Abstract
The Hospital Readmissions Reduction Program (HRRP) was developed and implemented by the Centers for Medicare & Medicaid Services to curb the rate of 30-day hospital readmissions for certain common, high-impact conditions. In October 2014, COPD became a target condition for which hospitals were penalized for excess readmissions. The appropriateness, utility, and potential unintended consequences of the metric have been a topic of debate since it was first enacted. Nevertheless, there is evidence that hospital policies broadly implemented in response to the HRRP may have been responsible for reducing the rate of readmissions following COPD hospitalizations even before it was added as a target condition. Since the addition of the COPD condition to the HRRP, several predictive models have been developed to predict COPD survival and readmissions, with the intention of identifying modifiable risk factors. A number of interventions have also been studied, with mixed results. Bundled care interventions using the electronic health record and patient education interventions for inhaler education have been shown to reduce readmissions, whereas pulmonary rehabilitation, follow-up visits, and self-management programs have not been consistently shown to do the same. Through this program, COPD has become recognized as a public health priority. However, 5 years after COPD became a target condition for HRRP, there continues to be no single intervention that reliably prevents readmissions in this patient population. Further research is needed to understand the long-term effects of the policy, the role of competing risks in measuring quality, the optimal postdischarge care for patients with COPD, and the integrated use of predictive modeling and advanced technologies to prevent COPD readmissions.
Key Words: COPD, Hospital Readmission Reduction Program, quality of care, readmissions, transitions of care
Abbreviations: CMS, Centers for Medicaid & Medicare Services; HRRP, Hospital Readmissions Reduction Program
In October 2014, the Centers for Medicaid & Medicare Services (CMS) added COPD to the list of conditions targeted by the Hospital Readmission Reduction Program (HRRP).1 The intent of the HRRP, first legislated by the Patient Protection and Affordable Care Act in 2010, was to financially incentivize health-care systems to provide high-quality, patient-centered care to reduce 30-day readmissions for several common and high-impact conditions.2 Since its inception, the HRRP has been criticized as an accountability measure for a range of reasons, including the lack of evidence that reducing readmissions improves patient outcomes and its potential to widen health disparities by overpenalization of safety net hospitals.3, 4, 5 In the case of COPD, additional concerns included difficulty identifying COPD hospitalizations using administrative data and the lack of evidence-based interventions to prevent readmission at the time the penalty was applied.3 Despite these criticisms, the HRRP has increased recognition of the public health importance of COPD and encouraged hospital administrators, clinicians, and researchers to increase efforts to improve COPD care quality and outcomes.
In this review of the literature, we discuss the significant public health burden of COPD, as well as the potential unintended consequences of the HRRP measure. Recent advances resulting from the addition of COPD to the HRRP, including the development of prediction tools and evidence-based interventions to reduce readmissions, are reviewed. We conclude with suggestions for areas of future research.
Materials and Methods
The aim of this review of the literature was to identify best practices and current innovations postimplementation of the CMS HRRP. The medical literature was searched by using PubMed and Google Scholar for articles related to “COPD readmissions” and “HRRP.” The primary focus of this review was to determine updated findings and best practices post-HRRP. However, to set these results within the scope, the pre-HRRP and peri-HRRP evidence is summarized. Because the HRRP is a US federal policy, the primary focus was on studies in the United States.
Public Health Burden of COPD
In the United States, COPD results in 923,000 ED visits and nearly 700,000 hospitalizations each year.6,7 Annual costs of treating COPD are estimated to be $50 billion,8 with 70% of this expense attributed to treating exacerbations requiring hospitalization.9 Approximately 20% of patients discharged following an exacerbation of COPD are readmitted for any reason within 30 days,10, 11, 12, 13 making it the third leading cause of readmissions in the United States.10 The majority of readmissions occur in the first week and are for respiratory-related symptoms.10,11,13 Early evidence suggested that among patients admitted to hospital for an exacerbation of COPD, more than one-third did not receive recommended care, contributing to concerns that the high rates of readmission reflected poor care quality.14,15
COPD as a Target Condition in the HRRP
COPD became a target condition for the HRRP in October 2014 as the second wave of penalties. At the initiation of the penalty, several concerns were raised.3,16,17 First, COPD is notoriously difficult to study at the population level due to the high prevalence of both underdiagnosis and overdiagnosis18 and low sensitivity of detection using administrative codes. Underdiagnosis and overdiagnosis occur due to a lack of use of spirometry to confirm the presence of fixed airflow obstruction and the cumbersome nature of obtaining lung function tests.19 The lack of a validated definition to identify COPD exacerbations using administrative data is problematic, with rates of hospitalization varying widely depending on the administrative definition chosen and sensitivities of various algorithms only 12% to 25%.20,21
Second, due to a significant lack of evidence on how to successfully intervene, there was concern that the true focus of the metric was to reduce cost without an obvious solution to improve the quality of patient care3 (Table 1).22, 23, 24, 25, 26 At the time that the penalty was introduced, individual interventions had shown some promise, such as a “teach-to-goal” patient education intervention for inhaler technique, which had significantly fewer acute care events within 30 days compared with a brief instruction intervention.25 However, other likely intervention targets such as postdischarge follow-up visits, improved guideline-concordance with medication prescribing, and increasing physical activity had less evidence.22,24,26,27 For instance, a randomized study of > 300 participants of an early pulmonary rehabilitation intervention to reduce readmissions had inconclusive findings, whereas a smaller randomized study of 60 patients found reduced acute care visits within 3 months.23,24 Another nested cohort study found missed opportunities to provide standard-of-care controller medications to prevent exacerbations.28 Furthermore, very little was known about programs of care that specifically targeted reducing readmissions, especially within 30 days. Studies examining the effectiveness of various bundles of interventions yielded mixed results. For example, one study conducted in Spain of an “integrated care intervention” that included education, care coordination, and improved access to a specialty case manager reported improved self-management but no differences in medical care.22 Given that approximately 50% of readmissions were due to nonrespiratory-related causes, there was additional concern that interventions targeting only COPD-specific care would be ineffective in reducing all-cause readmissions.29 In a systematic review of interventions to reduce readmissions after hospitalization for COPD exacerbations, the authors argued that there was inadequate evidence available to recommend any specific intervention, calling into question the validity of the penalty itself.27
Table 1.
Interventions to Reduce COPD Readmissions Pre-HRRP
| Author | Publication Year/Countrya/No. of Patients | Intervention | Outcomes | Study Design |
|---|---|---|---|---|
| Bundled programs to reduce readmissions | ||||
| Garcia-Aymerich et al22 | 2007/Spain/113 patients | Integrated care intervention: education + care coordination + improved access | Improved self-management score such as early exacerbation treatment (90% vs 66%) | RCT |
| Nonbundled interventions to reduce readmissions | ||||
| Seymour et al23 | 2010/United Kingdom/60 patients | Intervention: postexacerbation pulmonary rehabilitation | Reduced acute care visits with 3 mo (7% intervention vs 33% usual care; P = .02) | RCT |
| Eaton et al24 | 2009/New Zealand/397 patients | Pulmonary rehabilitation: early inpatient-outpatient rehabilitation | Inconclusive findings | RCT |
| Press et al25 | 2012/50 patients | Inhaler education: teach-to-goal vs brief instructions | Improved inhaler technique and fewer all-cause acute care visits within 30 d | RCT |
| Fidahussein et al26 | 2014/839 patients | Follow-up visits: postdischarge visit within 30 d | Reduced mortality (hazard ratio, 0.28; 95% CI, 0.15-0.5) but not 30 d readmissions | Retrospective cohort |
BPCI = bundled payment for care improvement; HRRP = Hospital Readmissions Reduction Program; RCT = randomized controlled trial.
United States unless specified.
Third, there were concerns that a large proportion of COPD readmissions were not truly preventable. In real time, most systems can identify admissions that are “nonelective” but are not yet sophisticated enough to distinguish admissions that are “preventable” from those that are not. As a result, resources may be directed toward averting readmissions that are not preventable. In 2011, a systematic review reported that the median proportion of hospital readmissions that were found to be “avoidable” was only 27%.30 This was approximately three times lower than originally anticipated.
Fourth, the metric did not account for ways in which lower readmission was misleading. For instance, there is a “competing risk” of death. Those who are most ill may die prior to being readmitted. Furthermore, as a result of this program, patients may be denied admission because the program may unintentionally incentivize hospitals not to readmit patients with target conditions. Either of these issues could produce falsely low readmission rates but not in a patient-centered way.
Finally, there were concerns about the penalty’s potential to widen health disparities.4,5 Safety net hospitals, which provide a significant level of care to underinsured and low-income vulnerable populations, had been shown to have higher rates of readmission and to incur high penalties for other targeted conditions, including acute myocardial infarction, pneumonia, and congestive heart failure.31,32 Among patients hospitalized for COPD, patients who were black, living in poverty, and receiving care at minority-serving institutions had higher rates of readmission than those living in high-income areas, receiving care at nonminority serving institutions, and belonging to other racial groups.33,34 Despite these findings, CMS did not initially include race or socioeconomic status in their case-mix adjustments.
The Impact of the HRRP
Half a decade after the penalty was introduced, debate continues about the utility, appropriateness, and unintended consequences of the measure.35 Several studies have examined the clinical and financial impacts of the HRRP. One study showed that readmissions for COPD decreased in 2013 before it even became a target condition.12 The off-target reduction was believed to be due to the fact that readmission prevention strategies focused on physician behavior and that hospital discharge practices were broadly implemented to all patients, rather than only those with target conditions. Studies have shown an association between the HRRP implementation and increased death following hospitalization for heart failure and pneumonia. One study found this to be true also for COPD.36,37
In addition to reducing readmissions, another objective of the HRRP was to lower cost and thereby increase the value of care provided. However, few intervention studies in the post-HRRP period have analyzed costs. The limited published studies showed mixed results.38 One study of a bundled intervention program found that readmissions and costs were reduced,39 whereas another study of a bundled payment for care innovation program found no significant reductions in either readmissions or costs.40
What Have We Learned About COPD Readmissions Post-HRRP Implementation?
Identifying Predictors of COPD Readmissions
To prevent readmissions, researchers have sought to identify risk factors, preferably modifiable ones, that are associated with readmissions following a hospitalization for COPD. Some risk factors for readmission following COPD were identified across multiple time points, including 30 days, 90 days, and 1 year. Key factors identified included dyspnea severity, prior admissions, pneumonia, physical activity, and depression.41, 42, 43 A systematic review from 2007 that identified risk factors for hospitalization and re-hospitalizations, not necessarily specific to 30 days, found three predictive factors (prior hospitalization, dyspnea, and being prescribed oral corticosteroids).44 However, following the introduction of HRRP, an increasing focus on readmission risk factors has led to further research in this area, particularly around 30 days. Novel risk factors have emerged, including longer length of stay during the index hospitalization,45 functional status prior to discharge from index admission,46 dual enrollment in Medicaid and Medicare,13 and higher blood eosinophil levels.47 Frailty has been identified as a predictor of 90-day readmissions.48 Despite the identification of factors associated with higher risk of readmission, many are not modifiable, and patients often have multiple risk factors making it difficult to use any single lever to influence care. As a result, focus has shifted to multi-factor prediction tools and bundled interventions.
Risk Prediction Tools to Identify High Risk Patients and Target Interventions
Many studies published in the last decade have tried to identify which patients with COPD would be readmitted, when, for what reason, and whether the readmission was preventable (Table 2).47, 48, 49, 50, 51 Much of this work was done outside the United Sates, despite the penalties being relevant only to hospitals contracting with CMS. The aim of these studies was to identify tool(s) that could help triage the right care to the right patients at the right time to prevent readmissions. To date, a widely accepted prediction tool does not exist, although several have been developed, some that are specific to COPD and others that target a broader population.49, 50, 51, 52, 53
Table 2.
Models and Predictive Analytic Tools to be Used to Decrease Readmissions
| Author | Model | Publication Year/Countrya | Target Cohort/No. of Patients | Predictors | Outcomes | Method of Derivation/Performance for Predictive Model |
|---|---|---|---|---|---|---|
| 30-day readmission prediction tools not specific to COPD | ||||||
| Donzé et al49 | HOSPITAL score | 2013 | Mixed/117,065 patients | Low hemoglobin at discharge (< 12 g/dL), Discharge from an oncology service, sodium <135 mEq/L, procedure during hospital stay, index admission type urgent or emergent, number of hospital admissions during the previous year (0-1, 2-5, > 5), length of stay ≥ 5 d | 30 d readmission | Logistic regression/AUC, 0.72 |
| van Walraven et al50 | LACE Index | 2010/Canada | Mixed/4,812 patients | Length of stay (< 1 d to ≥ 14 d), acute or emergent admission, Charlson Comorbidity Index score, visit to ED in previous 6 mo (0 to ≥ 4) | 30 d readmission | Logistic regression/AUC, 0.68 |
| COPD-specific readmission prediction tool | ||||||
| Echevarria et al51 | PEARL score | 2017/United Kingdom | COPD/2,417 patients | Previous admissions, eMRCD score, age, right-sided heart failure, left-sided heart failure | 90 d readmission | Logistic regression/AUC, 0.70 in external validation cohort |
| COPD-specific predictors/risk factors | ||||||
| Bernabeu-Mora et al48 | Frailty | 2017/Spain | COPD/102 patients with prospective observation | Frailty (mild, moderate, severe), age, number of hospitalizations because of exacerbations in the previous year, length of hospital stay | 90 d readmission | Logistic regression |
| Couillard et al47 | Eosinophils | 2017/Canada | COPD/167 patients | Eosinophils on admission (≥ 200 cells/μL and/or ≥2% of WBC count) Variables available for selection: age, sex, comorbidities, Charlson Comorbidity Index, smoking status, FEV1, FEV1/FVC ratio, GOLD stage, hospitalized for COPD in previous year, home oxygen use, inhaler use, WBC count on admission, inpatient therapy with steroid/antibiotic |
365 d readmission all-cause and COPD specific | Logistic regression with stepwise selection and Cox proportional hazards regression |
AUC = area under the curve; eMRCD = Extended Medical Research Council Dyspnea Score; GOLD = Global Initiative for Chronic Obstructive Lung Disease.
United States unless specified.
Two of the general 30-day readmission risk scores include the HOSPITAL score and the LACE Index.49,50 The HOSPITAL score was validated specifically to identify preventable hospital readmissions agnostic to condition for use following hospital discharge.49 The accuracy of the HOSPITAL score was tested for use among general medical patients, with a c-statistic of 0.71 in the validation cohort; follow-up testing found similar accuracy for use among patients with COPD.54 The PEARL score was specifically developed to predict 90-day readmission for patients with COPD.51 It incorporates previous admissions, Extended Medical Research Council Dyspnea score, and age, as well as presence of right-sided heart failure and left-sided heart failure. Examining 2,417 consecutive admissions, the PEARL score has a c-statistic of 0.70 in the external validation cohort. Higher PEARL scores were associated with shorter time to readmission. There is a need for scores that predict shorter term outcomes and are amenable to being embedded into the electronic health record for real-time use. The ability to tailor therapies to individual patients using prediction algorithms would allow us to approach precision medicine for patients with COPD.52,55 Moreover, Baechle et al56 propose that incorporation of costs into models of readmission prevention will be an important expansion of current models.
Interventions to Reduce COPD readmissions
Half a decade has passed since CMS implemented the HRRP for COPD. Many, if not most, hospitals across the United States have adjusted their care practices to improve COPD care and reduce readmissions (Table 3).22,39,40,57, 58, 59, 60, 61 However, few successful programs have been identified, due to a lack of rigorous evaluation of most programs as well as mixed results of randomized interventions. Program-level reports of success were identified in a recent American Thoracic Society Workshop Report published in 2019.55 Elements that were similar across several multicomponent readmission reduction programs included bundled interventions and interventions across transition of care settings. Several of the programs identified a core team member, such as an advanced practice nurse, who led the intervention bundle.
Table 3.
Interventions to Reduce COPD Readmissions Post-HRRP
| Author | Publication Year/Countrya/No. of Patients | Intervention | Outcomes | Study Design |
|---|---|---|---|---|
| Bundled programs | ||||
| Jennings et al58 | 2015/172 patients | Predischarge bundle: education + smoking cessation + co-morbidity screening + 48 h telephone calls | No significant differences in 30 d readmissions | RCT |
| Parikh et al39b | 2016/44 patients | EHR-based COPD care bundle: standardized nursing protocols, medications, education, postdischarge visits | Significant reductions in 30 d readmissions (9.1% vs 54.4% in control subjects); significant reductions in 90 d hospital costs ($7,652 vs $19,954 in control subjects) | Prospective cohort |
| Zafar et al57b | 2017/204 patients in intervention phase | Care bundle: 5- component COPD care bundle | Significant reductions in 30 d readmissions (22.7% to 14.7% over time); reductions increased with more components provided | QI |
| Johnson-Warrington et al60 | 2016/United Kingdom/78 patients | Activity, coping, education | Multilevel self-management program: no differences in readmissions or mortality at 3 mo | RCT |
| Bhatt et al40 | 2017/78 consecutive Medicare patients | Bundled payment for care innovation: transition of care interventions, including postdischarge follow-up phone calls, vaccines, home health care, pulmonary rehabilitation, pulmonary clinic visits | No differences in 30 d readmissions or costs; improved quality of care provided | BPCI/QI |
| Aboumatar et al59 | 2019/240 patients | Long-term self-management and transitional care: transitional care and long-term self-management | Unexpected increase in 6 mo ED/hospital revisits without improvement in quality of care | RCT |
| Nonbundled interventions | ||||
| Press et al25 | 2016/120 patients | Patient education: teach-to-goal inhaler education | Reduced 30 d acute care visits (ED and/or hospital) compared to brief instructions (17% vs 36%) but not at 90 d | RCT |
| Budde et al61 | 2019/2,653 patients | Follow-up visits: postdischarge visit within 10 d | No difference in 30 d readmissions | Retrospective |
Post-HRRP is 2016 and later. BPCI = bundled payment for care improvement; HER = electronic health record; HRRP = Hospital Readmissions Reduction Program; QI = quality improvement; RCT = randomized controlled trial.
United States unless specified.
HRRP specific.
HRRP-Specific Programs
Only limited studies have been published about programs specifically focused on reducing 30-day readmissions directly in response to the HRRP. These include a nonrandomized study of an electronic COPD care bundle (ie, order set) vs usual care that found significantly reduced readmissions within 30 days.39 This care bundle focused on standardizing nursing protocols, medications, and patient education, as well as postdischarge follow-up visits. This study also found significant reductions in 90-day hospital costs (intervention, $7,652; control subjects, $19,954). Another study of a five-component COPD care bundle found that readmission rates decreased from 23% to 15% and that receiving more components of the bundle led to greater reductions.57 The five components included: (1) appropriate inhaler regimen; (2) 30-day supply of inhalers (insurance compatible); (3) personalized inhaler education; (4) discharge instructions with COPD education; and (5) follow-up within 15 days.
Non-HRRP Specific Programs
There were also studies on intervention programs that did not specifically target their response to HRRP but could shed light on the topic. For instance, published just at the conclusion of the first year of the HRRP, Jennings et al58 published a study on a predischarge bundle. This randomized study of a “predischarge bundle” that included patient education, smoking cessation counseling, co-morbidity screening, and 48 h postdischarge calls found no significant reductions in readmission within 30 days of discharge. In another study, Bhatt et al40 evaluated a Bundled Payment for Care Innovation program and found that although patients in this program were more likely to receive postdischarge follow-up telephone calls, vaccines, home health care, pulmonary rehabilitation, and pulmonary clinic care, there was no reduction in all-cause 30-day readmissions compared with a historical group in 2012. In addition, no differences in costs were found. In a randomized controlled trial of transitional care and long-term self-management for patients with COPD, acute care utilization (ED visits, hospitalization) actually increased within 6 months in the intervention group, and no improvements in quality of care were found.59 A randomized study of a self-management program that focused on activity, coping, and education found no reduced readmissions or mortality at 3 months; however, twice the number of control (n = 10) vs intervention (n = 5) participants were readmitted within 30 days (P = not significant).60 A study with a larger sample size is needed to see if there are short-term (30-day) effects of this intervention.
Individual Interventions
Finally, there has been some additional evidence published on individual interventions focused on post-hospital ambulatory care that may help improve COPD care and reduce readmissions. Regarding early postdischarge follow-up, one retrospective study did not find that this intervention reduced 30-day readmissions.61 However, based on the study by Zafar et al57 that showed multiple care bundle components led to reduced readmissions, follow-up visits may be a necessary but insufficient intervention for reducing 30-day readmissions. Also with respect to ambulatory care, Department of Veterans Affairs vs non-Department of Veterans Affairs care use was not associated with decreased 30-day readmissions.62 Finally, with respect to inhaler education, another study re-demonstrated that teach-to-goal is more effective than “brief instructions” for improving inhaler technique and reducing all-cause acute care visits. However, effects were only seen in the short term, with technique waning by 30 days and effects on acute care utilization waning by 90 days.22
Policy Change Regarding Health Disparities
As noted earlier, when the HRRP was first implemented, there were concerns about widening health disparities, as there was very minimal risk adjustment. Subsequent research showed that lower resourced institutions were facing greater penalties and potentially realizing widening health disparities.63, 64, 65, 66 Due to ongoing concerns, starting in fiscal year 2019, CMS began stratifying according to hospitals treating Medicare populations with similar poverty levels, a move that is projected to substantially decrease subsequent penalties among safety net hospitals.67,68 Equity is identified as an important domain of quality health care by the Institute of Medicine,69 and widening health disparities through a quality incentive program would be an unfortunate and unintended consequence. Further work is needed to ensure that the change in policy was effective at mitigating the disparity.
Future Research Needs
Despite the introduction of HRRP to galvanize efforts to reduce COPD readmissions, much work remains to improve the quality of care for patients with COPD in the peri-hospitalization period. The factors leading to the initial admission and subsequent readmission(s) are multifactorial and interrelated; COPD is a multimorbid disease.70 We need ongoing rigorous and thoughtful research to identify tools, interventions, and methods of implementation that can improve the quality of care provided to patients with COPD throughout their care, not only at the time of discharge but in the postdischarge period. Consideration of comorbidities, including cardiac comorbidities, anxiety, and depression, is critical for managing this population on a broad scale. Prior reviews, including one published in CHEST in 2016, have identified several emerging interventions requiring further evaluation, including telehealth, “meds to beds” approaches, and “hospital-at-home.”71 For instance, Levine et al72 found lower costs and hospitalizations among participants receiving care through a hospital-at-home program, many of whom had a primary diagnosis of COPD.
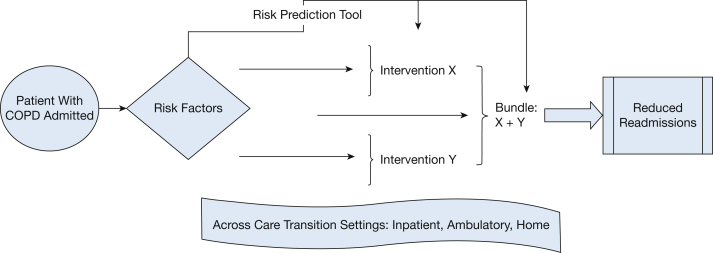
We present a conceptual framework to outline future research needs related to COPD readmissions (Fig 1). This framework considers the spectrum of tools and interventions that can be used to intervene to reduce readmissions. Built into this framework is the concept that these tools and interventions can be developed, evaluated, and implemented across the continuum of care.
Figure 1.
Conceptual model of preventing readmissions.
Transitions of care remain a focal point in preventing readmissions. There are several published hospital-to-home transition-of-care mechanisms that could be used to improve care, across which tools and interventions can be used to reduce readmissions.73 These include the Better Outcomes for Older Adults Through Safe Transitions,74 Care Transitions Intervention,75 Project Re-engineered Discharge,76 and Transitional Care Model.77 Common components of these models include addressing medications (eg, medication reconciliation and/or education), clarifying discharge instructions, patient education, and patient-centered care. Some of the models also address the need to identify high-risk patients, schedule postdischarge follow-up visits, and/or to incorporate at-home interventions such as telephone calls and/or home visits. All four transitions of care frameworks have had success with reducing readmissions and costs while also improving patient outcomes. Furthermore, Burke et al78 suggest an approach to improving transitional care that looks beyond readmissions and the readmission penalties. This is important when thinking about the relationship between quality of care and the readmission metric. Kangovi and Grande79 propose a framework that expands the hospital readmission burden from inpatient to both inpatient and outpatient care.
Future research endeavors could use advanced techniques in bioinformatics, such as machine learning and natural language processing, to improve our ability to predict not only readmissions but also COPD exacerbations.80,81 Understanding the timing and trajectories of patients having exacerbations as an inpatient or outpatient could allow resources to be deployed appropriately to treat patients at home or over the telephone if safe. Embedding such risk prediction tools into the electronic health record instead of relying on manual calculation of risk could allow for wider dissemination and use in real time.
Ongoing testing of individual and bundled interventions is also needed. When testing bundled interventions, all efforts should be made to evaluate which components and/or how many components are required to be most effective. Efforts should also be made to test interventions that could be implemented at scale as well as develop implementation approaches that are evidence based. For instance, evaluation of whether interventions are best provided in-person or via Web-based technology, such as video-visits, on-line learning modules, apps, or even games, is needed.
Finally, it is critical that combined quality and cost analyses are conducted to understand whether the HRRP had impact in these two domains. Because the value of care incorporates both quality and costs of care, understanding if overall value of care has improved is critical to continue to iterate on the current HRRP program and/or develop new policy.
Conclusions
The HRRP brought significant attention to the field of COPD and has expanded our knowledge about interventions and prediction models to prevent COPD readmissions. Although heavily criticized, the HRRP did effectively reduce COPD readmissions even before COPD became a target condition. However, studies aimed at reducing readmissions for COPD have shown mixed results, and no gold standard has emerged for how to reduce COPD readmissions effectively on a population level. Questions remain as to whether COPD was an optimal target condition and if other quality metrics for COPD may have better traction for improving care. In addition, despite reductions in readmissions since the HRRP penalty went into effect, increased mortality following discharge for COPD admissions has also been recently published.37 Further research is necessary to understand the full extent of the policy, including longer term effects, the role of competing risks in measuring quality, the optimal postdischarge care for patients with COPD, and the integrated use of predictive modeling and advanced technologies to prevent COPD readmissions. Interventions should be prioritized that focus on improving patients’ health, not solely decreasing cost, as well as those that can feasibly be implemented at scale. As more rigorous and comprehensive data become available about effective tools and interventions, it will be important to use this evidence to inform future policy to improve health-care quality for patients with COPD.
Acknowledgments
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: V. G. P. has received funding from National Institutes of Health (NIH) R03 and R01 and ALA and has consulted for Vizient Inc and Humana. L. C. F. has received funding from VA-I01 HX002499. None declared (L. C. M.).
References
- 1.Centers for Medicare and Medicaid Services Value based programs. Hospital Readmissions Reduction Program (HRRP) https://www.cms.gov/Medicare/Quality-Initiatives-Patient-Assessment-Instruments/Value-Based-Programs/HRRP/Hospital-Readmission-Reduction-Program
- 2.Centers for Medicare and Medicaid Services Acute inpatient PPS. Hospital Readmissions Reduction Program (HRRP) https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program
- 3.Feemster L.C., Au D.H. Penalizing hospitals for chronic obstructive pulmonary disease readmissions. Am J Respir Crit Care Med. 2014;189(6):634–639. doi: 10.1164/rccm.201308-1541PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joynt K.E., Jha A.K. Thirty-day readmissions—truth and consequences. N Engl J Med. 2012;366(15):1366–1369. doi: 10.1056/NEJMp1201598. [DOI] [PubMed] [Google Scholar]
- 5.Berenson J., Shih A. Higher readmissions at safety-net hospitals and potential policy solutions. Issue Brief Commonw Fund. 2012;34:1–16. [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention FastStats. Chronic obstructive pulmonary disease (COPD) https://www.cdc.gov/nchs/fastats/copd.htm
- 7.Ford E.S. Hospital discharges, readmissions, and ED visits for COPD or bronchiectasis among US adults. Chest. 2015;147(4):989–998. doi: 10.1378/chest.14-2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torabipour A., Hakim A., Ahmadi Angali K., Dolatshah M., Yusofzadeh M. Cost analysis of hospitalized patients with chronic obstructive pulmonary disease: a state-level cross-sectional study. Tanaffos. 2016;15(2):75–82. [PMC free article] [PubMed] [Google Scholar]
- 9.Sullivan S.D., Ramsey S.D., Lee T.A. The economic burden of COPD. Chest. 2000;117(suppl 2):5S–9S. doi: 10.1378/chest.117.2_suppl.5s. [DOI] [PubMed] [Google Scholar]
- 10.Jencks S.F., Williams M.V., Coleman E.A. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360(14):1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 11.Goto T., Faridi M.K., Camargo C.A., Hasegawa K. Time-varying readmission diagnoses during 30 days after hospitalization for COPD exacerbation. Med Care. 2018;56(8):673–678. doi: 10.1097/MLR.0000000000000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Myers L.C., Faridi M.K., Hasegawa K., Hanania N.A., Camargo C.A. The Hospital Readmissions Reduction Program and readmissions for chronic obstructive pulmonary disease, 2006-2015. Ann Am Thorac Soc. 2020;17(4):450–456. doi: 10.1513/AnnalsATS.201909-672OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah T., Churpek M.M., Coca Perraillon M., Konetzka R.T. Understanding why patients with COPD get readmitted: a large national study to delineate the Medicare population for the readmissions penalty expansion. Chest. 2015;147(5):1219–1226. doi: 10.1378/chest.14-2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lindenauer P.K., Pekow P., Gao S., Crawford A.S., Gutierrez B., Benjamin E.M. Quality of care for patients hospitalized for acute exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. 2006;144(12):894–903. doi: 10.7326/0003-4819-144-12-200606200-00006. [DOI] [PubMed] [Google Scholar]
- 15.Mularski R.A., Asch S.M., Shrank W.H. The quality of obstructive lung disease care for adults in the United States as measured by adherence to recommended processes. Chest. 2006;130(6):1844–1850. doi: 10.1378/chest.130.6.1844. [DOI] [PubMed] [Google Scholar]
- 16.Joynt K.E., Jha A.K. A path forward on Medicare readmissions. N Engl J Med. 2013;368(13):1175–1177. doi: 10.1056/NEJMp1300122. [DOI] [PubMed] [Google Scholar]
- 17.Shah T., Press V.G., Huisingh-Scheetz M., White S.R. COPD readmissions: addressing COPD in the era of value-based health care. Chest. 2016;150(4):916–926. doi: 10.1016/j.chest.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Diab N., Gershon A.S., Sin D.D. Underdiagnosis and overdiagnosis of chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2018;198(9):1130–1139. doi: 10.1164/rccm.201804-0621CI. [DOI] [PubMed] [Google Scholar]
- 19.Press V.G., Cifu A.S., White S.R. Screening for chronic obstructive pulmonary disease. JAMA. 2017;318(17):1702–1703. doi: 10.1001/jama.2017.15782. [DOI] [PubMed] [Google Scholar]
- 20.Stein B.D., Bautista A., Schumock G.T. The validity of International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis codes for identifying patients hospitalized for COPD exacerbations. Chest. 2012;141(1):87–93. doi: 10.1378/chest.11-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stein B.D., Charbeneau J.T., Lee T.A. Hospitalizations for acute exacerbations of chronic obstructive pulmonary disease: how you count matters. COPD. 2010;7(3):164–171. doi: 10.3109/15412555.2010.481696. [DOI] [PubMed] [Google Scholar]
- 22.Garcia-Aymerich J., Hernandez C., Alonso A. Effects of an integrated care intervention on risk factors of COPD readmission. Respir Med. 2007;101(7):1462–1469. doi: 10.1016/j.rmed.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Seymour J.M., Moore L., Jolley C.J. Outpatient pulmonary rehabilitation following acute exacerbations of COPD. Thorax. 2010;65(5):423–428. doi: 10.1136/thx.2009.124164. [DOI] [PubMed] [Google Scholar]
- 24.Eaton T., Young P., Fergusson W. Does early pulmonary rehabilitation reduce acute health-care utilization in COPD patients admitted with an exacerbation? A randomized controlled study. Respirol Carlton Vic. 2009;14(2):230–238. doi: 10.1111/j.1440-1843.2008.01418.x. [DOI] [PubMed] [Google Scholar]
- 25.Press V.G., Arora V.M., Trela K.C. Effectiveness of interventions to teach metered-dose and Diskus inhaler techniques. A randomized trial. Ann Am Thorac Soc. 2016;13(6):816–824. doi: 10.1513/AnnalsATS.201509-603OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fidahussein S.S., Croghan I.T., Cha S.S., Klocke D.L. Posthospital follow-up visits and 30-day readmission rates in chronic obstructive pulmonary disease. Risk Manag Healthc Policy. 2014;7:105–112. doi: 10.2147/RMHP.S62815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Prieto-Centurion V., Markos M.A., Ramey N.I. Interventions to reduce rehospitalizations after chronic obstructive pulmonary disease exacerbations. A systematic review. Ann Am Thorac Soc. 2014;11(3):417–424. doi: 10.1513/AnnalsATS.201308-254OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Melzer A.C., Feemster L.M., Uman J.E., Ramenofsky D.H., Au D.H. Missing potential opportunities to reduce repeat COPD exacerbations. J Gen Intern Med. 2013;28(5):652–659. doi: 10.1007/s11606-012-2276-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Press V.G., Miller B.J. The Hospital Readmissions Reduction Program and COPD: more answers, more questions. J Hosp Med. 2020;15(2):e1–e2. doi: 10.12788/jhm.3362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Walraven C., Bennett C., Jennings A., Austin P.C., Forster A.J. Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ. 2011;183(7):E391–E402. doi: 10.1503/cmaj.101860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Joynt K.E., Jha A.K. Characteristics of hospitals receiving penalties under the Hospital Readmissions Reduction Program. JAMA. 2013;309(4):342–343. doi: 10.1001/jama.2012.94856. [DOI] [PubMed] [Google Scholar]
- 32.Joynt K.E., Orav E.J., Jha A.K. Thirty-day readmission rates for Medicare beneficiaries by race and site of care. JAMA. 2011;305(7):675–681. doi: 10.1001/jama.2011.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Elixhauser A., Au D.H., Podulka J. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Agency for Healthcare Research and Quality (US); 2006. Readmissions for Chronic Obstructive Pulmonary Disease, 2008: Statistical Brief #121.http://www.ncbi.nlm.nih.gov/books/NBK65392/ [PubMed] [Google Scholar]
- 34.Prieto-Centurion V., Gussin H.A., Rolle A.J., Krishnan J.A. Chronic obstructive pulmonary disease readmissions at minority-serving institutions. Ann Am Thorac Soc. 2013;10(6):680–684. doi: 10.1513/AnnalsATS.201307-223OT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rinne S.T., Press V.G. Moving the bar on chronic obstructive pulmonary disease readmissions before and after the Hospital Readmission Reduction Program: myth or reality? Ann Am Thorac Soc. 2020;17(4):423–425. doi: 10.1513/AnnalsATS.202001-010ED. [DOI] [PubMed] [Google Scholar]
- 36.Wadhera R.K., Joynt Maddox K.E., Wasfy J.H., Haneuse S., Shen C., Yeh R.W. Association of the Hospital Readmissions Reduction Program with mortality among Medicare beneficiaries hospitalized for heart failure, acute myocardial infarction, and pneumonia. JAMA. 2018;320(24):2542–2552. doi: 10.1001/jama.2018.19232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.PueblaNeira DA, Hsu ES, Kuo YF, Ottenbacher KJ, Sharma G. Readmissions Reduction Program, mortality and readmissions for chronic obstructive pulmonary disease [published online ahead of print September 1, 2020]. Am J Respir Crit Care Med. https://doi.org/10.1164/rccm.202002-0310OC. [DOI] [PMC free article] [PubMed]
- 38.Press V.G., Konetzka R.T., White S.R. Insights about the economic impact of chronic obstructive pulmonary disease readmissions post implementation of the Hospital Readmission Reduction Program. Curr Opin Pulm Med. 2018;24(2):138–146. doi: 10.1097/MCP.0000000000000454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Parikh R., Shah T.G., Tandon R. COPD exacerbation care bundle improves standard of care, length of stay, and readmission rates. Int J Chron Obstruct Pulmon Dis. 2016;11:577–583. doi: 10.2147/COPD.S100401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bhatt S.P., Wells J.M., Iyer A.S. Results of a Medicare bundled payments for care improvement initiative for chronic obstructive pulmonary disease readmissions. Ann Am Thorac Soc. 2017;14(5):643–648. doi: 10.1513/AnnalsATS.201610-775BC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steer J., Norman E.M., Afolabi O.A., Gibson G.J., Bourke S.C. Dyspnoea severity and pneumonia as predictors of in-hospital mortality and early readmission in acute exacerbations of COPD. Thorax. 2012;67(2):117–121. doi: 10.1136/thoraxjnl-2011-200332. [DOI] [PubMed] [Google Scholar]
- 42.Chawla H., Bulathsinghala C., Tejada J.P., Wakefield D., ZuWallack R. Physical activity as a predictor of thirty-day hospital readmission after a discharge for a clinical exacerbation of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11(8):1203–1209. doi: 10.1513/AnnalsATS.201405-198OC. [DOI] [PubMed] [Google Scholar]
- 43.Coventry P.A., Gemmell I., Todd C.J. Psychosocial risk factors for hospital readmission in COPD patients on early discharge services: a cohort study. BMC Pulm Med. 2011;11:49. doi: 10.1186/1471-2466-11-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bahadori K., FitzGerald J.M. Risk factors of hospitalization and readmission of patients with COPD exacerbation—systematic review. Int J Chron Obstruct Pulmon Dis. 2007;2(3):241–251. [PMC free article] [PubMed] [Google Scholar]
- 45.Rinne S.T., Graves M.C., Bastian L.A. Association between length of stay and readmission for COPD. Am J Manag Care. 2017;23(8):e253–e258. [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen H.Q., Rondinelli J., Harrington A. Functional status at discharge and 30-day readmission risk in COPD. Respir Med. 2015;109(2):238–246. doi: 10.1016/j.rmed.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 47.Couillard S., Larivée P., Courteau J., Vanasse A. Eosinophils in COPD exacerbations are associated with increased readmissions. Chest. 2017;151(2):366–373. doi: 10.1016/j.chest.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 48.Bernabeu-Mora R., García-Guillamón G., Valera-Novella E., Giménez-Giménez L.M., Escolar-Reina P., Medina-Mirapeix F. Frailty is a predictive factor of readmission within 90 days of hospitalization for acute exacerbations of chronic obstructive pulmonary disease: a longitudinal study. Ther Adv Respir Dis. 2017;11(10):383–392. doi: 10.1177/1753465817726314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Donzé J., Aujesky D., Williams D., Schnipper J.L. Potentially avoidable 30-day hospital readmissions in medical patients: derivation and validation of a prediction model. JAMA Intern Med. 2013;173(8):632–638. doi: 10.1001/jamainternmed.2013.3023. [DOI] [PubMed] [Google Scholar]
- 50.van Walraven C., Dhalla I.A., Bell C. Derivation and validation of an index to predict early death or unplanned readmission after discharge from hospital to the community. CMAJ. 2010;182(6):551–557. doi: 10.1503/cmaj.091117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Echevarria C., Steer J., Heslop-Marshall K. The PEARL score predicts 90-day readmission or death after hospitalisation for acute exacerbation of COPD. Thorax. 2017;72(8):686–693. doi: 10.1136/thoraxjnl-2016-209298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Press V.G. Is it time to move on from identifying risk factors for 30-day chronic obstructive pulmonary disease readmission? A call for risk prediction tools. Ann Am Thorac Soc. 2018;15(7):801–803. doi: 10.1513/AnnalsATS.201804-246ED. [DOI] [PubMed] [Google Scholar]
- 53.Press V.G., Gershon A., Blagev D.P. Predicting COPD and lung function decline among a general population: too good to be true? Chest. 2020;157(3):481–483. doi: 10.1016/j.chest.2019.11.028. [DOI] [PubMed] [Google Scholar]
- 54.Burke R.E., Schnipper J.L., Williams M.V. The HOSPITAL score predicts potentially preventable 30-day readmissions in conditions targeted by the Hospital Readmissions Reduction Program. Med Care. 2017;55(3):285–290. doi: 10.1097/MLR.0000000000000665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Press V.G., Au D.H., Bourbeau J. Reducing chronic obstructive pulmonary disease hospital readmissions. An official American Thoracic Society Workshop Report. Ann Am Thorac Soc. 2019;16(2):161–170. doi: 10.1513/AnnalsATS.201811-755WS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baechle C., Agarwal A., Behara R., Zhu X. In: Proceedings from the 2017 IEEE EMBS International Conference on Biomedical Health Informatics (BHI) Jeju Island; Korea: July 11, 2017. A cost sensitive approach to predicting 30-day hospital readmission in COPD patients; pp. 317–320. [Google Scholar]
- 57.Zafar M.A., Panos R.J., Ko J. Reliable adherence to a COPD care bundle mitigates system-level failures and reduces COPD readmissions: a system redesign using improvement science. BMJ Qual Saf. 2017;26(11):908–918. doi: 10.1136/bmjqs-2017-006529. [DOI] [PubMed] [Google Scholar]
- 58.Jennings J.H., Thavarajah K., Mendez M.P., Eichenhorn M., Kvale P., Yessayan L. Predischarge bundle for patients with acute exacerbations of COPD to reduce readmissions and ED visits: a randomized controlled trial. Chest. 2015;147(5):1227–1234. doi: 10.1378/chest.14-1123. [DOI] [PubMed] [Google Scholar]
- 59.Aboumatar H., Naqibuddin M., Chung S. Effect of a hospital-initiated program combining transitional care and long-term self-management support on outcomes of patients hospitalized with chronic obstructive pulmonary disease: a randomized clinical trial. JAMA. 2019;322(14):1371–1380. doi: 10.1001/jama.2019.11982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnson-Warrington V., Rees K., Gelder C., Morgan M.D., Singh S.J. Can a supported self-management program for COPD upon hospital discharge reduce readmissions? A randomized controlled trial. Int J Chron Obstruct Pulmon Dis. 2016;11:1161–1169. doi: 10.2147/COPD.S91253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Budde J., Agarwal P., Mazumdar M., Braman S.S. Follow-up soon after discharge may not reduce COPD readmissions. Chronic Obstr Pulm Dis. 2019;6(2):129–131. doi: 10.15326/jcopdf.6.2.2018.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rinne S.T., Elwy A.R., Bastian L.A., Wong E.S., Wiener R.S., Liu C.F. Impact of multisystem health care on readmission and follow-up among veterans hospitalized for chronic obstructive pulmonary disease. Med Care. 2017;55:S20. doi: 10.1097/MLR.0000000000000708. [DOI] [PubMed] [Google Scholar]
- 63.Chaiyachati K.H., Qi M., Werner R.M. Changes to racial disparities in readmission rates after Medicare’s hospital readmissions reduction program within safety-net and non–safety-net hospitals. JAMA Netw Open. 2018;1(7) doi: 10.1001/jamanetworkopen.2018.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Figueroa J.F., Zheng J., Orav E.J., Epstein A.M., Jha A.K. Medicare program associated with narrowing hospital readmission disparities between black and white patients. Health Aff (Millwood) 2018;37(4):654–661. doi: 10.1377/hlthaff.2017.1034. [DOI] [PubMed] [Google Scholar]
- 65.Sheingold S.H., Zuckerman R., Shartzer A. Understanding Medicare hospital readmission rates and differing penalties between safety-net and other hospitals. Health Aff (Millwood) 2016;35(1):124–131. doi: 10.1377/hlthaff.2015.0534. [DOI] [PubMed] [Google Scholar]
- 66.Gilman M., Hockenberry J.M., Adams E.K., Milstein A.S., Wilson I.B., Becker E.R. The financial effect of value-based purchasing and the hospital readmissions reduction program on safety-net hospitals in 2014: a cohort study. Ann Intern Med. 2015;163(6):427–436. doi: 10.7326/M14-2813. [DOI] [PubMed] [Google Scholar]
- 67.Gabay M. 21st Century Cures Act. Hosp Pharm. 2017;52(4):264–265. doi: 10.1310/hpj5204-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Joynt Maddox K.E., Reidhead M., Qi A.C., Nerenz D.R. Association of stratification by dual enrollment status with financial penalties in the Hospital Readmissions Reduction Program. JAMA Intern Med. 2019;179(6):769–776. doi: 10.1001/jamainternmed.2019.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Agency for Healthcare Research and Quality Six domains of health care quality. http://www.ahrq.gov/talkingquality/measures/six-domains.html [DOI] [PubMed]
- 70.Hughes M.J., McGettrick H.M., Sapey E. Shared mechanisms of multimorbidity in COPD, atherosclerosis and type-2 diabetes: the neutrophil as a potential inflammatory target. Eur Respir Rev. 2020;29(155):190102. doi: 10.1183/16000617.0102-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Freedman N. Reducing COPD readmissions: strategies for the pulmonologist to improve outcomes. Chest. 2019;156(4):802–807. doi: 10.1016/j.chest.2019.06.005. [DOI] [PubMed] [Google Scholar]
- 72.Levine D.M., Ouchi K., Blanchfield B. Hospital-level care at home for acutely ill adults: a randomized controlled trial. Ann Intern Med. 2020;172(2):77–85. doi: 10.7326/M19-0600. [DOI] [PubMed] [Google Scholar]
- 73.The Agency for Healthcare Research and Quality Care transitions models. https://www.aaacn.org/sites/default/files/documents/SummaryComparisonTables.pdf [DOI] [PubMed]
- 74.Society of Hospital Medicine Advancing Successful Care Transitions to Improve Outcomes. https://www.hospitalmedicine.org/clinical-topics/care-transitions/
- 75.The Care Transitions Program®. https://caretransitions.org/
- 76.Project RED (Re-Engineered Discharge) http://www.bu.edu/fammed/projectred/
- 77.NYU Hartford Institute of Geriatric Nursing Transitional Care. https://hign.org/consultgeri/resources/protocols/transitional-care
- 78.Burke R.E., Kripalani S., Vasilevskis E.E., Schnipper J.L. Moving beyond readmission penalties: creating an ideal process to improve transitional care. J Hosp Med. 2013;8(2):102–109. doi: 10.1002/jhm.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kangovi S., Grande D. Hospital readmissions—not just a measure of quality. JAMA. 2011;306(16) doi: 10.1001/jama.2011.1562. [DOI] [PubMed] [Google Scholar]
- 80.Goto T., Camargo C.A., Faridi M.K., Yun B.J., Hasegawa K. Machine learning approaches for predicting disposition of asthma and COPD exacerbations in the ED. Am J Emerg Med. 2018;36(9):1650–1654. doi: 10.1016/j.ajem.2018.06.062. [DOI] [PubMed] [Google Scholar]
- 81.Agarwal A., Baechle C., Behara R., Zhu X. A natural language processing framework for assessing hospital readmissions for patients with COPD. IEEE J Biomed Health Inform. 2018;22(2):588–596. doi: 10.1109/JBHI.2017.2684121. [DOI] [PubMed] [Google Scholar]