Abstract
The spreading of the Severe Acute Respiratory Syndrome Coronavirus (COVID-19) pandemic could be associated with psychiatric implications. After COVID-19, depression was reported in 40% of patients at one-, three-, and six-months follow-up. Emerging literature suggests anti-inflammatory and antiviral properties of antidepressants in the treatment of SARS-CoV-2. We aim to investigate the efficacy of Selective Serotonin Reuptake Inhibitor (SSRI) in treating post-COVID depression.
We included 60 patients affected by a major depressive episode and treated with SSRI in the six months following recovery from COVID. The severity of depression was rated at baseline and after four weeks on the Hamilton Depression Rating Scale (HDRS). Response to treatment was considered when the patients achieved a 50% HDRS reduction. To investigate changes of depressive symptomatology over time, repeated measures ANOVAs according to clinical variables were performed.
We found that 55 (92%) patients showed a clinical response to antidepressant. Patients showed a significant decrease over time of HDRS score (baseline HDRS = 23.37 ± 3.94, post-treatment HDRS = 6.71±4.41, F = 618.90, p < 0.001), irrespectively of sex, previous psychiatric history, previous history of mood disorder, and SSRI type.
This is the first study to explore the SSRI efficacy in post-COVID depression, suggesting rapid antidepressant effects in most patients. SSRIs treatment could contribute to the rapid antidepressant response by directly targeting the neuroinflammation triggered by SARS-CoV-2. We suggest screening psychopathology of COVID-19 survivors to diagnose emergent depression and pharmacologically treat it to reduce the disease burden and related years of life lived with disability.
Keywords: COVID-19, Depression, Mental health, Psychopathology, Antidepressive agents, Serotonin uptake inhibitors
1. Introduction
Since the pandemic outbreak, coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has resulted in more than 160 million confirmed cases and more than 3 million deaths. SARS-CoV-2 infection is a multi-organ disease with a broad spectrum of manifestations ranging from asymptomatic to severe or fatal forms (Wiersinga et al., 2020). Apart from acute symptomatology, increasing scientific and clinical evidences, as previously observed in the SARS and MERS outbreak (Rogers et al., 2020), highlight the long-term sequelae of COVID-19 which can affect multiple organ systems (Gupta et al., 2020). The most prevalent long-term neuropsychiatric sequelae were found to be depression, anxiety, fatigue, sleep difficulties, and cognitive impairments, warranting consensus on specific neuropsychiatric needs in the interdisciplinary management of post-acute COVID-19 patients. Clinically significant depression was reported in approximately 30–40% of patients at one-, three-, and six-months follow-up following COVID-19 (Mazza et al., 2020; Mazza et al., 2021; Taquet et al., 2021). Interestingly, at three months follow-up we observed persistent depressive symptomatology and a parallel decrease of PTSD, anxiety and insomnia suggesting specific long lasting depressive sequelae in COVID-19 survivors (Mazza et al., 2021) . Post-COVID depression was found to possibly affect survivor's cognitive performances (Mazza et al., 2020; Mazza et al., 2021), fatigue symptoms (Manning et al., 2021), and daily functioning (Talman et al., 2021), enhancing the high-burden non-communicable condition associated with psychiatric disability.
The host immune response to SARS-CoV-2 infection and the related severe systemic inflammation seems to be the main mechanism contributing to the development of post-COVID depression. Severity of post-COVID depression associated with higher systemic inflammation at one and three months follow-up (Mazza et al., 2020; Mazza et al., 2021; Yuan et al., 2020), while the treatment with cytokine-blocking agents during acute COVID-19 showed a protective effect against depression, proportional to the dampening of systemic inflammation (Benedetti et al., 2021). Of note, also in the absence of known triggering factors, abnormal immune-inflammatory setpoints are currently considered an underpinning pathogenetic mechanisms for ‘endogenous’ depressive psychopathology (Gibney and Drexhage, 2013). Emerging literature suggests anti-inflammatory and antiviral properties of several antidepressants in the treatment of SARS-CoV-2 infection mainly mediated by their action on sigma-1-receptor and acid sphingomyelinase acid/ceramide system (Gulbins et al., 2013; Hoertel et al., 2021; Lenze et al., 2020).
Despite the impressive rates of affected patients, no report is available about the efficacy of pharmacological treatment of post-COVID depression. Here we report the effects of Selective serotonin reuptake inhibitors (SSRI) treatment, in the naturalistic setting of our post-acute COVID integrated outpatient care, in a homogeneous cohort of patients affected by a major depressive episode in the first 6 months after acute COVID-19.
2. Methods
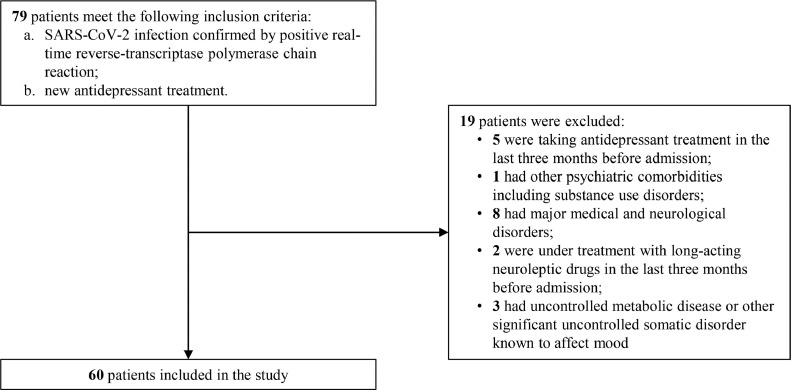
We studied 60 patients (27 males, mean age 54.82 ± 14.17), affected by a major depressive episode (DSM-5 criteria) in the 6 months following recovery from COVID-19 and clearance of SARS-CoV-2. Patients were evaluated in the context of psychiatric outpatient clinic at IRCCS San Raffaele Hospital in Milan. Inclusion criteria were SARS-CoV-2 infection confirmed by positive real-time reverse-transcriptase polymerase chain reaction; new antidepressant treatment. We excluded patients if they: were taking antidepressant treatment in the last three months before admission; had other psychiatric comorbidities including substance use disorders; were pregnant; had major medical and neurological disorders; were under treatment with long-acting neuroleptic drugs in the last three months before admission; had uncontrolled metabolic disease or other significant uncontrolled somatic disorder known to affect mood. The flowchart of sample inclusion and exclusion criteria is reported in Fig. 1 . The psychiatrist in charge of the patients (MGM, RZ, and FB) performed the clinical evaluation, administered antidepressant treatment upon clinical need, and rated the severity of the depression on the 21-items Hamilton Depression Rating Scale (HDRS). Specific SSRI molecules were selected according to our standard treatment protocols and to the NICE guidelines. Sociodemographic and clinical data were collected using a data extraction form, including age, sex, history of psychiatric disorder, history of mood disorder, need of hospitalization for COVID-19, time between COVID-19 and depression diagnosis, type of SSRI. Severity of depression according to HDRS was rated at baseline evaluation and after 4 weeks from the start of the treatment.
Fig. 1.
Flowchart of sample inclusion and exclusion criteria.
Our primary outcome was to evaluate the efficacy of antidepressant treatment in post-COVID depression. Response to treatment was considered when the patients achieved a 50% HDRS reduction after 4 weeks of treatment.
Statistical analyses to compare group means and frequencies (Student's t-test, Pearson χ2 test) exploring effects clinical and demographic variable on antidepressant response were performed. To investigate changes of depressive symptomatology over time, repeated measures ANOVAs (according to sex, history of psychiatric disorder, history of mood disorder, and SSRI type) were performed, considering HDRS scores at baseline and after four-weeks of antidepressant treatment. All the statistical analyses were performed with a commercially available software package (StatSoft Statistica 12, Tulsa, OK, USA) and following standard computational procedures in the context of the General Linear Model. After complete description of the study to the subjects, a written informed consent was obtained. The local ethical committee approved the study protocol in accordance with the principles in the Declaration of Helsinki.
3. Results
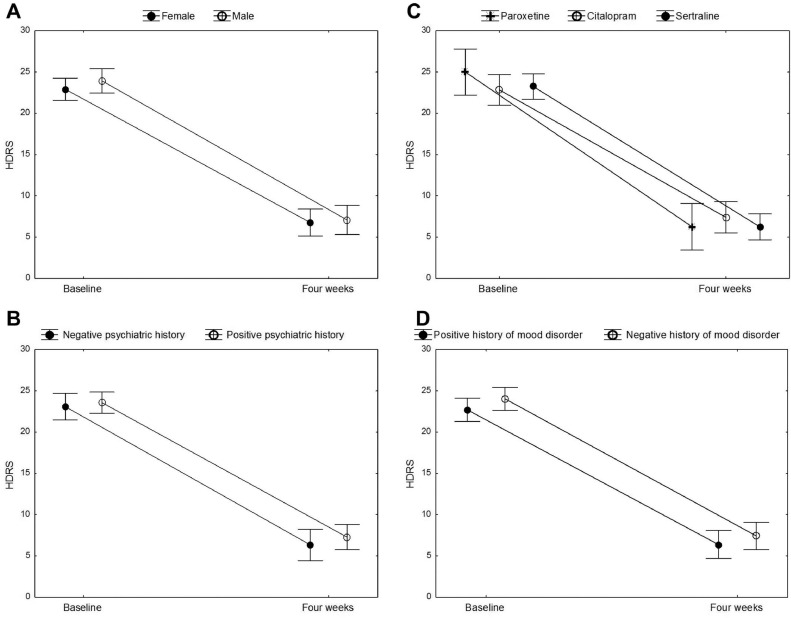
Patients were treated with sertraline (n = 26), citalopram (n = 18), paroxetine (n = 10), fluvoxamine (n = 4), and fluoxetine (n = 2) at a mean fluoxetine-equivalent dose of 30.66 ± 7.27 mg. A positive psychiatric history was found in 36 patients, 30 of them were previously diagnosed with a mood disorder (24 major depressive disorder and 6 bipolar disorder) and six with anxiety disorder (3 panic attack disorder and 3 generalized anxiety disorder). We found that 55 (92%) out of 60 patients showed a clinical response to antidepressant treatment. Clinical and demographic characteristics of the patients did not affect the response rate (Table 1 ). From the baseline to the four week follow-up, patients showed a significant decrease over time of HDRS score (baseline HDRS = 23.37 ± 3.94, post-treatment HDRS = 6.71 ± 4.41, F = 618.90, p < 0.001), irrespectively of sex (time x sex interaction: F = 0.78, p = 0.381), previous psychiatric history (time x psychiatric history interaction: F = 0.01, p = 0.917), previous history of mood disorder (interaction F = 0.26, p = 0.609), and SSRI type (interaction F = 1.18, p = 0.316) (Fig. 2 ).
Table 1.
Socio-demographic and clinical characteristics of the sample divided according to antidepressant response and levels of significance of the observed differences (Student's t test and Chi-square). HDRS, Hamilton Depression Rating Scale.
| Total (n = 60) |
HDRS reduction > 50% |
||||
|---|---|---|---|---|---|
| Yes (n 55) | No (n = 5) | t or χ2 | p-value | ||
| Age | 54.82 ± 14.17 | 54.58 ± 14.03 | 57.4 ± 17.21 | -0.42 | 0.674 |
| Sex (males - %) | 27–45% | 25–44% | 2–40% | 0.06 | 0.814 |
| Psychiatric history (positive - %) | 36–60% | 32–58% | 4–80% | 0.91 | 0.34 |
| Mood disorders history (positive - %) | 30–50% | 26–47% | 4–80% | 1.96 | 0.161 |
| Hospitalization for COVID-19 (yes - %) | 29–48% | 26–47% | 3–60% | 0.28 | 0.585 |
| Days between COVID-19 and depression diagnosis (mean±SD) | 79.25 ± 52.56 | 80.27 ± 53.93 | 64 ± 23.43 | 0.51 | 0.609 |
Fig. 2.
Changes of Hamilton Depression Rating Scale (HDRS) score over time. Points are means, whiskers are 95% confidence intervals. Patients have been divided according to sex (A), previous psychiatric history (B), administered antidepressant (C), previous history of mood disorders (D). The decrease of HDRS scores was always significant, with no significant interaction with these variables.
4. Discussion
We observed rapid antidepressant effects of SSRI treatment in the majority of patients with a post-COVID major depressive episode. In detail, the response rate was 89% and 95% in patients with or without a previous psychiatric history, respectively, thus suggesting that and recurrent episodes of major depression triggered by COVID-19 share the same good response to standard antidepressant treatment. On the contrary, common knowledge highlights that among antidepressant-treated depressed patients response rates are moderate (40–60%), and remission is achieved in a minority of patients (30–45%) with history of mood disorders (Fava, 2003).
Depression has been reported in 30–40% of COVID-19 survivors and was found to persist over time (Nalbandian et al., 2021). The pathophysiology of post-COVID neuropsychiatric sequelae mainly entails severe systemic inflammation and subsequent neuroinflammation (Troyer et al., 2020). While there is not yet compelling evidence of direct SARS-CoV-2 neuroinvasion, post mortem studies have shown that SARS-CoV-2 related systemic inflammation can induce brain parenchyma and vessels damages disrupting blood–brain (BBB) and blood–cerebrospinal fluid barriers, thus driving inflammation in central nervous system (Reichard et al., 2020). Inflammation is known to be associated to depression inducing microglia activation, neurotransmission alteration, indoleamine 2,3-dioxygenase 1 (IDO) activation and subsequent serotonin depletion, and oxidative stress (Miller and Raison, 2016) . Of note, depressed patients are characterized by higher levels of inflammatory markers such as peripheral cytokines and chemokine, acute phase protein (PCR), and inflammatory blood count ratios (Mazza et al., 2018). In this context, SARS-CoV-2 viral infections can trigger chronic inflammation causing long-lasting depressive symptomatology. Consistently, we have previously found that, one and three months after COVID-19, the severity of depression was predicted by the baseline systemic immune-inflammation index (SII) (Mazza et al., 2020; Mazza et al., 2021) . This result was confirmed by Yuan et al., who reported higher depression in convalescent COVID-19 patients with higher NLR (Yuan et al., 2020). Furthermore, we found a protective effect of the Interleukin (IL)-1β and IL-6 receptor antagonist against post-COVID depression possibly associated with their effect in dampening SII (Benedetti et al., 2021) . As such, we surmise that COVID-19 could result in prolonged systemic inflammation that could lead to the development of persistent depression.
By potentiating 5-HT neurotransmission, SSRI could directly counteract the IDO-mediated detrimental effects of inflammation in reducing 5-HT neurotransmission, also possibly modulating tryptophan metabolism and reducing levels of the excitotoxin quinolinic acid as suggested by animal models of depression-like behavior associated with inflammation (Eskelund et al., 2017). Moreover, mounting evidence suggests that antidepressant drug treatment may decrease peripheral markers of inflammation including IL-10, Tumor Necrosis Factor(TNF)-α, CC Chemokine Ligand-2, IL-6, notably associated with COVID-19 severity (Köhler et al., 2018). Antidepressants seem to be potentially useful in treatment of COVID-19 reducing the risk of intubation or death independently of patient characteristics, clinical and biological markers of disease severity, and other psychotropic medications (Hoertel et al., 2021; Lenze et al., 2020). SSRI and non-SSRI antidepressants may inhibit the sphingomyelinase acid/ceramide system (Gulbins et al., 2013), thus preventing the infection of epithelial cells with SARS-CoV-2 (Carpinteiro et al., 2020) and reducing the risk of intubation or death in individuals hospitalized for severe COVID-19 (Hoertel et al., 2021). Fluvoxamine, by stimulating the σ-1 receptor, reduce the systemic inflammation and may prevent the cytokine storm observed in severe COVID-19 thus improving patients prognosis (Lenze et al., 2020). Preclinical studies also suggest that fluoxetine and fluvoxamine may exert direct antiviral effects on SARS-CoV-2 maybe via its lysosomotropic properties (Zimniak et al., 2021). SSRI are also involved in the inhibition of platelets activation (Schlienger and Meier, 2003).
All these mechanisms could contribute to the rapid antidepressant benefit from SSRIs treatment observed in our cohort, by directly targeting the neuro-inflammation triggered by SARS-CoV-2 associated with post-COVID depression, and its detrimental effects on serotonergic neurotransmission.
Our results should be taken in the context of the following limitations. First, the observational design of the study limits the generalizable of our findings. Furthermore, the lack of a control group of antidepressant-treated depressed patients not previously affected by COVID-19, the small sample size, and the monocentric setting of the study suggest that our results should be regarded as preliminary. Multicentric larger case-control studies are needed. Finally, we were not able to assess the inflammatory status of patients during treatment, thus not being able to confirm if antidepressant response could be sustained by a reduction of the systemic inflammation.
In conclusion, given the alarming prevalence of post-COVID depression, and considering that untreated depression associates with poor outcomes, reduced antidepressant response, and increased risk of all-cause mortality, we suggest to routinely asses psychopathology of COVID-19 survivors in order to promptly diagnose emergent depression, and to pharmacologically treat it with the aim of reducing the disease burden and related years of life lived with disability.
Declaration of Competing Interest
None.
Acknowledgments
Aknowledgments
None.
Funding
None.
Contributors
FB and MGM conceived the study. MGM, RZ, and FB contributed to the inclusion of patients and acquisition of the data. MGM designed the analysis, with input from FB. MGM carried out the analysis and interpreted the data, with contributions from FB, RZ, MP, and PRQ. MGM, and RZ wrote the initial draft of the manuscript. All authors contributed to the final version, gave final approval of the version to be published and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved
References
- Benedetti F., Mazza M., Cavalli G., Ciceri F., Dagna L., Rovere-Querini P. Can cytokine blocking prevent depression in COVID-19 survivors? J. Neuroimmun. Pharmacol. 2021;16:1–3. doi: 10.1007/s11481-020-09966-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpinteiro A., Edwards M.J., Hoffmann M., Kochs G., Gripp B., Weigang S., Adams C., Carpinteiro E., Gulbins A., Keitsch S. Pharmacological inhibition of acid sphingomyelinase prevents uptake of SARS-CoV-2 by epithelial cells. J. Cell Reports Med. 2020;1 doi: 10.1016/j.xcrm.2020.100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskelund A., Li Y., Budac D.P., Muller H.K., Gulinello M., Sanchez C., Wegener G. Drugs with antidepressant properties affect tryptophan metabolites differently in rodent models with depression-like behavior. J. Neurochem. 2017;142:118–131. doi: 10.1111/jnc.14043. [DOI] [PubMed] [Google Scholar]
- Fava M. Diagnosis and definition of treatment-resistant depression. J. Biol. Psychiatry. 2003;53:649–659. doi: 10.1016/s0006-3223(03)00231-2. [DOI] [PubMed] [Google Scholar]
- Gibney S.M., Drexhage H.A. Evidence for a dysregulated immune system in the etiology of psychiatric disorders. J. Neuroimmun. Pharmacol. 2013;8:900–920. doi: 10.1007/s11481-013-9462-8. [DOI] [PubMed] [Google Scholar]
- Gulbins E., Palmada M., Reichel M., Lüth A., Böhmer C., Amato D., Müller C.P., Tischbirek C.H., Groemer T.W., Tabatabai G. Acid sphingomyelinase–ceramide system mediates effects of antidepressant drugs. J. Nat. Med. 2013;19:934–938. doi: 10.1038/nm.3214. [DOI] [PubMed] [Google Scholar]
- Gupta A., Madhavan M.V., Sehgal K., Nair N., Mahajan S., Sehrawat T.S., Bikdeli B., Ahluwalia N., Ausiello J.C., Wan E.Y. Extrapulmonary manifestations of COVID-19. J. Nat. Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PubMed] [Google Scholar]
- Hoertel N., Sánchez-Rico M., Vernet R., Beeker N., Jannot A.-S., Neuraz A., Salamanca E., Paris N., Daniel C., Gramfort A. Association between antidepressant use and reduced risk of intubation or death in hospitalized patients with COVID-19: results from an observational study. J. Mole. Psychiatry. 2021:1–14. doi: 10.1038/s41380-021-01021-4. [DOI] [PubMed] [Google Scholar]
- Köhler C.A., Freitas T.H., Stubbs B., Maes M., Solmi M., Veronese N., de Andrade N.Q., Morris G., Fernandes B.S., Brunoni A.R. Peripheral alterations in cytokine and chemokine levels after antidepressant drug treatment for major depressive disorder: systematic review and meta-analysis. J. Mole. Neurobiol. 2018;55:4195–4206. doi: 10.1007/s12035-017-0632-1. [DOI] [PubMed] [Google Scholar]
- Lenze E.J., Mattar C., Zorumski C.F., Stevens A., Schweiger J., Nicol G.E., Miller J.P., Yang L., Yingling M., Avidan M.S. Fluvoxamine vs placebo and clinical deterioration in outpatients with symptomatic COVID-19: a randomized clinical trial. J JAMA. 2020;324:2292–2300. doi: 10.1001/jama.2020.22760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning K., Zvolensky M.J., Garey L., Long L.J., Gallagher M.W. The explanatory role of fatigue severity in the relation between COVID-19 perceived stress and depression, anxiety, and panic severity. J. Cogn. Behav. Therapy. 2021:1–11. doi: 10.1080/16506073.2021.1874503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M.G., De Lorenzo R., Conte C., Poletti S., Vai B., Bollettini I., Melloni E.M.T., Furlan R., Ciceri F., Rovere-Querini P. Anxiety and depression in COVID-19 survivors: Role of inflammatory and clinical predictors. J. Brain, Behav. Immunity. 2020;89:594–600. doi: 10.1016/j.bbi.2020.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M.G., Lucchi S., Tringali A.G.M., Rossetti A., Botti E.R., Clerici M. Neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in mood disorders: a meta-analysis. J. Progress Neuro-Psychopharmacol. 2018;84:229–236. doi: 10.1016/j.pnpbp.2018.03.012. Biological Psychiatry. [DOI] [PubMed] [Google Scholar]
- Mazza M.G., Palladini M., De Lorenzo R., Magnaghi C., Poletti S., Furlan R., Ciceri F., Rovere-Querini P., Benedetti F., group, C.-B.O.C.S. Persistent psychopathology and neurocognitive impairment in COVID-19 survivors: effect of inflammatory biomarkers at three-month follow-up. J. Brain, Behav. Immun. 2021;94:138–147. doi: 10.1016/j.bbi.2021.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A.H., Raison C.L. The role of inflammation in depression: from evolutionary imperative to modern treatment target. J. Nat. Rev. Immunol. 2016;16:22. doi: 10.1038/nri.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., Cook J.R., Nordvig A.S., Shalev D., Sehrawat T.S. Post-acute COVID-19 syndrome. J. Nat. Med. 2021:1–15. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichard R.R., Kashani K.B., Boire N.A., Constantopoulos E., Guo Y., Lucchinetti C.F. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. J. Acta. Neuropathol. 2020;140:1–6. doi: 10.1007/s00401-020-02166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J.P., Chesney E., Oliver D., Pollak T.A., McGuire P., Fusar-Poli P., Zandi M.S., Lewis G., David A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. The Lancet Psychiatry. 2020;7:611–627. doi: 10.1016/S2215-0366(20)30203-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlienger R.G., Meier C.R. Effect of selective serotonin reuptake inhibitors on platelet activation: can they prevent acute myocardial infarction? Am. J. Cardiovasc. Drugs. 2003;3:149–162. doi: 10.2165/00129784-200303030-00001. [DOI] [PubMed] [Google Scholar]
- Talman S., Boonman-de Winter L., de Mol M., Hoefman E., van Etten R., De Backer I.J.R.M. Pulmonary function and health-related quality of life after COVID-19 pneumonia. J. Respir. Med. 2021;176 doi: 10.1016/j.rmed.2020.106272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taquet M., Geddes J.R., Husain M., Luciano S., Harrison P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: a retrospective cohort study using electronic health records. The Lancet Psychiatry. 2021;8:416–427. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troyer E.A., Kohn J.N., Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. J. Brain. 2020 doi: 10.1016/j.bbi.2020.04.027. behavior,immunity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, transmission, diagnosis, and treatment of coronavirus disease 2019 (COVID-19): a review. J. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- Yuan B., Li W., Liu H., Cai X., Song S., Zhao J., Hu X., Li Z., Chen Y., Zhang K. Correlation between immune response and self-reported depression during convalescence from COVID-19. Brain Behav. Immun. 2020;88:39–43. doi: 10.1016/j.bbi.2020.05.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimniak M., Kirschner L., Hilpert H., Geiger N., Danov O., Oberwinkler H., Steinke M., Sewald K., Seibel J., Bodem J. The serotonin reuptake inhibitor Fluoxetine inhibits SARS-CoV-2 in human lung tissue. J. Sci. Reports. 2021;11:1–5. doi: 10.1038/s41598-021-85049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]