Abstract
Bacteroides forsythus strains recovered from cat and dog bite wound infections in humans (n = 3), monkey oral strains (n = 3), and the human oral ATCC 43037 type strain were characterized by using phenotypic characteristics, enzymatic tests, whole cell fatty acid analysis, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis, PCR fingerprinting, and 16S rDNA (genes coding for rRNA) sequencing. All three bite wound isolates grew on brucella agar supplemented with 5% sheep blood, vitamin K1, and hemin. These strains, unlike the ATCC strain and previously described monkey oral and human clinical strains, did not require N-acetylmuramic acid supplementation for growth as pure cultures. However, their phenotypic characteristics, except for catalase production, were similar to those of previously identified strains. PCR fingerprinting analysis showed differences in band patterns from the ATCC strain. Also, SDS-PAGE and whole cell fatty acid analysis indicated that the dog and cat bite wound strains were similar but not identical to the human B. forsythus ATCC 43037 type strain and the monkey oral strains. The rDNA sequence analysis indicated that the three bite wound isolates had 99.93% homology with each other and 98.9 and 99.22% homology with the human ATCC 43037 and monkey oral strains, respectively. These results suggest that there are host-specific variations within each group.
Bacteroides forsythus is found in the subgingival, gingival, and periodontal pockets of humans and is known to cause periodontal disease. This organism was initially grown as part of mixed cultures from periodontal lesions and appeared as satellite colonies around other species. The fastidious nature of this organism had deterred extensive study until it was determined that B. forsythus requires exogenous N-acetylmuramic acid (NAM) for optimal growth as a pure culture (15, 16). B. forsythus was also recently found in monkey subgingival plaque samples. These strains (from Macaca fasciculans) have been described as a biotype of B. forsythus (1). In this study, we describe the characteristics of three strains isolated from infected cat and dog bites in humans as part of a nationwide study on the bacteriology of such wounds (14). In each of these patient specimens, other species of oral flora were also present. Surprisingly, these bite wound strains did not require NAM supplementation for growth as pure cultures. Therefore, we characterized these strains by phenotypic, biochemical, whole cell wall fatty acid analysis, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), PCR fingerprinting, and rDNA (genes coding for rRNA) sequencing of the 16S rRNA gene. These results showed that these strains are B. forsythus. To our knowledge, this is the first report of isolation of B. forsythus from a source other than the oral cavity (14).
MATERIALS AND METHODS
Bacterial strains and culture conditions.
Three clinical isolates recovered from dog (n = 2) and cat (n = 1) bite wounds in humans, three monkey oral strains, and the type strain of B. forsythus, ATCC 43037, a human oral isolate, were studied. All strains were taken from stock cultures frozen in 20% skim milk at −70°C and subcultured twice onto brucella agar supplemented with 5% sheep blood, vitamin K1, and hemin (Anaerobe Systems, San Jose, Calif.), except that the monkey strains and ATCC strain required 0.001% NAM (Sigma Chemical, St. Louis, Mo.) as an additional supplement. All strains were incubated in an anaerobic chamber (Anaerobe Systems) at 37°C for 5 to 7 days.
The NAM-supplemented medium was prepared by first preparing brucella agar as described by the manufacturer (Difco, Detroit, Mich.) and then adding vitamin K1 (1 μg/ml) and hemin (5 μg/ml), prior to autoclaving. After the agar was cooled in a 50°C water bath, defibrinated sheep blood (5%) and filter-sterilized stock NAM solution (final concentration, 0.001%) were added. Plates containing approximately 20 ml were prepared.
Substrate tests.
The RapID Ana II kit (Remel, Norcross, Ga.) was used for biochemical analysis of all strains. In addition, WEE-TAB triple-substrate tubes (Key Scientific, Round Rock, Tex.) were used to test for trypsin-like activity and chymotrypsin. The two kits were inoculated, and the results were interpreted according to the manufacturer’s instructions. The test for esculin hydrolysis was performed by using prereduced anaerobically sterilized (PRAS) medium (Remel). These tubes were inoculated with 0.1 ml of a suspension of bacteria with a McFarland standard of 0.5 and incubated at 37°C for 5 to 7 days. For the monkey strains and the human ATCC strains of B. forsythus, 0.1 ml of the 1% stock solution of NAM was added to each tube prior to incubation. The results were interpreted according to the manufacturer’s instructions. All isolates were tested for catalase production with 10% hydrogen peroxide, for spot indole with para-dimethyl-aminocinnamaldehyde reagent, and for susceptibility to special potency antibiotic disks by standard procedures (12).
Whole cell fatty acid analysis.
The isolates were referred to the Anaerobe Laboratory at the Veterinary Medicine Teaching Hospital at the University of California at Davis. Whole cell fatty acid analysis was performed by standard procedures as previously described (12).
PCR fingerprinting analysis.
Several bacterial colonies of each isolate were suspended in 1 ml of sterile distilled water to a turbidity approximately equal to a McFarland standard of 3. Two hundred microliters of the cell suspension was removed and pelleted, resuspended in 100 to 200 μl of InstaGene Matrix (Bio-Rad, Hercules, Calif.), and then incubated at 50°C for 15 to 30 min. Cell solutions were vortexed and then heated for 8 to 10 min at 100°C. After the cell solutions were vortexed, cell lysates containing the DNA extract were centrifuged to remove cellular debris. Cell lysates were stored at −20°C until use. Thirty-five microliters of each cell lysate supernatant was subjected to PCR amplification using a single primer derived from the tDNA intergenic spacer region (T3B [5′-AGGTCGCGGGTTCGAATCC-3′]) (2, 17). An ultrapure preparation of Escherichia coli B DNA (Sigma) was used as a positive control for PCR amplification. The negative control had no DNA added. Amplification reactions and absorbance profile analyses were performed as previously described (2). Briefly, amplification was performed as follows: 35 cycles, with each cycle consisting of 30 s at 94°C, 30 s at 50°C, and 2 min at 72°C.
SDS-PAGE analysis.
The bite wound and monkey strains were compared to the B. forsythus ATCC type strain by SDS-PAGE of whole-cell protein profiles. This is an established technique for the definitive phenotypic identification of strains as known species (5, 6, 8, 11). Briefly, a dense suspension of each strain in distilled water was sonicated, mixed with an equal volume of treatment buffer containing SDS and mercaptoethanol, and boiled for 5 min. A drop of phenol red tracking dye was then added to each preparation. An aliquot (2 μl) of each preparation was loaded into a well of a 10% acrylamide Ready Gel (Bio-Rad). Electrophoresis was run at a constant current of 30 mA, and the gels were stained by using the Silver Stain Plus kit (Bio-Rad). The protein profiles of both sets of the isolates were visually compared with that of the B. forsythus ATCC type strain run on the same gel.
16S rDNA sequencing.
The MicroSeq 16S rRNA Gene Kit (PE Applied Biosystems, Foster City, Calif.) was used following the manufacturer’s recommended procedures. The kit uses six forward (F) and six reverse (R) primers, giving on average fourfold coverage of the 16S rDNA. Cells of each strain harvested from 3-day-old cultures on NAM-supplemented medium were extracted by using Gene Releaser following the manufacturer’s microwave protocol. The 16S rDNA was PCR amplified from each extract with the 5F forward primer and E94 reverse primer (R1540 reverse primer modified with an additional wobble base). Following spin column purification, amplification products were cycle sequenced using the kit reagents, and the products were analyzed on an automated sequencer (model 377; PE Applied Biosystems).
16S rRNA data analysis.
The consensus sequence for each strain was assembled with the Auto Assembler software (PE Applied Biosystems) and analyzed with a custom program set for sequence editing, alignment, secondary structure comparison, similarity matrix generation, and dendrogram construction for 16S rDNA data written in Microsoft Quick BASIC (9). DNA sequences were aligned as previously described (9), and similarity matrices were constructed, correcting for multiple base changes at single positions (4). Phylogenetic trees were constructed by the neighbor-joining method (10).
RESULTS
In this study, we characterized three presumptive B. forsythus strains isolated from cat and dog bite wounds in humans. The bite wound strains grew on brucella agar supplemented with 5% sheep blood, vitamin K1, and hemin. After 5 to 7 days of incubation at 37°C under anaerobic conditions, all strains grew to a diameter of 1.0 mm and were gray-to-tan, circular, entire, opaque colonies.
Biochemical profiles.
The three bite wound strains showed similar biochemical profiles on both the RapID Ana II and WEE TAB systems (Table 1). They were catalase positive, susceptible to special potency disks of kanamycin and colistin, resistant to vancomycin, and positive for trypsin-like activity. The colony morphology and biochemical profiles identified these three bite wound strains as B. forsythus. However, they differed from the type strain because they did not require NAM supplementation and were catalase positive. They differed from the monkey oral strains by testing negative for phenylalanine aminopeptidase (PAL), whereas the monkey oral strains were catalase negative and positive for PAL (Table 1).
TABLE 1.
Biochemical reactions of B. forsythus
| B. forsythus strain | Source | Biochemical reactiona
|
|||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Urea | BLTS | aARA | ONPG | aGLU | aGAL | aFUC | NAG | PO4 | GLY | PRO | PAL | LGY | ARG | ESCb | TRYb | CHYb | bGAL | SER | PYR | CATb | IND | ||
| RMA 7251 | Bite (dog) | − | − | − | + | + | − | + | + | + | − | − | − | + | + | + | + | − | + | + | − | + | + |
| RMA 8286 | Bite (dog) | − | + | − | + | + | − | + | + | + | − | − | − | + | + | + | + | − | + | − | − | + | + |
| RMA 8360 | Bite (cat) | − | + | − | + | + | − | + | + | + | − | − | − | + | + | + | + | − | + | + | − | + | + |
| RMA 8464 | Monkey oral | − | wk+ | − | + | + | − | + | + | + | − | − | + | + | + | + | + | − | + | + | − | − | + |
| RMA 8562 | Monkey oral | − | wk+ | − | + | + | − | + | + | + | − | − | + | + | + | + | + | − | + | + | − | − | + |
| RMA 8563 | Monkey oral | − | + | − | + | + | − | + | + | + | + | − | + | + | + | + | + | − | + | + | − | − | + |
| ATCC 43037 | Human oral | − | − | − | − | + | − | + | + | + | − | − | − | + | + | + | + | − | + | − | − | − | + |
Abbreviations: BLTS, β-d-disaccharidase; aARA, alpha-l-arabinosidase; ONPG, β-d-galactosidase; aGLU, alpha-d-glucosidase; aGAL, alpha-d-galactosidase; aFUC, alpha-l-fucosidase, NAG, n-acetyl-β-d-glucosaminidase; PO4, phosphatase; GLY, glycine aminopeptidase; PRO, proline aminopeptidase; LGY, leucyl-aminopeptidase; ARG, arginine aminopeptidase; ESC, esculin; TRY, trypsin; CHY, chymotrypsin; bGAL, β-galactosidase; SER, serine aminopeptidase; PYR, pyrrolidonyl aminopeptidase; CAT, catalase; IND, indole. Symbols: −, negative; +, positive; wk+, weakly positive.
Substrates not included in the RapID Ana II System.
Whole cell fatty acid analysis.
The whole cell wall fatty acid analysis indicated that although all strains had similarities, there were differences in some of the major fatty acid constituents (Table 2). The major constituent for both the three bite wound and the human oral ATCC type strain was 15:0 anteiso fatty acid methyl ester (15:0 anteiso FAME) (12-methyl tetradecanoic acid). The three monkey oral strains differed from the other strains in that they contained 15:0 anteiso FAME to a lesser extent. In addition, they contained larger amounts of the fatty acids 18:2 cis-9,12 FAME, 18:1 cis-9-FAME, 16:0 FAME, and 18:0 FAME.
TABLE 2.
Cellular fatty acid composition of B. forsythus isolates
| Fatty acid | Composition (%)a in B. forsythus isolates
|
||
|---|---|---|---|
| Bite wound | Monkey oral | ATCC 43037 | |
| 15:0 anteiso FAME | 49.2 (41.1–55.5) | 12.2 (9.0–14.3) | 47.1 |
| 16:0 FAME | − | 13.0 (10.6–14.7) | |
| 16:0 3OH FAME | 8.6 (4.5–13.7) | − | 8.5 |
| 17:0 ante 3OH FAME | 8.1 (8.0–8.7) | − | − |
| 18:0 FAME | − | 20.8 (17.1–23.9) | |
| 18:2 cis-9-FAME | − | 17.6 (16.7–19.3) | − |
| 18:1 cis-9-FAME | − | 15.6 (13.5–18.0) | |
| Summed feature 11b | 15.3 (9.0–26.2) | − | 17.8 |
| Summed feature 3b | 8.5 | ||
Values are expressed as mean percentages of the total acids; the range of values for species in the genus is given in parentheses. The compounds included are those that occurred at levels of ≥5% of the total in one or more of the species. −, not present.
Either the sum of two products that elute closely together or a sum of the two peaks forms a more stable product and cannot be distinguished from each other. Summed feature 11 is 17:0 iso OH FAME or 18:2 DMA; summed feature 3 is 15:0 isoacid or aldehyde.
SDS-PAGE.
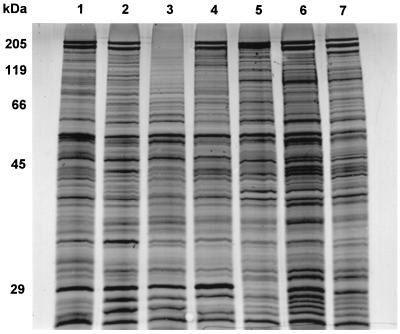
The protein profiles of the monkey and bite wound isolates were compared to that of the human ATCC type strain of B. forsythus (Fig. 1). All of the isolates had protein profiles very similar to each other but not identical to that of the human type strain. All of the nonhuman isolates (except monkey oral isolate RMA 8563) showed the prominent 200-kDa S-layer protein bands. The three monkey isolates were most similar to each other, and the three bite wound strains were most similar to each other.
FIG. 1.
SDS-PAGE of whole-cell proteins of B. forsythus type strain and isolates from monkey dental plaque and animal bite wounds. Lane 1, human strain, B. forsythus ATCC 43037; lane 2, monkey isolate RMA 8464, lane 3, monkey isolate RMA 8563; lane 4, monkey isolate RMA 8562; lane 5, dog bite wound isolate RMA 7251; lane 6, dog bite wound isolate RMA 8286; lane 7, cat bite wound isolate RMA 8360.
PCR fingerprinting.
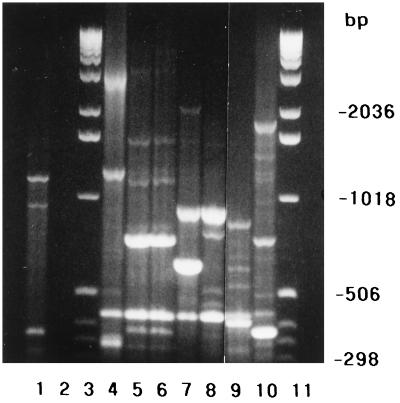
PCR of the seven B. forsythus strains using the single primer T3B revealed significant differences in the banding patterns among these strains (Fig. 2). Bite wound strain RMA 8286 (lane 8) and RMA 8360 (lane 7) had a band in common at 896 bp, which was not shared by any of the other isolates. A single band at 420 bp was common to all isolates. Only monkey oral strains RMA 8562 (lane 5) and RMA 8563 (lane 6) had other bands in common with the human oral strain ATCC 43037 (1,158 and 731 bp). These two monkey strains appeared to have identical PCR fingerprint profiles, with common bands at 1,571, 1,158, 731, 420, and 371 bp. Monkey oral strain RMA 8464 (lane 4) had no bands in common with the other isolates except for the band at 420 bp. Other than the monkey oral strains RMA 8562 and RMA 8563, each of the strains analyzed had a unique PCR fingerprint pattern. None of the fingerprints was identical to that of the human oral strain ATCC 43037.
FIG. 2.
PCR fingerprint analysis. Seven B. forsythus isolates were analyzed by PCR fingerprinting using the single primer T3B. Lane 1, ultrapure preparation of E. coli B DNA; lane 2, negative control; lanes 3 and 11, molecular size ladder; lane 4, RMA 8464 (monkey oral strain); lane 5, RMA 8562 (monkey oral strain); lane 6, RMA 8563 (monkey oral strain); lane 7, RMA 8360 (cat bite strain); lane 8, RMA 8286 (dog bite strain); lane 9, RMA 7251 (dog bite strain); lane 10, ATCC 43037 (human oral strain).
16S rDNA sequencing.
The relationships among the 16S rDNA from the monkey isolates, the bite wound isolates, and the human oral ATCC type strain of B. forsythus were examined (Fig. 3). The seven strains differed in 21 base positions in the 16S rDNA. The animal bite wound isolates clustered together with a similarity of 99.93%. They were 98.9% similar to the human B. forsythus ATCC strain and 99.22% similar to the monkey isolates. The monkey isolates clustered together with a similarity of 99.95% and were more similar (98.96%) to the human type strain than the bite strains were.
FIG. 3.
Dendrogram of 16S rRNA gene sequence relationships between the B. forsythus type strain and the monkey and bite wound isolates. The closest phylogenetic relatives of B. forsythus, Bacteroides distasonis and Bacteroides merdae, are included for reference. The bar represents 10% sequence difference.
DISCUSSION
Three strains from dog and cat bite wounds in humans were studied by conventional tests and were found to be similar in their colonial morphology and various biochemical activities to previously described strains of B. forsythus. For example, they produced a trypsin-like enzyme, and were α-fucosidase and esculin hydrolase positive (1, 3, 7, 13, 15). The strains were additionally identified as B. forsythus by SDS-PAGE and whole cell fatty acid analysis. Furthermore, PCR fingerprinting analysis showed that a single band was found to be common to all strains. The genetic relatedness of these strains was further established by DNA sequence analysis. It was found that the 16S rDNA sequences of the bite wound strains were 98.9% similar to the human oral ATCC type strain.
Although the three cat and dog bite strains were isolated from human wounds, presumably the strains originated from the oral flora of dogs and cats. B. forsythus has been isolated from the oral cavity of primates but has not been previously reported as occurring in other sites or species. These three strains were also unusual in that they did not require NAM supplementation for optimal growth in pure culture as previously described for B. forsythus (15, 16). This is important because a requirement for NAM has been one of the main criteria for identification of this species. Other differences were that the bite wound strains of B. forsythus were catalase positive and the SDS-PAGE showed some differences, while the PCR fingerprinting data showed band patterns that were quite diverse from that of the human oral type strain ATCC 43037.
For comparison, we studied three monkey strains of B. forsythus and found that, overall, our results were similar to published results for other monkey strains (1). One major difference from the bite wound and human strains was that the monkey strains were positive for PAL. The monkey strains required NAM supplementation for growth, like the human strain. In addition, the whole cell wall fatty acid analysis and PCR fingerprinting shared some similarities with the bite wound and human strains but were more diverse in their fatty acid constituents. The comparison of the 16S rDNA sequence analysis showed that the monkey strains were 98.96 and 99.22% similar to the human and bite wound strains, respectively.
The high similarity (≥98.9%) of 16S rDNA sequences from all the strains examined suggests they belong to a single species; however, DNA-DNA homology studies will be necessary for this to be confirmed. Although there is a high degree of similarity between the 16S rDNA sequences of all the B. forsythus strains, the sequences can be grouped according to their host species, suggesting the presence of host-specific subtypes within the species.
B. forsythus has been studied extensively and found to be a key organism associated with human periodontal disease (3, 13, 15). Previous studies have indicated that, due to the fastidious nature of these organisms, the incidence of these strains has probably been underestimated (3, 13, 15). In our previous study, we had isolated for the first time B. forsythus strains from dog and cat bite wounds in humans (14). The presence of these strains in an extraoral infection indicates that the periodontal pocket is not the only site in which this organism can colonize and potentially contribute to the infection process. In addition, the isolation of these strains from nonprimate origins shows that this organism is associated not just with humans or primates, as had been previously described (1, 3, 13, 15), but also exists in the oral cavities of dogs and cats.
ACKNOWLEDGMENTS
We thank Spencer Jang, Veterinary Medicine Teaching Hospital, University of California at Davis, for performing whole cell wall fatty acid analysis on these strains. Pamela Braham, University of Washington, kindly provided the monkey oral strains.
REFERENCES
- 1.Braham P H, Moncla B. Rapid presumptive identification and further characterization of Bacteroides forsythus. J Clin Microbiol. 1992;30:649–654. doi: 10.1128/jcm.30.3.649-654.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Claros M C, Citron D M, Hunt Gerardo S, Goldstein E J C, Schönian G, Montag T, Hampel B, Rodloff A C. Characterization of indole-negative Bacteroides fragilis group species with use of polymerase chain reaction fingerprinting and resistance profiles. Clin Infect Dis. 1996;23:66–72. doi: 10.1093/clinids/23.supplement_1.s66. [DOI] [PubMed] [Google Scholar]
- 3.Gersdorf H, Meissner A, Pelz K, Krekeler G, Göbel U B. Identification of Bacteroides forsythus in subgingival plaque from patients with advanced periodontitis. J Clin Microbiol. 1993;31:941–946. doi: 10.1128/jcm.31.4.941-946.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jukes T H, Cantor C R. Evolution of protein molecules. In: Munro H N, editor. Mammalian protein metabolism III. New York, N.Y: Academic Press; 1969. pp. 21–132. [Google Scholar]
- 5.Kinder S A, Kornman K S, Holt S C. Characterization of selected gram-negative oral microorganisms by SDS-PAGE. Oral Microbiol Immunol. 1989;4:52–56. doi: 10.1111/j.1399-302x.1989.tb00407.x. [DOI] [PubMed] [Google Scholar]
- 6.Maiden M F, Tanner A. Identification of oral yeasts by polyacrylamide gel electrophoresis. Oral Microbiol Immunol. 1991;6:187–190. doi: 10.1111/j.1399-302x.1991.tb00475.x. [DOI] [PubMed] [Google Scholar]
- 7.Maiden M F J, Tanner A, Macuch P J. Rapid characterization of periodontal bacterial isolates by using fluorogenic substrate tests. J Clin Microbiol. 1996;34:376–384. doi: 10.1128/jcm.34.2.376-384.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore W E C, Hash D E, Holdeman L V, Cato E P. Polyacrylamide slab gel electrophoresis of soluble proteins for studies of bacterial floras. Appl Environ Microbiol. 1980;39:900–907. doi: 10.1128/aem.39.4.900-907.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paster B J, Dewhirst F E. Phylogeny of Campylobacter, Wolinella, Bacteroides gracilis, and Bacteroides ureolyticus by 16S ribosomal ribonucleic acid sequencing. Int J Syst Bacteriol. 1988;38:56–62. [Google Scholar]
- 10.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 11.Slayne M A, Aldred M J, Wade W G. A rapid, semi-automated SDS-PAGE identification system for oral anaerobic bacteria. J Appl Bacteriol. 1990;68:391–395. doi: 10.1111/j.1365-2672.1990.tb02889.x. [DOI] [PubMed] [Google Scholar]
- 12.Summanen P, Baron E J, Citron D M, Strong C A, Wexler H M, Finegold S M. Wadsworth anaerobic bacteriology manual. 5th ed. Belmont, Calif: Star Publishing Company; 1993. [Google Scholar]
- 13.Takemoto T, Kurihara H, Dahlen G. Characterization of Bacteroides forsythus isolates. J Clin Microbiol. 1997;35:1378–1381. doi: 10.1128/jcm.35.6.1378-1381.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Talan D A, Citron D M, Abrahamian F M, Moran G J, Goldstein and Emergency Medicine Animal Bite Infection Study Group E J C. Bacteriologic analysis of infected dog and cat bites. N Engl J Med. 1999;340:85–92. doi: 10.1056/NEJM199901143400202. [DOI] [PubMed] [Google Scholar]
- 15.Tanner A C R, Listgarten M A, Ebersole J L, Strzempko M N. Bacteroides forsythus sp. nov., a slow-growing, fusiform Bacteroides sp. from the human oral cavity. Int J Syst Bacteriol. 1986;36:213–221. [Google Scholar]
- 16.Weiss C. Dependence of proliferation of Bacteroides forsythus on exogenous N-acetylmuramic acid. Infect Immun. 1989;57:1757–1759. doi: 10.1128/iai.57.6.1757-1759.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Welsh J, McClelland M. Genome fingerprints produced by PCR with consensus tRNA gene primers. Nucleic Acids Res. 1991;19:861–866. doi: 10.1093/nar/19.4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]