Abstract
Background: Gallbladder (GB) polyps are raised lesions from the GB wall and projected into its lumen. The prevalence of GB polyps ranged between 4.3% and 12.3%. The clinical presentation of GB polypoid lesions vary, can be nonspecific and vague, and may be asymptomatic. Identifying malignant and premalignant polyps is important to provide treatment early and prevent cancer spread or development of malignancy. Ultrasonography (US) is the first imaging modality widely used in abdominal imaging. It is a noninvasive, rapid, painless, and safe imaging technique, with no radiation; thus, it is considered the best available examination with good sensitivity and specificity for GB polyps.
Aim of the work: This study aimed to determine the relative frequency of the GB polyps and its risk factors among patients who underwent abdominal US in Primary Health Care Corporation, Qatar.
Materials and methods: This was quantitative multicenter observational case–control study nested in a cross-sectional design. For the cross-sectional top-level study, the first step was to assess available abdominal ultrasound studies for the presence of GB polyps and stones. The second step was to perform a case–control study with three groups (a case group and two control groups; first, participants without GB stones and GB polyps; second, patients with GB stones but without GB polyps).
Results: The study evaluated the GB images of 7156 individuals. The overall prevalence of GB polyps was 7.4% in the study population. Specifically, the overall prevalence of solitary GB polyp was 4.2% and that of multiple GB polyps was 3.2%. Regarding the size distribution of GB polyps in positive cases, 89.4% were < 6 mm, 9.3% were 69 mm, and 1.3% were ≥ 10 mm. Prevalence rate of selected comorbidities were as follows: liver disease, 1.8%; diabetes mellitus, 25.5%; hypertension, 25.5%; and dyslipidemia, 29.8%. The prevalence in male and female patients was 7.7% and 7%, respectively. The prevalence of GB polyps was higher in south-eastern patients (21.4% of positive cases) and was the highest in the overweight group (8.8%). A higher prevalence was noted in the hypertensive group (hypertensive group, 9.8%; non-hypertensive group, 6.6%) and dyslipidemia group (dyslipidemia group, 7.8%; no dyslipidemia group, 7.2%). Moreover, a higher prevalence was noted in hepatitis B surface (HBS)-positive group (15%) than in the HBS-negative group (8.2%) and slightly higher in Helicobacter pylori antigen positive group than in the negative group.
Conclusion: Abdominal US is an important and commonly used imaging modality in the detection of GB polyps. In this study, the prevalence of GB polyps was approximately 7.4%, with higher prevalence in participants who were overweight and had diabetes mellitus, hypertension, and dyslipidemia.
Keywords: Gallbladder, polyp, prevalence, risk factor, stone, ultrasound
Background
Gallbladder (GB) polyps are raised lesions from the GB wall and projected into its lumen. Most of them are asymptomatic and incidentally discovered during abdominal ultrasonography (US) performed for other reasons. GB polyps can be classified into benign and malignant polyps (carcinoma). While a great proportion of GB polyps are benign, a big concern is always regarding the presence of malignant change because most patients have late presentation, diagnosed post-cholecystectomy, and have late stage disease and poor prognosis.1-6
In literature, the prevalence of GB polyps varies, but it ranges between 4.3% and 12.3% in many studies.7-11 In a large Japanese study, GB polyps were reported in 5.6% with a male predominance.9,10 In Chinese patients, GB polyps were identified in 6.7% of the patients.8,9 Other studies have reported GB polyps in 4.6% of male and 4.3% of female individuals in Denmark,11 1.5% in Germany,12 and 0.32% in India.13
Many studies have investigated the risk factors for GB polyps. Demographic variations were observed, such as a high prevalence of GB polyps in the third to fifth decades of life.5,9,11 Some studies have shown a higher prevalence of GB polyps in men than in women,5,9,12,14 while no gender predominance was detected in other studies.8,11 Several earlier studies have identified some risk factors for GB polyps, including obesity, diabetes mellitus (DM), chronic hepatitis, serum cholesterol level, and metabolic syndrome.15–19
The clinical presentation of GB polypoid lesions vary, can be nonspecific and vague (such as nausea, vomiting, and occasional pain in the right hypochondrium), may be asymptomatic, and incidentally noted during abdominal US performed for other reasons.3,19,20 However, no significant difference was found in the presenting symptoms of benign and malignant polyps,18
The potential malignant transformation of GB polyps is a big concern; therefore, early detection and management of malignant ones are critical for treatment and long-term survival. Many studies have reported that GB polyps progress into cancer and adenomatous polyps containing carcinoma in situ.21-24 Adenomatous polyps appear to have the highest risk of malignant transformation. In a previous study of 300 randomly selected GBs at cholecystectomy, 19% of sessile adenomas showed small foci of moderate cellular atypia and 31% of them were positive for carcinoembryonic antigen.23 Another study of 1605 resected GBs also supported the adenoma–adenocarcinoma sequence.21
More than 178,000 new cases of GB cancers are diagnosed annually, making it the 20th most common cancer worldwide. The highest incidence of GB cancer is reported in South America and Asia, while the lowest is reported in North America and United Kingdom.5 Survival in GB cancer significantly varies. However, the 5-year survival rate can be as high as 80% in patients with in situ disease and can be at 8% and 2% in cases with lymph node involvement and stage 4b, respectively. These demonstrate the importance of identifying malignant and premalignant polyps to enable early treatment and thus prevent cancer spread or development of malignancy.5,21-32
Qatar is experiencing fast development with mega projects that led to high influxes of migrant workers and professionals from different countries, resulting in a multi-ethnic young adult population with continuous and high demographic turnover.25 This would reflect substantially on the epidemiology and clinicopathological characteristics of diseases prevalent in Qatar.26
US is the first imaging modality widely used in abdominal imaging. It is a noninvasive, rapid, painless, and safe imaging technique, with no radiation27; thus, it is considered the best readily available examination with good sensitivity and specificity to GB polyps. In some previous studies, the sensitivity of abdominal US in the diagnosis of GB polyps is higher than that of computed tomography (CT).3,27,28,29
The vision of the Primary Health Care Corporation (PHCC) is to be the leader in transforming the health and well-being of the people's lives in Qatar. At present, PHCC provides abdominal ultrasound service that can detect GB polyps with high sensitivity. However, to date, no data are available on the actual incidence and epidemiology of GB polyps in Qatar.26
Aim Of The Work
This study aimed to determine the relative frequency of GB polyps and its association with several risk factors among patients who underwent abdominal US in PHCC.
Materials And Methods
Study settings
PHCC is a publicly funded primary care provider in Qatar. Majority of the country's population is registered with PHCC. It has 27 health centers across Qatar that uses an integrated electronic medical record (EMR) system. Every Qatar resident is eligible to register with a PHCC health center.
Study design
This is a quantitative multicenter analytic cross-sectional study.
Study population
The study population included all EMRs of PHCC-registered individuals (CERNER) who underwent an abdominal US in the PHCC Radiology Departments for any reason. A valid GB ultrasound image should be available on the official PHCC EMR system (RIS PACS system) during the 1-year study period from January 1, 2020, to December 31, 2020. Excluded from this group are individuals with a history of surgery on the GB or any biliary intervention.
Study variables
The following variables were extracted from the electronic health record system for the targeted study population: The sociodemographic variables (age, gender, and nationality), personal history of biliary interventions, first available body mass index (BMI) measurement, presence of comorbidities (including type II DM, dyslipidemia, and arterial hypertension), smoking habit, past history of chronic liver disease, gastric Helicobacter pylori test, viral hepatitis, and viral hepatitis status (hepatitis surface antigen [HBS] and hepatitis C virus [HCV] antibodies). The list of comorbid conditions was identified in the EMR using preselected SNOMED codes.
Data collection
The PHCC EMR system uses SNOMED codes, which are systematically organized computer processable collections of medical terms providing codes, terms, synonyms, and definitions used in clinical documentation and reporting. These codes are quality controlled and reviewed by the Business Health Intelligence (BHI) department of PHCC. BHI is responsible for translating SNOMED codes into International Classification of Disease the Tenth Revision codes and continuously updating the coding manual with any new code used in the organizational database at a monthly interval. BHI provided a full list of variables for the study population using filters created for the purpose of the study.
The study team examined the available abdominal ultrasound scans for the presence of GB polyps and stones. The polyps were identified by a combination of the following characteristics on US images: a fixed hyperechoic material protruding from the GB wall into the lumen with no acoustic shadow and does not shift with positional change.30 The number and diameter of the largest polypoid lesion were recorded.
Data cleaning
Duplicates were removed. A total of 82 individuals had two sets of abdominal ultrasound scans performed during the study period. In these individuals, both sets of US images were evaluated to exclude inconsistencies between findings, and only one record per study participant was included. US images of 7645 participants were extracted from the records. Moreover, 489 of the remaining patients had no images of the GB that can be used for the assessment, and these images were also deleted from the database. The US images of the GB of the remaining 7156 individuals were evaluated.
Data analysis
SPSS version 23 (IBM Corp., Armonk, NY, USA) was used for the statistical analysis of data. Data cleaning involved a range of checks (e.g., checking dates to be within the specified study time and checking for completeness of visual triage questionnaire). The frequencies of the selected variables were assessed first. The prevalence ratio (PR) was used to measure the strength of the association between two categorical variables. The significance of these associations was assessed using Chi-square test of independence. The level of significance was assumed at p < 0.05.
The 1-year prevalence (per 100 persons) of GB polyps was calculated using the following formula, and the same applies for the calculation of the prevalence of GB stones:

The PR was used to measure the strength of the association between a dichotomous independent variable (a specific group compared with a reference group) and a dichotomous outcome variable (positive for polyp). The PR is equal to the ratio between the prevalence of an outcome (GB polyps) among those with risk factors divided by the prevalence among those without the risk factor (comparison group). The logarithm method was used in the calculation of confidence intervals for the PR.
A multiple logistic regression model with selected factors as independent variables and depression as the dependent variable was used. The model assessed the risk of having the outcome for each explanatory variable after adjusting for the effect of other confounders included in the model. The model provides the following parameters:
• P value for the model: To generalize the results obtained, the model should be significant.
• Predictive power of the model: The overall predictive power was expressed as a percentage of the study participants being classified correctly based on the calculated parameters.
• Adjusted odds ratio (OR)): This was defined as the risk of having the outcome in the presence of a specific risk factor. Each OR was adjusted for the effect of other explanatory variables included in the model to represent a net effect of each factor on the risk of having the outcome. The OR for different explanatory variables in a participant is additive; in other words, the risk of having the outcome in a specific participant is the sum of the OR for the whole set of explanatory variables.
• P value for OR: This reflects the significance of the calculated OR.31
Quality control measures
In the preparation phase of the study, an extensive review of literature was undertaken. The authors were responsible for the data collection in collaboration with the BHI.
Ethical considerations
The study presented a minimal risk of harm to its participants, and the data collected were anonymized by the BHI. Overall, the study was conducted with integrity according to generally accepted ethical principles. The research proposal was approved by PHCC's Research Sub-Committee (PHCC/DCR/2020/01/006).
Results
In total, GB US images of 7156 individuals were evaluated in this study (Figures 1–5). Female participants constituted 54.8% of the study population. Only 4.8% of the study sample was 65 years of age and older, while 5.6% were < 18 years old. The majority of the participants (70.2%) were young adults. More than a third of the recruited samples were overweight (35.8%), and another 44% were obese. In addition, those who ever smoked constituted 17.4% of the study population (Table 1).
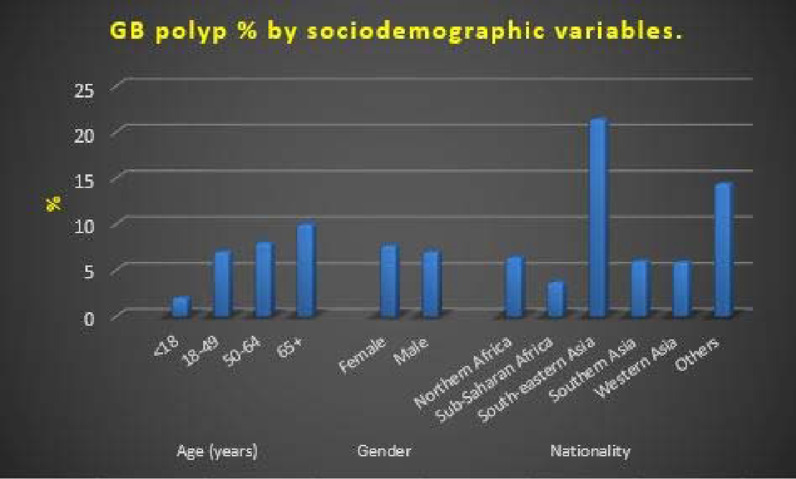
Figure 1.
Frequency distribution of gallbladder polyp in the study sample by sociodemographic variables
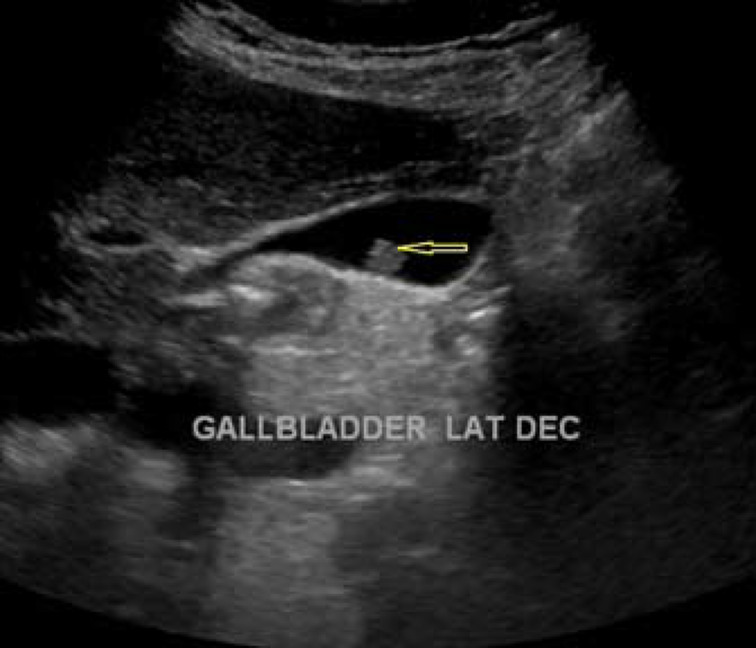
Figure 5.
Ultrasound image showing gallbladder polyp (yellow arrow) and stones (blue arrow)
Table 1.
Frequency distribution of gallbladder polyp in the study sample by sociodemographic variables.
| N | % | |
|
| ||
| Age (years) | ||
|
| ||
| < 18 | 398 | 5.6 |
|
| ||
| 18–49 | 5023 | 70.2 |
|
| ||
| 50–64 | 1389 | 19.4 |
|
| ||
| 65+ | 346 | 4.8 |
|
| ||
| Total | 7156 | 100.0 |
|
| ||
| Gender | ||
|
| ||
| Female | 3920 | 54.8 |
|
| ||
| Male | 3236 | 45.2 |
|
| ||
| Total | 7156 | 100.0 |
|
| ||
| BMI categories (kg/m2)-adults only | ||
|
| ||
| Abnormally low ( < 18.5) | 59 | 1.1 |
|
| ||
| Acceptable (18.5–24.9) | 1056 | 18.8 |
|
| ||
| Overweight (25–29.9) | 2005 | 35.8 |
|
| ||
| Obese I (30–34.9) | 1514 | 27.0 |
|
| ||
| Obese II (35–39.9) | 635 | 11.3 |
|
| ||
| Obese III (40+) | 338 | 6.0 |
|
| ||
| Total | 5607 | 100.0 |
|
| ||
| Nationality groups | ||
|
| ||
| Northern Africa | 1628 | 22.8 |
|
| ||
| Sub-Saharan Africa | 191 | 2.7 |
|
| ||
| South-eastern Asia | 537 | 7.5 |
|
| ||
| Southern Asia | 2049 | 28.6 |
|
| ||
| Western Asia | 2564 | 35.8 |
|
| ||
| Miscellaneous others | 187 | 2.6 |
|
| ||
| Total | 7156 | 100.0 |
|
| ||
| Ever smoked cigarettes | ||
|
| ||
| Negative | 4615 | 82.6 |
|
| ||
| Positive | 973 | 17.4 |
|
| ||
| Total | 5588 | 100.0 |
|
| ||
PHCC service users are multinationals. Three of the nationality groups constituted 87.2% of the total sample: Western Asia, Southern Asia, and Northern Africa (35.8%, 28.6%, and 22.8% respectively) (Table 1).
The overall prevalence of GB polyps was 7.4% (95% confidence level in the reference population ranged from 6.8% to 8%). In addition, GB stones were detected in 9.7% of the recruited sample (the 95% confidence level in the reference population ranged from 9% to 10.4%) (Tables 2 and 3).
Table 2.
Description of gallbladder polyps in the present study.
| n | % | 95% confidence interval for the prevalence rate | |
|
| |||
| Gallbladder polyps | |||
|
| |||
| None | 6627 | 92.6 | (92 to 93.2) |
|
| |||
| Single | 300 | 4.2 | (3.7 to 4.7) |
|
| |||
| Multiple | 229 | 3.2 | (2.8 to 3.6) |
|
| |||
| Total | 7156 | 100.0 | |
|
| |||
| Size of the largest gallbladder polyp | |||
|
| |||
| < 6 mm | 473 | 89.4 | (86.6 to 91.8) |
|
| |||
| 6–9 mm | 49 | 9.3 | (7 to 12) |
|
| |||
| ≥ 10 mm | 7 | 1.3 | (0.6 to 2.6) |
|
| |||
| Total | 529 | 100.0 | |
|
| |||
Table 3.
Prevalence of positive gallbladder findings.
| Gallbladder findings (n=7156) | n | % | 95% confidence interval for prevalence rate |
|
| |||
| Gallbladder Polyp | 529 | 7.4 | (6.8 to 8) |
|
| |||
| Gallbladder stones | 693 | 9.7 | (9 to 10.4) |
|
| |||
Solitary and multiple GB polyps were found in 4.2% and 3.2% of the study participants, respectively. Regarding the size, 89.4% of the GB polyps (considering the largest in case of multiple polyps) were < 6 mm, while only 1.3% of the polyps were >10 mm (Table 2).
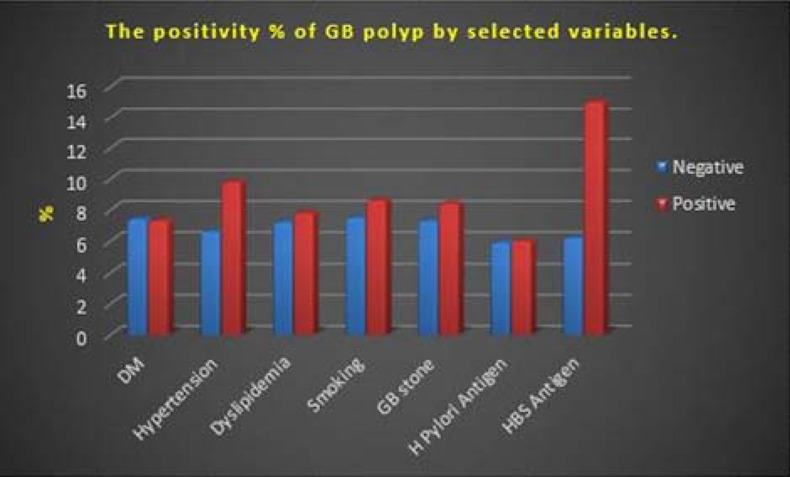
As shown in Table 4, the prevalence of selected comorbidities was 1.8% for chronic liver disease, 25.5% for DM, 25.5% for hypertension, and 29.8% for dyslipidemia. In addition, the positivity rates of selected test results were as follows: H. pylori antigen (7.9%), HBS antigen (0.3%), and HCV antibodies (0.8%) (Table 5).
Table 4.
Prevalence of selected comorbidities in patients with polypoid lesions of the gallbladder.
| (n=7156) | N | % |
|
| ||
| Comorbid conditions | ||
|
| ||
| Liver disease | 128 | 1.8 |
|
| ||
| Diabetes mellitus | 1826 | 25.5 |
|
| ||
| Hypertension | 1824 | 25.5 |
|
| ||
| Dyslipidemia | 2131 | 29.8 |
|
| ||
Table 5.
Positivity rates of selected test results in patients with polypoid lesions of the gallbladder.
| (n=7156) | N | % |
|
| ||
| Positive test results | ||
|
| ||
| H. pylori antigen | 562 | 7.9 |
|
| ||
| Hepatitis B surface antigen | 20 | 0.3 |
|
| ||
| Hepatitis C antibodies | 55 | 0.8 |
|
| ||
The risk of having GB polyps was assessed for selected independent variables (Table 6). The risk of having GB polyps is significantly increased with advanced age, with 4.5 times higher risk in those aged 65+ years than in those aged < 18 years. All nationality groups were associated with an increment in risk when compared with the Sub-Saharan Africans, which had the lowest prevalence rate of GB polyps (3.7%). Southern Asians were significantly associated with the highest increase in risk of 5.8 times when compared with Sub-Saharan Africans. Among the remaining explanatory variables, only hypertension was associated with a significant increase in the risk of having GB polyps by 48%. In addition, testing positive for HBS antigens was associated with an obvious (but insignificant) increase in risk of GB by 2.4 times. All other independent variables tested were not significantly or were significantly associated with an increase in GB polyp risk. These variables include, gender, BMI, liver disease, DM, dyslipidemia, GB stones, ever smoking, H. pylori antigen, and HCV antibodies.
Table 6.
Prevalence ratio for having gallbladder polyp by selected explanatory variables.
| Gallbladder polyps | |||||||||
|
| |||||||||
| Negative | Positive | Total | 95% CI for | ||||||
|
| |||||||||
| N | % | N | % | N | % | PR | PR | P | |
|
| |||||||||
| Age (years) | |||||||||
|
| |||||||||
| < 18 | 389 | 97.7 | 9 | 2.3 | 398 | 100 | Ref | ||
|
| |||||||||
| 18–49 | 4648 | 92.5 | 375 | 7.5 | 5023 | 100 | 3.26 | (1.7–6.26) | 0.002 |
|
| |||||||||
| 50–64 | 1280 | 92.2 | 109 | 7.8 | 1389 | 100 | 3.39 | (1.73–6.63) | 0.001 |
|
| |||||||||
| 65+ | 310 | 89.6 | 36 | 10.4 | 346 | 100 | 4.52 | (2.21–9.25) | < 0.001 |
|
| |||||||||
| Nationality groups | |||||||||
|
| |||||||||
| Sub-Saharan Africa | 184 | 96.3 | 7 | 3.7 | 191 | 100 | Ref | ||
|
| |||||||||
| Western Asia | 2412 | 94.1 | 152 | 5.9 | 2564 | 100 | 1.59 | (0.76–3.34) | 0.64[NS] |
|
| |||||||||
| Southern Asia | 1926 | 94 | 123 | 6 | 2049 | 100 | 1.62 | (0.77–3.42) | 0.63[NS] |
|
| |||||||||
| Northern Africa | 1523 | 93.6 | 105 | 6.4 | 1628 | 100 | 1.73 | (0.82–3.66) | 0.51[NS] |
|
| |||||||||
| South-eastern Asia | 422 | 78.6 | 115 | 21.4 | 537 | 100 | 5.78 | (2.74 − 12.17) | < 0.001 |
|
| |||||||||
| Miscellaneous others | 160 | 85.6 | 27 | 14.4 | 187 | 100 | 3.89 | (1.74–8.71) | 0.004 |
|
| |||||||||
| Gender | |||||||||
|
| |||||||||
| Male | 3008 | 93 | 228 | 7 | 3236 | 100 | Ref | ||
|
| |||||||||
| Female | 3619 | 92.3 | 301 | 7.7 | 3920 | 100 | 1.1 | (0.93–1.3) | 0.79[NS] |
|
| |||||||||
| BMI categories (kg/m2) | |||||||||
|
| |||||||||
| Acceptable ( < 25) | 1022 | 91.7 | 93 | 8.3 | 1115 | 100 | Ref | ||
|
| |||||||||
| Overweight (25–29.9) | 1829 | 91.2 | 176 | 8.8 | 2005 | 100 | 1.06 | (0.83–1.35) | 0.98[NS] |
|
| |||||||||
| Obese (30+) | 2325 | 93.5 | 162 | 6.5 | 2487 | 100 | 0.78 | (0.61–1) | 0.27[NS] |
|
| |||||||||
| Liver disease | |||||||||
|
| |||||||||
| Negative | 6503 | 92.5 | 525 | 7.5 | 7028 | 100 | Ref | ||
|
| |||||||||
| Positive | 124 | 96.9 | 4 | 3.1 | 128 | 100 | 0.41 | (0.16–1.08) | 0.33[NS] |
|
| |||||||||
| Diabetes mellitus | |||||||||
|
| |||||||||
| Negative | 4934 | 92.6 | 396 | 7.4 | 5330 | 100 | Ref | ||
|
| |||||||||
| Positive | 1693 | 92.7 | 133 | 7.3 | 1826 | 100 | 0.99 | (0.82–1.2) | 1[NS] |
|
| |||||||||
| Hypertension | |||||||||
|
| |||||||||
| Negative | 4982 | 93.4 | 350 | 6.6 | 5332 | 100 | Ref | ||
|
| |||||||||
| Positive | 1645 | 90.2 | 179 | 9.8 | 1824 | 100 | 1.48 | (1.25–1.76) | < 0.001 |
|
| |||||||||
| Dyslipidemia | |||||||||
|
| |||||||||
| Negative | 4663 | 92.8 | 362 | 7.2 | 5025 | 100 | Ref | ||
|
| |||||||||
| Positive | 1964 | 92.2 | 167 | 7.8 | 2131 | 100 | 1.08 | (0.91–1.29) | 0.83[NS] |
|
| |||||||||
| Ever smoked cigarettes | |||||||||
|
| |||||||||
| Negative | 4269 | 92.5 | 346 | 7.5 | 4615 | 100 | Ref | ||
|
| |||||||||
| Positive | 889 | 91.4 | 84 | 8.6 | 973 | 100 | 1.15 | (0.92–1.44) | 0.69[NS] |
|
| |||||||||
| Gallbladder stones | |||||||||
|
| |||||||||
| Negative | 5992 | 92.7 | 471 | 7.3 | 6463 | 100 | Ref | ||
|
| |||||||||
| Positive | 635 | 91.6 | 58 | 8.4 | 693 | 100 | 1.15 | (0.89–1.49) | 0.78[NS] |
|
| |||||||||
| H. pylori antigen | |||||||||
|
| |||||||||
| Negative | 983 | 94.1 | 62 | 5.9 | 1045 | 100 | Ref | ||
|
| |||||||||
| Positive | 528 | 94 | 34 | 6 | 562 | 100 | 1.02 | (0.68–1.53) | 1[NS] |
|
| |||||||||
| Hepatitis B surface antigen | |||||||||
|
| |||||||||
| Negative | 1435 | 93.8 | 95 | 6.2 | 1530 | 100 | Ref | ||
|
| |||||||||
| Positive | 17 | 85 | 3 | 15 | 20 | 100 | 2.42 | (0.84–6.99) | 0.46[NS] |
|
| |||||||||
| Hepatitis C antibodies | |||||||||
|
| |||||||||
| Negative | 1397 | 93.9 | 91 | 6.1 | 1488 | 100 | Ref | ||
|
| |||||||||
| Positive | 53 | 96.4 | 2 | 3.6 | 55 | 100 | 0.59 | (0.15–2.33) | 0.9[NS] |
|
| |||||||||
A multiple logistic regression model was used to assess the net and independent effect of a set of explanatory variables on the risk of having GB polyps (Table 7). The model was significant and associated with an overall predictive accuracy of 92.2%. Only age, South-eastern Asia nationality, and hypertension were significantly associated with the risk of having GB polyps after adjusting for the possible confounding effects of other explanatory variables included in the model (gender, liver disease, DM, dyslipidemia, BMI categories, GB stones, and smoking status). Belonging to an older age group (65+ years) is associated with a significant increase in the risk of having GB polyps by 63%. Being a South-eastern Asian is associated with a significant increase in risk of having GB polyps by 3.7 times, when compared with the remaining nationality groups. Having hypertension significantly increased the risk of having GB polyps by 39% after controlling for other explanatory variables included in the model.
Table 7.
Multiple logistic regression model with the risk of having GB polyps as the dependent (response) variable and selected explanatory variables.
| Partial OR | 95% confidence interval | P | |
|
| |||
| Age (years) | 0.11[NS] | ||
|
| |||
| 50–64 years compared with 18–49 years | 1.06 | (0.79 to 1.43) | 0.68[NS] |
|
| |||
| 65+ years compared with 18–49 years | 1.63 | (1.03 to 2.59) | 0.038 |
|
| |||
| South-eastern Asia nationality compared with the remaining nationality groups | 3.76 | (2.72 to 5.19) | < 0.001 |
|
| |||
| Males compared with females | 1.00 | (0.78 to 1.3) | 0.98[NS] |
|
| |||
| Liver disease | 0.37 | (0.12 to 1.18) | 0.09[NS] |
|
| |||
| Diabetes mellitus | 0.83 | (0.62 to 1.1) | 0.2[NS] |
|
| |||
| Hypertension | 1.39 | (1.05 to 1.83) | 0.021 |
|
| |||
| Dyslipidemia | 0.91 | (0.69 to 1.19) | 0.47[NS] |
|
| |||
| BMI categories (kg/m2) | 0.19[NS] | ||
|
| |||
| Overweight (25–29.9) compared with acceptable ( < 25) | 1.13 | (0.84 to 1.53) | 0.41[NS] |
|
| |||
| Obese (30+) compared with acceptable ( < 25) | 0.90 | (0.66 to 1.23) | 0.51[NS] |
|
| |||
| Having gallbladder stones | 0.95 | (0.67 to 1.35) | 0.78[NS] |
|
| |||
| Ever smoked cigarettes | 1.30 | (0.96 to 1.75) | 0.09[NS] |
|
| |||
| Constant | 0.07 | (0 to 0) | < 0.001 |
|
| |||
P (Model) < 0.001; Overall predictive accuracy = 92.2%
Discussion
Abdominal US is an important and commonly used imaging modality in the detection of GB polyps. The incidence of GB polyps detected by abdominal US increased, which can be attributed to the increased and widespread use of abdominal US (main). Additionally, this increase in the incidence can be partially attributed to the higher prevalence of metabolic syndrome.33-34
This study included a total of 7156 individuals.
Providing the accurate prevalence of GB polyps is quite difficult, as most GB polyps are incidentally discovered in ultrasound studies. GB polyps were reported in 5.6% in a large Japanese study, while it was 6.7% in Chinese participants.8,9 More recent studies have shown a higher prevalence, i.e., 8.5%35 and 9.96%,1 and this can be attributed to the wider use of US in health checkups.1 In the present study, the overall prevalence of GB polyps was comparable with those in previous reports. In addition, GB stones were detected in 9.7% of the recruited sample.
The present study showed that the risk of having GB polyps significantly increased with advancing age, reaching 4.5 times higher risk in those aged 65+ years than in those aged < 18 years. In addition, differences in the relative frequency of GB polyps were found among nationality groups. Southern Asians were associated with the highest increase in risk of 5.8 times when compared with Sub-Saharan Africans.
Other studies have reported extreme variations in the prevalence of GB polyps among nationalities. A prevalence of 4.3%–4.6% was reported in Denmark,11 1.5% in Germany,12 and 0.32% in India.13 In a study of Korean individuals who underwent health screening examinations, the estimated average prevalence of GB polyps was 2.94%.1,36
In the current study, the prevalence of GB polyps was slightly higher in female than in male (7.7 versus 7%, respectively) individuals. Similarly, small differences were reported between male and female individuals in Denmark11 (4.6% in male versus 4.3% for female individuals) and Korea36 (3.63% in male versus 2.09% in female individuals).
In the present study, the prevalence of the selected comorbidities was as follows: 1.8%, chronic liver disease; 25.5%, DM; 25.5%, hypertension; and 29.8%, dyslipidemia. In addition, the positivity rates of selected test results were as follows: H. pylori antigen (7.9%), HBS antigen (0.3%), and HCV antibodies (0.8%).
The most common GB polypoidal lesions are those containing cholesterol, and this was explained by some authors in that cholesterolosis is the result of the direct deposition of cholesterol from the blood,14 with cholesterol metabolism changes in the liver and impaired mucosal esterification of free sterols from the bile. Cholesterol polyps may be detached and causes GB-like clinical presentation such as biliary colic or obstruction.18
Based on this assumption, previous studies have investigated whether blood lipids are possible risk factors for GB polypoid lesions and concluded that blood cholesterol concentration is not an independent risk factor.5,7,8,12,16 In the present study, patients with dyslipidemia had slightly higher incidence for GB polyps (7.8 %) than patients without dyslipidemia (7.2%). This finding matches with earlier study37 that reported the association between dyslipidemias and GB polyp formations.
Among the remaining explanatory variables, only hypertension was associated with a significant increase in the risk of having GB polyps by 48%. In addition, testing positive for HBS antigen was associated with a remarkable (but insignificant) increase in the risk of GB by 2.4 times. All the other tested independent variables had no significant or obvious association with an increase in GB polyp risk. These variables include gender, BMI, liver disease, DM, dyslipidemia, GB stones, smoking status, H. pylori antigen and HCV antibodies.
Some studies have shown the role of hyperglycemia in the formation of biliary stones by inhibiting GB contraction and liver secretion of bile38–39; however, some authors could not confirm whether hyperglycemia is a risk factor for GB polypoid lesions.1 In the present study, patients with DM did not have a higher prevalence of GB polyps than patients without DM.
While chronic hepatitis B (CHB) can cause abnormal GB changes such as altered GB volume and wall thickening, the association of CHB with GB polypoid lesions is still not clear. While some authors have suggested the direct effect of hepatitis B virus in bile on the GB mucosa, others suggested that hepatic parenchyma inflammatory changes can affect the GB mucosa.1 Some earlier studies have reported CHB as a risk factor for GB polypoid lesions.1,40-41 The results of the present study were in agreement with those of previous studies because CHB was found to be an independent risk factor for GB polypoid lesions. In the present study, a higher prevalence of GB polypoid lesions was noted in patients positive for HBS antigen (15%) than in those negative for it (6.2%)
Gastric H. pylori is known to be commonly associated with formations of GB stones41,42; a previous study1 also showed higher rate of H. pylori infection in patients with GB stones and polypoid lesions, which support the finding that H. pylori infection is related to stone formation rather than GB polypoid lesions. In the present study, no significant higher prevalence of GB polyps was identified in positive H. pylori cases (6%) when compared with H. pylori-negative cases (5.9%).
Small GB polyps are considered of lower malignant potential than larger polyps,33 and this was confirmed by Kubota et al.43 who showed that 2/7 of GB polyps < 5 mm found by cholecystectomy were adenomas.
In the present study, solitary GB polyp was found in 4.2% of the study participants, while multiple ones were found in 3.2% of the study participants. Regarding the size, majority (89.4%) of GB polyps (considering the largest in case of multiple polyps) were < 6 mm, only 1.3% of the polyps were >10 mm, and polyps of 6–9 mm were found in 9.3% in participants with GB polyps.
Compared with multiple polyps, a solitary GB polypoid lesion is assumed to be more liable for the malignant changes42,44; however, the size of the polyps may play a more important role in malignant changes.1 In the present study, solitary GB polyp was more common than multiple ones (4.2% versus 3.2%).
A multiple logistic regression model was used in the present study to assess the net and independent effects of a set of explanatory variables on the risk of having GB polyps. The model was significant and associated with an overall predictive accuracy of 92.2%. Only age, South-eastern Asian nationality, and hypertension showed a significant association with the risk of having GB polyps after adjusting for the possible confounding effects of other explanatory variables included in the model (gender, liver disease, DM, dyslipidemia, BMI categories, GB stones, and smoking status). Being in an older age group (65+ years) is associated with a significant increase in the risk of having GB polyps by 63%. Compared with the remaining nationality groups, being a South-eastern Asian is associated with a significant increase in the risk of GB polyps by 3.7 times. Having hypertension significantly increases the risk of having GB polyps by 39% after controlling for the other explanatory variables included in the model.
This study has some limitations. The study included individuals who were referred for US, and this may result in the difference when compared with the prevalence in the general population.
Conclusion
Abdominal US are an important and commonly used imaging modality in the detection of GB polyps. In the present study, the prevalence of GB polyps was approximately 7.4%, with a higher prevalence in those with overweight, DM, hypertension, and dyslipidemia. Follow-up studies in high-risk groups are recommended for the early detection of any malignant changes.
Competing interests
The authors declare that they have no competing interests.
Ethical considerations
The study was approved by the Primary Health Care Corporation Research Committee and the Research Section in the Department of Clinical Affairs.
Consent for publication
All authors read and approved the final manuscript.
Availability of data and materials
The data that support the findings of this study are available on request from the corresponding author.
Acknowledgment
We wish to acknowledge the Primary Health Care Corporation (Qatar) as a research funding agency for this article, and we wish to acknowledge the help provided by Research Department of the PHCC.
Figure 2.
Prevalence of selected comorbidities in patients with polypoid lesions of the gallbladder
Figure 3.
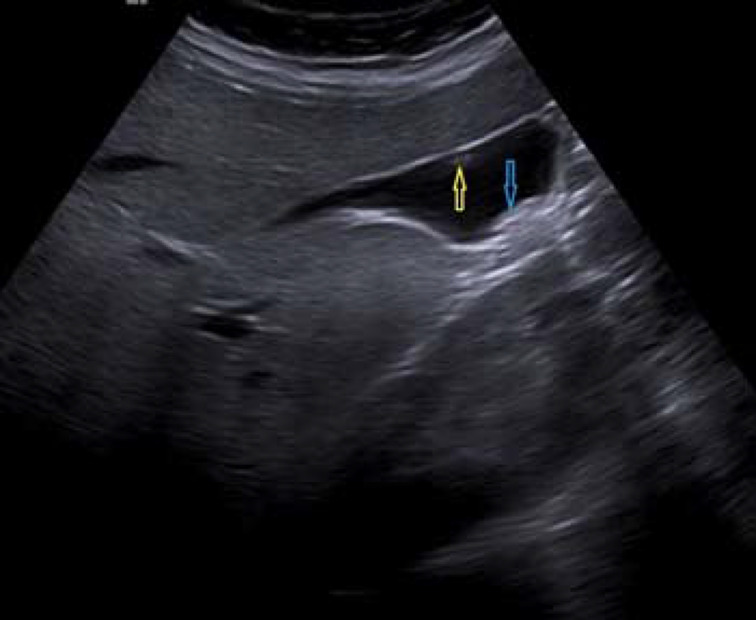
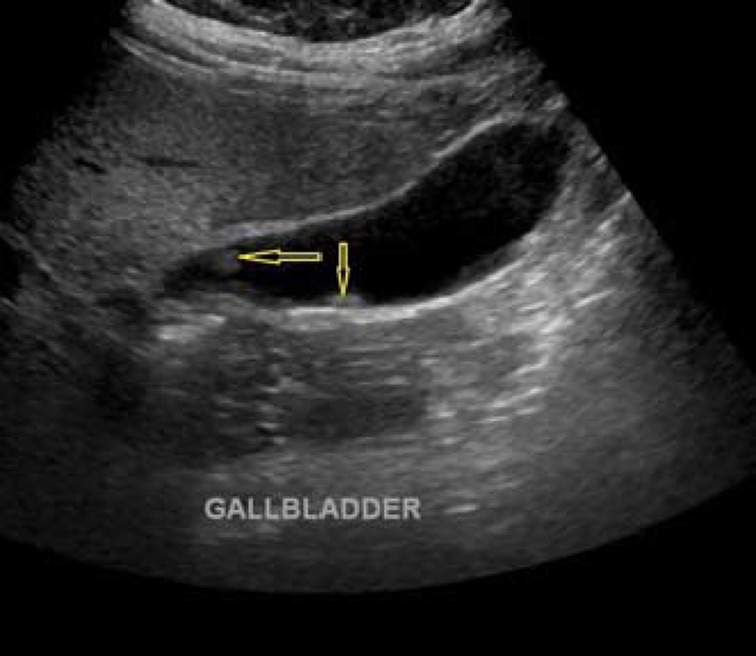
Ultrasound image showing a gallbladder polyp (yellow arrow)
Figure 4.
Ultrasound image showing multiple gallbladder polyps (yellow arrows)
References
- 1. Choi YS, Do JH, Seo SW, Lee SE, Oh HC, Min YJ, Kang H. Prevalence and risk factors of gallbladder polypoid lesions in a healthy population. Yonsei Med J. 2016;57:1370–1375. [DOI] [PMC free article] [PubMed]
- 2. Choi JH, Yun JW, Kim YS, Lee EA, Hwang ST, Cho YK, et al. Pre-operative predictive factors for gallbladder cholesterol polyps using conventional diagnostic imaging. World J Gastroenterol. 2008;14:6831–6834. [DOI] [PMC free article] [PubMed]
- 3. Sandberg AA. Diagnosis and management of gallbladder polyps. North Am J Med Sci. 2012;4:203–2011. [DOI] [PMC free article] [PubMed]
- 4. Matos AS, Baptista HN, Pinheiro C, Martinho F. Gallbladder polyps: how should they be treated and when? Rev Assoc Med Bras. 2010;56:318–321. [DOI] [PubMed]
- 5. Stephen RM, Diamond A, Jones C, and Coleman HG. Current practices and future prospects for the management of gallbladder polyps: a topical review. World J Gastroenterol. 2018;24:2844–2852. [DOI] [PMC free article] [PubMed]
- 6. Kratzer W, Gräter T, Schmidberger J. Gallbladder polyps – a follow-up study after 11 years. BMC Gastroenterol. 2019:42. [DOI] [PMC free article] [PubMed]
- 7. G. Randi, S. Franceschi, C. La Vecchia, Gallbladder cancer worldwide: geographical distribution and risk factors. Int. J. Cancer. 2006;118:1591–1602. [DOI] [PubMed]
- 8. Inui K, Yoshino J, Miyoshi H. Diagnosis of gallbladder tumors. Intern Med. 2011;50:1133–1136. [DOI] [PubMed]
- 9. Moriguchi H, Tazawa J, Hayashi Y, et al. Natural history of polypoid lesions in the gallbladder. Gut. 1996;39:860–862. [DOI] [PMC free article] [PubMed]
- 10. Segawa K, Arisawa T, Niwa Y, et al. Prevalence of gallbladder polyps among apparently healthy Japanese: ultrasonographic study. Am J Gastroenterol. 1992;87:630–633. [PubMed]
- 11. Okamoto M, Okamoto H, Kitahara F, et al. Ultrasonographic evidence of association of polyps and stones with gallbladder cancer. Am J Gastroenterol .1999;94:446–450. [DOI] [PubMed]
- 12. Jorgensen T, Jensen KH. Polyps in the gallbladder. A prevalence study. Scand J Gastroenterol. 1990;25:281-286. [PubMed]
- 13. Pandey M, Khatri AK, Sood BP, et al. Cholecystosonographic evaluation of the prevalence of gallbladder diseases. A university hospital experience. Clin Imaging. 1996;20:269–272. [DOI] [PubMed]
- 14. Collett JA, Allan RB, Chisholm RJ, et al. Gallbladder polyps: prospective study. J Ultrasound Med. 1998;17:207–211. [DOI] [PubMed]
- 15. Kwon W, Jang JY, Lee SE, Hwang DW, Kim SW. Clinicopathologic features of polypoid lesions of the gallbladder and risk factors of gallbladder cancer. J Korean Med Sci. 2009; 24:481–487. [DOI] [PMC free article] [PubMed]
- 16. Cantürk Z, Sentürk O, Cantürk NZ, Anik YA. Prevalence and risk factors for gallbladder polyps. East Afr Med J. 2007;84:336-341. [PubMed]
- 17. Park JY, Hong SP, Kim YJ, Kim HJ, Kim HM, Cho JH, et al. Long-term follow up of gallbladder polyps. J Gastroenterol Hepatol. 2009;24:219–222. [DOI] [PubMed]
- 18. Gallahan WC, Conway JD. Diagnosis and management of gallbladder polyps. Gastroenterol Clin North Am. 2010;39:359–367. [DOI] [PubMed]
- 19. Kwon W, Jang JY, Lee SE, Hwang DW, Kim SW. Clinicopathologic features of polypoid lesions of the gallbladder and risk factors of gallbladder cancer. J Korean Med Sci. 2009;24:481–487. [DOI] [PMC free article] [PubMed]
- 20. Zielinski MD, Atwell TD, Davis PW, Kendrick ML, Que FG. Comparison of surgically resected polypoid lesions of the gallbladder to their pre-operative ultrasound characteristics. J Gastrointest Surg. 2009;13:19–25. [DOI] [PubMed]
- 21. Rawla P, Sunkara T, Chaitanya KT, Barsouk A. Epidemiology of gallbladder cancer. Clin Exp HEPATOL 2019;5:93–102. [DOI] [PMC free article] [PubMed]
- 22. American Cancer Society: Cancer Facts and Figures 2020.Atlanta, Ga: American Cancer Society, 2020.Available online. Last accessed January 17, 2020.
- 23. Miranda AF, Piñeros M, Ferreccio C, Koshiol J, et al. Gallbladder and extrahepatic bile duct cancers in the Americas: incidence and mortality patterns and trends. Intern J Canc 2020. DOI:10.1002/ijc.32863. [DOI] [PMC free article] [PubMed]
- 24. Yamamoto M, Nakajo S, Tahara E. Histological classification of epithelial polypoid lesions of the gallbladder. Acta Pathol Joun. 1988;38:181–192. [DOI] [PubMed]
- 25. Ministry of Development Planning and Statistics State Qatar, Qatar Monthly Statistics Report, August 2016, (2016) Doha.
- 26. Sulieman I, Elmoghazy W, Ansari W, Elaffandi A and Khalaf H. Gallbladder cancer: 7-Year experience from Qatar. Ann Med Surg 2019;44:33–38. [DOI] [PMC free article] [PubMed]
- 27. Bennett, G.L. and Blathazar, E.J. Ultrasound and CT Evaluation of emergent gallbladder pathology. Radiol Clin North Am. 2003;41:1203–1216. [DOI] [PubMed]
- 28. Escalona A, León F, Bellolio F, Pimentel F, Guajardo M, Gennero R, et al. Gallbladder polyps: Correlation between ultrasonographic and histopathological findings. Rev Med Chil. 2006;134:1237–1242. [DOI] [PubMed]
- 29. Ito H, Hann LE, D'Angelica M, Allen P, Fong Y, Dematteo RP, et al. Polypoid lesions of the gallbladder: Diagnosis and followup. J Am Coll Surg. 2009;208:570–575. [DOI] [PubMed]
- 30. Myers RP, Shaffer EA, Beck PL. Gallbladder polyps: epidemiology, natural history and management. Can J Gastroenterol. 2002;16:187–194. [DOI] [PubMed]
- 31. Kleinbaum D G. Sullivan K M, Bark N D, 2007. A Pocket Guide to Epidemiology. Springer Science + Business Media, NewYork, USA.)
- 32. World Health Organization. Report of a WHO consultation: definition of metabolic syndrome in definition, diagnosis and classification of diabetes mellitus. World Health Organization, Department of Non-communicable Disease Surveillance, Geneva, 1999.
- 33. Pedersen MR, Dam C, Rafaelsen SR (2012) Ultrasound follow-up for gallbladder polyps less than 6 mm may not be necessary. Dan Med J. 59:A4503. [PubMed]
- 34. Hong AR, Lim S. Clinical characteristics of metabolic syndrome in Korea, and its comparison with other Asian countries. J Diabetes Investig. 2015;6:508–515. [DOI] [PMC free article] [PubMed]
- 35. Lee YJ, Park KS, Cho KB, Kim ES, Jang BK, Chung WJ, et al. Shifting prevalence of gallbladder polyps in Korea. J Korean Med Sci. 2014;29:1247–1252. [DOI] [PMC free article] [PubMed]
- 36. Shim SG, Lee KT, Lee JK, Park JH, Ryu JH, Rhee PL, et al. Prevalence and risk factors of gallbladder polyps in health screening subjects. Korean J Med. 1999;57:1014–1020.
- 37. Yamin Z, Xuesong B, Zhen Z, Yue H, Liwei L, Li Fei F. Correlation of dyslipidemias and gallbladder polyps. A large retrospective study among Chinese population. Asian J Surg. 2020 43,181–185. [DOI] [PubMed]
- 38. Chen CY, Lu CL, Chang FY, Lee SD. Risk factors for gallbladder polyps in the Chinese population. Am J Gastroenterol. 1997;92:2066–2068. [PubMed]
- 39. Everson GT. Gallbladder function in gallstone disease. Gastroenterol Clin North Am. 1991; 20:85–110. [PubMed]
- 40. Kim SY, Lee HS, Lee YS, Chung KW, Jang BK, Chung WJ, et al. [Prevalence and risk factors of gallbladder polyp in adults living in Daegu and Gyeongbuk provinces]. Korean J Gastroenterol. 2006;48:344–350. [PubMed]
- 41. Takahashi Y, Yamamichi N, Shimamoto T, Mochizuki S, Fujishiro M, Takeuchi C, et al. Helicobacter pylori infection is positively associated with gallstones: a large-scale cross-sectional study in Japan. J Gastroenterol. 2014;49:882–9. [DOI] [PubMed]
- 42. Mishra RR, Tewari M, Shukla HS. Association of Helicobacter pylori infection with inflammatory cytokine expression in patients with gallbladder cancer. Indian J Gastroenterol. 2013;32:232–235. [DOI] [PubMed]
- 43. Kubota K, Bandai Y, Noie T, Ishizaki Y, Teruya M, Makuuchi M. How should polypoid lesions of the gallbladder be treated in the era of laparoscopic cholecystectomy? Surgery. 1995;117:481–487. [DOI] [PubMed]
- 44. Sheth S, Bedford A, Chopra S. Primary gallbladder cancer: recognition of risk factors and the role of prophylactic cholecystectomy. Am J Gastroenterol. 2000;95:1402–410. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.