Abstract
Poor sleep is associated with increased hypertension risk, but few studies have evaluated multiple sleep dimensions or investigated racial/ethnic disparities in this association among women. We investigated multiple sleep dimensions (sleep duration, inconsistent weekly sleep patterns, sleep debt, frequent napping and difficulty falling or staying asleep) and hypertension risk among women, and determined modification by age, race/ethnicity and menopausal status. We used data from the Sister Study, a national cohort of 50 884 women who had sisters diagnosed with breast cancer in the United States enrolled at baseline (2003–2009) and followed through September 2018. Of 33 497 women without diagnosed hypertension at baseline (mean age±standard deviation: 53.9±8.8 years; 88.7% White, 6.4% Black and 4.9% Hispanic/Latina), 23% (n = 7686) developed hypertension over a median follow-up of 10.1 years [interquartile range: 8.2–11.9 years]. Very short, short or long sleep duration, inconsistent weekly sleep patterns, sleep debt, frequent napping, insomnia, insomnia symptoms as well as short sleep and cumulative, explorative poor sleep score were associated with incident hypertension after adjustment for demographics factors. After additional adjustment for lifestyle and clinical factors, insomnia [hazard ratio = 1.09, 95% confidence interval (95% CI): 1.03–1.15] and insomnia symptoms plus short sleep (hazard ratio = 1.13, 95% CI: 1.05–1.21) remained associated with incident hypertension. These associations were stronger in younger (age<54 vs. ≥54 years) and premenopausal vs. postmenopausal women (all P-interaction < 0.05). Associations did not differ by race/ethnicity (all P-interaction > 0.05). Thus, screening for multiple sleep dimensions and prioritizing younger and premenopausal women may help identify individuals at high risk for hypertension.
Keywords: ethnic groups, health status disparities, hypertension, race factors, sleep
INTRODUCTION
More than 70 million adults in the United States (US) experience habitually poor sleep [1]. Studies in the general population consistently link poor sleep health and sleep disorders with unfavourable health outcomes, including the development of hypertension [2,3]. Poor habitual sleep dimensions that have emerged as independent risk factors for hypertension include short sleep duration and insomnia [4]; but the role of other sleep dimensions on hypertension risk is unclear. Recent studies link irregular sleep pattern, sleep debt and frequent day-time napping with a higher prevalence of hypertension [5], suggesting that other less recognized sleep dimensions may also contribute to hypertension development. Yet, few studies have investigated the role of multiple sleep dimensions on hypertension risk.
The role of certain sleep dimensions as risk factors for the development of hypertension may differ according to age, sex, race/ethnicity and menopausal status [6–14]. Women, for instance, are generally more likely to report poor habitual sleep than men, especially before menopause. Consequently, studies have found stronger associations between poor habitual sleep and hypertension risk in premenopausal women vs. both postmenopausal women and men [6,14]. Likewise, both short sleep duration and hypertension are disproportionately more prevalent in Black vs. non-Black populations [11,12,15]. However, the relative importance of multiple sleep dimensions in hypertension risk across demographic subgroups is understudied.
Comorbid factors such as depression and use of certain medications are associated with both poor habitual sleep patterns and an increased risk for hypertension [16,17]. As such, these comorbidities can potentially confound the association between poor habitual sleep and incidence of hypertension in observational studies. We used detailed data from a large national cohort of US women enrolled in the Sister Study to examine the association of multiple sleep dimensions with risk of hypertension incidence over follow-up; examined potential modification of these associations by race/ethnicity, age, and menopausal status; and adjusted for a myriad of comorbidities.
MATERIALS AND METHODS
Study population
The Sister Study is a prospective cohort of 50 884 women in the US and Puerto Rico enrolled between 2003 and 2009. As described in detail elsewhere [18], the Sister Study recruited women who had a sister diagnosed with breast cancer through multimedia campaigns and using a network of breast cancer professionals and advocates [19]. Targeted recruitment strategies were employed to increase racial/ethnic, age and socioeconomic diversity within the cohort. Those who were residents of the US or Puerto Rico, 35–74 years of age and free of breast cancer at enrolment were eligible to participate in the study. Participants completed a comprehensive computer-assisted telephone interview at baseline and were subsequently followed annually via brief health assessments and every 2 to 3 years via detailed follow-up questionnaires. For this analysis, we used the Data Release 8.0., which is the most recent update of the Sister Study data with follow-up through 23 September, 2018.
Of the 50 884 participants enrolled in the Sister Study, participants who self-reported a diagnosis of hypertension at baseline or had unknown timing of hypertension diagnosis (n = 16 502) and those who did not self-identify as White or Black/African–American race or Hispanic/Latina ethnicity (n = 885) were excluded. The final analytic sample comprised 33 497 participants (Fig. 1). Institutional and Independent Review Boards at the National Institute of Environmental Health Sciences and the Copernicus Group approved the study. All participants provided verbal consent at first contact and written informed consent at the home visit.
FIGURE 1.
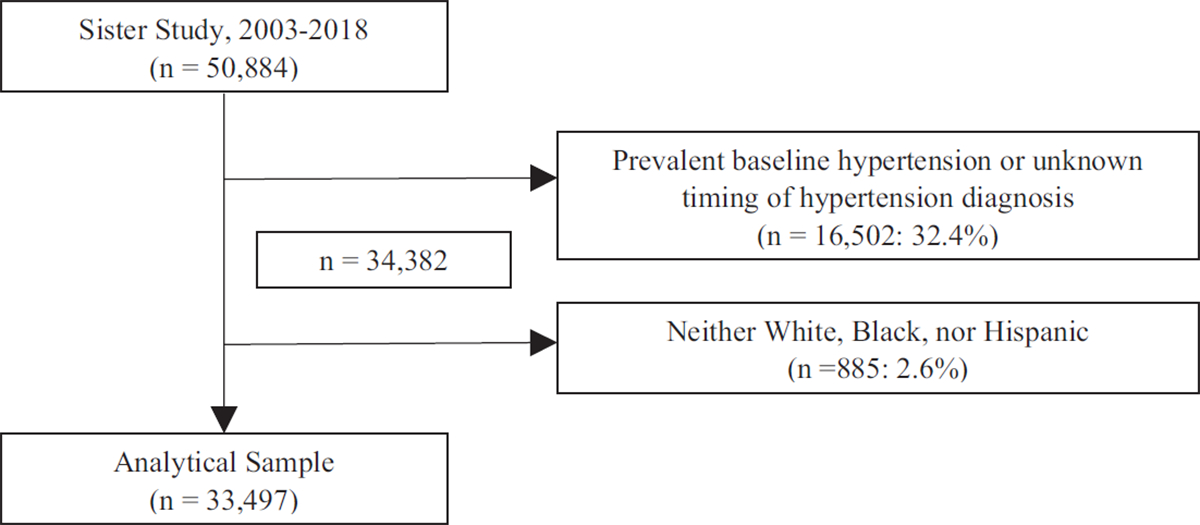
Flow chart diagram of final analytic sample derivation.
Assessment of sleep dimensions
Women self-reported their habitual sleep pattern as part of the baseline Sister Study interview. This analysis included data on sleep duration, inconsistent weekly sleep patterns, sleep debt, napping and insomnia. Sleep duration was a continuous measure based on participants’ response to the question, ‘About how many hours and/or minutes of sleep per night/day do you get on average?’ We categorized sleep duration into very short (≤5 h), short (>5 to <7h), recommended (7–9 h) and long (>9 h), based on National Sleep Foundation recommendations suggesting that 7–9 h may be optimal for health [20]. For all sleep duration analyses, we excluded participants with implausible average self-reported sleep duration values of 2 h or less or at least 23 h (n = 31). Weekly sleep patterns were based on participant-reported wake-up times and bedtimes during the prior 6 weeks. We categorized sleep patterns as consistent if they were stable from week-to-week (even if they varied day-to-day) and as inconsistent otherwise. For participants with consistent weekly sleep patterns, we directly calculated longest and shortest sleep durations from their reported daily bedtimes and wake-up times. For participants with inconsistent weekly sleep patterns, we utilized participant self-report of their longest and shortest average sleep duration. Sleep debt was defined as the difference between the longest and shortest sleep durations and was dichotomized at 2 h. For all sleep debt analyses, we excluded participants with 2 h or less or at least 23 h for their longest or shortest sleep duration (n = 504). Frequent napping was defined as at least three naps per week. Difficulty falling asleep was defined as taking at least 30 min to fall asleep on average, and difficulty staying asleep was defined as waking up at least three times per night on at least three nights per week. Women who reported experiencing difficulty falling asleep or difficulty staying asleep were considered to have insomnia symptoms. We also assessed the combination of short sleep duration and insomnia symptoms. Participants with long sleep (n = 305) Were excluded from the derivation of this variable to avoid lumping the long sleep category with the recommended sleep category, as long sleep has been shown to be associated with adverse health [21]. Lastly, we created a cumulative, explorative poor sleep score (range: 0–5), calculated as the sum of the following symptoms of poor sleep: short sleep or long sleep, inconsistent weekly sleep patterns, sleep debt ≥2 h, napping at least three times per week and insomnia symptoms.
Ascertainment of hypertension incidence
We defined incident hypertension as a new diagnosis of hypertension over follow-up among those without prevalent hypertension at baseline. Doctor-diagnosed hypertension (and date of diagnosis) was self-reported via annual update questionnaires and detailed follow-up questionnaires (completed every 3 years).
Demographics, lifestyle/health behaviours and clinical factors
Participants reported their demographic characteristics, lifestyle, and past medical history as part of the comprehensive computer-assisted telephone interviews administered at Demographic characteristics included age, self-identified race and ethnicity, marital status, household annual income, employment status and educational attainment. Lifestyle factors included smoking status alcohol consumption, physical activity based on metabolic equivalent of tasks (METs) [22,23] and healthy eating index-2015 calculated from a modified Block 1998 Food Frequency Questionnaire [24]. Past medical history was self-reported by participants and comprised menopausal status, cardiovascular disease, hypercholesterolemia, diabetes, depression, sleep medication use (including melatonin) and antidepressant use.
Premenopausal status was defined as having at least one menstrual cycle in the 12 months prior to the baseline assessment. Trained study personnel measured body anthropometrics, including height, weight and waist and hip circumference during a home visit at baseline. These measures were used to calculate BMI (in kg/m2) and waist-to-hip ratio.
Statistical analysis
We described baseline characteristics of participants overall and by incident hypertension status using frequency and proportion for categorical variables and mean ± standard deviation (SD) for continuous variables. We examined the association of sleep dimensions with incident hypertension using Cox Proportional Hazards models and reported findings as hazard ratios with 95% confidence intervals (95% CIs). Models were sequentially adjusted for demographics (Model 1: age, race/ethnicity, marital status, household income, employment status and educational attainment); lifestyle factors (Model 2: Model 1 covariates and smoking status, alcohol use, binge drinking, physical activity and healthy eating index score); and clinical characteristics (Model 3: Model 2 covariates and BMI, waist-to-hip ratio, diabetes and antidepressant use). We additionally adjusted sleep debt analyses for inconsistent weekly sleep patterns. To determine whether age (dichotomized at the median age of 54 years), race/ethnicity or menopausal status modified the aforementioned associations, we added interaction terms for sleep measures and the potential modifiers to the fully adjusted Cox models and tested for effect modification using likelihood ratio tests.
We conducted several sensitivity analyses. To determine if menopausal status was a proxy for age in the modification analyses (or vice versa), we assessed the interaction between age and sleep separately among premenopausal and postmenopausal women. We also stratified analyses by use of sleep medication and melatonin at study baseline. In addition, to determine if our analyses using self-reported data were sensitive to misclassification bias, we employed an alternative definition of prevalent hypertension using measured blood pressure (BP): SBP at least 130 mmHg, DBP at least 80 mmHg or use of antihypertensive medication at baseline. This approach excluded an additional 8097 women, which resulted in an analytic population of 25 400. Techniques for BP measurements employed in this sensitivity analysis followed standardized protocols and have been previously detailed elsewhere [25].
All hypothesis tests were two-sided at the 5% α level. Age was used as the time scale of the Cox Proportional Hazards models and participants were right-censored on the last date of follow-up (23 September 2018) or the date of diagnosis of hypertension, whichever came first. We assessed and confirmed that the Cox models did not violate the proportionality assumption of the hazard function using Schoenfeld residuals. We used STATA v.15 (STATA Corp., College Station, Texas, USA) for all analyses.
RESULTS
Baseline characteristics of study participants
Among the 33 497 included participants, mean age ± SD at baseline was 53.9 ± 8.8 years; 29 720 (88.7%) were white, 2151 (6.4%) black and 1626 (4.9%) Hispanic/Latina; and 14 299 (42.7%) were current or former cigarette smokers.
A majority of participants reported having the recommended 7–9 h of sleep per 24 h (n = 23 997; 71.8%). Overall, 7819 (23.8%) participants had sleep debt at least 2 h; 2980 (8.9%) reported napping at least three times per week; and 8332 (24.9%) had at least one insomnia symptom (Table 1).
TABLE 1.
Sociodemographic, health behaviour and clinical characteristics of eligible Sister Study Participants at baseline (2003–2009), overall and stratified by incident hypertension status through a follow-up period of 23 September 2018
| Baseline characteristics | Overall | Incident hypertension status |
|
|---|---|---|---|
| Yes | No | ||
|
| |||
| No. of participants (%) | 33 497 | 7686 (23.0) | 25 811 (77.1) |
| Sociodemographic characteristics | |||
| Mean age, years | 53.9 ± 8.8 | 55.8 ± 8.8 | 53.4 ± 8.7 |
| Age <54 years | 17147 (51.2) | 3333 (43.4) | 13814 (53.5) |
| Age ≥54 years | 16350 (48.8) | 4353 (56.6) | 11 997 (46.5) |
| Race/Ethnicity | |||
| Non-Hispanic White | 29 720 (88.7) | 6660 (86.7) | 23 060 (89.3) |
| Non-Hispanic Black | 2151 (6.4) | 666 (8.7) | 1485 (5.8) |
| Hispanic/Latina | 1626 (4.9) | 360 (4.7) | 1266 (4.9) |
| Marital status | |||
| Single/Never married | 1774 (5.3) | 444 (5.8) | 1330 (5.2) |
| Married/living as married | 25 871 (77.2) | 5702 (74.2) | 20 169 (78.2) |
| Separated/widowed/divorced | 5848 (17.5) | 1539 (20.0) | 4309 (16.7) |
| Educational attainment | |||
| ≤high school | 4487 (13.4) | 1218 (15.9) | 3269 (12.7) |
| >high school | 29007 (86.6) | 6467 (84.2) | 22 540 (87.9) |
| Annual household income a | 42.9 ± 27.2 | 41.5 ± 26.6 | 43.4 ± 27.4 |
| Unemployed (yes) | 9988 (30.1) | 2502 (32.9) | 7486 (29.3) |
| Work hours | |||
| <20 h/week | 11 865 (35.8) | 2912 (38.3) | 8953 (35.1) |
| ≥20 h/week | 21 282 (64.2) | 4695 (61.7) | 16 587 (65.0) |
| Health behaviours | |||
| Smoker status | |||
| Never | 19193 (57.3) | 4174 (54.3) | 15019 (58.2) |
| Former | 11 623 (34.7) | 2811 (36.6) | 8812 (34.1) |
| Current | 2676 (7.9) | 699 (9.1) | 1977 (7.7) |
| Alcohol consumption | |||
| Current/former | 32 418 (96.8) | 7409 (96.4) | 25 009 (96.9) |
| Binge drinking b | 6318 (18.9) | 1403 (18.3) | 4915 (19.1) |
| Physical activity, MET hours/week | 15.7 ± 18.7 | 13.1 ± 16.3 | 16.4 ± 19.3 |
| Healthy Eating Index score c | 72.1 ± 9.6 | 71.1 ± 9.8 | 72.4 ± 9.5 |
| Ever use of sleep medication, melatonin, or antihistamines (yes) | 11 149 (33.8) | 2787 (36.8) | 8362 (32.9) |
| Antidepressant use | 3938 (11.9) | 926 (14.0) | 3012 (11.3) |
| Never/no baseline depression | 27 248 (81.4) | 6079 (79.1) | 21 169 (82.1) |
| Former | 2235 (6.7) | 535 (6.8) | 1710 (6.6) |
| Current | 3987 (11.9) | 1077 (14.0) | 2910 (11.3) |
| Clinical characteristics | |||
| BMI (kg/m2) | 26.5 ± 5.5 | 28.4 ± 6.0 | 25.9 ± 5.2 |
| Underweight: <18.5 kg/m2 | 496 (1.5) | 63 (0.8) | 433 (1.7) |
| Normal: 18.5–24.9 kg/m2 | 15253 (45.6) | 2449 (31.9) | 12 804 (49.6) |
| Overweight: 25–29.9 kg/m2 | 10540 (31.5) | 2674 (34.8) | 7866 (30.5) |
| Obese: ≥30 kg/m2 | 7196 (21.4) | 2498 (32.5) | 4698 (18.2) |
| Waist-to-hip ratio | 0.80 ± 0.08 | 0.81 ± 0.07 | 0.79 ± 0.08 |
| Menopausal status | |||
| Premenopausal | 13 537 (40.4) | 2560 (33.3) | 10 977 (42.5) |
| Postmenopausal | 19946 (59.6) | 5121 (66.7) | 14825 (57.5) |
| SBP (mmHg) | 111.3 ± 12.3 | 118.9 ± 12.4 | 109.1 ± 11.3 |
| DBP (mmHg) | 70.7 ± 8.3 | 75.0 ± 8.3 | 69.5 ± 7.8 |
| Cardiovascular disease (yes) | 6297 (19.6) | 1578 (21.7) | 4719 (19.0) |
| Hypercholesterolemia (yes) | 8943 (27.0) | 2440 (32.3) | 6503 (25.4) |
| Diabetes (yes) | 834 (2.5) | 294 (3.9) | 540 (2.1) |
| Clinical depression (yes) | 6619 (22.0) | 1699 (25.0) | 4920 (21.2) |
| Sleep characteristics | |||
| Sleep duration | 7.1 ± 1.0 | 7.1 ± 1.1 | 7.1 ± 1.0 |
| ≤5 h | 1965 (5.9) | 534 (7.0) | 1431 (5.6) |
| >5 to <7h | 7153 (21.4) | 1742 (22.7) | 5411 (21.0) |
| 7–9 h | 23 997 (71.8) | 5298 (69.1) | 18 699 (72.6) |
| >9h | 313 (0.9) | 91 (1.2) | 222 (0.9) |
| Inconsistent weekly sleep patterns d | 4587 (13.7) | 1185 (15.4) | 3402 (13.2) |
| Sleep debt ≥2h e | 7819 (23.8) | 1862 (24.7) | 5957 (23.5) |
| Frequent napping ≥3 times/week | 2980 (8.9) | 797 (10.4) | 2183 (8.5) |
| Insomnia symptomsf | 8332 (24.9) | 2144 (28.0) | 6188 (24.0) |
| Difficulty falling asleep g | 5381 (16.1) | 1427 (18.6) | 3954 (15.3) |
| Difficulty staying asleep h | 4238 (12.7) | 1090 (14.2) | 3148 (12.2) |
| Insomnia symptoms and short sleep | 3528 (10.7) | 963 (12.7) 2565 (10.1) | |
| Cumulative poor sleep score i | 1.00 ± 1.14 | 1.10 ± 1.19 0.97 ± 1.12 | |
| 0 | 14 427 (43.3) | 3033 (39.7) 11 394 (44.4) | |
| 1 | 9751 (29.3) | 2287 (30.0) 7464 (29.1) | |
| 2 | 5296 (15.9) | 1286 (16.8) 4010 (15.6) | |
| 3 | 2471 (7.4) | 628 (8.2) 1843 (7.2) | |
| 4 | 1233 (3.7) | 351 (4.6) 882 (3.4) | |
| 5 | 141 (0.4) | 51 (0.7) 90 (0.4) | |
Data are presented as n (%) or mean ±standard deviation.
BP, blood pressure; MET, metabolic equivalent of task.
Annual income scaled to 1000 US Dollars.
Binge drinking: ≥4 per sitting.
Healthy Eating Index scores range from 0 to 100 with higher scores indicating a healthier diet.
Inconsistent weekly sleep patterns (yes vs. no) were defined as participants reporting inconsistent wake-up times and bedtimes from week-to-week during the prior 6 weeks.
Sleep debt was defined as the difference in sleep hours between the nights with the longest and shortest sleep durations and was dichotomized at ≥2h.
Insomnia defined as difficulty falling asleep or difficulty staying asleep.
Difficulty falling asleep defined as taking ≥30 min to fall asleep.
Difficulty staying asleep defined as waking up at least three times per night at least three nights per week.
Cumulative poor sleep score is the summation of the following symptoms of poor sleep: short sleep or long sleep, inconsistent weekly sleep patterns, sleep debt ≥2 h, napping at least three times per week and insomnia.
Compared with black or Hispanic participants, white participants were more likely to be postmenopausal (60.8% for white vs. 48.8% for black vs. 50.9% for Hispanic participants), to have the recommended sleep duration of 7–9 h (74.0 vs. vs. 64.0%, respectively) and to have lower cumulative poor sleep score (0.95 vs. 1.52 vs. 1.33, respectively) (Appendix eTable 1, http://links.lww.com/HJH/B707).
Cumulative incidence of hypertension
Over a median follow-up of 10.1 years [(interquartile range (IQR): 8.2–11.9], 7686 (23%) participants developed hypertension. Compared with those who did not develop hypertension, those who did develop hypertension were older (mean age 55.8 vs. 53.4 years) and more likely to identify as black (8.7 vs. 5.8%), to be current or former smokers (45.7 vs. 41.8%), and to use sleep medications (%) (Table 1).
Sleep dimensions and hypertension risk
In models adjusted for demographic factors, all poor sleep dimensions were associated with greater incidence of hypertension. In fully adjusted models, insomnia symptoms (difficulty falling or staying asleep) (hazard ratio = 1.09, 95% CI: 1.03–1.15) and insomnia symptoms in combination with short sleep duration (hazard ratio = 1.13, 95% CI: 1.05–1.21) were associated with greater hypertension risk (Table 2).
TABLE 2.
Hazard ratios for multiple sleep dimensions and hypertension risk in the Sister Study (March 2003–September 2018), N = 33497
| Model 1: Adjusted for demographics a |
Model 2: Adjusted for demographics and lifestyle b |
Model 3: Fully adjusted c |
|||||
|---|---|---|---|---|---|---|---|
| Sleep dimensions | Number of events/person-years | HR (95% CI)d | P | HR (95% CI) | P | HR (95% CI) | P |
|
| |||||||
| Sleep duration | 0.248 | ||||||
| ≤5 h | 483/16 311 | 1.14 (1.04–1.26) | 1.10 (1.00–1.22) | 1.05 (0.95–1.16) | |||
| >5 to <7 h | 1612/63 535 | 1.07 (1.01–1.13) | 1.04 (0.98– 1.11) | 1.03 (0.97–1.09) | |||
| 7 to <9 h | 4951/221 500 | 1.00 | 1.00 | 1.00 | |||
| >9 h | 82/2540 | 1.38 (1.10–1.72) | 0.001 | 1.27 (1.02–1.60) | 0.033 | 1.21 (0.96–1.52) | |
| Inconsistent weekly sleep patterns | 0.354 | ||||||
| No | 6072/264 565 | 1.00 | 1.00 | 1.00 | |||
| Yes | 1066/39 362 | 1.08 (1.01–1.15) | 0.033 | 1.03 (0.96–1.10) | 0.467 | 0.97 (0.90–1.04) | |
| Sleep debt >2 h | 0.132 | ||||||
| No | 5274/229 963 | 1.00 | 1.00 | 1.00 | |||
| Yes | 1728/69 779 | 1.13 (1.05–1.20) | 0.001 | 1.09 (1.02–1.16) | 0.015 | 1.05 (0.98–1.13) | |
| Napping at least three times/week | 0.890 | ||||||
| No | 6422/278 742 | 1.00 | 1.00 | 1.00 | |||
| Yes | 724/25 629 | 1.09 (1.01– 1.18) | 0.033 | 1.07 (0.99–1.16) | 0.105 | 1.01 (0.93–1.09) | |
| Insomnia symptoms | 0.002 | ||||||
| No | 5156/231 749 | 1.00 | 1.00 | 1.00 | |||
| Yes | 1976/72 014 | 1.15 (1.09–1.22) | <0.001 | 1.11 (1.06–1.18) | <0.001 | 1.09 (1.03– 1.15) | |
| Difficulty falling asleep | 0.003 | ||||||
| No | 5815/258 258 | 1.00 | 1.00 | 1.00 | |||
| Yes | 1324/45 721 | 1.19 (1.11–1.26) | <0.001 | 1.14 (1.07–1.21) | <0.001 | 1.10 (1.03–1.17) | |
| Difficulty staying asleep | 0.005 | ||||||
| No | 6133/267 001 | 1.00 | 1.00 | 1.00 | |||
| Yes | 1006/37 157 | 1.14 (1.06–1.22) | <0.001 | 1.11 (1.04–1.19) | 0.003 | 1.11 (1.03–1.19) | |
| Insomnia symptoms and short sleep e | 0.002 | ||||||
| No | 6142/271 074 | 1.00 | 1.00 | 1.00 | |||
| Yes | 891/29 826 | 1.19 (1.10–1.28) | <0.001 | 1.15 (1.07–1.24) | <0.001 | 1.13 (1.05–1.21) | |
| Cumulative poor sleep score f | 1.06 (1.04–1.09) | <0.001 | 1.04 (1.02–1.06) | <0.001 | 1.02 (1.00–1.04) | 0.093 | |
CI, confidence interval; HR, hazard ratio.
Model 1: Demographic factors (age, race/ethnicity, marital status, income level, employment status and educational attainment) and inconsistent weekly sleep patterns (for sleep debt analyses only).
Model 2: Model 1 + smoking status, alcohol use, binge drinking, physical activity and health eating index score.
Model 3: Model 2 + comorbidities (BMI, waist-to-hip ratio, and diabetes) and antidepressant use.
Bolded estimates and p-values are significant at the 0.05 significance level.
Excludes those with long sleep duration.
Number of events/person-years for all values of the cumulative poor sleep score: Score of 0 = 2861 events/135 542 person-years; 1 = 2112/88 089; 2 = 1192/46 811; 3 = 571/21 294; 4 = 322/10 086; 5 = 45/1091
Sleep dimensions and hypertension risk by potential moderators
Age
The association of sleep with incident hypertension was evident only among younger (age < 54 years) participants for the following poor sleep dimensions. Short (>5 to <7 h) vs. recommended sleep duration: hazard ratio = 1.09, 95% CI: 1.00–1.19; P-interaction = 0.022. Napping at least three vs. less than three times per week: hazard ratio = 1.13, 95% CI: 0.99–1.29; P-interaction < 0.001. Insomnia symptoms: hazard ratio = 1.19, 95% CI: 1.10–1.29; P-interaction < 0.001. Insomnia symptoms in combination with short sleep duration: hazard ratio = 1.28, 95% CI: 1.15–1.42; P-interaction < 0.001. 0.001. Cumulative poor sleep score: hazard ratio = 1.06, 95% CI: 1.02–1.09; P-interaction < 0.001 (Table 3).
TABLE 3.
Hazard ratios for multiple sleep dimensions and hypertension risk by age group in the Sister Study (March 2003–September 2018)a
| Age <54 years |
Age ≥54 years |
||||
|---|---|---|---|---|---|
| Sleep dimensions | No. with event/person-years | HR (95% CI)b | No. with event/person-years | HR (95% CI) | P for interaction |
|
| |||||
| Sleep duration | 0.0216 | ||||
| ≤5 h | 247/8687 | 1.12 (0.97–1.29) | 236/7624 | 1.00 (0.86–1.15) | |
| >5 to <7 h | 773/34 052 | 1.09 (1.00–1.19) | 839/29 483 | 0.98 (0.90–1.07) | |
| 7 to <9 h | 2102/117 663 | 1.00 | 2849/103 837 | 1.00 | |
| >9h | 37/1189 | 1.40 (0.99–1.97) | 45/1350 | 1.10 (0.81– 1.49) | |
| Inconsistent weekly sleep patterns | 0.3066 | ||||
| No | 2751/143 645 | 1.00 | 3321/120920 | 1.00 | |
| Yes | 411/17 853 | 0.99 (0.88–1.10) | 655/21 509 | 0.96 (0.87–1.05) | |
| Sleep debt ≥2h | 0.2886 | ||||
| No | 2187/117 787 | 1.00 | 3087/112 176 | 1.00 | |
| Yes | 904/41 743 | 1.06 (0.97–1.16) | 824/28 036 | 1.04 (0.93–1.15) | |
| Napping ≥3 times/week | 0.0066 | ||||
| No | 2874/150 567 | 1.00 | 3548/128175 | 1.00 | |
| Yes | 291/11 252 | 1.13 (0.99–1.29) | 433/14 378 | 0.93 (0.83–1.03) | |
| Insomnia symptoms | 0.0005 | ||||
| No | 2261/126143 | 1.00 | 2895/105 606 | 1.00 | |
| Yes | 901/35 542 | 1.19 (1.10–1.29) | 1078/36 696 | 1.01 (0.94–1.09) | |
| Difficulty falling asleep | 0.0050 | ||||
| No | 2539/138 525 | 1.00 | 3276/119 734 | 1.00 | |
| Yes | 625/23 172 | 1.18 (1.08–1.30) | 699/22 549 | 1.03 (0.95–1.13) | |
| Difficulty staying asleep | 0.0056 | ||||
| No | 2726/144 513 | 1.00 | 3407/122 488 | 1.00 | |
| Yes | 437/17 218 | 1.22 (1.10–1.36) | 569/19 939 | 1.02 (0.93–1.12) | |
| Short sleep and insomnia symptoms | 0.0001 | ||||
| No | 2668/145 049 | 1.00 | 3474/126 025 | 1.00 | |
| Yes | 450/15 192 | 1.28 (1.15–1.42) | 441/14 633 | 1.00 (0.90–1.11) | |
| Cumulative poor sleep score | 1.06 (1.02–1.09) c | 0.99 (0.96–1.02)d | <0.0001 | ||
CI, confidence interval; HR, hazard ratio.
All models adjusted for age, race/ethnicity, marital status, income level, employment status, educational attainment, smoking status, alcohol use, binge drinking, physical activity, healthy eating index score, BMI, waist-to-hip ratio and diabetes), antidepressant use and inconsistent weekly sleep patterns (for sleep debt analyses only).
Bolded estimates and P-values are significant at the 0.05 significance level.
Number of events/person-years for all values of the cumulative poor sleep score for those age <54 years: Score of 0 = 1202 events/72 991 person-years; 1 = 941/437 085; 2 = 561/24 765; 3 = 272/101 008; 4 = 150/4629; 5 = 24/617.
Number of events/person-years for all values of the cumulative poor sleep score for those age ≥54 years: Score of 0 = 1528 events/62 552 person-years; 1 = 1171/41 004; 2 = 631/22 047; 3 = 299/10 286; 4 = 172/5457; 5 = 21/474.
Race/ethnicity
There was no evidence of interactions between race/ethnicity and any of the sleep dimensions on risks for hypertension (all P-interaction > 0.05) (Table 4).
TABLE 4.
Hazard ratios for multiple sleep dimensions and hypertension risk by race/ethnicity in the Sister Study (March 2003–September 2018)a
| White |
Black |
Hispanic/Latina |
|||||
|---|---|---|---|---|---|---|---|
| Sleep dimensions | No. with event/person-years | Hazard ratio (95% CI)b | No. with event/person-years | Hazard ratio (95% CI) | No. with event/person-years | Hazard ratio (95% CI) | P for interaction |
|
| |||||||
| Sleep duration | 0.4845 | ||||||
| ≤5 h | 323/12 312 | 1.03 (0.91–1.16) | 127/2734 | 1.16 (0.92–1.46) | 33/1265 | 0.91 (0.62–1.34) | |
| >5 to <7 h | 1327/54987 | 1.03 (0.97–1.10) | 202/5459 | 1.00 (0.82–1.22) | 83/3089 | 1.06 (0.80–1.39) | |
| 7 to <9 h | 4478/205 562 | 1.00 | 269/7441 | 1.00 | 204/8497 | 1.00 | |
| >9 h | 74/2356 | 1.18 (0.93–1.50) | 6/85 | 2.11 (0.93–4.80) | 2/99 | 0.46 (0.06–3.32) | |
| Inconsistent weekly sleep patterns | 0.8845 | ||||||
| No | 5327/241 180 | 1.00 | 486/12 766 | 1.00 | 259/10 619 | 1.00 | |
| Yes | 876/34000 | 0.97 (0.90–1.05) | 124/3002 | 0.94 (0.75–1.17) | 66/2360 | 0.99 (0.54–1.82) | |
| Sleep debt ≥2 h | 0.8338 | ||||||
| No | 4670/210831 | 1.00 | 390/10 277 | 1.00 | 214/8855 | 1.00 | |
| Yes | 1426/61 064 | 1.05 (0.98–1.14) | 198/4904 | 1.02 (0.83–1.26) | 104/3812 | 1.06 (0.80–1.42) | |
| Napping ≥3 times/week | 0.8642 | ||||||
| No | 5602/253121 | 1.00 | 537/14 069 | 1.00 | 283/11 551 | 1.00 | |
| Yes | 608/22 440 | 1.01 (0.92–1.10) | 74/1749 | 0.93 (0.71–1.22) | 42/1441 | 1.02 (0.72–1.46) | |
| Insomnia symptoms | 0.4004 | ||||||
| No | 4532/211 954 | 1.00 | 409/10 989 | 1.00 | 215/8805 | 1.00 | |
| Yes | 1669/63 300 | 1.10 (1.04–1.17) | 201/4785 | 1.01 (0.83–1.21) | 109/4153 | 1.02 (0.79–1.31) | |
| Difficulty falling asleep | 0.1668 | ||||||
| No | 5134/236 778 | 1.00 | 440/11 806 | 1.00 | 241/9674 | 1.00 | |
| Yes | 1069/38452 | 1.12 (1.05–1.21) | 171/3971 | 1.04 (0.86, 1.26) | 84/3297 | 0.93 (0.71–1.22) | |
| Difficulty staying asleep | 0.2631 | ||||||
| No | 5322/241 385 | 1.00 | 539/14 251 | 1.00 | 272/11 365 | 1.00 | |
| Yes | 884/34021 | 1.10 (1.02–1.18) | 70/1535 | 0.96 (0.72, 1.27) | 52/1601 | 1.46 (1.07–2.01) | |
| Insomnia symptoms and short sleep | 0.6297 | ||||||
| No | 5419/247 837 | 1.00 | 459/12 506 | 1.00 | 264/10 732 | 1.00 | |
| Yes | 698/24 656 | 1.14 (1.05–1.24) | 138/3085 | 1.09 (0.88–1.34) | 55/2085 | 1.01 (0.74–1.37) | |
| Cumulative poor sleep score | 1.02 (1.00–1.05)c | 0.99 (0.92–1.07)d | 0.99 (0.90–1.10)e | 0.6726 | |||
CI, confidence interval.
All models adjusted for age, marital status, income level, employment status, educational attainment, smoking status, alcohol use, binge drinking, physical activity, healthy eating index score, BMI, waist-to-hip ratio, diabetes, antidepressant use and inconsistent weekly sleep patterns (for sleep debt analyses only).
Bolded estimates and P-values are significant at the 0.05 significance level.
Number of events/person-years for all values of the cumulative poor sleep score for white participants: Score of 0 = 2623 events/127 320 person-years; 1 = 1839/79385; 2 = 965/ 40463; 3 = 460/17 963; 4 = 260/8448; 5 = 34/824.
Number of events/person-years for all values of the cumulative poor sleep score for black participants: Score of 0 = 135 events/3890 person-years; 1 = 187/4904; 2 = 155/3712; 3 = 74/1974; 4 = 41/972; 5 = 9/164.
Number of events/person-years for all values of the cumulative poor sleep score for Hispanic participants: Score of 0 = 103 events/4332 person-years; 1 = 86/3800; 2 = 72/2636; 3 = 37/1357; 4 = 21/666; 5 = 2/103.
Menopausal status
There was evidence for interaction between menopausal status and several sleep dimensions on hypertension risk. Associations of poor sleep dimensions with incident hypertension were evident among premenopausal but not postmenopausal women for insomnia symptoms (hazard ratio = 1.23, 95% CI: 1.11–1.35; P-interaction < 0.001); insomnia symptoms in combination with short sleep duration (hazard ratio = 1.27, 95% CI: 1.12–1.45; P-interaction = 0.002); and cumulative poor sleep score (hazard ratio = 1.05, 95% CI: 1.02–1.09; P-interaction < 0.001) (Table 5).
TABLE 5.
Hazard ratios for multiple sleep dimensions and hypertension risk by menopausal status in the Sister Study (March 2003–September 2018)a
| Premenopausal |
Postmenopausal |
||||
|---|---|---|---|---|---|
| Sleep dimensions | No. with event/person-years | HR (95% CI)b | No. with event/person-years | HR (95% CI) | P for interaction |
|
| |||||
| Sleep duration | 0.1129 | ||||
| ≤5 h | 162/5821 | 1.08 (0.91–1.29) | 319/10 462 | 1.03 (0.91–1.17) | |
| >5 to <7 h | 570/25 917 | 1.09 (0.98–1.20) | 1041/37 593 | 1.00 (0.93–1.07) | |
| 7 to <9 h | 1661/95311 | 1.00 | 3288/126 099 | 1.00 | |
| >9h | 30/1004 | 1.31 (0.89–1.92) | 52/1536 | 1.15 (0.86–1.52) | |
| Inconsistent weekly sleep patterns | 0.5923 | ||||
| No | 2134/114 342 | 1.00 | 3933/150 092 | 1.00 | |
| Yes | 291/13590 | 0.95 (0.83–1.09) | 775/25 761 | 0.98 (0.90–1.06) | |
| Sleep debt ≥2h | 0.5141 | ||||
| No | 1682/93 043 | 1.00 | 3590/136 823 | 1.00 | |
| Yes | 697/33 577 | 1.06 (0.96–1.17) | 1028/36169 | 1.04 (0.95–1.14) | |
| Napping ≥3 times/week | 0.0078 | ||||
| No | 2209/119 609 | 1.00 | 4209/159 026 | 1.00 | |
| Yes | 217/8591 | 1.14 (0.98–1.32) | 506/17 009 | 0.95 (0.86–1.05) | |
| Insomnia symptoms | 0.0002 | ||||
| No | 1779/102 474 | 1.00 | 3375/129 204 | 1.00 | |
| Yes | 647/25 645 | 1.23 (1.11–1.35) | 1329/46 522 | 1.03 (0.96–1.10) | |
| Difficulty falling asleep | 0.0072 | ||||
| No | 1980/111293 | 1.00 | 3831/146 861 | 1.00 | |
| Yes | 447/16855 | 1.20 (1.07– 1.34) | 876/28 828 | 1.06 (0.98–1.15) | |
| Difficulty staying asleep | 0.0057 | ||||
| No | 2121/1 16223 | 1.00 | 4009/150 679 | 1.00 | |
| Yes | 305/11 919 | 1.26 (1.11–1.43) | 699/25 195 | 1.05 (0.96–1.14) | |
| Insomnia symptoms and short sleep | 0.0019 | ||||
| No | 2085/1 16 416 | 1.00 | 4054/154 565 | 1.00 | |
| Yes | 307/10 535 | 1.27 (1.12–1.45) | 582/19 241 | 1.06 (0.97–1.16) | |
| Cumulative poor sleep score | 1.05 (1.02–1.09) c | 1.00 (0.97–1.03)d | 0.0002 | ||
CI, confidence interval.
All models adjusted for age, race/ethnicity, marital status, income level, employment status, educational attainment, smoking status, alcohol use, binge drinking, physical activity, healthy eating index score, BMI, waist-to-hip ratio, diabetes, antidepressant use and inconsistent weekly sleep patterns (for sleep debt analyses only).
Bolded estimates and P-values are significant at the 0.05 significance level.
Number of events/person-years for all values of the cumulative poor sleep score for premenopausal participants: Score of 0 = 962 events/59 955 person-years; 1 = 730/36 679; 2 = 403/18 994; 3 = 204/8387; 4 = 101/3253; 5 = 18/390.
Number of events/person-years for all values of the cumulative poor sleep score for postmenopausal participants: Score of 0 = 1898 events/75 543 person-years; 1 = 1382/51 373; 2 = 786/27 780; 3 = 367/12 906; 4 = 220/6822; 5 = 27/689.
Sensitivity analysis
The interactions between age and poor sleep dimensions with incident hypertension risk were generally not evident after stratification by menopausal status. The exceptions were insomnia symptoms in combination with short sleep duration (P-interaction = 0.401 among premenopausal and 0.021 among postmenopausal women) and cumulative sleep score (P-interaction = 0.583 among premenopausal and 0.003 among postmenopausal women) (Appendix eTable 2, http://links.lww.com/HJH/B707). Sleep medication and melatonin use did not modify the association of sleep dimensions and hypertension risk (all P-interaction > 0.05), with the exception of short sleep duration (P-interaction = and (P-interaction = 0.042) (Appendix eTable 3, http://links.lww.com/HJH/B707). Results from the main analyses were generally consistent when prevalent hypertension was defined using measured BP (Appendix eTable 4, http://links.lww.com/HJH/B707) in combination with medication use.
DISCUSSION
In this large cohort of women from across the US and Puerto Rico, multiple dimensions of poor habitual sleep were associated with an increased risk of incident hypertension. Overall, insomnia symptoms and insomnia symptoms in combination with short sleep were associated with an increased risk for hypertension incidence after adjustment for demographic factors, lifestyle and comorbidities. Napping at least three times per week, insomnia symptoms, difficulty falling/staying asleep, insomnia symptoms combined with short sleep and cumulative poor sleep score were associated with an increased risk for hypertension incidence specifically in premenopausal and younger women. Findings support menopausal status more so than age as a modifier of the association between poor habitual sleep and development of hypertension.
The robust association between insomnia symptoms in combination with short sleep and risk for incident hypertension in our study underpins a growing literature that consistently links this phenotype of insomnia with independent risk for unfavourable cardiovascular outcomes such as hypertension incidence [4,26–28]. Several potential mechanisms underlying this link have emerged, and physiological hyperarousal is the most extensively studied [4]. This hyperarousal state activates the hypothalamic-pituitary-adrenal (HPA) axis [29,30] and is thought to potentiate development of hypertension by blunting nocturnal BP dipping, which has been associated with an increased risk for hypertension [31]. Another potential underlying mechanism that was recently demonstrated in one study is chronic low-grade endothelial inflammation, defined in that study as greater nuclear translocation of NFκB in endothelial cells [32]. Moving forward, this compelling empirical evidence warrants screening for severe phenotypes of insomnia such as its cooccurrence with short sleep to offset hypertension risk in women.
The current findings highlight important heterogeneity in the association of poor sleep with hypertension risk between demographic subgroups, which has clinically relevant implications for screening efforts and sleep interventions. Foremost, the data from the current study suggest younger and premenopausal women as subpopulations in whom unfavourable sleep patterns disproportionately confers increased hypertension risk. These findings were evident despite hypertension incidence being relatively higher among older (vs. Younger) and postmenopausal (vs. premenopausal) women. Thus, lending to the growing recognition of early adulthood as a sensitive period during which poor sleep particularly affects health [3]. In prior studies, the association between poor habitual sleep and hypertension risk has been robust among young adults [3], but this relationship is not evident in similarly designed studies among older adults [33]. Likewise, prior studies link poor habitual sleep with hypertension risk specifically in premenopausal, compared with postmenopausal, women [13]. The underlying reasons for this relative importance of poor sleep in younger and premenopausal women warrants further investigation, including the potential role of physiological and psychosocial factors that might potentiate the consequences of poor sleep in these groups [34,35]. However, it is worth noting that changes in sex hormones that accompany menopause do not appear to play a role in this relation [36].
Although differences in the association of sleep dimensions and hypertension risk across race/ethnicity did not reach statistical significance, these trends were generally consistent with prior studies [11,35]. Further, it is worth recognizing the limitations inherent in assessing effect modification by interaction terms alone, especially when assessing racial health disparities [37]. Thus, although the associations of interest did not vary by race/ethnicity in the current study, this does not necessarily indicate that sleep is not a mechanism that perpetuates racial disparities in incident hypertension. To this point, there were racial/ethnic disparities in the exposures and outcome of interest, whereby, compared with white women, black and Hispanic/Latina women had a higher prevalence of most dimensions of poor habitual sleep and black women had a greater incidence of hypertension.
STRENGTHS AND LIMITATIONS
This study has several limitations. Our findings might not be generalizable to the broader US population given that the Sister Study enrolled a targeted cohort of women whose sisters had a diagnosis of breast cancer. We used self-reported sleep dimensions [38,39]. Although it is a more feasible approach to assess sleep in epidemiologic studies compared with objective assessments such as using polysomnography and actigraphy, self-report only moderately correlates with objective measurements [38]. Future work should, therefore, replicate our findings using objective sleep measures. The hypertension outcome in this study was defined based on participant self-reports of physician diagnosis, which can be problematic given that many US adults have undiagnosed hypertension. However, our findings remained largely unchanged in a sensitivity analysis in which prevalent hypertension status at baseline was ascertained based on measured BP instead of self-reports of physician diagnosis. In addition, unmeasured confounding by factors such as psychosocial stressors (other than depression) remain possible, especially given the observational design of the Sister Study. Despite these limitations, our study had strengths. The inclusion of more than 33 000 community-dwelling women from across the US population without prevalent hypertension at baseline and detailed assessment of demographic, lifestyle and clinical factors, and follow-up with more than 90% retention rate in the Sister Study allowed for comprehensive assessment of the association of multiple sleep dimensions and hypertension risk.
PERSPECTIVE
Insomnia symptoms and insomnia symptoms in combination with short sleep were associated with increased incident hypertension risk in this multiethnic sample of middle-aged and older-aged women across the US and Puerto Rico. These poor habitual sleep dimensions were associated with increased hypertension risk specifically in younger and premenopausal women. Thus, screening for poor sleep and interventions aimed at improving sleep health to prevent hypertension may need to include various dimensions of poor habitual sleep and should prioritize specific subgroups of the population to maximize benefits.
Supplementary Material
NOVELTY and SIGNIFICANCE.
What is new?
We examined the association of multiple dimensions of poor sleep health with hypertension risk in women.
What is relevant?
Among 33 497 women who were free of hypertension at baseline, insomnia symptoms and insomnia symptoms in combination with short sleep were associated with an increased risk of hypertension over a median 10.1 years of follow-up, after adjustment for demographic, lifestyle and comorbid characteristics. These associations were stronger among younger and premenopausal women but did not differ by race/ethnicity.
Summary:
Markers of poor sleep were associated with hypertension risk in women, especially among those who were less than 54 years and premenopausal.
ACKNOWLEDGEMENTS
We sincerely thank the staff and participants of the Sister Study.
The Intramural Program of the National Institute of Health, National Institute of Environmental Health Sciences (Z1A ES103325-01 to CLJ and Z01 ES044005 to DPS) funded this work. N.A.B. is supported by NHLBI under award K01HL140146.
Abbreviations:
- HPA
Hypothalamus-Pituitary-Adrenal axis
- MET
Metabolic Equivalent of Tasks
- NFκB
Nuclear Factor Kappa B
Footnotes
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Colten HR, Altevogt BM, editors. Sleep disorders and sleep deprivation: an unmet public health problem. Washington, DC; 2006. [PubMed] [Google Scholar]
- 2.Calhoun DA, Harding SM. Sleep and hypertension. Chest 2010; 138:434–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knutson KL, Van Cauter E, Rathouz PJ, Yan LL, Hulley SB, Liu K, Lauderdale DS. Association between sleep and blood pressure in midlife: the CARDIA sleep study. Arch Intern Med 2009; 169:1055–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Y, Vgontzas AN, Fernandez-Mendoza J, Bixler EO, Sun Y, Zhou J, et al. Insomnia with physiological hyperarousal is associated with hypertension. Hypertension 2015; 65:644–650. [DOI] [PubMed] [Google Scholar]
- 5.Ramos AR, Weng J, Wallace DM, Petrov MR, Wohlgemuth WK, Sotres-Alvarez D, et al. Sleep patterns and hypertension using actigraphy in the Hispanic community Health Study/Study of Latinos. Chest 2018; 153:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cappuccio FP, Stranges S, Kandala NB, et al. Gender-specific associations of short sleep duration with prevalent and incident hypertension: the Whitehall II Study. Hypertension 2007; 50:693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carnethon MR, De Chavez PJ, Zee PC, Kim KY, Liu K, Goldberger JJ, et al. Disparities in sleep characteristics by race/ethnicity in a population-based sample: Chicago Area Sleep Study. Sleep Med 2016; 18:50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Wang R, Zee P, Lutsey PL, Javaheri S, Alcántara C, et al. Racial/ethnic differences in sleep disturbances: the Multi-Ethnic Study of Atherosclerosis (MESA). Sleep 2015; 38:877–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curtis DS, Fuller-Rowell TE, El-Sheikh M, Carnethon MR, Ryff CD. Habitual sleep as a contributor to racial differences in cardiometabolic risk. Proc Natl Acad Sci U S A 2017; 114:8889–8894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gaston SA, Park YM, McWhorter KL, Sandler DP, Jackson CL. Multiple poor sleep characteristics and metabolic abnormalities consistent with metabolic syndrome among white, black, and Hispanic/Latina women: modification by menopausal status. Diabetol Metab Syndr 2019; 11:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pandey A, Williams N, Donat M, Ceide M, Brimah P, Ogedegbe G, et al. Linking sleep to hypertension: greater risk for blacks. Int J Hypertens 2013; 2013:436502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rasmussen-Torvik LJ, De Chavez PJD, Kershaw KN, Montag SE, Knutson KL, Kim KA, et al. The mediation of racial differences in hypertension by sleep characteristics: Chicago Area Sleep Study. Am J Hypertens 2016; 29:1353–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stranges S, Dorn JM, Cappuccio FP, Donahue RP, Rafalson LB, Hovey KM, et al. A population-based study of reduced sleep duration and hypertension: the strongest association may be in premenopausal women. J Hypertens 2010; 28:896–902. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Mei H, Jiang YR, et al. Relationship between duration of sleep and hypertension in adults: a meta-analysis. J Clin Sleep Med 2015; 11:1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson CL, Redline S, Emmons KM. Sleep as a potential fundamental contributor to disparities in cardiovascular health. Annu Rev Public Health 2015; 36:417–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gangwisch JE, Malaspina D, Posner K, et al. Insomnia and sleep duration as mediators of the relationship between depression and hypertension incidence. Am J Hypertens 2010; 23:62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas SJ, Calhoun D. Sleep, insomnia, and hypertension: current findings and future directions. J Am Soc Hypertens 2017; 11:122–129. [DOI] [PubMed] [Google Scholar]
- 18.Sandler DP, Hodgson ME, Deming-Halverson SL, Juras PS, D’Aloisio AA, Suarez LM, et al. The Sister Study Cohort: baseline methods and participant characteristics. Environ Health Perspect 2017; 125:127003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinberg CR, Shore DL, Umbach DM, Sandler DP. Using risk-based sampling to enrich cohorts for endpoints, genes, and exposures. Am J Epidemiol 2007; 166:447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hirshkowitz M, Whiton K, Albert SM, Alessi C, Bruni O, DonCarlos L, et al. National Sleep Foundation’s sleep time duration recommendations: methodology and results summary. Sleep Health 2015; 1:40–43. [DOI] [PubMed] [Google Scholar]
- 21.Jike M, Itani O, Watanabe N, Buysse DJ, Kaneita Y. Long sleep duration and health outcomes: a systematic review, meta-analysis and metaregression. Sleep Med Rev 2018; 39:25–36. [DOI] [PubMed] [Google Scholar]
- 22.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Montoye HJ, Sallis JF, PaffenbargerRS. Compendiumofphysicalactivities: classificationofenergy costs of human physical activities. Med Sci Sports Exerc 1993; 25:71–80. [DOI] [PubMed] [Google Scholar]
- 23.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000; 32 (9 Suppl):S498–504. [DOI] [PubMed] [Google Scholar]
- 24.Krebs-Smith SM, Pannucci TE, Subar AF, Kirkpatrick SI, Lerman JL, Tooze JA, et al. Update of the Healthy Eating Index: HEI-2015. J Acad Nutr Diet 2018; 118:1591–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muntner P, Shimbo D, Carey RM, Charleston JB, Gaillard T, Misra S, et al. Measurement of blood pressure in humans: a scientific statement From the American Heart Association. Hypertension 2019; 73:e35–e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bertisch SM, Pollock BD, Mittleman MA, Buysse DJ, Bazzano LA, Gottlieb DJ, Redline S. Insomnia with objective short sleep duration and risk of incident cardiovascular disease and all-cause mortality: Sleep Heart Health Study. Sleep 2018; 41:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez-Mendoza J, Vgontzas AN, Liao D, Shaffer ML, Vela-Bueno A, Basta M, Bixler EO. Insomnia with objective short sleep duration and incident hypertension: the Penn State Cohort. Hypertension 2012; 60:929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vgontzas AN, Liao D, Bixler EO, Chrousos GP, Vela-Bueno A. Insomnia with objective short sleep duration is associated with a high risk for hypertension. Sleep 2009; 32:491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohammadi H, Rezaei M, Amiri SM, Rahimi Z, Mansouri K, Khazaie H. Sleep architecture and hypothalamic-pituitary-adrenal activity in paradoxical and psychophysiological insomnia. Basic Clin Neurosci 2018; 9:397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vgontzas AN, Bixler EO, Lin HM, Prolo P, Mastorakos G, Vela-Bueno A, et al. Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab 2001; 86:3787–3794. [DOI] [PubMed] [Google Scholar]
- 31.Lanfranchi PA, Pennestri MH, Fradette L, Dumont M, Morin CM, Montplaisir J. Nighttime blood pressure in normotensive subjects with chronic insomnia: implications for cardiovascular risk. Sleep 2009; 32:760–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aggarwal B, Makarem N, Shah R, Emin M, Wei Y, St-Onge MP, Jelic S. Effects of inadequate sleep on blood pressure and endothelial inflammation in women: findings from the American Heart Association Go Red for Women Strategically Focused Research Network. J Am Heart Assoc 2018; 7:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohammadi H, Rezaei M, Faghihi F, Khazaie H. Hypothalamic-pituitary-gonadal activity in paradoxical and psychophysiological insomnia. J Med Signals Sens 2019; 9:59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kravitz HM, Zhao X, Bromberger JT, et al. Sleep disturbance during the menopausal transition in a multiethnic community sample of women. Sleep 2008; 31:979–990. [PMC free article] [PubMed] [Google Scholar]
- 35.Pien GW, Sammel MD, Freeman EW, Lin H, DeBlasis TL. Predictors of sleep quality in women in the menopausal transition. Sleep 2008; 31:991–999. [PMC free article] [PubMed] [Google Scholar]
- 36.Lopez-Garcia E, Faubel R, Guallar-Castillon P, Leon-Munoz L, Banegas JR, Rodriguez-Artalejo F. Self-reported sleep duration and hypertension in older Spanish adults. J Am Geriatr Soc 2009; 57:663–668. [DOI] [PubMed] [Google Scholar]
- 37.Ward JB, Gartner DR, Keyes KM, Fliss MD, McClure ES, Robinson WR. How do we assess a racial disparity in health? Distribution, interaction, and interpretation in epidemiological studies. Ann Epidemiol 2019; 29:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Girschik J, Fritschi L, Heyworth J, Waters F. Validation of self-reported sleep against actigraphy. J Epidemiol 2012; 22:462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jackson CL, Patel SR, Jackson WB 2nd, Lutsey PL, Redline S. Agreement between self-reported and objectively measured sleep duration among white, black, Hispanic, and Chinese adults in the United States: Multi-Ethnic Study of Atherosclerosis. Sleep 2018; 41:. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


