Abstract
Pre-operative chemoradiotherapy (CRT) followed by surgical resection is still the standard treatment for locally advanced low rectal cancer. Nowadays new strategies are emerging to treat patients with a complete response to pre-operative treatment, rendering the optimal management still controversial and under debate. The primary aim of this study was to obtain a snapshot of tumor regression grade (TRG) distribution after standard CRT. Second, we aimed to identify a correlation between clinical tumor stage (cT) and TRG, and to define the accuracy of magnetic resonance imaging (MRI) in the restaging setting. Between January 2017 and June 2019, a cross sectional multicentric study was performed in 22 referral centers of colon-rectal surgery including all patients with cT3-4Nx/cTxN1-2 rectal cancer who underwent pre-operative CRT. Shapiro–Wilk test was used for continuous data. Categorical variables were compared with Chi-squared test or Fisher’s exact test, where appropriate. Accuracy of restaging MRI in the identification of pathologic complete response (pCR) was determined evaluating the correspondence with the histopathological examination of surgical specimens.
In the present study, 689 patients were enrolled. Complete tumor regression rate was 16.9%. The “watch and wait” strategy was applied in 4.3% of TRG4 patients. A clinical correlation between more advanced tumors and moderate to absent tumor regression was found (p = 0.03). Post-neoadjuvant MRI had low sensibility (55%) and high specificity (83%) with accuracy of 82.8% in identifying TRG4 and pCR.
Our data provided a contemporary description of the effects of pre-operative CRT on a large pool of locally advanced low rectal cancer patients treated in different colon-rectal surgical centers.
Keywords: Rectal cancer, Pathologic complete response, Neoadjuvant therapy, Tumor regression grade
Introduction
Colorectal cancer represents the third most common cancer and the second cause of cancer-related mortality worldwide [1]. In about 35% of cases, patients present with rectal cancer [2] and, in case of locally advanced low rectal lesions, pre-operative chemoradiotherapy (CRT) followed by surgical resection with total mesorectal excision is currently the standard treatment, reducing local recurrence from 25% to 5–10% and significantly improving overall survival (OS) [3–9].
Response to neoadjuvant CRT is evaluated on the surgical specimen by assessing the stage and tumor regression grade (TRG). Among the many TRG systems which aim to categorize the amount of regressive changes after CRT, the Dworak classification is one of the most commonly used for rectal cancer [10–12]. Response rate to pre-operative CRT can be quite variable in the literature [10–12], with pathological complete response (pCR) reported in 8–21% of patients and partial responses reported in about 40% of patients [4, 13–15]. Correct assessment of TRG, as well as thorough evaluation of the tumor and nodal status (and, in case, of the metastatic status) on the surgical specimen (i.e. ypTNM) [16], are of paramount importance to predict prognosis as long-term oncological outcomes are significantly better in patients with complete regression compared to those with partial or absent regression [3, 4, 17–20].
According to these considerations, in 2004 Habr-Gama proposed the “watch and wait” strategy for patients with clinical complete response (cCR), demonstrating that it was possible to avoid surgical resection and its related morbidity and long-term sequelae without affecting OS and disease-free survival (DFS) [21]. Nevertheless, more recent studies have failed to reach the same results [22]. The main challenge remains the correct identification of patients eligible for this approach. In fact, cCR does not always correspond to pCR, thus determining an increased risk of tumor regrowth in poorly selected patients [18, 20, 23], although salvage surgery can still be performed without reduction in survival [17, 18]. Consequently, current ESMO guidelines endorse a “watch and wait” strategy only for cCR cases that are poor surgical candidates, recommending at any rate that patients should be warned about the slightly increased risk of pelvic relapse and distant metastases [2]. Another topic of debate is the fact that patients undergoing a “watch and wait” strategy demand a strict follow-up protocol, but no agreement has been reached on the timing and type of evaluations (i.e. clinical, endoscopic, and radiological) required [24].
As a matter of fact, the therapeutic approach to rectal cancer is constantly changing and defining the best treatment for each patient is currently one of the biggest challenges in clinical oncology. The primary aim of this study was to obtain a snapshot of TRG distribution after standard CRT in some European referral centers of colorectal surgery. Second, we aimed to identify a correlation between the clinical stage of the tumor (cT) and TRG, and to define the accuracy of magnetic resonance imaging (MRI) in the restaging setting. Finally, we aimed to present the current attitudes of colorectal surgeons towards cCR patients (i.e. surgery vs. conservative treatment).
Methods
Study design
A cross sectional multicentric study was performed in 14 Italian and 8 European referral centers of colorectal surgery between January 2017 and June 2019. All the clinical and pathological data were drawn together in a single anonymous database. The manuscript adheres to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement [25].
Inclusion criteria were: locally advanced rectal carcinoma (cT3–cT4 and/or cN1–cN2 at diagnosis); neoadjuvant long-course CRT; surgical resection with curative intent or “watch and wait” strategy with either full-thickness or endoscopic biopsy. Patients were excluded in case of: stage IV at diagnosis; neoadjuvant short-course radiotherapy (RT); neoadjuvant chemotherapy (CT) without RT; lack of significant data (i.e. pre-operative c-stage and/or yc-stage, histology, TRG, type of pre-operative regimen).
After being diagnosed with rectal cancer, all patients underwent a staging MRI. Primary tumor (T) and nodal involvement (N) were registered according to the American Joint Committee on Cancer (AJCC), 7th edition [16]. Infiltration of the mesorectal fascia, and distance between primary tumor and mesorectal fascia were recorded. The mesorectal fascia was considered infiltrated in case of tumor distance ≤ 5 mm and non-infiltrated in case of tumor distance ≥ 6 mm. The same parameters were recorded in the restaging setting, where available.
Different long-course CRT regimens were reported. After pre-operative treatment, patients underwent surgical resection with curative intent. The “watch and wait” strategy was considered an option for patients with cCR, according to either surgeon’s or patient’s preference. Retrospective analysis of different surgeon’s attitudes was performed.
All surgical specimens were classified according to the AJCC staging system, 7th edition [16]. Data about regressive changes after CRT, infiltration of the mesorectal fascia, and distance between primary tumor and mesorectal fascia were recorded for all patients. Tumor regression grading was evaluated according to the Dworak classification [10].
Statistical analysis
Continuous data were summarized by median and range (minimum–maximum) and were tested for normality with the Shapiro–Wilk test. Post-hoc tests and pairwise comparisons through Mann–Whitney test were conducted and p value adjusted using Holm method. Categorical variables were reported as absolute and relative frequencies (percentages) and compared with Chi-Squared test or Fisher’s exact test where appropriate.
The capability of restaging MRI at correctly identifying pCR patients was evaluated with sensibility, specificity, and accuracy. True positive results were considered in case of ycT0 patients at restaging MRI corresponding to TRG 4 specimens on histopathological examination.
Statistical analysis was conducted with R version 3.5.0 and STATA 14.2 (StataCorp, College Station, TX, USA). All p values (p) were two-tailed and differences were considered statistically significant when p < 0.05.
Results
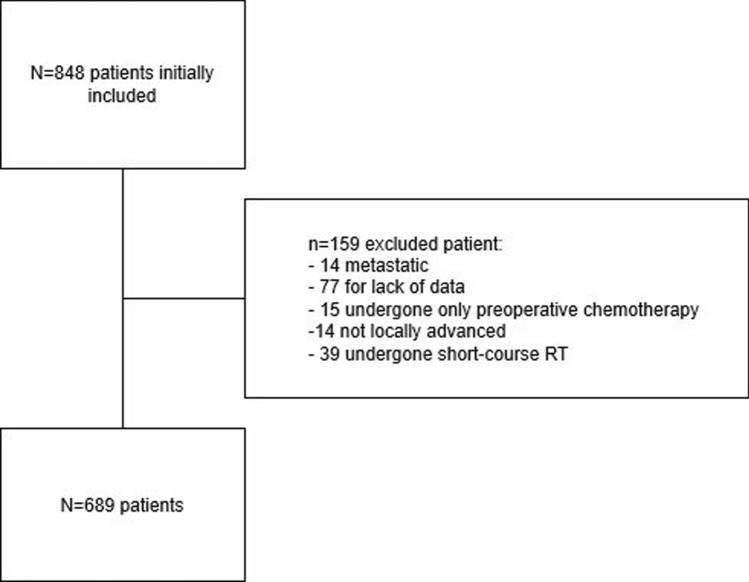
A total of 848 patients from 22 European referral centers of colorectal surgery were initially considered for the present study. Of these, 159 were excluded for the following reasons: 14 were metastatic, 14 were not locally advanced, 15 underwent only neoadjuvant chemotherapy (CT), 39 underwent short-course radiotherapy (RT), and 77 were missing significant data (i.e. pre-operative c-Stage and/or yc-Stage, histology, TRG, type of pre-operative regimen). Overall, 689 patients were included for final analysis (Fig. 1). Population distribution and type of long-course CRT regimens are summarized in Tables 1 and 2.
Fig. 1.
Patients selection
Table 1.
Population distribution
| Centre | Total number (%) |
|---|---|
| Italy (14 centres) | 442 (64.2%) |
| Switzerland (3 centres) | 89 (12.9%) |
| Belgium (1 center) | 72 (10.4%) |
| Netherlands (2 centres) | 39 (5.7%) |
| Spain (1 center) | 25 (3.6%) |
| Slovenia (1 center) | 22 (3.2%) |
| Total (22 center) | 689 |
Table 2.
Pre-operative CRT regimens
| Chemotherapic agent | No. (%) |
|---|---|
| Capecitabine | 474 (68.8%) |
| Folfox | 32 (4.6%) |
| 5-FU | 30 (4.3%) |
| Folfiri + panitumumab | 2 (0.3%) |
| Folfox + bevacizumab | 2 (0.3%) |
| Folfoxiri | 2 (0.3%) |
| Oxaliplatino | 2 (0.3%) |
| 5-FU + levofolinile | 1 (0.15%) |
| Docetaxel | 1 (0.15%) |
| Folfiri | 1 (0.15%) |
| Folfirinox | 1 (0.15%) |
| Atezolizumab | 1 (0.15%) |
| Capox + bevacizumab | 1 (0.15%) |
| Missing data | 139 (20.2%) |
TRG distribution
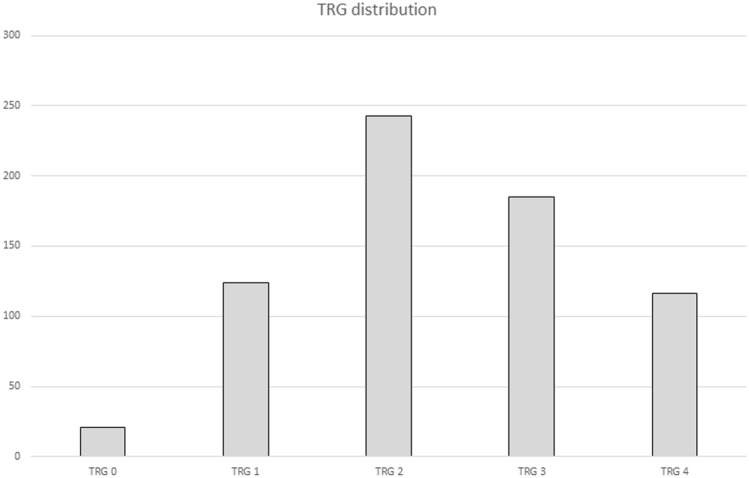
Overall, TRG 0 was recorded in 21 (3%) patients, TRG 1 in 124 (18%) patients, TRG 2 in 243 (35.3%) patients, TRG 3 in 185 (26.8%) patients, and TRG 4 in 116 (16.9%) patients. Results are displayed in Fig. 2.
Fig. 2.
TRG distribution
Surgeon’s attitude in case of TRG 4 patients
A retrospective analysis of the therapeutic strategy in case of TRG 4 patients was performed. Overall, 111 (95.7%) patients underwent surgical resection and 5 (4.3%) patients underwent full-thickness biopsy as part of a “watch and wait” strategy. The reason behind the choice was reported in 42 cases and it was predominantly made by the surgeon (i.e. surgeon’s choice in 40 cases vs. patient’s choice in two cases).
Among TRG 4 patients, despite complete tumor response (ypT0), a non-complete nodal response was observed in 11 (9.9%) cases on surgical specimen.
Association between TRG and clinical T-stage (cT)
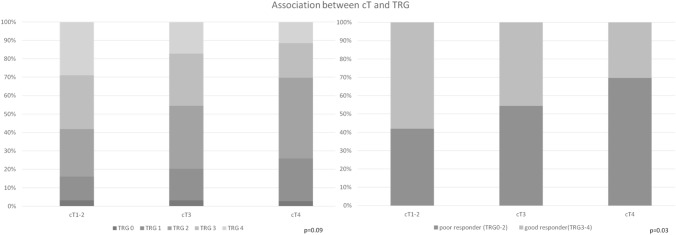
Considering the clinical stage of the tumor at diagnosis (i.e. cT), more advanced lesions were less likely to achieve complete regression after neoadjuvant CRT. Indeed, TRG 4 rate was 29.0% for cT1–cT2 tumors, 17.2% for cT3 tumors, and 11.6% for cT4 tumors, respectively (p = 0.09). Near-complete (i.e. TRG 3) and complete (i.e. TRG 4) regression significantly correlated with tumor size, as it was reported in 58.1% of cT1–cT2 tumors, 45.6% of cT3 tumors, and 30.4% of cT4 tumors (p = 0.003).
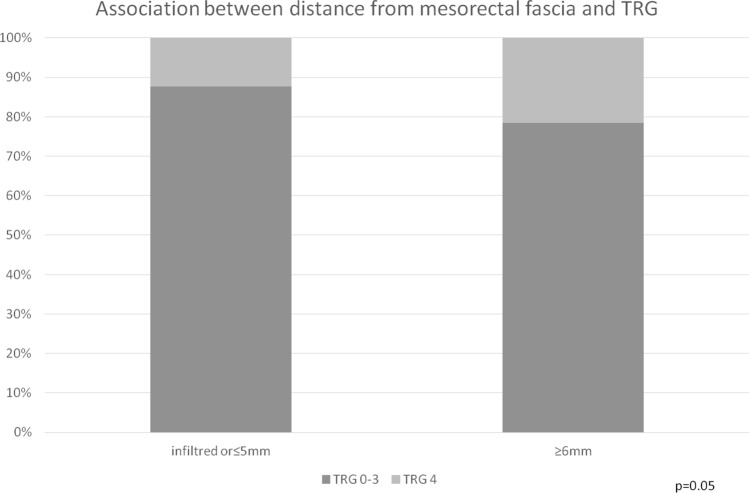
Data on the distance between the tumor and the mesorectal fascia were available for 538 (78.1%) patients. Tumors close to or infiltrating the mesorectal fascia (≤ 5 mm) were less likely to achieve TRG 4 response (p = 0.005).
Results are summarized in Figs. 3 and 4 and Tables 3 and 4.
Fig. 3.
Association between cT and TRG
Fig. 4.
Association between distance from mesorectal fascia and TRG
Table 3.
Association between cT and TRG
| TRG 0 | TRG 1 | TRG 2 | TRG 3 | TRG 4 | Tot | ||
|---|---|---|---|---|---|---|---|
| cT1-2 | 1 (3.2%) | 4 (12.9%) | 8 (25.8%) | 9 (29.05%) | 9 (29.05%) | 31 | p = 0.09 |
| cT3 | 17 (3,1%) | 94 (17.2%) | 186 (34.1%) | 155 (28.4%) | 94 (17.2%) | 546 | |
| cT4 | 3 (2.7%) | 26 (23.2%) | 49 (43.8%) | 21 (18.7%) | 13 (11.6%) | 112 | |
| Tot | 21 | 124 | 243 | 185 | 116 | 689 |
| Poor responder (TRG0–2) | Good responder (TRG3–4) | Tot | ||
|---|---|---|---|---|
| cT1–2 | 13 (41.9%) | 18 (58.1%) | 31 | p = 0.003 |
| cT3 | 297 (54.4%%) | 249 (45.6%) | 546 | |
| cT4 | 78 (69.6%) | 34 (30.4%) | 112 | |
| Tot | 388 | 301 | 689 |
Table 4.
Association between distance from mesorectal fascia and TRG
| TRG 0–3 | TRG 4 | Total | ||
|---|---|---|---|---|
| Infiltrated or ≤ 5 mm | 317 (87.6%) | 45 (12.4%) | 362 | p = 0.005 |
| ≥ 6 mm | 138 (78.4%) | 38 (21.6%) | 176 | |
| Total | 455 | 83 | 538 |
Accuracy of restaging MRI
Restaging MRI was performed in 607 (88.1%) patients. In 82 (11.9%) patients, it was omitted according to center protocol. All patients, in whom restaging MRI was omitted according to center protocol, underwent surgical resection, including TRG4 patients (i.e. 10 cases).
Overall, MRI showed a 55% sensibility, 83% specificity, and 82.8% accuracy for the correct identification of TRG 4 patients (p < 0.001). When analyzing the nodal status, MRI showed similar results, with a 52% sensibility, 85% specificity, and 84.5% accuracy for correct identification of lymph node involvement (p < 0.001).
Generally, MRI correctly identify ypT and ypN stages in 49.8 and 66.2% of cases, respectively. Overestimation occurred in 41.3% of patients for T stage and 25.4% of patients for N stage, respectively. Underestimation occurred in 8.9% of patients for T stage and in 8.4% of patients for N stage, respectively.
Discussion
The present paper evaluated the factors associated with tumor regression, analyzed the accuracy of restaging MRI, and described real-life therapeutic strategies in case of complete response to CRT among a sample of Italian and European surgeons.
Overall, our results are consistent with literature data. Specifically, TRG 4 was recorded in 16.9% of patients. Of these, the majority underwent surgical resection; whereas, a “watch and wait” strategy was performed only in 4.3% of cases. This could be due to lack of standardized protocols, but a major concern is certainly represented by the fact that complete tumor regression does not always correspond to complete response in the lymph nodes. In the present series, 9.9% of TRG 4 patients presented residual nodal disease (i.e. ypN+) at histological examination of the surgical specimen. This is consistent with literature data, reporting that up to 10% of ypT0 patients still present metastatic lymph nodes at the time of surgery, thus determining an increased risk of local recurrence and lower 5-year DFS and OS rates [20, 23, 26, 28].
Tumor regression grade was also found to significantly correlate with clinical T stage and distance of the tumor from the mesorectal fascia. According to our results, early clinical T stages and tumors with a distance ≥ 6 mm from the mesorectal fascia were more likely to achieve complete tumor regression (i.e. TRG 4). This is in accordance with the literature. Several studies have analyzed pCR predictive factors and clinical T stage has been widely recognized as a strong predictive factor of tumor response. Tan et al. [27] have recently reported that only 12% of cT4 patients achieve pCR compared to 21% of cT3, 23% of cT3, and 27% of cT1 patients, respectively (p < 0.001). Therefore, neoadjuvant treatment may play a role also in early rectal cancers, and specifically cT2N0 lesions. Recent studies comparing surgery alone versus CRT and local excision for cT2N0 patients showed similar oncologic results [28–30]; however, further studies with longer follow-up periods and larger series are required to draw definitive conclusions.
A tailored therapeutic approach strongly relies on accurate pre-operative imaging and MRI should be highly effective in predicting the pathologic state of rectal cancer patients undergoing neoadjuvant treatment. In this study, restaging MRI showed a low sensitivity (55%) but high specificity (83%) in the identification of ypT0 tumors, with an accuracy of 82.8%. Similar results were obtained for the nodal status and overall, restaging MRI correctly identified 49.8% of T stages and 66.2% of N stages, respectively. As reported by other studies, stage overestimation was more common than stage underestimation. In 2014, Lee et al. [31] analyzed 150 patients with locally advanced rectal cancer undergoing post-CRT MRI and observed that pathologic T stage-matched restaging MRI findings in 64.7% of patients, with 24.0% of cases being over-staged. Similarly, pathologic N stage-matched restaging MRI findings in 56.6% of patients, with 36.0% of cases being over-staged. The authors concluded that restaging MRI has low accuracy for the prediction of pathologic T and N classifications in patients-receiving pre-operative CRT. More recently, Cho et al. [34] reported that magnetic resonance TRG (mrTRG) has a sensibility of 37.9%, a specificity of 76.5%, and an accuracy of 66.3%, in the identification of ypT0 tumors. Furthermore, Sclafani et al. [32] reported a low agreement between mrTRG and pTRG, although sensibility and specificity were high (74.4 and 62.8%, respectively).
MRI accuracy in the assessment of TRG can be increased by diffusion-weighted imaging (DWI). However, over-staging and under-staging remains a problem, as the main limitation of MRI lies in the difficulty at discriminating between residual tumor, fibrosis, edema, and inflammation. Moreover, small residual cells may not be seen on radiological evaluation [33, 34]. Some authors believe that the addition of endoscopic ultrasound (EUS) imaging to MRI might increase the accuracy of post-treatment staging; however, a satisfactory agreement has yet to be reached [33, 35]. In this setting, radiomic seems to be a promising technique. Preliminary results, recently published by the MSKCC group, showed a better performance of radiomic in the identification of a complete response compared to T2-weighted MRI and DWI sequences [35], thus representing a significant potential guidance in the individualization of the most appropriate therapeutic approach for each patient [35–37].
The present study had several limitations. First, it was retrospective and not fully representative of European surgical attitudes in rectal cancers, although several referral centers were included in the analysis. Second, the study evaluated only some predictive factors of complete tumor response, such as clinical stage, neoadjuvant therapy, and distance of the tumor from the mesorectal fascia. However, other factors, including molecular pathways and tumoral markers, may play a role as well. Third, the population was not homogeneous and included patients who underwent different schemes of chemotherapy, thus potentially influencing outcomes.
Nevertheless, this is one of the first studies to offer some sort of contemporary description of the effects of pre-operative CRT on a large pool of locally advanced low rectal cancer patients treated in different Italian and European colorectal surgical centers. Moreover, despite being retrospective, the relatively large sample size collected in a short and recent period allowed to obtain clinically significant results and all data were drawn out from primary referral colorectal centers. Therefore, we believe that the results of this study could be useful for the definition of an ever more tailored approach and the analysis of current critical issues could promote further prospective multicentric evaluations on multimodal therapy for rectal cancer.
Acknowledgements
TRG Snapshot Study Group: Gabriele Bellio1,2, Cristiana Iacuzzo1,2, Annalisa Zucca1,2, Pio Corleone1,2, Fabiola Giudici1, Silvia Palmisano1,2, Michele Carvello3, Christophe Remue4, Radu Bachmann4, Nicolas Lombard4, Christine Pirlet4, Andries Ryckx4, Simonetta Massaron6, Luigi Pugliese11, Roberto Coppola12, Cecilia Ferrari13, Simone Castiglioni15, Elisa Ponte20, Serena Concina23, Arthur Piveteau25, Yongbo An17, Emanuela Cagnazzo22, Marina Troian23
Funding
Open access funding provided by Università degli Studi di Trieste within the CRUI-CARE Agreement. The authors declare that they have no financial ties to disclose.
Availability of data and materials
The data will not be deposited.
Code availability
Not aplicable.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in this study were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in this study.
Footnotes
The members of the TRG Snapshot Study Group are listed in Acknowledgements section.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Paola Germani, Email: paolagermani1987@gmail.com.
the TRG Snapshot Study Group:
Gabriele Bellio, Cristiana Iacuzzo, Annalisa Zucca, Pio Corleone, Fabiola Giudici, Silvia Palmisano, Michele Carvello, Christophe Remue, Radu Bachmann, Nicolas Lombard, Christine Pirlet, Andries Ryckx, Simonetta Massaron, Luigi Pugliese, Roberto Coppola, Cecilia Ferrari, Simone Castiglioni, Elisa Ponte, Serena Concina, Arthur Piveteau, Yongbo An, Emanuela Cagnazzo, and Marina Troian
References
- 1.(2018) https://www.uicc.org/news/new-global-cancer-data-globocan-2018
- 2.Glynne-Jones R, Wyrwicz L, Tiret E, et al. Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(Supplement 4):iv22–iv40. doi: 10.1093/annonc/mdx224. [DOI] [PubMed] [Google Scholar]
- 3.Kim SH, Chang HJ, Kim DY, et al. What is the ideal tumor regression grading system in rectal cancer patients after preoperative chemoradiotherapy? Cancer Res Treat. 2016;48(3):998–1009. doi: 10.4143/crt.2015.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Song C, Chung JH, Kang SB, et al. Impact of tumor regression grade as a major prognostic factor in locally advanced rectal cancer after neoadjuvant chemoradiotherapy: a proposal for a modified staging system. Cancers (Basel). 2018;10(9):319. doi: 10.3390/cancers10090319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gollins S, Sebag-Montefiore D. Neoadjuvant treatment strategies for locally advanced rectal cancer. Clin Oncol. 2016;28(2):146–151. doi: 10.1016/j.clon.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Peeters KCMJ, Marijnen CAM, Nagtegaal ID, et al. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246(5):693–701. doi: 10.1097/01.sla.0000257358.56863.ce. [DOI] [PubMed] [Google Scholar]
- 7.Gérard JP, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3–4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24(28):4620–4625. doi: 10.1200/JCO.2006.06.7629. [DOI] [PubMed] [Google Scholar]
- 8.Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355(11):1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 9.de Manzini N et al (2013) Rectal cancer. In: Updates in surgery. 10.1007/978-88-470-2670-4
- 10.Dworak O, Keilholz L, Hoffmann A. Pathological features of rectal cancer after preoperative radiochemotherapy. Int J Colorectal Dis. 1997;12(1):19–23. doi: 10.1007/s003840050072. [DOI] [PubMed] [Google Scholar]
- 11.Fokas E, Ströbel P, Fietkau R, et al. Tumor regression grading after preoperative chemoradiotherapy as a prognostic factor and individual-level surrogate for disease-free survival in rectal cancer. J Natl Cancer Inst. 2017;109(12):1–10. doi: 10.1093/jnci/djx095. [DOI] [PubMed] [Google Scholar]
- 12.Erlandsson J, Lörinc E, Ahlberg M, et al. Tumour regression after radiotherapy for rectal cancer—results from the randomised Stockholm III trial. Radiother Oncol. 2019;135:178–186. doi: 10.1016/j.radonc.2019.03.016. [DOI] [PubMed] [Google Scholar]
- 13.Dayde D, Tanaka I, Jain R, Tai MC, Taguchi A. Predictive and prognostic molecular biomarkers for response to neoadjuvant chemoradiation in rectal cancer. Int J Mol Sci. 2017;18(3):573. doi: 10.3390/ijms18030573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Park IJ, You YN, Agarwal A, et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol. 2012;30(15):1770–1776. doi: 10.1200/JCO.2011.39.7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith KD, Tan D, Das P, et al. Clinical significance of acellular mucin in rectal adenocarcinoma patients with a pathologic complete response to preoperative chemoradiation. Ann Surg. 2010;251(2):261–264. doi: 10.1097/SLA.0b013e3181bdfc27. [DOI] [PubMed] [Google Scholar]
- 16.Edge S, Byrd D, Compton C, Fritz A, Greene F, Trotti A (2010) AJCC cancer staging manual, 7th edn, vol 28. Springer
- 17.Mullaney TG, Lightner AL, Johnston M, Keck J, Wattchow D. ‘Watch and wait’ after chemoradiotherapy for rectal cancer. ANZ J Surg. 2018;88(9):836–841. doi: 10.1111/ans.14352. [DOI] [PubMed] [Google Scholar]
- 18.Kong JC, Guerra GR, Warrier SK, Ramsay RG, Heriot AG. Outcome and salvage surgery following “Watch and Wait” for rectal cancer after neoadjuvant therapy: a systematic review. Dis Colon Rectum. 2017;60(3):335–345. doi: 10.1097/DCR.0000000000000754. [DOI] [PubMed] [Google Scholar]
- 19.Li Y-H, Li J-L, Zhu X-G et al (2017) Associations of tumor regression grade with outcomes in patients with locally advanced rectal cancer treated with preoperative two-week course of radiotherapy. http://www.impactjournals.com/oncotarget [DOI] [PMC free article] [PubMed]
- 20.Wee IJY, Cao HM, Ngu JCY. The risk of nodal disease in patients with pathological complete responses after neoadjuvant chemoradiation for rectal cancer: a systematic review, meta-analysis, and meta-regression. Int J Colorectal Dis. 2019 doi: 10.1007/s00384-019-03327-w. [DOI] [PubMed] [Google Scholar]
- 21.Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240(4):711–718. doi: 10.1097/01.sla.0000141194.27992.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith JJ, Strombom P, Chow OS, et al. Assessment of a watch-and-wait strategy for rectal cancer in patients with a complete response after neoadjuvant therapy. JAMA Oncol. 2019;5(4):e185896. doi: 10.1001/jamaoncol.2018.5896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baucom RB, Maguire LH, Kavalukas SL, et al. Nodal disease in rectal cancer patients with complete tumor response after neoadjuvant chemoradiation: danger below calm waters. Dis Colon Rectum. 2017;60(12):1260–1266. doi: 10.1097/DCR.0000000000000947. [DOI] [PubMed] [Google Scholar]
- 24.Huh JW, Maeda K, Liu Z, Wang X, Roslani AC, Lee WY. Current status of “Watch-and-Wait” rectal cancer treatment in Asia-Pacific Countries. Ann Coloproctol J. 2020;36:37–41. doi: 10.3393/ac.2020.01.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 26.Erkan A, Mendez A, Trepanier M, et al. Impact of residual nodal involvement after complete tumor response in patients undergoing neoadjuvant (chemo)radiotherapy for rectal cancer. Surgery. 2019;166:648–654. doi: 10.1016/j.surg.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 27.Tan Y, Fu D, Li D, et al. Predictors and risk factors of pathologic complete response following neoadjuvant chemoradiotherapy for rectal cancer: a population-based analysis. Front Oncol. 2019 doi: 10.3389/fonc.2019.00497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee L, Kelly J, Nassif GJ, et al. Chemoradiation and local excision for T2N0 rectal cancer offers equivalent overall survival compared to standard resection: a national cancer database analysis. J Gastrointest Surg. 2017;21(10):1666–1674. doi: 10.1007/s11605-017-3536-5. [DOI] [PubMed] [Google Scholar]
- 29.Lezoche G, Baldarelli M, Mario, et al. A prospective randomized study with a 5-year minimum follow-up evaluation of transanal endoscopic microsurgery versus laparoscopic total mesorectal excision after neoadjuvant therapy. Surg Endosc Other Interv Tech. 2008;22(2):352–358. doi: 10.1007/s00464-007-9596-y. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Aguilar J, Renfro LA, Chow OS, et al. Organ preservation for clinical T2N0 distal rectal cancer using neoadjuvant chemoradiotherapy and local excision (ACOSOG Z6041): results of an open-label, single-arm, multi-institutional, phase 2 trial. Lancet Oncol. 2015;16(15):1537–1546. doi: 10.1016/S1470-2045(15)00215-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JH, Jang HS, Kim JG, et al. Prediction of pathologic staging with magnetic resonance imaging after preoperative chemoradiotherapy in rectal cancer: pooled analysis of KROG 10-01 and 11-02. Radiother Oncol. 2014;113(1):18–23. doi: 10.1016/j.radonc.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 32.Sclafani F, Brown G, Cunningham D, et al. Comparison between MRI and pathology in the assessment of tumour regression grade in rectal cancer. Br J Cancer. 2017;117(10):1478–1485. doi: 10.1038/bjc.2017.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho MS, Kim H, Han YD, et al. Endoscopy and magnetic resonance imaging-based prediction of ypT stage in patients with rectal cancer who received chemoradiotherapy. Medicine (Baltimore) 2019;98(35):e16614. doi: 10.1097/md.0000000000016614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Den Broek JJ, Van Der Wolf FSW, Lahaye MJ, et al. Accuracy of MRI in restaging locally advanced rectal cancer after preoperative chemoradiation. Dis Colon Rectum. 2017;60(3):274–283. doi: 10.1097/DCR.0000000000000743. [DOI] [PubMed] [Google Scholar]
- 35.Horvat N, Veeraraghavan H, Khan M, et al. Mr imaging of rectal cancer: radiomics analysis to assess treatment response after neoadjuvant therapy. Radiology. 2018;287(3):833–843. doi: 10.1148/radiol.2018172300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meng X, Xia W, Xie P, et al. Preoperative radiomic signature based on multiparametric magnetic resonance imaging for noninvasive evaluation of biological characteristics in rectal cancer. Eur Radiol. 2019;29(6):3200–3209. doi: 10.1007/s00330-018-5763-x. [DOI] [PubMed] [Google Scholar]
- 37.Nie K, Shi L, Chen Q, et al. Rectal cancer: assessment of neoadjuvant chemoradiation outcome based on radiomics of multiparametric MRI. Clin Cancer Res. 2016;22(21):5256–5264. doi: 10.1158/1078-0432.CCR-15-2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data will not be deposited.
Not aplicable.