Abstract
MELD-Na appears to disadvantage women awaiting liver transplant by underestimating their mortality rate. Fixing this problem involves: (1) estimating the magnitude of this disadvantage separately for each MELD-Na, (2) designing a correction for each MELD-Na, and (3) evaluating corrections to MELD-Na using simulated allocation. Using Kaplan-Meier modeling, we calculated 90-day without-transplant survival for men and women, separately at each MELD-Na. For most scores between 15 and 35, without-transplant survival was higher for men by 0 to 5 percentage points. We tested two proposed corrections to MELD-Na (MELD-Na-MDRD and MELD-GRAIL-Na), and one correction we developed (MELD-Na-Shift) to target the differences we quantified in survival across the MELD-Na spectrum. In terms of without-transplant survival, MELD-Na-MDRD overcorrected sex differences while MELD-GRAIL-Na and MELD-Na-Shift eliminated them. Estimating the impact of implementing these corrections with the liver simulated allocation model, we found that MELD-Na-Shift alone eliminated sex disparity in transplant rates (p=0.4044) and mortality rates (p=0.7070); transplant rates and mortality rates were overcorrected by MELD-Na-MDRD (p=0.0025, p=0.0006) and MELD-GRAIL-Na (p=0.0079, p=0.0005). We designed a corrected MELD-Na that eliminates sex disparities in without-transplant survival, but allocation changes directing smaller livers to shorter candidates may also be needed to equalize womens’ access to liver transplant.
1. INTRODUCTION
Women are more likely than men to die on the liver transplant waitlist, more likely to be removed from the waitlist for being “too sick” for transplant, and less likely to receive a transplant (1-6). Some of these sex differences might stem from lower serum creatinine (and hence lower MELD-Na) for women versus men with similar renal dysfunction (1, 3, 5, 7-12). However, the contribution of creatinine to MELD-Na varies across the score spectrum because creatinine measurements are rounded up to 1.0 and capped at 4.0 mg/dL (13), so it is likely that sex differences also vary across the MELD-Na score spectrum. While both Myers et al. and Locke et al. (5, 14) have estimated the average difference (across all MELD-Na scores) between men and women in without-transplant survival, correcting MELD-Na scores to resolve sex disparities requires estimating these differences separately for each MELD-Na score.
There have been recent calls to develop and study policy changes to mitigate sex disparities in liver transplantation (15, 16). An early approach was to correct serum creatinine based on the modification of diet in renal disease (MDRD) formula for estimated GFR (eGFR) (7). This resulted in 65% of women having an increase of 2 or 3 points under the MDRD correction, but unfortunately this correction to MELD was no better than MELD at predicting 3-, 6-, 9-, or 12-month mortality for women (10), with similar results in subsequent studies of this approach (5, 17, 18). Asrani et al. refit MELD-Na to include eGFR via the glomerular filtration rate in liver disease (GRAIL) formula. They found that MELD-GRAIL-Na was better than MELD-Na at predicting waitlist mortality for women (19, 20), but only tested this on average (versus for each MELD-Na score), and did not test this in real-world simulations of allocation. A systems engineering approach might be to separately shift each woman’s MELD-Na according to observed sex differences in survival at that MELD-Na, effectively “reverse engineering” the disparities so that men and women with similar without-transplant survival will have the same score; we will present such an approach called MELD-Na-Shift.
Regardless of whether a corrected MELD-Na score yields an unbiased estimate of without-transplant survival for men and women, the ultimate goal is to remedy all disadvantages women have faced awaiting transplant. Only a real-world, clinically detailed simulated allocation model that includes disease etiology, donor and candidate size, accept/decline decisions, and uncertainty in disease progression and organ availability can answer the question of whether a corrected score would additionally mitigate sex disparity in transplant and mortality rates. None of the previously proposed corrections have been tested in a simulated allocation model. Unfortunately, the Liver Simulated Allocation Model (LSAM) underestimates the magnitude of the sex disparity in transplant rates and does not explicitly model decreased acceptance of larger livers for candidates of shorter stature, which limits the use of this tool to address height and size mismatch as a driver of sex disparity in transplantation. We therefore limit our main inferences to whether we can correct the sex bias in estimated without-transplant survival.
To explore ways to fix the sex disparity in MELD-Na, we quantified sex differences in without-transplant survival, independent of allocation and separately at each MELD-Na score, using Kaplan-Meier modeling in contrast to standard Cox regression. We then re-calculated without-transplant survival for women using two previously proposed corrections to MELD-Na (MELD-Na-MDRD and MELD-GRAIL-Na), and one correction of our own design that shifts each MELD-Na score for women to the MELD-Na of men with similar without-transplant survival (MELD-Na-Shift). Finally, we applied these MELD-Na corrections in a simulated allocation model to determine whether sex disparities in transplant and mortality rates could be reduced.
2. METHODS
2.1. Data Source
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donor, wait-listed candidates, and transplant recipients in the U.S., submitted by the members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors. This dataset has previously been described elsewhere (21).
2.2. Study Population
For the transplant-censored survival analysis, we studied all adult liver transplant candidates on the waitlist from 01/01/2003 – 01/01/2019. We excluded candidates who received an exception score or status 1A. For each candidate, status updates missing values required for the calculation of MELD-Na or any of the MELD-Na corrections were imputed with the values from the candidate’s previous status update if available, otherwise they were removed. For the simulated allocation modeling, we used the SRTR’s Liver Simulated Allocation Model, using data on all liver transplant candidates and recovered deceased donor livers from 07/01/2011 – 06/30/2016.
2.3. Survival Analysis
Kaplan-Meier analysis gives a nonparametric estimate of the probability of survival up to any given time, given a data set of independent censored survival times. We recently described a framework for performing Kaplan-Meier survival analysis which utilizes MELD-Na throughout a candidate’s history on the waitlist as opposed to MELD-Na at listing alone (22). We computed bias-corrected (23) Kaplan-Meier estimates of the 90-day without-transplant survival by MELD-Na. Because transplants tend to go to candidates at high MELD-Na, censoring by transplant introduces bias in survival estimation. We corrected for this using inverse probability censoring weights, like what was described in Robbins and Finkelstein (23) (See Appendix for more details).
For each MELD-Na score, we found all candidates who ever had that score during our study period. If a candidate had multiple updates at the same MELD-Na, the first update was selected, such that the model estimates the probability of surviving 90 days without a transplant since first arriving at a certain MELD-Na. The time from that update to an event – either death or censoring – was recorded. Candidates with removal code 8 (“Died”), 5 (“Medically unsuitable”) or 13 (“Candidate condition deteriorated, too sick for transplant”) were counted as death, unless the cause of death was suicide, which we treated as censoring. All other candidates were censored, either by transplant, by the conclusion of the study period, or by waitlist removal for any other reason.
2.4. MELD-Na Corrections
We investigated three changes to MELD-Na as possible corrections for the sex bias we found in without-transplant survival. For MELD-Na and each correction, we calculated the 90-day without-transplant survival for men and compared it that for women. The distribution of MELD-Na (and each correction) was assessed for our study population based on the candidate’s score at listing.
2.4.1. MELD-Na-MDRD
The first correction involved adjusting female serum creatinine based on the modification of diet in renal disease (MDRD) formula for eGFR as others have done (5, 7, 18). Because age and race are not directly accounted for, the adjustment simplifies to approximately 1.295 times each woman’s serum creatinine. The adjusted serum creatinine was constrained to the usual bounds of a minimum of 1.0 and a maximum of 4.0 mg/dl, and the current coefficients of MELD-Na were applied without refitting. Policy additionally dictates that a candidate who received two or more dialysis treatments in the prior seven days, or who received 24 hours of continuous veno-venous hemodialysis within the prior seven days, is assigned a serum creatinine of 4.0 mg/dl for the purpose of calculating MELD-Na. For such a candidate, we left her serum creatinine at 4.0 mg/dl.
2.4.2. MELD-GRAIL-Na
The second correction involved using MELD-GRAIL-Na, a recently proposed modification to MELD-Na that replaces serum creatinine with eGFR via the glomerular filtration rate in liver disease (GRAIL) formula (19). GRAIL takes into account serum creatinine, age, sex, race, albumin, and blood urea nitrogen (20). MELD-GRAIL-Na was scaled to a minimum of 6 and a maximum of 50 and then capped at 40.
2.4.3. MELD-Na-Shift
The final correction involved shifting female MELD-Na to the corresponding male MELD-Na that equalized 90-day without-transplant survival. For example, we found that women at MELD-Na of 25 had a 90-day without-transplant survival rate of 0.740, whereas men at the same MELD-Na had a survival rate of 0.766. The MELD-Na at which men had a 90-day without-transplant survival rate closest to 0.740 was 26, where their survival rate was 0.725. Therefore we shifted the MELD-Na of women at MELD-Na of 25 to 26.
2.5. Simulating MELD-Na Corrections in LSAM
We used LSAM to simulate the effect of our MELD-Na corrections for liver allocation on outcomes for men and women on the liver waitlist. We ran 10 iterations of the acuity circles allocation system from 07/01/2013 – 06/30/2016, first with MELD-Na and then with each of the three MELD-Na corrections. The LSAM input files were adjusted as follows. For each correction, we calculated a corrected score for every candidate older than 12 throughout the simulation. The corrected score was then used as the match MELD for each candidate, unless the candidate had an exception with an exception score higher than the corrected score, in which case the exception score was retained as the match MELD. Output metrics were calculated for adult candidates only, and differences in output metrics were evaluated using the matched-pairs t test.
3. Results
3.1. Study Population
Our study population consisted of 73,846 men and 45,478 women. Table 1 shows demographic data comparing the men and women in our cohort. Women had lower serum creatinine and lower MELD-Na at listing, and women were also less likely to be listed with hepatitis C, alcoholic cirrhosis, and alcoholic cirrhosis with hepatitis C, and more likely to be listed with NASH. Table 2 shows the distribution of MELD-Na (or MELD-Na correction) scores in our study population, stratified according to the acuity circles allocation system.
Table 1.
Demographics of Study Population at Listing – Quantitative variables (e.g. Age) are reported as mean (standard deviation) and categorical variables (e.g. Hepatitis C) are reported as n (%).
| Male (n = 73,846) | Female (n = 45,478) | |
|---|---|---|
| Age (Years) | 53.8 (9.9) | 53.8 (11.0) |
| Race | ||
| White | 54,542 (73.9) | 31,803 (69.9) |
| Hispanic | 10,687 (14.5) | 7,191 (15.8) |
| Black | 5,437 (7.4) | 4,393 (9.7) |
| Asian | 2,365 (3.2) | 1,392 (3.1) |
| Other | 815 (1.1) | 699 (1.5) |
| ABO | ||
| A | 27,935 (37.8) | 16,900 (37.2) |
| O | 34,263 (46.4) | 21,501 (47.3) |
| B | 8,835 (12.0) | 5,438 (12.0) |
| AB | 2,813 (3.80) | 1,639 (3.6) |
| Disease Etiology | ||
| Hepatitis C | 19,074 (25.8) | 9,549 (21.0) |
| Alcoholic Cirrhosis | 19,514 (26.4) | 7,191 (15.8) |
| NASH | 6,913 (9.4) | 6,990 (15.4) |
| Alcoholic Cirrhosis with Hepatitis C | 5,317 (7.2) | 1,285 (2.8) |
| Other | 23,028 (31.2) | 20,463 (45.0) |
| INR | 1.70 (0.8) | 1.68 (0.9) |
| Bilirubin | 6.58 (9.3) | 6.70 (9.1) |
| Serum Creatinine | 1.57 (1.5) | 1.34 (1.2) |
| Serum Sodium | 135.60 (5.0) | 136.05 (5.0) |
| MELD-Na | 19.5 (9.7) | 17.8 (10.1) |
Table 2.
Distribution of MELD-Na (Or MELD-Na Correction) Score for Study Population, Stratified According to the Acuity Circles Allocation System.
| Score >= 37 | 33 <= Score < 37 | 29 <= Score < 33 | 15 <= Score < 29 | Score < 15 | |
|---|---|---|---|---|---|
| MELD-Na | 8391 | 6342 | 8728 | 59572 | 36291 |
| MELD-Na-MDRD | 9064 | 6880 | 9388 | 63288 | 30704 |
| MELD-GRAIL-Na | 9666 | 6158 | 7911 | 55071 | 40518 |
| MELD-Na-Shift | 8391 | 6342 | 9609 | 58691 | 36291 |
3.2. Survival Analysis by MELD-Na and MELD-Na Corrections
3.2.1. MELD-Na
The 90-day without-transplant survival for men is higher than survival for women at most MELD-Na scores between 15 and 35, although the difference is small, at most about 5 percentage points (Figures 1 (a) and 2 (a)).
Figure 1.
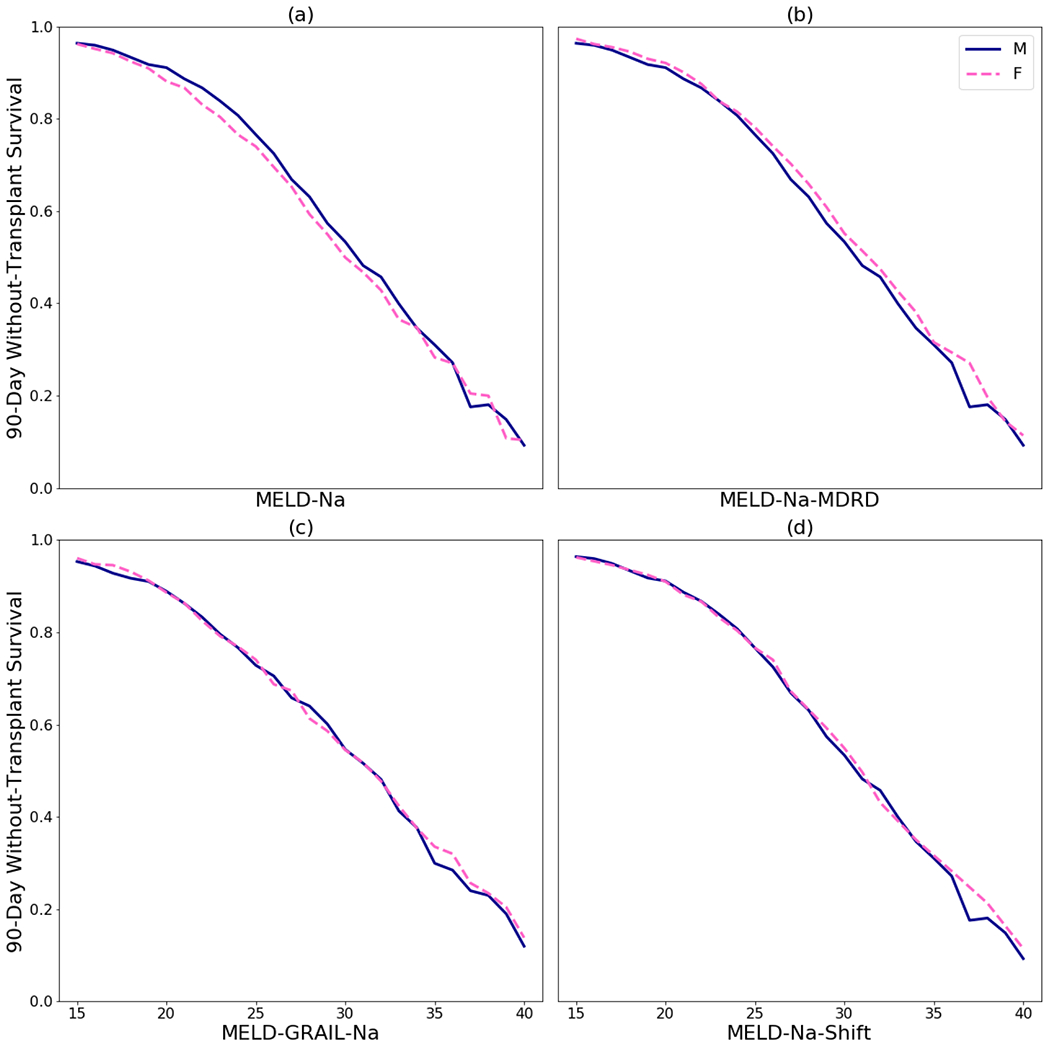
90-day without-transplant survival for men and women: (a) by MELD-Na, (b) by MELD-Na for men and MELD-Na-MDRD for women, (c) MELD-GRAIL-Na, and (d) by MELD-Na for men and MELD-Na-Shift for women. Scores below 15 were omitted because those candidates are rarely transplanted and because survival differences were always negligible.
Figure 2.
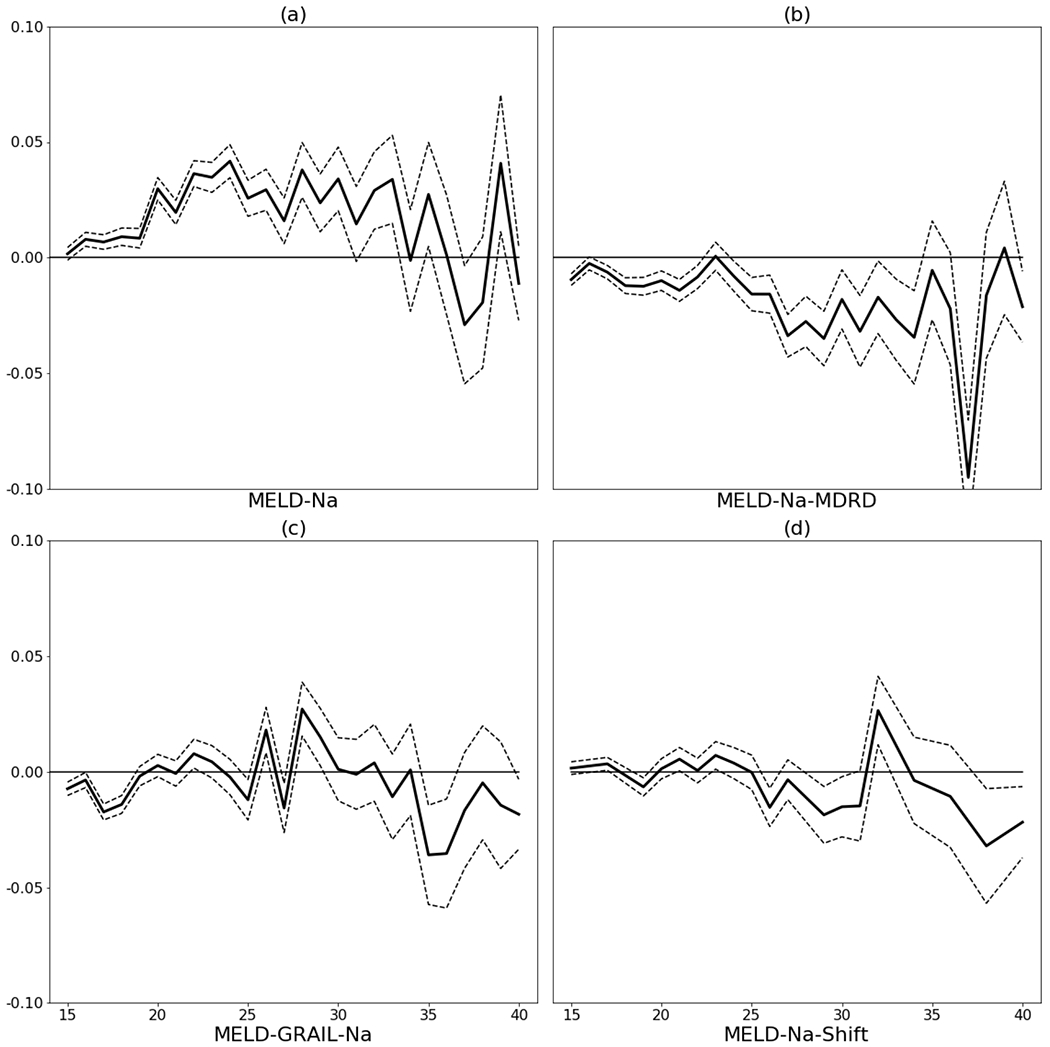
Difference in 90-day without transplant survival (men – women) with marginal 95% confidence intervals: (a) by MELD-Na, (b) by MELD-Na for men and MELD-Na-MDRD for women, (c) MELD-GRAIL-Na, and (d) by MELD-Na for men and MELD-Na-Shift for women. Scores below 15 were omitted because those candidates are rarely transplanted and because survival differences were always negligible.
3.2.2. MELD-Na-MDRD
The MELD-Na-MDRD correction increases female MELD-Na from 0 to 3 points depending on her serum creatinine, bilirubin, INR, and serum sodium. Under this correction, survival for women tends to be higher than survival for men at most MELD-Na/MELD-Na-MDRD scores. For most scores between 15 and 35, women had significantly higher 90-day without-transplant survival than men, although again the difference is small (Figures 1 (b) and 2 (b)). This indicates that using the MELD-Na-MDRD correction for women would be an overcorrection.
3.2.3. MELD-GRAIL-Na
Using MELD-GRAIL-Na, men do not consistently have higher 90-day without-transplant survival compared to women, nor vice versa. MELD-GRAIL-Na seems to correct the without-transplant survival differences between men and women seen in MELD-Na (Figures 1 (c) and 2 (c)).
3.2.4. MELD-Na-Shift
To equalize the 90-day without-transplant survival rate based on our 3.2.1 above, women had their MELD-Na score shifted according to Table 3. There are some scores for women under MELD-Na-Shift which cannot be obtained, because after shifting there are no longer any women at that MELD-Na score (e.g. 16). Without-transplant survival and differences for those scores are interpolated from survival of the neighboring scores (e.g. 15 and 17).
Table 3.
MELD-Na-Shift. Women at each MELD-Na score had their MELD-Na shifted by the corresponding value.
| MELD-Na | Shift |
|---|---|
| <15 | 0 |
| 16 | +1 |
| 17 | 0 |
| 18-26 | +1 |
| 27 | 0 |
| 28-31 | +1 |
| 32 | 0 |
| 33 | +1 |
| 34 | 0 |
| 35 | +1 |
| 36 | 0 |
| 37 | +1 |
| 38 | 0 |
| 39 | +1 |
| 40 | 0 |
With MELD-Na-Shift, men do not consistently have higher 90-day without-transplant survival compared to women, nor vice versa. MELD-Na-Shift seems to correct the without-transplant survival differences between men and women seen in MELD-Na (Figures 1 (d) and 2 (d)).
3.3. Simulation Study
To test the impact of the above approaches in the real world, we simulated using each of the three corrections to MELD-Na for allocation in LSAM under the acuity circles allocation system. All proposed corrections would increase the number of transplants and decrease the number of waitlist deaths for women, and consequently would decrease the number of transplants and increase the number of waitlist deaths for men (Table 4).
Table 4.
LSAM Simulation Results – Average number of transplants and waitlist deaths by sex for MELD-Na and each MELD-Na correction. An asterisk indicates the number is statistically significantly different than the MELD-Na case with p < 0.05.
| Male Transplants | Female Transplants | Male Deaths | Female Deaths | |
|---|---|---|---|---|
| MELD-Na | 10672 | 5871 | 4936 | 2925 |
| MELD-Na-MDRD | 10286* | 6275* | 5047* | 2764* |
| MELD-GRAIL-Na | 10340* | 6189* | 5011* | 2775* |
| MELD-Na-Shift | 10531* | 6026* | 4982* | 2860* |
Transplant rates for men and women using MELD-Na and each MELD-Na correction are recorded in Table 5. Using MELD-Na, women were transplanted at 94.50% of the rate of men (37.27 vs 35.22 transplants per 100 patient-years, p < 0.0001). Using the corrections, MELD-Na-MDRD resulted in women being transplanted at 108.11% of the rate of men (35.51 vs 38.35 transplants per 100 patient-years, p = 0.0025), MELD-GRAIL-Na resulted in women being transplanted at 105.25% of the rate of men (35.71 vs 37.57 transplants per 100 patient-years, p = 0.0079), and MELD-Na-Shift resulted in women being transplanted at 99.44% of the rate of men (36.61 vs 36.40 transplants per 100 patient-years, p = 0.4044). MELD-Na-MDRD and MELD-GRAIL-Na overcorrected the difference in transplant rates between men and women, whereas MELD-Na-Shift eliminated the difference.
Table 5.
LSAM Simulation Results – Average transplant rate (transplants per 100 patient-years) by sex for MELD-Na and each MELD-Na correction.
| Male Rate | Female Rate | Percent of Male Rate | p | |
|---|---|---|---|---|
| MELD-Na | 37.27 | 35.22 | 94.50 | <0.0001 |
| MELD-Na-MDRD | 35.51 | 38.35 | 108.11 | 0.0025 |
| MELD-GRAIL-Na | 35.71 | 37.57 | 105.25 | 0.0079 |
| MELD-Na-Shift | 36.61 | 36.40 | 99.44 | 0.4044 |
Mortality rates for men and women using MELD-Na and each MELD-Na correction are recorded in Table 6. Using MELD-Na, women died on the waitlist at 101.80% of the rate of men (17.24 vs 17.55 deaths per 100 patient-years, p = 0.0255). Using the corrections, MELD-Na-MDRD resulted in women dying on the waitlist at 96.90% of the rate of men (17.42 vs 16.88 deaths per 100 patient-years, p = 0.0006), MELD-GRAIL-Na resulted in women dying on the waitlist at 97.28% of the rate of men (17.31 vs 16.84 deaths per 100 patient-years, p = 0.0005), and MELD-Na-Shift resulted in women dying on the waitlist at 99.71% of the rate of men (17.32 vs 17.27 deaths per 100 patient-years, p = 0.7070). MELD-Na-MDRD and MELD-GRAIL-Na overcorrected the difference in mortality rates between men and women, whereas MELD-Na-Shift eliminated the difference.
Table 6.
LSAM Simulation Results – Average mortality rate (waitlist deaths per 100 patient-years) by sex for MELD-Na and each MELD-Na correction.
| Male Rate | Female Rate | Percent of Male Rate | p | |
|---|---|---|---|---|
| MELD-Na | 17.24 | 17.55 | 101.80 | 0.0255 |
| MELD-Na-MDRD | 17.42 | 16.88 | 96.90 | 0.0006 |
| MELD-GRAIL-Na | 17.31 | 16.84 | 97.28 | 0.0005 |
| MELD-Na-Shift | 17.32 | 17.27 | 99.71 | 0.7070 |
4. Discussion
In this national study of the liver transplant waitlist, we found that women had lower 90-day without-transplant survival compared to men at most MELD-Na scores between 15 and 35, but not at the highest MELD-Na scores. The difference, although statistically significant, was small, varying from about 0 to 5 percentage points depending on MELD-Na. We investigated two proposed MELD-Na corrections (MELD-Na-MDRD and MELD-GRAIL-Na) and one correction of our own design that shifted female MELD-Na to the corresponding male MELD-Na that equalized 90-day without-transplant survival (MELD-Na-Shift). MELD-Na-MDRD overcorrected the differences in without-transplant survival, whereas MELD-GRAIL-Na and MELD-Na-Shift eliminated the differences. We tested these MELD-Na corrections in a simulated allocation model to determine their effect on men and women in the presence of transplant. In simulation, MELD-Na-MDRD and MELD-GRAIL-Na overcorrected the differences in transplant and mortality rates between men and women, whereas MELD-Na-Shift eliminated the differences in transplant and mortality rates.
Our decision to censor transplants in a Kaplan-Meier framework was deliberate and distinguishes our analysis from others on this topic that use competing risk methodology. Kaplan-Meier analysis estimates survival in the absence of transplant while competing risk analysis estimates survival in the presence of transplant. For an excellent elaboration on the appropriate uses as well as the drawbacks of each method, see the discussion in Kim et. al (24). Accordingly, our research answers the question of whether womens’ without-transplant survival is systematically overestimated by the MELD-Na score. We do not address the question of whether women receive fewer transplants because their smaller size forces them to decline more livers.
Our methodology is novel because we separately estimated the sex disparity in without-transplant survival for each MELD-Na score, allowing us to reverse-engineer a novel MELD-Na-Shift correction which targets specific MELD-Na scores where there is a disparity, and because our methodology is fully non-parametric and uses data from every new MELD-Na update rather than only at listing. Although it may seem more intuitive to increase the MELD-Na score of all female candidates equally regardless of score, doing so would overcorrect the disparity in without-transplant survival even with an increase of only one point (see Supporting Document). Additionally, we used inverse probability censoring weights to overcome one of the major drawbacks mentioned by Kim et al., namely the questionable assumption of noninformative censoring (See Appendix for more details).
Our findings are consistent with those of others who also censor for transplant, such as Myers et al. and Locke et al. (5, 14). Myers et al. showed that a Cox model with various baseline variables and MELD had a hazard ratio for female sex of 1.07 (indicating moderate bias in favor of males), whereas a similar model with INR, bilirubin, and eGFR instead of serum creatinine had a hazard ratio for female sex of 0.85 (indicating overcorrection). Locke et al. showed that accounting for MELD-Na by weighting a Cox model increased the sex disparity in waitlist survival (the hazard ratio changed from a baseline of 1.09 to 1.14) and slightly decreased the sex disparity in the probability of receiving a transplant (hazard ratio changed from 0.86 to 0.87).
Other researchers including Lai et al. and Allen et al. have used a competing risk framework, which estimates waitlist mortality in the presence of transplant, combining liver disease risk with the effects of allocation (1, 25). Lai et al. found that much of the disparity between men and women in mortality can be explained by differences in height. Allen et al. used Cox modeling with competing risks to find that differences in height and exception scores accounted for most of the disparity between men and women in transplant rates. Their Cox model suggested that giving women 1 or 2 additional MELD-Na points would substantially increase the number of women who would receive liver transplants. They called for further exploration using sophisticated simulation software, a call we have answered. Using LSAM, we found that each of our proposed corrections to MELD-Na resulted in a significant increase in the number of transplants for women over our three year simulation: 155 for MELD-Na-Shift, 318 for MELD-GRAIL-Na, and 404 for MELD-Na-MDRD.
Bowring et al. showed that offered livers tend to be too large for female candidates to accept, which might explain the differences in transplant rates (26). Darden et al. showed that differences in size account for 19% of the disparity in transplant rates between men and women (27). Our simulation results agree that men are transplanted at a higher rate than women and that women die on the waitlist at a higher rate than men. However, LSAM underestimated the magnitude of these differences. Using LSAM to model share 35 (the allocation system in effect during our simulation period), we estimated that women were transplanted at 90.57% of the rate of men and died on the waitlist at 103% of the rate of men, whereas in reality women were transplanted at around 83% of the rate of men and died on the waitlist at around 105% of the rate of men (6). As a result, it is not entirely clear whether MELD-Na-MDRD and MELD-GRAIL-Na would truly be over-corrections to transplant and mortality rates. For this reason, additional policy changes aimed at the sex disparity in transplant rates (such as donor-candidate size matching) may be warranted.
We hypothesize two reasons that LSAM might underestimate the gender gap in transplant and mortality rates. First, LSAM’s accept/decline models do take sex into account (such that men are slightly more likely to accept an offer than women), but the models do not incorporate candidate height. This may partially explain why LSAM underestimated male transplant rate and overestimated female transplant rate. Second, LSAM’s candidate generator imputes disease trajectories for candidates who were transplanted in real life. The candidate generator does this by matching status updates via a linear predictor for candidate mortality. This mortality predictor, however, does not account for candidate sex. The imputed disease trajectories for women might actually be disease trajectories from male candidates, and thus may underestimate women’s true risk of mortality.
We purposefully did not account for disease etiology when estimating without-transplant survival, as MELD-Na is identically defined for all etiologies. However, we found that certain disease etiologies are more prevalent in men than in women (and vice versa). Looking at without-transplant survival for candidates with NASH, and for candidates with hepatitis C, men still had higher without-transplant survival than women for most MELD-Na scores. For candidates with alcoholic cirrhosis, men did not consistently have higher without-transplant survival compared to women, nor vice versa. This suggests that the difference in without-transplant survival between men and women is not due to differences in disease etiology, however small sample sizes make it difficult to draw conclusions from these analyses (see Supporting Document).
We found that women are disadvantaged on the liver waitlist as evidenced by lower without-transplant survival when compared to men at most MELD-Na scores between 15 and 35, and by lower transplant rates and higher mortality rates. Both MELD-GRAIL-Na and our MELD-Na-Shift corrections eliminated the sex disparity in without-transplant survival. Only our MELD-Na-Shift correction eliminated the sex disparity in transplant rates and mortality rates in simulated allocation modeling, however limitations in LSAM’s ability to capture the magnitude of this disparity leaves open MELD-GRAIL-Na as another possibly effective correction. Allocating livers via MELD-Na-Shift or MELD-GRAIL-Na could reduce the disadvantage women face awaiting transplant due to the use of serum creatinine in MELD-Na, however additional allocation changes may be needed to address the issue of size mismatch for women and candidates of shorter stature. (28).
5. Appendix
5.1. Handling Informative Censoring in Transplant Data
Traditional Kaplan-Meier analysis involves an assumption that transplant candidates removed from a study (i.e. “censored”) are similar in terms of expected survival to those who remain in the study. This assumption is met is when the reason for censoring has nothing to do with a candidate’s survival - for example the administrative censoring that happens when a study ends. Such candidates are no different in terms of their expected survival than those who had been at risk for the same amount of time during the middle of the study. This kind of censoring is known as non-informative censoring.
A problem occurs when candidates are censored from a study for reasons that are related to their chance of survival, such as transplant. Livers are allocated to candidates with the highest MELD-Na score because they are at the greatest risk of death without a transplant. Therefore censoring these candidates from the study at the time of transplant and conducting traditional Kaplan-Meier analysis results in a biased estimate of without-transplant survival probability. This kind of censoring is known as informative censoring.
We therefore adjust the Kaplan-Meier analysis to take into account candidates’ unequal chances of getting transplanted based on MELD-Na. For example, suppose a person is alive at some time t and has a probability of remaining untransplanted until time t of 1/4. We can envision three other prognostically similar candidates (“ghosts” in the language of Robbins and Finklestein (23)) who would have survived up to time t had they not been transplanted. Similarly, for a candidate who dies at time t with a probability of remaining untransplanted until time t of 1/4, we can envision three other ghosts who also would have died at time t had they not been transplanted. To account for candidates’ unequal probability of transplant, we simply include these unobserved ghosts in the Kaplan-Meier calculations. This reduces the bias in our estimation of the without-transplant survival probability.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by grant numbers R01DK111233 (PI: Dorry Segev) and K24DK101828 (PI: Dorry Segev) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the U.S. Government.
The data reported here have been supplied by the Hennepin Healthcare Research Institute (HHRI) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the U.S. Government.
Abbreviations:
- (eGFR)
Estimated Glomerular Filtration Rate
- (GFR)
Glomerular Filtration Rate
- (MELD-Na)
Model for End-Stage Liver Disease
- (MDRD)
Modification of Diet in Renal Disease
- (LSAM)
Liver Simulated Allocation Model
- (SRTR)
Scientific Registry of Transplant Recipients
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Allen AM, Heimbach JK, Larson JJ, Mara KC, Kim WR, Kamath PS et al. Reduced Access to Liver Transplantation in Women: Role of Height, MELD Exception Scores, and Renal Function Underestimation. Transplantation 2018;102(10):1710–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cullaro G, Sarkar M, Lai JC. Sex-based disparities in delisting for being “too sick” for liver transplantation. Am J Transplant 2018;18(5):1214–1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mindikoglu AL, Regev A, Seliger SL, Magder LS. Gender disparity in liver transplant waiting-list mortality: the importance of kidney function. Liver Transpl 2010;16(10):1147–1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moylan CA, Brady CW, Johnson JL, Smith AD, Tuttle-Newhall JE, Muir AJ. Disparities in liver transplantation before and after introduction of the MELD score. JAMA 2008;300(20):2371–2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myers RP, Shaheen AA, Aspinall AI, Quinn RR, Burak KW. Gender, renal function, and outcomes on the liver transplant waiting list: assessment of revised MELD including estimated glomerular filtration rate. J Hepatol 2011;54(3):462–470. [DOI] [PubMed] [Google Scholar]
- 6.Kwong AJ, Kim WR, Lake JR, Smith JM, Schladt DP, Skeans MA et al. OPTN/SRTR 2019 Annual Data Report: Liver. American Journal of Transplantation 2021;21(S2):208–315. [DOI] [PubMed] [Google Scholar]
- 7.Cholongitas E, Marelli L, Kerry A, Goodier DW, Nair D, Thomas M et al. Female liver transplant recipients with the same GFR as male recipients have lower MELD scores--a systematic bias. Am J Transplant 2007;7(3):685–692. [DOI] [PubMed] [Google Scholar]
- 8.Cholongitas E, Thomas M, Senzolo M, Burroughs AK. Gender disparity and MELD in liver transplantation. J Hepatol 2011;55(2):500–501. [DOI] [PubMed] [Google Scholar]
- 9.Durand F The quest for equity in liver transplantation: another lesson learned from women. J Hepatol 2011;54(3):401–402. [DOI] [PubMed] [Google Scholar]
- 10.Huo SC, Huo TI, Lin HC, Chi CW, Lee PC, Tseng FW et al. Is the corrected-creatinine model for end-stage liver disease a feasible strategy to adjust gender difference in organ allocation for liver transplantation? Transplantation 2007;84(11):1406–1412. [DOI] [PubMed] [Google Scholar]
- 11.Oloruntoba OO, Moylan CA. Gender-based disparities in access to and outcomes of liver transplantation. World J Hepatol 2015;7(3):460–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez-Castro KI, De Martin E, Gambato M, Lazzaro S, Villa E, Burra P. Female gender in the setting of liver transplantation. World J Transplant 2014;4(4):229–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huo TI, Hsu CY, Lin HC, Lee PC, Lee JY, Lee FY et al. Selecting an optimal cutoff value for creatinine in the model for end-stage liver disease equation. Clin Transplant 2010;24(2):157–163. [DOI] [PubMed] [Google Scholar]
- 14.Locke JE, Shelton BA, Olthoff KM, Pomfret EA, Forde KA, Sawinski D et al. Quantifying Sex-Based Disparities in Liver Allocation. JAMA Surg 2020;155(7):e201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ge J, Wood N, Segev D, Lai JC, Gentry S. Implementing a Height-Based Rule for Allocation of Pediatric Donor Livers to Adults – A Liver Simulated Allocation Model (LSAM) Study. Liver Transplantation;To Appear. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheikh SS, Locke JE. Leveraging Frailty to Mitigate Sex-Based Disparities in Access to Liver Transplant: Justice in Allocation. JAMA Surgery 2020. [DOI] [PubMed] [Google Scholar]
- 17.Mariante-Neto G, Marroni CP, Fleck Junior AM, Marroni CA, Zanotelli ML, Cantisani G et al. Impact of creatinine values on MELD scores in male and female candidates for liver transplantation. Ann Hepatol 2013;12(3):434–439. [PubMed] [Google Scholar]
- 18.Leithead JA, MacKenzie SM, Ferguson JW, Hayes PC. Is estimated glomerular filtration rate superior to serum creatinine in predicting mortality on the waiting list for liver transplantation? Transpl Int 2011;24(5):482–488. [DOI] [PubMed] [Google Scholar]
- 19.Asrani SK, Jennings LW, Kim WR, Kamath PS, Levitsky J, Nadim MK et al. MELD-GRAIL-Na: Glomerular Filtration Rate and Mortality on Liver-Transplant Waiting List. Hepatology 2020;71(5):1766–1774. [DOI] [PubMed] [Google Scholar]
- 20.Asrani SK, Jennings LW, Trotter JF, Levitsky J, Nadim MK, Kim WR et al. A Model for Glomerular Filtration Rate Assessment in Liver Disease (GRAIL) in the Presence of Renal Dysfunction. Hepatology 2019;69(3):1219–1230. [DOI] [PubMed] [Google Scholar]
- 21.Massie AB, Kucirka LM, Segev DL. Big data in organ transplantation: registries and administrative claims. Am J Transplant 2014;14(8):1723–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.VanDerwerken D, Wood NL, Segev D, Gentry S. The Precise Relationship Between MELD and Survival Without a Liver Transplant. Hepatology;n/a(n/a). [DOI] [PubMed] [Google Scholar]
- 23.Robins JM, Finkelstein DM. Correcting for noncompliance and dependent censoring in an AIDS Clinical Trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics 2000;56(3):779–788. [DOI] [PubMed] [Google Scholar]
- 24.Kim WR, Therneau TM, Benson JT, Kremers WK, Rosen CB, Gores GJ et al. Deaths on the liver transplant waiting list: an analysis of competing risks. Hepatology 2006;43(2):345–351. [DOI] [PubMed] [Google Scholar]
- 25.Lai JC, Terrault NA, Vittinghoff E, Biggins SW. Height contributes to the gender difference in wait-list mortality under the MELD-based liver allocation system. Am J Transplant 2010;10(12):2658–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bowring MG, Ruck JM, Haugen CE, Massie AB, Segev DL, Gentry SE. Deceased-Donor Liver Size and the Sex-Based Disparity in Liver Transplantation. Transplantation 2017;101(11):e329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darden M, Parker G, Anderson E, Buell JF. Persistent sex disparity in liver transplantation rates. Surgery 2021;169(3):694–699. [DOI] [PubMed] [Google Scholar]
- 28.Verna EC, Lai JC. Time for Action to Address the Persistent Sex-Based Disparity in Liver Transplant Access. JAMA Surg 2020;155(7):545–547. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


