Abstract
Fluorinated compounds are widely used in the fields of molecular imaging, pharmaceuticals, and materials. Fluorinated natural products in nature are rare, and the introduction of fluorine atoms into organic compound molecules can give these compounds new functions and make them have better performance. Therefore, the synthesis of fluorides has attracted more and more attention from biologists and chemists. Even so, achieving selective fluorination is still a huge challenge under mild conditions. In this review, the research progress of enzymatic synthesis of fluorinated compounds is summarized since 2015, including cytochrome P450 enzymes, aldolases, fluoroacetyl coenzyme A thioesterases, lipases, transaminases, reductive aminases, purine nucleoside phosphorylases, polyketide synthases, fluoroacetate dehalogenases, tyrosine phenol-lyases, glycosidases, fluorinases, and multienzyme system. Of all enzyme-catalyzed synthesis methods, the direct formation of the C-F bond by fluorinase is the most effective and promising method. The structure and catalytic mechanism of fluorinase are introduced to understand fluorobiochemistry. Furthermore, the distribution, applications, and future development trends of fluorinated compounds are also outlined. Hopefully, this review will help researchers to understand the significance of enzymatic methods for the synthesis of fluorinated compounds and find or create excellent fluoride synthase in future research.
Key points
• Fluorinated compounds are distributed in plants and microorganisms, and are used in imaging, medicine, materials science.
• Enzyme catalysis is essential for the synthesis of fluorinated compounds.
• The loop structure of fluorinase is the key to forming the C-F bond.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00253-021-11608-0.
Keywords: Enzymatic synthesis, Fluorinase, C-F bonds, Fluorinated compounds, Applications of fluorides
Introduction
Fluorine has different physical and chemical properties from other elements, including bond length, van der Waals radius, van der Waals volume of the atom, and electronegativity of elements (Meanwell 2018). Unlike other halogens (chlorine, bromine, iodine, astatine, and tennessine), non-polarizable fluorine does not participate in halogen bonding. Due to its non-polarizability, strong electrostatic interactions will be formed, resulting in attractiveness or repulsion (Yang et al. 2015). Moreover, fluorine can change the pKa, affinity, dipole moment, stability, lipophilicity, and bioavailability of adjacent groups (Dos Santos et al. 2020; Khosravan et al. 2017; Muller et al. 2007). Therefore, the target compounds doped with fluorine are endowed with stronger activity and stability, longer half-life, and better bioabsorbability (Moschner et al. 2019; Ni and Hu 2016; Reichel and Karaghiosoff 2020), especially in the fields of pharmaceutical intermediates (Gillis et al. 2015; Swallow 2015; Zhdankin et al. 2017; Zou et al. 2019), cancer treatment (Meanwell et al. 2020), antiviral agents (Pomeisl et al. 2019), photovoltaics, diagnostic probes and bioinspired materials (Moschner et al. 2019). Besides, natural proteins incorporating fluorinated amino acids showed also many unique characteristics, which are used in the biotherapeutics protein–protein interaction and the synthesis of high value-added chemicals (Arias et al. 2020; Awad and Ayoup 2020; Bucci et al. 2019; Liu et al. 2019; Mei et al. 2020a; Remete et al. 2018; Sisila et al. 2021; Vaughan et al. 2016; Wang and Matthews 2020; Won et al. 2019).
Unfortunately, fluorinated natural products are extremely rare in nature, which limits the application of fluorine-containing compounds. Accordingly, the artificial synthesis of fluorinated compounds has become an important alternative in modern society. However, the formation of C-F bonds through nucleophilic fluorination reactions has been progressing slowly. The main reason is that fluorinated compounds react poorly and fluoride ion is easily solvated (△G° > 450 kJ/mol) in protic media (Liang et al. 2017; Markakis et al. 2020; Mennie et al. 2018; Scheidt et al. 2018). Hence, they have the strongest hydration of halide so that the huge kinetic inertia needs to be overcome to promote this reaction. To realize the artificial synthesis of fluorinated compounds, many chemical catalysis methods have been developed and used to synthesize various fluorine-containing compounds including [18F]6-fluoro-l-3,4-dihydroxy-phenylalanine (Mossine et al. 2020), 18F-fluoro-α-methyl tyrosine, 18F-prostate-specific membrane antigen (PSMA)-1007 (Giesel et al. 2017), and 18F-fluoro-α-methyl phenylalanine (18F-FAMP) (Hanaoka et al. 2019; Yamaguchi et al. 2020).
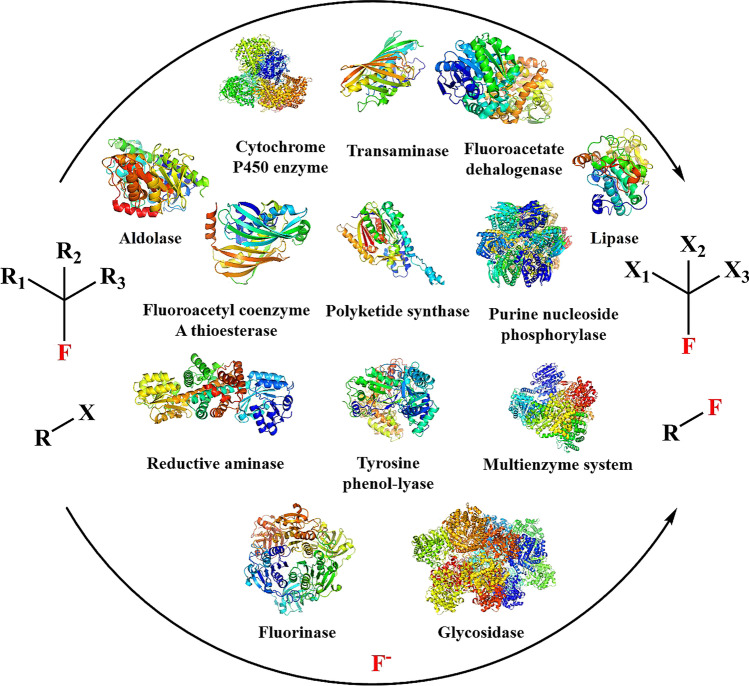
Nonetheless, these conventional chemical synthesis methods required precious metals, toxic and contaminating chemical reagents, high temperature, high pressure, extreme conditions, and the protection and deprotection of groups. This is not conducive to the sustainable development of green chemicals and the global economy. In contrast, the biosynthesis methods are good partners/supplements/alternatives in the field of chemical synthesis, especially for fluorinated compounds. The biosynthesis methods are divided into fermentation methods and enzyme-based biocatalysis methods. Through fermentation methods, various fluorinated compounds can be synthesized by engineered microorganisms that integrated substrate transporters, product degradation pathways, fluoride ion channels, fluoride-responsive riboswitch, orthogonal T7 RNA polymerase, or chromosome (Calero et al. 2020; Jiang et al. 2018; Markakis et al. 2020; Rivera-Chavez et al. 2017; Sester et al. 2020; Thuronyi et al. 2017). However, some factors limit its application, including complex regulatory networks and the toxic effects of fluoride on living microorganisms. For enzyme-based biocatalysis, it has the advantages of energy-saving, environmental protection, safety, high selectivity, atomic economy, convenient operation, and mild reaction conditions (Council et al. 2020; Fryszkowska and Devine 2020; Hauer 2020; Honig et al. 2017; Latham et al. 2018; Menon et al. 2020; Minges and Sewald 2020; O'Hagan and Deng 2015; Odar et al. 2015; Sandoval and Hyster 2020; Tong et al. 2019; Wu et al. 2020a). Hence, it has attracted more and more attention from biologists and chemists. In this review, diverse methods are introduced for the synthesis of fluorinated compounds catalyzed by different enzymes in recent years, especially fluorinase that can form the C-F bond with the selective catalytic mechanism (Fig. 1). Combined with the distribution, enzymatic synthesis methods, and applications of fluorinated compounds, this review will attract more scientists to participate in the field of fluorobiochemistry.
Fig. 1.
Enzymatic synthesis of fluorinated compounds
Distribution of fluorinated natural products
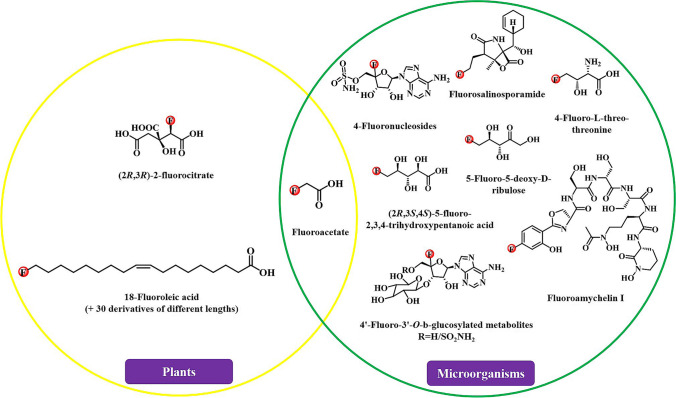
Since the discovery of the first fluorinated natural product, it has been more than 70 years. Fluorinated natural products are only distributed in fewer tissues. Although fluorides have also been found in animals, there is no direct evidence that fluorinated natural products can be synthesized by animals (Harper and O'Hagan 1994). Fluorides are obtained by animals mainly by eating foods containing fluorinated natural products. Plants and microorganisms have developed a huge ability to biosynthesize secondary metabolites, some of which are fluorinated natural products of various structures. However, there are still very few fluorinated natural products found in nature. The main known fluorinated natural products in plants and microorganisms are as follows (Fig. 2).
Fig. 2.
Known fluorinated natural products
Fluorinated natural products in plants
Fluoroacetate was first discovered from plant gifblaar (Dichapetalum cymosum) in 1943 (Toit 1943). It is also the most common fluorinated natural product and has been identified in more than 40 higher plants. These plants that produce fluoroacetate mainly come from the families of Malpighiaceae, Fabaceae, Dichapetalaceae, Rubiaceae, and Bignoniaceae (Grobbelaar and Marion Meyer 1990; Lee et al. 2014). Fluoroacetate-containing plants grow all over the world, especially in Africa, Australia, and South America (Leong et al. 2017). Due to the toxicity of fluoroacetate, it is generally believed that these plants produce fluoroacetate for the defense of herbivores (Leong et al. 2017). Especially Dichapetalum braunii, its seeds contain up to 8000 ppm of fluoroacetate, which is extremely defensive and even lethal (O’Hagan and B. Harper 1999). In addition, (2R,3R)-2-fluorocitrate was also found to be present in the West African shrub Dichapetalum toxicarium and forage plants (soya bean, alfalfa, and crested wheatgrass) (Carvalho and Oliveira 2017; Harper and O'Hagan 1994; O'Hagan and Deng 2015; Ren et al. 2020; Zhang et al. 2019c). Despite the great interest, the physiological role of fluorinated natural products is not fully understood in higher plants. If fluoroacetate is for self-defense, are other natural products of fluorination involved in the synthesis of metabolites or proteins? There are still many areas to be explored.
Fluorinated natural products in microorganisms
Fluoroacetate is also produced by various microorganisms, including S. cattleya, Streptomyces sp. MA37, Norcardia brasiliensis, Actinoplanes sp. N902-109, Actinopolyspora mzabensis, and Streptomyces xinghaiensis NRRL B24674 (Deng et al. 2014; Huang et al. 2014; Ren et al. 2020; Sanada et al. 1986; Sooklal et al. 2020). In addition to fluoroacetate, fluorinated threonine is another fluorinated natural product derived from fluoride-producing microorganisms. Moreover, it is also the only fluorinated amino acid found in nature. It should be noted that a new type of fluorometabolite ((2R3S4S)-5-fluoro-2,3,4-trihydroxypentanoic acid) was identified in Streptomyces sp. MA37 except for fluoroacetate and fluorinated threonine (Ma et al. 2015). This also showed that there is a unique fluoride metabolism pathway in Streptomyces sp. MA37. In addition to the above fluorinated natural products, microorganisms also produce other fluorinated natural products mainly including 4´-fluoro-3´-O-β-glucosylated metabolites (F-Mets I and II) (Feng et al. 2019), 4-fluoronucleosides (Zhang et al. 2019c), fluorosalinosporamide (O'Hagan and Deng 2015), and 5-fluoro-5-deoxy-D-ribulose (5-FDRul) (Wu et al. 2020b). Like plants, the physiological role and mechanism of fluorinated natural products are still a mystery in microorganisms.
Enzymatic synthesis of fluorinated compounds
Cytochrome P450 enzymes
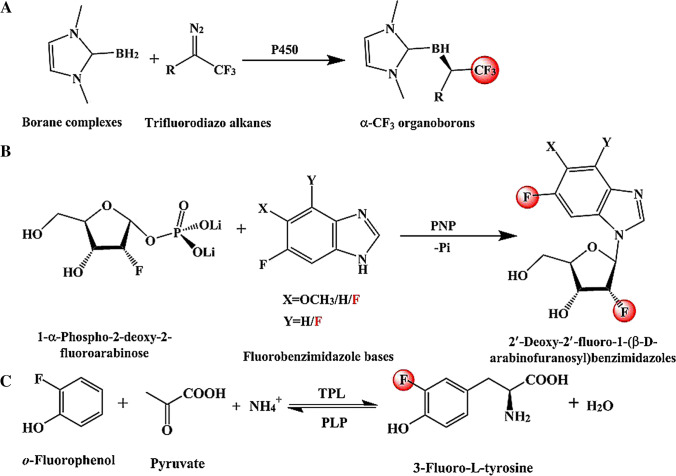
Cytochrome P450 enzymes (P450s) belong to the superfamily of heme monooxygenases and are involved in the synthesis and degradation of many metabolites in actinomycetes. P450s can catalyze a variety of different reactions, mainly including dehydrogenation, epoxidation, dealkylation, C–C bond formation or cleavage, C-N bond formation, and hydroxylation (Chen and Arnold 2020; Iizaka et al. 2021). Therefore, various fluorinated compounds can be synthesized through different types of reactions mediated by P450s. This is an effective strategy to incorporate fluoroalkyl groups through the conversion of C(sp3)-H bonds to fluorocarbon alkyl bonds (McAtee et al. 2018). To synthesize organic fluorocarbon alkanes with α-CF3, a biocatalytic platform was developed based on cytochrome P450 (Fig. 3A) (Huang et al. 2019). Through this platform, diverse α-CF3 organoborons were obtained from 400 mM Lewis-base borane complexes and trifluorodiazo alkanes (ee = 85–99%). Similarly, various organofluorine compounds with S-stereoselectivity were also obtained from 10 mM N-aryl pyrrolidine and 20 mM 2,2,2-trifluoro-1-diazoethane (ee > 99%) (Zhang et al. 2019b). Notably, engineered P450 completely reversed the enantioselectivity of the products through directed evolution (R-stereoselectivity, ee > 99%). Moreover, fluorinated compounds can also be synthesized by P450 through nitrification reaction. Aromatic nitration is an important industrial process for the production of many chemicals. Cytochrome P450 enzyme from Streptomyces scabies 87.22 TxtE is the only enzyme that can directly catalyze the nitrification reaction so far (Zuo et al. 2016). To expand its application potential, fluorinated nitro-tryptophan analogs were obtained by P450TxtE from 1.5 mM 4-F-dl-tryptophan and 5-F-l-tryptophan in a 10 mL reaction system.
Fig. 3.
The fluorinated compounds were obtained by combination reactions. A) The P450-mediated biocatalytic platform for the synthesis of α-CF3 organoborons; B) Various modified 2´-deoxy-2´-fluoro-β-d-arabinofuranosyl benzimidazoles were synthesized by the chemo-enzymatic method based on PNP (Pi = inorganic phosphate); C) The biosynthetic of 3-fluoro-l-tyrosine catalyzed by TPL
Among all the reaction types catalyzed by P450, the hydroxylation reaction is the most important type of reaction. Thus, the fluorinated derivative of N-(1-adamantyl)-1-pentyl-1H-indazole-3-carboxamide (5F-AKB48) was obtained in vitro hydroxylation reaction (Pinson et al. 2020). Moreover, the 5F-AKB48 is the main component of cannabinoids (SCs), and the experiments showed that P450s from humans can oxidize 5F-AKB48 to fluoride with different hydroxyl groups. Interestingly, fluorine-containing compounds are also activators in the P450-mediated hydroxylation process. With the assistance of perfluorinated decoy molecules (PFC), the hydroxylation rate was significantly enhanced for unnatural substrate cycloalkanes by P450 from Bacillus megaterium (Dezvarei et al. 2018). In addition to bioenzymatic methods, P450 can also realize the biosynthesis of fluorides through chemoenzymatic methods. For common organic molecules, one or more non-activated sites were selectively fluorinated by P450 and nucleophilic fluorination reagent diethylaminosulfur trifluoride (DAST) (Figure S1) (Obach et al. 2016; Rentmeister et al. 2009). In this process, monofluorine and polyfluorine compounds were synthesized through a two-step chemical enzymatic method. Especially for cyclic fluorides (steroidal analogs), high regioselectivity (55–100%) and yields (> 60%) could be achieved by various P450 mutants and DAST. It is just that the use of chemical reagents makes the entire catalytic process less economical and environmentally friendly. It is worth noting that the ability of P450s to synthesize fluorides is also their physiological function in some organisms. Such as 6:2 fluorotelomer sulfonic acid (6:2 FTSA) is widely used in water-based film-forming foam as a new type of perfluorooctane sulfonic acid. In earthworms (Eisenia fetida), studies have shown that P450 is involved in the biotransformation of 6:2 FTSA (Zhao et al. 2021). Similarly, this function is also proven in pumpkin (Cucurbita maxima L.) (Zhao et al. 2019a).
As such, various fluorinated compounds can be obtained through diverse reactions mediated by P450. However, it is still necessary to further improve the issues of thermal stability, regional and stereo specificity of P450. Moreover, P450 must co-express the electron transport chain to perform its biological functions. These disadvantages are being overcome by experts in the field of synthetic biology. Synthetic biology is a cross-discipline that combines biology and engineering. It aims to achieve the goal of de novo synthesis of organisms through genetic design and construction of new biological elements and artificial gene circuits. In the future, synthetic biology is expected to combine interdisciplinary disciplines to use P450 for the de novo synthesis of fluorinated compounds.
Purine nucleoside phosphorylases
Purine nucleoside phosphorylase (E.C. 2.4.2.1, PNP) is a well-known drug target including cancer and immunodeficiency diseases (Pant et al. 2021; Parker et al. 2020). PNP can reversibly catalyze phosphorolysis of purine nucleosides to form glycosidic bonds (Holanda et al. 2020; Stachelska-Wierzchowska and Wierzchowski 2020; Timofeev et al. 2020). Therefore, PNP is a significant catalyst in the metabolic pathway of purines and nucleosides, especially 2-fluoroadenosine and 2-fluorocordycepin (Kayushin et al. 2021). To obtain fluorinated nucleoside analogs with antiviral activity, a chemo-enzymatic method was developed based on PNP (Fig. 3B) (Kharitonova et al. 2016). In this process, four fluorides were synthesized from 5 mM 1-α-phospho-2-deoxy-2-fluoroarabinose and 1 mM fluorobenzimidazole bases (Conversions of bases = 46–88%; Yields = 40–55%). This provides an effective route for the synthesis of fluorinated nucleoside analogs. Although there is no need for any coenzymes or cofactors to participate, the use of chemical reagents increases the pollution of the reaction. This does not meet the requirements of the green economy and will increase the pressure on subsequent environmental protection treatments. Accordingly, an important challenge of this route is to develop new biocatalysts without chemical reagents.
Tyrosine phenol-lyases
PLP-dependent tyrosine phenol-lyases (EC 4.1.99.2, TPLs) can reversibly synthesize l-tyrosine from phenol, pyruvate, and ammonia (Phillips et al. 2019; Rocha et al. 2019). Thus, a TPL-based process has been developed to obtain the fluorinated l-tyrosine analogs, a potential drug for the treatment of Parkinson's disease (Zhu et al. 2020a). Through the process, o-fluorophenol was transform into 3-fluoro-l-tyrosine by TPL and the space–time yield reached 4.2 g/L/h in fed-batch fermentation (Conversion = 90.2%; Yield = 95.1%) (Fig. 3C). Similarly, other fluorinated l-tyrosine derivatives were also synthesized through TPL-catalyzed synthetic biological systems (Dennig et al. 2015; Li et al. 2020a; Won et al. 2019). Moreover, the donor substrates were generated in situ. In this process, l-lactate oxidase (LOX) and P450BM3 were usually used to generate pyruvate and fluorinated phenol from l-lactate and fluorinated aromatic hydrocarbon, respectively. As such, this method is very promising for the large-scale production of fluorinated l-tyrosine and its derivatives. Moreover, this biocatalytic method is also an important measure to upgrade cheap bulk chemicals to high value-added chiral fluorides. Unfortunately, the low catalytic efficiency requires more machine learning-assisted directed evolution to improve.
Aldolases
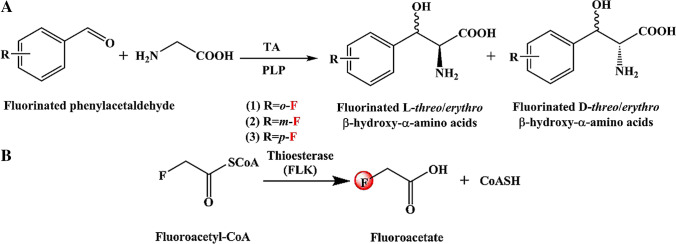
β-Hydroxy-α-amino acids and their fluorinated derivatives are key intermediates for many drugs, which were used for the treatment of Parkinson’s disease immunodeficiency and inflammation (Rocha et al. 2019; Scott et al. 2017). Pyridoxal 5´-phosphate (PLP)-dependent aldolases are regarded as the key biocatalysts for the formation of C–C bonds and have become powerful tools for the sustainable synthesis of complex molecules, especially fluorinated β-hydroxy-α-amino acids through aldol condensation (Chen et al. 2019a; Fang et al. 2019; Zheng et al. 2020, 2021). The formation of two chiral stereocenters is the main feature of this reaction. Moreover, it is difficult to control the stereoselectivity of the two chiral centers at the same time. The introduction of the special fluorine element further increases this difficulty, so that the first stereoselective fluorine-aldol condensation reaction was established in 2016 (Saadi and Wennemers 2016). Subsequently, fluorine-substituted β-hydroxy-α-amino acids are synthesized by d/l-threonine aldolases (d/l-TAs) (Fig. 4A) (Chen et al. 2017, 2019a). In these cases, fluorine is not in the chiral center but the side chain. Various fluorinated d/l-threo/erythro-β-hydroxy-α-amino acids were obtained from 5–100 mM aromatic fluorine substituted aldehydes and 10 equivalents of glycine (Conversions of aldehydes = 29.8–92.8%). Similarly, fluorinated l-threo-threonine was also biosynthesized by 4-fluorothreonine transaldolase (FTase) from l-threo-threonine and fluoroacetaldehyde (Figure S2) (Wu et al. 2020c). Besides, chiral organofluorines have also been synthesized through a chemo-enzymatic method based on type II pyruvate aldolase (Fang et al. 2019). The donor binding and enolization can only be activated by divalent metal cations. In this process, 50 mM fluoropyruvate and twelve aldehydes were synthesized corresponding to β-fluoro-α-hydroxy compounds by pyruvate aldolase, 1 mM MgCl2, and H2O2 (Conversions of polar aldehydes = 80–100%; Conversions of nonpolar aldehydes = 10–70%) (Figure S3). In all reactions catalyzed by aldolases, control of regio and stereoselectivity is the key to the reaction. This is also one of the future development directions of aldolases.
Fig. 4.
The fluorinated compounds with hydroxyl group were obtained. A) Synthesis of fluorinated β-hydroxy-α-amino acids employing threonine aldolase; B) Biosynthesis of fluoroacetate catalyzed by FLK
Fluoracetyl coenzyme A thioesterases
Fluoroacetyl coenzyme A thioesterases (EC 3.1.2.29, FLKs) can catalyze the decomposition of fluoroacetyl coenzyme A (fluoroacetyl-CoA) into fluoroacetate and CoA, and does not catalyze acetyl-CoA (Fig. 4B) (Huang et al. 2006; Thuronyi and Chang 2015). FLK has extremely high specificity for fluorinated substrates, and its hydrolysis efficiency is 10,000-fold that of non-fluorinated substrates (Weeks and Chang 2012). To understand the mechanism of selective fluorination, a comprehensive strategy was implemented for FLK including thermodynamic, kinetic, and biophysical experiments. These studies indicated that entropy drive mediated by hydrophobicity and solvation is the key to selective fluorination recognition, especially the substrate-binding controlled by the active site Phe36 (Figure S4) (Weeks et al. 2018). Moreover, the selectivity to the fluoroacetyl-CoA substrate not only depends on the polarization provided by the negatively charged fluorine substitution but also on the molecular recognition of fluorine during the formation of acyl-FLK intermediate (Weeks et al. 2014). It should be noted that FLK itself also has important physiological functions. FLK can prevent the further metabolism of fluoroacetyl-CoA to 4-hydroxy-trans-arachidic acid ester, a lethal inhibitor of the tricarboxylic acid cycle (Dias et al. 2010). The specific catalytic activity of FLK has great advantages in physiology. In addition, it also has extremely high selectivity for the synthesis of specific fluorinated products. However, more directed evolution engineering should be carried out to obtain fluorinated acetic acid analogs with diverse structures requires. Moreover, the rational use of CoASH is also an issue worth considering in the process of biosynthesis.
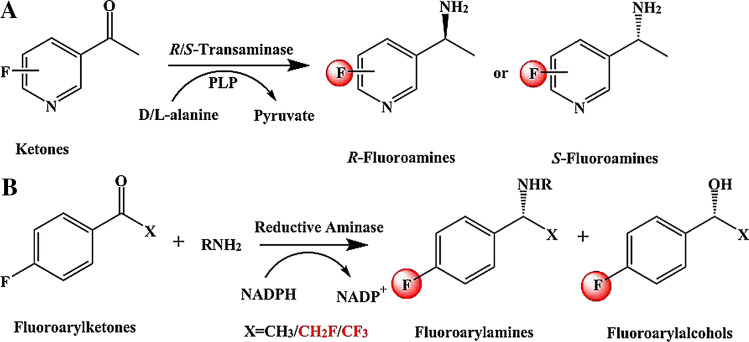
Transaminases
PLP-dependent transaminases are important biocatalysts for the synthesis of chiral amines (Patil et al. 2018). For fluorinated chiral amines, an asymmetric amination method was established based on transaminase (Fig. 5A) (López-Iglesias et al. 2017). R-fluoroamines or S-fluoroamines were obtained from 50 mM 1-(5/6-fluoropyridin-3-yl)ethan-1-one or 1-(6-(trifluoromethyl)pyridin-3-yl)ethan-1-one by R/S-selective transaminase (Conversions = 75–94%; eeR/S > 99%). d/l-Alanine (250 mM) was used as amino donors for transaminase. Similarly, methyl/trifluoromethyl diketone was also catalyzed by transaminase to fluoroamines (Figure S5) (González‐Martínez et al. 2020b). Although the amination of two carbonyl groups will form three products in theory, transaminase only selectively modified smaller methyl ketones in practice. Consequently, the product 1-(4-(1-aminoethyl)phenyl)-2,2,2-trifluoroethan-1-one was obtained by amination of a carbonyl group (ee = 98- > 99%). To further produce chiral fluorinated hydroxylamine, alcohol dehydrogenase was subjected to a hydrogenation reaction to obtain the substrate of hydroxyketones. The hydroxyketones obtained were then converted chiral fluorinated hydroxylamines by transaminase (conversions = 84–90%) (Figure S6). Since the reaction catalyzed by transaminase is reversible, the kinetic resolution reaction can be achieved to obtain fluoroamines from the corresponding racemate. Interestingly, the kinetic resolution reaction catalyzed by transaminase is promiscuous with hydrodefluorination and deamination (Cuetos et al. 2016). In the unconventional process mediated by catalytic promiscuity, a series of aromatic β-fluoroamines were obtained by kinetic resolution through transaminase-mediated hydrodefluorination/deamination reactions (Yields = 75–80%, eeS > 99%) (Figure S7). Notably, this protocol is the first case of using transaminase to catalyze promiscuousness and does not require amino acceptors. Even so, transaminases still require optically pure d/l-amino acids as amino donors. Expensive amino donors increase the cost of biocatalysis and bioconversion. The converted amino donors also increase the difficulty of separating the final products. Moreover, the limited recycling of the cofactor PLP also restricts the further promotion of this method.
Fig. 5.
The fluorinated compounds were obtained by reductive amination. A) Asymmetric amination of ketones to fluorinated amines catalyzed by the transaminase; B) The reaction catalyzed by reductive aminase
Reductive aminases
Chiral fluoroamines are the key components of drug molecules, which can improve efficacy and slow down metabolism. In addition to transaminases, chiral fluoroamines can also be synthesized by reductive aminases, which belong to the imine reductase family (France et al. 2018). Reductive aminase showed high activity for the reductive amination of ketones for the synthesis of fluorinated amines (Aleku et al. 2017). Similar to transaminase, reductive aminase also exhibited a promiscuous reaction to reduce ketones to amines and alcohols (Fig. 5B) (González‐Martínez et al. 2020a; Yang et al. 2021). Through reductive aminase, primary and secondary β-fluoroarylamines and fluoroarylalcohols were obtained from 5 mM fluoroarylketones (Conversions > 90%, ee = 85–99%). As such, reductive aminase provides a sustainable and highly economical biocatalysis toolbox, which can synthesize fluorinated chiral amines and fluorinated chiral alcohols from readily available prochiral substrates. Moreover, inexpensive inorganic ammonium salts provide a lot of convenience as amino donors and do not produce by-products. Nonetheless, the high cost of the coenzyme NADPH is a major disadvantage. Therefore, various coenzyme circulation systems are usually integrated through glucose dehydrogenase or formate dehydrogenase. But it will undoubtedly be more perfect if the coenzyme preference of expensive NADPH is changed to cheap NADH.
Polyketide synthases
Polyketides are a class of complex natural products with multiple structures and biological activities, which are prolific sources of numerous different scaffolds (Maglangit et al. 2019; Tao et al. 2021). Polyketide synthases (PKSs) are multidomain megasynthetases and participate in the synthesis of numerous polyketide natural products in bacteria and fungi (Adrover‐Castellano et al. 2021; Weissman 2016; Zhu et al. 2021). The introduction of fluorine-containing modules into the PKS system can bring fluorine into the scaffold of natural products, which is an important strategy for the synthesis of complex organic fluorides. Here, acetate is the basic element for the formation of polyketide natural products. Hence, the preparation of fluorinated polyketide has been investigated using PKS from fluoroacetate (Fig. 6) (Hong et al. 2008; Klopries et al. 2014; Walker et al. 2013; Wu et al. 2020a). Similarly, fluorinated polyketones can also be obtained from fluoromalonate and fluorobutyrate, which are derived from the basic building blocks malonate and butyric acid, respectively (Klopries et al. 2014; Thuronyi and Chang 2015; Thuronyi et al. 2017).
Fig. 6.
The complex fluorinated compounds were obtained from fluoroacetate as the building block
PKS has three necessary domains, including the ketone synthase (KS) domain, acyltransferase (AT) domain, and acyl carrier protein (ACP) domain (Robbins et al. 2016; Wang et al. 2021). The catalytic process showed that the inactivation of the AT domain can eliminate the selectivity to the fluorine-containing extension unit in the fluorinated polyketones biosynthesis system and initiate the C–C bond formation mode independent of ACP (Ad et al. 2017). Subsequently, the trans-AT complementary domain was incorporated to synthesize both monofluorinated and difluorinated products. Consequently, the use of the PKS module is expected to achieve a stable production method of fluorinated full-length polyketide and other complex products at a specific location. Due to the complexity of the structure of fluorinated polyketones, the selective synthesis mechanism of PKS remains to be elucidated. Therefore, this is a huge challenge for the selective synthesis of various fluorinated substituted polyketones. To explore the catalytic mechanism, more work needs to be done in the field of crystal structure analysis and mutation in the future.
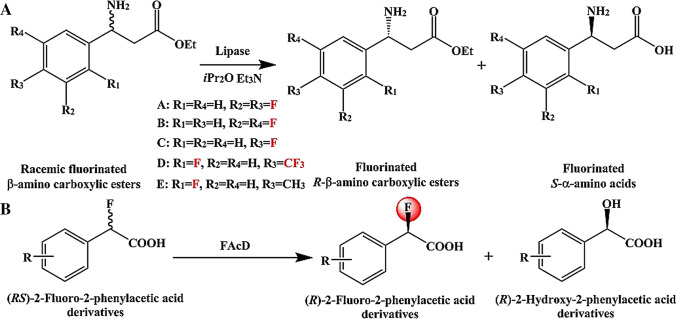
Lipases
Lipases (EC 3.1.1.3) belong to the family of hydrolases. In the presence of organic solvents (such as stearic acids, triacylglycerides, and fatty acids), many substrates can be effectively catalyzed by lipases for acylation (Cavalcante et al. 2021; Huang et al. 2021; Marquez-Rodriguez et al. 2021). Besides, lipases are also excellent biocatalysts to obtain fluorinated compounds in the presence of organic solvents. For fluorinated 1-(pyridin-3-yl)ethanamines, ethyl methoxyacetate, and tetrahydrofuran were used as acylating agents and cosolvent, respectively (López-Iglesias et al. 2017). In this reaction, 100 mM fluorinated racemic amines were converted to corresponding R-isomers and S-isomers through kinetic resolution (Conversions = 50%; eeS = 90–96%; eeR = 99%). Similar to kinetic resolution, lipases can also catalyze enantioselective hydrolysis reactions to obtain β-fluorinated amino acids, which are key components of many natural products, drugs, and biologically active peptides (Shahmohammadi et al. 2020; Zhang et al. 2020b). In the presence of iPr2O, Et3N, and H2O, 25 mM racemic fluorinated β-amino carboxylic ester hydrochloride salts were hydrolyzed into fluorinated R-β-amino carboxylic ester enantiomers (Yields = 38–49%, ee = 94- > 99%) and fluorinated S-β-amino acids (Yields = 48–49%, ee > 99%) (Fig. 7A). Likewise, many other types of fluorinated compounds were also obtained by lipases, like 2,2,2-trifluoroethyl trifluoromethyl sulfate, fluorinated polyesters, and meso-2,4,6-trifluoro-1,3,5,7-tetrahydroxyheptane-1,7-diacetate (Bentler et al. 2019; Snoch et al. 2019; Zhao et al. 2019b). Importantly, lipase has good resistance to organic solvents in addition to being able to catalyze cheap ester substrates. Thus, lipase is an excellent choice for catalytic reactions that require organic solvents to help dissolve substrates. Notably, the pollution of organic solvents to the environment must be carefully considered in this process. The organic solvent recycling device may become an alternative in the future.
Fig. 7.
The fluorinated compounds were obtained by kinetic resolution. A) Fluorinated β-amino acids/carboxylic esters were obtained by lipase; B) 2-Fluorocarboxylic acid derivatives were obtained by fluoroacetate dehalogenase
Fluoroacetate dehalogenases
According to the “Stockholm Convention,” all persistent organic pollutants are halogenated by fluorine, chlorine, or bromine. Therefore, dehalogenation is an important measure to control environmental pollution (Yue et al. 2020). Fluoroacetate dehalogenases (EC 3.8.1.3, FAcDs) are a class of enzymes that can cleave carbon-halogen bonds initiated by SN2 substitution (Kim et al. 2017; Miranda-Rojas et al. 2018; Wang et al. 2017). Accordingly, FAcD is an effective tool to control pollution through enzymatic biodegradation (Li et al. 2019). Interestingly, FAcD can not only degrade fluoride but also obtain fluoride by kinetic resolution of racemates (Fig. 7B) (Zhang et al. 2020a). In this process, 500 mM (RS)-2-fluoro-2-phenylacetic acid derivatives were transformed to corresponding R-isomers and (R)-2-hydroxy-2-phenylacetic acid derivatives by FAcD (Conversion = 50%, Yields of R-fluorocarboxylic acids = 58–97%, ee > 97%). Hence, FAcD can not only eliminate environmental pollution but also effectively synthesize high value-added organic fluorides. It is just that the maximum theoretical yield of kinetic resolution is only 50% and there are by-products. These limit the application of FAcD in the field of fluorides biosynthesis. Therefore, it will be a huge improvement if by-products are converted into products or a two-phase separation system can be developed for products and by-products.
Glycosidases: β-mannosynthase and β-glucosidase
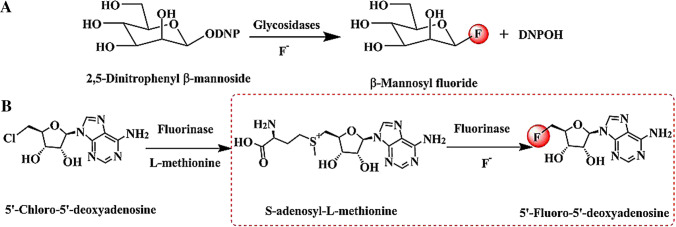
Typical glycosidases can hydrolyze sugars at specific locations and types (Li and Fan 2020). Excitingly, the C-F bonds can be formed by glycosidases (β-mannosynthase and β-glucosidase) through nucleophilic fluorination (Fig. 8A) (Nashiru et al. 2001; Zechel et al. 2001). This is the first enzyme catalyst with selective fluorination for C-F bonds discovered. Utilizing glycosidase, 23 mM 2,5-dinitrophenyl β-mannoside and 2 M KF were successfully transformed to β-mannosyl fluoride. Unfortunately, this fluorinated compound has not been isolated. This is because the fluorinated compound formed can also serve as a glycosyl donor and continue to participate in the transglycosylation reaction to form new glycosidic bonds. This process will cause the formed C-F bond to be degraded. Therefore, it is difficult to accumulate fluorinated products. This is why this enzyme has not been widely used to synthesize fluorinated compounds. In the future, it may become possible to use this enzyme for the biocatalytic synthesis of fluoride by controlling the progress of the reaction. Besides, a specific fluorinated product separation device may be necessary to utilize glycosidases.
Fig. 8.
The fluorinated compounds were obtained through the formation of C-F bond. A) β-Mannosyl fluoride was formed from 2,4-dinitrophenyl β-glycoside catalyzed by glycosidases (DNP = 2,5-dinitrophenyl); B) Two-step synthesis of fluorinated compounds by transhalogenation reaction catalyzed by fluorinase
Fluorinases
In 2002, a fluorination enzyme (fluorinase) was isolated and identified from the soil bacterium Streptomyces cattelya for the first time (O'Hagan et al. 2002). The fluorinase (EC 2.5.1.63) is also called 5´-fluoro-5´-deoxyadenosine (5´-FDA) synthase, which participates in the secondary metabolism of fluorinated natural products. The fluorinase can catalyze the synthesis of 5´-FDA from S-adenosyl-l-methionine (SAM) and fluoride ion through nucleophilic attack to form a C-F bond (Fig. 8B, red dashed frame). As such, fluorinase has become an important biocatalyst for the synthesis of fluorinated nucleosides and their derivatives. Although fluorinase from S. cattleya had been applied to catalyze the non-natural substrates, a major drawback is that it exhibited reduced catalytic activity for unnatural substrates (Fraley and Sherman 2018). To overcome this obstacle, the directed evolution strategy was first implemented on fluorinase in 2016 (Sun et al. 2016; Thomsen et al. 2013). In this process, 23 sites were selected within 5 Å of the substrate, and the best mutant F213Y/A279L was obtained after saturation mutation and high-throughput screening (1932 clones). The catalytic efficiency of mutant F213Y/A279L was significantly increased by 25-fold compared to that of wild type for the non-natural substrate 5´-chloro-5´-deoxyadenosine (5´-ClDA). In addition, the efficiency in synthesizing radioactive 5'-[18F] FDA was also tripled compared to that of wild type. To expand the synthetic range of fluorinase, a new class of fluorinated nucleoside receptor agonists was synthesized from 0.1–0.2 mM chlorinated precursors, 50 mM KF, and 0.075 mM l-SeMet (Conversion = 10%, Yield = 3.5%) (Lowe et al. 2017). The agonist belongs to the analogs of 5´-FDA with a terminal functionalized ethynyl unit substituted at the C2 position. Similarly, the fluorinated RGD peptides and prostate-specific membrane antigen were also synthesized by fluorinase through transhalogenation reaction (Lowe et al. 2019b; Thompson et al. 2016a, 2015; Zhang et al. 2016). The above-mentioned reaction was a two-step process in nature. In the first step, 5´-ClDA was transformed into SAM in the presence of fluorinase and l-Met. In the next step, the producing SAM was transformed into 5´-FDA in the presence of fluorinase and fluoride ions (Fig. 8B).
Moreover, the first reaction is the rate-limiting step for the synthesis of fluorinated compounds. To overcome the rate-limiting step, two novel SAM-dependent chlorinases were discovered to improve the efficiency of the transhalogenation reaction in the first step reaction. Finally, a chlorinase-fluorinase coupling system was developed. Through this system, 5´-FDA and 5´-fluorodeoxy-2-ethynyladenosine (5´-FDEA) were synthesized efficiently from 0.2 mM 5´-ClDA/5´-chlorodeoxy-2-ethynyladenosine (5´-ClDEA), 0.1 mM l-SeMet, and 80 mM NaF (98.04 ± 0.27% and 91.60 ± 0.13% yields, respectively) (Sun et al. 2018). Surprisingly, Phillip T. Lowe et al. found that intermediate products SAM and l-methionine are not necessary for synthesizing 5´-FDA through fluorinase (Lowe et al. 2019a). Fluorinase can directly catalyze the synthesis of 5´-FDA from 5´-ClDA, 5´-bromo-5´-deoxyadenosine (5´-BrDA), and 5´-iodo-5´-deoxyadenosine (5´-IDA) in the presence of fluoride ion without SAM and l-Met (Finkelstein Reaction). Additionally, the highest conversion efficiency was obtained for the substrate 5´-BrDA (35%) compared with 5´-IDA (8%) and 5´-ClDA (5%).
In addition to the catalytic process in vitro, the metabolic pathways of fluoride in microorganisms have also been elucidated, and related fluorinated natural products and biocatalysts have been confirmed (Figure S8). Unfortunately, there are few sources of fluorinases, which limits the development of effective biocatalysts for the synthesis of fluorinated compounds. Before 2016, only 5 fluorinases were identified and characterized from S. cattleya, Streptomyces sp MA37, Norcardia brasiliensis, Actinoplanes sp N902-109 and Streptomyces xinghaiensis NRRL B24674 (Deng et al. 2014; Ma et al. 2016; Ren et al. 2020). Until recently, the sixth fluorinase was identified from Actinopolyspora mzabensis (AmFLA) (Sooklal et al. 2020). Due to AmFLA was an inclusion body, it was refolded into soluble protein through 8 M urea. The soluble AmFLA showed the specific activity of 0.44 ± 0.03 µM/min/mg for SAM at pH 7.2 and 65 °C (both the optimal conditions). Besides, AmFLA also exhibited comparatively thermostability that it still retained 26% catalytic activity at 25 °C for 2 months and > 70% catalytic activity at 80 °C for 20 min. Surprisingly, the specific activity of AmFLA was increased by 9.5% with the help of 1 mM Mg2+. This is the first time that fluorinase was found to be metal ion-dependent. To improve the activity and stability, fluorinase was immobilized with fluoridated hydroxyapatite nanoflowers (Li et al. 2020b). Under the same conditions, the activity of immobilized fluorinase is twice as high as the free enzyme. After 8 h of incubation at 30 °C, 80% of activity was retained for the immobilized fluorinase and only 48% for free fluorinase. Apart from immobilization methods, protein self-assembly is also an effective strategy to improve activity and stability (Tu et al. 2020). With the help of self-assembled tags, fluorinase was formed into a novel artificial enzyme with nanometer dimensions. Strikingly, the novel artificial fluorinase exhibited higher enzyme activity, thermal stability, and reusability than the original fluorinase. Notably, fluorinase is currently the only biocatalyst widely used in the synthesis of fluorinated compounds that can form the C-F bond. In particular, fluorinase has great application potential for the synthesis of radioactive fluorinated imaging tracers. However, only limited potential has been exploited for fluorinase. Therefore, there is still much work to be done as soon as possible, especially considering that there are only six fluorinase sources, only two fluorinase crystal structures, low enzyme activity and narrow substrate range.
Multienzyme system
Not all products of interest can be obtained through a single biocatalyst as desired. Inspired by the natural multi-enzyme catalytic system, many high value-added products have been obtained through the artificial multi-enzymatic system, especially fluorinated compounds (Table 1). Accordingly, the artificial multi-enzymatic system was established for fluorinated aromatic compounds, including l-amino acid deaminase (LAAD), α-keto acid decarboxylase (ARO10), and aldehyde dehydrogenase (ALDH) (Figure S9) (Mao et al. 2020). Through the system, 10 mM m-fluoro-d/l-phenylalanine was transformed to m-fluoro-phenylacetic acid with a conversion of 48% in 24 h. The artificial multi-enzymatic system constructed has been proved to be effective in synthesizing non-natural fluorinated aromatic compounds without transferring intermediate products. Similarly, a multi-enzymatic system was performed with aromatic amino acid transaminase and hydroxymandelate synthase (HMS), producing fluoro-l-mandelic acid from 5 mM fluoro-l-phenylalanine (Yield = 65 ± 1.4%, ee > 86%) (Figure S10) (Youn et al. 2020). Moreover, ortho and meta fluorinated mandelic acids were also synthesized in the same method (Yields = 37 ± 0.4% and 47 ± 1.8%). Also starting from substrate fluoro-l-phenylalanine, fluoro-benzyl alcohol was successfully synthesized in a multi-enzymatic system of five enzymes, including LAAD, HMS, l-mandelate dehydrogenase (LMDH), benzoylformate decarboxylase (BFD), and phenylacetaldehyde reductase (PAR) (Yield = 633.17 mg/L) (Figure S11) (Liu et al. 2020).
Table 1.
The multi-enzymatic systems of fluorinated compounds
| Entry | Substrates | Multi-enzyme catalyst | Products |
|---|---|---|---|
| 1 | m-fluoro-d/l-phenylalanine |
l-amino acid deaminase α-keto acid decarboxylase aldehyde dehydrogenase |
m-fluoro-phenylacetic acid |
| 2 | o/m/p-fluoro-l-phenylalanine |
aromatic amino acid transaminase hydroxymandelate synthase |
o/m/p-fluoro-l-mandelic acids |
| 3 | fluoro-l-phenylalanine |
l-amino acid deaminase hydroxymandelate synthase l-mandelate dehydrogenase benzoylformate decarboxylase phenylacetaldehyde reductase |
fluoro-benzyl alcohol |
| 4 |
fluorophenols pyruvate |
tyrosine phenol-lyase tyrosine ammonia-lyase |
p-hydroxyfluorocinnamic acids |
| 5 |
4-deoxy-4-fluoro-N-acetylhexosamine pyruvate |
N-acyl-d-glucosamine 2-epimerase sialic acid aldolase CMP sialic acid synthetase inorganic pyrophosphatase sialyltransferase |
(7F-)sialyl galactoside |
Besides, a catalytic system coupled with TPL and tyrosine ammonia-lyase (TAL) was established to obtain the fluorinated unsaturated carboxylic acid (Figure S12) (Busto et al. 2016). This overcomes the shortcomings of by-products produced in the direct alkenylation process. During the TPL-TAL coupling process, 23 mM fluorophenols and 46 mM pyruvate were converted into fluoro-l-tyrosines by TPL. Subsequently, the intermediate products obtained were transformed to p-hydroxyfluorocinnamic acids by TAL (Yield > 95%). Moreover, fluorinated galactoside was also synthesized by the multi-enzyme catalysis method (Figure S13) (Geissner et al. 2021). This is mainly because fluorinated galactoside can improve the stability of glycoprotein, and stable glycoprotein plays a vital role in the persistence of the therapeutic effect. In this process, 100 mM 4-deoxy-4-fluoro-N-acetylhexosamine and 500 mM pyruvate were transformed to 7-modified sialic acid through porcine N-acyl-d-glucosamine 2-epimerase and sialic acid aldolase (Conversion = 95%, Yield = 91%). Subsequently, the 7-modified sialic acid obtained was converted to 7-modified CMP-sialic acid (CMP = cytidine monophosphate) by CMP sialic acid synthetase and inorganic pyrophosphatase (Yield = 86%). Ultimately, the 7-modified CMP-sialic acid obtained was transformed to (7F-)sialyl galactoside by sialyltransferase and alkaline phosphatase (Conversion = 100%, Yield = 64%). The final results indicated that the half-life of the therapeutic glycoprotein was significantly provided by the incorporation of (7F-)sialyl galactoside synthesized.
The multi-enzyme synthesis of fluorine compounds can not only expand the application potential of enzymes as catalysts but also provide new ideas for the preparation of complex fluorinated products. Multi-enzyme catalysis can effectively shorten the transfer distance of intermediate products and accelerate the reaction rate. Moreover, the synthetic route can be realized from cheap substrates as starting substrates. However, there are still many challenges facing the development of the multi-enzymatic synthesis of fluorine compounds. First, the cost of the catalyst is increased due to the preparation of multiple enzymes. Second, the compatibility needs to be considered for multiple enzyme reaction conditions (for example, temperature, pH, metal ions, inhibition of other enzymes by intermediate products or coenzymes). Finally, it is necessary to balance the activity and stability of multiple enzymes at the same time.
Structure and catalytic mechanism of fluorinases
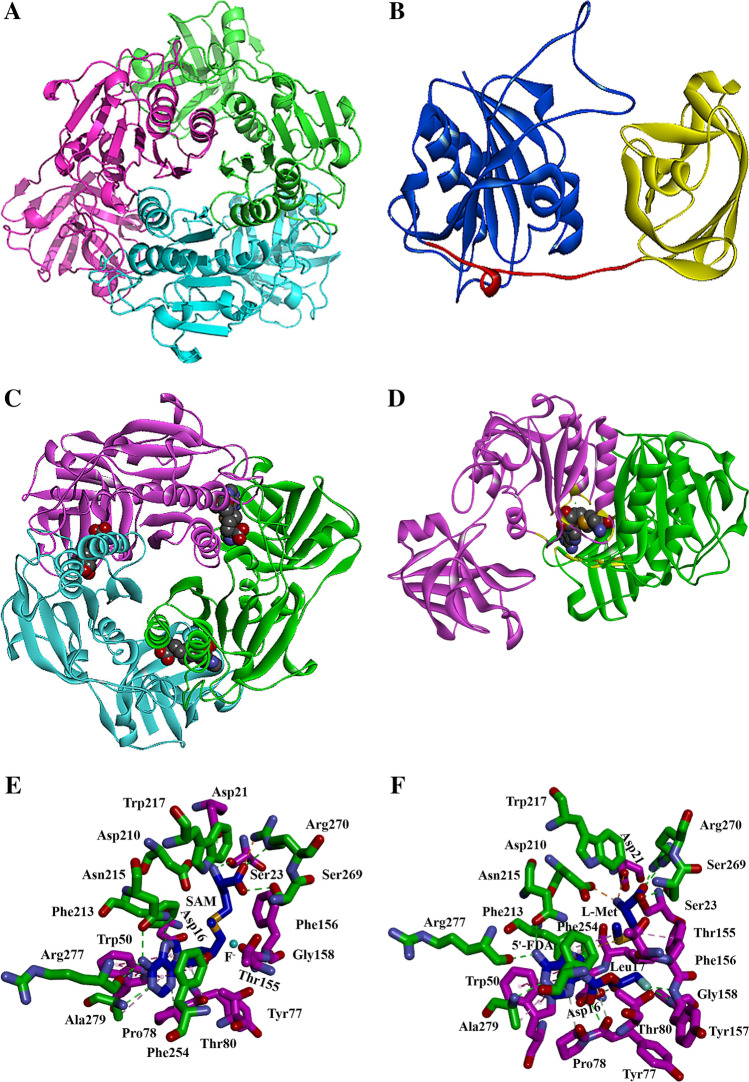
As an important biocatalyst for the formation of the C-F bond, the structure and catalytic mechanism of fluorinase have always been a topic of concern in the field of biocatalysis and biotransformation. There is a close relationship between protein structure and function. Therefore, studying the structure of fluorinase is essential for understanding the catalytic mechanism of enzymes. To confirm the conserved amino acids, the multiple sequence alignment of six known fluorinases was constructed using DNAMAN (Figure S14). 182 conserved residues were identified, and the identity is as high as 85.11%. This indicated that the six known fluorinases have a high degree of homology and evolutionary origin from the primary structure of the protein. These conserved regions are the key to the catalytic function of fluorinases. Moreover, the crystal structures of fluorinases have been obtained only from two microbial sources, Streptomyces cattleya, and Streptomyces sp. MA37. The first crystal structure of fluorinase from S. cattleya was resolved to 1.9 Å resolution (Cobb et al. 2006; Dong et al. 2003, 2004). Through gel filtration analysis, the single frozen protein is a symmetrical hexamer (186 kDa) composed of two trimers. The trimer is composed of three identical monomers (Fig. 9A). The monomer has two domains: the amino-terminal domain (residues 1–180) and the carboxy-terminal domain (residues 195–298), and a loop structure that connects the two domains (residues 181–194) (Fig. 9B). The amino-terminal domain is composed of two antiparallel β-sheets with 4 and 5 helices. The carboxy-terminal domain has a 7-stranded β-sheet, which is sandwiched between the parallel and antiparallel α-helix strands. However, a single crystal structure cannot completely analyze the catalytic mechanism of fluorinase. As such, the crystal structure of fluorinase bound to substrate and product was obtained (Fig. 9C). In this structure, SAM was located between the C-terminal of a monomer and the N-terminal of a monomer in the trimer (Fig. 9D). The catalytic function of fluorinase must rely on the interactions of two monomers. As shown in Fig. 9E and F, the active sites of the fluorinase are distributed around the substrates or products and at the ends of two adjacent monomers. When combined, SAM is completely buried by fluorinase, which suggests that the fluorinase has other open conformations during the catalysis process. Comparing the binding status of substrate and product with fluorinase showed that 5´-FDA and l-methionine occupied the binding site of SAM after the reaction. Also, the difference between the two states is very small except for the bond formation/fracture area (Deng et al. 2004). This indicates that the key structures and regions are similar to the substrates and products of fluorinase and located between the two domains. Furthermore, the reaction is activated by changes in these key structures and regions.
Fig. 9.
The structures of fluorinase. A) The trimer structure formed by three monomers; B) The monomer structure formed by the amino-terminal domain (blue), carboxy-terminal domain (yellow), and loop linker (red); C) The co-crystal structure of trimer and substrate SAMs; The SAM molecules are displayed in CPK format; D) The stereogram of SAM, fluorine (Replaced by chlorine in actual experiments) and interacting monomers during the catalysis process; E) The three-dimensional structure of the substrates and active sites complex; The SAM is displayed as a stick model with center carbon in blue; The fluorine is displayed in light blue; F) The three-dimensional structure of the products and active sites complex; The 5´-FDA and l-Met are displayed as a stick model with center carbon in blue. Note: Classical hydrogen bond: green dashed line; Non-classical hydrogen bond: gray dashed line; Electrostatic interaction: orange dashed line; Hydrophobic interaction: purple dashed line
In the field of organic chemistry, SN2 nucleophilic substitution is a paradigm reaction (Xie and Hase 2016). Through structure and function analysis, asymmetric SN2 nucleophilic fluorination is the key to the formation of C-F bonds for fluorinase (Pupo et al. 2018). Moreover, the SN2 reaction mechanism of fluorinase was also supported strongly by QM/MM calculations (Senn et al. 2005). In the process of forming the C-F bond, the reaction is thermodynamically driven due to the slow reaction Kcat and the equilibrium of the reaction lies in the products. The SN2 nucleophilic attack will provide a hydrogen bond for the anion, which can stabilize the desolvated F− and is very important for the selective fluorination of sp3 carbon centers (Lee et al. 2016). This indicates that the positively charged SAM sulfur is a crucial factor in stabilizing the partial desolvation and transition state. Consequently, the detailed molecular mechanism of fluorinase was further revealed (Zhu et al. 2007). During the catalysis process, solvated fluorine first binds to the unoccupied pockets and exchanges the hydrogen bonds of the water molecules to the polar groups of the protein (amide NH and side-chain OH of S158). The SAM is then bound, and the remaining water molecules enter the hydrophobic binding pocket to dissociate the fluorine. Remarkably, SAM is a competitive inhibitor of fluoride ion binding to fluorinase during the transition state. Afterwards, fluorine SN2 attacks C5 of SAM and replaces l-Met of SAM. After the conformation of loop (A95-Q102) changes, l-Met is released. Moreover, the loop participates in the combination of l-Met but not directly combined with l-Met. And then, the conformation of a loop (T75-R85) is changed to release 5´-FDA, which the loop forms the binding region for the adenine ring and ribose of SAM and 5´-FDA derivatives.
To further explore the catalytic mechanism of fluorinases, a series of different types of substrates and mutants were analyzed and compared. When analyzing the crystal structure of fluorinase, an extensive hydrogen bond network was formed through water molecules and the carboxylate groups of l-Met with amino acids (T155, W217, Y266, backbone amide of S269) (Thomsen et al. 2013). The two water molecules were tightly bound at the active site of the fluorinase. This indicated that the instability of these water molecules may promote the accommodation of sterically bulky substrates. Therefore, site-directed mutagenesis was performed at these sites by introducing small hindered amino acids and disrupting the hydrogen bond network. Y266F and T155S exhibited significantly higher catalytic efficiency than that of the wild type. The results showed that the hydrogen bond network of the active center is an important factor for fluorinase to accommodate large sterically hindered substrates. The two amino acid residues 266 and 155 play a significant role in the formation of the hydrogen bond network. For substrates modified at the C2 (R1 substitution) and C6 (R2 substitution) positions of the adenine ring, the substrates catalytic range was also studied (Figure S15) (Yeo et al. 2017). The results showed that substrates with hydrogen donors at the C2 position of the adenine group interact better with the fluorinase, obtaining a high yield. Besides, amino acid residue 279 plays an important role in promoting binding to ethynyl of the substrate at the C2 position. The C2 position is sensitive to large steric hindrances. Unfortunately, all substitutions at the C6 position caused catalysis difficulties, except for chlorine and hydrogen. Similarly, the directed evolution was also implemented in the process of catalyzing 5´-ClDA to SAM to 5´-FAD (Sun et al. 2016). Mutants F213Y/A279L have been shown to increase the yield of 5´-FDA. Specifically, F213Y/A279L can increase the turnover from 5´-ClDA to SAM but decrease the turnover from SAM to 5´-FAD. These mutations did not affect the affinity of the enzyme to 5´-ClDA but increase the affinity to SAM. The movement of the loop (277–281) is closely related to the binding of fluorinase and adenine. When position 279 is mutated to a large hindered amino acid, the movement of the loop is restricted, resulting in a tighter binding. Moreover, the hydrophobic environment facilitates the binding of substrates in the substrate-binding pocket. Leu279 and Tyr213 participate in favorable hydrophobic contact. The former improves the hydrophobicity of the environment through van der Waals forces with aromatic carbon of adenosine. The latter acts as a clamp between protein monomers across the ribose ring. Additionally, the reverse reaction of fluorinase was also applied to study the catalytic mechanism of the substrate with the difluoromethyl group (Thompson et al. 2016b). Regrettably, no reaction was observed in the presence of 5´,5´-difluoro-5´-deoxyadenosine 7 (F2DA) and l-Met/l-SeMet. As such, the co-crystal structure of fluorinase and F2DA, and tartrate was obtained to analyze the reason (1.8 Å). Structural analysis showed that the difluoromethyl bridged the interaction known to be essential for the activation of single fluorine in 5´-FDA. The bridging reaction was combined with the strong C-F bonds inherent in the difluoromethyl group, and there was an abnormal hydrogen bond interaction between the hydrogen of the difluoromethyl group and hydroxyl oxygen of the tartrate ligand. This indicated that the geometry of the bond is no longer suitable for the attack of sulfur or selenium and form higher response barriers. This explains why no response was observed for F2DA. Thus, the hydrophobic environment and steric hindrance of the substrate-binding pocket are essential for the catalytic reaction of fluorinase. In addition, the movement of the loop structure is also essential to regulating the catalytic function around the substrate binding pocket.
Applications of fluorinated compounds
PET tracers
Positron emission tomography (PET) is a non-invasive molecular imaging technique that relies on compounds labeled with positron emitters (da Silva et al. 2018; Preshlock et al. 2016). Nowadays, PET has become a valuable tool for detecting pathology in the clinical field. Fluorine is a typical positron emitter due to its long half-life (t1/2 = 110 min), high isotope abundance, large gyromagnetic ratio, sensitivity, and specificity (Clark and O’Hagan 2017; da Silva et al. 2018; Lu et al. 2019; Troelsen et al. 2020; Yamamoto et al. 2019). Since 2-fluorinated glycosides are more stable than glycosides, they can be used as active probes (Sadurni and Gilmour 2018). In traditional PET technology, 2-18F-fluoro-2-deoxy-d-glucose (18F-FDG) was the most commonly used tracer in the diagnosis of most diseases. Nevertheless, 18F-FDG-PET is far from meeting the requirements of modern disease detection technology. 18F-FDG is based on glucose, but most disease tissues contain a lot of glucose which interfere with accuracy (de Zwart et al. 2020; Langen et al. 2017; Lohmann et al. 2019). Therefore, more and more fluorinated PET tracers are being developed. For example, the 18F-fluoro-l-3,4-dihydroxy-phenylalanine (18F-DOPA) was used as an amino acid tracer in PET imaging (Christine et al. 2019; Fraioli et al. 2020; Mossine et al. 2020). This (18F-DOPA) PET could be applied to assess the volume of remaining gliomas in the patient, Parkinson's syndrome, and focal hyperinsulinemia in infants, which was a perfect complement to the traditional detection technology. Except that, other types of fluorinated compounds were also used as PET tracers for cancer and tumor detection, such as 18F-PSMA-1007 (Dietlein et al. 2020), l-2-18F-FAMP, d-2/3/4-18F-FAMP and l-3-18F-FAMT (Hanaoka et al. 2019; Mahendra et al. 2020; Shimizu et al. 2019), (18F-FBPA) (Romanov et al. 2020), 18F-fluoro-ethyl-l-tyrosine (18F-FET) (Fuenfgeld et al. 2020; Marner et al. 2019), 6-18F-fluoro-meta-tyrosine (18F-FMT) (Badin et al. 2019; Kojima et al. 2019; Miyamoto et al. 2020), N-[18F]-fluoropropylJDTic (Schmitt et al. 2017), 2-(3-(1-carboxy-5-[(6- 18F-fluoro-pyridine-3-carbonyl)-amino]-pentyl)-ureido)-pentanedioic acid (18F-DCFPyL) (Jansen et al. 2019; Rousseau et al. 2019; Rowe et al. 2020a, 2020b), 5-18F-fluoro-l-amino suberate (18F-FASu) (Alluri et al. 2020; Colovic et al. 2019), 4-borono-2-18F-fluoro-l-phenylalanine fluorine substituted 3-azabicyclo hexane (Chen et al. 2019b), and [18F]FDA-PEG-biotin/tetrazine (Lowe et al. 2018). Therefore, PET tracers based on new fluorinated compounds will play an increasing role in the field of visual detection technology.
Antitumor inhibitors
To effectively treat the tumor, inhibiting key enzymes of tumor cells is regarded as an important treatment. Hence, many fluorinated compounds have been developed as novel antitumor inhibitors, considering the problems of drug resistance, solubility, and toxicity. For example, diverse fluorinated cyclohexene derivatives were used to inhibit the activity of hOAT to treat HCC, because human ornithine aminotransferase (hOAT) plays an essential role in metabolic pathways of hepatocellular carcinoma (HCC) (Zhu et al. 2020b). Especially (1R,3S,4S)-3-amino-4,4-difluoro cyclopentane-1-ene-1-carboxylate and (1R,3S,4S)-3-amino-4-fluoro cyclopentane-1-ene-1-carboxylate, they showed the total different inactivation mechanisms from previous inhibitors. Moreover, tyrosine kinase (TK) is the epidermal growth factor receptor (EGFR) of A549 and Hela cancer cells. Four fluorinated quinazolinone derivatives showed excellent anti-proliferative properties against A549 and Hela cancer cells by inhibiting TK (IC50 = 0.462–7.568 µM; IC50 = 0.175–7.096 µM) (Le et al. 2020).
In addition to better resistance, fluorine-containing inhibitors have a stronger inhibitory effect than traditional non-fluorine anti-tumor inhibitors. Such as, steroid sulfatase (STS) is an important enzyme to regulate metabolism in breast cancer cells, which is a malignant tumor with the highest incidence among women (Armstrong et al. 2020; Maltais et al. 2020). Therefore, various inhibitors of STS have been developed gradually. To improve the inhibitory effect, C-F bonds were introduced into STS inhibitors. Their inhibitory efficacy was ten-fold that of traditional inhibitor (coumarin-7-O-sulfamate), especially 3-(3,4-difluorophenyl)-2-oxo-2H-chromen-7-yl sulfamate, 2-oxo-3-(3,4,5-trifluorophenyl)-2H-chromen-7-yl sulfamate, 3-(2-(2,5-bis(trifluoromethyl)phenyl)acetamido)-2-oxo-2H-chromen-7-yl sulfamate, and 3-(3,4-difluorobenzamido)-2-oxo-2H-chromen-7-yl sulfamate (IC50 = 0.18–0.27 µM) (Dasko et al. 2017; Demkowicz et al. 2016). Correspondingly, tautomerase is human macrophage migration inhibitory factor (MIF), which is also a pro-inflammatory cytokine associated with many tumors. Studies showed that the growth of the tumor can be effectively inhibited by MIF inhibitors and the inhibitory effect can be significantly enhanced by adding fluorine atoms (Dziedzic et al. 2015). Similarly, other fluorinated compounds also exhibited stronger inhibitory effects than non-fluorinated compounds for distinct tumor cells, such as fluorinated green tea polyphenols, fluorinated largazole, fluorinated 4-thiazolidinone, fluorinated griseofulvin, fluorinated l-threonine, fluorinated aminophosphonite, and fluorinated docetaxel (Zhang et al. 2019a) (Stadlbauer et al. 2018) (Makki et al. 2019; Olgun 2019; Paguigan et al. 2017; Sudileti et al. 2019; Tang et al. 2016). These results indicated that the incorporation of fluorine-substituted fragments into the inhibitor is an effective means to enhance the therapeutic effect of tumors.
Antiviral inhibitors
In addition to being novel anti-tumor inhibitors, fluorinated compounds are also effective against viruses. Coxsackie virus B (CVB) is a common pathogen for humans, causing aseptic meningitis, pericarditis, and myocarditis. To counter CVB, seven fluorinated pyrimidine nucleoside analogs were synthesized and tested for their antiviral activity (Tao et al. 2020). The results showed that 4-N-(2′-amino-glutarate-1′-methylester)-1-(2′-deoxy-2′-β-fluoro-4′-azido)-furanosyl-cytosine has the most effective activity of anti-CVB (IC50 = 9.3 µM). Similarly, 1-(2´-Deoxy-2´-fluoro-β-d -arabinofuranosyl)benzimidazoles were also proven to have anti-herpes virus activity (Kharitonova et al. 2016). As for antiviral activity, the global health system is currently being destroyed by the novel coronavirus (COVID-19). Although diverse vaccines have been developed, they cannot produce a therapeutic effect on patients who have been infected with COVID-19. As such, it is crucial to find special drugs for COVID-19. As we all know, protease Mpro is an important drug target for COVID-19. Through molecular simulation, antiviral N-heterocycles (2’-fluoro-2’-deoxycytidine) was evaluated for its inhibitory effect on protease and showed good affinity for target receptors (Hagar et al. 2020). So far, there is no specific drug that can treat COVID-19 through experimental verification. But, it is believed that fluorinated compounds have this potential. Moreover, more and more fluorinted compounds will be artificially synthesized and used to fight viruses in the future of fluorinated biochemistry.
Pharmaceutical intermediates
Traditional pharmaceutical intermediates are increasingly unable to meet the needs of the current pharmaceutical industry. The development of novel pharmaceutical intermediates has become more and more urgent and necessary. Selective fluorination is an effective strategy to improve pharmaceutical efficacy, half-life, lipophilicity, absorption rate, and the permeability of cell membranes (Sudileti et al. 2019; Zhou et al. 2016). Therefore, the exploration has never stopped for the role of fluorine in drug design since the first fluorinated drug (9α-fluoro-substituted corticosteroid fludrocortisone) was approved in the 1950s (Fried and Sabo 1954). Nowadays, fluorine functional groups are contained in more than 25–30% of drugs, such as mefloquine, teriflunomide, citalopram, sorafenib, efavirenz, tedizolid phosphate, donepezil, and vorapaxar (Adler et al. 2019; Izquierdo et al. 2019; Khosravan et al. 2017; Markakis et al. 2020; Mei et al. 2019, 2020b; Palmer-Brown et al. 2017; Zhang et al. 2020b).
A typical example is the use of fluorinated compounds as drug intermediates for the treatment of Alzheimer's disease (AD). AD is associated with changes in cAMP (cyclic adenosine monophosphate) and cGMP (cyclic guanosine monophosphate) signals (Nakashima et al. 2019). Accordingly, regulating the levels of cAMP and cGMP in the brain has become an effective means of treating AD. It is known that phosphodiesterase 2A (PDE2A) is involved in the hydrolysis of cAMP and cGMP. Therefore, fluorine-substituted carboxamide analog as PDE2A inhibitors (TAK-915) was used to test the effect of treating AD. The results showed that TAK-915 was the most effective in treating AD when taken orally at 10 mg/kg. In addition, acetylcholinesterase is also an important target of AD. The activity of acetylcholinesterase can be inhibited by piperine. To overcome the poor water solubility and photostability of piperine, a threo-difluoropiperine analog was synthesized and showed higher potency and selectivity than piperine for the treatment of AD (Lizarme-Salas et al. 2020). Similarly, fluorinated compounds are also the key building blocks of antibiotic, antimalarial, antiinflammatory, asthma, antagonists, migraine, and central nervous system drugs, such as 2,2-bis(6-fluoro-1H-indol-3-yl)ethan-1-amine, fluorinated steroidal, Trifluorothymidine and fluorouracil polytoxin, fluorinated cholesterol, fluorinated galegine, fluorinated bastimolide A, 6(R/S)-fluoropenibruguieramine, fluorinated cyclopropanecarboxylic acid derivatives, aryl-fluoro sulfates, 3-fluoro-4-aminopiperidine, and β-fluoramines (Adler et al. 2019; Bakhotmah and Abdel-Rahman 2017; Campana et al. 2020; Frank et al. 2016; Fujino et al. 2017; Gambini et al. 2019; Liu et al. 2018; Molinaro et al. 2019; Munck Af Rosenschold et al. 2019; Pupo et al. 2019; Quintard et al. 2018; Shao et al. 2015; Wu et al. 2020d). Accordingly, fluorinated compounds will pay more and more attention to the research and development of new pharmaceutical intermediates. Many intractable diseases are also expected to be cured.
Functional materials
As we all know, white spot lesion (WSL) is the main cause of dental caries. The main measure of WSL management is to prevent the formation of new lesions. The fluorinated compounds can harden the surface layer of minerals, thereby preventing the occurrence of lesions and the development of dental caries (Decha et al. 2019; Lena Sezici et al. 2021; Raskin et al. 2021; Shah et al. 2018). Therefore, fluorinated compounds can be used to synthesize dental care materials, especially silver diamine fluoride, quaternary ammonium fluoride salt. Besides, the fluorinated membrane also displayed anti-pollution and anti-wetting properties in the field of industrial wastewater treatment (Abdulkarem et al. 2021; Cheng et al. 2021; Ji et al. 2021; Kang et al. 2021; Koh and Lee 2021; Li et al. 2021; Nayak and Tripathi 2021; Xiao et al. 2021; Zhong et al. 2021). Due to this unique performance, the fluorinated membrane can be used to treat wastewater with high salinity and hardness, remove heavy metal ions, separate oil, water, and as a flexible dielectric material and air filter with antibacterial properties. Moreover, the performance of traditional materials can also be enhanced by fluorine substitution. For instance, acrylate is the main component of rubber; Fluorinated acrylate is usually used to improve the compatibility and toughness of rubber (Yimmut et al. 2018). Similarly, fluorographene is a two-dimensional material with a high surface-to-mass ratio. Unlike graphene, fluorographene material is a wide band gap insulator because it lacks a conjugated network of π-conjugated electrons. As one of the thinnest insulators, fluorographene is most commonly used in the battery field. Fluorographene also plays an important role in industrial lubrication due to its delamination nature, low surface energy, and easy peeling characteristics (Chronopoulos et al. 2017; Liang et al. 2021). As such, fluorinated compounds are gradually becoming important sources of novel functional materials.
Conclusions and perspectives
Fluorine is a distinctive element and fluorine-containing organic compounds are playing an increasingly important role in the fields of molecular imaging, pharmaceuticals, and materials. This has also led to high demand for reagents capable of selectively introducing fluorine into organics, especially biological enzyme catalysts. In this review, 13 enzymatic pathways are introduced. There are two main strategies for the enzymatic synthesis of fluorinated compounds: (1) C-F bonds are directly formed to obtain fluorine-containing compounds. (2) Complex fluorinated compounds are synthesized from simple fluorine-containing modules. Of all the current enzymatic methods, the catalytic route mediated by fluorinase has the most potential for applications. Nevertheless, the artificial green synthesis of fluorinated compounds still has a long way to go. Since C-F is a strong and inert chemical bond, it is extremely challenging to artificially synthesize compounds containing the C-F bond, especially the selective incorporation of fluorine into biologically active molecules.
Therefore, many future efforts will be made by our laboratory and other counterparts in the following aspects. (I) More and more new fluorinated natural products will be discovered and their biosynthetic pathways will be explored. Based on these biosynthetic pathways, artificial synthetic systems will be reconstructed to synthesize fluorinated natural products and their analogs. (II) Directed evolution based on machine learning is a mainstream tool for fluorinase, which will improve the efficiency and range of fluoride synthesis. Through this process, fluorinated nucleoside derivatives and non-nucleoside fluorides will have the opportunity to be obtained. (III) New enzymes that can directly form C-F bonds will be identified through metagenomics and gene mining methods. At that time, various fluorinated organic compounds will be easily obtained by direct fluorination at different positions and different groups. (IV) To introduce fluorine into valuable natural products, fluorine-containing building blocks will be introduced into complex natural product biosynthesis pathways through in vivo and in vitro synthetic biology methods. Besides, the toxicity of fluorides will also be overcome for synthetic biology in vivo based on the understanding of the mechanism of fluoride detoxification and detoxification of organisms.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contribution
XC collected literature data, conceived the idea and wrote the manuscript. LM conceived the idea and revised the manuscript. All authors read and approved the final version of the paper.
Funding
This work was financially supported by the National Natural Science Foundation of China (No. 21672161), Tianjin Municipal Science and Technology Commission (18PTSYJC00140), State Key Laboratory of Food Nutrition and Safety, Tianjin University of Science & Technology (19PTSYJC00060), Scientific Research Project of Tianjin Education Commission (2019KJ239).
Declarations
Ethics approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdulkarem E, Ibrahim Y, Kumar M, Arafat HA, Naddeo V, Banat F, Hasan SW. Polyvinylidene fluoride (PVDF)-alpha-zirconium phosphate (alpha-ZrP) nanoparticles based mixed matrix membranes for removal of heavy metal ions. Chemosphere. 2021;267:128896. doi: 10.1016/j.chemosphere.2020.128896. [DOI] [PubMed] [Google Scholar]
- Ad O, Thuronyi BW, Chang MC. Elucidating the mechanism of fluorinated extender unit loading for improved production of fluorine-containing polyketides. Proc Natl Acad Sci U S A. 2017;114(5):E660–E668. doi: 10.1073/pnas.1614196114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adler P, Teskey CJ, Kaiser D, Holy M, Sitte HH, Maulide N. α-Fluorination of carbonyls with nucleophilic fluorine. Nat Chem. 2019;11(4):329–334. doi: 10.1038/s41557-019-0215-z. [DOI] [PubMed] [Google Scholar]
- Adrover-Castellano ML, Schmidt JJ, Sherman DH. Biosynthetic cyclization catalysts for the assembly of peptide and polyketide natural products. ChemCatChem. 2021;13(9):2095–2116. doi: 10.1002/cctc.202001886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleku GA, France SP, Man H, Mangas-Sanchez J, Montgomery SL, Sharma M, Leipold F, Hussain S, Grogan G, Turner NJ. A reductive aminase from Aspergillus oryzae. Nat Chem. 2017;9(10):961–969. doi: 10.1038/nchem.2782. [DOI] [PubMed] [Google Scholar]
- Alluri SR, Pitman KE, Malinen E, Riss PJ. Synthesis, radiosynthesis, and positron emission tomography neuroimaging using 5-[18F]fluoro-L-amino suberate. J Labelled Comp Radiopharm. 2020;63(1):6–14. doi: 10.1002/jlcr.3814. [DOI] [PubMed] [Google Scholar]
- Arias M, Aramini JM, Riopel ND. Vogel HJ (2020) Fluorine-19 NMR spectroscopy of fluorinated analogs of tritrpticin highlights a distinct role for Tyr residues in antimicrobial peptides. Biochim Biophys Acta Biomembr. 1862;6:183260. doi: 10.1016/j.bbamem.2020.183260. [DOI] [PubMed] [Google Scholar]
- Armstrong CM, Liu C, Liu L, Yang JC, Lou W, Zhao R, Ning S, Lombard AP, Zhao J, D'Abronzo LS, Evans CP, Li PK, Gao AC. Steroid sulfatase stimulates intracrine androgen synthesis and is a therapeutic target for advanced prostate cancer. Clin Cancer Res. 2020;26(22):6064–6074. doi: 10.1158/1078-0432.CCR-20-1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awad LF, Ayoup MS. Fluorinated phenylalanines: synthesis and pharmaceutical applications. Beilstein J Org Chem. 2020;16:1022–1050. doi: 10.3762/bjoc.16.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badin RA, Binley K, Van Camp N, Jan C, Gourlay J, Robert C, Gipchtein P, Fayard A, Stewart H, Ralph GS, Lad Y, Kelleher M, Loader J, Hosomi K, Palfi S, Mitrophanous KA, Hantraye P. Gene therapy for parkinson's disease: Preclinical evaluation of optimally configured TH:CH1 fusion for maximal dopamine synthesis. Mol Ther Methods Clin Dev. 2019;14:206–216. doi: 10.1016/j.omtm.2019.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakhotmah D, Abdel-Rahman R. Synthesis and structural determination of novel fluorinated steroidal spiro(pyrazolo[4,3-e][1,2,4]triazin-3'-yl) derivatives as affecting enzymatic agents. Lett Org Chem. 2017;14(2):134–140. doi: 10.2174/1570178614666161230143228. [DOI] [Google Scholar]
- Bentler P, Bergander K, Daniliuc CG, Muck-Lichtenfeld C, Jumde RP, Hirsch AKH, Gilmour R. Inverting small molecule-protein recognition by the fluorine gauche effect: Selectivity regulated by multiple H→F bioisosterism. Angew Chem Int Ed Engl. 2019;58(32):10990–10994. doi: 10.1002/anie.201905452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucci R, Contini A, Clerici F, Beccalli EM, Formaggio F, Maffucci I, Pellegrino S, Gelmi ML. Fluoro-aryl substituted alpha, beta(2,3)-peptides in the development of foldameric antiparallel beta-sheets: A conformational study. Front Chem. 2019;7:192. doi: 10.3389/fchem.2019.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busto E, Gerstmann M, Tobola F, Dittmann E, Wiltschi B, Kroutil W. Systems biocatalysis: para-alkenylation of unprotected phenols. Catal Sci Technol. 2016;6(22):8098–8103. doi: 10.1039/c6cy01947a. [DOI] [Google Scholar]
- Calero P, Volke DC, Lowe PT, Gotfredsen CH, O'Hagan D, Nikel PI. A fluoride-responsive genetic circuit enables in vivo biofluorination in engineered Pseudomonas putida. Nat Commun. 2020;11(1):5045. doi: 10.1038/s41467-020-18813-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campana R, Mangiaterra G, Tiboni M, Frangipani E, Biavasco F, Lucarini S, Citterio B. A fluorinated analogue of marine bisindole alkaloid 2,2-bis(6-bromo-1H-indol-3-yl)ethanamine as potential anti-biofilm agent and antibiotic adjuvant against Staphylococcus aureus. Pharmaceuticals (basel) 2020;13(9):210. doi: 10.3390/ph13090210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho MF, Oliveira RS. Natural production of fluorinated compounds and biotechnological prospects of the fluorinase enzyme. Crit Rev Biotechnol. 2017;37(7):880–897. doi: 10.1080/07388551.2016.1267109. [DOI] [PubMed] [Google Scholar]
- Cavalcante FTT, Neto FS, de Aguiar R, Falcão I, da Silva E, Souza J, de Moura Junior LS, da Silva SP, Rocha TG, de Sousa IG, de Lima Gomes PH, de Souza MCM, dos Santos JCS. Opportunities for improving biodiesel production via lipase catalysis. Fuel. 2021;288:119577. doi: 10.1016/j.fuel.2020.119577. [DOI] [Google Scholar]
- Chen K, Arnold FH. Engineering new catalytic activities in enzymes. Nat Catal. 2020 doi: 10.1038/s41929-019-0385-5. [DOI] [Google Scholar]
- Chen Q, Chen X, Cui Y, Ren J, Lu W, Feng J, Wu Q, Zhu D. A new D-threonine aldolase as a promising biocatalyst for highly stereoselective preparation of chiral aromatic β-hydroxy-α-amino acids. Catal Sci Technol. 2017;7(24):5964–5973. doi: 10.1039/c7cy01774j. [DOI] [Google Scholar]
- Chen Q, Chen X, Feng J, Wu Q, Zhu D, Ma Y. Improving and inverting Cβ-stereoselectivity of threonine aldolase via substrate-binding-guided mutagenesis and a stepwise visual screening. ACS Catal. 2019;9(5):4462–4469. doi: 10.1021/acscatal.9b00859. [DOI] [Google Scholar]
- Chen Z, Mori W, Fu H, Schafroth MA, Hatori A, Shao T, Zhang G, Van RS, Zhang Y, Hu K, Fujinaga M, Wang L, Belov V, Ogasawara D, Giffenig P, Deng X, Rong J, Yu Q, Zhang X, Papisov MI, Shao Y, Collier TL, Ma JA, Cravatt BF, Josephson L, Zhang MR, Liang SH. Design, synthesis, and evaluation of 18F-labeled monoacylglycerol lipase inhibitors as novel positron emission tomography probes. J Med Chem. 2019;62(19):8866–8872. doi: 10.1021/acs.jmedchem.9b00936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Sun H, Xu J, Yu C, Xiao H, Wang R, Xu L, Zeng Z, Liang S. Emulation of synaptic behavior by organic ferroelectric tunnel junctions. Phys Lett A. 2021;392:127138. doi: 10.1016/j.physleta.2021.127138. [DOI] [Google Scholar]
- Christine CW, Bankiewicz KS, Van Laar AD, Richardson RM, Ravina B, Kells AP, Boot B, Martin AJ, Nutt J, Thompson ME, Larson PS. Magnetic resonance imaging-guided phase 1 trial of putaminal AADC gene therapy for Parkinson's disease. Ann Neurol. 2019;85(5):704–714. doi: 10.1002/ana.25450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chronopoulos DD, Bakandritsos A, Pykal M, Zboril R, Otyepka M. Chemistry, properties, and applications of fluorographene. Appl Mater Today. 2017;9:60–70. doi: 10.1016/j.apmt.2017.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J, O’Hagan D. Strategies for radiolabelling antibody, antibody fragments and affibodies with fluorine-18 as tracers for positron emission tomography (PET) J Fluorine Chem. 2017;203:31–46. doi: 10.1016/j.jfluchem.2017.08.001. [DOI] [Google Scholar]
- Cobb SL, Deng H, McEwan AR, Naismith JH, O'Hagan D, Robinson DA. Substrate specificity in enzymatic fluorination. The fluorinase from Streptomyces cattleya accepts 2′-deoxyadenosine substrates. Org Biomol Chem. 2006;4(8):1458. doi: 10.1039/b600574h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colovic M, Yang H, Merkens H, Colpo N, Benard F, Schaffer P. The effect of chirality on the application of 5-[18F]fluoro-aminosuberic acid ([18F]FASu) for oxidative stress imaging. Mol Imaging Biol. 2019 doi: 10.1007/s11307-019-01450-2. [DOI] [PubMed] [Google Scholar]
- Council CE, Kilpin KJ, Gusthart JS, Allman SA, Linclau B, Lee SS. Enzymatic glycosylation involving fluorinated carbohydrates. Org Biomol Chem. 2020;18(18):3423–3451. doi: 10.1039/d0ob00436g. [DOI] [PubMed] [Google Scholar]
- Cuetos A, Garcia-Ramos M, Fischereder EM, Diaz-Rodriguez A, Grogan G, Gotor V, Kroutil W, Lavandera I. Catalytic promiscuity of transaminases: Preparation of enantioenriched beta-fluoroamines by formal tandem hydrodefluorination/deamination. Angew Chem Int Ed Engl. 2016;55(9):3144–3147. doi: 10.1002/anie.201510554. [DOI] [PubMed] [Google Scholar]
- da Silva ES, Gomez-Vallejo V, Lopez-Gallego F, Llop J. Biocatalysis in radiochemistry: Enzymatic incorporation of PET radionuclides into molecules of biomedical interest. J Labelled Comp Radiopharm. 2018;61(4):332–354. doi: 10.1002/jlcr.3592. [DOI] [PubMed] [Google Scholar]
- Dasko M, Przybylowska M, Rachon J, Maslyk M, Kubinski K, Misiak M, Skladanowski A, Demkowicz S. Synthesis and biological evaluation of fluorinated N-benzoyl and N-phenylacetoyl derivatives of 3-(4-aminophenyl)-coumarin-7-O-sulfamate as steroid sulfatase inhibitors. Eur J Med Chem. 2017;128:79–87. doi: 10.1016/j.ejmech.2017.01.028. [DOI] [PubMed] [Google Scholar]
- de Zwart PL, van Dijken BRJ, Holtman GA, Stormezand GN, Dierckx R, Jan van Laar P, van der Hoorn A. Diagnostic accuracy of PET tracers for the differentiation of tumor progression from treatment-related changes in high-grade glioma: A systematic review and metaanalysis. J Nucl Med. 2020;61(4):498–504. doi: 10.2967/jnumed.119.233809. [DOI] [PubMed] [Google Scholar]
- Decha N, Talungchit S, Iawsipo P, Pikulngam A, Saiprasert P, Tansakul C. Synthesis and characterization of new hydrolytic-resistant dental resin adhesive monomer HMTAF. Des Monomers Polym. 2019;22(1):106–113. doi: 10.1080/15685551.2019.1615789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demkowicz S, Dasko M, Kozak W, Krawczyk K, Witt D, Maslyk M, Kubinski K, Rachon J. Synthesis and biological evaluation of fluorinated 3-phenylcoumarin-7-O-sulfamate derivatives as steroid sulfatase inhibitors. Chem Biol Drug Des. 2016;87(2):233–238. doi: 10.1111/cbdd.12652. [DOI] [PubMed] [Google Scholar]
- Deng H, Ma L, Bandaranayaka N, Qin Z, Mann G, Kyeremeh K, Yu Y, Shepherd T, Naismith JH, O'Hagan D. Identification of fluorinases from Streptomyces sp MA37, Norcardia brasiliensis, and Actinoplanes sp N902–109 by genome mining. ChemBioChem. 2014;15(3):364–368. doi: 10.1002/cbic.201300732. [DOI] [PubMed] [Google Scholar]
- Deng H, O'Hagan D, Schaffrath C. Fluorometabolite biosynthesis and the fluorinase from Streptomyces cattleya. Nat Prod Rep. 2004;21(6):773–784. doi: 10.1039/b415087m. [DOI] [PubMed] [Google Scholar]
- Dennig A, Busto E, Kroutil W, Faber K. Biocatalytic one-pot synthesis of L-tyrosine derivatives from monosubstituted benzenes, pyruvate, and ammonia. ACS Catal. 2015;5(12):7503–7506. doi: 10.1021/acscatal.5b02129. [DOI] [Google Scholar]
- Dezvarei S, Onoda H, Shoji O, Watanabe Y, Bell SG. Efficient hydroxylation of cycloalkanes by co-addition of decoy molecules to variants of the cytochrome P450 CYP102A1. J Inorg Biochem. 2018;183:137–145. doi: 10.1016/j.jinorgbio.2018.03.001. [DOI] [PubMed] [Google Scholar]
- Dias MV, Huang F, Chirgadze DY, Tosin M, Spiteller D, Dry EF, Leadlay PF, Spencer JB, Blundell TL. Structural basis for the activity and substrate specificity of fluoroacetyl-CoA thioesterase FlK. J Biol Chem. 2010;285(29):22495–22504. doi: 10.1074/jbc.M110.107177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietlein F, Kobe C, Hohberg M, Zlatopolskiy BD, Krapf P, Endepols H, Tager P, Hammes J, Heidenreich A, Persigehl T, Neumaier B, Drzezga A, Dietlein M. Intraindividual comparison of 18F-PSMA-1007 with renally excreted PSMA ligands for PSMA PET imaging in patients with relapsed prostate cancer. J Nucl Med. 2020;61(5):729–734. doi: 10.2967/jnumed.119.234898. [DOI] [PubMed] [Google Scholar]
- Dong C, Deng H, Dorward M, Schaffrath C, O'Hagan D, Naismith JH. Crystallization and X-ray diffraction of 5′-fluoro-5′-deoxyadenosine synthase, a fluorination enzyme fromStreptomyces cattleya. Acta Crystallogr, Sect d: Biol Crystallogr. 2003;59(12):2292–2293. doi: 10.1107/s0907444903019826. [DOI] [PubMed] [Google Scholar]
- Dong C, Huang F, Deng H, Schaffrath C, Spencer JB, O'Hagan D, Naismith JH. Crystal structure and mechanism of a bacterial fluorinating enzyme. Nature. 2004;427(6974):561–565. doi: 10.1038/nature02280. [DOI] [PubMed] [Google Scholar]
- Dos Santos LM, Bernard FL, Polesso BB, Pinto IS, Frankenberg CC, Corvo MC, Almeida PL, Cabrita E, Einloft S. Designing silica xerogels containing RTIL for CO2 capture and CO2/CH4 separation: Influence of ILs anion, cation and cation side alkyl chain length and ramification. J Environ Manage. 2020;268:110340. doi: 10.1016/j.jenvman.2020.110340. [DOI] [PubMed] [Google Scholar]
- Dziedzic P, Cisneros JA, Robertson MJ, Hare AA, Danford NE, Baxter RH, Jorgensen WL. Design, synthesis, and protein crystallography of biaryltriazoles as potent tautomerase inhibitors of macrophage migration inhibitory factor. J Am Chem Soc. 2015;137(8):2996–3003. doi: 10.1021/ja512112j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang J, Hait D, Head-Gordon M, Chang MCY. Chemoenzymatic platform for synthesis of chiral organofluorines based on type II aldolases. Angew Chem Int Ed Engl. 2019;58(34):11841–11845. doi: 10.1002/anie.201906805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng X, Bello D, Lowe PT, Clark J, O'Hagan D. Two 3'-O-beta-glucosylated nucleoside fluorometabolites related to nucleocidin in Streptomyces calvus. Chem Sci. 2019;10(41):9501–9505. doi: 10.1039/c9sc03374b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraioli F, Shankar A, Hyare H, Ferrazzoli V, Militano V, Samandouras G, Mankad K, Solda F, Zaccagna F, Mehdi E, Lyasheva M, Bomanji J, Novruzov F. The use of multiparametric 18F-fluoro-L-3,4-dihydroxy-phenylalanine PET/MRI in post-therapy assessment of patients with gliomas. Nucl Med Commun. 2020;41(6):517–525. doi: 10.1097/MNM.0000000000001184. [DOI] [PubMed] [Google Scholar]
- Fraley AE, Sherman DH. Halogenase engineering and its utility in medicinal chemistry. Bioorg Med Chem Lett. 2018;28(11):1992–1999. doi: 10.1016/j.bmcl.2018.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- France SP, Howard RM, Steflik J, Weise NJ, Mangas-Sanchez J, Montgomery SL, Crook R, Kumar R, Turner NJ. Identification of novel bacterial members of the imine reductase enzyme family that perform reductive amination. ChemCatChem. 2018;10(3):510–514. doi: 10.1002/cctc.201701408. [DOI] [Google Scholar]
- Frank DJ, Zhao Y, Wong SH, Basudhar D, De Voss JJ, Ortiz de Montellano PR. Cholesterol analogs with degradation-resistant alkyl side chains are effective Mycobacterium tuberculosis growth inhibitors. J Biol Chem. 2016;291(14):7325–7333. doi: 10.1074/jbc.M115.708172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fried J, Sabo EF. 9α-Fluoro derivatives of cortisone and hydrocortisone. J Am Chem Soc. 1954;76(5):1455–1456. doi: 10.1021/ja01634a101. [DOI] [Google Scholar]
- Fryszkowska A, Devine PN. Biocatalysis in drug discovery and development. Curr Opin Chem Biol. 2020;55:151–160. doi: 10.1016/j.cbpa.2020.01.012. [DOI] [PubMed] [Google Scholar]
- Fuenfgeld B, Machler P, Fischer DR, Esposito G, Rushing EJ, Kaufmann PA, Stolzmann P, Huellner MW. Reference values of physiological 18F-FET uptake: Implications for brain tumor discrimination. PLoS ONE. 2020;15(4):e0230618. doi: 10.1371/journal.pone.0230618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino H, Nagatomo M, Paudel A, Panthee S, Hamamoto H, Sekimizu K, Inoue M. Unified total synthesis of polyoxins J, L, and fluorinated analogues on the basis of decarbonylative radical coupling reactions. Angew Chem Int Ed Engl. 2017;56(39):11865–11869. doi: 10.1002/anie.201706671. [DOI] [PubMed] [Google Scholar]
- Gambini L, Baggio C, Udompholkul P, Jossart J, Salem AF, Perry JJP, Pellecchia M. Covalent inhibitors of protein-protein interactions targeting lysine, tyrosine, or histidine residues. J Med Chem. 2019;62(11):5616–5627. doi: 10.1021/acs.jmedchem.9b00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissner A, Baumann L, Morley TJ, Wong AKO, Sim L, Rich JR, So PPL, Dullaghan EM, Lessard E, Iqbal U, Moreno M, Wakarchuk WW, Withers SG. 7-Fluorosialyl glycosides are hydrolysis resistant but readily assembled by sialyltransferases providing easy access to more metabolically stable glycoproteins. ACS Cent Sci. 2021;7(2):345–354. doi: 10.1021/acscentsci.0c01589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giesel FL, Hadaschik B, Cardinale J, Radtke J, Vinsensia M, Lehnert W, Kesch C, Tolstov Y, Singer S, Grabe N, Duensing S, Schafer M, Neels OC, Mier W, Haberkorn U, Kopka K, Kratochwil C. F-18 labelled PSMA-1007: biodistribution, radiation dosimetry and histopathological validation of tumor lesions in prostate cancer patients. Eur J Nucl Med Mol Imaging. 2017;44(4):678–688. doi: 10.1007/s00259-016-3573-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis EP, Eastman KJ, Hill MD, Donnelly DJ, Meanwell NA. Applications of fluorine in medicinal chemistry. J Med Chem. 2015;58(21):8315–8359. doi: 10.1021/acs.jmedchem.5b00258. [DOI] [PubMed] [Google Scholar]
- González-Martínez D, Cuetos A, Sharma M, García-Ramos M, Lavandera I, Gotor-Fernández V, Grogan G. Asymmetric synthesis of primary and secondary β-fluoro-arylamines using reductive aminases from fungi. ChemCatChem. 2020;12(9):2421–2425. doi: 10.1002/cctc.201901999. [DOI] [Google Scholar]
- González-Martínez D, Gotor V, Gotor-Fernández V. Chemo- and stereoselective synthesis of fluorinated amino alcohols through one-pot reactions using alcohol dehydrogenases and amine transaminases. Adv Synth Catal. 2020;362(23):5398–5410. doi: 10.1002/adsc.202000798. [DOI] [Google Scholar]
- Grobbelaar N, Marion Meyer JJ. Fluoroacetate production by Dichapetalum cymosum. J Plant Physiol. 1990;135(5):550–553. doi: 10.1016/s0176-1617(11)80634-6. [DOI] [Google Scholar]
- Hagar M, Ahmed HA, Aljohani G, Alhaddad OA (2020) Investigation of some antiviral N-heterocycles as COVID 19 drug: Molecular docking and DFT calculations. Int J Mol Sci 21(11). 10.3390/ijms21113922 [DOI] [PMC free article] [PubMed]
- Hanaoka H, Ohshima Y, Yamaguchi A, Suzuki H, Ishioka NS, Higuchi T, Arano Y, Tsushima Y. Novel 18F-labeled alpha-methyl-phenylalanine derivative with high tumor accumulation and ideal pharmacokinetics for tumor-specific imaging. Mol Pharm. 2019;16(8):3609–3616. doi: 10.1021/acs.molpharmaceut.9b00446. [DOI] [PubMed] [Google Scholar]
- Harper DB, O'Hagan D. The fluorinated natural products. Nat Prod Rep. 1994;11(2):123–133. doi: 10.1039/np9941100123. [DOI] [PubMed] [Google Scholar]
- Hauer B. Embracing nature's catalysts: a viewpoint on the future of biocatalysis. ACS Catal. 2020;10(15):8418–8427. doi: 10.1021/acscatal.0c01708. [DOI] [Google Scholar]
- Holanda RJ, Deves C, Moreira-Dill LS, Guimaraes CL, Marttinelli LKB, Fernandes CFC, Medeiros PSM, Pereira SS, Honda ER, Stabeli RG, Santos DS, Soares AM, Pereira da Silva LH. Plasmodium falciparum purine nucleoside phosphorylase as a model in the search for new inhibitors by high throughput screening. Int J Biol Macromol. 2020;165(Part B):1832–1841. doi: 10.1016/j.ijbiomac.2020.10.062. [DOI] [PubMed] [Google Scholar]
- Hong H, Spiteller D, Spencer JB. Incorporation of fluoroacetate into an aromatic polyketide and its influence on the mode of cyclization. Angew Chem Int Ed Engl. 2008;47(32):6028–6032. doi: 10.1002/anie.200801100. [DOI] [PubMed] [Google Scholar]
- Honig M, Sondermann P, Turner NJ, Carreira EM. Enantioselective chemo- and biocatalysis: Partners in retrosynthesis. Angew Chem Int Ed Engl. 2017;56(31):8942–8973. doi: 10.1002/anie.201612462. [DOI] [PubMed] [Google Scholar]
- Huang F, Haydock SF, Spiteller D, Mironenko T, Li TL, O'Hagan D, Leadlay PF, Spencer JB. The gene cluster for fluorometabolite biosynthesis in Streptomyces cattleya: a thioesterase confers resistance to fluoroacetyl-coenzyme A. Chem Biol. 2006;13(5):475–484. doi: 10.1016/j.chembiol.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Huang S, Ma L, Tong MH, Yu Y, O'Hagan D, Deng H. Fluoroacetate biosynthesis from the marine-derived bacterium Streptomyces xinghaiensis NRRL B-24674. Org Biomol Chem. 2014;12(27):4828–4831. doi: 10.1039/c4ob00970c. [DOI] [PubMed] [Google Scholar]
- Huang X, Garcia-Borras M, Miao K, Kan SBJ, Zutshi A, Houk KN, Arnold FH. A biocatalytic platform for synthesis of chiral alpha-trifluoromethylated organoborons. ACS Cent Sci. 2019;5(2):270–276. doi: 10.1021/acscentsci.8b00679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z, Guo Z, Xie D, Cao Z, Chen L, Wang H, Jiang L, Shen Q. Rhizomucor miehei lipase-catalysed synthesis of cocoa butter equivalent from palm mid-fraction and stearic acid: Characteristics and feasibility as cocoa butter alternative. Food Chem. 2021;343:128407. doi: 10.1016/j.foodchem.2020.128407. [DOI] [PubMed] [Google Scholar]
- Iizaka Y, Sherman DH, Anzai Y. An overview of the cytochrome P450 enzymes that catalyze the same-site multistep oxidation reactions in biotechnologically relevant selected actinomycete strains. Appl Microbiol Biotechnol. 2021;105(7):2647–2661. doi: 10.1007/s00253-021-11216-y. [DOI] [PubMed] [Google Scholar]
- Izquierdo J, Jain AD, Abdulkadir SA, Schiltz GE. Palladium-catalyzed coupling reactions on functionalized 2-trifluoromethyl-4-chromenone scaffolds. Synthesis of highly functionalized trifluoromethyl-heterocycles. Synthesis (Stuttg) 2019;51(6):1342–1352. doi: 10.1055/s-0037-1610669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen BHE, Yaqub M, Voortman J, Cysouw MCF, Windhorst AD, Schuit RC, Kramer GM, van den Eertwegh AJM, Schwarte LA, Hendrikse NH, Vis AN, van Moorselaar RJA, Hoekstra OS, Boellaard R, Oprea-Lager DE. Simplified methods for quantification of 18F-DCFPyL uptake in patients with prostate cancer. J Nucl Med. 2019;60(12):1730–1735. doi: 10.2967/jnumed.119.227520. [DOI] [PubMed] [Google Scholar]
- Ji D, Xiao C, Chen K, Zhou F, Gao Y, Zhang T, Ling H. Solvent-free green fabrication of PVDF hollow fiber MF membranes with controlled pore structure via melt-spinning and stretching. J Membr Sci. 2021;621:118953. doi: 10.1016/j.memsci.2020.118953. [DOI] [Google Scholar]
- Jiang G, Zhang Y, Powell MM, Zhang P, Zuo R, Zhang Y, Kallifidas D, Tieu AM, Luesch H, Loria R, Ding Y (2018) High-yield production of herbicidal thaxtomins and thaxtomin analogs in a nonpathogenic Streptomyces strain. Appl Environ Microbiol 84(11). 10.1128/AEM.00164-18 [DOI] [PMC free article] [PubMed]
- Kang D, Shao H, Chen G, Dong X, Qin S. Fabrication of highly permeable PVDF loose nanofiltration composite membranes for the effective separation of dye/salt mixtures. J Membr Sci. 2021;621:118951. doi: 10.1016/j.memsci.2020.118951. [DOI] [Google Scholar]
- Kayushin AL, Tokunova JA, Fateev IV, Arnautova AO, Berzina MY, Paramonov AS, Lutonina OI, Dorofeeva EV, Antonov KV, Esipov RS, Mikhailopulo IA, Miroshnikov AI, Konstantinova ID (2021) Radical dehalogenation and purine nucleoside phosphorylase E. coli: How does an admixture of 2',3'-anhydroinosine hinder 2-fluoro-cordycepin synthesis. Biomolecules 11(4). 10.3390/biom11040539 [DOI] [PMC free article] [PubMed]
- Kharitonova M, Antonov K, Fateev I, Berzina M, Kaushin A, Paramonov A, Kotovskaya S, Andronova V, Konstantinova I, Galegov G, Charushin V, Miroshnikov A. Chemoenzymatic synthesis of modified 2′-deoxy-2′-fluoro-β-D-arabinofuranosyl benzimidazoles and evaluation of their activity against herpes simplex virus type 1. Synthesis. 2016;49(05):1043–1052. doi: 10.1055/s-0036-1588625. [DOI] [Google Scholar]
- Khosravan A, Marani S, Sadeghi Googheri MS. The effects of fluorine substitution on the chemical properties and inhibitory capacity of donepezil anti-Alzheimer drug; density functional theory and molecular docking calculations. J Mol Graph Model. 2017;71:124–134. doi: 10.1016/j.jmgm.2016.11.013. [DOI] [PubMed] [Google Scholar]
- Kim TH, Mehrabi P, Ren Z, Sljoka A, Ing C, Bezginov A, Ye L, Pomes R, Prosser RS, Pai EF (2017) The role of dimer asymmetry and protomer dynamics in enzyme catalysis. Science 355(6322). 10.1126/science.aag2355 [DOI] [PubMed]
- Klopries S, Koopmans KR, Sanchez-Garcia E, Schulz F. Biosynthesis with fluorine. ChemBioChem. 2014;15(4):495–497. doi: 10.1002/cbic.201300750. [DOI] [PubMed] [Google Scholar]
- Koh E, Lee YT. Preparation of an omniphobic nanofiber membrane by the self-assembly of hydrophobic nanoparticles for membrane distillation. Sep Purif Technol. 2021;259:118134. doi: 10.1016/j.seppur.2020.118134. [DOI] [Google Scholar]
- Kojima K, Nakajima T, Taga N, Miyauchi A, Kato M, Matsumoto A, Ikeda T, Nakamura K, Kubota T, Mizukami H, Ono S, Onuki Y, Sato T, Osaka H, Muramatsu SI, Yamagata T. Gene therapy improves motor and mental function of aromatic L-amino acid decarboxylase deficiency. Brain. 2019;142(2):322–333. doi: 10.1093/brain/awy331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langen KJ, Galldiks N, Hattingen E, Shah NJ. Advances in neuro-oncology imaging. Nat Rev Neurol. 2017;13(5):279–289. doi: 10.1038/nrneurol.2017.44. [DOI] [PubMed] [Google Scholar]
- Latham J, Brandenburger E, Shepherd SA, Menon BRK, Micklefield J. Development of halogenase enzymes for use in synthesis. Chem Rev. 2018;118(1):232–269. doi: 10.1021/acs.chemrev.7b00032. [DOI] [PubMed] [Google Scholar]
- Le Y, Gan Y, Fu Y, Liu J, Li W, Zou X, Zhou Z, Wang Z, Ouyang G, Yan L. Design, synthesis and in vitro biological evaluation of quinazolinone derivatives as EGFR inhibitors for antitumor treatment. J Enzyme Inhib Med Chem. 2020;35(1):555–564. doi: 10.1080/14756366.2020.1715389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JW, Oliveira MT, Jang HB, Lee S, Chi DY, Kim DW, Song CE. Hydrogen-bond promoted nucleophilic fluorination: concept, mechanism and applications in positron emission tomography. Chem Soc Rev. 2016;45(17):4638–4650. doi: 10.1039/c6cs00286b. [DOI] [PubMed] [Google Scholar]
- Lee ST, Cook D, Pfister JA, Allen JG, Colegate SM, Riet-Correa F, Taylor CM. Monofluoroacetate-containing plants that are potentially toxic to livestock. J Agric Food Chem. 2014;62(30):7345–7354. doi: 10.1021/jf500563h. [DOI] [PubMed] [Google Scholar]
- Lena Sezici Y, Yetkiner E, Aykut Yetkiner A, Eden E, Attin R. Comparative evaluation of fluoride varnishes, self-assembling peptide-based remineralization agent, and enamel matrix protein derivative on artificial enamel remineralization in vitro. Prog Orthod. 2021;22(1):4. doi: 10.1186/s40510-020-00345-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong LEX, Khan S, Davis CK, Denman SE, McSweeney CS. Fluoroacetate in plants-a review of its distribution, toxicity to livestock and microbial detoxification. J Anim Sci Biotechnol. 2017;8:55. doi: 10.1186/s40104-017-0180-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Yun Y, Wang M, Li C, Yang W, Li J, Liu G. Superhydrophobic polymer membrane coated by mineralized β-FeOOH nanorods for direct contact membrane distillation. Desalination. 2021;500:114889. doi: 10.1016/j.desal.2020.114889. [DOI] [Google Scholar]
- Li G, Lian J, Xue H, Jiang Y, Ju S, Wu M, Lin J. Yang L (2020a) Biocascade synthesis of L-tyrosine derivatives by coupling a thermophilic tyrosine phenol-lyase and L-lactate oxidase. Eur J Org Chem. 2020;8:1050–1054. doi: 10.1002/ejoc.202000061. [DOI] [Google Scholar]
- Li N, Hu B, Wang A, Li H, Yin Y, Mao T, Xie T. Facile bioinspired preparation of fluorinase@fluoridated hydroxyapatite nanoflowers for the biosynthesis of 5′-fluorodeoxy adenosine. Sustainability. 2020;12(1):431. doi: 10.3390/su12010431. [DOI] [Google Scholar]
- Li WN, Fan DD. Biocatalytic strategies for the production of ginsenosides using glycosidase: current state and perspectives. Appl Microbiol Biotechnol. 2020;104(9):3807–3823. doi: 10.1007/s00253-020-10455-9. [DOI] [PubMed] [Google Scholar]
- Li Y, Yue Y, Zhang H, Yang Z, Wang H, Tian S, Wang JB, Zhang Q, Wang W. Harnessing fluoroacetate dehalogenase for defluorination of fluorocarboxylic acids: in silico and in vitro approach. Environ Int. 2019;131:104999. doi: 10.1016/j.envint.2019.104999. [DOI] [PubMed] [Google Scholar]
- Liang H, Hu Z, Zhao Z, Chen D, Zhang H, Wang H, Wang X, Li Q, Guo X, Li H. Dendrite-structured FeF2 consisting of closely linked nanoparticles as cathode for high-performance lithium-ion capacitors. J Energy Chem. 2021;55:517–523. doi: 10.1016/j.jechem.2020.07.031. [DOI] [Google Scholar]
- Liang S, Hammond GB, Xu B. Hydrogen bonding: Regulator for nucleophilic fluorination. Chemistry. 2017;23(71):17850–17861. doi: 10.1002/chem.201702664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Li S, Aslam NA, Zheng F, Yang B, Cheng R, Wang N, Rozovsky S, Wang PG, Wang Q, Wang L. Genetically encoding photocaged quinone methide to multitarget protein residues covalently in vivo. J Am Chem Soc. 2019;141(24):9458–9462. doi: 10.1021/jacs.9b01738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Zhu Y, Chen Y, Chen H, Fan C, Mo Q, Yuan J. One-pot cascade biotransformation for efficient synthesis of benzyl alcohol and its analogs. Chem Asian J. 2020;15(7):1018–1021. doi: 10.1002/asia.201901680. [DOI] [PubMed] [Google Scholar]
- Liu T, Yan N, Zhao H, Wang Z-X, Hu X-G. Synthesis and antibacterial activity of 6(R)- and 6(S)-fluoropenibruguieramine As: Fluorine as a probe for testing the powerfulness of memory of chirality (MOC) J Fluorine Chem. 2018;207:18–23. doi: 10.1016/j.jfluchem.2018.01.002. [DOI] [Google Scholar]
- Lizarme-Salas Y, Ariawan AD, Ratnayake R, Luesch H, Finch A, Hunter L. Vicinal difluorination as a C=C surrogate: an analog of piperine with enhanced solubility, photostability, and acetylcholinesterase inhibitory activity. Beilstein J Org Chem. 2020;16:2663–2670. doi: 10.3762/bjoc.16.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohmann P, Werner JM, Shah NJ, Fink GR, Langen KJ, Galldiks N (2019) Combined amino acid positron emission tomography and advanced aagnetic resonance imaging in glioma patients. Cancers (Basel) 11(2). 10.3390/cancers11020153 [DOI] [PMC free article] [PubMed]
- López-Iglesias M, González-Martínez D, Rodríguez-Mata M, Gotor V, Busto E, Kroutil W, Gotor-Fernández V. Asymmetric biocatalytic synthesis of fluorinated pyridines through transesterification or transamination: Computational insights into the reactivity of transaminases. Adv Synth Catal. 2017;359(2):279–291. doi: 10.1002/adsc.201600835. [DOI] [Google Scholar]
- Lowe PT, Cobb SL, O'Hagan D. An enzymatic Finkelstein reaction: fluorinase catalyses direct halogen exchange. Org Biomol Chem. 2019;17(32):7493–7496. doi: 10.1039/c9ob01625b. [DOI] [PubMed] [Google Scholar]
- Lowe PT, Dall'Angelo S, Devine A, Zanda M, O'Hagan D. Enzymatic fluorination of biotin and tetrazine conjugates for pretargeting approaches to positron emission tomography imaging. ChemBioChem. 2018;19(18):1969–1978. doi: 10.1002/cbic.201800234. [DOI] [PubMed] [Google Scholar]
- Lowe PT, Dall'Angelo S, Fleming IN, Piras M, Zanda M, O'Hagan D. Enzymatic radiosynthesis of a 18F-Glu-Ureido-Lys ligand for the prostate-specific membrane antigen (PSMA) Org Biomol Chem. 2019;17(6):1480–1486. doi: 10.1039/c8ob03150a. [DOI] [PubMed] [Google Scholar]
- Lowe PT, Dall'Angelo S, Mulder-Krieger T, AP IJ, Zanda M, O'Hagan D, A new class of fluorinated A2A adenosine receptor agonist with application to last-step enzymatic [18F]fluorination for PET imaging. ChemBioChem. 2017;18(21):2156–2164. doi: 10.1002/cbic.201700382. [DOI] [PubMed] [Google Scholar]
- Lu X, Skomski D, Thompson KC, McNevin MJ, Xu W, Su Y. Three-dimensional NMR spectroscopy of fluorinated pharmaceutical solids under ultrafast magic angle spinning. Anal Chem. 2019;91(9):6217–6224. doi: 10.1021/acs.analchem.9b00884. [DOI] [PubMed] [Google Scholar]
- Ma L, Bartholome A, Tong MH, Qin Z, Yu Y, Shepherd T, Kyeremeh K, Deng H, O'Hagan D. Identification of a fluorometabolite from Streptomyces sp. MA37: (2R3S4S)-5-fluoro-2,3,4-trihydroxypentanoic acid. Chem Sci. 2015;6(2):1414–1419. doi: 10.1039/c4sc03540b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Li Y, Meng L, Deng H, Li Y, Zhang Q, Diao A. Biological fluorination from the sea: discovery of a SAM-dependent nucleophilic fluorinating enzyme from the marine-derived bacterium Streptomyces xinghaiensis NRRL B24674. RSC Adv. 2016;6(32):27047–27051. doi: 10.1039/c6ra00100a. [DOI] [Google Scholar]
- Maglangit F, Fang Q, Leman V, Soldatou S, Ebel R, Kyeremeh K, Deng H (2019) Accramycin A, a new aromatic polyketide, from the soil bacterium, Streptomyces sp. MA37. Molecules 24(18). 10.3390/molecules24183384 [DOI] [PMC free article] [PubMed]
- Mahendra I, Hanaoka H, Yamaguchi A, Amartuvshin T, Tsushima Y. Diagnosis of bladder cancer using 18F-labeled alpha-methyl-phenylalanine tracers in a mouse model. Ann Nucl Med. 2020;34(5):329–336. doi: 10.1007/s12149-020-01452-z. [DOI] [PubMed] [Google Scholar]
- Makki MST, Abdel-Rahman RM, Alshammari NAH. Synthesis of novel fluorine compounds substituted-4-thiazolidinones derived from rhodanine drug as highly bioactive probes. Curr Org Synth. 2019;16(3):413–422. doi: 10.2174/1570179416666190312150046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltais R, Ngueta Djiemeny A, Roy J, Barbeau X, Lambert JP, Poirier D. Design and synthesis of dansyl-labeled inhibitors of steroid sulfatase for optical imaging. Bioorg Med Chem. 2020;28(7):115368. doi: 10.1016/j.bmc.2020.115368. [DOI] [PubMed] [Google Scholar]
- Mao Z, Liu L, Zhang Y, Yuan J. Efficient synthesis of phenylacetate and 2-phenylethanol by modular cascade biocatalysis. ChemBioChem. 2020 doi: 10.1002/cbic.202000182. [DOI] [PubMed] [Google Scholar]
- Markakis K, Lowe PT, Davison-Gates L, O'Hagan D, Rosser SJ, Elfick A. An engineered E. coli strain for direct in vivo fluorination. Chembiochem. 2020;21(13):1856–1860. doi: 10.1002/cbic.202000051. [DOI] [PubMed] [Google Scholar]
- Marner L, Nysom K, Sehested A, Borgwardt L, Mathiasen R, Henriksen OM, Lundemann M, Munck Af Rosenschold P, Thomsen C, Bogeskov L, Skjoth-Rasmussen J, Juhler M, Kruse A, Broholm H, Scheie D, Lauritsen T, Forman JL, Wehner PS, Hojgaard L, Law I. Early postoperative 18F-FET PET/MRI for pediatric brain and spinal cord tumors. J Nucl Med. 2019;60(8):1053–1058. doi: 10.2967/jnumed.118.220293. [DOI] [PubMed] [Google Scholar]
- Marquez-Rodriguez AS, Guimaraes M, Mateus N, de Freitas V, Ballinas-Casarrubias ML, Fuentes-Montero ME, Salas E, Cruz L. Disaccharide anthocyanin delphinidin 3-O-sambubioside from Hibiscus sabdariffa L.: Candida antarctica lipase B-catalyzed fatty acid acylation and study of its color properties. Food Chem. 2021;344:128603. doi: 10.1016/j.foodchem.2020.128603. [DOI] [PubMed] [Google Scholar]
- McAtee RC, Beatty JW, McAtee CC, Stephenson CRJ. Radical chlorodifluoromethylation: Providing a motif for (Hetero)arene diversification. Org Lett. 2018;20(12):3491–3495. doi: 10.1021/acs.orglett.8b01249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meanwell M, Silverman SM, Lehmann J, Adluri B, Wang Y, Cohen R, Campeau LC, Britton R. A short de novo synthesis of nucleoside analogs. Science. 2020;369(6504):725–730. doi: 10.1126/science.abb3231. [DOI] [PubMed] [Google Scholar]
- Meanwell NA. Fluorine and fluorinated motifs in the design and application of bioisosteres for drug design. J Med Chem. 2018;61(14):5822–5880. doi: 10.1021/acs.jmedchem.7b01788. [DOI] [PubMed] [Google Scholar]
- Mei H, Han J, Fustero S, Medio-Simon M, Sedgwick DM, Santi C, Ruzziconi R, Soloshonok VA. Fluorine-containing drugs approved by the FDA in 2018. Chemistry. 2019;25(51):11797–11819. doi: 10.1002/chem.201901840. [DOI] [PubMed] [Google Scholar]
- Mei H, Han J, Klika KD, Izawa K, Sato T, Meanwell NA, Soloshonok VA. Applications of fluorine-containing amino acids for drug design. Eur J Med Chem. 2020;186:111826. doi: 10.1016/j.ejmech.2019.111826. [DOI] [PubMed] [Google Scholar]
- Mei H, Remete AM, Zou Y, Moriwaki H, Fustero S, Kiss L, Soloshonok VA, Han J. Fluorine-containing drugs approved by the FDA in 2019. Chin Chem Lett. 2020;31(9):2401–2413. doi: 10.1016/j.cclet.2020.03.050. [DOI] [Google Scholar]
- Mennie KM, Banik SM, Reichert EC, Jacobsen EN. Catalytic diastereo- and enantioselective fluoroamination of alkenes. J Am Chem Soc. 2018;140(14):4797–4802. doi: 10.1021/jacs.8b02143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon BRK, Richmond D, Menon N (2020) Halogenases for biosynthetic pathway engineering: Toward new routes to naturals and non-naturals. Catalysis Reviews:1–59. 10.1080/01614940.2020.1823788
- Minges H, Sewald N. Recent advances in synthetic application and engineering of halogenases. ChemCatChem. 2020;12(18):4450–4470. doi: 10.1002/cctc.202000531. [DOI] [Google Scholar]
- Miranda-Rojas S, Fernández I, Kästner J, Toro-Labbé A, Mendizábal F. Unraveling the nature of the catalytic power of fluoroacetate dehalogenase. ChemCatChem. 2018;10(5):1052–1063. doi: 10.1002/cctc.201701517. [DOI] [Google Scholar]
- Miyamoto M, Miyamoto T, Saitou J, Sato T. Longitudinal study of striatal aromatic L-amino acid decarboxylase activity in patients with idiopathic rapid eye movement sleep behavior disorder. Sleep Med. 2020;68:50–56. doi: 10.1016/j.sleep.2019.09.013. [DOI] [PubMed] [Google Scholar]
- Molinaro C, Phillips EM, Xiang B, Milczek E, Shevlin M, Balsells J, Ceglia S, Chen J, Chen L, Chen Q, Fei Z, Hoerrner S, Qi J, de Lera RM, Tan L, Wan B, Yin J. Synthesis of a CGRP receptor antagonist via an asymmetric synthesis of 3-fluoro-4-aminopiperidine. J Org Chem. 2019;84(12):8006–8018. doi: 10.1021/acs.joc.9b00569. [DOI] [PubMed] [Google Scholar]
- Moschner J, Stulberg V, Fernandes R, Huhmann S, Leppkes J, Koksch B. Approaches to obtaining fluorinated alpha-amino acids. Chem Rev. 2019;119(18):10718–10801. doi: 10.1021/acs.chemrev.9b00024. [DOI] [PubMed] [Google Scholar]
- Mossine AV, Tanzey SS, Brooks AF, Makaravage KJ, Ichiishi N, Miller JM, Henderson BD, Erhard T, Bruetting C, Skaddan MB, Sanford MS, Scott PJH. Synthesis of high-molar-activity [18F]6-fluoro-L-DOPA suitable for human use via Cu-mediated fluorination of a BPin precursor. Nat Protoc. 2020;15(5):1742–1759. doi: 10.1038/s41596-020-0305-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller K, Faeh C, Diederich F. Fluorine in pharmaceuticals: looking beyond intuition. Science. 2007;317(5846):1881–1886. doi: 10.1126/science.1131943. [DOI] [PubMed] [Google Scholar]
- Munck Af Rosenschold M, Johannesson P, Nikitidis A, Tyrchan C, Chang HF, Ronn R, Chapman D, Ullah V, Nikitidis G, Glader P, Kack H, Bonn B, Wagberg F, Bjorkstrand E, Andersson U, Swedin L, Rohman M, Andreasson T, Bergstrom EL, Jiang F, Zhou XH, Lundqvist AJ, Malmberg A, Ek M, Gordon E, Pettersen A, Ripa L, Davis AM. Discovery of the oral leukotriene C4 synthase inhibitor (1S,2S)-2-({5-[(5-Chloro-2,4-difluorophenyl)(2-fluoro-2-methylpropyl)amino]-3-methoxypyrazin-2-yl}carbonyl)cyclopropanecarboxylic acid (AZD9898) as a new treatment for asthma. J Med Chem. 2019;62(17):7769–7787. doi: 10.1021/acs.jmedchem.9b00555. [DOI] [PubMed] [Google Scholar]
- Nakashima M, Suzuki N, Shiraishi E, Iwashita H. TAK-915, a phosphodiesterase 2A inhibitor, ameliorates the cognitive impairment associated with aging in rodent models. Behav Brain Res. 2019;376:112192. doi: 10.1016/j.bbr.2019.112192. [DOI] [PubMed] [Google Scholar]
- Nashiru O, Zechel DL, Stoll D, Mohammadzadeh T, Warren RAJ, Withers SG. β-Mannosynthase: Synthesis of β-mannosides with a mutant β-mannosidase. Angew Chem Int Ed. 2001;113(2):431–434. doi: 10.1002/1521-3757(20010119)113:2<431::AID-ANGE431>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Nayak K, Tripathi BP. Molecularly grafted PVDF membranes with in-air superamphiphilicity and underwater superoleophobicity for oil/water separation. Sep Purif Technol. 2021;259:118068. doi: 10.1016/j.seppur.2020.118068. [DOI] [Google Scholar]
- Ni C, Hu J. The unique fluorine effects in organic reactions: recent facts and insights into fluoroalkylations. Chem Soc Rev. 2016;45(20):5441–5454. doi: 10.1039/c6cs00351f. [DOI] [PubMed] [Google Scholar]
- O'Hagan D, Deng H. Enzymatic fluorination and biotechnological developments of the fluorinase. Chem Rev. 2015;115(2):634–649. doi: 10.1021/cr500209t. [DOI] [PubMed] [Google Scholar]
- O'Hagan D, Schaffrath C, Cobb SL, Hamilton JT, Murphy CD. Biochemistry: biosynthesis of an organofluorine molecule. Nature. 2002;416(6878):279. doi: 10.1038/416279a. [DOI] [PubMed] [Google Scholar]
- O’Hagan D, Harper BD. Fluorine-containing natural products. J. Fluorine Chem. 1999;100(1–2):127–133. doi: 10.1016/s0022-1139(99)00201-8. [DOI] [Google Scholar]
- Obach RS, Walker GS, Brodney MA. Biosynthesis of fluorinated analogs of drugs using human cytochrome P450 enzymes followed by deoxyfluorination and quantitative nuclear magnetic resonance spectroscopy to improve metabolic stability. Drug Metab Dispos. 2016;44(5):634–646. doi: 10.1124/dmd.116.069310. [DOI] [PubMed] [Google Scholar]
- Odar C, Winkler M, Wiltschi B. Fluoro amino acids: A rarity in nature, yet a prospect for protein engineering. Biotechnol J. 2015;10(3):427–446. doi: 10.1002/biot.201400587. [DOI] [PubMed] [Google Scholar]
- Olgun A. Selective targeting of signet ring cell adenocarcinomas. Med Hypotheses. 2019;133:109380. doi: 10.1016/j.mehy.2019.109380. [DOI] [PubMed] [Google Scholar]
- Paguigan ND, Al-Huniti MH, Raja HA, Czarnecki A, Burdette JE, Gonzalez-Medina M, Medina-Franco JL, Polyak SJ, Pearce CJ, Croatt MP, Oberlies NH. Chemoselective fluorination and chemoinformatic analysis of griseofulvin: Natural vs fluorinated fungal metabolites. Bioorg Med Chem. 2017;25(20):5238–5246. doi: 10.1016/j.bmc.2017.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer-Brown W, Dunne B, Ortin Y, Fox MA, Sandford G, Murphy CD. Biotransformation of fluorophenyl pyridine carboxylic acids by the model fungus Cunninghamella elegans. Xenobiotica. 2017;47(9):763–770. doi: 10.1080/00498254.2016.1227109. [DOI] [PubMed] [Google Scholar]
- Pant P, Pathak A, Jayaram B. Symmetric nucleosides as potent purine nucleoside phosphorylase inhibitors. J Phys Chem B. 2021;125(11):2856–2862. doi: 10.1021/acs.jpcb.0c10553. [DOI] [PubMed] [Google Scholar]
- Parker WB, Allan PW, Waud WR, Hong J, Gilbert-Ross M, Achyut BR, Joshi D, Behbahani T, Rab R, Ealick SE, Sorscher EJ. The use of Trichomonas vaginalis purine nucleoside phosphorylase to activate fludarabine in the treatment of solid tumors. Cancer Chemother Pharmacol. 2020;85(3):573–583. doi: 10.1007/s00280-019-04018-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil MD, Grogan G, Bommarius A, Yun H. Oxidoreductase-catalyzed synthesis of chiral amines. ACS Catal. 2018;8(12):10985–11015. doi: 10.1021/acscatal.8b02924. [DOI] [Google Scholar]
- Phillips RS, Craig S, Kovalevsky A, Gerlits O, Weiss K, Iorgu AI, Heyes DJ, Hay S. Pressure and temperature effects on the formation of aminoacrylate intermediates of tyrosine phenol-lyase demonstrate reaction dynamics. ACS Catal. 2019;10(3):1692–1703. doi: 10.1021/acscatal.9b03967. [DOI] [Google Scholar]
- Pinson A, Yarbrough AL, Bush JM, Cabanlong CV, Shoeib A, Jackson BK, Fukuda S, Gogoi J, Fantegrossi WE, McCain K, Prather PL, Fujiwara R, Radominska-Pandya A. Metabolism, CB1 cannabinoid receptor binding and in vivo activity of synthetic cannabinoid 5F-AKB48: Implications for toxicity. Pharmacol Biochem Behav. 2020;195:172949. doi: 10.1016/j.pbb.2020.172949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomeisl K, Krecmerova M, Pohl R, Snoeck R, Andrei G. Synthesis of fluorinated acyclic nucleoside phosphonates with 5-azacytosine base moiety. Tetrahedron. 2019;75(39):130529. doi: 10.1016/j.tet.2019.130529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preshlock S, Tredwell M, Gouverneur V. 18F-labeling of arenes and heteroarenes for applications in positron emission tomography. Chem Rev. 2016;116(2):719–766. doi: 10.1021/acs.chemrev.5b00493. [DOI] [PubMed] [Google Scholar]
- Pupo G, Ibba F, Ascough DMH, Vicini AC, Ricci P, Christensen KE, Pfeifer L, Morphy JR, Brown JM, Paton RS, Gouverneur V. Asymmetric nucleophilic fluorination under hydrogen bonding phase-transfer catalysis. Science. 2018;360(6389):638–642. doi: 10.1126/science.aar7941. [DOI] [PubMed] [Google Scholar]
- Pupo G, Vicini AC, Ascough DMH, Ibba F, Christensen KE, Thompson AL, Brown JM, Paton RS, Gouverneur V. Hydrogen bonding phase-transfer catalysis with potassium fluoride: Enantioselective synthesis of beta-fluoroamines. J Am Chem Soc. 2019;141(7):2878–2883. doi: 10.1021/jacs.8b12568. [DOI] [PubMed] [Google Scholar]
- Quintard A, Sperandio C, Rodriguez J. Modular enantioselective synthesis of an advanced pentahydroxy intermediate of antimalarial bastimolide A and of fluorinated and chlorinated analogues. Org Lett. 2018;20(17):5274–5277. doi: 10.1021/acs.orglett.8b02213. [DOI] [PubMed] [Google Scholar]
- Raskin SE, Tranby EP, Ludwig S, Okunev I, Frantsve-Hawley J, Boynes S. Survival of silver diamine fluoride among patients treated in community dental clinics: a naturalistic study. BMC Oral Health. 2021;21(1):35. doi: 10.1186/s12903-020-01379-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel M, Karaghiosoff K. Reagents for selective fluoromethylation: A challenge in organofluorine chemistry. Angew Chem Int Ed Engl. 2020;59(30):12268–12281. doi: 10.1002/anie.201913175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remete AM, Nonn M, Fustero S, Fülöp F, Kiss L. Synthesis of fluorinated amino acid derivatives through late-stage deoxyfluorinations. Tetrahedron. 2018;74(44):6367–6418. doi: 10.1016/j.tet.2018.09.021. [DOI] [Google Scholar]
- Ren S, Cheng X, Zhang Y, Zhuang J, Ma L. Advances in biosynthesis of fluorinated products. Acta Microbiol Sin. 2020;61(3):524–538. doi: 10.13343/j.cnki.wsxb.20200284. [DOI] [Google Scholar]
- Rentmeister A, Arnold FH, Fasan R. Chemo-enzymatic fluorination of unactivated organic compounds. Nat Chem Biol. 2009;5(1):26–28. doi: 10.1038/nchembio.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera-Chavez J, Raja HA, Graf TN, Burdette JE, Pearce CJ, Oberlies NH. Biosynthesis of fluorinated peptaibols using a site-directed building block incorporation approach. J Nat Prod. 2017;80(6):1883–1892. doi: 10.1021/acs.jnatprod.7b00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins T, Liu YC, Cane DE, Khosla C. Structure and mechanism of assembly line polyketide synthases. Curr Opin Struct Biol. 2016;41:10–18. doi: 10.1016/j.sbi.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha JF, Pina AF, Sousa SF, Cerqueira NMFSA. PLP-dependent enzymes as important biocatalysts for the pharmaceutical, chemical and food industries: a structural and mechanistic perspective. Catal Sci Technol. 2019;9(18):4864–4876. doi: 10.1039/c9cy01210a. [DOI] [Google Scholar]
- Romanov V, Isohashi K, Alobthani G, Beshr R, Horitsugi G, Kanai Y, Naka S, Watabe T, Shimosegawa E, Hatazawa J. Evaluation of the total distribution volume of 18F-FBPA in normal tissues of healthy volunteers by non-compartmental kinetic modeling. Ann Nucl Med. 2020;34(3):155–162. doi: 10.1007/s12149-019-01427-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau E, Wilson D, Lacroix-Poisson F, Krauze A, Chi K, Gleave M, McKenzie M, Tyldesley S, Goldenberg SL, Benard F. A prospective study on 18F-DCFPyL PSMA PET/CT imaging in biochemical recurrence of prostate cancer. J Nucl Med. 2019;60(11):1587–1593. doi: 10.2967/jnumed.119.226381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe SP, Campbell SP, Mana-Ay M, Szabo Z, Allaf ME, Pienta KJ, Pomper MG, Ross AE, Gorin MA. Prospective evaluation of PSMA-targeted 18F-DCFPyL PET/CT in men with biochemical failure after radical prostatectomy for prostate cancer. J Nucl Med. 2020;61(1):58–61. doi: 10.2967/jnumed.119.226514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe SP, Li X, Trock BJ, Werner RA, Frey S, DiGianvittorio M, Bleiler JK, Reyes DK, Abdallah R, Pienta KJ, Gorin MA, Pomper MG. Prospective comparison of PET imaging with PSMA-targeted 18F-DCFPyL versus Na18F for bone lesion detection in patients with metastatic prostate cancer. J Nucl Med. 2020;61(2):183–188. doi: 10.2967/jnumed.119.227793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadi J, Wennemers H. Enantioselective aldol reactions with masked fluoroacetates. Nat Chem. 2016;8(3):276–280. doi: 10.1038/nchem.2437. [DOI] [PubMed] [Google Scholar]
- Sadurni A. Gilmour R (2018) Stereocontrolled synthesis of 2-fluorinated C-glycosides. European J Org Chem. 2018;27–28:3684–3687. doi: 10.1002/ejoc.201800618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanada M, Miyano T, Iwadare S, Williamson JM, Arison BH, Smith JL, Douglas AW, Liesch JM, Inamine E. Biosynthesis of fluorothreonine and fluoroacetic acid by the thienamycin producer Streptomyces Cattleya. J Antibiot (tokyo) 1986;39(2):259–265. doi: 10.7164/antibiotics.39.259. [DOI] [PubMed] [Google Scholar]
- Sandoval BA, Hyster TK. Emerging strategies for expanding the toolbox of enzymes in biocatalysis. Curr Opin Chem Biol. 2020;55:45–51. doi: 10.1016/j.cbpa.2019.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheidt F, Schafer M, Sarie JC, Daniliuc CG, Molloy JJ, Gilmour R. Enantioselective, catalytic vicinal difluorination of alkenes. Angew Chem, Int Ed. 2018;57(50):16431–16435. doi: 10.1002/anie.201810328. [DOI] [PubMed] [Google Scholar]
- Schmitt S, Delamare J, Tirel O, Fillesoye F, Dhilly M, Perrio C. N-[18F]-FluoropropylJDTic for kappa-opioid receptor PET imaging: Radiosynthesis, pre-clinical evaluation, and metabolic investigation in comparison with parent JDTic. Nucl Med Biol. 2017;44:50–61. doi: 10.1016/j.nucmedbio.2016.09.005. [DOI] [PubMed] [Google Scholar]
- Scott TA, Heine D, Qin Z, Wilkinson B. An L-threonine transaldolase is required for L-threo-beta-hydroxy-alpha-amino acid assembly during obafluorin biosynthesis. Nat Commun. 2017;8:15935. doi: 10.1038/ncomms15935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senn HM, O'Hagan D, Thiel W. Insight into enzymatic C-F bond formation from QM and QM/MM calculations. J Am Chem Soc. 2005;127(39):13643–13655. doi: 10.1021/ja053875s. [DOI] [PubMed] [Google Scholar]
- Sester A, Stuer-Patowsky K, Hiller W, Kloss F, Lutz S, Nett M. Biosynthetic plasticity enables production of fluorinated aurachins. ChemBioChem. 2020;21(16):2268–2273. doi: 10.1002/cbic.202000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah M, Paramshivam G, Mehta A, Singh S, Chugh A, Prashar A, Chugh VK. Comparative assessment of conventional and light-curable fluoride varnish in the prevention of enamel demineralization during fixed appliance therapy: a split-mouth randomized controlled trial. Eur J Orthod. 2018;40(2):132–139. doi: 10.1093/ejo/cjx037. [DOI] [PubMed] [Google Scholar]
- Shahmohammadi S, Fulop F, Forro E (2020) Efficient synthesis of new fluorinated beta-amino acid enantiomers through lipase-catalyzed hydrolysis. Molecules 25(24). 10.3390/molecules25245990 [DOI] [PMC free article] [PubMed]
- Shao CL, Linington RG, Balunas MJ, Centeno A, Boudreau P, Zhang C, Engene N, Spadafora C, Mutka TS, Kyle DE, Gerwick L, Wang CY, Gerwick WH. Bastimolide A, a potent antimalarial polyhydroxy macrolide from the marine cyanobacterium Okeania hirsuta. J Org Chem. 2015;80(16):7849–7855. doi: 10.1021/acs.joc.5b01264. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Kaira K, Higuchi T, Hisada T, Yokobori T, Oyama T, Asao T, Tsushima Y, Shirabe K. Relationship between tumor immune markers and fluorine-18-alpha-methyltyrosine ([18F]FAMT) uptake in patients with lung cancer. Mol Imaging Biol. 2019 doi: 10.1007/s11307-019-01456-w. [DOI] [PubMed] [Google Scholar]
- Sisila V, Puhazhselvan P, Aarthy M, Sakkeeshyaa G, Saravanan P, Kamini NR, Ayyadurai N. Esterification of polymeric carbohydrate through congener cutinase-like biocatalyst. Appl Biochem Biotechnol. 2021;193(1):19–32. doi: 10.1007/s12010-020-03415-6. [DOI] [PubMed] [Google Scholar]
- Snoch W, Stępień K, Prajsnar J, Staroń J, Szaleniec M, Guzik M. Influence of chemical modifications of polyhydroxyalkanoate-derived fatty acids on their antimicrobial properties. Catalysts. 2019;9(6):510. doi: 10.3390/catal9060510. [DOI] [Google Scholar]
- Sooklal SA, De Koning C, Brady D, Rumbold K. Identification and characterisation of a fluorinase from Actinopolyspora mzabensis. Protein Expr Purif. 2020;166:105508. doi: 10.1016/j.pep.2019.105508. [DOI] [PubMed] [Google Scholar]
- Stachelska-Wierzchowska A, Wierzchowski J. Non-typical nucleoside analogs as fluorescent and fluorogenic indicators of purine-nucleoside phosphorylase activity in biological samples. Anal Chim Acta. 2020;1139:119–128. doi: 10.1016/j.aca.2020.09.018. [DOI] [PubMed] [Google Scholar]
- Stadlbauer S, Steinborn C, Klemd A, Hattori F, Ohmori K, Suzuki K, Huber R, Wolf P, Grundemann C. Impact of green tea catechin ECG and its synthesized fluorinated analogue on prostate cancer cells and stimulated immunocompetent cells. Planta Med. 2018;84(11):813–819. doi: 10.1055/s-0044-102099. [DOI] [PubMed] [Google Scholar]
- Sudileti M, Nagaripati S, Gundluru M, Chintha V, Aita S, Wudayagiri R, Chamarthi N, Cirandur SR. rGO-SO3H catalysed green synthesis of fluoro-substituted aminomethylene bisphosphonates and their anticancer, molecular docking studies. ChemistrySelect. 2019;4(44):13006–13011. doi: 10.1002/slct.201903191. [DOI] [Google Scholar]
- Sun H, Yeo WL, Lim YH, Chew X, Smith DJ, Xue B, Chan KP, Robinson RC, Robins EG, Zhao H, Ang EL. Directed evolution of a fluorinase for improved fluorination efficiency with a non-native substrate. Angew Chem, Int Ed. 2016;55(46):14277–14280. doi: 10.1002/anie.201606722. [DOI] [PubMed] [Google Scholar]
- Sun H, Zhao H, Ang EL. A coupled chlorinase-fluorinase system with a high efficiency of trans-halogenation and a shared substrate tolerance. Chem Commun (camb) 2018;54(68):9458–9461. doi: 10.1039/c8cc04436h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swallow S. Fluorine in medicinal chemistry. Prog Med Chem. 2015;54:65–133. doi: 10.1016/bs.pmch.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Tang ML, Zhou L, Chang J, Hu ZH, Qin Y, Sun X. Differential metabolism of 3FDT and docetaxel in RLMs, rats, and HLMs. Eur J Med Chem. 2016;113:81–91. doi: 10.1016/j.ejmech.2016.02.007. [DOI] [PubMed] [Google Scholar]
- Tao H, Mori T, Wei X, Matsuda Y, Abe I. One polyketide synthase, two distinct products: Trans-acting enzyme-controlled product divergence in calbistrin biosynthesis. Angew Chem Int Ed. 2021;60(16):8851–8858. doi: 10.1002/anie.202016525. [DOI] [PubMed] [Google Scholar]
- Tao L, Li Y, Guo X, Dong L, Liu L, Wang Q, Yu X, Song C, Chang J. Synthesis and anti-CVB3 activity of 4-amino acid derivative substituted pyrimidine nucleoside analogues. Bioorg Med Chem Lett. 2020;30(1):126770. doi: 10.1016/j.bmcl.2019.126770. [DOI] [PubMed] [Google Scholar]
- Thompson S, Fleming IN, O'Hagan D. Enzymatic transhalogenation of dendritic RGD peptide constructs with the fluorinase. Org Biomol Chem. 2016;14(11):3120–3129. doi: 10.1039/c6ob00239k. [DOI] [PubMed] [Google Scholar]
- Thompson S, McMahon SA, Naismith JH, O'Hagan D. Exploration of a potential difluoromethyl-nucleoside substrate with the fluorinase enzyme. Bioorg Chem. 2016;64:37–41. doi: 10.1016/j.bioorg.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Thompson S, Onega M, Ashworth S, Fleming IN, Passchier J, O'Hagan D. A two-step fluorinase enzyme mediated 18F labelling of an RGD peptide for positron emission tomography. Chem Commun (camb) 2015;51(70):13542–13545. doi: 10.1039/c5cc05013h. [DOI] [PubMed] [Google Scholar]
- Thomsen M, Vogensen SB, Buchardt J, Burkart MD, Clausen RP. Chemoenzymatic synthesis and in situ application of S-adenosyl-L-methionine analogs. Org Biomol Chem. 2013;11(43):7606–7610. doi: 10.1039/c3ob41702f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuronyi BW, Chang MC. Synthetic biology approaches to fluorinated polyketides. Acc Chem Res. 2015;48(3):584–592. doi: 10.1021/ar500415c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuronyi BW, Privalsky TM, Chang MCY. Engineered fluorine metabolism and fluoropolymer production in living cells. Angew Chem Int Ed. 2017;56(44):13637–13640. doi: 10.1002/anie.201706696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timofeev VI, Fateev IV, Kostromina MA, Abramchik YA, Konstantinova ID, Volkov VV, Lykoshin DD, Mikheeva OO, Muravieva TI, Esipov RS, Kuranova IP (2020) The comparative analysis of the properties and structures of purine nucleoside phosphorylases from thermophilic bacterium Thermus thermophilus HB27. J Biomol Struct Dyn:1–16. 10.1080/07391102.2020.1848628 [DOI] [PubMed]
- Toit JMP. The isolation of the toxic principle "potassium cymonate" from "Gifblaar" Dichapetalum cymosum (Hook) Engl. 1943. Onderstepoort J Vet Sci Anim Ind. 1943;18:203–206. [Google Scholar]
- Tong W, Huang Q, Li M, Wang J-b. Enzyme-catalyzed C-F bond formation and cleavage. Bioresour Bioprocess. 2019;6(1):46. doi: 10.1186/s40643-019-0280-6. [DOI] [Google Scholar]
- Troelsen NS, Shanina E, Gonzalez-Romero D, Dankova D, Jensen ISA, Sniady KJ, Nami F, Zhang H, Rademacher C, Cuenda A, Gotfredsen CH, Clausen MH. The 3F library: Fluorinated Fsp3-rich fragments for expeditious 19F-NMR based screening. Angew Chem, Int Ed. 2020;59(6):2204–2210. doi: 10.1002/anie.201913125. [DOI] [PubMed] [Google Scholar]
- Tu C, Zhou J, Peng L, Man S, Ma L. Self-assembled nano-aggregates of fluorinases demonstrate enhanced enzymatic activity, thermostability and reusability. Biomater Sci. 2020;8(2):648–656. doi: 10.1039/c9bm00402e. [DOI] [PubMed] [Google Scholar]
- Vaughan MD, Su Z, Daub E, Honek JF. Intriguing cellular processing of a fluorinated amino acid during protein biosynthesis in Escherichia coli. Org Biomol Chem. 2016;14(38):8942–8946. doi: 10.1039/c6ob01690a. [DOI] [PubMed] [Google Scholar]
- Walker MC, Thuronyi BW, Charkoudian LK, Lowry B, Khosla C, Chang MC. Expanding the fluorine chemistry of living systems using engineered polyketide synthase pathways. Science. 2013;341(6150):1089–1094. doi: 10.1126/science.1242345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Liang J, Yue Q, Li L, Shi Y, Chen G, Li YZ, Bian X, Zhang Y, Zhao G, Ding X. Engineering the acyltransferase domain of epothilone polyketide synthase to alter the substrate specificity. Microb Cell Fact. 2021;20(1):86. doi: 10.1186/s12934-021-01578-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JB, Ilie A, Yuan S, Reetz MT. Investigating substrate scope and enantioselectivity of a defluorinase by a stereochemical probe. J Am Chem Soc. 2017;139(32):11241–11247. doi: 10.1021/jacs.7b06019. [DOI] [PubMed] [Google Scholar]
- Wang Z, Matthews H. Translational incorporation of modified phenylalanines and tyrosines during cell-free protein synthesis. RSC Adv. 2020;10(19):11013–11023. doi: 10.1039/d0ra00655f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks AM, Chang MC. Catalytic control of enzymatic fluorine specificity. Proc Natl Acad Sci U S A. 2012;109(48):19667–19672. doi: 10.1073/pnas.1212591109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks AM, Keddie NS, Wadoux RD, O'Hagan D, Chang MC. Molecular recognition of fluorine impacts substrate selectivity in the fluoroacetyl-CoA thioesterase FlK. Biochemistry. 2014;53(12):2053–2063. doi: 10.1021/bi4015049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weeks AM, Wang N, Pelton JG, Chang MCY. Entropy drives selective fluorine recognition in the fluoroacetyl-CoA thioesterase from Streptomyces cattleya. Proc Natl Acad Sci U S A. 2018;115(10):E2193–E2201. doi: 10.1073/pnas.1717077115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman KJ. Genetic engineering of modular PKSs: from combinatorial biosynthesis to synthetic biology. Nat Prod Rep. 2016;33(2):203–230. doi: 10.1039/c5np00109a. [DOI] [PubMed] [Google Scholar]
- Won Y, Jeon H, Pagar AD, Patil MD, Nadarajan SP, Flood DT, Dawson PE, Yun H. In vivo biosynthesis of tyrosine analogs and their concurrent incorporation into a residue-specific manner for enzyme engineering. Chem Commun (camb) 2019;55(100):15133–15136. doi: 10.1039/c9cc08503c. [DOI] [PubMed] [Google Scholar]
- Wu L, Maglangit F, Deng H. Fluorine biocatalysis. Curr Opin Chem Biol. 2020;55:119–126. doi: 10.1016/j.cbpa.2020.01.004. [DOI] [PubMed] [Google Scholar]
- Wu L, Tong MH, Kyeremeh K, Deng H (2020b) Identification of 5-fluoro-5-deoxy-ribulose as a shunt fluorometabolite in Streptomyces sp. MA37. Biomolecules 10(7). 10.3390/biom10071023 [DOI] [PMC free article] [PubMed]
- Wu L, Tong MH, Raab A, Fang Q, Wang S, Kyeremeh K, Yu Y, Deng H. An unusual metal-bound 4-fluorothreonine transaldolase from Streptomyces sp. MA37 catalyses promiscuous transaldol reactions. Appl Microbiol Biotechnol. 2020;104(9):3885–3896. doi: 10.1007/s00253-020-10497-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q, Cai J, Zhao F, Zhou Z, Yang D, Qin Z (2020d) Synthesis and insecticidal activity of the fluorinated galegine analogues. Nat Prod Res:1–5. 10.1080/14786419.2020.1836631 [DOI] [PubMed]
- Xiao Y, Wang Y, Zhu W, Yao J, Sun C, Militky J, Venkataraman M, Zhu G. Development of tree-like nanofibrous air filter with durable antibacterial property. Sep Purif Technol. 2021;259:118135. doi: 10.1016/j.seppur.2020.118135. [DOI] [Google Scholar]
- Xie J, Hase WL. Organic chemistry. Rethinking the SN2 reaction. Science. 2016;352(6281):32–33. doi: 10.1126/science.aaf5172. [DOI] [PubMed] [Google Scholar]
- Yamaguchi A, Hanaoka H, Higuchi T, Tsushima Y. Selective synthesis of L-2-[18F]fluoro-alpha-methylphenylalanine via copper-mediated 18F-fluorination of (mesityl)(aryl)iodonium salt. J Labelled Comp Radiopharm. 2020;63(8):368–375. doi: 10.1002/jlcr.3840. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Umegawa Y, Yamagami M, Suzuki T, Tsuchikawa H, Hanashima S, Matsumori N, Murata M. The perpendicular orientation of amphotericin B methyl ester in hydrated lipid bilayers supports the barrel-stave model. Biochemistry. 2019;58(17):2282–2291. doi: 10.1021/acs.biochem.9b00180. [DOI] [PubMed] [Google Scholar]
- Yang X, Wu T, Phipps RJ, Toste FD. Advances in catalytic enantioselective fluorination, mono-, di-, and trifluoromethylation, and trifluoromethylthiolation reactions. Chem Rev. 2015;115(2):826–870. doi: 10.1021/cr500277b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang ZY, Hao YC, Hu SQ, Zong MH, Chen Q, Li N. Direct reductive amination of biobased furans to N-substituted furfurylamines by engineered reductive aminase. Adv Synth Catal. 2021;363(4):1033–1037. doi: 10.1002/adsc.202001495. [DOI] [Google Scholar]
- Yeo WL, Chew X, Smith DJ, Chan KP, Sun H, Zhao H, Lim YH, Ang EL. Probing the molecular determinants of fluorinase specificity. Chem Commun (camb) 2017;53(17):2559–2562. doi: 10.1039/c6cc09213f. [DOI] [PubMed] [Google Scholar]
- Yimmut K, Homchoo K, Hinchiranan N. Poly(butyl acrylate-co-fluorinated acrylate)-graft-natural rubber: Synthesis and application as compatibilizer for natural rubber/poly(butyl acrylate-co-fluorinated acrylate) films. Colloids Surf A. 2018;540:11–22. doi: 10.1016/j.colsurfa.2017.12.062. [DOI] [Google Scholar]
- Youn J-W, Albermann C, Sprenger GA. In vivo cascade catalysis of aromatic amino acids to the respective mandelic acids using recombinant E. coli cells expressing hydroxymandelate synthase (HMS) from Amycolatopsis mediterranei. Mol Catal. 2020;483:110713. doi: 10.1016/j.mcat.2019.110713. [DOI] [Google Scholar]
- Yue Y, Chen J, Bao L, Wang J, Li Y, Zhang Q. Fluoroacetate dehalogenase catalyzed dehalogenation of halogenated carboxylic acids: A QM/MM approach. Chemosphere. 2020;254:126803. doi: 10.1016/j.chemosphere.2020.126803. [DOI] [PubMed] [Google Scholar]
- Zechel DL, Reid SP, Nashiru O, Mayer C, Stoll D, Jakeman DL, Warren RA, Withers SG. Enzymatic synthesis of carbon-fluorine bonds. J Am Chem Soc. 2001;123(18):4350–4351. doi: 10.1021/ja005855q. [DOI] [PubMed] [Google Scholar]
- Zhang B, Liu J, Gao D, Yu X, Wang J, Lei X. A fluorine scan on the Zn2+-binding thiolate side chain of HDAC inhibitor largazole: Synthesis, biological evaluation, and molecular modeling. Eur J Med Chem. 2019;182:111672. doi: 10.1016/j.ejmech.2019.111672. [DOI] [PubMed] [Google Scholar]
- Zhang H, Tian S, Yue Y, Li M, Tong W, Xu G, Chen B, Ma M, Li Y, Wang J-b. Semirational design of fluoroacetate dehalogenase RPA1163 for kinetic resolution of α-fluorocarboxylic acids on a gram scale. ACS Catal. 2020;10(5):3143–3151. doi: 10.1021/acscatal.9b04804. [DOI] [Google Scholar]
- Zhang J, Huang X, Zhang RK, Arnold FH. Enantiodivergent alpha-amino C-H fluoroalkylation catalyzed by engineered cytochrome P450s. J Am Chem Soc. 2019;141(25):9798–9802. doi: 10.1021/jacs.9b04344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Dall'Angelo S, Fleming IN, Schweiger LF, Zanda M, O'Hagan D. Last-step enzymatic [18F]-fluorination of cysteine-tethered RGD peptides using modified barbas linkers. Chemistry. 2016;22(31):10998–11004. doi: 10.1002/chem.201601361. [DOI] [PubMed] [Google Scholar]
- Zhang S, Klementz D, Zhu J, Makitrynskyy R, Ola Pasternak AR, Gunther S, Zechel DL, Bechthold A. Genome mining reveals the origin of a bald phenotype and a cryptic nucleocidin gene cluster in Streptomyces asterosporus DSM 41452. J Biotechnol. 2019;292:23–31. doi: 10.1016/j.jbiotec.2018.12.016. [DOI] [PubMed] [Google Scholar]
- Zhang XX, Gao Y, Hu XS, Ji CB, Liu YL, Yu JS. Recent advances in catalytic enantioselective synthesis of fluorinated α- and β-amino acids. Adv Synth Catal. 2020;362(22):4763–4793. doi: 10.1002/adsc.202000966. [DOI] [Google Scholar]
- Zhao S, Liang T, Zhu L, Yang L, Liu T, Fu J, Wang B, Zhan J, Liu L. Fate of 6:2 fluorotelomer sulfonic acid in pumpkin (Cucurbita maxima L.) based on hydroponic culture: Uptake, translocation and biotransformation. Environ Pollut. 2019;252(Pt A):804–812. doi: 10.1016/j.envpol.2019.06.020. [DOI] [PubMed] [Google Scholar]
- Zhao S, Liu T, Zhu L, Yang L, Zong Y, Zhao H, Hu L, Zhan J. Formation of perfluorocarboxylic acids (PFCAs) during the exposure of earthworms to 6:2 fluorotelomer sulfonic acid (6:2 FTSA) Sci Total Environ. 2021;760:143356. doi: 10.1016/j.scitotenv.2020.143356. [DOI] [PubMed] [Google Scholar]
- Zhao X, Noro J, Fu J, Silva C, Cavaco-Paulo A. Strategies for the synthesis of fluorinated polyesters. RSC Adv. 2019;9(4):1799–1806. doi: 10.1039/c8ra10341k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhdankin VV, Luzzio FA, Monsen PJ. Selective fluorination of natural products. Arkivoc. 2017;2017(1):117–147. doi: 10.24820/ark.5550190.p009.917. [DOI] [Google Scholar]
- Zheng W, Chen K, Wang Z, Cheng X, Xu G, Yang L, Wu J. Construction of a highly diastereoselective aldol reaction system with L-threonine aldolase by computer-assisted rational molecular modification and medium engineering. Org Lett. 2020;22(15):5763–5767. doi: 10.1021/acs.orglett.0c01792. [DOI] [PubMed] [Google Scholar]
- Zheng W, Yu H, Fang S, Chen K, Wang Z, Cheng X, Xu G, Yang L, Wu J. Directed evolution of L-threonine aldolase for the diastereoselective synthesis of β-hydroxy-α-amino acids. ACS Catal. 2021;11(6):3198–3205. doi: 10.1021/acscatal.0c04949. [DOI] [Google Scholar]
- Zhong J, Li W, Qian J, Fu C, Chu H, Xu J, Ran X, Nie W. Modulation of the interfacial architecture enhancing the efficiency and energy density of ferroelectric nanocomposites via the irradiation method. J Colloid Interface Sci. 2021;586:30–38. doi: 10.1016/j.jcis.2020.10.066. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Wang J, Gu Z, Wang S, Zhu W, Acena JL, Soloshonok VA, Izawa K, Liu H. Next generation of fluorine-containing pharmaceuticals, compounds currently in phase II-III clinical trials of major pharmaceutical companies: New structural trends and therapeutic areas. Chem Rev. 2016;116(2):422–518. doi: 10.1021/acs.chemrev.5b00392. [DOI] [PubMed] [Google Scholar]
- Zhu HQ, Tang XL, Zheng RC, Zheng YG. Purification and biochemical characterization of a tyrosine phenol-lyase from Morganella morganii. Appl Biochem Biotechnol. 2020 doi: 10.1007/s12010-020-03301-1. [DOI] [PubMed] [Google Scholar]
- Zhu W, Doubleday PF, Catlin DS, Weerawarna PM, Butrin A, Shen S, Wawrzak Z, Kelleher NL, Liu D, Silverman RB. A remarkable difference that one fluorine atom confers on the mechanisms of inactivation of human ornithine aminotransferase by two cyclohexene analogues of gamma-aminobutyric acid. J Am Chem Soc. 2020;142(10):4892–4903. doi: 10.1021/jacs.0c00193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Robinson DA, McEwan AR, O'Hagan D, Naismith JH. Mechanism of enzymatic fluorination in Streptomyces cattleya. J Am Chem Soc. 2007;129(47):14597–14604. doi: 10.1021/ja0731569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Wang J, Mou P, Yan Y, Chen M, Tang Y. Genome mining of cryptic tetronate natural products from a PKS-NRPS encoding gene cluster in Trichoderma harzianum t-22. Org Biomol Chem. 2021;19(9):1985–1990. doi: 10.1039/d0ob02545c. [DOI] [PubMed] [Google Scholar]
- Zou L, Ruan Y, Jiang W, Yan N, Liu DY, Yu CY, Hu XG. Switchable regioselectivity in the opening of aziridine by fluoride: DFT calculation and synthesis of fluorinated piperidines. ChemistrySelect. 2019;4(43):12683–12688. doi: 10.1002/slct.201903403. [DOI] [Google Scholar]
- Zuo R, Zhang Y, Huguet-Tapia JC, Mehta M, Dedic E, Bruner SD, Loria R, Ding Y. An artificial self-sufficient cytochrome P450 directly nitrates fluorinated tryptophan analogs with a different regio-selectivity. Biotechnol J. 2016;11(5):624–632. doi: 10.1002/biot.201500416. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.