Abstract
Objectives
Because there is uncertainty about the extent to which baseline blood pressure level or cardiovascular risk modifies the relationship between blood pressure variability (BPv) and cardiovascular disease, we comprehensively examined the role of BPv in cardiovascular disease risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial.
Methods
Using data from ACCORD, we examined the relationship of BPv with development of the primary CVD outcome, major coronary heart disease (CHD), and total stroke using time-dependent Cox proportional hazards models.
Results
BPv was associated with the primary CVD outcome and major CHD, but not stroke. The positive association with the primary CVD outcome and major CHD was more pronounced in low and high strata of baseline SBP (<120mmHg and >140mmHg) and DBP (<70mmHg and >80mmHg). The effect of BPv on CVD and CHD was more pronounced in those with both prior CVD history and low blood pressure. Dips, not elevations, in blood pressure appeared to drive these associations. The relationships were generally not attenuated by adjustment for mean blood pressure, medication adherence, or baseline comorbidities. A sensitivity analysis using CVD events from the long-term post-trial follow-up (ACCORDION) was consistent with the results from ACCORD.
Conclusions
In ACCORD, the effect of BPv on adverse cardiovascular (but not cerebrovascular) outcomes is modified by baseline blood pressure and prior CVD. Recognizing these more nuanced relationships may help improve risk stratification and blood pressure management decisions as well as provide insight into potential underlying mechanisms.
INTRODUCTION
A large body of epidemiologic evidence indicates that visit-to-visit blood pressure variability is associated with cardiovascular risk,[1–4] including among individuals with type 2 diabetes (T2D).[5] Such associations have been reported to be independent of average blood pressure level[1, 2, 6] and may be explained by several putative mechanisms such as reductions in aortic distensibility,[7] increased arterial stiffness,[8] impaired endothelial function,[9] or increasing global longitudinal strain.[10]
Recent studies indicate that the association between blood pressure variability and cardiovascular disease (CVD) may be modified by baseline cardiovascular history or blood pressure level. In post-hoc analyses of three studies—the Valsartan Antihypertensive Long-Term Use Evaluation (VALUE) trial, the PROspective Study of Pravastatin in the Elderly at Risk (PROSPER), and the Stabilization of Atherosclerotic Plaque by Initiation of Darapladib Therapy (STABILITY) trial—it was observed that the association between systolic or diastolic blood pressure (SBP or DBP) variability and CVD was strongest in those with blood pressure level below the median or in the lowest tertile.[11–13] Furthermore, dips rather than peaks in blood pressure in the STABILITY trial were shown to be more closely associated with cardiovascular mortality.[13]
While these new findings are noteworthy, there are some limitations to the work previously described. Although several of these studies examined the relationship between blood pressure variability and CVD outcomes in groups stratified by median blood pressure, rigorous examination by finer baseline blood pressure categories could help provide a clearer understanding of the risk associated with variability across the spectrum of blood pressure. It may also provide additional insight into the underlying mechanisms of cardiovascular risk due to blood pressure variability. Moreover, previous studies did not systematically explore the relationship between blood pressure categories and blood pressure variability on different cardiovascular outcomes.
One potential pathway proposed to account for heightened cardiovascular risk[13, 14] in those with increased blood pressure variability is an increased occurrence of coronary hypoperfusion. As coronary perfusion is dependent on diastolic blood flow in epicardial vessels, factors such as low blood pressure or restrictions in blood flow would be expected to exacerbate the effects of blood pressure variability. In addition, one would anticipate cardiac outcomes to be particularly sensitive to blood pressure variability.
To address several of these limitations and unanswered questions in this area, we performed a comprehensive post-hoc assessment of blood pressure variability and cardiovascular risk outcome measures in participants of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. This included among other approaches a more focused examination of background blood pressure levels, relevant patterns of blood pressure variability, prior CVD history, and type of incident CVD outcomes affected. With a large sample size, detailed medical history, frequent blood pressure measurements, and a broad group of adjudicated outcomes, ACCORD provides an ideal cohort to use to explore this research question.
METHODS
Study design, participants, and variability measures
The ACCORD trial was a double two-by-two factorial, parallel treatment trial in which patients were randomly assigned to receive intensive glucose lowering targeting an HbA1c concentration of < 6.0% or standard treatment targeting HbA1c of 7.0–7.9%. Participant recruitment began in 2001, and the goal of at least 10,000 participants recruited was reached in September 2005. The trial also included randomization to distinct blood pressure and lipid intervention arms. ACCORD included participants with T2D, HbA1c concentrations ≥ 7.5%, and who were aged 40–79 years with a history of CVD or 55–79 years with evidence of significant atherosclerosis, albuminuria, left ventricular hypertrophy, or at least two risk factors for CVD (dyslipidemia, hypertension, obesity, or smoking). Of the 10,251 participants in the entire ACCORD population, 4,733 were randomized to two blood pressure intervention arms: an intensive blood pressure arm with a goal of reducing SBP to <120 mmHg or a standard blood pressure arm with a goal of reducing SBP to <140 mmHg. Blood pressure measurements were available at baseline and 4-month intervals for all participants. After participants sat quietly for five minutes, blood pressures were measured by certified staff. Using the Omron Healthcare HEM-907, an automated oscillometric device, three SBP and DBP measurements were taken automatically at one-minute intervals. Blood pressure readings were recorded to the nearest digit, and the average of the three readings was the blood pressure value reported for a visit.[15, 16] Details of the overall design of the ACCORD trial,[17] as well as the rationale and treatment protocol for the blood pressure intervention,[18] have been reported previously.
This post-hoc analysis included those who did and did not participate in the blood pressure lowering intervention arms of the trial. We included longitudinal data from the standard 4-month visits in the current analysis for all participants. The baseline visit was excluded from the analysis to reduce the effect of rapid reduction in blood pressure at the early phase of the trial. Those who had two or fewer blood pressure measurements were also excluded. We report coefficient of variation (CV) of SBP and DBP in this analysis. Analyses of average real variability (ARV) of SBP and DBP were also conducted and are presented in supplemental tables.
Outcomes
The primary outcome in ACCORD, and this analysis, was a composite of first occurrence of a major cardiovascular event – cardiovascular death, non-fatal MI, or non-fatal stroke. Secondary outcomes evaluated were major coronary heart disease, CHD (a composite of fatal coronary artery disease events, non-fatal MI, or unstable angina) and total stroke. As described previously,[17] diagnosis of MI in ACCORD was based on occurrence of a clinical syndrome associated with diagnostic elevation in cardiac enzymes (e.g., troponin I or troponin T to a level indicative of myonecrosis). Total stroke (fatal and non-fatal) was diagnosed by autopsy, MRI, evidence of hemorrhage or brain infarction by CT, or a focal neurologic deficit lasting >24 hours.[17]
Statistical analysis
Data are expressed as means (SD) for continuous variables or as numbers and percentages for categorical variables. Differences between participants who did and did not develop an event were analyzed using the Wilcoxon test for continuous variables and chi-square test or Fisher’s exact test, as appropriate, for categorical variables. We display age-adjusted hazard ratios (HRs) for CVD outcomes across quartiles of CV-SBP and CV-DBP.
We used a time-dependent approach for assessing the effect of risk factor variability on cardiovascular outcomes that has been described in detail in previous post-hoc analyses of several clinical trials.[6, 19–21] Briefly, Cox proportional hazards models were fitted to evaluate time-dependent effects of blood pressure measures on CVD outcomes. HRs for all variables were standardized to a change of 1 SD. Three models for variability were reported. Model 1 adjusted for age only. Model 2 adjusted for age and those covariates reflecting significant baseline differences between those who did and did not develop the event in question. Model 3 adjusted for Model 2 covariates and added cumulative mean of blood pressure; this model tests whether variability measures provide additional information beyond overall blood pressure control. We tested the Cox model of SBP and DBP variability metrics and determined that there was no violation of the proportional hazards assumption.
Several subgroup analyses and sensitivity analyses were performed. To test, as previously hypothesized, whether risk of adverse events due to blood pressure variability depends on level of baseline blood pressure, we tested these associations across strata of baseline SBP (>140 mmHg, 120–139 mmHg, <120 mmHg) and baseline DBP (>80 mmHg, 70–79 mmHg, <70 mmHg). To determine whether variability above the mean blood pressure (‘elevations’) or variability below the mean blood pressure (‘dips’) conferred risk, we estimated this BP area as trapezoids based on consecutives visits (“variability area”) and tested for associations of this area with the cardiovascular outcomes. We examined the role of blood pressure variability by presence or absence of CVD history in development of the primary CVD outcome and of the secondary major CHD outcome. For the primary CVD outcome, we ran several additional sensitivity analyses: 1) we checked for consistency of results with prior blood pressure variability studies[11, 12] by examining whether the variability of SBP or DBP below the median (in ACCORD, 126 mmHg and 68 mmHg at baseline, respectively) was more strongly associated with risk; 2) we adjusted the effect of variability for use of blood pressure medications to assess the influence of adherence; 3) we adjusted for a modified Charlson Comorbidity Index to assess the effect of baseline comorbidities on risk due to blood pressure variability (point assignment in this index is described in Supplementary Table 1); 4) we used an alternative landmark period approach[2] that captured variability during the ACCORD trial, and reported continuous CV-SBP and CV-DBP HRs for risk of the primary CVD outcome during the long-term observational follow-up study (ACCORDION, 2005–2009)[22], to provide evidence that the associations reported within ACCORD are unlikely to be artifacts of reverse causality; 5) using the added number of events from both ACCORD and ACCORDION we tested effects of blood pressure variability on the primary CVD outcome using more granular subsets of baseline DBP and SBP; 6) we tested the role of variability in a subset of participants with no evidence of atrial fibrillation; 7) we assessed how limiting the time-dependent analysis to those with four or more visits with blood pressure measures, a more restrictive condition, may influence the CV result.
All statistical analyses were performed using R software 4.0.2 (http://www.r-project.com). A two-sided P < 0.05 was considered statistically significant.
RESULTS
After exclusion of those with two or fewer eligible visits, 9,856 participants were included in this post-hoc analysis. During the median 4.7-year ACCORD study period, a primary CVD outcome occurred in 879 individuals, a major CHD event occurred in 985 individuals, and a stroke occurred in 167 individuals. Our analysis included a mean of 12.7 (median of 13) blood pressure visit measures (SD = 3.8) and the cohort included a maximum of 21 visit measures per participant. Baseline characteristics among those who did or did not experience adverse events are presented in Table 1. Supplementary Figure 1 shows the mean SBP and DBP during the ACCORD study, depicting the protocol driven initial drop in blood pressures during the early phase of the trial.
Table 1.
Baseline characteristics in the ACCORD by primary outcome, major CHD, and total stroke.
| Primary CVD Outcome 1 | P-value | Major CHD 2 | P-value | Total stroke 3 | P-value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 62.6 (6.5) | 64.6 (7.1) | <0.001 | 62.6 (6.5) | 63.9 (7.2) | <0.001 | 62.7 (6.6) | 64.8 (7.0) | <0.001 |
| Intensive BP treatment (n, %) | 2,083 (23.4) | 175 (19.9) | 0.02 | 2,033 (23.3) | 225 (21.2) | 0.14 | 2,235 (23.1) | 31 (18.6) | 0.20 |
| Female (n, %) | 3,494 (39.2) | 265 (30.1) | <0.001 | 3,430 (39.3) | 329 (31.1) | <0.001 | 3,720 (38.4) | 58 (34.7) | 0.38 |
| Race (n, %) | 0.02 | <0.001 | 0.21 | ||||||
| Diabetes duration (years) | 10.6 (7.5) | 12.5 (8.4) | <0.001 | 10.6 (7.5) | 12.1 (8.3) | <0.001 | 10.8 (7.6) | 12.8 (8.1) | 0.001 |
| CVD History (n, %) | 2,914 (32.7) | 479 (54.5) | <0.001 | 2,771 (31.8) | 622 (58.7) | <0.001 | 3,361 (34.7) | 78 (46.7) | 0.002 |
| Smoker† (n, %) | 3,980 (51.2) | 437 (57.7) | 0.001 | 3,887 (51.0) | 530 (57.8) | <0.001 | 4,368 (51.7) | 79 (54.5) | 0.56 |
| BMI (kg/m 2 ) | 32.3 (5.4) | 32.0 (5.4) | 0.001 | 32.2 (5.4) | 32.4 (5.3) | 0.33 | 32.3 (5.4) | 31.2 (5.4) | 0.01 |
| DBP (mmHg) | 75 (10) | 73 (11) | <0.001 | 75 (10.5) | 73 (11.4) | <0.001 | 75 (10) | 75 (11) | 0.95 |
| SBP (mmHg) | 136 (17) | 138 (18) | 0.005 | 136 (16.9) | 137 (17.6) | 0.24 | 136 (17) | 141 (19) | <0.001 |
| HDL cholesterol (mg/dL) | 42 (11) | 40 (12.1) | <0.001 | 42 (11.6) | 40 (11.4) | <0.001 | 42 (11) | 41.5 (13) | 0.66 |
| LDL cholesterol (mg/dL) | 105 (34) | 106 (35) | 0.20 | 105 (33) | 105 (34) | 0.93 | 105 (34) | 108 (35) | 0.19 |
| Total cholesterol (mg/dL) | 183 (42) | 184 (44) | 0.44 | 183 (42) | 183 (42) | 0.70 | 183 (42) | 188 (49) | 0.12 |
| Triglycerides (mg/dL) | 189 (150) | 196 (134) | 0.21 | 189 (150) | 199 (136) | 0.04 | 190 (149) | 197 (149) | 0.54 |
| HbA1c (%) | 8.3 (1.0) | 8.5 (1.1) | <0.001 | 8.3 (1.1) | 8.4 (1.1) | 0.006 | 8.3 (1.0) | 8.8 (1.3) | <0.001 |
| Fasting glucose (mg/dL) | 174 (56) | 179 (58) | 0.02 | 175 (56) | 176 (57) | 0.40 | 175 (55.8) | 187 (64.8) | 0.09 |
| Albumin to creatinine ratio (mg/g) | 85.4 (312.1) | 197.3 (554.1) | <0.001 | 86.2 (315.0) | 173.1 (510.4) | <0.001 | 95.2 (353.0) | 239.4 (565.7) | <0.001 |
| eGFR (ml/min/1.73 m2) | 91.5 (27.2) | 86.9 (27.6) | <0.001 | 91.6 (27.2) | 87.5 (26.9) | <0.001 | 91.1 (27.2) | 87.5 (28.0) | 0.09 |
| Statin n (%) | 5,566 (62.5) | 515 (58.6) | 0.29 | 5,411 (62.0) | 609 (61.8) | <0.001 | 6,072 (62.7) | 89 (53.3) | 0.46 |
| Sulfonylurea n (%) | 4,080 (45.8) | 377 (42.9) | 0.46 | 4,004 (45.9) | 412 (41.8) | 0.82 | 4,440 (45.8) | 75 (44.9) | 0.31 |
| Total antihypertensives | 1.68 (1.20) | 1.97 (1.21) | <0.001 | 1.70 (1.20) | 1.92 (1.13) | 0.01 | 1.70 (1.20) | 1.89 (1.16) | 0.06 |
| Beta-blocker n (%) | 6,252 (70.2) | 457 (52.0) | <0.001 | 6,205 (71.1) | 481 (48.8) | <0.001 | 6,685 (69.0) | 100 (59.8) | 0.50 |
| Loop diuretic n (%) | 8,023 (90.1) | 672 (76.5) | <0.001 | 7,885 (90.4) | 747 (75.8) | <0.001 | 8,676 (89.5) | 128 (76.6) | 0.06 |
Data are means, SD or No. (%)
BMI: body mass index; DBP: diastolic blood pressure; SBP: systolic blood pressure; HDL: High-density lipoprotein; LDL: Low-density lipoprotein; CVD: Cardiovascular disease; eGFR: estimated glomerular filtration rate from four variable Modification of Diet in Renal Disease Study (MDRD) equation.
Primary composite CVD outcome is first occurrence of major cardiovascular event – cardiovascular death, non-fatal MI, or non-fatal stroke
Major CHD is a composite of fatal coronary artery disease events, non-fatal MI, or unstable angina
Total stroke includes both fatal and non-fatal stroke events
smoker: smoked more than 100 cigarettes during lifetime
Blood pressure variability and cardiovascular outcomes
To assess the relationship between blood pressure variability and outcomes, we determined age-adjusted HRs across quartiles of CV-SBP and CV-DBP. For the primary CVD outcome and major CHD (Figure 1), but not stroke, we observed a pattern of increasing risk with increasing quartiles of both CV-SBP and CV-DBP (p for trend < 0.001 for the primary CVD outcome and major CHD).
Figure 1:
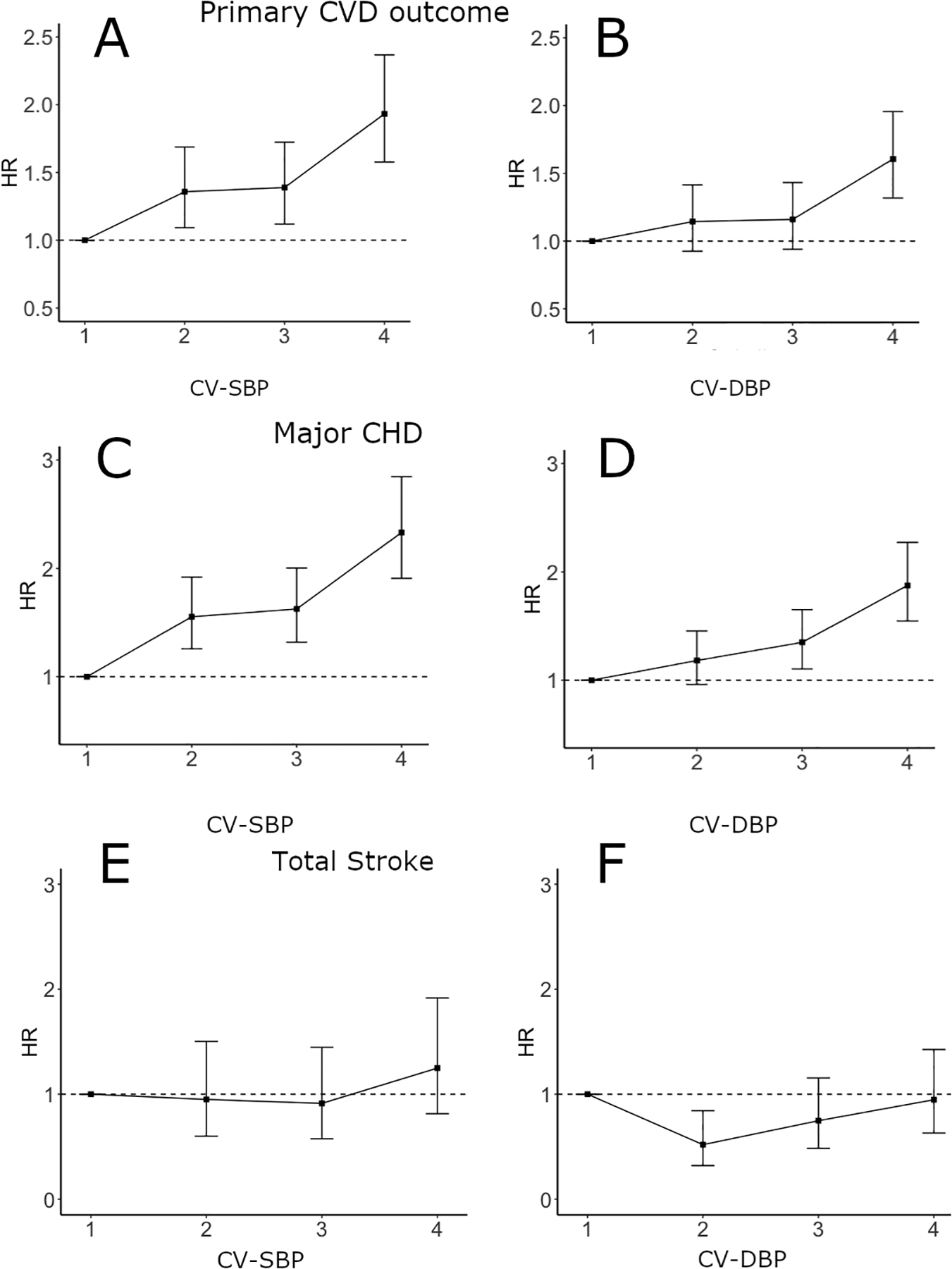
Age-adjusted hazard ratio (HR) estimates for risk of primary CVD outcome, major CHD, and total stroke by quartiles of blood pressure variability in ACCORD. Panels A and B show quartiles of CV-SBP and CV-DBP for the primary CVD outcome, respectively. Panels C and D show quartiles of CV-SBP and CV-DBP for major CHD, respectively. Panels E and F show quartiles of CV-SBP and CV-DBP for total stroke, respectively. Results of trend tests for panels A, B, C, and D are all p < 0.001.
After adjustment for age, variability of both SBP and DBP (as continuous variables) were significantly associated with the primary CVD outcome (Table 2) (CV-SBP: HR=1.13; CV-DBP: HR=1.19, p<0.001 for both). This association remained significant after adjusting for baseline covariates that differed between those who did and did not develop a primary outcome event (Model 2), as well as cumulative mean blood pressure (Model 3: CV-SBP, HR=1.07, p=0.002; CV-DBP, HR=1.13, p<0.001). A very similar pattern was observed in the analysis for major CHD (Model 3: CV-SBP HR=1.07, p=0.001; CV-DBP HR=1.13, p<0.001). In contrast, neither CV-SBP or CV-DBP were associated with risk of total stroke in adjusted models (Table 2). These major findings were consistent in the analyses using ARV-SBP and ARV-DBP (Supplementary Table 2). There were no significant differences by primary CVD event status for use of statins, sulfonylureas, or meglitinides. Adjustment for total number of antihypertensive medications, or use of beta-blockers or loop diuretics that differed between event groups, did not appreciably influence the HRs for variability. This was also true for adjustment for biguanides and platelet aggregation inhibitors. Associations between baseline covariates and SBP variability (CV-SBP) are presented in Supplementary Table 3.
Table 2.
Hazard ratios for the association of blood pressure variability with cardiovascular outcomes.
| Model-1 Age Adjustment |
Model-2 Multivariate Adjustment |
Model-3 Model 2 + Cumulative mean BP |
||||
|---|---|---|---|---|---|---|
| Primary CVD outcome (n = 879) | HR (95% C.I.) | P-value | HR (95% C.I.) | P-value | HR (95% C.I.) | P-value |
| CV-SBP | 1.13 (1.09–1.17) | <0.001 | 1.08 (1.04–1.13) | <0.001 | 1.07 (1.03–1.11) | 0.002 |
| CV-DBP | 1.19 (1.12–1.25) | <0.001 | 1.13 (1.05–1.20) | <0.001 | 1.13 (1.05–1.20) | <0.001 |
| Major CHD (n = 985) | ||||||
| CV-SBP | 1.14 (1.11–1.18) | <0.001 | 1.08 (1.04–1.12) | <0.001 | 1.07 (1.03–1.12) | 0.001 |
| CV-DBP | 1.22 (1.16–1.28) | <0.001 | 1.13 (1.06–1.20) | <0.001 | 1.13 (1.06–1.20) | <0.001 |
| Total stroke (n = 167) | ||||||
| CV-SBP | 1.11 (1.01–1.21) | 0.03 | 0.97 (0.85–1.10) | 0.63 | - | - |
| CV-DBP | 1.05 (0.92–1.21) | 0.46 | 1.00 (0.87–1.16) | 0.99 | - | - |
Hazard ratios (HR), (95% confidence interval (95% CI)), and p-values estimated by Cox proportional hazards model. Blood pressure variables were adjusted for age (Model 1), baseline factors from Table 1 that differed significantly between those who did and did not develop each distinct event (Model 2) and additionally for cumulative mean of blood pressure excluding the baseline BP. Model 3 was not computed if Model 2 HRs were not statistically significant. P-values <0.05 (bold font) are considered significant. CV: coefficient of variation. The primary outcome is a composite of CVD death, non-fatal MI and non-fatal stroke. Major CHD includes fatal coronary artery disease events, non-fatal MI, and unstable angina.
In a sensitivity analysis using blood pressure variability during ACCORD, we tested for associations with primary CVD outcomes (n=671) that developed during the subsequent ACCORDION follow-up study (2005–2009). In this landmark period approach, both SBP and DBP variability were again associated with risk of the primary CVD outcome in a fully adjusted model that included cumulative mean blood pressure (CV-SBP: HR=1.17; CV-DBP: HR=1.15, p<0.001 for both).
Blood pressure variability by strata of baseline blood pressure
To examine whether blood pressure levels might modify effects of blood pressure variability, we first tested for, and found, an interaction between baseline SBP and DBP blood pressure (above vs. below the median) and blood pressure variability for the primary CVD outcome (CV-SBP p-for-interaction = 0.0013; CV-DBP p-for-interaction = 0.021). We noted very similar contrasts in effects of blood pressure variability when we used median “on-study” blood pressure (CV-SBP p-for-interaction = 0.0008; CV-DBP p-for-interaction = 0.046).
We then examined effects of blood pressure variability across clinically relevant baseline blood pressure ranges. The CV-SBP was significantly associated with the primary CVD outcome in the <120 and >140 mmHg SBP strata (HRs 1.24 and 1.15, p=0.02 and p=0.01, respectively), but not in the 120–139 mmHg SBP strata (HR=1.06, p=0.07) (Table 3). Similarly, a significant association of CV-DBP with the primary CVD outcome was found in the <70 mmHg and >80 mmHg baseline DBP strata (respectively, HRs 1.25 and 1.23, p=0.002 and p<0.001) but not in the 70–79 mmHg baseline DBP stratum (HR=1.12, p=0.09). Results for the major CHD outcome by strata of baseline SBP and DBP were very similar (Table 3). In contrast, the CV-SBP or CV-DBP were not associated with risk of total stroke in any of the baseline blood pressure strata (Table 3). The patterns of risk by strata described for the primary CVD outcome and major CHD were consistent in the analysis of ARV-SBP and ARV-DBP (Supplementary Table 4).
Table 3.
Hazard ratios for the association of blood pressure variability with cardiovascular outcomes by baseline blood pressure levels.
| Model-1 Age Adjustment |
Model-2 Multivariate Adjustment |
Model-3 Model 2 + Cumulative mean BP |
|||||
|---|---|---|---|---|---|---|---|
| Primary CVD outcome | No. of events | HR (95% C.I.) | P-value | HR (95% C.I.) | P-value | HR (95% C.I.) | P-value |
| CV-SBP | |||||||
| SBP > 140 mmHg | n = 355 | 1.09 (0.99–1.19) | 0.07 | 1.14 (1.02–1.28) | 0.02 | 1.15 (1.02–1.29) | 0.02 |
| SBP 120–139 mmHg | n = 361 | 1.12 (1.07–1.16) | <0.001 | 1.07 (1.01–1.12) | 0.01 | 1.06 (0.99–1.12) | 0.07 |
| SBP < 120 mmHg | n = 134 | 1.38 (1.21–1.57) | <0.001 | 1.25 (1.06–1.48) | 0.008 | 1.24 (1.05–1.46) | 0.01 |
| CV-DBP | |||||||
| DBP > 80 mmHg | n = 230 | 1.20 (1.07–1.35) | 0.002 | 1.19 (1.05–1.34) | 0.005 | 1.23 (1.08–1.40) | 0.002 |
| DBP 70–79 mmHg | n = 280 | 1.21 (1.10–1.33) | <0.001 | 1.10 (0.97–1.25) | 0.13 | 1.12 (0.98–1.27) | 0.09 |
| DBP < 70 mmHg | n = 339 | 1.32 (1.22–1.43) | <0.001 | 1.25 (1.15–1.36) | <0.001 | 1.25 (1.15–1.36) | <0.001 |
| Major CHD | |||||||
| CV-SBP | |||||||
| SBP > 140 mmHg | n = 382 | 1.15 (1.06–1.25) | 0.001 | 1.12 (1.01–1.25) | 0.04 | 1.13 (1.02–1.26) | 0.03 |
| SBP 120–139 mmHg | n = 425 | 1.12 (1.08–1.15) | <0.001 | 1.05 (0.99–1.11) | 0.06 | 1.05 (0.99–1.11) | 0.08 |
| SBP < 120 mmHg | n = 150 | 1.39 (1.23–1.57) | <0.001 | 1.22 (1.04–1.43) | 0.01 | 1.21 (1.03–1.42) | 0.02 |
| CV-DBP | |||||||
| DBP > 80 mmHg | n = 236 | 1.26 (1.13–1.40) | <0.001 | 1.21 (1.08–1.34) | <0.001 | 1.25 (1.11–1.41) | <0.001 |
| DBP 70–79 mmHg | n = 326 | 1.18 (1.09–1.29) | <0.001 | 1.11 (0.99–1.24) | 0.06 | 1.10 (0.98–1.23) | 0.11 |
| DBP <70 mmHg | n = 387 | 1.32 (1.22–1.42) | <0.001 | 1.23 (1.12–1.35) | <0.001 | 1.23 (1.12–1.35) | <0.001 |
| Total stroke | |||||||
| CV-SBP | |||||||
| SBP > 140 mmHg | n = 82 | 0.98 (0.82–1.18) | 0.86 | 0.94 (0.81–1.11) | 0.48 | 0.99 (0.85–1.18) | 0.99 |
| SBP 120–139 mmHg | n = 61 | 1.09 (0.99–1.19) | 0.07 | 1.08 (0.97–1.20) | 0.18 | 1.02 (0.90–1.15) | 0.78 |
| SBP < 120 mmHg | n = 24 | 1.32 (0.98–1.76) | 0.07 | 1.26 (0.95–1.68) | 0.11 | 1.05 (0.75–1.48) | 0.75 |
| CV-DBP | |||||||
| DBP > 80 mmHg | n = 57 | 1.11 (0.95–1.30) | 0.18 | 1.11 (0.94–1.29) | 0.21 | 1.11 (0.96–1.29) | 0.17 |
| DBP 70–79 mmHg | n = 48 | 1.02 (0.79–1.32) | 0.86 | 0.95 (0.71–1.28) | 0.76 | 1.01 (0.76–1.33) | 0.93 |
| DBP < 70 mmHg | n = 57 | 1.13 (0.90–1.40) | 0.27 | 1.09 (0.87–1.37) | 0.46 | 1.12 (0.91–1.36) | 0.49 |
Hazard ratios (HR), (95% confidence interval (95% CI)), and p-values estimated by Cox proportional hazards model. Blood pressure variables were adjusted for age (Model 1), baseline factors that differed significantly between those who did and did not develop each event (Model 2) and additionally for cumulative mean of blood pressure excluding the baseline BP. P-values <0.05 (bold font) are considered significant. CV: coefficient of variation.
We also combined ACCORD and ACCORDION follow-up periods to examine the effect of baseline blood pressure categories over an extended time period. Hazard ratios for the primary CVD outcome in those with DBP <70 mmHg (n=596; CV-DBP HR = 1.22, p<0.001) and DBP >80 mmHg (n=438; CV-DBP HR = 1.15, p=0.002) were similar to effects observed in our primary ACCORD analysis. In this combined analysis, the association we noted in the analysis limited to ACCORD was attenuated in those with high baseline SBP. However, this analysis, which had greater power to examine a broader range of blood pressure, revealed that even more extreme low levels of baseline SBP exacerbated the relationship between CV-SBP and the primary CVD outcome (e.g. for baseline SBP < 110 mmHg, Model 3 CV-SBP HR = 1.46, p < 0.001) (Supplementary Table 5).
To explore the hypothesis that effects of variability may be due in part to reduced coronary perfusion during diastole, we examined whether prior CVD history influenced CV-DBP associations, and whether this was further strengthened by low baseline blood pressure (defined as <120 mmHg SBP and <70 mmHg DBP) (Table 4). Interestingly, associations of CV-DBP with CVD risk were generally weaker in those without CVD history, even in those with low baseline blood pressures. Conversely, CV-DBP was strongly associated with risk of the primary CVD outcome or a major CHD in those with CVD history, and the association was heightened further in those with low baseline blood pressures (Table 4). The strongest association was observed between CV-DBP and major CHD in those with CVD history and low baseline blood pressure (HR=1.40 in the fully adjusted model, p<0.0001).
Table 4.
Hazard ratios for the association of diastolic blood pressure variability with the primary outcome and major CHD, in those with CVD history at baseline, those with CVD history and low baseline blood pressure (<120 mmHg SBP & <70 mmHg DBP), and those without CVD history at baseline in each category.
| Model-1 Age adjustment |
Model-2 Multivariate adjustment |
Model-3 Model 2 + cumulative mean BP |
||||
|---|---|---|---|---|---|---|
| Primary CVD outcome | ||||||
| With CVD history | ||||||
| All (n=479) | 1.20 (1.12–1.28) | <0.0001 | 1.20 (1.11–1.30) | <0.0001 | 1.21 (1.12–1.30) | <0.0001 |
| Low BP (n=73) | 1.33 (1.15–1.53) | 0.0001 | 1.33 (1.15–1.53) | 0.0001 | 1.29 (1.13–1.48) | 0.0002 |
| No CVD history | ||||||
| All (n = 400) | 1.07 (0.98–1.18) | 0.10 | 1.09 (0.98–1.20) | 0.11 | 1.09 (0.98–1.20) | 0.10 |
| Low BP (n=36) | 1.22 (0.93–1.62) | 0.14 | 1.25 (0.95–1.64) | 0.12 | 1.19 (0.90–1.57) | 0.22 |
| Major CHD | ||||||
| With CVD history | ||||||
| All (n=572) | 1.22 (1.14–1.29) | <0.0001 | 1.22 (1.14–1.30) | <0.0001 | 1.22 (1.13–1.31) | <0.0001 |
| Low BP (n=86) | 1.42 (1.26–1.59) | <0.0001 | 1.40 (1.22–1.60) | <0.0001 | 1.40 (1.22–1.61) | <0.0001 |
| No CVD history | ||||||
| All (n=413) | 1.17 (1.07–1.27) | 0.0004 | 1.14 (1.03–1.25) | 0.01 | 1.14 (1.03–1.25) | 0.01 |
| Low BP (n=37) | 1.06 (0.79–1.43) | 0.69 | 1.04 (0.77–1.40) | 0.79 | 1.02 (0.75–1.38) | 0.89 |
Hazard ratios (HR), (95% confidence interval (95% CI)), and p-values estimated by Cox proportional hazards model for coefficient of variation of DBP. Blood pressure variables were adjusted for age (Model 1), baseline factors that differed significantly between those who did and did not develop each event (Model 2) and additionally for cumulative mean of blood pressure excluding the baseline BP. P-values <0.05 (bold font) are considered significant.
Sensitivity analyses
If decreased coronary perfusion was a contributor to CVD risk conveyed by blood pressure variability, “dips” in blood pressure would be expected to be more harmful. We therefore calculated “variability area” above or below participants’ mean blood pressure preceding an event. For SBP, dips in blood pressure were associated with risk of the primary CVD outcome, major CHD, and total stroke (p<0.001, p<0.001, and p=0.022, respectively), but elevations in blood pressure were not (p > 0.6 for all). Dips in DBP were also related to the primary CVD outcome, major CHD, and total stroke (p<0.001 for all), but we did not observe an association with elevations in DBP (p > 0.4 for all).
To test whether adherence to blood pressure medication might account for the relationship between blood pressure variability and CVD, we also included in fully adjusted models the reported adherence with these medications during the ACCORD study. This adjustment had little effect on the associations of blood pressure variability with the primary CVD outcome. Similarly, when added to Model 3, adjustment for various baseline comorbidities in a modified comorbidity index also had a negligible effect.
When restricting the ACCORD population to those participants who had a normal echocardiogram and thus no evidence of atrial fibrillation or flutter, a primary CVD outcome occurred in 694 participants. Even in this subset free of atrial fibrillation, CV-SBP and CV-DBP were significant predictors of risk of the primary CVD outcome (Model 3, CV-SBP: HR = 1.08, p < 0.001; CV-DBP: HR = 1.12, p = 0.002). Finally, in a subset of the cohort that had four or more office visits of blood pressure, the significant associations we reported held (age-adjusted CV-SBP: HR = 1.14, p < 0.001; CV-DBP: HR = 1.25, p < 0.001).
DISCUSSION
In this comprehensive post-hoc evaluation in the ACCORD trial, blood pressure variability was significantly associated with risk for a spectrum of cardiovascular events, with an especially prominent relationship with coronary heart disease events. Consistent with reports from other large cohort studies,[1, 2, 5] the effect was robust to adjustment for baseline risk factors and cumulative mean blood pressure. Importantly, our analysis of SBP and DBP variability by levels of baseline blood pressure provided novel evidence of more robust effects of blood pressure variability at low baseline blood pressures. These associations were independent of baseline comorbidities and blood pressure medication adherence during the trial. The significant relationships also persisted in a sensitivity analysis including long-term observational ACCORDION follow-up, reducing the likelihood that these findings are due to reverse causality.
The extent to which baseline or on-study blood pressure level modifies the association between blood pressure variability and adverse outcomes has been scrutinized in some recent studies using cohort-specific threshold values. In the VALUE trial, Mehlum and colleagues report a significantly stronger association between SBP variability and cardiovascular risk in participants with treatment SBP below the median,[11] though the same test was not performed for DBP. Moreover, in the PROSPER trial, DBP variability was more strongly associated with vascular mortality among those with SBP below the median.[12] However, these trials did not address whether the relationship between blood pressure variability and CVD risk differs in clinically defined blood pressure categories, nor explore potential mechanisms underlying these findings. In our prior analysis using ACCORD data, we observed a stepwise increase in risk of heart failure for both SBP and DBP variability in those with lower SBP and DBP levels.[6] The present finding that variability in ACCORD participants with lower DBP or SBP was associated with elevated risk of the primary CVD outcome and CHD events compared with those in the higher but normal blood pressure stratum provides novel evidence for the dependence of this association on absolute blood pressure level categories.
Insofar as risk from increased blood pressure variability was also elevated in participants with high SBP and DBP, our study appears to be the first to report a potential U-shaped pattern for variability effects by baseline blood pressure level. As this may have implications for both understanding mechanisms of blood pressure variability risk and treatment approaches, these results should be confirmed in other populations. However, in a recent investigation by Ferreira and colleagues of patients in the Eplerenone Post-Acute Myocardial Infarction with Heart Failure Efficacy and Survival Study (EPHESUS), both low and high levels of blood pressure variability appeared to be linked to increased risk of cardiovascular mortality.[23] These data, along with our current and prior findings[6] of a blood pressure level and blood pressure variability interaction, suggest that the relationship of visit-to-visit blood pressure variability with cardiovascular risk is more complex than previously reported and possibly non-linear.[23, 24] Developing clinical strategies for evaluating blood pressure variability, if proven to be causally linked with CVD, will depend on a clearer understanding of these more nuanced risk patterns.
Our finding that dips, rather than elevations, in SBP and DBP appear to be driving the association between variability and CVD is consistent with patterns observed previously in two clinical trials. In the STABILITY trial, dips in blood pressure appeared to be driving the effect on adverse cardiovascular outcomes. Also, DBP variability in the STABILITY trial was most potent in those in the lowest tertile of DBP.[13] Our findings also echo data on blood pressure instability in the Health, Aging, and Body Composition Study, where episodes of decreased DBP were associated with a 30% increase in cardiovascular mortality.[25]
Reductions in aortic distensibility,[7] increased arterial stiffness,[26] autonomic dysfunction,[27] impaired endothelial function,[9] and aggravation of atherosclerosis[28, 29] have been posited as potential explanations for increased cardiovascular risk in those with increased blood pressure variability. The enhancing effect of low blood pressure levels on this association suggests other mechanisms. One possibility is that blood pressure variability—particularly transient decreases in DBP—may put cardiac tissue at increased risk of coronary hypoperfusion[13] and exacerbate myocardial ischemia. Notably, in the Atherosclerosis Risk in Communities (ARIC) Study, McEvoy and colleagues reported that low DBP in healthy individuals was associated with damage to myocardium as estimated by increased levels of high-sensitivity cardiac troponin-T.[14] In a recent investigation from the SPRINT group, DBP variability was specifically linked to potential hypoperfusion-related adverse events, including acute kidney injury.[30] This hypoperfusion hypothesis is further supported by the fact that 1) in the present study, the effects of blood pressure variability were greatest at low levels of blood pressure, 2) the risk was enhanced in those with low DBP and prior CVD—consistent with the key role of coronary artery anatomic and/or functional narrowing in coronary hypoperfusion[31]; 3) low DBP was associated with cardiac events but not with risk of stroke in the ARIC study;[14] and 4) blood pressure variability showed no significant interaction with blood pressure level for stroke events in the current analysis of ACCORD.
Prior investigators have speculated that differences between relationships of variability with coronary outcomes versus stroke might be attributable to chance, inconsistent follow-up duration, different covariates included in the model,[32] or uneven histories of myocardial infarction.[11] Alternatively, these differences may in fact reflect physiologic distinctions in these two vascular beds. If the mechanisms linking blood pressure variability with risk of stroke or cardiac outcomes were similar, we would expect low blood pressure levels to also exacerbate the relationship between variability and stroke. The lack of an association with stroke in the current ACCORD data suggests that hypoperfusion may be particularly relevant for cardiac outcomes. This is not surprising as cerebral tissue may be uniquely protected from transient declines in blood pressure[33] via autoregulatory mechanisms that maintain cerebral blood flow,[34, 35] even in the setting of low blood pressure levels.
The current study has several strengths. Besides careful adjudication of outcomes and frequent blood pressure measurements, the ACCORD trial involved detailed collection of data on comorbidities and medication use. Neither of these potential confounders influenced the relationship between blood pressure variability and CVD outcomes. The large sample size allowed testing of associations between blood pressure variability and CVD outcomes across standard blood pressure categories compared to cohort-dependent thresholds in prior studies. In addition, patterns we reported for CV were confirmed in analysis using the ARV metric. Use of time-dependent estimates in Cox modeling as our major analysis approach is another important advantage of our statistical design; this method permits inclusion of blood pressure measures up to the time of the event in question and has been deemed an effective approach in visit-to-visit variability analysis.[36] Moreover, this time-dependent approach avoids some pitfalls that have been identified in prior blood pressure variability analysis,[37] including conditioning on the future.
One limitation of the current project is that the ACCORD cohort enrolled persons with advanced T2D and high CVD risk, so we do not know if these findings are generalizable to the broad diabetes population. Despite detailed collection of comorbidities, the information on certain serious conditions such as cancer was not collected and therefore not included in a modified comorbidity index. Since terminal digit rounding was employed in the collection of blood pressure measures in ACCORD, this is another potential limitation, as this practice can in some cases misclassify the prevalence of hypertension in a study sample;[38] however, preferred use of an automatic blood pressure device as in ACCORD mitigates the influence[39] of this practice. Reverse causality has been noted as a potential bias in variability studies: e.g., arterial stiffness and target organ damage presage cardiovascular complications but can also lead to increased blood pressure variability.[40] However, we found similar results using the landmark approach in predicting observational ACCORDION events, which signifies that reverse causality is unlikely to explain our findings. Although there were several advantages to including ACCORDION data to confirm key findings in ACCORD, we did not incorporate ACCORDION outcomes in the main analysis because of small number of additional blood pressure measurements with substantially longer between-visit intervals. Furthermore, the trial-defined CVD risk factor intervention was discontinued during the ACCORDION follow-up period. As the ACCORD participants were highly adherent to trial medications, our ability to test nuances in medication adherence in our variability model was somewhat limited by this relatively homogenous level of adherence.
In conclusion, our findings provide further evidence of a link between visit-to-visit variability of both systolic and diastolic blood pressure and cardiovascular risk. Our demonstration that blood pressure variability was heightened in those with low blood pressure, particularly in those with prior CVD, and was related to dips in blood pressure provides support for the hypothesis that blood pressure variability could contribute to cardiac injury by exacerbating coronary hypoperfusion. These data appear to support cautions raised by both the American Diabetes Association[41] and the European Society of Hypertension[42] concerning increased risk of cardiovascular events in some individuals undergoing more ambitious blood pressure lowering targets (i.e. SBP < 120 mmHg), suggesting that teasing out the role of blood pressure variability in this excess risk may facilitate a more personalized blood pressure target. Our finding that blood pressure variability may also be associated with CVD risk at higher levels of blood pressure is novel and needs confirmation in additional cohorts. These results contribute to increased understanding of the potential relevance of blood pressure variability in CVD risk and may inform future recommendations for achieving optimal blood pressure control.
Supplementary Material
Acknowledgements
The contents of this study do not represent the views of the Department of Veterans Affairs or the United States Government. The contents are the sole responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Funding
Research reported in this publication was supported by grants from the National Institutes of Health / National Heart, Lung, and Blood Institute (1F32HL156626 to D.S.N.; 1R21HL150374 to J.J.Z., P.D.R.; 1R21HL150268 to J.K., P.D.R.).
Footnotes
Other presentations
The abstract of this project will be presented at the 81st Scientific Sessions of the American Diabetes Association (June 2021).
Conflicts of interest
The authors report no relevant financial conflicts of interest.
REFERENCES
- 1.Stevens SL, Wood S, Koshiaris C, Law K, Glasziou P, Stevens RJ, McManus RJ (2016) Blood pressure variability and cardiovascular disease: Systematic review and meta-analysis. BMJ. 10.1136/bmj.i4098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Muntner P, Whittle J, Lynch AI, et al. (2015) Visit-to-visit variability of blood pressure and coronary heart disease, stroke, heart failure, and mortality a cohort study. Ann Intern Med 163:329–338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gosmanova EO, Mikkelsen MK, Molnar MZ, Lu JL, Yessayan LT, Kalantar-Zadeh K, Kovesdy CP (2016) Association of Systolic Blood Pressure Variability With Mortality, Coronary Heart Disease, Stroke, and Renal Disease. J Am Coll Cardiol. 10.1016/j.jacc.2016.06.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rothwell PM, Howard SC, Dolan E, O’Brien E, Dobson JE, Dahlöf B, Sever PS, Poulter NR (2010) Prognostic significance of visit-to-visit variability, maximum systolic blood pressure, and episodic hypertension. Lancet. 10.1016/S0140-6736(10)60308-X [DOI] [PubMed] [Google Scholar]
- 5.Chiriacò M, Pateras K, Virdis A, et al. (2019) Association between blood pressure variability, cardiovascular disease and mortality in type 2 diabetes: A systematic review and meta‐analysis. Diabetes, Obes Metab. 10.1111/dom.13828 [DOI] [PubMed] [Google Scholar]
- 6.Nuyujukian DS, Koska J, Bahn G, Reaven PD, Zhou JJ (2020) Blood Pressure Variability and Risk of Heart Failure in ACCORD and the VADT. Diabetes Care dc192540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shimbo D, Shea S, McClelland RL, Viera AJ, Mann D, Newman J, Lima J, Polak JF, Psaty BM, Muntner P (2013) Associations of aortic distensibility and arterial elasticity with long-term visit-to-visit blood pressure variability: The multi-ethnic study of atherosclerosis (MESA). Am J Hypertens. 10.1093/ajh/hpt040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou TL, Henry RMA, Stehouwer CDA, Van Sloten TT, Reesink KD, Kroon AA (2018) Blood pressure variability, arterial stiffness, and arterial remodeling the Maastricht study. Hypertension. 10.1161/HYPERTENSIONAHA.118.11325 [DOI] [PubMed] [Google Scholar]
- 9.Diaz KM, Veerabhadrappa P, Kashem MA, Feairheller DL, Sturgeon KM, Williamson ST, Crabbe DL, Brown MD (2012) Relationship of visit-to-visit and ambulatory blood pressure variability to vascular function in African Americans. Hypertens Res. 10.1038/hr.2011.135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nwabuo CC, Yano Y, Moreira HT, et al. (2020) Association Between Visit-to-Visit Blood Pressure Variability in Early Adulthood and Myocardial Structure and Function in Later Life. JAMA Cardiol. 10.1001/jamacardio.2020.0799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mehlum MH, Liestøl K, Kjeldsen SE, Julius S, Hua TA, Rothwell PM, Mancia G, Parati G, Weber MA, Berge E (2018) Blood pressure variability and risk of cardiovascular events and death in patients with hypertension and different baseline risks. Eur Heart J 39:2243–2251 [DOI] [PubMed] [Google Scholar]
- 12.Poortvliet RKE, Ford I, Lloyd SM, et al. (2012) Blood Pressure Variability and Cardiovascular Risk in the PROspective Study of Pravastatin in the Elderly at Risk (PROSPER). PLoS One. 10.1371/journal.pone.0052438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vidal-Petiot E, Stebbins A, Chiswell K, et al. (2017) Visit-to-visit variability of blood pressure and cardiovascular outcomes in patients with stable coronary heart disease. Insights from the STABILITY trial. Eur Heart J. 10.1093/eurheartj/ehx250 [DOI] [PubMed] [Google Scholar]
- 14.McEvoy JW, Chen Y, Rawlings A, Hoogeveen RC, Ballantyne CM, Blumenthal RS, Coresh J, Selvin E (2016) Diastolic Blood Pressure, Subclinical Myocardial Damage, and Cardiac Events: Implications for Blood Pressure Control. J Am Coll Cardiol 68:1713–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Espeland M, Probstfield J, Hire D, et al. (2015) Systolic blood pressure control among individuals with type 2 diabetes: A comparative effectiveness analysis of three interventions. Am J Hypertens 28:995–1009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aronow WS (2016) Orthostatic Hypotension in Diabetics in the ACCORD (Action to Control Cardiovascular Risk in Diabetes) Blood Pressure Trial. Hypertension 68:851–852 [DOI] [PubMed] [Google Scholar]
- 17.Buse JB, Friedewald WT, Bigger JT, et al. (2007) Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial: Design and methods. Am J Cardiol. 10.1016/j.amjcard.2007.03.003 [DOI] [PubMed] [Google Scholar]
- 18.Cushman WC, Grimm RH, Cutler JA, et al. (2007) Rationale and Design for the Blood Pressure Intervention of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial. Am J Cardiol. 10.1016/j.amjcard.2007.03.005 [DOI] [PubMed] [Google Scholar]
- 19.Zhou JJ, Schwenke DC, Bahn G, Reaven P (2018) Glycemic variation and cardiovascular risk in the Veterans Affairs Diabetes trial. Diabetes Care 41:2187–2194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou JJ, Koska J, Bahn G, Reaven P (2019) Glycaemic variation is a predictor of all-cause mortality in the Veteran Affairs Diabetes Trial. Diabetes Vasc Dis Res 16:178–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou JJ, Coleman R, Holman RR, Reaven P (2020) Long-term glucose variability and risk of nephropathy complication in UKPDS, ACCORD and VADT trials. Diabetologia. 10.1007/s00125-020-05273-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerstein HC, Beavers DP, Bertoni AG, et al. (2016) Nine-year effects of 3.7 years of intensive glycemic control on cardiovascular outcomes. Diabetes Care. 10.2337/dc15-2283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferreira JP, Duarte K, Pitt B, Dickstein K, McMurray JJV, Zannad F, Rossignol P (2018) Visit-to-visit blood pressure variation is associated with outcomes in a U-shaped fashion in patients with myocardial infarction complicated with systolic dysfunction and/or heart failure: Findings from the EPHESUS and OPTIMAAL trials. J Hypertens. 10.1097/HJH.0000000000001742 [DOI] [PubMed] [Google Scholar]
- 24.Rosei EA, Chiarini G, Rizzoni D (2020) How important is blood pressure variability? Eur Hear J Suppl. 10.1093/eurheartj/suaa061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu C, Shlipak MG, Stawski RS, Peralta CA, Psaty BM, Harris TB, Satterfield S, Shiroma EJ, Newman AB, Odden MC (2017) Visit-to-Visit Blood Pressure Variability and Mortality and Cardiovascular Outcomes Among Older Adults: The Health, Aging, and Body Composition Study. Am J Hypertens. 10.1093/ajh/hpw106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee HT, Lim YH, Kim BK, et al. (2011) The relationship between ambulatory arterial stiffness index and blood pressure variability in hypertensive patients. Korean Circ J. 10.4070/kcj.2011.41.5.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Spallone V (2018) Blood Pressure Variability and Autonomic Dysfunction. Curr Diab Rep. 10.1007/s11892-018-1108-z [DOI] [PubMed] [Google Scholar]
- 28.Nagai M, Hoshide S, Ishikawa J, Shimada K, Kario K (2011) Visit-to-visit blood pressure variations: New independent determinants for carotid artery measures in the elderly at high risk of cardiovascular disease. J Am Soc Hypertens 5:184–192 [DOI] [PubMed] [Google Scholar]
- 29.Okada R, Okada A, Okada T, Nanasato M, Wakai K (2014) Visit-to-visit blood pressure variability is a marker of cardiac diastolic function and carotid atherosclerosis. BMC Cardiovasc Disord. 10.1186/1471-2261-14-188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mezue K, Goyal A, Pressman GS, Matthew R, Horrow JC, Rangaswami J (2018) Blood pressure variability predicts adverse events and cardiovascular outcomes in SPRINT. J Clin Hypertens. 10.1111/jch.13346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bache RJ, Schwartz JS (1982) Effect of perfusion pressure distal to a coronary stenosis on transmural myocardial blood flow. Circulation. 10.1161/01.CIR.65.5.928 [DOI] [PubMed] [Google Scholar]
- 32.Hata J, Arima H, Rothwell PM, et al. (2013) Effects of visit-to-visit variability in systolic blood pressure on macrovascular and microvascular complications in patients with type 2 diabetes mellitus: The advance trial. Circulation. 10.1161/CIRCULATIONAHA.113.002717 [DOI] [PubMed] [Google Scholar]
- 33.Silverman A, Petersen NH (2020) Physiology, Cerebral Autoregulation. [PubMed]
- 34.Harms MPM, Finucane C, Pérez-Denia L, Jurachek S, van Wijnen VK, Lipsitz LA, van Lieshout JJ, Wieling W (2021) Systemic and cerebral circulatory adjustment within the first 60 s after active standing: An integrative physiological view. Auton Neurosci Basic Clin. 10.1016/j.autneu.2020.102756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castro P, Azevedo E, Sorond F (2018) Cerebral Autoregulation in Stroke. Curr Atheroscler Rep. 10.1007/s11883-018-0739-5 [DOI] [PubMed] [Google Scholar]
- 36.De Courson H, Leffondré K, Tzourio C (2018) Blood pressure variability and risk of cardiovascular event: Is it appropriate to use the future for predicting the present? Eur Heart J 39:4220. [DOI] [PubMed] [Google Scholar]
- 37.Torp-Pedersen C, Mortensen RN, Jeppesen J, Gerds TA (2018) Blood pressure and the uncertainty of prediction using hazard ratio. Eur Heart J 39:4219. [DOI] [PubMed] [Google Scholar]
- 38.Wen SW, Kramer MS, Hoey J, Hanley JA, Usher RH (1993) Terminal digit preference, random error, and bias in routine clinical measurement of blood pressure. J Clin Epidemiol. 10.1016/0895-4356(93)90118-K [DOI] [PubMed] [Google Scholar]
- 39.Greiver M, Kalia S, Voruganti T, et al. (2019) Trends in end digit preference for blood pressure and associations with cardiovascular outcomes in Canadian and UK primary care: A retrospective observational study. BMJ Open 9:e024970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hansen TW, Li Y, Staessen JA (2009) Blood pressure variability remains an elusive predictor of cardiovascular outcome. Am J Hypertens. 10.1038/ajh.2008.322 [DOI] [PubMed] [Google Scholar]
- 41.De Boer IH, Bangalore S, Benetos A, Davis AM, Michos ED, Muntner P, Rossing P, Zoungas S, Bakris G (2017) Diabetes and hypertension: A position statement by the American diabetes association. Diabetes Care. 10.2337/dci17-0026 [DOI] [PubMed] [Google Scholar]
- 42.Williams B, Mancia G, Spiering W, et al. (2018) 2018 ESC/ESH Guidelines for the management of arterial hypertension. The task force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens 36:1953–2041 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


