Abstract
Neuropathological research suggests the tau pathology of Alzheimer’s disease may originate in brainstem nuclei, yet it remains unknown whether tau-mediated degeneration of brainstem nuclei influences cognitive impairment in prodromal Alzheimer’s disease. The present study examined cognitive domains impacted in prodromal Alzheimer’s disease and brainstem substructure volume in cognitively normal older adults (n = 814) and those with mild cognitive impairment (n = 542). Subsamples of cognitively normal (n = 112) and mild cognitive impairment (n = 202) also had cerebrospinal fluid Alzheimer’s disease biomarker characterization. Region-of-interest and voxel-level analyses related whole brainstem, midbrain, pons, and locus coeruleus volumes to cognition with multiple linear regression models corrected for age, sex, education, apolipoprotein-ε4 carrier status, and MRI magnet strength. Within mild cognitive impairment participants, smaller midbrain and locus coeruleus volumes were significantly related to poorer performance on tests of attention and executive function, and the relationship between locus coeruleus volume and executive abilities remained significant in the mild cognitive impairment subsample with biomarker-confirmed Alzheimer’s disease. A brainstem-masked voxel-wise regression further demonstrated an association between locus coeruleus volume and executive abilities. Brainstem volumes were not significantly related to memory processes. Study findings implicate midbrain and locus coeruleus volume in attention and executive deficits in mild cognitive impairment. Together with prior neuropathological studies, our data suggest a link between Alzheimer’s disease-related degeneration of brainstem nuclei and cognitive deficits in prodromal Alzheimer’s disease.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11682-021-00459-y.
Keywords: Alzheimer’s disease, Brainstem, Cognition, Locus coeruleus, Magnetic resonance imaging
Introduction
Recent updated Braak staging of Alzheimer’s disease (AD) implicates the brainstem as the first site of tau-related pathology, with the locus coeruleus (LC) the first nucleus to demonstrate signs of pretangles (i.e., precursors to neurofibrillary tangle pathology) (Braak and Del Tredici 2015). Although the origin of tau seeding activity remains controversial, recent histopathological studies demonstrated the presence of tau cytoskeletal pathology in the LC prior to allocortical cytoskeletal changes (Heinsen and Grinberg 2018; Kaufman et al. 2018; Rüb et al. 2016; Stratmann et al. 2016). The LC is the noradrenergic epicenter of the brain and helps regulate autonomic and neurovascular function and modulate aspects of cognition. Human and animal studies reveal the LC-noradrenergic system modulates attentional shifts, executive function, cognitive control and memory processes (Aston-Jones and Cohen 2005; Mather 2020; Mather et al. 2016; Sara 2009). Recent efforts have highlighted the importance of characterizing LC integrity in aging and neurodegenerative disease (Mather 2020; Mather and Harley 2016), and neuroimaging studies have employed T1-weighted neuromelanin-sensitive scans to approximate LC structural integrity in vivo (Betts et al. 2019; Liu et al. 2017). Neuroimaging studies using these specialized scans have demonstrated associations between LC integrity and episodic memory encoding for stimuli of varying salience (Dahl et al. 2019; Hämmerer et al. 2018; Liu et al. 2020; Olivieri et al. 2019). However, to our knowledge no studies have evaluated associations between cognition and LC volume derived from standard structural T1-weighted scans.
Prior studies examining brainstem volumetrics with standard structural T1-weighted scans in AD populations found volume differences in rostral midbrain and pons regions in AD relative to cognitively normal (CN) individuals (Ji et al. 2020; Lee et al. 2015). Furthermore, we recently demonstrated volumetric differences specific to the midbrain and LC in the prodromal phase of AD, mild cognitive impairment (MCI), compared to CN individuals, and at an earlier preclinical stage in asymptomatic CN individuals who later received a diagnosis of AD dementia (Dutt et al. 2020). The methodology from this study adjusted for total brainstem volume and found overlap with prior LC masks, demonstrating that functionally-relevant LC volume estimates can be quantified from standard T1-weighted MRI scans. Thus, brainstem substructures, and the LC in particular, experience volumetric loss detectible on traditional MRI sequences during the early preclinical phase of AD pathophysiology. However, no studies have evaluated whether AD-related brainstem volume changes are associated with cognitive deficits. The present study investigated how neuropsychological deficits associated with brainstem substructure volume in prodromal AD, building upon our prior efforts to detail brainstem substructure volumes across the AD spectrum (Dutt et al. 2020). Based on the growing literature linking LC integrity with cognition, we hypothesized that smaller brainstem substructure volumes, and smaller LC volumes in particular, would be linked to worse performance on tests of attention, executive function and episodic memory encoding.
Methods
Study design
Data were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) online database. The ADNI is a multisite natural history study that has collected clinical, biomarker, and neuropsychological data since 2003 to measure progression of normal aging, MCI, and AD. Detailed study information is available online (http://adni.loni.usc.edu/). 1356 participants with a baseline clinical diagnosis of CN or MCI and available neuropsychological and structural neuroimaging data were included from the ADNI1, ADNI GO, and ADNI2 cohorts. Participant data represented a subset of a larger study of brainstem volumetrics in preclinical and prodromal AD (Dutt et al. 2020). This study was conducted in accordance with the Helsinki Declaration and approved by all local Institutional Review Boards.
Neuropsychological testing
Participants completed a standardized battery of neuropsychological tests at baseline. Trail Making Test parts A & B assessed attentional/executive abilities (visual attention & set-shifting, respectively). Rey Auditory Verbal Learning Test (RAVLT) delayed free recall and recognition assessed memory consolidation/retrieval abilities. RAVLT trial 1 performance assessed auditory attention and working memory, while RAVLT trials 1–5 total score indexed episodic memory encoding. Category fluency (Animals) tested both language (semantic retrieval) and executive abilities, while the Boston Naming Test (BNT) assessed language (confrontation naming) specific to lexical-semantic retrieval abilities.
Cluster-derived diagnoses
We entered all participants clinically diagnosed as MCI at baseline into a cluster analysis to address previously described high rates of MCI misclassification (Bondi et al. 2014; Clark et al. 2013; Delano-Wood et al. 2009; Edmonds et al. 2015). First, participants diagnosed as CN by ADNI and who remained CN throughout enrollment were designated the normal reference group. Linear regression models predicted cognitive performance on six tests (Trails A, Trails B, RAVLT free recall, RAVLT recognition, Animals fluency, Boston Naming Test) from age and education within this normal reference group. Expected cognitive performance of MCI participants based on their age and education was calculated using the resulting regression coefficients from these models, and the expected scores were used along with the MCI participants’ observed performance to calculate age- and education-adjusted z-scores. Finally, z-scores were entered into a hierarchical cluster analysis using Ward’s method and a forced 4-cluster solution. An emergent cluster-derived CN group was combined with the ADNI-diagnosed CN group to form the CN group (n = 814), while the remaining three MCI sub-groups (amnestic, dysnomic, and dysexecutive) formed the MCI group (n = 542).
Neuroimaging acquisition & analyses
T1-weighted structural images were collected from all ADNI participants using either a 3D-MPRAGE or 3D IR-SPGR sequence. Sequence parameters are available online (http://adni.loni.usc.edu/methods/documents/mri-protocols/). MRI scans from 1.5 T and 3 T magnetic field strengths were combined for analyses, an approach previously shown to be feasible in voxel-based analyses of the ADNI dataset (Dutt et al. 2020; Jack et al. 2015; Marchewka et al. 2014). Images were downloaded from the ADNI-LONI database, checked for image quality, and manually reoriented in SPM12 within MATLAB (http://www.fil.ion.ucl.ac.uk/spm/). Images were processed using the voxel-based morphometry (VBM) pipeline via segmentation into tissue classes, creation of and alignment to a study-specific DARTEL template, spatial normalization, modulation, and 8 mm smoothing (Ashburner and Friston 2000). Region-of-interest (ROI) masks for midbrain, pons, and whole brainstem were derived from previously published atlases (Iglesias et al. 2015; Mazziotta et al. 2001). We used a pre-existing LC ROI mask that averaged peak voxel coordinates from studies that localized the LC on functional MRI and neuromelanin-sensitive T1-weighted scans (https://rcweb.dartmouth.edu/CANlab/brainstemwiki/doku.php/lc.html) (Astafiev et al. 2010; Keren et al. 2009). To adjust for whole brain volume and facilitate comparisons, we divided ROI volumes by total intracranial volume and multiplied them by 103 (midbrain, pons, whole brainstem) or 104 (LC) (Whitwell et al. 2001).
CSF biomarkers
MCI participants who were both amyloid-β (Aβ) and phosphorylated tau (pTau) positive based on pre-established cutoffs (Hansson et al. 2018) comprised the MCI due to AD group (MCIAβ + pTau+, n = 202). Aβ-positive and pTau-positive CN participants comprised the preclinical AD group (CNAβ + pTau+, n = 112). For detailed information on CSF biomarker quantification, see Supplemental Methods.
Statistical analyses
For all ROI volumes and cognitive measures, Pearson correlations were first examined to confirm the presence or absence of zero-order relationships (Keith 2014; Kraha et al. 2012), followed by multiple linear regression models with TIV-adjusted brainstem ROI volume as independent variable, neuropsychological test as dependent variable, and age, sex, education, apolipoprotein-ε4 (APOE-ε4) carrier status, and MRI magnet strength as covariates. In order to demonstrate that our substructural findings were independent of total brainstem volume changes, we repeated analyses with an additional covariate for total brainstem volume. False discovery rate (FDR) correction via the Benjamini-Hochberg procedure (Glickman et al. 2014) was controlled at 0.10 to address multiple comparisons, similar to prior AD studies (Readhead et al. 2018; Yew and Nation 2017). Further information regarding statistical analyses is available in Supplemental Methods.
For all significant multiple regressions, we conducted exploratory voxel-wise regression analyses in SPM12 with neuropsychological test of interest as independent variable and segmented white matter map as dependent variable, consistent with prior studies (Dutt et al. 2016, 2020; Nigro et al. 2014). An explicit mask of the midbrain and pons constrained analyses to rostral brainstem regions, and age, sex, education, APOE-ε4 carrier status, MRI magnet strength, and total intracranial volume were included as covariates. Voxel-wise analyses were repeated with an additional covariate for pons volume to determine regional specificity. Results were examined at family-wise error (FWE)-corrected p < 0.05 and uncorrected p < 0.05.
Results
Demographic, clinical, and cognitive variables
Descriptive statistics for demographic, cognitive, and neuroimaging variables are displayed in Table 1.
Table 1.
Descriptive statistics for demographic, cognitive, and neuroimaging variables
| Total Sample | Prodromal AD Subsets | |||
|---|---|---|---|---|
| CN | MCI | CNAβ + pTau+ | MCIAβ + pTau+ | |
| Demographics | ||||
| n | 814 | 542 | 112 | 202 |
| Age | 73.49 (6.76) | 73.54 (7.35) | 74.75 (6.18) | 73.61 (7.13) |
| Sex (M/F) | 417/397 | 332/210 | 59/53 | 112/90 |
| Education | 16.29 (2.65) | 15.85 (2.92) | 15.90 (2.66) | 15.98 (2.86) |
| APOE-ε4 (0/1/2 ε4) | 536/246/32 | 249/221/72 | 39/61/12 | 57/104/41 |
| MRI Scanner (1.5 T/3 T) | 304/510 | 305/237 | 34/78 | 91/111 |
| Cognitive Testing | ||||
| Trails A | −1.53 (0.14) | −1.60 (0.17) | −1.56 (0.14) | −1.63 (0.17) |
| Trails B | −1.91 (0.17) | −2.06 (0.23) | −1.97 (0.19) | −2.08 (0.21) |
| RAVLT Trial 1 | 5.23 (1.78) | 4.14 (1.41) | 4.81 (1.62) | 4.01 (1.38) |
| RAVLT Encoding | 43.47 (10.42) | 30.10 (8.14) | 39.88 (9.56) | 28.71 (7.39) |
| RAVLT Recall | 7.26 (3.88) | 2.16 (2.62) | 6.11 (3.39) | 1.55 (2.23) |
| RAVLT Recognition | 13.05 (2.20) | 8.93 (3.21) | 12.97 (2.09) | 8.58 (3.07) |
| Animals Fluency | 20.13 (5.26) | 15.73 (4.72) | 19.22 (4.60) | 15.46 (4.59) |
| BNT | −0.37 (0.28) | −0.65 (0.34) | −0.42 (0.27) | −0.67 (0.32) |
| Neuroimaging | ||||
| TIV | 1499.92 (146.93) | 1518.99 (159.50) | 1488.19 (146.29) | 1505.68 (166.34) |
| LC | 1.22 (0.12) | 1.20 (0.12) | 1.24 (0.13) | 1.21 (0.11) |
| Midbrain | 3.88 (0.31) | 3.82 (0.31) | 3.92 (0.30) | 3.85 (0.30) |
| Pons | 7.70 (0.75) | 7.60 (0.75) | 7.81 (0.81) | 7.67 (0.73) |
| Brainstem | 13.32 (1.18) | 13.14 (1.20) | 13.50 (1.23) | 13.26 (1.17) |
Means (SD) are reported for continuous variables unless otherwise noted. Biomarker-positive groups are subsets of respective diagnostic groups. ROI volumes (LC, midbrain, pons, brainstem) were normalized via division by TIV. Scores for Trails A, Trails B, and BNT were log-transformed and reflected
Aβ, amyloid-β; APOE-ε4, apolipoprotein ε4; BNT, Boston Naming Test; CN, cognitively normal; LC, locus coeruleus; MCI, mild cognitive impairment; pTau, phosphorylated tau; RAVLT, Rey Auditory Verbal Learning Test; ROI, region of interest; TIV, total intracranial volume
Memory
Multiple linear regression models predicting memory performance (RAVLT trials 1–5, delayed recall, and recognition) from ROI volumes were not significant within CN, MCI, CNAβ + pTau+, or MCIAβ + pTau+.
Attention and executive function measures
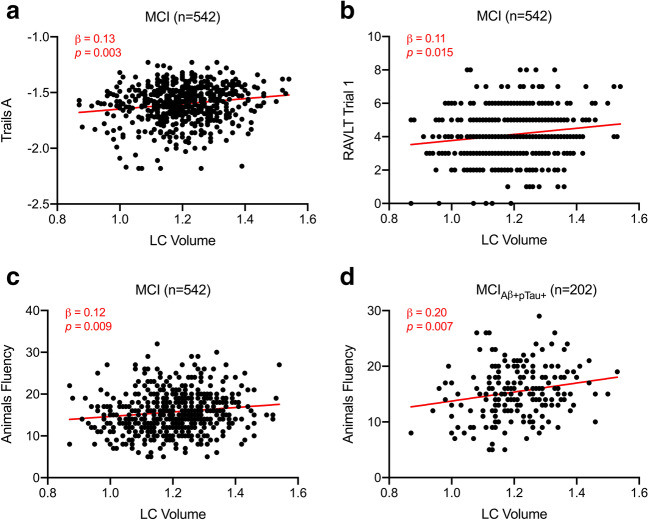
Within the overall MCI group, multiple linear regression models indicated smaller LC volume predicted worse performance on Trails A (β = 0.13, p = 0.003; Fig. 1a), RAVLT trial 1 (β = 0.11, p = 0.015; Fig. 1b), and Animals fluency (β = 0.12, p = 0.009; Fig. 1c). When including an additional covariate for whole brainstem volume, the relationship between LC volume and Animals fluency (β = 0.29, p = 0.008) remained significant. When constraining analyses to AD biomarker-positive MCIAβ + pTau+ participants, smaller LC volume predicted worse performance on Animals fluency (β = 0.20, p = 0.007; Fig. 1d), and this finding remained significant with an additional covariate for whole brainstem volume (β = 0.48, p = 0.007).
Fig. 1.
Regression analyses predicting cognition from locus coeruleus volume. Scatter plots and regression lines showing associations between TIV-normalized LC volume and (a) Trails A performance, (b) RAVLT trial 1 performance, and (c) category fluency performance in the MCI group (n = 542), and (d) between LC volume and category fluency performance in the MCIAβ + pTau+ group (n = 202). Plotted data are unadjusted values, and red text indicates β and p value corresponding to multiple linear regression models with ROI volume as independent variable, cognitive test as dependent variable, and age, sex, education, APOE-ε4 carrier status, and MRI magnet strength as covariates. Abbreviations: Aβ = amyloid-β, APOE-ε4 = apolipoprotein ε4, LC = locus coeruleus, MCI = mild cognitive impairment, pTau = phosphorylated tau, RAVLT = Rey Auditory Verbal Learning Test, ROI = region of interest, TIV = total intracranial volume.
Within the overall MCI group, smaller midbrain volume predicted worse performance on Trails A (β = 0.13, p = 0.004; Fig. 2a), Trails B (β = 0.10, p = 0.022; Fig. 2b), RAVLT trial 1 (β = 0.11, p = 0.011; Fig. 2c), and Animals fluency (β = 0.11, p = 0.02; Fig. 2d), while smaller whole brainstem volume (β = 0.10, p = 0.02) and smaller pons volume (β = 0.09, p = 0.031) predicted worse performance on Trails A. When correcting for whole brainstem volume, smaller midbrain volume predicted worse performance on Trails B (β = 0.28, p = 0.016) and RAVLT trial 1 (β = 0.26, p = 0.026). Within AD biomarker-positive MCIAβ + pTau+ participants, midbrain, pons, or whole brainstem volumes were not associated with neuropsychological testing. Regression models predicting attention and executive function performance from ROI volumes were not significant within the CN or CNAβ + pTau+ groups.
Fig. 2.
Regression analyses predicting cognition from midbrain volume. Scatter plots and regression lines showing associations between TIV-normalized midbrain volume and (a) Trails A performance, (b) Trails B performance, (c) RAVLT trial 1 performance, and (d) category fluency performance in the MCI (n = 542) group. Plotted data are unadjusted values, and red text indicates β and p value corresponding to multiple linear regression models with ROI volume as independent variable, cognitive test as dependent variable, and age, sex, education, APOE-ε4 carrier status, and MRI magnet strength as covariates. Abbreviations: Aβ = amyloid-β, APOE-ε4 = apolipoprotein ε4, LC = locus coeruleus, MCI = mild cognitive impairment, pTau = phosphorylated tau, RAVLT = Rey Auditory Verbal Learning Test, ROI = region of interest, TIV = total intracranial volume
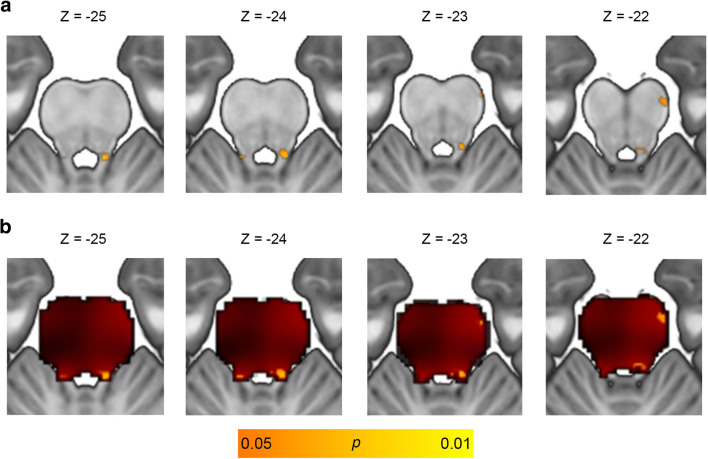
Brainstem-masked voxel-wise regressions relating brain volume to neuropsychological tests within the overall MCI group were not significant at FWE-corrected p < 0.05. At a less stringent threshold of uncorrected p < 0.05, worse Animals fluency correlated with smaller volume of clusters overlapping the bilateral LC and right anterolateral midbrain (Fig. 3; Table 2), and a similar cluster emerged when including an additional covariate for total pons volume (Supp Fig. 1, Supp Table 1).
Fig. 3.
Voxel-wise correlation between category fluency and locus coeruleus volume. Results of voxel-wise multiple regression correlating brain volume with category fluency performance in the MCI (n = 542) group with covariates for total intracranial volume, age, sex, education, APOE-ε4 carrier status, and MRI magnet strength. (a) Significant clusters emerged overlapping the bilateral locus coeruleus and right lateral midbrain at an uncorrected height threshold of p < 0.05. (b) Significant clusters at p < 0.05 (orange) overlaid on an unthresholded statistical map (red). Explicit mask comprising the midbrain and pons was applied to limit search volume to rostral brainstem structures. Images are shown in neurological orientation. Text indicates MNI coordinates of corresponding axial slices. Abbreviations: APOE-ε4 = apolipoprotein ε4, MCI = mild cognitive impairment, MNI = Montreal Neurological Institute
Table 2.
MNI coordinates from voxel-wise correlation between category fluency and locus coeruleus volume
| A | |||||||
| Set-level | Cluster-level | Peak-level | |||||
| p | puncorr | kE | puncorr | T | x | y | z |
| 0.032 | 0.983 | 19 | 0.023 | 1.99 | 8 | −40 | −24 |
| 0.994 | 4 | 0.036 | 1.79 | −8 | −40 | −22 | |
| 0.987 | 13 | 0.043 | 1.70 | 14 | −21 | −21 | |
| B | |||||||
| MNI x-range | MNI y-range | MNI z-range | |||||
| Voxel-wise correlation with Animals fluency | 8 to −9 | −38 to −41 | −21 to −27 | ||||
| MCI < CN (Dutt et al. 2020) | 8 to −8 | −39 to −42 | −21 to −28 | ||||
| AD < CN (Dutt et al. 2020) | 9 to −6 | −33 to −41 | −17 to −26 | ||||
| Converters < Non-Converters (Dutt et al. 2020) | 8 to −8 | −39 to −41 | −21 to −26 | ||||
| LC mask (Keren et al. 2009) | 9 to −9 | −36 to −39 | −18 to −33 | ||||
| LC mask (Betts et al. 2017) | 9 to −9 | −36 to −43 | −15.5 to −37.5 | ||||
| LC mask (Dahl et al. 2019) | 8 to −10 | −29 to −42 | −18 to −38 | ||||
| NC vs. AD peak coordinates (Ji et al. 2020) | −6, −9 | −36, −36 | −24, −29 | ||||
(A) Coordinates from voxel-wise multiple regression in MCI (n = 542) group regressing category fluency onto brain volume with an explicit mask comprising the midbrain + pons and covariates for total intracranial volume, age, sex, education, APOE-ε4 carrier status, and MRI magnet strength. (B) MNI coordinate range for significant clusters from present study and from prior brainstem VBM studies and established locus coeruleus masks
AD, Alzheimer’s disease; CN, cognitively normal; kE, cluster size; LC, locus coeruleus; MCI, mild cognitive impairment; NC, normal controls; MNI, Montreal Neurological Institute; uncorr, uncorrected
Language
Multiple regression models predicting BNT performance from ROI volumes were not significant within participant subgroups (CN, MCI, CNAβ + pTau+, MCIAβ + pTau+).
Discussion
The present study found that MCI patients with smaller midbrain and LC volumes performed worse on tests of visual attention (Trails A), verbal attention (RAVLT trial 1), executive function (Trails B), and category fluency (Animals), suggesting brainstem substructural volumes may be related to underlying attention, processing speed, and executive abilities. In MCI patients with biomarker-confirmed AD, the relationship between LC volume and Animals fluency remained significant in the presence of prodromal AD pathology. Whole brainstem, midbrain, pons and LC volumes were not associated with episodic memory (RAVLT encoding, delayed recall, and recognition) or a confrontation naming test of language ability (BNT), highlighting the specific association between brainstem substructure volumes and measures of attention, processing speed, and executive function. This is the first study to report associations between cognition and brainstem substructure volumes in MCI populations. We provided preliminary evidence that well-documented relationships between the LC noradrenergic system and attention (Aston-Jones et al. 1999; Mather et al. 2020; Sara 2009) are detectible when examining LC volume in the prodromal phase of AD.
The critical MCI phase preceding AD dementia may be a window when neural and cognitive reserve in brainstem regions are integral to maintaining optimal cognitive function. Within this prodromal period, we found that individuals with smaller midbrain and LC volumes performed worse on tasks of executive function and visual and verbal attention. This echoes the neuropathology literature demonstrating that individuals with greater pathological burden (i.e., greater subcortical tau deposition) exhibit diminished volumes of nuclei known to contain the first signs of AD-related pathology and perform worse on corresponding cognitive tests (Braak and Del Tredici 2015; Grudzien et al. 2007). Alternatively, our findings could reflect that greater premorbid LC volume supports better performance on attentional tasks. This supports a previously theorized buffering role of the LC, due to its high lifetime noradrenergic turnover and neuronal density, in protecting against the detrimental effects of accumulating AD-related pathology (Clewett et al. 2015; Mather and Harley 2016; Robertson 2013). Although the exact role of brainstem degeneration in cognitive dysfunction is not well-understood, degeneration of the LC appears to be related to cognitive function in normal aging (Dahl et al. 2019; Langley et al. 2020) and correlates with cognitive abilities and pathological protein accumulation in animal models of AD (Chalermpalanupap et al. 2017; James et al. 2020; Kelberman et al. 2020). Of note, we found attenuated brain-behavior relationships in the biomarker-confirmed MCI due to AD group compared to the overall MCI group, likely due to the smaller sample size. Interestingly, we did not observe relationships between brainstem structure and cognition in the CN group, despite observable first signs of tau pathology in postmortem adult cognitively normal samples (Braak and Del Tredici 2015). We previously demonstrated that LC structural abnormalities are observable using MRI with cognitively normal participants (Dutt et al. 2020); however, the current findings suggest these individuals do not yet exhibit cognitive decline that correlates with brainstem structure. Future studies will be necessary to clarify whether LC function, as opposed to structure, in the early preclinical AD phase better correlates with cognition.
The category fluency task was the cognitive test most strongly associated with midbrain and LC volumes in MCI and biomarker-confirmed MCI due to AD. The category fluency task, though often broadly categorized under the domain of language processing, also requires executive abilities subserved by frontal-subcortical systems, including monitoring, shifting, and inhibition (Shao et al. 2014). Furthermore, the category fluency task is similar to other tests from the present study (e.g., Trails A & B) because it represents a timed test requiring adequate attention and processing speed to complete successfully (Auriacombe et al. 2001; Baddeley and Della Sala 1996). Subcortical dementias experience specific impairments in attention, executive function, and processing speed (Cummings 1986; Salmon and Filoteo 2007), and our findings may similarly reflect subcortical contributions to cognitive impairment in prodromal AD.
The present study did not find relationships between brainstem volumes and episodic verbal memory encoding, which contrasts with associations observed in studies of LC signal intensity and memory encoding performance during verbal learning and immediate recall tasks in older adults and AD populations (Dahl et al. 2019; Olivieri et al. 2019). Memory performance on the immediate recall trial and across the encoding trials is linked to an individual’s ability to engage attention during the presentation of stimuli and store items in working memory (Buckner et al. 2000), and our study findings suggest a role of brainstem volume in attention and working memory. Interestingly, relationships between brainstem volumes and measures of episodic verbal memory abilities linked to integrity of medial temporal and hippocampal structures (Squire and Zola-Morgan 1991), were not observed. Our approach did not include hippocampal and medial temporal structures, as these areas are well-studied and known to experience profound atrophy in AD neurodegenerative processes (Jack et al. 1998; Mori et al. 1997). Our study was not designed to determine if brainstem substructures are better predictors of cognition than medial temporal and hippocampal regions, but rather to independently assess relationships between brainstem substructure volumes and cognition. Our findings complement a growing body of evidence supporting the role of LC structural integrity (as measured by neuromelanin-sensitive T1-weighted imaging) and functional activity (as measured by fMRI) in diverse memory processes when the stimuli involved are particularly salient or emotionally charged (Clewett et al. 2018; Hämmerer et al. 2018; Jacobs et al. 2020; Liu et al. 2020). The relative neutrality of word stimuli in the present study may partially explain why no relationships between brainstem volumes and recall or recognition were observed, yet a recent diffusion-weighted imaging study found an association between LC microstructure and RAVLT delayed recall performance in healthy older adults (Langley et al. 2020). More multimodal neuroimaging work is needed in MCI populations to disentangle the specific associations between brainstem substructures and memory for stimuli of varying emotional arousal.
A study limitation is the use of segmented structural T1 images to estimate volumes of deep brainstem nuclei, which inherently lack information regarding the boundaries of structures such as the LC. Although prior studies have demonstrated an ability to detect structural brainstem differences between disease groups with a similar method (Dutt et al. 2020), our approach should be further validated in cohorts with MRI sequences specialized for assessment of LC structure (Betts et al. 2019). Another limitation is the racially homogeneous and highly educated nature of the ADNI cohort, which limits the generalizability of our findings. Future studies should examine diverse populations. Given the cross-sectional study design, directionality of brainstem-cognition relationships cannot be determined. Other limitations include the overlaid ROI approach to volume extraction as opposed to individual structural segmentation and the high variability in individual subject history and instrumentation between sites, all of which should be addressed in follow-up studies.
Conclusions
The present study examined relationships between brainstem volumes and cognition by quantifying VBM-estimated brainstem substructure and LC volumes from structural MRI images in individuals with normal cognition, biomarker-confirmed preclinical AD, neuropsychologically-confirmed MCI, and biomarker-confirmed MCI due to AD. Midbrain and LC volumes were associated with measures of attention, processing speed, and executive function, but not with episodic memory performance or confrontation naming. A growing number of studies have implicated subcortical brainstem structures as the earliest sites of AD-related tau pathology, and MRI-measured volumes of these regions appear to correlate strongest with tasks that require greater executive control and attention in the MCI phase preceding the later onset of dementia.
Supplementary Information
(DOC 142 kb)
Acknowledgements
We would like to thank the participants and their families, investigators, and researchers from the ADNI study. Data used in preparation of this article were obtained from the ADNI database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
Authors’ contributions
The listed authors contributed to the present manuscript in the following ways: study concept and design (SD, YL, MM, DAN), analysis and interpretation of data (SD, YL), statistical analyses (SD), drafting the manuscript (SD), critical revision of the manuscript (YL, MM, DAN), and final approval of and accountability for the manuscript (SD, YL, MM, DAN).
Funding
Author funding for this study was obtained through grants from the National Institutes of Health (D.N., grant numbers R01AG060049, R01AG64228, P01AG052350, P50AG016573), (M.M., grant number R01AG025340); the Alzheimer’s Association (D.N., grant number AA008369); and the National Science Foundation (S.D., grant number DGE1418060). Data collection and sharing for this project was funded by the Alzheimer’s Disease Neuroimaging Initiative (ADNI) (National Institutes of Health Grant U01 AG024904) and DOD ADNI (Department of Defense award number W81XWH-12-2-0012). ADNI is funded by the National Institute on Aging, the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from the following: AbbVie, Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen; Bristol-Myers Squibb Company; CereSpir, Inc.; Cogstate; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly and Company; EuroImmun; F. Hoffmann-La Roche Ltd. and its affiliated company Genentech, Inc.; Fujirebio; GE Healthcare; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development LLC.; Lumosity; Lundbeck; Merck & Co., Inc.; Meso Scale Diagnostics, LLC.; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals Corporation; Pfizer Inc.; Piramal Imaging; Servier; Takeda Pharmaceutical Company; and Transition Therapeutics. The Canadian Institutes of Health Research is providing funds to support ADNI clinical sites in Canada. Private sector contributions are facilitated by the Foundation for the National Institutes of Health (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education, and the study is coordinated by the Alzheimer’s Therapeutic Research Institute at the University of Southern California. ADNI data are disseminated by the Laboratory for Neuroimaging at the University of Southern California.
Data availability
All data used in the present study are publicly available via the ADNI website (http://adni.loni.usc.edu/) and the ADNI-LONI Image & Data Archive (https://ida.loni.usc.edu/login.jsp).
Declarations
Conflicts of interest/competing interests
The authors have no disclosures or conflicts of interest to report.
Ethics approval
The present study was conducted in accordance with the Helsinki Declaration and approved at all ADNI sites by local Institutional Review Boards.
Consent to participate
Written informed consent was obtained from all participants.
Footnotes
Data used in preparation of this article were obtained from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ashburner J, Friston KJ. Voxel-based Morphometry—The methods. NeuroImage. 2000;11(6):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Astafiev SV, Snyder AZ, Shulman GL, Corbetta M. Comment on “Modafinil shifts human locus coeruleus to low-tonic, high-phasic activity during functional MRI” and “Homeostatic sleep pressure and responses to sustained attention in the suprachiasmatic area”. Science. 2010;328(5976):309. doi: 10.1126/science.1177200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annual Review of Neuroscience. 2005;28(1):403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J. Role of locus coeruleus in attention and behavioral flexibility. Biological Psychiatry. 1999;46(9):1309–1320. doi: 10.1016/S0006-3223(99)00140-7. [DOI] [PubMed] [Google Scholar]
- Auriacombe S, Fabrigoule C, Lafont S, Amieva H, Jacqmin-Gadda H, Dartigues JF. Letter and category fluency in normal elderly participants: A population-based study. Aging, Neuropsychology, and Cognition. 2001;8(2):98–108. doi: 10.1076/anec.8.2.98.841. [DOI] [Google Scholar]
- Baddeley AD, Della Sala S. Working memory and executive control. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 1996;351(1346):1397–1404. doi: 10.1098/rstb.1996.0123. [DOI] [PubMed] [Google Scholar]
- Betts MJ, Cardenas-Blanco A, Kanowski M, Jessen F, Düzel E. In vivo MRI assessment of the human locus coeruleus along its rostrocaudal extent in young and older adults. NeuroImage. 2017;163:150–159. doi: 10.1016/j.neuroimage.2017.09.042. [DOI] [PubMed] [Google Scholar]
- Betts MJ, Kirilina E, Otaduy MCG, Ivanov D, Acosta-cabronero J, Callaghan MF, et al. Locus coeruleus imaging as a biomarker for noradrenergic dysfunction in neurodegenerative diseases. Brain. 2019;142(9):2558–2571. doi: 10.1093/brain/awz214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondi MW, Edmonds EC, Jak AJ, Clark LR, Delano-Wood L, McDonald CR, et al. Neuropsychological criteria for mild cognitive impairment improves diagnostic precision, biomarker associations, and progression rates. Journal of Alzheimer’s Disease. 2014;42(1):275–289. doi: 10.3233/JAD-140276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. The preclinical phase of the pathological process underlying sporadic Alzheimer’s disease. Brain. 2015;138(10):2814–2833. doi: 10.1093/brain/awv236. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Logan J, Donaldson DI, Wheeler ME. Cognitive neuroscience of episodic memory encoding. Acta Psychologica. 2000;105(2–3):127–139. doi: 10.1016/s0001-6918(00)00057-3. [DOI] [PubMed] [Google Scholar]
- Chalermpalanupap T, Weinshenker D, Rorabaugh JM. Down but not out: The consequences of Pretangle tau in the locus Coeruleus. Neural Plasticity. 2017;2017:1–9. doi: 10.1155/2017/7829507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LR, Delano-Wood L, Libon DJ, Mcdonald CR, Nation DA, Bangen KJ, et al. Are empirically-derived subtypes of mild cognitive impairment consistent with conventional subtypes? Journal of the International Neuropsychological Society. 2013;19(6):635–645. doi: 10.1017/S1355617713000313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewett DV, Lee TH, Greening S, Ponzio A, Margalit E, Mather M. Neuromelanin marks the spot: Identifying a locus coeruleus biomarker of cognitive reserve in healthy aging. Neurobiology of Aging. 2015;37:117–126. doi: 10.1016/j.neurobiolaging.2015.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewett DV, Huang R, Velasco R, Lee TH, Mather M. Locus coeruleus activity strengthens prioritized memories under arousal. Journal of Neuroscience. 2018;38(6):1558–1574. doi: 10.1523/JNEUROSCI.2097-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings JL. Subcortical dementia neuropsychology, neuropsychiatry, and pathophysiology. British Journal of Psychiatry. 1986;149(6):682–697. doi: 10.1192/bjp.149.6.682. [DOI] [PubMed] [Google Scholar]
- Dahl MJ, Mather M, Düzel S, Bodammer NC, Lindenberger U, Kühn S, Werkle-Bergner M. Rostral locus coeruleus integrity is associated with better memory performance in older adults. Nature Human Behaviour. 2019;3:1203–1214. doi: 10.1038/s41562-019-0715-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delano-Wood L, Bondi MW, Sacco J, Abeles N, Jak AJ, Libon DJ, Bozoki A. Heterogeneity in mild cognitive impairment: Differences in neuropsychological profile and associated white matter lesion pathology. Journal of the International Neuropsychological Society. 2009;15(6):906–914. doi: 10.1017/S1355617709990257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutt S, Binney RJ, Heuer HW, Luong P, Marx GA, Elofson J, et al. Progression of brain atrophy in PSP and CBS over 6 months and 1 year. Neurology. 2016;87(19):2016–2025. doi: 10.1212/WNL.0000000000003305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutt S, Li Y, Mather M, Nation DA. Brainstem volumetric integrity in preclinical and prodromal Alzheimer’s disease. Journal of Alzheimer’s Disease. 2020;77(4):1579–1594. doi: 10.3233/JAD-200187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmonds EC, Delano-Wood L, Clark LR, Jak AJ, Nation DA, McDonald CR, Libon DJ, Au R, Galasko D, Salmon DP, Bondi MW, Alzheimer's Disease Neuroimaging Initiative Susceptibility of the conventional criteria for mild cognitive impairment to false-positive diagnostic errors. Alzheimer’s and Dementia. 2015;11(4):415–424. doi: 10.1016/j.jalz.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman ME, Rao SR, Schultz MR. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. Journal of Clinical Epidemiology. 2014;67(8):850–857. doi: 10.1016/j.jclinepi.2014.03.012. [DOI] [PubMed] [Google Scholar]
- Grudzien A, Shaw P, Weintraub S, Bigio E, Mash DC, Mesulam MM. Locus coeruleus neurofibrillary degeneration in aging, mild cognitive impairment and early Alzheimer’s disease. Neurobiology of Aging. 2007;28(3):327–335. doi: 10.1016/j.neurobiolaging.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Hämmerer D, Callaghan MF, Hopkins A, Kosciessa J, Betts M, Cardenas-Blanco A, Kanowski M, Weiskopf N, Dayan P, Dolan RJ, Düzel E. Locus coeruleus integrity in old age is selectively related to memories linked with salient negative events. Proceedings of the National Academy of Sciences of the United States of America. 2018;115(9):2228–2233. doi: 10.1073/pnas.1712268115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson O, Seibyl J, Stomrud E, Zetterberg H, Trojanowski JQ, Bittner T, Lifke V, Corradini V, Eichenlaub U, Batrla R, Buck K, Zink K, Rabe C, Blennow K, Shaw LM, for the Swedish BioFINDER study group. Alzheimer's Disease Neuroimaging Initiative CSF biomarkers of Alzheimer’s disease concord with amyloid-β PET and predict clinical progression: A study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimer’s & Dementia. 2018;14(11):1470–1481. doi: 10.1016/j.jalz.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinsen H, Grinberg LT. On the origin of tau seeding activity in Alzheimer’s disease. Acta Neuropathologica. 2018;136(5):815–817. doi: 10.1007/s00401-018-1890-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglesias JE, Van Leemput K, Bhatt P, Casillas C, Dutt S, Schuff N, et al. 2Bayesian segmentation of brainstem structures in MRI. NeuroImage. 2015;113:184–195. doi: 10.1016/j.neuroimage.2015.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Petersen RC, Xu Y, O’Brien PC, Smith GE, Ivnik RJ, et al. Rate of medial temporal lobe atrophy in typical aging and Alzheimer’s disease. Neurology. 1998;51(4):993–999. doi: 10.1212/WNL.51.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Barnes J, Bernstein MA, Borowski BJ, Brewer J, Clegg S, et al. Magnetic resonance imaging in Alzheimer’s Disease Neuroimaging Initiative 2. Alzheimer’s and Dementia. 2015;11(7):740–756. doi: 10.1016/j.jalz.2015.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs HIL, Priovoulos N, Poser BA, Pagen LHG, Ivanov D, Verhey FRJ, Uludağ K. Dynamic behavior of the locus coeruleus during arousal-related memory processing in a multi-modal 7T fMRI paradigm. eLife. 2020;9:1–30. doi: 10.7554/eLife.52059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James, T., Kula, B., Choi, S., Khan, S. S., Bekar, L. K., & Smith, N. A. (2020). Locus coeruleus in memory formation and Alzheimer’s disease. European Journal of Neuroscience.10.1111/ejn.15045. [DOI] [PMC free article] [PubMed]
- Ji X, Wang H, Zhu M, He Y, Zhang H, Chen X, et al. Brainstem atrophy in the early stage of Alzheimer’s disease: A voxel-based morphometry study. Brain Imaging and Behavior. 2020;15:49–59. doi: 10.1007/s11682-019-00231-3. [DOI] [PubMed] [Google Scholar]
- Kaufman SK, Del Tredici K, Braak H, Diamond MI. Rebuttal to Drs. Grinberg and Heinsen. Acta Neuropathologica. 2018;136(5):819–819. doi: 10.1007/s00401-018-1917-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keith, T. Z. (2014). Multiple regression and beyond: An introduction to multiple regression and structural equation modeling. Routledge.
- Kelberman M, Keilholz S, Weinshenker D. What’s that (blue) spot on my MRI ? Multimodal neuroimaging of the locus Coeruleus in neurodegenerative disease. Frontiers in Neuroscience. 2020;14:1–17. doi: 10.3389/fnins.2020.583421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keren NI, Lozar CT, Harris KC, Morgan PS, Eckert MA. In vivo mapping of the human locus coeruleus. NeuroImage. 2009;47(4):1261–1267. doi: 10.1016/j.neuroimage.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraha A, Turner H, Nimon K, Zientek LR, Henson RK. Tools to support interpreting multiple regression in the face of multicollinearity. Frontiers in Psychology. 2012;3:1–16. doi: 10.3389/fpsyg.2012.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langley J, Hussain S, Flores JJ, Bennett IJ, Hu X. Characterization of age-related microstructural changes in locus coeruleus and substantia nigra pars compacta. Neurobiology of Aging. 2020;87:89–97. doi: 10.1016/j.neurobiolaging.2019.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Ryan J, Andreescu C, Aizenstein H, Lim HK. Brainstem morphological changes in Alzheimer’s disease. Neuroreport. 2015;26(7):411–415. doi: 10.1097/WNR.0000000000000362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KY, Marijatta F, Hämmerer D, Acosta-Cabronero J, Düzel E, Howard RJ. Magnetic resonance imaging of the human locus coeruleus: A systematic review. Neuroscience and Biobehavioral Reviews. 2017;83:325–355. doi: 10.1016/j.neubiorev.2017.10.023. [DOI] [PubMed] [Google Scholar]
- Liu KY, Kievit RA, Tsvetanov KA, Betts MJ, Düzel E, Rowe JB, et al. Noradrenergic-dependent functions are associated with age-related locus coeruleus signal intensity differences. Nature Communications. 2020;11(1):1712. doi: 10.1038/s41467-020-15410-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchewka A, Kherif F, Krueger G, Grabowska A, Frackowiak R, Draganski B. Influence of magnetic field strength and image registration strategy on voxel-based morphometry in a study of Alzheimer’s disease. Human Brain Mapping. 2014;35(5):1865–1874. doi: 10.1002/hbm.22297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather, M. (2020). The locus coeruleus-norepinephrine system role in cognition and how it changes with aging. (D. Poeppel, G. Mangun, & M. Gazzaniga, Eds.) The Cognitive Neurosciences. Cambridge, MA: MIT Press.
- Mather M, Harley CW. The locus Coeruleus: Essential for maintaining cognitive function and the aging brain. Trends in Cognitive Sciences. 2016;20(3):214–226. doi: 10.1016/j.tics.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Clewett D, Sakaki M, Harley CW. Norepinephrine ignites local hotspots of neuronal excitation: How arousal amplifies selectivity in perception and memory. Behavioral and Brain Sciences. 2016;39(2016):e200. doi: 10.1017/S0140525X15000667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Huang R, Clewett D, Nielsen SE, Velasco R, Tu K, Han S, Kennedy BL. Isometric exercise facilitates attention to salient events in women via the noradrenergic system. NeuroImage. 2020;210:116560. doi: 10.1016/j.neuroimage.2020.116560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazziotta J, Toga A, Evans A, Fox P, Lancaster J, Zilles K, Woods R, Paus T, Simpson G, Pike B, Holmes C, Collins L, Thompson P, MacDonald D, Iacoboni M, Schormann T, Amunts K, Palomero-Gallagher N, Geyer S, Parsons L, Narr K, Kabani N, Goualher GL, Boomsma D, Cannon T, Kawashima R, Mazoyer B. A probabilistic atlas and reference system for the human brain: International consortium for brain mapping (ICBM) Philosophical Transactions of the Royal Society B: Biological Sciences. 2001;356(1412):1293–1322. doi: 10.1098/rstb.2001.0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori E, Yoneda Y, Yamashita H, Hirono N, Ikeda M, Yamadori A. Medial temporal structures relate to memory impairment in Alzheimer’s disease: An MRI volumetric study. Journal of Neurology, Neurosurgery & Psychiatry. 1997;63(2):214–221. doi: 10.1136/jnnp.63.2.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigro S, Cerasa A, Zito G, Perrotta P, Chiaravalloti F, Donzuso G, Fera F, Bilotta E, Pantano P, Quattrone A, the Alzheimer's Disease Neuroimaging Initiative Fully automated segmentation of the pons and midbrain using human T1 MR brain images. PLoS One. 2014;9(1):e85618. doi: 10.1371/journal.pone.0085618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivieri P, Lagarde J, Lehericy S, Valabrègue R, Michel A, Macé P, Caillé F, Gervais P, Bottlaender M, Sarazin M. Early alteration of the locus coeruleus in phenotypic variants of Alzheimer’s disease. Annals of Clinical and Translational Neurology. 2019;6(7):1345–1351. doi: 10.1002/acn3.50818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Readhead B, Haure-Mirande JV, Funk CC, Richards MA, Shannon P, Haroutunian V, Sano M, Liang WS, Beckmann ND, Price ND, Reiman EM, Schadt EE, Ehrlich ME, Gandy S, Dudley JT. Multiscale analysis of independent Alzheimer’s cohorts finds disruption of molecular, genetic, and clinical networks by human Herpesvirus. Neuron. 2018;99(1):64–82. doi: 10.1016/j.neuron.2018.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson IH. A noradrenergic theory of cognitive reserve: Implications for Alzheimer’s disease. Neurobiology of Aging. 2013;34(1):298–308. doi: 10.1016/j.neurobiolaging.2012.05.019. [DOI] [PubMed] [Google Scholar]
- Rüb U, Stratmann K, Heinsen H, Del Turco D, Seidel K, den Dunnen W, Korf H-W. The brainstem tau cytoskeletal pathology of Alzheimer’s disease: A brief historical overview and description of its anatomical distribution pattern, evolutional features, pathogenetic and clinical relevance. Current Alzheimer Research. 2016;13(10):1178–1197. doi: 10.2174/1567205013666160606100509. [DOI] [PubMed] [Google Scholar]
- Salmon DP, Filoteo JV. Neuropsychology of cortical versus subcortical dementia syndromes. Seminars in Neurology. 2007;27(1):7–21. doi: 10.1055/s-2006-956751. [DOI] [PubMed] [Google Scholar]
- Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nature Reviews Neuroscience. 2009;10(3):211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- Shao Z, Janse E, Visser K, Meyer AS. What do verbal fluency tasks measure? Predictors of verbal fluency performance in older adults. Frontiers in Psychology. 2014;5:1–10. doi: 10.3389/fpsyg.2014.00772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253(5026):1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Stratmann K, Heinsen H, Korf HW, Del Turco D, Ghebremedhin E, Seidel K, et al. Precortical phase of Alzheimer’s disease (AD)-related tau cytoskeletal pathology. Brain Pathology. 2016;26(3):371–386. doi: 10.1111/bpa.12289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwell JL, Crum WR, Watt HC, Fox NC. Normalization of cerebral volumes by use of intracranial volume: Implications for longitudinal quantitative MR imaging. American Journal of Neuroradiology. 2001;22(8):1483–1489. [PMC free article] [PubMed] [Google Scholar]
- Yew B, Nation DA. Cerebrovascular resistance: Effects on cognitive decline, cortical atrophy, and progression to dementia. Brain. 2017;140(7):1987–2001. doi: 10.1093/brain/awx112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 142 kb)
Data Availability Statement
All data used in the present study are publicly available via the ADNI website (http://adni.loni.usc.edu/) and the ADNI-LONI Image & Data Archive (https://ida.loni.usc.edu/login.jsp).