In the early phases of the COVID-19 pandemic, amid shortages for essential medicines, reagents, and equipment, the European Commission (EC) struggled to develop a coordinated and comprehensive European response [1,2]. The EC now seeks to strengthen its role in preparedness planning and response through, among other measures, the establishment of the European Union (EU) Health Emergency Preparedness and Response Authority (HERA) [3]. While the remit and scope of HERA is yet to be formally agreed, it is envisaged to play a similar role to the US Biomedical Advanced Research and Development Authority (BARDA) and be responsible for:[4]
-
•
horizon scanning of major threats to health and potential medical countermeasures,
-
•
funding research and development,
-
•
supporting manufacturing capacity, and
-
•
stockpiling of essential medical supplies, and equipment.
The proposed focus is broad, and HERA will be expected to operate among a multiplicity of agents already responsible for preparedness planning and response in Europe. To stimulate debate and discussion, we consider potential functions of HERA and how they may complement the roles and responsibilities of pre-existing EC agencies and institutions.
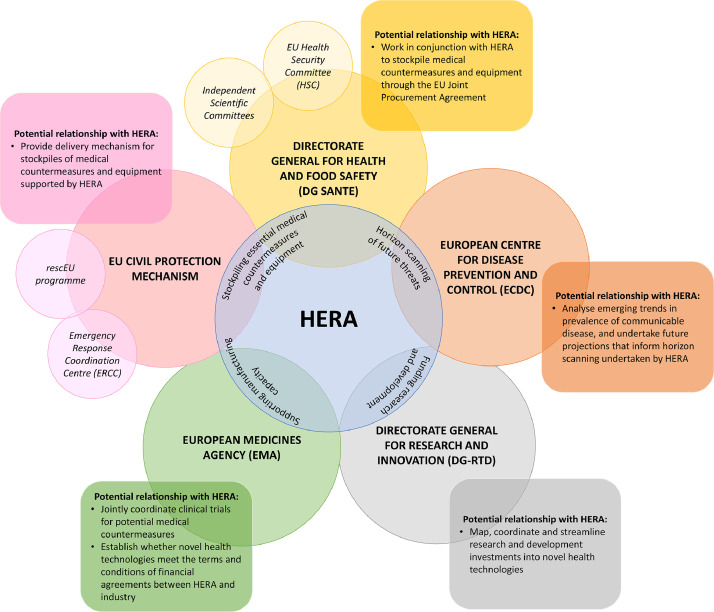
To understand how HERA could position itself in an emerging and increasingly complex European landscape for preparedness planning and response (Table 1), it is important to outline which key players are already involved. The Directorate-General for Health and Food Safety (DG-SANTE), is the EC department responsible for EU policy on health, and hosts the Health Security Committee (HSC), and independent scientific committees, that provide forums to coordinate and share best practice between member states and non-member observer states for preparedness and response activities. DG-SANTE has also played a key role in utilising the EU Joint Procurement Agreement (JPA) to negotiate contracts for the procurement of vaccines, medicines, and protective equipment throughout the COVID-19 pandemic [5]. The Directorate-General for Research and Innovation (DG-RTD) is responsible for allocating and coordinating EU research and development investments for medical countermeasures, and diagnostics through programmes such as Horizon 2020, which allocated €469 million of funding to a portfolio of 105 research projects relevant to COVID-19 in 2020 [6]. Whereas, the European Centre for Disease Prevention and Control (ECDC) (https://www.ecdc.europa.eu/en/about-ecdc) is the agency responsible for communicable disease surveillance, provision of scientific advice on communicable disease epidemiology, prevention and control, and training of public health professionals. The European Medicines Agency (EMA) (https://www.ema.europa.eu/en/about-us) is responsible for assessing available evidence on the safety and effectiveness of novel health technologies including vaccines, and medicines. However, the EMA is not responsible for assessing evidence on the safety and effectiveness of medical devices, and despite the importance of medical devices such as diagnostics in pandemic response, the EU lacks a single organisational responsible for this task. Finally, the EU Civil Protection Mechanism responds to requests for assistance from countries that experience emergencies or disasters, channelled through the Emergency Response Coordination Centre (ERCC), and carried out through the rescEU programme (https://ec.europa.eu/echo/what/civil-protection/resceu_en), which deploys a pool of assets such as aircraft, medical equipment, and field hospitals. While the EU Civil Protection Mechanism was originally envisaged as predominantly responding to environmental disasters such as forest fires or flash flooding, it has played a key role in coordinating the delivery of medical countermeasures and equipment to countries in need during the COVID-19 pandemic.
Table 1.
Pre-existing European Union (EU) agencies and institutions with responsibilities for preparedness and planning
| Agency/Institution | Current roles and responsibilities |
|---|---|
| Directorate-General for Health and Food Safety (DG-SANTE) |
|
| The Directorate-General for Research and Innovation (DG-RTD) |
|
| European Centre for Disease Prevention and Control (ECDC) |
|
| European Medicines Agency (EMA) |
|
| EU Civil Protection Mechanism (including the Emergency Response Coordination Centre and rescEU programme) |
|
Looking ahead to the next major threat to health, HERA will be expected to undertake both short-term and long-term horizon scanning to guide policy. This will be a challenging task, involving predicting where, when and how communicable disease threats will re-emerge over the next decades and considering how to exploit genomic “big data” spanning entire viral families to transform future medical countermeasure development, enabling quicker identification and response to future outbreaks. Beyond pandemics, the impeding threats of climate change, and antimicrobial resistance will also guide thinking over the next decades. Increasingly, these issues are acknowledged as interlinked, with environmental disruption driving zoonotic events that have the potential to cause pandemics [7], and climate change impacting the proliferation and dissemination of antimicrobial resistance [8]. To fully understand future and impeding threats to health, HERA will be required to take a One-Health approach [9], and foster closer collaboration across human, animal, and environmental health disciplines and international organisations including the World Health Organisation (WHO), the World Organisation for Animal Health (OIE), and the Food and Agriculture Organization (FAO). To understand emerging trends in prevalence of communicable disease, HERA will need to closely collaborate with the ECDC, and other centres for disease control and prevention (CDC) across the globe. HERA may also choose to draw upon and develop platforms such as the EU Early Warning and Response System (EWRS) (https://www.ecdc.europa.eu/en/publications-data/early-warning-and-response-system-european-union-ewrs)/), a rapid alert system that allows public health authorities to share data on serious cross-border threats to health. To identify potential pathogens of interest which may become responsible for future pandemics, HERA will need to collaborate with international initiatives such as the Global Virome Project (GVP) (https://www.globalviromeproject.org/our-approach), and repositories of genomic sequencing data such as the Global Initiative on Sharing Avian Influenza Data (GISAID) (https://www.gisaid.org).
A major task for HERA will be to develop and coordinate a consistent and strategic European approach to funding research and development that stimulates innovation and supports manufacturing capacity for novel and diverse health technologies, including vaccines, medicines, and diagnostics. A first step will be mapping, coordinating, and streamlining the many disparate funding programmes across Europe with similar objectives, and HERA may choose to collaborate with the DG-RTD for this task. This approach will need to be adaptable to future threats such as another pandemic or current threats such as COVID-19 and antimicrobial resistance. BARDA has demonstrated this versatility by supporting a variety of initiatives, including the CARB-X programme (https://carb-x.org/about/overview/), which focuses on stimulating innovation into novel antibiotics and diagnostics. HERA can build upon the HERA incubator, to develop vaccines against new variants of COVID-19 by funding research projects, supporting manufacturing capacity, and speeding up regulatory approval [10].
However, HERA will face significant challenges in achieving these objectives. First, HERA will need to work in conjunction with the EMA to develop infrastructure that can coordinate mid- to large-size clinical trials for potential medical countermeasures across Europe and beyond. HERA has begun this work by launching the VACCELERATE clinical trials network (https://vaccelerate.eu/), that involves 16 EU member states and 5 associated countries. The EMA has also been given a strengthened mandate to coordinate multi-national clinical trials during public health crises by the EC through the establishment of an Emergency Task Force that will provide scientific advice on clinical trial design and product development [11]. From the offset, HERA and the EMA will need to forge close relationships with academic institutions across Europe, and leverage pre-existing networks such as the European Global Health Research Institutes Network (https://eghrin.eu/) for this purpose. VACCELERATE can also learn from the UK's RECOVERY trial, where trialists focused on simplifying processes such as recruitment procedures, informed consent, and data collection, to reduce bureaucracy and accelerate development [12]. Second, is whether HERA will be allocated sufficient resources to support research and development. The annual budget of HERA is yet to be decided, although it is assumed its budget will be from the EU4Health programme, which has been allocated €5.3 billion of funding between 2021 and 2027 [13]. While this is over a ten-fold increase on the €450 million the EU spent on health priorities in the previous seven years [14], there will be multiple competing demands for funding. Therefore, the annual budget of HERA is likely to be substantially below the annual budget for BARDA ($1.4 billion/ €1.18 billion in FY 2021) [15]. Third, it is unclear how HERA can target investments in a manner that stimulates innovation across the development pathway. So far, the EC has indicated the HERA incubator will utilise advance purchase agreements, a form of pull incentives, to encourage investment in manufacturing capacity for vaccine candidates already in clinical trial stages [10]. However to incentivise activity across the development pathway, HERA may wish to utilise a combination of push and pull incentives, that fund both basic science research and clinical trial phases. There are several such proposed financial mechanisms [16], including the options market for vaccines [17], and antibiotics [18], which encourage funders to invest at different stages of the development pathway and, in return, secure rights to purchase successful vaccine or antibiotic candidates at discounted prices. This, in effect, provides a mechanism for public and private funders to share the risk of investing in novel health technologies. Once financial agreements are in place, HERA will need to closely collaborate with the EMA to ensure available evidence on the safety and effectiveness of novel health technologies is reviewed rapidly to facilitate timely market access and to establish whether they meet the terms and conditions of milestone payments. Finally, HERA will need to consider carefully how it can maximise manufacturing capacity in Europe and globally. One strategy is to encourage the use of flexible technology transfer agreements between pharmaceutical and medical device companies that involve transferring technical knowledge on how to manufacture effective health technologies to maximise the use of available capacity [19]. Another is to support investment in expanding manufacturing capacity, although HERA will need to decide to what degree it will prioritise strengthening manufacturing capacity in Europe or internationally, including in low and middle-income countries (LMICs). The latter would have the dual benefits of producing health technologies at low-cost and improving access in countries that typically experience unmet need. HERA may wish to recommend that the Directorate-General for International Partnerships (DG INTPA) supports investment in manufacturing capacity in Africa, particularly as India and China are already well-developed manufacturing hubs.
It is also expected that HERA will be responsible for maintaining a stockpile of essential medical countermeasures and equipment ready to deploy against future major threats to health. This will require mapping and monitoring of pre-existing resources among member states, and modelling future requirements under alternative scenarios or threats. Throughout the pandemic, DG-SANTE has leveraged the joint procurement agreement between member states to purchase protective equipment, medicines, and vaccines [5]. The technical and legal expertise utilised to negotiate these contracts will be an essential asset for HERA to draw upon when building this stockpile. Mapping and monitoring pre-existing resources across Europe will also require providing recommendations on which stockpiles should be maintained by member states independently and by HERA centrally. Once the stockpile is developed, HERA will need a delivery mechanism to ensure it is deployed rapidly when countries are in need. The EU Civil Protection Mechanism, discussed above, including the ERCC and rescEU programme, is readily available for this purpose. Although, a framework needs to be agreed that outlines specific roles and responsibilities in relation to stockpiling and delivery between HERA, ERCC, and the rescEU programme, particularly as there is a risk of overlap in functions, since rescEU has pre-existing responsibilities for stockpiling.
As well as navigating relationships within Europe, HERA will be expected to have a global focus that is aligned with the EU commitment to ensure fair and equitable access to medical countermeasures in LMICs [20]. Despite being established to respond to US national security threats such as bioterrorism, BARDA has repeatedly demonstrated its willingness to take a global focus through programmes related to threats such as Ebola virus [21], and Zika virus [22]. Doing something similar with HERA will require working with international partners and funders such as the World Health Organisation (WHO), Gavi, and the Coalition for Epidemic Preparedness Innovations (CEPI), and contributing to initiatives such as the WHO Global Observatory on Health Research and Development (https://www.who.int/observatories/global-observatory-on-health-research-and-development), and COVID-19 Vaccines Global Access (COVAX) scheme (https://www.gavi.org/covax-facility). HERA will also have to establish working relationships with national initiatives and agencies outside of the EU that may have similar goals, such as BARDA in the US, the Health Security Agency (HSC) in the UK and the Africa CDC. It will be important to coordinate several activities internationally, including surveillance of communicable disease, identifying synergies in research and development investments, stockpiling medical countermeasures and equipment, and subsequent distribution mechanisms.
The establishment of HERA is a welcome development that has the potential to strengthen preparedness and resilience in Europe against major threats to health. Moreover, it represents a fundamental shift in the approach of the EC to member states’ health systems, an area where the EC was previously reluctant to be involved [23,24]. HERA can capitalise upon by this by issuing policy guidance to member states on required standards for preparedness planning and response, and providing an overarching coordination mechanism for the activities of pre-existing EC agencies and institutions related to health emergency preparedness planning and response (Fig. 1). While the EC itself will inevitably continue to take responsibility for coordination through DG-SANTE, the HSC, and independent scientific committees, the development of HERA could enable a more coordinated and comprehensive European response to future major threats to health. However, the early legitimacy and success of HERA will be very dependent upon its allocated funding and how clearly its stated objectives and relationship with pre-existing EC agencies and institutions are defined.
Fig. 1.
The role of HERA in coordinating European responses to major threats to health. HERA: Health Emergency Preparedness and Response Authority. Four proposed functions of HERA are outlined in the blue circle. The potential relationship between pre-existing EU agencies and institutions are outlined within squares with rounded edges.
Author contributions
EM and MA designed the study. MA produced the first draft of this manuscript. All authors critically revised the manuscript for important intellectual content.
Declaration of interests
The authors of this manuscript have no conflicts of interest to declare.
References
- 1.Anderson M, Mckee M, Mossialos E. Covid-19 exposes weaknesses in European response to outbreaks. BMJ. 2020;368 doi: 10.1136/bmj.m1075. [DOI] [PubMed] [Google Scholar]
- 2.Forman R, Atun R, McKee M, Mossialos E. 12 Lessons learned from the management of the coronavirus pandemic. Health Policy. 2020;124:577–580. doi: 10.1016/j.healthpol.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villa S, Leeuwen R van, Gray CC. HERA: a new era for health emergency preparedness in Europe? Lancet North Am Ed. 2021;397:2145–2147. doi: 10.1016/S0140-6736(21)01107-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.European Commission . 2020. Building a European Health Union: Reinforcing the EU's resilience for cross-border health threats.https://ec.europa.eu/info/sites/default/files/communication-european-health-union-resilience_en.pdf (accessed July 16, 2021) [Google Scholar]
- 5.Mcevoy E, Ferri D. The role of the Joint Procurement Agreement during the COVID-19 Pandemic: Assessing Its usefulness and discussing its potential to support a European Health Union. European Journal of Risk Regulation. 2020;11:851–863. [Google Scholar]
- 6.European Commission . 2021. EU research and innovation in action against the coronavirus: funding, results and impact.https://ec.europa.eu/info/sites/default/files/research_and_innovation/research_by_area/documents/ec_rtd_eu-research-innovation-against-covid.pdf (accessed July 30, 2021) [Google Scholar]
- 7.Waugh C, Lam SS, Sonne C. One Health or Planetary Health for pandemic prevention? Lancet North Am Ed. 2020;396:1882. doi: 10.1016/S0140-6736(20)32387-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burnham JP. Climate change and antibiotic resistance: a deadly combination. Therapeutic Advances in Infection. 2021;8 doi: 10.1177/2049936121991374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Renwick MJ, Simpkin V, Mossialos E. Targeting innovation in antibiotic drug discovery and development: The need for a One Health – One Europe – One World Framework. European Observatory on Health Systems and Policies; Copenhagen (Denmark): 2016. Targeting innovation in antibiotic drug discovery and development: The need for a One Health – One Europe – One World Framework.http://www.ncbi.nlm.nih.gov/books/NBK447337/ (accessed July 16, 2021) [PubMed] [Google Scholar]
- 10.European Commission . 2021. Questions and Answers: HERA Incubator - Anticipating together the threat of COVID-19 variants.https://ec.europa.eu/commission/presscorner/detail/en/qanda_21_642 (accessed July 16, 2021) [Google Scholar]
- 11.European Commission . 2020. Proposal for a regulation of the European parliament and of the council on a reinforced role for the European Medicines Agency in crisis preparedness and management for medicinal products and medical devices.https://ec.europa.eu/transparency/documents-register/detail?ref=COM(2020)725&lang=en (accessed July 30, 2021) [Google Scholar]
- 12.Mather N. How we accelerated clinical trials in the age of coronavirus. Nature. 2020;584:326. doi: 10.1038/d41586-020-02416-z. [DOI] [PubMed] [Google Scholar]
- 13.EU4Health 2021-2027 – a vision for a healthier European Union. https://ec.europa.eu/health/funding/eu4health_en (accessed July 16, 2021).
- 14.European Commission. The Third Health Programme 2014-2020 Funding Health Initiatives. https://ec.europa.eu/chafea/health/programme/documents/factsheet-hp_en.pdf (accessed July 16, 2021).
- 15.Office of the Assistant Secretary for Preparedness a nd Response . 2021. Fiscal Year 2021 Budget-In-Brief.https://www.phe.gov/about/aspr/Pages/aspr-fy2021-bib.aspx (accessed July 16, 2021) [Google Scholar]
- 16.Forman R, Shah S, Jeurissen P, Jit M, Mossialos E. COVID-19 vaccine challenges: What have we learned so far and what remains to be done? Health Policy. 2021;125:553–567. doi: 10.1016/j.healthpol.2021.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forman R, Anderson M, Jit M, Mossialos E. Ensuring access and affordability through COVID-19 vaccine research and development investments: A proposal for the options market for vaccines. Vaccine. 2020;38:6075–6077. doi: 10.1016/j.vaccine.2020.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brogan DM, Mossialos E. Systems, not pills: The options market for antibiotics seeks to rejuvenate the antibiotic pipeline. Soc Sci Med. 2016;151:167–172. doi: 10.1016/j.socscimed.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 19.O'Sullivan C, Rutten P, Schatz C. 2020. Why tech transfer may be critical to beating COVID-19.https://www.mckinsey.com/industries/pharmaceuticals-and-medical-products/our-insights/why-tech-transfer-may-be-critical-to-beating-covid-19 (accessed July 16, 2021) [Google Scholar]
- 20.European Commission. International Partnerships: Good health and well-being. https://ec.europa.eu/international-partnerships/sdg/good-health-and-well-being_en (accessed July 16, 2021).
- 21.Espeland EM, Tsai C-W, Larsen J, Disbrow GL. Safeguarding against Ebola: Vaccines and therapeutics to be stockpiled for future outbreaks. PLoS Negl Trop Dis. 2018;12 doi: 10.1371/journal.pntd.0006275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abbasi J. HHS Moves to Accelerate Zika Vaccine Development. JAMA. 2016;316:808. [Google Scholar]
- 23.Anderson M, Mossialos E. Time to strengthen capacity in infectious disease control at the European level. Int J Infect Dis. 2020;99:263–265. doi: 10.1016/j.ijid.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McKee M, Mossialos E, Belcher P. The Influence of European Law On National Health Policy. Journal of European Social Policy. 1996;6:263–286. [Google Scholar]