Abstract
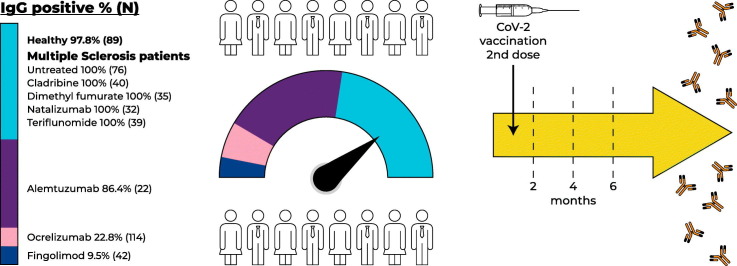
Appropriate immune response following COVID-19 vaccination is important in the context of disease-modifying treatments (DMTs). In a prospective cross-sectional study, we determined SARS-COV-2 IgG response up to 6 months following PfizerBNT162b2 vaccination in 414 multiple sclerosis (MS) patients and 89 healthy subjects. Protective response was demonstrated in untreated MS patients (N = 76, 100%), treated with Cladribine (N = 48, 100%), Dimethyl fumarate (N = 35, 100%), Natalizumab (N = 32, 100%), and Teriflunomide (N = 39, 100%), similarly to healthy subjects (N = 89, 97.8%). Response was decreased in Fingolimod (N = 42, 9.5%), Ocrelizumab (N = 114, 22.8%) and Alemtuzumab (N = 22, 86.4%) treated patients. IgG response can help tailor adequate vaccine guidelines for MS patients under various DMTs.
Keywords: Multiple sclerosis, COVID-19, Vaccination, IgG antibody, Humoral immunity, Disease modifying treatments
Graphical abstract
1. Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-COV-2), the causative agent of coronavirus disease 2019 (COVID-19), has caused a devastating disease with high rates of morbidity and mortality spreading all over the world (Seyed Hosseini et al., 2020). The lipid nanoparticle-formulated Pfizer-BioNTech COVID-19 (BNT162b2) vaccine is a new type of vaccine based on nucleoside-modified mRNA vector vaccine encoding the pre-fusion spike glycoprotein of SARS-COV-2 (Polack et al., 2020).
In Israel, a nationwide mass vaccination setting was initiated as early as Dec 2020 leading to full vaccination of a large population by the novel BNT162b2 mRNA vaccine (Dagan et al., 2021). As the process of vaccination against COVID-19 is currently spreading all over the world, it is of importance to assess the duration of the humoral immune response. BNT162b2 induced high titers of neutralizing antibodies against the SARS-COV-2 and its variants including B.1.1.7, B.1.351, and P.1., in the majority of vaccinees (Barros-Martins et al., 2021).
Effective vaccination both humoral and cellular against COVID-19 is critical especially in susceptible patients with chronic autoimmune diseases treated with immune suppressive medications as patients with multiple sclerosis (MS). We have previously shown that the humoral immune response within the early stage post-COVID-19 vaccination was impaired in patients treated with fingolimod and patients treated with ocrelizumab, while patients treated with cladribine developed protective antibody response 4 months after the last dosing (Achiron et al., 2021).
It is still unknown whether COVID-19 vaccination will induce long-term immunity that results in a protective humoral response in MS patients treated with other DMTs, and to what extent the immune response will be sustained. Specifically, many MS patients treated with teriflunomide, dimethyl fumarate, and natalizumab have low peripheral blood lymphocyte counts (Fox et al., 2019; Schweitzer et al., 2021), that could interfere with adequate induction of an immune response, and the question of whether these patients will develop appropriate immunogenicity to COVID-19 vaccine needs to be approached. In accordance, in the current study, we evaluated long-term humoral immune response in a large cohort of MS vaccinees using a targeted quantitative SARS-COV-2 IgG antibody assay reported to highly correlate with neutralizing antibodies (Mendrone-Junior et al., 2021). Our findings are of importance to the MS community in the aim of establishing an appropriate immunization policy in relation to DMTs.
2. Methods
Study design: Prospective observational study.
Ethics approval: The study was approved by Sheba Institutional Review Board Committee (Sheba.SMC-8182-21).
Consent to participate: Written informed consent was obtained from all participants. Each participant record was coded anonymously to ensure confidentiality during statistical analyses.
2.1. Participants
Patients with MS followed at the Sheba MS Center either untreated or under treatment with DMTs and fully vaccinated against COVID-19 were included in the study. A group of vaccinated healthy subjects served as controls. Timing of vaccination was between December 2020 and February 2021. Demographic, clinical, DMTs, and vaccine related data were obtained from the Sheba MS Center Computerized Database and further verified with each subject. Patients underwent neurological examination and an updated disability score by the Extended Disability Status Scale (EDSS) was performed. Blood for SARS-COV-2 serology and lymphocyte count was collected at least 28 days following the second vaccine dose.
In a subgroup of healthy subjects, untreated MS patients and patients treated with fingolimod and ocrelizumab, blood samples were tested for SARS-COV-2 specific B-cell memory response and SARS-COV-2 specific T cell memory response, 2 to 4 months following the second COVID-19 vaccine dose.
Inclusion criteria: (1) MS diagnosed according to McDonald criteria 2010, (Polman et al., 2011); (2) Age ≥ 18 years; (3) Received PfizerBNT162b2 two vaccine doses, 21 days apart; (3) Signed written informed consent.
Exclusion Criteria: (1) COVID-19 disease prior to the study confirmed by a nasopharyngeal PCR examination.
2.2. COVID-19 vaccination
Subjects received two intramuscular injections, 21 days apart, delivered in the deltoid muscle. Each injection contained 30 μg of PfizerBNT162b2 (0.3 ml volume per dose).
2.3. Immunoassay for detection of anti-SARS-COV-2 IgG
Serum samples were examined for anti-SARS-COV-2 IgG using ELISA kit based on the recombinant S1 protein from the SARS-COV-2 spike protein (Euroimmun, Lubeck, Germany). Index values (signal to cut-off ratios) >1.1 were considered positive (EUROIMMUN. Anti-SARS-COV-2 ELISA IgG, [Package Insert], Moutain Lakes, NJ: EUROIMMUN US, 2020). Absolute lymphocyte count (ALC, cells/mm3) in the peripheral blood were collected at the same date of IgG serology and determined by a DxI hematology analyzer (Beckman Coulter USA).
2.4. SARS-COV-2 specific memory B-cells
Analysis of SARS-COV-2 specific memory B-cells was performed by FluoroSpot assay detecting receptor-binding domain (RBD) memory B cell following polyclonal B cell stimulation. We used reversed antigen human IgG SARS-COV-2 RBD ELISpotPLUS (Mabtech, Sweden), according to the manufacturer instructions. Briefly, peripheal blood mononuclar cells (PBMCs) were incubated (250,000 cells/well) on an anti-IgG FluoroSpot plate after stimulation with a mixture of R848 (1mkg/ml) and IL2 (10 ng/ml) (B-Cell stimpack, Mabtech, Sweden). The number of SARS-COV-2 specific IgG secreting B-cells was measured as Spot Forming Units (SFU) using Mabtech IRIS™ reader. The results were expressed as the number SFU per 250,000 seeded cells after subtracting the background of unstimulated cells. Positive cut-off value was set above the 90% confidence interval in healthy non-vaccinated subjects, (n = 10, cutoff = 5.0 SFU), Suppl Fig. 1A.
Fig. 1.
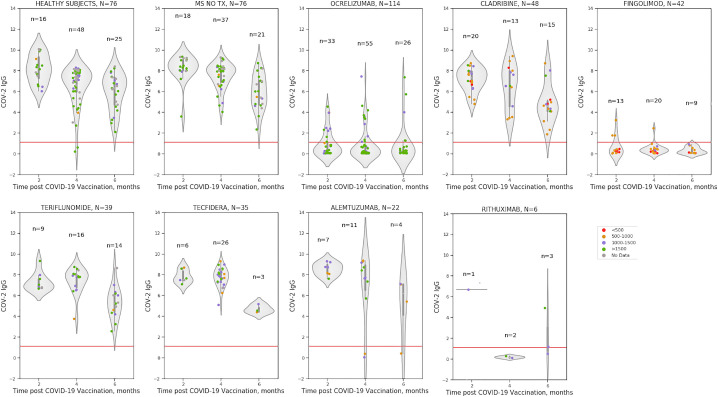
Post-vaccination COV-2 IgG antibody titer by DMTs in relation absolute lymphocyte count presented as grading >1500 cells/mm3 (green circles), between 1000 and 1500/cells mm3 (purple), between 500 and 1000/cells mm3 (orange), <500/mm3 (red), no data (grey). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.5. SARS-COV-2 specific memory T-cells
IFNg and IL2 secreting memory T cells were detected using SARS-COV-2 FluoroSpotPLUS kit according to the manufacture's protocol (Mabtech AB, Sweden). We used pre-coated plates with captured monoclonal anti-IFNγ and anti-IL2 incubated overnight in RPMI-1640 medium containing 10% FCS supplemented with SARS-COV-2 peptide pools (Mabtech AB, Sweden), covering the S1 domain (166 peptides) and the SNMO domains (47 synthetic peptides derived from the S, N, M ORF3a and ORF7a viral proteins), (Ahmed et al., 2020). Anti-CD3 response served as positive control for polyclonal T-cell activation.
Results for any memory T-cell response to any spike peptides (S1, SNMO) were expressed as the number of SFU per 250,000 seeded cells after subtracting the background spots of healthy non-vaccinated subjects (n = 10, cutoff for S1 = 3.0 SFU, for SNMO = 2.0 SFU), Suppl Fig. 1B, C.
Results of ELISpot and Fluorospot assays were evaluated using IRIS-reader and analyzed by IRIS software version 1.1.9 (Mabtech AB).
2.6. Statistical analysis
Categorical variables are described giving sample size absolute and relative frequency by treatment group. Continuous variables are reported by sample size, arithmetic mean, standard deviation, range, median, and 95% confidence interval (CI) for means of variables, by treatment group. Analysis of variance (ANOVA) model was applied for analyzing the effect of absolute lymphocyte count (ALC), and time between vaccination and the anti-SARS-COV-2 IgG test on the level of anti-SARS-COV-2 IgG adjusted for possible confounders by treatment group. Chi-square was applied to test the statistical significance of the difference in the distribution of categorical variables between treatment groups. t-test was applied to test the statistical significance of the difference in continuous variables means between ocrelizumab-treated MS patients that developed positive humoral response and ocrelizumab-treated patients that failed. ANOVA model using Dunnett method was applied for testing the differences in anti-SARS-COV-2 IgG between each treatment group and the healthy subjects group adjusted to possible covariates and for assessing the length of time of post-vaccination humoral immune response. Pearson coefficients were calculated to test the relationship between anti-SARS-COV-2 IgG and ALC, and between anti-SARS-COV-2 IgG and the time from last dose to first vaccine dose for relevant DMTs. All tests were two-tailed, and a p-value of <0.5% or less was considered statistically significant. The data was analyzed using SAS® version 9.4 (SAS Institute, Cary North Carolina) and Python version 3.0 software.
3. Results
3.1. Participants
The study population included 503 subjects, 89 healthy subjects and 414 MS patients, all completed COVID-19 vaccination and received 2 doses of PfizerBNT162b2 m-RNA vaccine. Of the 414 MS patients, 76 (18.4%) were untreated and 338 (81.6%) were treated with various DMTs including alemtuzumab (N = 22), cladribine (N = 48), dimethyl fumarate (N = 35), fingolimod (N = 42), natalizumab (N = 32), ocrelizumab (N = 114), rituximab (N = 6), and teriflunomide (N = 39). The demographics and clinical data of the study cohort by specific DMTs are presented in Table 1 .
Table 1.
Demographic, clinical, and COVID-19 vaccine immune-related variables.
| Study population N = 503 MS patients N = 414 |
Gender, n (%) Females Males |
Age, years | Disease duration, years | Disability by EDSS |
|---|---|---|---|---|
| Healthy subjects | 64 (71.9) | 53.5 ± 15.4 | _______ | ______ |
| N = 89 | 25 (28.1) | 56.6 (50.2–56.7) | ||
| Untreated MS | 50 (65.8) | 45.1 ± 15.2 | 12.1 ± 11.6 | 2.7 ± 2.4 |
| N = 76, 18.4% | 26 (34.2) | 44.7 (41.6–48.6) | 8.8 (9.5–14.7) | 2.0 (2.1–3.2) |
| Alemtuzumab | 17 (77.3) | 39.7 ± 8.1 | 13.6 ± 6.9 | 4.2 ± 2.4 |
| N = 22 | 5 (22.7) | 39.7 (36.1–43.3) | 11.3 (10.5–16.6) | 4.5 (3.2–5.3) |
| Cladribine | 39 (81.3) | 42.7 ± 8.1 | 14.8 ± 9.4 | 2.9 ± 1.8 |
| N = 48 | 9 (18.8) | 43.2 (40.3–45.0) | 11.2 (12.1–17.5) | 2.8 (2.4–3.2) |
| Dimethyl fumarate | 32 (91.4) | 47.0 ± 8.6 | 14.3 ± 10.6 | 2.2 ± 1.6 |
| N = 35 | 3 (8.6) | 47.3 (44.0–49.9) | 13.8 (10.7–18.0) | 2.0 (1.6–2.8) |
| Fingolimod | 24 (57.1) | 45.2 ± 11.1 | 16.0 ± 7.4 | 2.3 ± 1.7 |
| N = 42 | 18 (42.9) | 44.8 (41.7–48.6) | 16.4 (13.7–18.3) | 2.0 (1.8–2.9) |
| Natalizumab | 25 (78.1) | 41.1 ± 10.9 | 12.6 ± 7.2 | 2.5 ± 1.7 |
| N = 32 | 7 (21.9) | 43.4 (37.2–45.0) | 12.0 (10.0–15.2) | 2.3 (1.9–3.1) |
| Ocrelizumab | 61 (53.5) | 41.1 ± 10.9 | 16.3 ± 10.9 | 5.2 ± 1.5 |
| N = 114 | 53 (46.5) | 43.4 (37.2–45.0) | 15.6 (14.3–18.4) | 5.5 (4.9–5.5) |
| Rituximab | 6 (100) | 51.7 ± 6.3 | 20.5 ± 2.6 | 2.9 ± 2.8 |
| N = 6 | 48.3 (45.1–58.3) | 21.5 (17.8–23.2) | 1.3 (0–5.8) | |
| Teriflunomide | 28 (71.8) | 47.6 ± 10.5 | 15.0 ± 10.4 | 2.7 ± 1.7 |
| N = 39 | 11 (28.2) | 49.0 (44.2–51.1) | 10.5 (11.6–18.4) | 2.0 (2.2–3.2) |
Data are presented as mean ± SD, median (95% CI).
3.2. Anti-SARS-COV-2 IgG humoral immune response
Humoral immune response by quantitative anti-SARS-COV-2 IgG index, the percent of patients with positive anti-SARS-COV-2 IgG response by various DMTs, and related variables including ALC and time from the last treatment dose to vaccine for relevant DMTs are presented in Table 2 . Positive humoral response was demonstrated in 97.8% of healthy subjects, 100% of untreated MS patients, patients treated with cladribine, dimethyl fumarate, natalizumab, and teriflunomide, 86.4% of MS patients treated with alemtuzumab, 22.8% of MS patients treated with ocrelizumab, and 9.5% of MS patients treated with fingolimod. There were no significant changes regarding the level of SARS-COV-2 IgG antibodies between the healthy vaccinated subjects, untreated MS patients and patients treated with various DMTs groups, except for ocrelizumab and fingolimod treated patients that had significantly lower antibody level as compared with healthy subjects (p < 0.001 for both).
Table 2.
Humoral immune response following COVID-19 vaccination in MS patients.
| Mean ± SD Median (95% CI) Range |
Time from 2nd vaccine dose, days | SARS-COV-2 IgG Index | SARS-COV-2 positive N, % |
ALC cells/mm3 | Time from last dosing, months |
|---|---|---|---|---|---|
| Healthy | 89.9 ± 39.3 75.0 (81.7–98.2) 28–165 |
6.7 ± 1.9 7.1 (6.2–7.1) 0.2–10.1 |
87/89 97.8 |
2090 ± 812 2130 1747–2432 840–4020 |
_____ |
| N = 89 | |||||
| Untreated MS | 89.4 ± 39.9 77.0 (80.3–98.5) 28–167 |
7.3 ± 1.6 7.9 (9.5–14.7) 2.3–9.3 |
76/76 100 |
2378 ± 1418 2100 (2002–2755) 580–8480 |
_____ |
| N = 76, 18.4% | |||||
| Alemtuzumab | 85.8 ± 42.0 73.5 (67.1–104.4) 28–171 |
7.0 ± 2.9 7.9 (5.7–8.3) 0–9.3 |
19/22 86.4 |
1215 ± 348 1085 (1051–1378) 800–2080 |
21.6 ± 13.7 17.4 (14.3–28.9) 3.6–50.1 |
| N = 22 | |||||
| Cladribine | 89.3 ± 42.8 66.5 (76.9–101.7) 33–167 |
6.3 ± 1.9 6.6 (5.7–6.8) 1.9–9.4 |
48/48 100 |
1029 ± 390 950 (916–1142) 440–1930 |
8.5 ± 4.8 6.8 (7.1–9.9) 2.8–22.6 |
| N = 48 | |||||
| Dimethyl fumarate | 76.4 ± 29.4 64.0 (66.3–86.5) 43.0–169.0 |
7.5 ± 1.2 7.6 (7.1–7.9) 4.4–9.3 |
35/35 100 |
1505 ± 559 1390 (1303–1707) 830–2900 |
_____ |
| N = 35 | |||||
| Fingolimod | 88.0 ± 39.5 76.0 (75.7–100.4) 28–175 |
0.5 ± 0.7 0.3 (0.3–0.7) 0–3.2 |
4/42 9.5 |
606 ± 227 600 (535–677) 270–1220 |
_____ |
| N = 42 | |||||
| Natalizumab | 69.1 ± 30.4 63.0 (58.1–80.1) 28–172 |
7.2 ± 1.7 7.4 (6.6–7.8) 1.7–9.1 |
32/32 100 |
3562 ± 1333 3630 (3081–4042) 900–6130 |
_____ |
| N = 32 | |||||
| Ocrelizumab | 87.4 ± 37.2 70.0 (80.5–94.3) 48–169 |
0.9 ± 1.5 0.2 (0.6–1.2) 0.0–7.4 |
26/114 22.8 |
1834 ± 641 1750 (1714–1953) 850–4650 |
4.3 ± 1.8 4.1 4.0–4.6 1.0–12.0 |
| N = 114 | |||||
| Rituximab | 100 ± 46.5 110.5 (51.2–148.8) 28–155 |
2.3 ± 2.8 0.8 (−0.7–5.2) 0.1–6.7 |
2/6 33 |
1405 ± 316 1285 (1072–1737) 1150–1890 |
29.2 ± 25.2 16.4 (−33.4–91.7) 12.9–58.2 |
| N = 6 | |||||
| Teriflunomide | 91.6 ± 40.9 71.0 (78.3–104.9) 35–161 |
6.6 ± 1.6 6.8 (6.1–7.2) 2.6–9.3 |
39/39 100 |
1666 ± 551 1580 (1464–1868) 870–3470 |
_____ |
| N = 39 |
*Time from 2nd vaccine dose (days) and Time from last treatment dosing (months) were calculated as the time difference to the date of SARS-COV-2 IgG test.
3.3. Assessment of possible covariates that affect post-COVID-19 vaccination anti-SARS-COV-2 IgG response in ocrelizumab treated MS patients
Comparison within ocrelizumab-treated MS patients that developed positive humoral response (26/114, 22.8%) and ocrelizumab-treated patients that failed (88/114, 77.2%), showed that patients that developed positive humoral response were significantly younger at the time of vaccination (mean ± SD age 48.8 ± 13.13 vs. 55.4 ± 12.78 years, p = 0.012), the time from last ocrelizumab dosing was significantly longer (mean ± SD 5.2 ± 2.6 vs. 4.0 ± 1.37 months, p = 0.001), and the ALC was higher (mean ± SD 1893 ± 680 vs. 1636 ± 444 cells/mm3, p = 0.037). Disease duration and neurological disability by the EDSS score did not differ between ocrelizumab-treated MS patients that mounted a protective immune response and those that did not.
3.4. Duration of post-vaccination SARS-COV-2 IgG humoral immune response
Humoral IgG immune response was assessed within a median range of 2.3 to 6.3 months following vaccination in MS patients treated with various DMTs. Analysis of IgG response by the time in months following the second vaccine dose is demonstrated in Fig. 1.
Comparison of the longevity of post-vaccination SARS-COV-2 IgG humoral immune response assessing the time interval from vaccination to SARS-COV-2 IgG test, between various DMTs, untreated MS and healthy subjects demonstrated that post-vaccination SARS-COV-2 IgG was significantly lower in fingolimod (p < 0.0001), ocrelizumab (p < 0.0001), and rituximab (p < 0.0001) treated MS patients after adjustment for age and gender. No significant statistical differences were found in post-vaccination SARS-COV-2 IgG between healthy subjects and untreated MS patients or patients treated with alemtuzumab, cladribine, dimethyl fumarate, natalizumab and teriflunomide.
3.5. Effect of ALC on post-vaccination SARS-COV-2 IgG humoral immune response
ALC (cells/mm3) were within the normal range in healthy subjects, untreated MS patients and patients treated with dimethyl fumarate, natalizumab, ocrelizumab, rituximab, and teriflunomide. In patients treated with alemtuzumab, cladribine and fingolimod ALC was lower (median 1085, 980, 600, cells/mm3, respectively). However, only in fingolimod-treated patients ALC significantly correlated with post-vaccination SARS-COV-2 IgG (r = 0.338, p = 0.028). This correlation held true after adjustment to age, gender and neurological disability by the EDSS score, Fig. 1.
3.6. Effect of the time from last dosing on post-vaccination SARS-COV-2 IgG
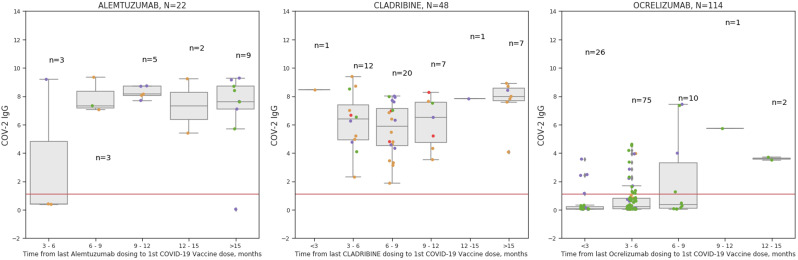
Linear regression model evaluating the effect of time from last treatment dosing to first vaccine dose on post-vaccination SARS-COV-2 IgG, demonstrated significant correlation for alemtuzumab (p < 0.0001) and for ocrelizumab (p < 0.0001), and non-significant correlation for cladribine (p = 0.0879) that could be accounted to limited heterogeneity in the time from last dosing to first vaccine dose, Fig. 2 .
Fig. 2.
Time in months from the last DMT dosing to COVID-19 vaccination for MS patients treated with Alemtuzumab, Cladribine or Ocrelizumab in relation to post-vaccination SARS-COV-2 IgG.
Post-vaccination SARS-COV-2 IgG antibody titer by time (months) from last DMT dosing is shown in relation to absolute lymphocyte count presented as grading >1500 cells/mm3 (green circles), between 1000 and 1500/cells mm3 (purple), between 500 and 1000/cells mm3 (orange), <500/mm3 (red), no data (grey). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
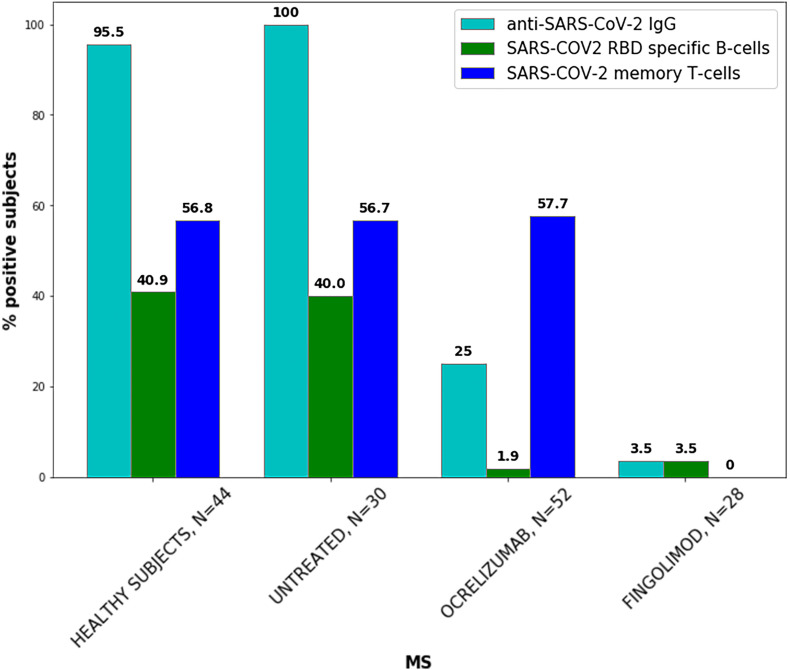
3.7. SARS-COV-2 specific cellular immune responses following COVID-19 vaccination
The percent of subjects that developed memory B-cells specific for SARS-COV-2 RBD and memory T-cells secreting IFN-γ and IL-2 in response to SARS-COV-2 peptides, 2 to 4 months post the second vaccine dose are presented in Fig. 3 . In healthy subjects (N = 44) and untreated MS patients (N = 30) specific SARS-COV-2 memory B cells were detected in 40.9% and 40.0% of subjects, respectively. Specific SARS-COV-2 memory T cells were found in 56.8% and 56.7% of healthy subjects and untreated MS patients, respectively. In ocrelizumab-treated MS patients (N = 52) specific SARS-COV-2 memory B cells was detected in 1.9% of patients, while 57.7% developed specific SARS-COV-2 memory T cells responses. These findings were obtained within median (IQR) 4.3 (3.3–5.1) months from the last treatment dosing with a maximal dosing interval of 7 months. In fingolimod-treated MS patients (N = 28) specific SARS-COV-2 memory B cells were seen in 3.5% of patients, while none developed SARS-COV-2 memory T- cell responses.
Fig. 3.
SARS-COV-2 specific humoral and cellular immune responses following COVID-19 vaccination in healthy subjects and MS patients.
4. Discussion
In the current study, we demonstrated that humoral immunity has been achieved post-COVID-19 vaccination in the majority of MS patients as well as in healthy subjects. As the COVID-19 pandemic is going on, our findings are encouraging showing that MS patients either untreated or treated with various DMTs successfully developed antibodies up to 6 months following the vaccination. It is of note that the timing of the vaccinations could result in different immunity states in relation to different variants, as mRNA vaccines were reported to provide lower protection against SARS-COV-2 infection after the Delta variant (Nanduri et al., 2021). Our findings are innovative indicating that humoral immune response was achieved in a large cohort of vaccinated MS patients treated with various DMTs including alemtuzumab, cladribine, dimethyl fumarate, natalizumab, and teriflunomide up to 6 months following m-RNA PfizerBNT162b2 vaccination. The present findings further validated our previous study showing that the majority of patients treated with fingolimod and ocrelizumab failed to mount this protective IgG response (Achiron et al., 2021). In the current study we have shown for the first time that MS patients treated with ocrelizumab that successfully developed humoral immunity as compared to ocrelizumab-treated MS patients that failed, were younger at the time of vaccination by a mean of 7 years, were vaccinated after a longer period of time from the last ocrelizumab dosing, and also had higher ALC. Taken together these findings suggest that by one month extension over the 6 months from the last dosing, successful humoral immunity was achieved. This extension resulted in higher ALC and contributed to CD20 lymphocyte repopulation and thus the ability to generate IgG from plasma cells changed the immune outcome.
In addition to the practical challenge of vaccination, it is important for treating neurologists to have data related to vaccine-related immune responses that could impact the prioritization of patients to be vaccinated and to advocate an appropriate time frame from the last dosing of specific DMTs for the best interest of the patients. In accordance, it is important to note that not all vaccinated healthy subjects, similarly to untreated MS patients, developed cellular immunity within 2 to 4 months following the second vaccine dose. Only ~40% of vaccinated healthy subjects and untreated MS patients, all with positive anti-SARS-COV-2 IgG, developed SARS-COV-2 RBD specific memory B-cells, and only ~55% demonstrated memory T-cells secreting IFN-γ and/or IL-2 in response to SARS-COV-2 peptides. These findings imply that an additional booster dose might be needed to induce immune memory in higher percentages of the population. Similarly to our data, SARS-COV-2 specific memory B cells were found in ~50% of 33 healthy subjects, one week post the second vaccine dose (Goel et al., 2021), and CD4 T cell response against RBD was observed in 5/9 (55.6%) of healthy vaccinees, 7 days after PfizerBNT162b1 first vaccine dose (Sahin et al., 2020). Ocrelizumab-treated MS patients failed to mount cellular B-cell response but developed specific T-cell memory response similarly to vaccinated healthy subjects and untreated MS patients. These findings extend a recent study (Apostolidis et al., 2021) that reported attenuated antibody responses to SARS-COV-2 mRNA vaccine in 20 relapsing-remitting MS patients, 19 treated with ocrelizumab and 1 treated with rithuximab, within 25–30 days following the second vaccine dose. Similarly to our findings only a minority of patients generated detectable memory B cells while T cell response was preserved.
Although the exact immunological protection provided by this T-cell cellular response is unknown, it suggests the possibility that if infected these subjects will have only a mild disease. MS patients treated with fingolimod, failed to mount either B-cell or T-cell cellular immune responses following the vaccination. It is conceivable to suggest that in the absence of adequate number of peripheral blood lymphocytes, vaccination failed to elicit the required immune process. The disconnect between the overall lack of immune responses in fingolimod-treated MS patients, and the apparent lack of increased risk of morbidity or mortality to SARS-COV-2 infection is intriguing. It may suggest that despite severe lymphopenia and impaired adaptive immune responses, an innate response consisting of macrophages, granulocytes and lung dendritic cells provides immune protection.
There are several limitations to our study. The first is the cross-sectional design, although useful for establishing the prevalence of SARS-COV-2 IgG after vaccination, it does not enable to determine how the antibody titer changes over time in the same individuals. Moreover, lack of SARS-COV-2 binding IgG does not confer lack of immunity to COVID-19, though a recent study demonstrated that among fully vaccinated health care workers, the occurrence of breakthrough infections with SARS-COV-2 correlated with neutralizing antibody titers during the peri-infection period (Bergwerk et al., 2021). For this purpose, a longitudinal study with a longer follow-up is needed. Second, we did not use a neutralizing antibody assay but rather an assay with high correlation to a neutralizing antibody assay. Finally, we evaluated SARS-COV-2 specific memory B-cell and T-cell cellular immune responses only in a sub-group of subjects and only at one time point post-vaccination.
Our findings suggest that COVID-19 vaccination leads to IgG humoral immune response in the majority of MS patients treated with DMTs. For patients treated with ocrelizumab or fingolimod we found that these treatments might prevent the development of a favorable immune response. SARS-COV-2 IgG antibody testing following the vaccination can provide immunization knowledge about a possible risk for COVID-19 infection. The absence of SARS-COV-2 immune response signifies these patients are not protected from the viewpoint of public health and should be considered for a booster vaccine dose.
The following are the supplementary data related to this article.
Supplementary Fig. 1.
SARS-COV-2 specific immune responses following COVID-19 vaccination. A. Memory B-cells specific for SARS-COV-2 RBD, left panel positive result, right panel negative result; B. T-cells secreting IFN-γ, left panel positive control, middle panel positive result, right panel negative result; C. T-cells secreting IL-2, left panel positive control, middle panel positive result, right panel negative result.
Funding
This study was supported by research grants from Sheba MS Center, and the Laura Schwarz-Kipp Research Fund for Autoimmune Diseases, Sackler Faculty of Medicine, Tel-Aviv University, Israel.
Sapir Dreyer-Alster is supported by a research grant from the Clair and Amedee Maratier Institute for the study of Blindness and Visual Disorders, Sackler Faculty of Medicine, Tel-Aviv University, Israel.
Availability of data and material
All data generated or analyzed during this study are included in the tables and figures.
Author contributions
Conceptualization: Anat Achiron, Michael Gurevich, Rina Falb, Mathilda Mandel; Data curation: Sapir Dreyer-Alster, Anat Achiron, Gil Harari, Michael Gurevich, Mathilda Mandel; Investigation: David Magalashvili, Mark Dolev, Shay Menascu, Shlomo Flechter, Diana Guber, Uri Givon, Michael Polliack, Anat Achiron; Methodology: Michael Gurevich, Rina Falb, Polina Sonis; Formal analysis: Gil Harari, Sapir Dreyer-Alster; Writing - original draft: Anat Achiron, Mathilda Mandel; Writing - review & editing: All authors.
Declaration of Competing Interest
AA, MD, SM, DM, SF received research support from Bristol Myers Squibb, Roche, Biogen Idec, Sanofi-Genzyme, and Merck, none related to this study. MM, SDA, GH, UG, DG, PS, RZF, MG report no conflict of interest.
References
- Achiron A., Mandel M., Dreyer-Alster S., Harari G., Magalashvili D., Sonis P., Dolev M., Menascu S., Flechter S., Falb R., Gurevich M. Humoral immune response to COVID-19 mRNA vaccine in patients with multiple sclerosis treated with high-efficacy disease-modifying therapies. Ther. Adv. Neurol. Disord. 2021;14 doi: 10.1177/17562864211012835. (17562864211012835) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S.F., Quadeer A.A., McKay M.R. Preliminary identification of potential vaccine targets for the COVID-19 coronavirus (SARS-CoV-2) based on SARS-CoV immunological studies. Viruses. 2020;12:254. doi: 10.3390/v12030254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apostolidis S.A., Kakara M., Painter M.M., Goel R.R., Mathew D., Lenzi K., Rezk A., Patterson K.R., Espinoza D.A., Kadri J.C., Markowitz D.M., Markowitz E., Mexhitaj I., Jacobs D., Babb A., Betts M.R., Prak E., Weiskopf D., Grifoni A., Lundgreen K.A., Gouma S., Sette A., Bates P., Hensley S.E., Greenplate A.R., Wherry E.J., Li R., Bar-Or A. Cellular and humoral immune responses following SARS-CoV-2 mRNA vaccination in patients with multiple sclerosis on anti-CD20 therapy. Nat. Med. 2021 doi: 10.1038/s41591-021-01507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros-Martins J., Hammerschmidt S.I., Cossmann A., Odak I., Stankov M.V., Morillas Ramos G., Dopfer-Jablonka A., Heidemann A., Ritter C., Friedrichsen M., Schultze-Florey C., Ravens I., Willenzon S., Bubke A., Ristenpart J., Janssen A., Ssebyatika G., Bernhardt G., Münch J., Hoffmann M., Pöhlmann S., Krey T., Bošnjak B., Förster R., Behrens G.M.N. Immune responses against SARS-CoV-2 variants after heterologous and homologous ChAdOx1 nCoV-19/BNT162b2 vaccination. Nat. Med. 2021 Jul 14 doi: 10.1038/s41591-021-01449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergwerk M., Gonen T., Lustig Y., Amit S., Lipsitch M., Cohen C., Mandelboim M., Levin E.G., Rubin C., Indenbaum V., Tal I., Zavitan M., Zuckerman N., Bar-Chaim A., Kreiss Y., Regev-Yochay G. Covid-19 breakthrough infections in vaccinated health care workers. N Engl J Med. 2021 doi: 10.1056/NEJMoa2109072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., Hernán M.A., Lipsitch M., Reis B., Balicer R.D. BNT162b2 mRNA Covid-19 vaccine in a Nationwide mass vaccination setting. N. Engl. J. Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E.J., Buckle G.J., Singer B., Singh V., Boster A. Lymphopenia and DMTs for relapsing forms of MS: considerations for the treating neurologist. Neurol Clin Pract. 2019;9:53–63. doi: 10.1212/CPJ.0000000000000567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goel R.R., Apostolidis S.A., Painter M.M., Mathew D., Pattekar A., Kuthuru O., Gouma S., Hicks P., Meng W., Rosenfeld A.M., Dysinger S., Lundgreen K.A., Kuri-Cervantes L., Adamski S., Hicks A., Korte S., Oldridge D.A., Baxter A.E., Giles J.R., Weirick M.E., McAllister C.M., Dougherty J., Long S., D'Andrea K., Hamilton J.T., Betts M.R., Luning Prak E.T., Bates P., Hensley S.E., Greenplate A.R., Wherry E.J. Distinct antibody and memory B cell responses in SARS-CoV-2 naïve and recovered individuals following mRNA vaccination. Sci Immunol. 2021;6:eabi6950. doi: 10.1126/sciimmunol.abi6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendrone-Junior A., Dinardo C.L., Ferreira S.C., Ferreira S.C., Nishya A., Salles N.A., de Almeida Neto C., Hamasaki D.T., Facincani T., de Oliveira Alves L.B., Machado R.R.G., Araujo D.B., Durigon E.L., Rocha V., Sabino E.C. Correlation between SARS-COV-2 antibody screening by immunoassay and neutralizing antibody testing. Transfusion. 2021;61:1181–1190. doi: 10.1111/trf.16268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanduri S., Pilishvili T., Derado G., Soe M.M., Dollard P., Wu H., Li Q., Bagchi S., Dubendris H., Link-Gelles R., Jernigan J.A., Budnitz D., Bell J., Benin A., Shang N., Edwards J.R., Verani J.R., Schrag S.J. Effectiveness of Pfizer-BioNTech and Moderna Vaccines in Preventing SARS-CoV-2 Infection Among Nursing Home Residents Before and During Widespread Circulation of the SARS-CoV-2 B.1.617.2 (Delta) Variant - National Healthcare Safety Network, March 1–August 1, 2021. MMWR. 2021;70(34):1163–1166. doi: 10.15585/mmwr.mm7034e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., Bailey R., Swanson K.A., Roychoudhury S., Koury K., Li P., Kalina W.V., Cooper D., Frenck R.W., Jr., Hammitt L.L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D.B., Mather S., Dormitzer P.R., Şahin U., Jansen K.U., Gruber W.C., C4591001 Clinical Trial Group C4591001 Clinical Trial Group. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polman C.H., Reingold S.C., Banwell B., Clanet M., Cohen J.A., Filippi M., Fujihara K., Havrdova E., Hutchinson M., Kappos L., Lublin F.D., Montalban X., O’Connor P., Sandberg-Wollheim M., Thompson A.J., Waubant E., Weinshenker B., Wolinsky J.S. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann. Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin U., Muik A., Derhovanessian E., Vogler I., Kranz L.M., Vormehr M., Baum A., Pascal K., Quandt J., Maurus D., Brachtendorf S., Lörks V., Sikorski J., Hilker R., Becker D., Eller A.K., Grützner J., Boesler C., Rosenbaum C., Kühnle M.C., Luxemburger U., Kemmer-Brück A., Langer D., Bexon M., Bolte S., Karikó K., Palanche T., Fischer B., Schultz A., Shi P.Y., Fontes-Garfias C., Perez J.L., Swanson K.A., Loschko J., Scully I.L., Cutler M., Kalina W., Kyratsous C.A., Cooper D., Dormitzer P.R., Jansen K.U., Türeci Ö. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- Schweitzer F., Laurent S., Fink G.R., Barnett M.H., Hartung H.P., Warnke C. Effects of disease-modifying therapy on peripheral leukocytes in patients with multiple sclerosis. J. Neurol. 2021;268:2379–2389. doi: 10.1007/s00415-019-09690-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seyed Hosseini E., Riahi Kashani N., Nikzad H., Azadbakht J., Hassani Bafrani H., Haddad Kashani H. The novel coronavirus disease-2019 (COVID-19): mechanism of action, detection and recent therapeutic strategies. Virology. 2020;551:1–9. doi: 10.1016/j.virol.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in the tables and figures.