Abstract
An enzyme-linked immunosorbent assay (ELISA) based on the recombinant Toscana virus nucleoprotein (rN) has been developed. Its sensitivity and specificity for the detection of virus-specific immunoglobulins G and M in human sera were similar to those of the ELISA that is based on an antigen extracted from infected mouse brain and that is routinely used for serodiagnosis.
Toscana (TOS) virus is a member of the sandfly fever group (genus Phlebovirus of the Bunyaviridae family). It has been associated with acute neurological diseases, mainly aseptic meningitis, which resolves itself with no neurological sequelae. TOS virus infection with the involvement of the central nervous system has been reported not only in people living in regions of endemicity (9) but also in tourists returning from Mediterranean countries (3, 5, 11).
The clinical symptoms of TOS virus infection such as fever, headache, vomiting, photophobia, and neck rigidity are not unique to TOS virus infection, which can be diagnosed either by the isolation of the virus or by the detection of specific antibodies in patient sera. Isolation of virus from both blood and cerebrospinal fluid (CSF) from acutely ill patients is rare and requires long and laborious procedures. Recently, the presence of viral RNA in CSF has been demonstrated by PCR (13).
Among the several assays used for serodiagnosis of TOS virus infection, the enzyme-linked immunosorbent assay (ELISA) has proved to be the most sensitive (9). This ELISA is based on viral antigen extracted from infected suckling mouse brain by a laborious procedure that includes a sucrose-acetone (SA) extraction step (4), followed by capture (10) with purified antibodies specific to the TOS virus. In this paper we report on the development of an ELISA based on the recombinant viral nucleoprotein (rN) as the antigen. The viral N protein has been shown to be the major viral immunodominant antigen (8, 12), like in other viruses of the Bunyaviridae family (7, 14).
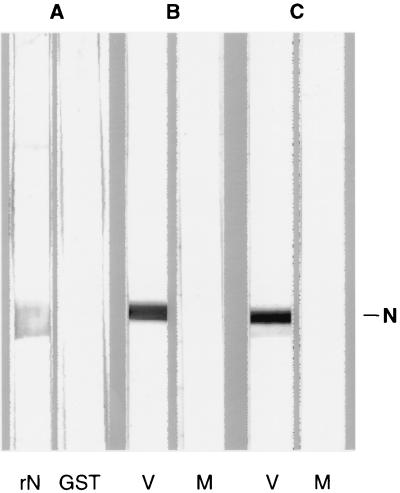
The genomic sequences coding for the N protein (6) were inserted in an expression plasmid (4a). rN, which contains a histidine-tail at its NH2 terminus, was expressed in Escherichia coli and was purified by affinity chromatography by a nondenaturing method (QIAexpressionist; Qiagen). The immunological properties of rN were tested by immunoblot analysis with sera from TOS virus-infected patients and from hyperimmune mouse sera raised against the protein itself. As shown in Fig. 1, the serum from infected patients reacted with the rN but not with the glutathione S-transferase protein used as the heterologous control (Fig. 1A) and the mouse anti-rN sera specifically recognized the intracellular N protein (Fig. 1B and C), indicating that the N protein expressed by the bacteria retained the antigenic properties of the viral N protein.
FIG. 1.
Western blot analysis of purified rN (lane rN) and glutathione S-transferase (GST) as heterologous antigen with serum from a TOS virus-infected patient (A) and cell lysates from infected (V) and uninfected (M) Vero cells with sera from two different mice injected with purified rN (B and C).
The purified rN was used to replace the viral SA antigen in the ELISA currently used in our laboratory for the serodiagnosis of TOS virus infection (1, 2). The specificity and sensitivity of the rN-based ELISA (rN-ELISA) were evaluated by testing several human serum samples for the presence of TOS virus-specific immunoglobulin G (IgG) and IgM and comparing the results with those obtained by the SA-based ELISA (SA-ELISA).
Indirect ELISA for IgG detection was performed in wells of polystyrene plates coated with a predetermined optimum quantity of either SA antigen or rN protein (1 μg/well) in 50 mM NaHCO3 (pH 9.6) buffer overnight at 4°C. The following reagents were subsequently added: a blocking solution containing 1% bovine serum albumin (BSA), human serum diluted 1:50 in PBS-TB (phosphate-buffer saline, 0.05% Tween 20, 0.5% BSA), and alkaline phosphatase-conjugated goat anti-human IgG (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, Md.). The reaction color was developed by adding a substrate solution containing p-nitrophenylphosphate (Sigma). At each step the reaction mixture was incubated for 1 h at 37°C and was extensively washed with PBS-TB. The reaction was stopped by adding NaOH at a final concentration of 1 N. The optical density (OD) of each sample was read at a wavelength of 405 nm.
Detection of IgM was performed by a μ-capture ELISA adopted to avoid common sources of false-positive results like the presence of rheumatoid factor or high levels of IgG antibodies. The wells of the microtiter plates were coated with goat anti-human IgM antibodies (μ-chain specific; Cappel Laboratories, ICN, Costa Mesa, Calif.). Human serum (diluted 1:50), antigen (either SA or rN), affinity-purified mouse anti-TOS virus antibodies, and alkaline phosphatase-conjugated anti-mouse IgG were subsequently added. Each reaction step was done as described above for IgG detection.
Each serum sample was tested in duplicate, and BSA was used as a heterologous antigen. The OD value for each sample was obtained by determining the difference between the ODs measured with the specific and the heterologous antigens. The cutoff value for each assay was calculated as the mean OD for 60 negative sera plus 3 standard deviations. The negative control sera were from healthy people living in Trentino, Italy, which is a mountainous region where the TOS virus does not circulate. The calculated cutoff values for IgG and IgM were 0.100 for both the rN- and SA-ELISAs. Samples with OD values above the cutoff were considered positive.
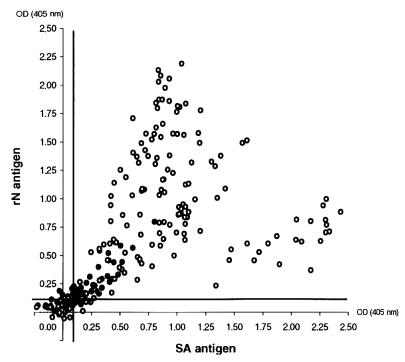
The rN- and SA-ELISAs were used in parallel to detect virus-specific IgG in 168 serum samples from people hospitalized (patients) with symptoms of meningitis and 86 serum samples from healthy people living in regions of endemicity for TOS virus. For the patient group, 96% of the serum samples had the same results by both assays, while for the healthy population, the proportion was lower (90%). The proportion of IgG-positive sera for the latter group was 44%. An inspection of the OD values for the samples with discrepant results showed that they were very close to the cutoff value. As expected, for the healthy population the number of samples with OD values close to the cutoff point appeared to be greater, because the IgG level in the sera from the healthy population is lower than that in the sera from patients. The correlation between the ODs for each sample by the two assays is shown in Fig. 2. A better correlation seems to be achieved for the samples from the healthy population rather than for the samples from the patient group.
FIG. 2.
TOS virus-specific IgG detection in sera from a healthy population (black circles) and patients (open circles). The correlation between the OD values obtained by the SA- and rN-ELISAs, reported on the abscissa and the ordinate, respectively, is shown. The bold lines indicate the cutoff value for each assay.
Because of the high prevalence of TOS virus IgG in the healthy population from regions of endemicity (this study; 1), the serological diagnosis of TOS virus-associated meningitis requires the detection of IgM antibodies, whose presence is assumed to indicate a recent infection.
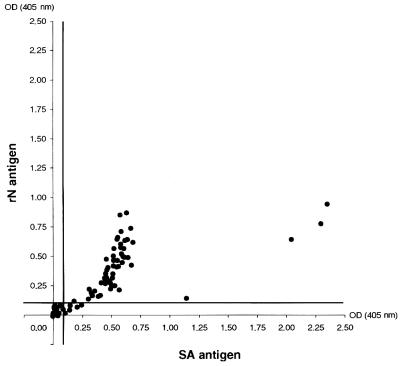
Eighty-three serum samples from patients hospitalized with a diagnosis of meningitis or meningoencephalitis were analyzed for the presence of virus-specific IgM; 90% of the sera had the same results by both assays. Only five samples with weak positivity by SA-ELISA were negative by rN-ELISA. The correlation between the ODs for each serum sample by the two assays was good for all except four serum samples, which had a higher OD by the SA-ELISA than by the rN-ELISA (Fig. 3).
FIG. 3.
TOS virus-specific IgM detection in sera from patients. The correlation between the OD values obtained by SA- and rN ELISAs, reported on the abscissa and the ordinate, respectively, is shown. The bold lines indicate the cutoff value for each assay.
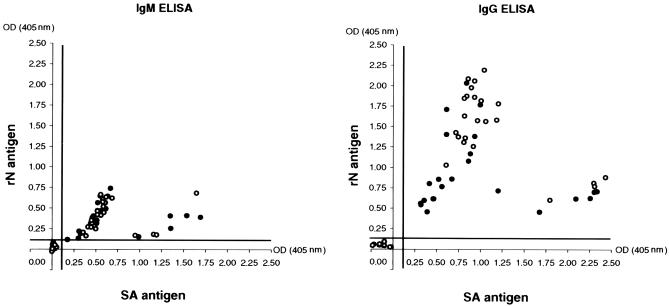
IgG and IgM in acute- and convalescent-phase sera from 28 patients hospitalized with clinical symptoms of neurological disease were also evaluated by the rN- and SA-ELISAs. The interval of collection between the first and second serum samples ranged from 11 to 29 days. The results obtained by the two assays were the same. Acute- and convalescent-phase sera from 22 patients were found to be positive for both IgG and IgM. The second serum sample from one patient whose first serum sample was IgG positive and IgM negative was found to be IgM positive. Both serum samples from the other patients were IgG and IgM negative. The correlation between the OD values obtained for each sample by the rN- and SA-ELISAs is presented in Fig. 4.
FIG. 4.
Detection of IgM and IgG in acute-phase (black circles) and convalescent-phase (open circles) sera from patients. The correlation between the OD values obtained by the SA- and rN-ELISAs, reported on the abscissa and the ordinate, respectively, is shown. The bold lines indicate the cutoff value for each assay.
The results presented in this report demonstrate that the N protein expressed by bacterial cells can be used as the antigen in a diagnostic immunoassay for the detection of TOS virus-specific IgG and IgM antibodies without a loss of sensitivity and specificity. There are many advantages to the use of recombinant protein in place of viral native antigen. The rN protein is expressed at a high level in bacterial cells, it can be easily purified from cell lysates by a one-step protocol, and its production does not require mammalian cell cultures or the handling and use of animals. The new antigen is well characterized, which is not the case for the SA antigen, whose viral components have not been defined. The new assay can easily be set up and standardized, and therefore, its use could help greatly in the management of patients with meningitis induced by TOS virus infection and would avoid unnecessary invasive procedures.
Acknowledgments
We thank S. Tocchio for excellent secretarial assistance, R. Gilardi and P. Piccinini for assistance in computer artwork, and A. Cesolini for help in handling of the animals.
REFERENCES
- 1.Braito A, Corbisiero R, Corradini S, Marchi B, Sancasciani N, Fiorentini C, Ciufolini M G. Evidence of Toscana virus infections without central nervous system involvement. Eur J Epidemiol. 1997;13:761–764. doi: 10.1023/a:1007422103992. [DOI] [PubMed] [Google Scholar]
- 2.Braito A, Corbisiero R, Corradini S, Fiorentini C, Ciufolini M G. Toscana virus infections of the central nervous system in children: a report of 14 cases. J Pediatr. 1998;132:144–148. doi: 10.1016/s0022-3476(98)70500-1. [DOI] [PubMed] [Google Scholar]
- 3.Calisher C H, Weinberg A N, Muth D J, Lazuick J S. Toscana virus infection in United States citizen returning from Italy. Lancet. 1987;i(8525):165–166. doi: 10.1016/s0140-6736(87)92005-8. [DOI] [PubMed] [Google Scholar]
- 4.Clarke D H, Casals J. Techniques for hemagglutination and hemagglutination inhibition with arthropod-borne-viruses. Am J Trop Med Hyg. 1958;7:561–573. doi: 10.4269/ajtmh.1958.7.561. [DOI] [PubMed] [Google Scholar]
- 4a.Di Bonito, P., et al. Submitted for publication.
- 5.Ehrnst A, Peters C J, Niklasson B, Svedmayr A, Holmgren B. Neurovirulent Toscana virus (a sandfly fever virus) in Swedish man after visit to Portugal. Lancet. 1985;i(8439):1212–1213. doi: 10.1016/s0140-6736(85)92886-7. [DOI] [PubMed] [Google Scholar]
- 6.Giorgi C, Accardi L, Nicoletti L, Grò M C, Takehara K, Hildtch C, Bishop D H L. Sequences and coding strategies of the S RNAs of Toscana and Rift Valley fever viruses compared to those of Punta Toro, Sicilian sandfly fever and Unkuniemi viruses. Virology. 1991;180:738–753. doi: 10.1016/0042-6822(91)90087-r. [DOI] [PubMed] [Google Scholar]
- 7.Groen J, Jordans H G M, Clement J P G, Rooijakkers E J M, Uytdehaag F G C M, Dalrymple J, Van der Groen G, Osterhaus A D M E. Identification of hantavirus serotypes by testing of post-infection sera in immunofluorescence and enzyme-linked immunosorbent assays. J Med Virol. 1991;33:26–32. doi: 10.1002/jmv.1890330106. [DOI] [PubMed] [Google Scholar]
- 8.Magurano F, Nicoletti L. The humoral response in Toscana virus acute neurologic disease investigated by viral protein specific immunoassays. Clin Diagn Lab Immunol. 1999;6:55–60. doi: 10.1128/cdli.6.1.55-60.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nicoletti L, Verani P, Caciolli S, Ciufolini M G, Renzi A, Bartolozzi D, Paci P, Leoncini F, Padovani P, Traini E, Baldereschi M, Balducci M. Central nervous system involvement during infection by phlebovirus Toscana of residents in natural foci in Central Italy (1977–1988) Am J Trop Med Hyg. 1991;45:429–434. doi: 10.4269/ajtmh.1991.45.429. [DOI] [PubMed] [Google Scholar]
- 10.Niklasson B, Peters C J, Grandien M, Wood O. Detection of human immunoglobulins G and M antibodies to Rift Valley virus by enzyme-linked immunosorbent assay. J Clin Microbiol. 1984;19:225–229. doi: 10.1128/jcm.19.2.225-229.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schwarz T F, Gilch S, Jager G. Travel-related Toscana virus infection. Lancet. 1993;342:803. doi: 10.1016/0140-6736(93)91568-7. [DOI] [PubMed] [Google Scholar]
- 12.Schwarz T F, Gilch S, Pauli C, Jäger G. Immunoblot detection of antibodies to Toscana virus. J Med Virol. 1996;49:83–86. doi: 10.1002/(SICI)1096-9071(199606)49:2<83::AID-JMV2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 13.Valassina M, Cuppone A M, Bianchi S, Santini L, Cusi M G. Evidence of Toscana virus variants circulating in Tuscany, Italy, during the summers of 1995 to 1997. J Clin Microbiol. 1998;36:2103–2104. doi: 10.1128/jcm.36.7.2103-2104.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zoller L, Scholz J, Stohwasser R, Giebel L B, Sethi K K, Bautz E K F, Darai G. Immunoblot analysis of the serological response in hantavirus infections. J Med Virol. 1989;27:231–237. doi: 10.1002/jmv.1890270309. [DOI] [PubMed] [Google Scholar]