Abstract
Purpose
There are limited data on SARS-CoV-2 (COVID-19) infection in children with cancer or after haematopoietic stem cell transplant (HSCT). We describe the severity and outcomes of SARS-COV-2 in these patients and identify factors associated with severe disease.
Methods
This was a multinational, observational study of children (aged <19 years) with cancer or HSCT and SARS-CoV-2 confirmed by polymerase chain reaction. COVID-19 was classified as asymptomatic, mild, moderate, severe or critical (≥1 organ support). Exact polytomous regression was used to determine the relationship between clinical variables and disease severity.
Results
One hundred and thirty-one patients with COVID-19 across 10 countries were identified (median age 8 years). Seventy-eight (60%) had leukaemia/lymphoma, 48 (37%) had solid tumour and five had primary immunodeficiency and HSCT. Fever (71%), cough (47%) and coryza (29%) were the most frequent symptoms. The median duration of detectable virus was 16 days (range, 1–79 days). Forty-nine patients (37%) were hospitalised for COVID-19 symptoms, and 15 (11%) required intensive care unit–level care. Chemotherapy was delayed/modified in 35% of patients. COVID-19 was asymptomatic in 32% of patients, mild in 47%, moderate in 8%, severe in 4% and critical in 9%. In 124 patients (95%), a full recovery was documented, and four (3%) died due to COVID-19. Any comorbidity (odds ratio, 2.94; 95% confidence interval [CI], 1.81–5.21), any coinfection (1.74; 95% CI 1.03–3.03) and severe baseline neutropenia (1.82; 95% CI 1.13–3.09) were independently and significantly associated with increasing disease severity.
Conclusion
Although most children with cancer had asymptomatic/mild disease, 13% had severe COVID-19 and 3% died. Comorbidity, coinfection and neutropenia may increase the risk of severe disease. Our data may help management decisions in this vulnerable population.
Keywords: Child, Cancer, SARS-CoV-2, COVID-19, Chemotherapy, Haematopoietic stem cell transplantation
1. Introduction
The global COVID-19 pandemic caused by the novel SARS-CoV-2 has posed great challenges for patients with cancer. Internationally, the haematological-oncology community mobilised rapidly with the early release of recommendations for adjustments to cancer care to ensure the safety of this vulnerable population [[1], [2], [3], [4], [5], [6]]. Although data emerged early in the pandemic about the increased risks of adverse outcomes and mortality for adult cancer and haematopoietic stem cell transplant (HSCT) patients with COVID-19, the impact of the disease in children was less clear.
Overall, children are significantly less likely to develop severe or fatal COVID-19 compared with adults [7]. The exact mechanism for this remains unknown, however factors proposed include those thought to be protective in children such as a stronger innate immune response leading to more effective viral clearance, a weaker adaptive immunity leading to less hyperinflammation and pre-existing immunity because of exposure to commonly circulating coronaviruses [8]. Although increasing age and underlying comorbidities, including cancer, are well documented to confer an increased risk of severe disease in adult patients, there remains a paucity of paediatric data [[9], [10], [11]]. Furthermore, among children with cancer, the impact of underlying disease (haematological or solid tumour), treatment status (active or past) and even chemotherapy interruptions on COVID-19 severity and outcome is not clear.
The objectives of this study were to describe the severity and outcomes of SARS-COV-2 in children with cancer or after HSCT and to identify factors associated with severe disease.
2. Methods
This was a multinational, observational cohort study of children and adolescents (aged <19 years) with a diagnosis of cancer or after HSCT who had confirmed SARS-CoV-2 infection by polymerase chain reaction (PCR). Patients were included if they had a diagnosis of cancer, irrespective of treatment status, or had an HSCT for malignant or non-malignant diagnoses. For details of testing criteria of participating centres and COVID-19 country incidence see Appendix 1. The study protocol and database was developed and approved in March 2020 by the Umbrella research group, an international collaboration of paediatric oncology and infectious diseases clinicians.
Deidentified data were collected retrospectively by study site investigators and entered into REDCap. COVID-19 severity was classified as (1) asymptomatic, (2) mild (upper respiratory or gastrointestinal symptoms), (3) moderate (acute lower respiratory tract infection but no hypoxemia), (4) severe (hypoxia <92% without supplemental oxygen) or (5) critical (admission to intensive care unit [ICU], or equivalent, for organ support) [12]. Eligible patients were identified from microbiological reports, existing surveillance and notification systems and hospital records. Patients were followed up for a minimum of 30 days after infection diagnosis.
Active treatment was defined as any chemotherapy or radiotherapy within 30 days before SARS-CoV-2 infection or HSCT within 6 months before SARS-CoV-2 diagnosis. Intensive treatment was defined as chemotherapy more intensive than maintenance chemotherapy for acute lymphoblastic leukaemia [13]. Neutropenia was defined as absolute neutrophil count <500 cells/mm3 and lymphopenia as absolute lymphocyte count <1000 cells/mm [3]. Overweight was defined as body mass index >95th percentile [14].
Human research and ethics approval for the multisite study was obtained at Royal Children's Hospital, Melbourne, and site-specific ethics at participating centres as required. The study was conducted in accordance with the Declaration of Helsinki, and informed consent was obtained if requested by the local ethical committee.
2.1. Statistics
Associations of clinical factors with the severity of disease were analysed using univariate and multivariate exact polytomous regression, based on adjacent categories modelling [15]. Exact logistic regression was used to account for the smaller sample size [16]. Factors significantly associated in univariate analysis were used for stepwise forward multivariate analysis using LogXact 10 software from Cytel Software Corporation (Cambridge, MA, USA). Two-sided tests were used throughout and P-values <0.05 were considered significant.
3. Results
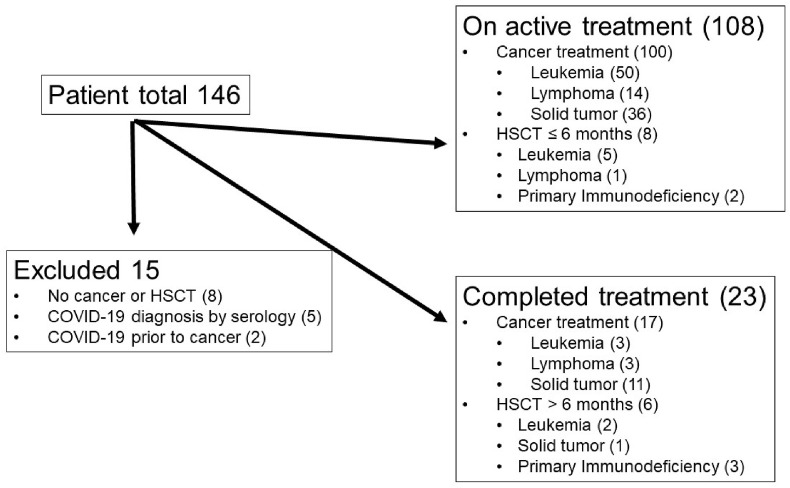
Twenty tertiary paediatric hospitals across 10 countries contributed data to the study from beginning of pandemic until the end of February 2021 applying different testing strategies (Appendix 1). Of the 146 patient episodes entered, 15 were excluded from this analysis (eight with non-cancer or HSCT-related immunosuppression, five with COVID-19 diagnosed on serology only, and two with COVID-19 before cancer diagnosis; Fig. 1 ). Of the remaining 131 episodes, 108 were on active treatment (including eight with HSCT within 6 months before SARS-CoV-2 diagnosis), and 23 had completed treatment (including six with HSCT >6 months before SARS-CoV-2 diagnosis; Fig. 1). Table 1 provides demographic information. For the 23 patients who had completed cancer treatment, the median time from their last dose of chemotherapy to infection was 1.5 years (range, 0.2–7.4 years).
Fig. 1.
Consort diagram on patients included in the registry and analysed. HSCT, haematopoietic stem cell transplantation.
Table 1.
Demographic data of children with cancer or after haematopoietic stem cell transplantation and SARS-CoV-2 infection confirmed by PCR.
| Demographic data | N = 131 |
|---|---|
| Median age, (IQR) | 8 (4–14) |
| Male sex, n (%) | 75 (57) |
| Country, n (%) | |
|
10 (8) |
|
26 (20) |
|
5 (4) |
|
2 (1.5) |
|
2 (1.5) |
|
41 (31) |
|
21 (16) |
|
12 (9) |
|
9 (7) |
|
3 (2) |
| Underlying diagnosis, n (%) | |
|
60 (46) |
|
18 (14) |
|
48 (37) |
|
5 (4) |
| Active cancer treatment, n (%) | 100 (76) |
|
31a |
| GVHD, n (%) | |
|
2 (2) |
|
2 (2) |
|
1 (0.8) |
| CAR-T treatment, n (%) | 3 (2) |
| No comorbidity, n (%) | 96 (73) |
| At least one comorbidity | 35 (27) |
|
8 |
|
7 |
|
5 |
|
4 |
|
4 |
|
2 |
|
2 |
|
2 |
|
7 |
| Bacterial coinfection (n = 5) | |
|
4 |
|
1 |
|
1 |
|
1 |
| Viral coinfection (n = 5) | |
|
2 |
|
1 |
|
1 |
|
1 |
| Invasive fungal infection (n = 3) | |
|
1 |
|
1 |
|
1 |
| Median neutrophil count, cells/mm3 (IQR) | 805 (155–2020) |
| Median lymphocyte count, cells/mm3 (IQR) | 530 (190–1030) |
IQR, interquartile range; GVHD, graft versus host disease; BMI, body mass index.
Unknown in n = 43.
Includes children with brain tumour affecting the pituitary stalk (n = 4) or long-term immunosuppression (n = 3).
All patients in whom the tumour has caused brain damage.
Includes patients with Down syndrome, psoriasis, xeroderma pigmentosum, Niemann-Pick disease, kidney transplantation, Kaposi sarcoma and Russel's syndrome.
Disease severity was categorised as asymptomatic in 42 patients (32%, 95% CI, 24–41%), mild in 61 (47%, 95% CI 38–55%), moderate in 11 (8%, 95% CI 4–14%), severe in 5 (4%, 95% CI 2–8%) and critical in 12 (9%, 95% CI 5–15%).
3.1. Acquisition, incubation and shedding
Virus acquisition was known in 93 episodes and included 79 (85%) with community-acquired and 14 (15%) with hospital-acquired infection. Fifty-eight (44%) infections were part of a SARS-CoV-2 family cluster. The median duration from known SARS-CoV-2 exposure to earliest onset of symptoms or positive PCR result for asymptomatic cases was 5 days (range, 1–22 days) in the 26 episodes where the date of exposure was known. In 42 asymptomatic patients, testing indication included confirmed contact (n = 14), pregeneral anaesthetic (n = 8), prechemotherapy (n = 8) and pre-HSCT (n = 6; unknown, n = 6).
The median duration of virus detection by PCR was 16 days (IQR, 9.5–27 days; range, 1–79 days) in the 51 patients (including 48 on active treatment) with two or more positive respiratory swabs.
3.2. Symptoms and signs
There was no difference in the proportion of patients with symptomatic infection who were on active cancer treatment (69%) or who had completed treatment (65%). In the 89 symptomatic patients, fever (≥38°C) was the most common symptom (Table 2 ). Chest computerised tomography was performed in 27 patients, with bilateral ground glass changes in 14 and bilateral consolidation in 6 (normal in 7). A coinfection was diagnosed at the time of diagnosis (±5 days) of SARS-CoV-2 in 16 patients (12%), with bacterial coinfection the most common (n = 7; Table 1).
Table 2.
Symptoms, treatment and outcomes of children with cancer or following haematopoietic stem cell transplantation and SARS-CoV-2 infection confirmed by PCR.
| Symptoms, treatment and outcome | N (%) |
|---|---|
| COVID-19 symptoms (n = 89) | |
|
63 (71) |
|
42 (47) |
|
26 (29) |
|
21 (24) |
|
17 (19) |
|
10 (11) |
|
6 (7) |
|
6 (7) |
|
4 (4) |
| COVID-19 treatment | |
| Any antiviral therapy (n = 11, 8%) | |
| Oseltamivir | 6 |
| Hydroxychloroquine | 3 |
| Ribavirin | 1 |
| Lopinavir/ritonavir | 1 |
| Remdesivir | 1 |
| Azithromycin | 17 |
| Any adjunctive therapy (n = 13, 10%) | |
| Corticosteroids | 11 |
| Immunoglobulin | 8 |
| Tocilizumab | 5 |
| Convalescent plasma | 3 |
| Anticoagulation | 1 |
| Supplemental O2 | 17 |
| Chemotherapy changes (n=100) | |
| Withheld | 30 (30) |
| Modified | 6 (6) |
| Unknown | 1 (1) |
| No change | 63 (63) |
| Outcome | |
| ICU admission | 15 (11) |
|
10 |
|
10 |
|
0 |
| Disease severity | |
|
42 (32) |
|
61 (47) |
|
11 (8) |
|
5 (4) |
|
12 (9) |
| Died | 5 (4) |
|
4 (3) |
|
1 (1) |
ICU, intensive care unit; ECMO, extracorporeal membrane oxygenation.
3.3. Management
Sixty-five (50%) patients were admitted to hospital at the time of infection, including 49 (37%) for COVID-19-related symptoms or management and 16 (12%) for chemotherapy or other non-COVID-19-related treatment. Of the 49 patients admitted because of COVID-19, the median hospital length of stay (LOS) was 9.3 days (IQR, 4.5–19.6 days). Of the 100 patients on active cancer treatment, 36 (36%) had chemotherapy interrupted or dose modified (Table 2). On univariate analysis, any chemotherapy modification (interrupted or dose modified) was not significantly associated with a reduced risk of severe COVID-19 (OR 0.72, 95% CI 0.46–1.05) in all patients or in the 31 patients on intensive chemotherapy (OR 0.62, 95% CI 0.20–1.39).
Specific treatments are documented in Table 2. Antiviral or other potentially COVID-19 directly therapy was used infrequently (8%). Children with severe or critical disease were more likely to receive antiviral therapy (47% versus 3%) or corticosteroids (52% versus 2%) compared with those with non-severe disease. Forty-three (32%) patients had broad-spectrum antibiotics (>1 agent in 32), most commonly an antipseudomonal beta-lactam or cephalosporin (n = 33).
Fifteen patients (11%, 95% CI, 7–18%) required ICU admission, including ten who required mechanical ventilation (median duration 7.5 days, IQR 3.8–11.8 days). The patients requiring ICU admission were treated for leukaemia/lymphoma (n = 10) and solid tumour (n = 5) in Brazil (n = 10), Russia (n = 2), Germany (n = 2) and Israel (n = 1), with a median age of 7 years (range, 5-16y). No SARS-CoV-2 variant was found in these patients. One patient had a coinfection with S. aureus/E. coli and cytomegalovirus, another patient a coinfection with Pneumocystis jirovecii. The median ICU LOS was 10 days, IQR 6–19 days, with 14 patients requiring prolonged (>3 days) admission.
3.4. Blood biomarkers
Full blood examination was available in 109 patients, of which 72 (66%) were lymphopenic and 24 (22%) were neutropenic at SARS-CoV-2 diagnosis. C-reactive protein was available in 59 (45%) patients with the median peak value significantly higher in those with severe/critical disease compared to non-severe/critical disease (126 [IQR, 66–287] versus 21 [4.6–57], P < 0.001).
3.5. Outcome
A total of 124 (95%) patients made a full recovery. Myocarditis was documented in two patients and encephalitis in one, all of whom recovered fully (all on active treatment). One patient, also on active treatment, had a protracted course and developed postinfectious bronchiolitis obliterans. Five patients died within 30 days, including 4 (3%; 95% CI, 1–7%) due to COVID-19 infection (Table 3 ). These four patients died between 6 and 21 days after the diagnosis of the infection. Of the four COVID-19-related deaths, one was on active treatment (1% of 108 patients on active treatment), and three had completed cancer treatment (13% of 23 with completed treatment). Final outcome unknown in two patients.
Table 3.
Clinical details of children with cancer and lethal outcome because of SARS-CoV-2 infection.
| Age (y)/sex | Country | Malignancy | Last anticancer treatment | Comorbidity | ANC/ALC at diagnosis of SARS-CoV-2 (per mm3) | Coinfection | Mechanical ventilation | Antiviral treatment | Time between SARS-CoV-2 diagnosis and death (days) |
|---|---|---|---|---|---|---|---|---|---|
| 6/female | Russia | Acute leukaemia | HSCT >6 months | Kaposi sarcoma | 3900/780 | No | Yes (ARDS) | Ribavirin, lopinavir, hydroxychloroquine | 18 |
| 9/female | Brazil | Acute leukaemia | 5 days before | Steroid-induced adrenal insufficiency | 0/0 | No | Yes (ARDS) | No | 21 |
| 11/female | Brazil | Brain tumour | >30 days before | Steroid-induced adrenal insufficiency, obesity | 3680/3320 | No | Yes (ARDS) | Oseltamivir | 9 |
| 11/female | Brazil | Brain tumour | >30 days before | Steroid-induced adrenal insufficiency, obesity, chronic neurological problems | 7080/2730 | No | Yes (ARDS) | No | 6 |
HSCT, haematopoietic stem cell transplantation; ANC, absolute neutrophil count; ALC, absolute lymphocyte count; ARDS, acute respiratory distress syndrome.
Factors significantly associated with increasing disease severity in univariate analysis included the presence of any comorbidity, chronic lung disease, any coinfection, bacterial coinfection and neutropenia. On multivariate analysis, comorbidity, any coinfection and neutropenia remained independently and significantly associated with increasing disease severity (Table 4 ).
Table 4.
Associations of patient characteristics with disease severity based on results of exact polytomous regression.
| Patient characteristics | Univariate |
Multivariate |
||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Europe versus non-Europe | 1.31 (0.93–1.92) | 0.13 | – | – |
| Age ≤7 versus >7 years | 1.28 (0.93–1.81) | 0.13 | – | – |
| Male versus female | 0.88 (0.65–1.20) | 0.45 | – | – |
| Leukaemia v solid tumour | 1.35 (0.99–1.87) | 0.06 | – | – |
| Intensive versus non-intensive treatment phase | 1.24 (0.88–1.73) | 0.23 | – | – |
| Radiotherapy versus no radiotherapy | 1.19 (0.83–1.67) | 0.35 | – | – |
| Relapse/progressive versus non-relapse/progressive malignancy | 1.17 (0.77–1.74) | 0.49 | – | – |
| HSCT versus no HSCT | 1.22 (0.80–1.79) | 0.36 | – | – |
| CAR-T versus non-CAR-T | 0.63 (0.06–2.11) | 0.82 | – | – |
| Active versus no active cancer treatment at SARS-CoV-2 diagnosis | 1.09 (0.73–1.59) | 0.70 | – | – |
| Any comorbidity versus no comorbidity | 1.90 (1.35–2.74) | <0.001 | 2.94 (1.81–5.21) | <0.001 |
| Chronic lung disease versus no | 2.68 (1.25–7.09) | 0.010 | NS | NS |
| Coinfection versus no coinfection at SARS-CoV-2 diagnosis | 1.44 (1.00–2.06) | 0.047 | 1.74 (1.03–3.03) | 0.036 |
| Bacterial coinfection versus no at SARS-CoV-2 diagnosis | 1.71 (1.05–2.76) | 0.032 | NS | NS |
| Neutropenia versus no neutropenia at SARS-CoV-2 diagnosis | 1.54 (1.07–2.25) | 0.021 | 1.82 (1.13–3.09) | 0.013 |
| Lymphopenia versus no lymphopenia at SARS-CoV-2 diagnosis | 1.19 (0.83–1.77) | 0.39 | NS | NS |
HSCT, haematopoietic stem cell transplantation; OR, odds ratio; CI, confidence interval; NS, non-significant.
4. Discussion
In the multinational study of children with cancer or HSCT, infection with SARS-CoV2 was asymptomatic in almost one-third of children, with fever, cough and coryza as the most common symptoms in the remaining patients. Although 95% of patients made a full recovery, severe or critical disease occurred in 13%, and 11% required prolonged ICU admission. Factors significantly associated with increasing COVID-19 severity included presence of at least one comorbidity, any coinfection and neutropenia. Overall mortality was 3% and higher in patients who had completed cancer treatment or had undergone HSCT (13%) compared with patients on active treatment (0.9%). For patients on active treatment, chemotherapy was modified/interrupted in 35%, and although no significant association of any adjustment with disease severity was seen for all patients on active treatment, the wide confidence intervals limit firm conclusions.
Since SARS-CoV-2 was first reported, marked variation in the incidence of severe or critical disease in children with cancer has been described, ranging from 0 through to 15% [[17], [18], [19], [20]]. Similarly, variation in infection-related mortality has also been described, with many large paediatric cancer centres reporting no deaths [18,20,21], whereas others, albeit small single-centre studies, reporting case fatality up to 28% [22]. This variation likely reflects the different populations included (e.g. hospitalised versus non-hospitalised), available resources to manage patients and COVID-19 testing criteria. The overall mortality of 3% in our study is in keeping with results of a systematic review of five small studies of COVID-19 in paediatric cancer (pooled mortality of 4%) [23], as well as the St Jude Global registry of COVID-19 (3.7%; https://global.stjude.org/). Mortality was higher in patients who had completed cancer treatment, compared with those on active treatment, although the small number limits firm conclusions. This observation is in contrast to adult patients, where patients on systemic anticancer therapy had a similar risk of death to patients on no treatment [23]. Factors such as increasing age, comorbidities and potentially a protective effect of corticosteroids in children on active treatment are some hypotheses that may explain these results [8].
Children with cancer are a heterogeneous population, with a spectrum of infection risk that varies both between cancer diagnoses and during the course of treatment [24]. Although paediatric cancer has not consistently been identified as a risk for severe or fatal COVID-19, most studies have grouped solid tumour, leukaemia, relapsed disease and HSCT together [23]. An understanding of the impact of these disease- and treatment-related factors is critical to informing paediatric cancer care pathways including modifying chemotherapy regimens and isolation restrictions. Although chemotherapy intensity, relapse-status, active treatment and baseline lymphopenia were not significantly associated with disease severity in our cohort, neutropenia at the time of infection with SARS-CoV-2 increased the odds of severe disease. Similar to adult literature, the presence of a comorbidity also increased odds of severe disease [25]. Not surprisingly, in our cohort, an increased odds of severe disease was observed in patients with coinfection at the time of SARS-CoV-2 diagnosis. To date, this association has not been well described in the paediatric oncology literature. Notably, the emerging reports of invasive mould coinfections, including aspergillosis [26,27] and mucomycoses [28], was not reflected in our data with only three invasive fungal infections (IFIs) documented, one of which was with Aspergillus spp.
Duration of viral shedding in the cancer population has important infection-control implications, given the necessity for frequent hospital attendance and potential for nosocomial transmission. A study in otherwise well children found the median period of viral shedding of COVID-19 was 15 days (IQR 11–20 d) and shorter in asymptomatic patients (11 versus 17 d) [29]. Similarly, the median duration of SARS-CoV-2 detected by PCR was 16 days in our study, although in one patient, virus was detected by PCR for 79 days. Although a theoretical risk of transmission remains, there are limited data on viability of the virus in patients with prolonged shedding or the potential to reactive and cause severe disease in subsequent chemotherapy cycles.
In our study, most known acquisitions occurred within the family home. In addition to the vaccination of treating physicians and nurses, vaccination of household contacts against COVID-19 may be another important protective measure. As adult cancer patients have an increased risk of severe disease and death, vaccination of this patient population is now a priority for many centres. Although an immune correlate of protection against COVID-19 has not been established to date, a study of adult cancer patient on active treatment found 90% exhibited antibody response to an messenger RNA vaccine, albeit significantly lower than in healthy controls [30]. Although vaccination of children has commenced in some regions, there are no data on vaccine efficacy in the paediatric cancer population, and clinical studies are urgently needed.
Highly effective antiviral or adjunctive treatment of COVID-19 in children continues to remain elusive. While relatively few patients received targeted treatment in our cohort, patients with more severe disease were more likely receive one or more antiviral or adjuvant treatments. The range of agents used also reflects the evolving evidence during the pandemic and includes medications such as hydroxychloroquine, ribavirin, remdesivir and azithromycin that are no longer recommended in this setting [31].
Notably, more than one-third of patients on active treatment had chemotherapy interruptions due to COVID-19, including cycles withheld in 30%, which may impact treatment response and risk of relapse. Corroborating our data, a study in 308 adult cancer patients demonstrated that continuing cytotoxic chemotherapy administration was not significantly associated with a severe or critical COVID-19 event [25].
Although one of the largest cohorts of COVID-19 in paediatric patients with cancer published to date, our data are retrospective, and variations in testing criteria between centres may impact the spectrum of disease severity reported. As only PCR-confirmed infections were included, this may have also led to an over estimation of disease severity. Included patients may have been entered into other international or country-based registries, which has important implications for interpreting data from meta-analyses. Finally, as data collection ended in February 2021, the impact of newer variants including Delta-variant on disease severity remains unknown.
Our study provides new insights into factors associated with COVID-19 disease severity, duration of shedding, symptoms and outcomes in children with cancer, which could be helpful in the clinical management of this patient population. Although almost all children made a full recovery, severe or critical disease still occurred in 15% of patients, and 3% died. A high awareness for increased COVID-19 severity is recommended in children with cancer and comorbidities, baseline coinfection or severe neutropenia. Ongoing surveillance is critical to monitor vaccine efficacy and impact of emerging COVID-19 variants in this population.
Funding source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author contributions
Gabrielle M Haeusler, Roland A Ammann, Fabianne Carlesse, Andreas H Groll, Dina Averbuch, Elio Castagnola, Philipp KA Agyeman, Bob Phillips, Lillian Sung, Thomas Lehrnbecher: Study design.
Gabrielle M Haeusler: Quality control of data and algorithms.
Gabrielle M Haeusler, Roland A Ammann, Thomas Lehrnbecher: Data analysis and interpretation.
Gabrielle M Haeusler, Roland A Ammann, Thomas Lehrnbecher, Lillian Sung: Statistical analysis.
All authors: Study concepts, Data acquisition, Manuscript preparation, Manuscript editing, Manuscript review.
Conflict of interest statement
A.H.G. has research support from Gilead Sciences, Merck Sharp and Dohme and Pfizer; is a consultant for Amplyx, Astellas, Basilea, F2G, Gilead Sciences, Merck Sharp and Dohme and Pfizer; and served at the speakers' bureau of Astellas, Basilea, F2G, Gilead Sciences, Merck Sharp and Dohme and Pfizer. A.A. is a consultant for Jazz Pharmaceuticals, Amgen, Novartis, Gilead Sciences and MSD/Merck and served at the speakers' bureau of Jazz Pharmaceuticals, Amgen and Novartis. T.L. has an unrestricted research support from Gilead Sciences; is a consultant for Gilead Sciences, Merck Sharp and Dohme, Pfizer, Astellas and Roche; and serves at the speakers' bureau of Gilead Sciences, Merck Sharp and Dohme, Astellas, Pfizer and GlaxoSmithKline. All the other authors do not have to declare a conflict of interest.
Acknowledgements
The authors would like to acknowledge the Australian and New Zealand Children's Haematology/Oncology (ANZCHOG) for support and binational endorsement of this study.
Footnotes
Part of the study has been presented at the 31st ECCMID meeting, July 9–12, 2021: abstract 1671.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ejca.2021.09.027.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Sullivan M., Bouffet E., Rodriguez-Galindo C., et al. The COVID-19 pandemic: a rapid global response for children with cancer from SIOP, COG, SIOP-E, SIOP-PODC, IPSO, PROS, CCI, and St Jude Global. Pediatr Blood Cancer. 2020;67 doi: 10.1002/pbc.28409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baruchel A., Bertrand Y., Boissel N., et al. COVID-19 and acute lymphoblastic leukemias of children and adolescents: first recommendations of the Leukemia committee of the French Society for the fight against Cancers and Leukemias in children and adolescents (SFCE) Bull Cancer. 2020;107:629–632. doi: 10.1016/j.bulcan.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Janssens G.O., Mandeville H.C., Timmermann B., et al. A rapid review of evidence and recommendations from the SIOPE radiation oncology working group to help mitigate for reduced paediatric radiotherapy capacity during the COVID-19 pandemic or other crises. Radiother Oncol. 2020;148:216–222. doi: 10.1016/j.radonc.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weinkove R., McQuilten Z.K., Adler J., et al. Managing haematology and oncology patients during the COVID-19 pandemic: interim consensus guidance. Med J Aust. 2020;212:481–489. doi: 10.5694/mja2.50607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ljungman P., Mikulska M., de la Camara R., et al. The challenge of COVID-19 and hematopoietic cell transplantation; EBMT recommendations for management of hematopoietic cell transplant recipients, their donors, and patients undergoing CAR T-cell therapy. Bone Marrow Transplant. 2020;55:2071–2076. doi: 10.1038/s41409-020-0919-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verbruggen L.C., Wang Y., Armenian S.H., et al. Guidance regarding COVID-19 for survivors of childhood, adolescent, and young adult cancer: a statement from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Pediatr Blood Cancer. 2020;67 doi: 10.1002/pbc.28702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bailey L.C., Razzaghi H., Burrows E.K., et al. Assessment of 135794 pediatric patients tested for severe acute respiratory syndrome coronavirus 2 across the United States. JAMA Pediatr. 2021;175:176–184. doi: 10.1001/jamapediatrics.2020.5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zimmermann P., Curtis N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch Dis Child. 2020 Dec 1 doi: 10.1136/archdischild-2020-320338. In press. [DOI] [PubMed] [Google Scholar]
- 9.Belsky J.A., Tullius B.P., Lamb M.G., et al. COVID-19 in immunocompromised patients: a systematic review of cancer, hematopoietic cell and solid organ transplant patients. J Infect. 2021;82:329–338. doi: 10.1016/j.jinf.2021.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai M., Liu D., Liu M., et al. Patients with cancer appear more vulnerable to SARS-COV-2: a multicenter study during the COVID-19 outbreak. Cancer Discov. 2020;10:783–791. doi: 10.1158/2159-8290.CD-20-0422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang W., Guan W., Chen R., et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21:335–337. doi: 10.1016/S1470-2045(20)30096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong Y., Mo X., Hu Y., et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145 doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 13.Ammann R.A., Bodmer N., Hirt A., et al. Predicting adverse events in children with fever and chemotherapy-induced neutropenia: the prospective multicenter SPOG 2003 FN study. J Clin Oncol. 2010;28:2008–2014. doi: 10.1200/JCO.2009.25.8988. [DOI] [PubMed] [Google Scholar]
- 14.Sung L., Aplenc R., Alonzo T.A., et al. Effectiveness of supportive care measures to reduce infections in pediatric AML: a report from the Children's Oncology Group. Blood. 2013;121:3573–3577. doi: 10.1182/blood-2013-01-476614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Agresti A. 2nd ed. John Wiley & Sons; New York: 2002. Categorical data analysis; pp. 286–287. [Google Scholar]
- 16.Ammann R.A. Defibrotide for hepatic VOD in children: exact statistics can help. Bone Marrow Transplant. 2004;34:277–278. doi: 10.1038/sj.bmt.1704571. [DOI] [PubMed] [Google Scholar]
- 17.Bisogno G., Provenzi M., Zama D., et al. Clinical characteristics and outcome of SARS-CoV-2 infection in Italian pediatric oncology patients: a study from the Infectious Diseases Working Group of the AIEOP. J Pediatric Infect Dis Soc. 2020;9:530–534. doi: 10.1093/jpids/piaa088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boulad F., Kamboj M., Bouvier N., et al. COVID-19 in children with cancer in New York city. JAMA Oncol. 2020;6:1459–1460. doi: 10.1001/jamaoncol.2020.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Andre N., Rouger-Gaudichon J., Brethon B., et al. COVID-19 in pediatric oncology from French pediatric oncology and hematology centers: high risk of severe forms? Pediatr Blood Cancer. 2020;67 doi: 10.1002/pbc.28392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Millen G.C., Arnold R., Cazier J.B., et al. Severity of COVID-19 in children with cancer: report from the United Kingdom paediatric coronavirus cancer monitoring project. Br J Cancer. 2021;124:754–759. doi: 10.1038/s41416-020-01181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hrusak O., Kalina T., Wolf J., et al. Flash survey on severe acute respiratory syndrome coronavirus-2 infections in paediatric patients on anticancer treatment. Eur J Cancer. 2020;132:11–16. doi: 10.1016/j.ejca.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arous R., Djillali I.S., Rouis N.O., et al. High mortality of COVID-19 in children with cancer in a single center in Algiers, Algeria. Pediatr Blood Cancer. 2021;68 doi: 10.1002/pbc.28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vijenthira A., Gong I.Y., Fox T.A., et al. Outcomes of patients with hematologic malignancies and COVID-19: a systematic review and meta-analysis of 3377 patients. Blood. 2020;136:2881–2892. doi: 10.1182/blood.2020008824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haeusler G.M., Thusky K.A., Slavin M., et al. Re-evaluating and recalibrating predictors of bacterial infection in children with cancer and febrile neutropenia. EClinicalMedicine. 2020;23:100394. doi: 10.1016/j.eclinm.2020.100394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jee J., Foote M.B., Lumish M., et al. Chemotherapy and COVID-19 outcomes in patients with cancer. J Clin Oncol. 2020;38:3538–3546. doi: 10.1200/JCO.20.01307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van de Veerdonk F.L., Bruggemann R.J.M., Vos S., et al. COVID-19-associated Aspergillus tracheobronchitis: the interplay between viral tropism, host defence, and fungal invasion. Lancet Respir Med. 2021;9(7):795–802. doi: 10.1016/S2213-2600(21)00138-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alanio A., Delliere S., Fodil S., et al. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir Med. 2020;8:e48–e49. doi: 10.1016/S2213-2600(20)30237-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raut A., Huy N.T. Rising incidence of mucormycosis in patients with COVID-19: another challenge for India amidst the second wave? Lancet Respir Med. 2021;9(8):E77. doi: 10.1016/S2213-2600(21)00265-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu Y., Li Y., Deng W., et al. Symptomatic infection is associated with prolonged duration of viral shedding in mild coronavirus disease 2019: a retrospective study of 110 children in Wuhan. Paediatr Infect Dis J. 2020;39:E95–E99. doi: 10.1097/INF.0000000000002729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Massarweh A., Eliakim-Raz N., Stemmer A., et al. Evaluation of seropositivity following BNT162b2 messenger RNA vaccination for SARS-CoV-2 in patients undergoing treatment for cancer. JAMA Oncol. 2021;7(8):1133–1140. doi: 10.1001/jamaoncol.2021.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tendal B., Vogel J.P., McDonald S., et al. Weekly updates of national living evidence-based guidelines: methods for the Australian living guidelines for care of people with COVID-19. J Clin Epidemiol. 2021;131:11–21. doi: 10.1016/j.jclinepi.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.