are shown in Table 5. Again, nine laboratories were evaluated, using 5-μg gatifloxacin disks and 60 replicates each, for a total of 540 results. The numbers of occurrences at each zone diameter were compared site by site and showed minimal variation for eight of the nine participants. Laboratory B had a series of larger zone diameters than the other participants, and for purposes of control range calculation, this laboratory’s results were omitted. Using the median zone diameter from the combined results (37 mm), a range of 3 mm on either side of this zone (34 to 40 mm) (Table 5) would encompass the median and modal values from each of the qualifying individual sites. This proposed quality control range of 34 to 40 mm contains slightly fewer than 95% of all reported zone diameters (excluding laboratory B) and was identical to the range calculated by using the statistical methods of the NCCLS (10) and Gavan et al. (2). By expanding this range by 1 mm on each extreme, the number of generated results within the control limits would increase to 99.0%, thus achieving ≥95% of results within the proposed ranges, which is listed as a criterion by the NCCLS (10).
TABLE 5.
Gatifloxacin disk diffusion quality control result for H. influenzae ATCC 49247 from a nine-laboratory trial (10)
| Gatifloxacin 5-μg disk zone diameter (mm) | No. of occurrences for laboratory:
|
Total no. of occur-rencesa | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | Bb | C | D | E | F | G | H | I | ||
| 32 | 1 | 1 | ||||||||
| 33a | 2 | 6 | 2 | 10 | ||||||
| 34a | 12 | 8 | 4 | 1 | 25 | |||||
| 35a | 1 | 26 | 12 | 3 | 2 | 6 | 14 | 64 | ||
| 36a | 4 | 10 | 13 | 16 | 8 | 13 | 16 | 17 | 97 | |
| 37a | 5 | 23 | 7 | 11 | 8 | 16 | 9 | 26 | 105 | |
| 38a | 4 | 14 | 6 | 10 | 24 | 11 | 2 | 71 | ||
| 39a | 18 | 12 | 12 | 11 | 5 | 8 | 66 (54) | |||
| 40a | 17 | 17 | 20 | 2 | 56 (39) | |||||
| 41a | 9 | 13 | 1 | 23 (10) | ||||||
| 42 | 3 | 11 | 1 | 15 (4) | ||||||
| 43 | 3 | 3 (0) | ||||||||
| 44 | 4 | 4 (0) | ||||||||
| Total | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 60 | 540 (480) |
The proposed quality control range calculated as the median ± 0.5 × the average zone range = 37 ± 3 mm, or 34 to 40 mm (10). This range contains only 94.8% of the generated zone diameters from qualifying sites and was expanded to 33 to 41 mm. Numbers in parentheses are the numbers of occurrences after deletion of results from laboratory B.
Results from laboratory B results were omitted because this site’s median zone diameter varied significantly from those of the other participants.
These results demonstrate a potent activity for gatifloxacin when tested against contemporary isolates of H. influenzae and M. catarrhalis. The MIC90s of gatifloxacin reported here for the E-test (0.023 μg/ml) and reference broth microdilution test (0.016 μg/ml) with HTM correspond to the results reported for 274 strains (MIC90 range, 0.013 to 0.016 μg/ml) isolated in Germany, Japan, and the United Kingdom (1, 3, 12, 13). All earlier studies failed to utilize the HTM reference/standardized method recommended by the NCCLS (7, 8), making these data unique. However, the results from all prior media utilized for gatifloxacin testing appear to be uniform and similar to those produced with HTM.
The suggested susceptibility breakpoint for gatifloxacin (≤2 μg/ml or ≥18 mm) for testing H. influenzae by NCCLS methods conforms to those criteria proposed for rapidly growing nonfastidious species and streptococci (5, 7, 8). Furthermore, peak levels of gatifloxacin in serum at 3.35 to 5.41 μg/ml (half-life, 7 to 8 h) can be achieved with single oral doses of 400 and 600 mg (6).
Quality control guidelines for the NCCLS-recommended H. influenzae ATCC 49247 control strain were easily established via a nine-laboratory study, which surpasses by two sites the number of laboratories required by the NCCLS for such studies (7, 8, 10). The proposed ranges of 0.004 to 0.03 μg/ml and 33 to 41 mm contained 99.0 to 100.0% of the results reported by the study participants. These proposals await final action by the NCCLS for addition into supplemental tables for routine clinical laboratory use (9) and by the Food and Drug Administration for placement in the product package insert.
ACKNOWLEDGMENTS
We express our appreciation of all of the participating technologists in the control trials and to Kay Meyer for her excellent support in manuscript preparation.
These investigations were funded by an educational/research grant from Bristol-Myers Squibb.
ADDENDUM IN PROOF
Since the acceptance of this publication, the NCCLS has accepted the proposed quality control guidelines for gatifloxacin.
REFERENCES
- 1.Bauernfeind A. Comparison of the antibacterial activities of the quinolones BAY 12-8039, gatifloxacin (AM-1155), trovafloxacin, clinafloxacin, levofloxacin, and ciprofloxacin. J Antimicrob Chemother. 1997;40:639–651. doi: 10.1093/jac/40.5.639. [DOI] [PubMed] [Google Scholar]
- 2.Gavan T L, Jones R N, Barry A L, Fuchs P C, Gerlach E H, Matsen J M, Reller L B, Thornsberry C, Thrupp L D. Quality control limits for ampicillin, carbenicillin, mezlocillin, and piperacillin disk diffusion susceptibility tests: a collaborative study. J Clin Microbiol. 1981;14:67–72. doi: 10.1128/jcm.14.1.67-72.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hosaka M, Kinoshita S, Toyama A, Otsuki M, Nishino T. Antibacterial properties of AM-1155, a new 8-methoxy quinolone. J Antimicrob Chemother. 1995;36:293–301. doi: 10.1093/jac/36.2.293. [DOI] [PubMed] [Google Scholar]
- 4.Ishida K, Kaku M, Irifune K, Mizukane R, Takemura H, Yoshida R, Tanaka H, Usui T, Tomono K, Suyama N. In vitro and in vivo activity of a new quinolone AM-1155 against Mycoplasma pneumoniae. J Antimicrob Chemother. 1994;34:875–883. doi: 10.1093/jac/34.6.875. [DOI] [PubMed] [Google Scholar]
- 5.Jones, R. N., K. C. Kugler, M. E. Erwin, D. J. Biedenbach, M. A. Pfaller, and The Quality Control Study Group. Gatifloxacin (AM-1155, CG5501) susceptibility testing interpretive criteria and quality control guidelines for dilution and disk (5-μg) diffusion methods. Diagn. Microbiol. Infect. Dis., in press. [DOI] [PubMed]
- 6.Nakashima M, Uematsu T, Kosuge K, Kusajima H, Dori T, Masuda Y, Ishida R, Uchida H. Single- and multiple-dose pharmacokinetics of AM-1155, a new 6-fluoro-8-methoxy quinolone, in humans. Antimicrob Agents Chemother. 1995;39:2635–2640. doi: 10.1128/aac.39.12.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial disk susceptibility tests, 6th ed. Approved standard M2-A6. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 8.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standard M7-A4. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing. Supplemental table M100-S8. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 10.National Committee for Clinical Laboratory Standards. Development of in vitro susceptibility testing criteria and quality control parameters. Tentative guideline M23-T3. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1998. [Google Scholar]
- 11.Soejima R, Miyashita N, Nakabayashi M, Kimura M, Hashiguchi K, Kishimoto T. In vitro activities of AM-1155 against Chlamydia spp. and M. avium complex. Drugs. 1993;45:181. [Google Scholar]
- 12.Wakahayashi E, Mitsuhashi S. In vitro antibacterial activity of AM-1155, a novel 6-fluoro-8-methoxy quinolone. Antimicrob Agents Chemother. 1994;38:594–601. doi: 10.1128/aac.38.3.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wise R, Brenwald N P, Andres J M, Boswell F. The activity of the methylpiperazinyl fluoroquinolone CG 5501: a comparison with other fluoroquinolones. J Antimicrob Chemother. 1997;39:447–452. doi: 10.1093/jac/39.4.447. [DOI] [PubMed] [Google Scholar]