Abstract
Background
The main goal of management in patients with non-small cell lung cancer (NSCLC) and malignant pleural effusion (MPE) is palliation. Patients with MPE and actionable mutations, because their disease is expected to respond quickly and markedly to targeted therapy, are less likely than those without actionable mutations to receive definitive MPE management. Whether such management is indicated in these patients is unclear.
Research Questions
What is the time to ipsilateral MPE recurrence requiring intervention in patients with metastatic NSCLC by mutation status? What are the risk factors for MPE recurrence?
Study Design and Methods
Retrospective cohort study of consecutive patients who underwent initial thoracentesis for MPE. We used a Fine-Gray subdistribution hazard model to calculate the time to ipsilateral MPE recurrence requiring intervention within 100 days of initial thoracentesis and to identify variables associated with time to pleural fluid recurrence.
Results
A total of 396 patients, comprising 295 (74.5%) without and 101 (25.5%) with actionable mutations, were included. Most patients with actionable mutations (90%) were receiving targeted treatment within 30 days of initial thoracentesis. On univariate analysis, patients with actionable mutations showed a significantly higher hazard of MPE recurrence. On multivariate analysis, this difference was not significant. Larger pleural effusion size on chest radiography (P < .001), higher pleural fluid lactate dehydrogenase (P < .001), and positive cytologic examination results (P = .008) were associated with an increased hazard of recurrence.
Interpretation
Our findings indicate that patients with actionable mutations have a similar risk of MPE recurrence when compared with patients without mutations and would benefit from a similar definitive management approach to MPE.
Key Words: ALK, EGFR, lung cancer, pleural effusion
Abbreviations: ALK, anaplastic lymphoma kinase; EGFR, epidermal growth factor receptor; LDH, lactate dehydrogenase; MPE, malignant pleural effusion; NSCLC, non-small cell lung cancer; SHR, subdistribution hazard ratio; TKI, tyrosine kinase inhibitor
Malignant pleural effusion (MPE) generally is considered a poor prognostic factor and is associated with a median survival duration of 3 to 12 months, depending on patient comorbidities and the underlying malignancy.1, 2, 3 Up to 15% of lung cancer patients have an MPE at diagnosis, and an even greater proportion demonstrate an MPE at some point in the course of the disease.4,5 Several studies have identified an association between EGFR-mutated non-small cell lung cancer (NSCLC) and MPE, which suggests that MPEs are common in patients with actionable mutations.4,6 More than 50% of MPEs will recur within 90 days.7 Recurrent MPEs may cause significant dyspnea, cough, and chest discomfort, resulting in poor quality of life. The main treatment goals in patients with MPEs are palliation of symptoms, limiting unnecessary pleural procedures, and minimizing the need for hospitalization, as well as decreasing hospital lengths of stay when required. Some MPE treatments, such as thoracentesis, achieve only temporary relief; more definitive treatments include placement of an indwelling pleural catheter, placement of a chest tube with chemical pleurodesis, and pleuroscopy with chemical pleurodesis. For patients who have recurrent, symptomatic MPE after initial thoracentesis, definitive pleural procedures are recommended,1 although repeat thoracentesis is reasonable for patients who have slow fluid re-accumulation, an expected rapid and marked response to treatment, or a very short life expectancy. However, in some patients, repeating a thoracentesis, rather than performing a definitive procedure, may increase pleural loculations, may complicate future management, or may prolong MPE-related symptoms.8
NSCLC with common actionable mutations such as EGFR and ALK markedly respond to EGFR tyrosine kinase inhibitors (TKIs) and ALK inhibitors compared with platinum-based chemotherapy. Despite EGFR TKIs and ALK inhibitors being clinically effective, approximately 15% of patients have no survival benefit.9,10 Currently, no guidelines formally recommend treating NSCLC before performing palliative procedures for MPE,1 but because patients with actionable mutations have marked responses to treatment, they often are less likely than those without actionable mutations to receive early definitive management for MPE symptom palliation (ie, indwelling pleural catheter placement or pleurodesis for first MPE recurrence).5,11
Whereas approximately 40% of patients with metastatic NSCLC and MPE also have EGFR mutations, only a few studies have evaluated the use of targeted monotherapy for the treatment of MPE in these patients.5,6 Despite a lack of evidence, many patients with NSCLC and MPE who have actionable mutations are more likely to be treated with targeted therapy and repeat thoracentesis, rather than early definitive procedural management, for pleural effusion.5,12 This may lead to persistent pleural effusion-related symptoms and at least a temporarily diminished quality of life. Further investigations to determine the optimal approach to management are indicated and prompted this study.
We sought to evaluate further whether the time to pleural effusion recurrence was different in patients with actionable mutations when compared with those without actionable mutations and to provide data that will assist in determining the optimal approach to management of MPE in patients with metastatic NSCLC and actionable mutations. The primary objective of this study was to evaluate the association between actionable mutation status and time to MPE recurrence. The secondary objectives were to estimate the survival time of patients with actionable mutations after initial thoracentesis and to identify risk factors for recurrent, symptomatic MPE.
Methods
We performed a retrospective cohort study of consecutive patients with metastatic NSCLC who underwent an initial thoracentesis for MPE at The University of Texas MD Anderson Cancer Center between January 1, 2014, and January 1, 2019. The study was approved by MD Anderson’s Institutional Review Board (Identifier: PA 2019-1050). Patients were identified from a Department of Pulmonary Medicine database and a database maintained by the Genomic Marker-Guided Therapy Initiative team at MD Anderson Cancer Center.
Eligible patients were at least 18 years of age and had either biopsy-proven metastatic NSCLC or strong clinical evidence of metastatic NSCLC, which was defined as imaging demonstrating multiple metastases in a typical clinical pattern, such as PET imaging or bone scintigraphy showing multiple bone metastases; CT scanning or MRI of the brain showing metastatic disease; or CT scanning of the chest, abdomen, or pelvis with sufficiently definitive findings to deem the patient as having metastatic disease. Patients were excluded if they were less than 18 years of age, were lost to follow-up immediately after initial thoracentesis, had no available chest radiographic images within 14 days before initial thoracentesis, previously had undergone fluid drainage at an outside institution, had a history of chest tube placement, or had a primary malignancy diagnosis other than lung cancer.
Patient records were reviewed for demographic information; pathology and cytology reports were reviewed documenting mutation analysis and laboratory values from pleural fluid analysis; and procedural reports were reviewed documenting date of first thoracentesis and subsequent procedures, as well as volume drained. Patient records also were reviewed for documentation of other medical diagnoses potentially contributing to the development of a pleural effusion (ie, congestive heart failure, abdominal ascites, or high clinical suspicion of pneumonia within 14 days of the first thoracentesis), timing and type of therapy received for the underlying malignancy, date of MPE recurrence requiring intervention, and details regarding the second procedure performed. The date of malignancy diagnosis was determined based on the first available pathology or cytology report documenting a primary pulmonary malignancy. Information regarding clinical follow-up and date of death also were recorded.
The size of each pleural effusion on the most recent chest radiograph performed within 2 weeks before initial thoracentesis was evaluated and categorized as described previously.13 Chest radiograph size 1 was defined as blunting of the costophrenic angle, with at least part of the diaphragm still visible; chest radiograph size 2 was defined as effusion of more than size 1 and up to the inferior border of the vascular pedicle; chest radiograph size 3 was defined as effusion more than size 2 and up to the top of the cardiac silhouette; and chest radiograph size 4 was defined as effusion more than size 3 and above the cardiac silhouette.
Descriptive statistics were used to summarize all variables stratified by actionable mutation status. A histogram of continuous variables with frequencies and overlaid with a normal-density curve was created. Those variables found to have normal distribution were expressed as mean ± SD. Categorical variables were expressed as frequencies and were compared using the χ 2 test or Fisher exact test. The Wilcoxon rank-sum test (Mann-Whitney U test) was used to analyze nonparametrically distributed data.
The primary outcome was time from initial thoracentesis to ipsilateral MPE recurrence requiring an intervention within 100 days. This was chosen as a clinically meaningful duration for the development of a recurrent, ipsilateral MPE when compared with indefinite time, given the low median survival in patients with NSCLC and MPE as well as the high expected MPE recurrence rate within 1 month of initial thoracentesis.14 In addition, if a patient were to have a recurrence after 100 days, this may not be considered as rapid, and management with a repeat thoracentesis would be reasonable. We used a Fine-Gray subdistribution hazard model to identify variables associated with time to MPE recurrence requiring intervention within 100 days of initial thoracentesis.15 Death was considered a competing risk. We used the Kaplan-Meier method and log-rank test to evaluate survival times.
All patients alive at the end of 100 days were censored at that time, as were patients lost to follow-up. Variables with P < .20 on univariate analysis were considered candidate variables for the multivariate regression models. Backward selection with P < .05 to remain in the model then was used to arrive at a parsimonious multivariate model. To check the proportional hazards assumption, we used standard methods, including complementary log-log plots and Shoenfeld residuals. All statistical analyses were performed with STATA version 14.2 software (StataCorp).
Results
We identified 3,681 patients who underwent a thoracentesis at MD Anderson Cancer Center between January 1, 2014, and January 1, 2019. Of these patients, 396 had lung cancer with biopsy-proven or strong clinical evidence of metastatic disease before the thoracentesis and were included in the final analysis. Of these 396 patients, 295 (74.5%) showed no identified actionable mutations, and 101 (25.5%) showed actionable mutations (Table 1). Of the 101 patients with actionable mutations, 86 showed EGFR mutations and 15 showed ALK mutations. Most of these patients (90%) were receiving targeted treatment within 30 days of the initial thoracentesis. Between patients with actionable mutations and those without, incidence proportions of MPE recurrence did not differ significantly (P = .371), nor did rates of definitive procedures (P = .688) (Table 1).
Table 1.
Clinical Characteristics of Patients With Metastatic NSCLC and Malignant Pleural Effusion by Actionable Mutation Status
| Characteristic | Actionable Mutation (n = 101) | No Actionable Mutation (n = 295) | P Value |
|---|---|---|---|
| Malignancy diagnosis | < .001a | ||
| Lung adenocarcinoma | 93 (92.08) | 181 (61.36) | |
| Lung squamous cell carcinoma | 1 (0.99) | 49 (15.25) | |
| Lung, unspecified | 7 (7) | 65 (22) | |
| Age, y | .020b | ||
| Mean (SD) | 61.55 (12.17) | 64.66 (11.3) | |
| Median (range) | 63 (30-83) | 66 (28-95) | |
| Sex | .044a | ||
| Male | 40 (39.6) | 151 (51.19) | |
| Female | 61 (60.4) | 144 (48.81) | |
| History of CHF | .031a | ||
| Yes | 1 (0.99) | 19 (6.44) | |
| No | 100 (99.01) | 276 (93.56) | |
| History of ascites | .049a | ||
| Yes | 0 (0) | 11 (3.73) | |
| No | 101 (100) | 284 (96.27) | |
| High clinical suspicion of pneumonia | .001a | ||
| Yes | 5 (4.95) | 56 (18.98) | |
| No | 96 (95.05) | 239 (81.02) | |
| Chemotherapy/targeted therapy within 30 d before initial drainage | < .001a | ||
| Yes | 76 (75.25) | 127 (43.05) | |
| No | 25 (24.75) | 168 (56.95) | |
| Radiation therapy within 30 d before initial drainage | .080a | ||
| Yes | 9 (8.91) | 47 (15.93) | |
| No | 92 (91.09) | 248 (84.07) | |
| Chemotherapy/targeted therapy within 30 d after initial drainage | < .001a | ||
| Yes | 88 (88.89) | 147 (51.4) | |
| No | 11 (11.11) | 139 (48.6) | |
| Laterality of pleural effusion of interest | .312a | ||
| Left | 35 (34.65) | 119 (40.34) | |
| Right | 66 (65.35) | 176 (59.66) | |
| Presence of contralateral effusion | .016a | ||
| Yes | 25 (24.75) | 112 (37.97) | |
| No | 76 (75.25) | 183 (62.03) | |
| Size of the effusion on chest radiography | .323a | ||
| Blunting of costophrenic angle | 8 (9.09) | 26 (12.87) | |
| Above 1 and up to the vascular pedicle | 40 (45.45) | 73 (36.14) | |
| Above 2 and up to the top of the cardiac silhouette | 31 (35.23) | 71 (35.15) | |
| Above the cardiac silhouette | 9 (10.23) | 32 (15.84) | |
| Fluid LDH, IU/L | .620b | ||
| Mean (SD) | 1,468.59 (2,585.22) | 1,310.11 (2,713.9) | |
| Median (range) | 654 (92-20,571) | 693 (104-39,722) | |
| Fluid protein, g/dL | .985b | ||
| Mean (SD) | 4.15 (1.24) | 4.14 (4.49) | |
| Median (range) | 4.2 (2-10.5) | 3.95 (1.4-77) | |
| Fluid cholesterol, mg/dL | .001b | ||
| Mean (SD) | 82.58 (32.17) | 72.72 (22.11) | |
| Median (range) | 82 (31-237) | 67 (32-144) | |
| Fluid triglycerides, mg/dL | .677b | ||
| Mean (SD) | 34.55 (15.81) | 33.49 (23) | |
| Median (range) | 28 (14-140) | 27.5 (11-362) | |
| Positive cytologic results | .002a | ||
| Yes | 81 (81) | 188 (63.95) | |
| No | 19 (19) | 106 (36.05) | |
| Recurrence of pleural effusion requiring intervention during entire follow-up | .371a | ||
| Yes | 64 (63.37) | 172 (58.31) | |
| No | 37 (36.63) | 123 (41.69) | |
| Definitive intervention vs repeat thoracentesis for first recurrence | .688a | ||
| Definitive interventionc | 51 (79.7) | 141 (81.9) | |
| Repeat thoracentesis | 13 (20.3) | 31 (18.1) | |
| Died during entire follow-up | .004a | ||
| Yes | 71 (70.3) | 246 (83.39) | |
| No | 30 (29.7) | 49 (16.61) |
Data are presented as No. (%), unless otherwise indicated. CHF = congestive heart failure; LDH = lactate dehydrogenase; NSCLC = non-small cell lung cancer.
χ 2 test.
t Test.
Indwelling pleural catheter or pleuroscopy with chemical pleurodesis or chest tube with chemical pleurodesis.
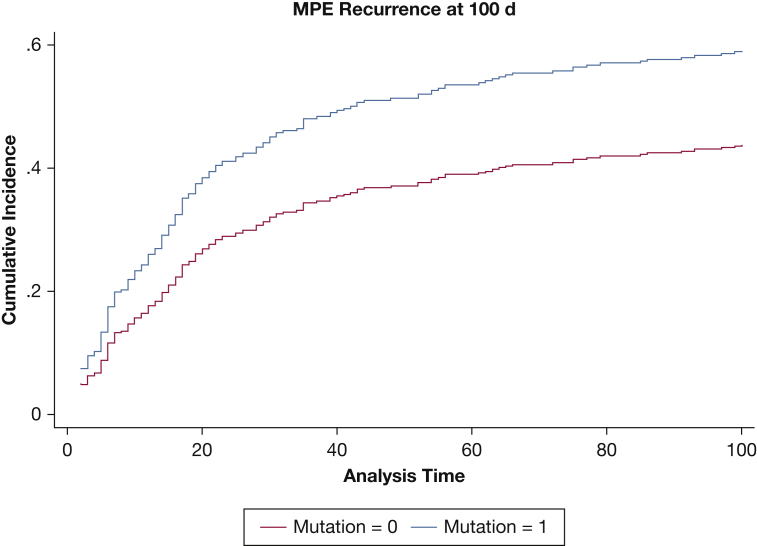
On univariate competing risk analysis, higher amount of pleural fluid drained, higher pleural fluid lactate dehydrogenase (LDH) level, and higher pleural protein level were associated with a higher hazard of symptomatic pleural fluid recurrence. In addition, larger pleural effusion size on chest radiography was also associated with an increased hazard of recurrence. Positive cytologic results and the presence of an actionable mutation also were associated with an increased hazard for recurrence (Table 2). Patients with actionable mutations showed a significantly higher hazard of recurrence after initial thoracentesis on univariate competing risk analysis (Fig 1).
Table 2.
Risk Factors for Pleural Effusion Recurrence Requiring Intervention in Patients With Biopsy-Proven or Strong Clinical Evidence of Metastatic Disease at 100 Days
| Covariate | Univariate Model |
Multivariate Model |
||||
|---|---|---|---|---|---|---|
| SHR | 95% CI | P Value | SHR | 95% CI | P Value | |
| Histologic findings | ||||||
| Lung adenocarcinoma | 1.000 | … | … | … | … | … |
| Lung squamous cell carcinoma | 0.918 | 0.576-1.465 | .723 | … | … | … |
| Lung, unspecified | 0.776 | 0.469-1.284 | .325 | … | … | … |
| Age | 0.999 | 0.9868188-1.012387 | .941 | … | … | … |
| Male sex | 1.104 | 0.822661-1.482548 | .509 | … | … | … |
| CHF present | 1.271 | 0.6618541-2.444204 | .471 | … | … | … |
| Ascites present | 1.659 | 0.6700529-4.10834 | .274 | … | … | … |
| High clinical suspicion of pneumonia | 1.153 | 0.769207-1.731177 | .489 | … | … | … |
| Chemotherapy within 30 d before thoracentesis | 1.241 | 0.9229143-1.670758 | .153 | … | … | … |
| Radiation within 30 d before thoracentesis | 0.948 | 0.6154011-1.462989 | .812 | … | … | … |
| Chemotherapy within 30 d after thoracentesis | 0.940 | 0.6935878-1.276578 | .696 | … | … | … |
| Contralateral effusion present | 0.794 | 0.5768333-1.094897 | .160 | … | … | … |
| Size of the effusion on chest radiography | ||||||
| Blunting of costophrenic angle | 1.000 | … | … | 1.000 | … | … |
| Above 1 and up to the vascular pedicle | 3.021041 | 1.358069-6.720344 | .007 | 2.670 | 1.1971-5.956 | .016 |
| Above 2 and up to the top of the cardiac silhouette | 5.221265 | 2.37956-11.45658 | < .001 | 4.218 | 1.9159-9.289 | .001 |
| Above the cardiac silhouette | 3.841847 | 1.603659-9.203818 | .003 | 3.631 | 1.5244-8.650 | .004 |
| Amount of fluid drained, 100-mL increments | 1.038 | 1.0111-1.0649 | .006 | … | … | … |
| Fluid LDH, 100-IU/L increments | 1.0066 | 1.0041-1.0091 | < .001 | 1.0069 | 1.0035-1.0103 | < .001 |
| Fluid protein, 1-g/dL increments | 1.01581 | 1.010328-1.021323 | < .001 | … | … | … |
| Fluid cholesterol, 1-mg/dL increments | .9991036 | 0.9934539-1.004785 | .757 | … | … | … |
| Fluid triglycerides, 10-mg/dL increments | .9968247 | 0.9892062-1.004502 | .417 | … | … | … |
| Cytologic results positive | 2.207 | 1.54658-3.149714 | < .001 | 1.804 | 1.1673-2.7883 | .008 |
| Mutation present | 1.487246 | 1.069992-2.067213 | .018 | 1.429558 | 0.9543335-2.141427 | .083 |
CHF = congestive heart failure; LDH = lactate dehydrogenase; SHR = subhazard ratio.
Figure 1.
Competing risk plot depicting the cumulative incidence of MPE recurrence at 100 d by actionable mutation status with death as a competing risk (subdistribution hazard ratio, 1.487; 95% CI, 1.07-2.07; P = .018). MPE = malignant pleural effusion.
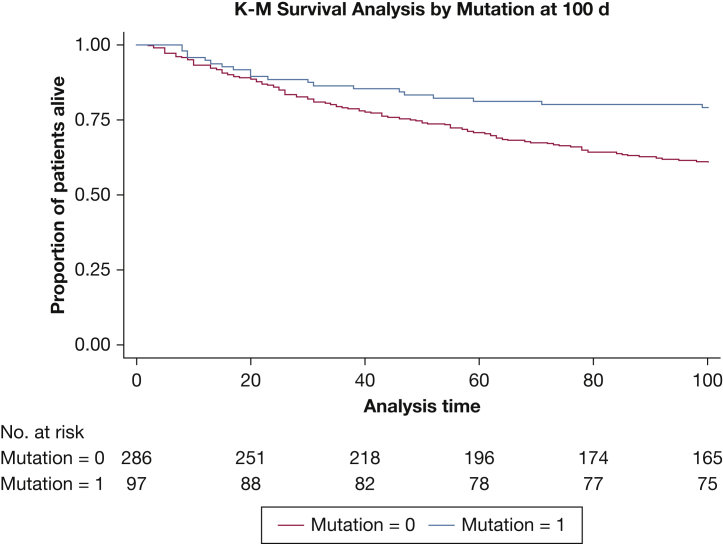
On multivariate analysis, pleural effusion size on chest radiography, higher pleural fluid LDH level, and positive cytologic results were associated with an increased hazard of recurrence (Table 2). On multivariate analysis, the presence of an actionable mutation was not associated with a significantly increased hazard of recurrence. In addition, patients with actionable mutations showed a higher survival rate when compared with those without actionable mutations (Fig 2).
Figure 2.
Kaplan-Meier survival plot at 100 d by actionable mutation status (P = .0026, log-rank test).
Discussion
To the best of our knowledge, this is the first study to evaluate time to recurrence of ipsilateral MPE in patients with actionable mutations to determine if a significant difference exists when compared with the time to recurrence in patients without an actionable mutation. Our findings showed that larger pleural effusion size on chest radiography, higher pleural fluid LDH level, and positive cytologic results are associated with a higher hazard of pleural fluid recurrence. On univariate analysis, patients with actionable mutations showed a higher hazard of MPE recurrence; however, this did not meet traditional levels of statistical significance on multivariate analysis.
Our data are consistent with and build on previous studies indicating that targeted treatment alone may not be sufficient for the management of MPE in patients with stage IV NSCLC and actionable mutations. For example, Yang et al16 evaluated the response rates to EGFR TKIs in patients who had lung adenocarcinoma and cytology-positive MPEs with and without EGFR mutations. They found that in patients with EGFR mutations, pleural effusions did not respond well to treatment with TKI therapy, whereas the solid tumor components demonstrated a more favorable response to therapy (61.5% vs 88.5%; P = .026). Approximately 75% of the patients in that study underwent at least two pleural drainage procedures before disease response evaluation, and thus the true MPE response rate may be even lower than reported. In addition, Lin et al6 performed a prospective study evaluating 76 patients with MPE and EGFR mutations who were treated with oral gefitinib. Although most patients showed an initial reduction in radiographic MPE size, 48 patients later experienced disease progression, with almost 70% demonstrating recurrent MPEs requiring palliative intervention.
In the present study, most patients were treated with targeted therapy within 30 days of the initial thoracentesis, but despite such treatment, they showed an increased hazard of early symptomatic pleural fluid recurrence when compared with patients without these mutations. Many MPEs will recur within 30 days of the initial thoracentesis,17 and this was reflected in patients with actionable mutations, approximately 50% of whom experienced recurrence by day 30. These findings indicate that patients with actionable mutations are similar to patients without actionable mutations in terms of MPE recurrence.
In contrast to our study, Kashiwabara et al12 evaluated patients with an EGFR mutation and MPE treated with EGFR TKI with (n = 18) and without pleurodesis (n = 12) and found that patients with MPE who received first-line EGFR TKI therapy without pleurodesis showed a better prognosis than the same subset of patients with pleurodesis. No difference was found in the MPE control rate; however, among patients without pleurodesis, more than 50% of patients were treated with repeat thoracentesis and 42% of patients underwent chest tube placement. In addition, Verma et al5 compared time to MPE recurrence in patients treated with EGFR TKI therapy alone (n = 20) with patients receiving EGFR TKI therapy and an early talc pleurodesis procedure (n = 14). No significant difference in time to MPE recurrence was identified, regardless of whether a pleurodesis procedure was performed (P = .59). Compared with patients without EGFR mutations, those with EGFR mutations more commonly demonstrated complete hemithorax opacification, and hence large effusions (P = .02). These findings are similar to those of Chen et al,18 who concluded that gefitinib monotherapy (n = 39) was at least as successful as pleurodesis followed by gefitinib (n = 17) in controlling MPE in patients with metastatic NSCLC, although several patients in that study had progressive disease despite previous chemotherapy and were receiving gefitinib as second- or third-line therapy. In addition, Chen et al found that among patients who did not undergo pleurodesis, those with larger effusions showed a poorer response to treatment than those with small to moderate MPEs. The present study also showed that larger effusion size on chest radiography is associated with a higher hazard of symptomatic pleural fluid recurrence. These findings indicate that mutation status and targeted therapy use alone should not determine whether patients receive early definitive therapy for MPE and that other clinical characteristics also must be considered. It is worth noting the small sample sizes of the available studies on this topic. Larger studies are needed to determine the effectiveness of TKI therapy without a procedure in preventing MPE recurrence.
We also found that larger pleural effusion size on chest radiography, higher pleural fluid LDH level, and positive cytologic results were associated with a higher hazard of pleural fluid recurrence. Although many studies have reported predictors of survival in patients with MPE, very few have reported predictors of recurrence. Our conclusions are concordant with data we published previously showing that increasing pleural effusion size on chest radiography, larger volume of drained pleural fluid, higher pleural fluid LDH level, and positive cytologic results each are associated with an increased hazard of MPE recurrence in NSCLC patients.13
We recognize that our study has limitations. Because the study was retrospective, it has the potential disadvantages associated with such a study design. Also, because our institution is an academic tertiary care hospital and serves as a referral center for patients with complex malignancies, the results of our study may not be broadly applicable. However, the results of the present study are similar to those of previously published studies of comparable patient populations, suggesting the patients enrolled in the current study indeed are representative of others with underlying metastatic NSCLC and MPE. We also did not formally evaluate the overall extent of disease in patients. Increasing disease burden, as identified on imaging, may affect treatment response negatively. The overall extent of disease in these patients may need to be evaluated in future studies.
Interpretation
In conclusion, patients with advanced metastatic NSCLC and large unilateral pleural effusions have a high probability of symptomatic, ipsilateral pleural fluid recurrence within 100 days after initial thoracentesis, regardless of actionable mutation status. Risk factors for recurrence include larger size of the pleural effusion as seen on chest radiography, increasing pleural fluid LDH level, and positive pleural fluid cytologic results. The results of our study, in conjunction with previously published literature, suggest that targeted therapy alone is not sufficient for managing MPEs in patients with metastatic NSCLC and actionable mutations. Our findings indicate that patients with actionable mutations have a similar risk of early MPE recurrence when compared with patients without actionable mutations and would benefit from a similar definitive management approach to MPE.
Take-home Points.
Study Question: What is the time to ipsilateral MPE recurrence requiring intervention in patients with metastatic NSCLC by mutation status and what are the risk factors for MPE recurrence?
Results: On univariate analysis, patients with actionable mutations showed a significantly higher hazard of MPE recurrence, but on multivariate analysis, this difference was not statistically significant. Larger pleural effusion size on chest radiography (P < .001), higher pleural fluid LDH (P < .001), and positive cytologic results (P = .008) were associated with an increased hazard of symptomatic, ipsilateral MPE recurrence.
Interpretation: Our findings indicate that patients with actionable mutations have a similar risk of MPE recurrence when compared with patients without mutations and would benefit from a similar definitive management approach to MPE.
Acknowledgments
Author contributions: A. J. S. and H. B. G. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. A. J. S. and H. B. G. participated in study design, data collection, data analysis, manuscript writing, and manuscript review. S. N. S. and H. D. L. G. participated in data collection and manuscript review. All other authors participated in manuscript review and revision and provided final approval for submission.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript. This research and manuscript have not been supported either directly or indirectly by tobacco companies.
Footnotes
FUNDING/SUPPORT: This study was supported by The University of Texas MD Anderson Lung Moon Shot Program and the MD Anderson Cancer Center Support Grant P30 CA01667.
References
- 1.Bibby A.C., Dorn P., Psallidas I. ERS/EACTS statement on the management of malignant pleural effusions. Eur Respir J. 2018;52(1):1–23. doi: 10.1183/13993003.00349-2018. [DOI] [PubMed] [Google Scholar]
- 2.Musani A.I., Haas A.R., Seijo L., Wilby M., Sterman D.H. Outpatient management of malignant pleural effusions with small-bore, tunneled pleural catheters. Respiration. 2004;71(6):559–566. doi: 10.1159/000081755. [DOI] [PubMed] [Google Scholar]
- 3.Putnam J.B., Jr., Light R.W., Rodriguez R.M. A randomized comparison of indwelling pleural catheter and doxycycline pleurodesis in the management of malignant pleural effusions. Cancer. 1999;86(10):1992–1999. [PubMed] [Google Scholar]
- 4.Zou J., Bella A.E., Chen Z. Frequency of EGFR mutations in lung adenocarcinoma with malignant pleural effusion: Implication of cancer biological behaviour regulated by EGFR mutation. J Int Med Res. 2014;42(5):1110–1117. doi: 10.1177/0300060514539273. [DOI] [PubMed] [Google Scholar]
- 5.Verma A., Chopra A., Lee Y.W. Can EGFR-tyrosine kinase inhibitors (TKI) alone without talc pleurodesis prevent recurrence of malignant pleural effusion (MPE) in lung adenocarcinoma. Curr Drug Disc Technol. 2016;13(2):68–76. doi: 10.2174/1570163813666160524142846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin J.B., Lai F.C., Li X. Sequential treatment strategy for malignant pleural effusion in non-small cell lung cancer with the activated epithelial grow factor receptor mutation. J Drug Target. 2017;25(2):119–124. doi: 10.1080/1061186X.2016.1200590. [DOI] [PubMed] [Google Scholar]
- 7.Feller-Kopman D.J., Reddy C.B., DeCamp M.M. Management of malignant pleural effusions. An official ATS/STS/STR Clinical Practice Guideline. Am J Respir Crit Care Med. 2018;198(7):839–849. doi: 10.1164/rccm.201807-1415ST. [DOI] [PubMed] [Google Scholar]
- 8.Chung C.L., Chen Y.C., Chang S.C. Effect of repeated thoracenteses on fluid characteristics, cytokines, and fibrinolytic activity in malignant pleural effusion. Chest. 2003;123(4):1188–1195. doi: 10.1378/chest.123.4.1188. [DOI] [PubMed] [Google Scholar]
- 9.Maemondo M., Inoue A., Kobayashi K. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- 10.Rosell R., Carcereny E., Gervais R. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 11.Shamblin C.J., Tanner N.T., Sanchez R.S., Woolworth J.A., Silvestri G.A. EGFR mutations in malignant pleural effusions from lung cancer. Curr Respir Care Rep. 2013;2(2):79–87. [Google Scholar]
- 12.Kashiwabara K., Fuji S., Tsumura S., Sakamoto K. Prognosis of EGFR-mutant lung adenocarcinoma patients with malignant pleural effusion receiving first-line EGFR-TKI therapy without pleurodesis: a single-institute retrospective study. Anticancer Res. 2020;40(2):1117–1121. doi: 10.21873/anticanres.14051. [DOI] [PubMed] [Google Scholar]
- 13.Grosu H.B., Molina S., Casal R. Risk factors for pleural effusion recurrence in patients with malignancy. Respirology. 2019;24(1):76–82. doi: 10.1111/resp.13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ost D.E., Niu J., Zhao H., Grosu H.B., Giordano S.H. Quality gaps and comparative effectiveness of management strategies for recurrent malignant pleural effusions. Chest. 2018;153(2):438–452. doi: 10.1016/j.chest.2017.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lau B., Cole S.R., Gange S.J. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170(2):244–256. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang J., Lee O.J., Son S.M. EGFR mutation status in lung adenocarcinoma-associated malignant pleural effusion and efficacy of EGFR tyrosine kinase inhibitors. Cancer Res Treat. 2018;50(3):908–916. doi: 10.4143/crt.2017.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burrows C.M., Mathews W.C., Colt H.G. Predicting survival in patients with recurrent symptomatic malignant pleural effusions: an assessment of the prognostic values of physiologic, morphologic, and quality of life measures of extent of disease. Chest. 2000;117(1):73–78. doi: 10.1378/chest.117.1.73. [DOI] [PubMed] [Google Scholar]
- 18.Chen C., Gow C., Yu C. Clinical response of gefitinib on malignant pleural effusions in patients with non-small cell lung cancer. Journal of Cancer Molecules. 2008;4(1):23–28. [Google Scholar]