Abstract
Background
Although asthma has been suggested as a risk factor for cardiovascular disease (CVD), robust longitudinal evidence of this relationship is limited.
Research Question
Using Framingham Offspring Cohort data, the goal of this study was to longitudinally examine the association between asthma and lifetime risk of CVD while controlling for cardiovascular risk factors included in the Framingham Risk Score.
Study Design and Methods
Data were analyzed from a prospective population-based cohort of 3,612 individuals, ages 17 to 77 years, who participated in Framingham Offspring Study examinations from 1979 to 2014. Asthma was defined based on physician diagnosis during study interviews. Incident CVD included myocardial infarction, angina, coronary insufficiency, stroke, transient ischemic attack, and heart failure. Time-dependent Cox regression models were used to evaluate the relationship between asthma and CVD incidence.
Results
Overall, 533 (15%) participants had a diagnosis of asthma and 897 (25%) developed CVD during the course of the study. Unadjusted analyses revealed that asthma was associated with increased CVD incidence (hazard ratio, 1.40; 95% CI, 1.17-1.68). Cox regression also showed an adjusted association between asthma and CVD incidence (hazard ratio, 1.28; 95% CI, 1.07-1.54) after controlling for established cardiovascular risk factors.
Interpretation
This prospective analysis with > 35 years of follow-up shows that asthma is a risk factor for CVD after adjusting for potential confounders. When assessing risk of cardiovascular disease, asthma should be evaluated and managed as a risk factor contributing to morbidity and mortality.
Key Words: asthma, cardiovascular disease, Framingham Offspring Cohort
Abbreviations: CVD, cardiovascular disease; FOS, Framingham Offspring Study; HR, hazard ratio
FOR EDITORIAL COMMENT, SEE PAGE 1311
In recent decades, asthma and cardiovascular disease (CVD) have seen a considerable increase in worldwide incidence and prevalence.1, 2, 3, 4 Amid the enormous social and economic burdens posed by these conditions, research is needed to better understand the interplay between the two diseases, particularly because a significant proportion of CVD cannot be explained solely by risk factors such as high blood pressure, hyperlipidemia, smoking, and diabetes.5,6 Asthma and CVD share a common etiology that is underlined by chronic systemic inflammation, which plays an important role in the chain of events culminating in poor asthma control and atherosclerotic or ischemic disease.7 Understanding the relationship between the two can help in developing new management models for preventive strategies against CVD in patients with asthma.
The existing literature on the association between asthma and CVD has been mixed. Some studies have found that asthma is associated with an increased incidence of CVD.8, 9, 10 Other analyses have shown that the increased risk of CVD conferred by asthma is limited to specific subgroups such as smokers5 and women.11, 12, 13 Conversely, several studies found no significant relationships between the two conditions.14,15
Although meta-analyses have evaluated the relationship between asthma and CVD, the conclusion of these reviews has been limited by the presence of high variability in study design, including heterogeneous patient populations, and the varied prevalence of cardiovascular risk factors in the population studied.13,16 The existing literature has also been limited by a reliance of self-reported rather than physician-diagnosed asthma.
The current study used data from the Framingham Offspring Study (FOS) cohort to prospectively assess the relationship of asthma and CVD incidence while adjusting for established CVD risk factors. The FOS is a population-based cohort followed up every 4 years for > 3 decades and includes detailed data on CVD risk factors, incident CVD events, and clinical diagnoses of asthma.
Patients and Methods
The FOS is a prospective cohort study following up offspring and offspring spouses of the original Framingham Heart Study participants.17 Although the FOS methods, selection criteria, sampling design, and response rates have been described elsewhere, the overarching aim of the study is to identify risk factors of CVD. Participants free of CVD at enrollment underwent in-person examinations every 4 to 8 years for a maximum of 35 years of follow-up. Each examination consisted of medical history, physical examination, and laboratory assessments of CVD risk factors using a standardized protocol.
For the current study, de-identified data from the National Institutes of Health Biologic Specimen and Data Repository Information Coordinating Center were used. Our analysis began with examination 2 (collected between 1979 and 1983), the first assessment that provided information about clinical diagnoses of asthma, and extended until examination 9 (collected between 2011 and 2014), the last available follow-up. FOS participants were excluded from this analysis if they had preexisting CVD at the time of examination 2 or missing data about their asthma status.
Predictor Variable and End Points
Asthma status at each follow-up was determined by a physician who interviewed and examined participants during study visits. For this study, participants were classified as having asthma if they received a physician diagnosis at any point during their 35 years of follow-up. As a result, patients in the asthma group included both those who had asthma at baseline as well as those who subsequently developed asthma during the follow-up period.
CVD events in the FOS cohort through 2013 included myocardial infarction, angina, coronary insufficiency, stroke, transient ischemic attack, and existing heart failure. Death attributable to any of these etiologies was also classified as a CVD event. As previously described, the FOS used examination interviews, hospital and death records, passive reporting between examinations, and monitoring of local newspaper obituaries to ascertain CVD events.18
Covariates
Demographic variables (age, sex, and education) were obtained via self-report during each examination survey. Obesity was defined by using World Health Organization cutoffs (BMI ≥ 30 kg/m2)19. Diabetes was defined as fasting blood sugar levels > 125 mg/dL or use of insulin/oral hypoglycemic medication; for participants with missing information on fasting blood sugar or treatment status, they were classified as diabetic if nonfasting blood glucose levels were > 200 mg/dL (or 126-200 mg/dL if the participants reported a history of diabetes). Systolic blood pressure was calculated based on the mean of the clinic physician’s two measurements while the participant was seated.1,17 Use of antihypertensive medication was obtained by self-report.
The Framingham Risk Score (FRS) and 10-year CVD risk was calculated by using patients’ age, sex, education status, systolic blood pressure and treatment for hypertension, current smoking status, high-density lipoprotein levels, total cholesterol levels, and presence of diabetes (defined as fasting blood sugar > 125 mg/dL or use of insulin/oral hypoglycemic medications).20 D’Agostino et al20 have validated the combination of these risk factors to assess CVD risk. Following current guidelines, participants were stratified into three categories based on their FRS’s corresponding 10-year CVD risk: high (≥ 20%), intermediate (10%-20%), or low (< 10%). Details on classification are given in Table 1.
Table 1.
Calculation of Framingham Risk Score
| CVD Risk Points | Age (y) | HDL (mg/100 mL) | TC (mg/100 mL) | No HTN Medicationsa | On HTN Medications | Diabetesb | Current Smoking |
|---|---|---|---|---|---|---|---|
| Male participants | |||||||
| –2 | HDL > 1.6 | SBP < 120 | |||||
| –1 | 1.6 ≥ HDL > 1.3 | ||||||
| 0 | Age < 35 | 1.3 ≥ HDL > 1.2 | TC ≤ 4.1 | 120 ≤ SBP < 130 | SBP < 120 | No | No |
| 1 | 1.2 ≥ HDL > 0.9 | 4.1 < TC ≤ 5.2 | 130 ≤ SBP < 140 | ||||
| 2 | 35 ≤ age < 40 | HDL < 0.9 | 5.2 < TC ≤ 6.2 | 140 ≤ SBP < 160 | 120 ≤ SBP < 130 | ||
| 3 | 6.2 < TC ≤ 7.2 | 160 ≤ SBP | 130 ≤ SBP < 140 | Yes | |||
| 4 | 7.2 < TC | 140 ≤ SBP < 160 | Yes | ||||
| 5 | 40 ≤ age < 45 | 160 ≤ SBP | |||||
| 6 | |||||||
| 7 | 45 ≤ age < 50 | ||||||
| 8 | 50 ≤ age < 55 | ||||||
| 9 | |||||||
| 10 | 55 ≤ age < 60 | ||||||
| 11 | 60 ≤ age < 65 | ||||||
| 12 | |||||||
| 13 | 65 ≤ age < 70 | ||||||
| 14 | 70 ≤ age < 75 | ||||||
| 15 | Age ≥ 75 | ||||||
| Female participants | |||||||
| –3 | SBP < 120 | ||||||
| –2 | HDL > 1.6 | ||||||
| –1 | 1.6 ≥ HDL > 1.3 | SBP < 120 | |||||
| 0 | Age < 35 | 1.3 ≥ HDL > 1.2 | TC ≤ 4.1 | 120 ≤ SBP < 130 | No | No | |
| 1 | 1.2 ≥ HDL > 0.9 | 4.1 < TC ≤ 5.2 | 130 ≤ SBP < 140 | ||||
| 2 | 35 ≤ age < 40 | HDL < 0.9 | 140 ≤ SBP < 150 | 120 ≤ SBP < 130 | |||
| 3 | 5.2 < TC ≤ 6.2 | 130 ≤ SBP < 140 | Yes | ||||
| 4 | 40 ≤ age < 45 | 6.2 < TC ≤ 7.2 | 150 ≤ SBP < 160 | Yes | |||
| 5 | 45 ≤ age < 50 | 7.2 < TC | 160 ≤ SBP | 140 ≤ SBP < 150 | |||
| 6 | 150 ≤ SBP < 160 | ||||||
| 7 | 50 ≤ age < 55 | 160 ≤ SBP | |||||
| 8 | 55 ≤ age < 60 | ||||||
| 9 | 60 ≤ age < 65 | ||||||
| 10 | 65 ≤ age < 70 | ||||||
| 11 | 70 ≤ age < 75 | ||||||
| 12 | Age ≥ 75 | ||||||
CVD risk points were assigned for each participant based on FRS components, as illustrated in the table. For each participant, the sum of individual CVD risk points across age, HDL, total cholesterol, HTN status, diabetes status, and current smoking status was calculated. This sum was subsequently converted to FRS risk percentages based on methods detailed in D’Agostino et al.20 Those with total risk points under 11 and 13, for male and female participants, respectively, were classified as low risk (FRS < 10%). Those with risk points between 11 and 15 (for male participants) and 13 and 18 (for female participants) were classified as intermediate risk (FRS, 10%-19%). Finally, those with risk points above 15 and 18, for male and female participants, were classified as high risk (FRS > 20%). All SBP values are given in millimeters of mercury. CVD = cardiovascular disease; FRS = Framingham Risk Score; HDL = high-density lipoprotein; HTN = hypertension; SBP = systolic blood pressure; TC = total cholesterol. (Adapted from Tables 5 and 7 in D’Agostino et al.20)
HTN medication status was ascertained by self-report.
Defined as fasting blood sugar levels > 125 mg/dL or use of insulin/oral hypoglycemic medications. For participants with missing information on fasting blood sugar or treatment status, nonfasting blood glucose levels > 200 mg/dL (or 126-200 mg/dL if the participants reported a history of diabetes) met criteria for diabetes.
Statistical Analysis
Baseline characteristics of participants with and without asthma were compared by using a Wilcoxon rank-sum or χ2 test, as appropriate. The unadjusted probability of developing CVD among participants with and without asthma was estimated by using the Kaplan-Meier method (observations were censored at the date of non-CVD-related death or at the date of the last follow-up visit if alive and free of CVD in 2014) and compared by using the log-rank test.
A time-dependent Cox proportional hazards model was used to examine the relationship between asthma status at each interview (included as a time-dependent covariate) and CVD risk, with models based on complete case analysis. Asthma was used as a time-dependent covariate to ensure that participants had an asthma diagnosis prior to having a CVD event. All models were adjusted for FRS, obesity, and socioeconomic variables. Because past literature has suggested that the increased risk of CVD conferred by asthma may be limited to subgroups such as women11, 12, 13 and smokers,5 stratified analyses according to sex and smoking status were also conducted.
All analyses were conducted by using SAS version 9.3 (SAS Institute, Inc.) with two-tailed P values < .05 regarded as significant. The study was approved by the Institutional Review Board of the Icahn School of Medicine at Mount Sinai. Framingham Heart Study participants have provided informed consent, with the study reviewed annually by the Institutional Review Board of Boston University Medical Center.
Results
Study Population
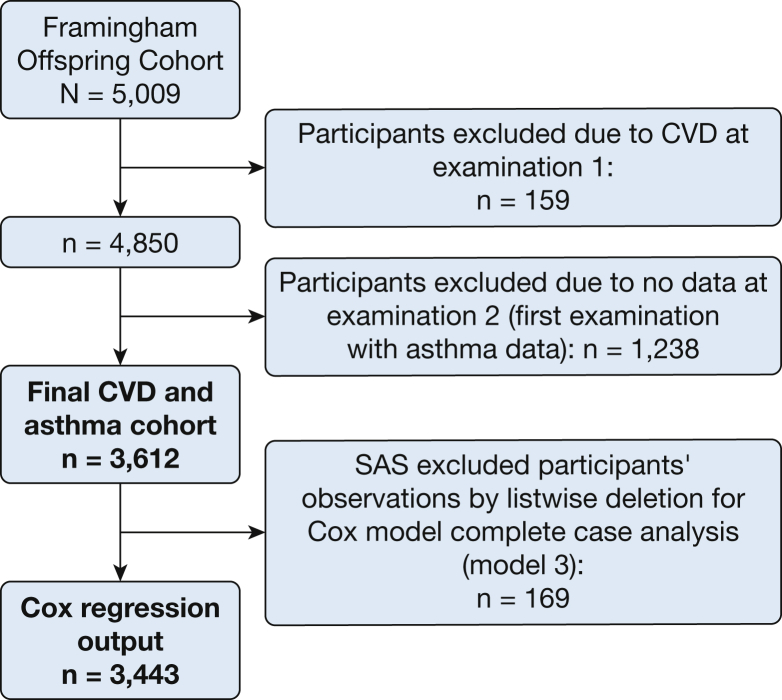
The FOS cohort is derived from the original Framingham Heart Study participants. From the original cohort, 5,009 of their offspring were selected for the FOS cohort. The current analysis was conducted on a subgroup of participants from the FOS cohort. Figure 1 presents a consortium diagram, a selection of participants leading to a study population of 3,612 individuals aged 17 to 77 years (72.1% of the original FOS cohort).
Figure 1.
Consortium diagram, selection of participants leading to a study population of 3,612 individuals (72.1% of the original Framingham Offspring Cohort). CVD = cardiovascular disease.
Baseline characteristics of study participants are depicted in Table 2. Overall, 533 (14.8%) participants had asthma before CVD. At baseline, 384 (10.6%) participants had asthma, while during the course of follow-up, 149 (4.1%) developed asthma prior to developing CVD. The average age of participants with asthma at baseline was 44.4 years compared with 40.4 and 43.9 years in those participants who developed asthma during the study and those without asthma, respectively (P < .0001). Asthma was associated with lower educational attainment, with 21.9% of participants with asthma endorsing college education or above compared with 30.9% and 32.5% of participants who developed asthma during the study and those without asthma (P < .0001). Asthma at baseline was associated with a lower prevalence of obesity (24.5%) compared with participants who developed asthma during the study (24.8%) but greater than those without asthma (19.2%) (P = .015).
Table 2.
Baseline Characteristics of Study Participants According to Asthma Status
| Characteristic | All |
Asthma at Baseline |
Asthma Following Baseline |
No Asthma |
P Value |
|---|---|---|---|---|---|
| (N = 3,612) | (n = 384) | (n = 149) | (n = 3,079) | ||
| Age, y | 43.8 ± 10.0 | 44.4 ± 10.0 | 40.4 ± 9.8 | 43.9 ± 9.9 | < .0001 |
| Male sex, No. (%) | 1,713 (47.4) | 195 (50.8) | 49 (32,9) | 1,469 (47.7) | .0007 |
| Education, No. (%) | < .0001 | ||||
| Less than high school | 258 (7.2) | 46 (12.0) | 9 (6,0) | 203 (6.6) | |
| High school | 1,238 (34.3) | 149 (38.9) | 50 (33.6) | 1,039 (33.8) | |
| Some college | 981 (27.2) | 104 (27.2) | 44 (29.5) | 833 (27.1) | |
| College or greater | 1,131 (31.4) | 84 (21.9) | 46 (30.9) | 1,001 (32.5) | |
| Annual income, US$, No. (%) | .284 | ||||
| < 20,000 | 1,026 (40.8) | 121 (47.1) | 50 (44.6) | 855 (39.8) | |
| 20,000-35,000 | 845 (33.6) | 79 (30.7) | 35 (31.3) | 731 (34.1) | |
| 35,000-50,000 | 372 (14.8) | 37 (14.4) | 17 (15.2) | 318 (14.8) | |
| > 50,000 | 273 (10.9) | 20 (7.8) | 10 (8.9) | 243 (11.3) | |
| Obesity, No. (%) | 721 (20.0) | 94 (24.5) | 37 (24.8) | 590 (19.2) | .015 |
| Framingham Risk Score Components | |||||
| HDL cholesterol, mg/100 mL | 48.7 ± 13.4 | 47.2 ± 13.3 | 49.7 ± 14.5 | 48.8 ± 13.4 | .053 |
| Total cholesterol, mg/100 mL | 202.7 ± 38.9 | 204.9 ± 36.8 | 194.4 ± 38.4 | 202.9 ± 39.1 | .006 |
| Taking antihypertensive medication, No. (%) | 343 (9.5) | 47 (12.2) | 10 (6.7) | 286 (9.3) | .088 |
| Systolic blood pressure, mm Hg | 121.8 ± 16.3 | 124.3 ± 17.3 | 116.8 ± 13.5 | 121.7 ± 16.2 | < .0001 |
| Diabetes, No. (%) | 84 (2.4) | 13 (3.5) | 2 (1.4) | 69 (2.3) | .288 |
| Currently smoking, No. (%) | 1,401 (38.8) | 220 (57.4) | 60 (40.3) | 1,121 (36.4) | < .0001 |
| Framingham Risk Score, No. (%) | < .0001 | ||||
| High | 282 (7.8) | 50 (13.0) | 5 (3.4) | 227 (7.4) | |
| Intermediate | 661 (18.3) | 79 (20.6) | 15 (10.1) | 567 (18.4) | |
| Low | 2,669 (73.9) | 255 (66.4) | 129 (86.6) | 2,285 (74.2) |
Data are presented as mean ± SD unless otherwise indicated. HDL = high-density lipoprotein.
The rates of high, low, and intermediate FRS 10-year CVD risk were 13.0%, 20.6%, and 66.4% for those who had asthma at baseline, 3.4%, 10.1%, and 86.6% for those who developed asthma during the study vs 7.4%, 18.4%, and 74.2% among participants without asthma, respectively (P < .0001). Currently smoking was more common among participants who had asthma at baseline compared with those who developed asthma during follow-up and those without asthma (57.4% and 40.3% vs 36.4%; P < .0001). Mean systolic blood pressure was higher for patients with asthma at baseline compared with those who developed asthma during the study and those without (124.3 mm Hg and 116.8 mm Hg vs 121.7 mm Hg; P < .0001). Total cholesterol levels were greater for participants who had asthma at baseline compared with those who developed asthma during follow-up and those without asthma (204.9 mg/100 mL, 194.4 mg/100 mL vs 202.9 mg/100 mL; P = .006).
Unadjusted Analyses
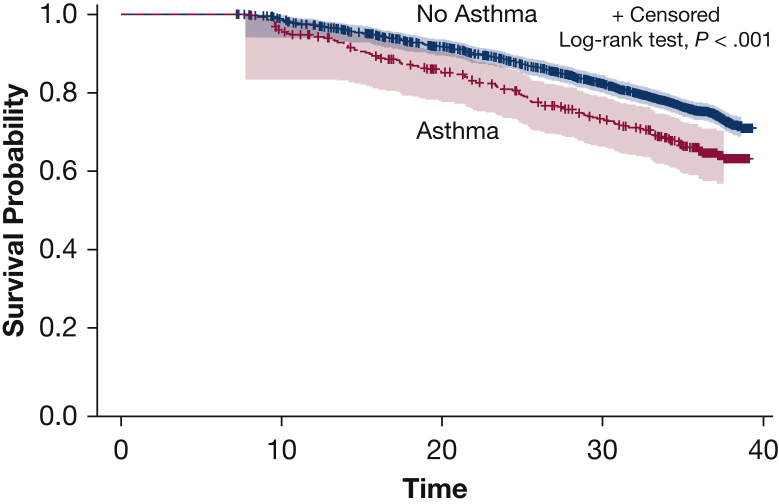
A total of 897 (25%) participants developed CVD during the course of the > 35 years of follow-up. The cumulative incidence of CVD among patients with asthma was 36.9% (95% CI, 31.4-42.4) compared with 29.1% (95% CI, 26.9-31.2) among FOS participants without asthma (Fig 2). Similarly, analysis using an unadjusted time-dependent Cox model showed that participants with asthma also had an increased risk for incident CVD (hazard ratio [HR], 1.40; 95% CI, 1.17-1.68).
Figure 2.
Cardiovascular disease incidence based on asthma status. The graph shows the cumulative incidence of cardiovascular events comparing participants with asthma vs those with no asthma over a period of about 32 years.
Adjusted Analyses
Adjusted analyses showed that asthma was associated with increased risk of CVD (HR, 1.31; 95% CI, 1.10-1.57) when using 10-year CVD risk, obesity, and educational attainment (Table 3). Asthma remained a significant predictor of increased CVD risk when also adjusting for obesity, educational attainment, and individual components of 10-year CVD risk (HR, 1.28; 95% CI, 1.07-1.54).
Table 3.
Relationship Between Asthma and Risk of Cardiovascular Disease
| Model | All Participants (N = 3,612) | Women (n = 1,899) | Men (n = 1,713) | Nonsmokers (n = 2,208) | Smokers (n = 1,401) |
|---|---|---|---|---|---|
| 1a | 1.40 (1.17-1.68) | 1.46 (1.11-1.92) | 1.35 (1.07-1.71) | 1.08 (0.81-1.50) | 1.52 (1.20-1.91) |
| 2b | 1.31 (1.10-1.57) | 1.37 (1.04-1.81) | 1.24 (0.98-1.58) | 1.07 (0.80-1.43) | 1.47 (1.16-1.86) |
| 3c | 1.28 (1.07-1.54) | 1.27 (0.95-1.70) | 1.26 (0.99-1.61) | 0.96 (0.71-1.31) | 1.53 (1.20-1.94) |
Data are presented as hazard ratios (95% CI).
Unadjusted.
Adjusted for Framingham Risk Score, obesity, and education.
Adjusted for age, sex, high-density lipoprotein cholesterol, total cholesterol, currently taking antihypertensive medications, systolic blood pressure, diabetes, currently smoking, obesity, and education.
In analyses stratified according to sex, the association of asthma with increased CVD risk was statistically significant among women (HR, 1.37; 95% CI, 1.04-1.80) but not men (HR, 1.22; 95% CI, 0.97-1.55). Analyses stratified according to smoking status showed that although asthma was associated with increased CVD risk among smokers (HR, 1.53; 95% CI, 1.20-1.94), no significant relationship was observed among nonsmokers (HR, 0.96; 95% CI, 0.71-1.31).
Discussion
Using data from a large population-based cohort, this study found that asthma is associated with a higher risk of CVD after carefully adjusting for other cardiovascular risk factors. This relationship seems to be strongest among women and smokers. Our study identified patients with asthma as a high-risk group that may benefit from targeted interventions to prevent onset of CVD. Our findings suggest a potential role for the need for personalized CVD prevention strategies and diagnostic testing among patients with asthma, and they may catalyze future investigation on the pathophysiology of systemic inflammation underlying these two conditions.
There is a growing body of research suggesting that asthma is associated with adverse cardiovascular consequences.6 Our work adds to this evidence by showing a robust prospective relationship between asthma and CVD,6,8,10 even following a detailed adjustment for CVD risk factors such as hypertension, BMI, smoking, diabetes, and socioeconomic status.9,21 Our study is also strengthened by the use of validated FRS measures to control for confounding, rigorously adjudicated CVD events in the Framingham dataset, diagnosis of asthma made following physician assessment during study visits, and time-dependent Cox regression enabling us to appropriately incorporate into the analyses participants who developed asthma during the follow-up period.
Investigators have suggested that common systemic inflammatory markers such as IL-6, C-reactive protein, fibrinogen, and D-dimer are potential mediators of the association between asthma and CVD.7,16 Shared inflammatory mechanisms of asthma and CVD from the 5-lipo-oxygenase enzymatic pathway7,22 may also contribute to concomitant long-term airway remodeling and atherosclerosis.6,12 Another proposed mechanism is increased blood pressure during and following acute asthma attacks and severe hypoxic episodes leading to stroke and related ischemic disease.15 Lastly, IL-6 and tumor necrosis factor alpha-α, two pro-inflammatory cytokines that are known to be elevated in asthma, are also believed to play a key role in driving atherosclerosis.11 Additional research is warranted to clarify the pathways by which asthma is associated with CVD, which may lead to novel prevention strategies.
The large number of CVD events over 35 years of follow-up in the FOS allowed us to evaluate the role of effect modification by smoking history in the asthma-CVD relationship. Previous studies have suggested that tobacco smoking is a major driver of cardiovascular comorbidities in people with asthma.5 In addition, smoking is a major contributor for CVD and cardiovascular mortality. Colak et al5 found that tobacco smokers with asthma have an increased risk of developing lung cancer, cardiovascular comorbidities, and death compared with nonsmokers with asthma. We confirm these findings, and our results support that asthma was more strongly associated with CVD among smokers. The reasons for these findings are unclear but may be related to a ceiling effect, if the contribution of asthma to CVD morbidity is only marginal among smokers given the strong effect of tobacco exposure on cardiovascular risk.
Our finding that the increased risk of CVD conferred by asthma is primarily observed among women is consistent with prior research.8 One pathway that may explain the differential effect of asthma according to sex is estrogen, which has been shown to modulate alterations in inflammatory cytokine and leukotriene regulation.12 Prior studies have suggested that estrogen plays a key role in modulating the release of proinflammatory cytokines from activated cells such as monocytes, macrophages, and vascular cells.13 Card and Zeldin23 suggested that female sex hormones increase allergic lung inflammation in animal models. The regulation of these cytokines by estrogen may trigger systemic inflammatory responses in women with asthma more intensely than in men, thus creating a proinflammatory state that predisposes women with asthma to have increased risk of developing CVD. In addition, Onufrak et al12 found that the incidence rate of asthma for women peaks and is temporally associated with shifts in estrogen levels. Other studies assessing the association of asthma with CVD have not revealed effect modification by sex, but these results may be partly due to limited statistical power.9 In a controlled mouse model, investigators showed that female mice had increased allergic airway inflammation, due in part to a lower number of naturally occurring regulatory T cells.23
The current study has some strengths and limitations. By using FOS data, this is one of the largest population-based analyses of the lifetime risk of CVD conferred by asthma. In addition, the FOS provides robust data about asthma diagnoses, detailed information about other CVD risk factors, and excellent ascertainment of cardiac events over a very long follow-up period. Furthermore, the FOS cohort is not an ethnically diverse population, with most of the participants being middle-aged, middle-class, and self-identifying as White, thus limiting the current study’s generalizability in terms of population level applications. Our study uses smoking data obtained as part of baseline examinations conducted during the 1980s and 1990s. Although cigarette smoking among US adults has steadily decreased during the last 2 decades, the elevated smoking rates in the study population represent the smoking patterns at the time of baseline examination. The progression and burden of disease of both asthma and cardiovascular disease vary widely across demographic characteristics. Our findings are most pertinent to settings that are similar in demographic characteristics to those studied within the town of Framingham, Massachusetts.
In addition, we did not have detailed information about lung function or standardized asthma control scores. Although data were collected on hospitalizations and acute care encounters during follow-up visits, to preserve participant confidentiality, these variables are not publicly available. Thus, we were unable to explore whether severity of disease plays a role in the relationship between asthma and CVD. Similarly, we had no detailed information about use of asthma medications during the 35 years of follow-up, limiting our ability to test if the impact of asthma is mediated, in part, by asthma treatments. Prior studies have suggested that asthma patients using oral corticosteroids are at elevated risk of CVD.8 Conversely, studies evaluating risk of cardiovascular events in patients with asthma receiving inhaled corticosteroids and long-acting bronchodilators have suggested that these medications have a safe cardiovascular profile.24 Unfortunately, we did not have access to detailed data on asthma medications and therefore could not assess if risk of CVD varied according to asthma medication regimen.
Conclusions
This study adds to the growing body of evidence that asthma is a risk factor for lifetime CVD events, both fatal and nonfatal, after adjusting for existing FRS predictors. Amid the rising prevalence of asthma and comorbid CVD, our data suggest that asthma may be an underrecognized contributor to CVD and should be a focus of targeted interventions in high-risk populations. In addition, the current study raises important questions about the potential for asthma treatments such as corticosteroids and biologic therapies for reducing CVD risk.
Acknowledgments
Author contributions: M. E. P. and K. Y. X. were involved in all aspects of the project, including but not limited to: literature search, figure creation, study design, data collection, data interpretation, writing. G. M. played a large role in figure creation, study design, data analysis and interpretation. E. G. F. was crucial in obtaining data access and data management. R. V. was involved in aspects of study design, data interpretation, and writing. F. H. was involved in aspects of study design, data interpretation, and writing. A. D. F. was involved in aspects of study design, data interpretation, and writing. J. P. W. was involved in all aspects of the project, including but not limited to study design, data interpretation, writing and served as the senior author on the project.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: J. P. W. has received consulting honorarium from Sanofi and Banook; and research grants from Aventis and Quorum. None declared (M. E. P., K. Y. X., G. M., E. G. F., R. V., P. B., F. H., A. D. F.).
Other contributions: The authors acknowledge the support of the research staff at NIH Biologic Specimen and Data Repository Information Coordinating Center.
Footnotes
FUNDING/SUPPORT: K. Y. X. is supported by National Institutes of Health [NIH R25 MH112473-01] and acknowledges the support of the Psychiatry Residency Research Education Program of Washington University.
References
- 1.Agha G., Murabito J.M., Lynch J.W., Abrahamowicz M., Harper S.B., Loucks E.B. Relation of socioeconomic position with ankle-brachial index. Am J Cardiol. 2011;108(11):1651–1657. doi: 10.1016/j.amjcard.2011.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akinbami L.J., Moorman J.E., Bailey C. Trends in asthma prevalence, health care use, and mortality in the United States, 2001-2010. NCHS Data Brief. 2012;(94):1–8. [PubMed] [Google Scholar]
- 3.Benjamin E.J., Blaha M.J., Chiuve S.E. Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146–e603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rudd R.A., Moorman J.E. Asthma incidence: data from the National Health Interview Survey, 1980-1996. J Asthma. 2007;44(1):65–70. doi: 10.1080/02770900601125896. [DOI] [PubMed] [Google Scholar]
- 5.Colak Y., Afzal S., Nordestgaard B.G., Lange P. Characteristics and prognosis of never-smokers and smokers with asthma in the Copenhagen General Population Study. A prospective cohort study. Am J Respir Crit Care Med. 2015;192(2):172–181. doi: 10.1164/rccm.201502-0302OC. [DOI] [PubMed] [Google Scholar]
- 6.Strand L.B., Tsai M.K., Wen C.P., Chang S.S., Brumpton B.M. Is having asthma associated with an increased risk of dying from cardiovascular disease? A prospective cohort study of 446 346 Taiwanese adults. BMJ Open. 2018;8(5) doi: 10.1136/bmjopen-2017-019992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tattersall M.C., Guo M., Korcarz C.E. Asthma predicts cardiovascular disease events: the Multi-Ethnic Study Of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35(6):1520–1525. doi: 10.1161/ATVBAHA.115.305452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iribarren C., Tolstykh I.V., Miller M.K., Sobel E., Eisner M.D. Adult asthma and risk of coronary heart disease, cerebrovascular disease, and heart failure: a prospective study of 2 matched cohorts. Am J Epidemiol. 2012;176(11):1014–1024. doi: 10.1093/aje/kws181. [DOI] [PubMed] [Google Scholar]
- 9.Tattersall M.C., Barnet J.H., Korcarz C.E., Hagen E.W., Peppard P.E., Stein J.H. Late-onset asthma predicts cardiovascular disease events: the Wisconsin Sleep Cohort. J Am Heart Assoc. 2016;5(9) doi: 10.1161/JAHA.116.003448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yun H.D., Knoebel E., Fenta Y. Asthma and proinflammatory conditions: a population-based retrospective matched cohort study. Mayo Clin Proc. 2012;87(10):953–960. doi: 10.1016/j.mayocp.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iribarren C., Tolstykh I.V., Eisner M.D. Are patients with asthma at increased risk of coronary heart disease? Int J Epidemiol. 2004;33(4):743–748. doi: 10.1093/ije/dyh081. [DOI] [PubMed] [Google Scholar]
- 12.Onufrak S.J., Abramson J.L., Austin H.D., Holguin F., McClellan W.M., Vaccarino L.V. Relation of adult-onset asthma to coronary heart disease and stroke. Am J Cardiol. 2008;101(9):1247–1252. doi: 10.1016/j.amjcard.2007.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang L., Gao S., Yu M., Sheng Z., Tan W. Association of asthma with coronary heart disease: a meta analysis of 11 trials. PLoS One. 2017;12(6) doi: 10.1371/journal.pone.0179335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellia V., Pedone C., Catalano F. Asthma in the elderly: mortality rate and associated risk factors for mortality. Chest. 2007;132(4):1175–1182. doi: 10.1378/chest.06-2824. [DOI] [PubMed] [Google Scholar]
- 15.Schanen J.G., Iribarren C., Shahar E. Asthma and incident cardiovascular disease: the Atherosclerosis Risk in Communities Study. Thorax. 2005;60(8):633–638. doi: 10.1136/thx.2004.026484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu M., Xu J., Yang X. Asthma and risk of cardiovascular disease or all-cause mortality: a meta-analysis. Ann Saudi Med. 2017;37(2):99–105. doi: 10.5144/0256-4947.2017.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feinleib M., Kannel W.B., Garrison R.J., McNamara P.M., Castelli W.P. The Framingham Offspring Study. Design and preliminary data. Prev Med. 1975;4(4):518–525. doi: 10.1016/0091-7435(75)90037-7. [DOI] [PubMed] [Google Scholar]
- 18.Pletcher M.J., Vittinghoff E., Thanataveerat A., Bibbins-Domingo K., Moran A.E. Young adult exposure to cardiovascular risk factors and risk of events later in life: the Framingham Offspring Study. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0154288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. Obesity: preventing and managing the global epidemic. 2000. https://www.who.int/nutrition/publications/obesity/WHO_TRS_894/en/. Accessed March 1, 2021. [PubMed]
- 20.D'Agostino R.B., Sr., Vasan R.S., Pencina M.J. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–753. doi: 10.1161/CIRCULATIONAHA.107.699579. [DOI] [PubMed] [Google Scholar]
- 21.Bang D.W., Wi C.I., Kim E.N. Asthma status and risk of incident myocardial infarction: a population-based case-control study. J Allergy Clin Immunol Pract. 2016;4(5):917–923. doi: 10.1016/j.jaip.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiu H., Gabrielsen A., Agardh H.E. Expression of 5-lipoxygenase and leukotriene A4 hydrolase in human atherosclerotic lesions correlates with symptoms of plaque instability. Proc Natl Acad Sci U S A. 2006;103(21):8161–8166. doi: 10.1073/pnas.0602414103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Card J.W., Zeldin D.C. Hormonal influences on lung function and response to environmental agents: lessons from animal models of respiratory disease. Proc Am Thorac Soc. 2009;6(7):588–595. doi: 10.1513/pats.200904-020RM. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Covelli H., Pek B., Schenkenberger I., Scott-Wilson C., Emmett A., Crim C. Efficacy and safety of fluticasone furoate/vilanterol or tiotropium in subjects with COPD at cardiovascular risk. Int J Chron Obstruct Pulmon Dis. 2016;11:1–12. doi: 10.2147/COPD.S91407. [DOI] [PMC free article] [PubMed] [Google Scholar]