Abstract
Objectives
We demonstrate the feasibility and safety of robotics-assisted left atrial appendage clip exclusion in clinical practice.
Methods
Analysis of a single center robotics-assisted left atrial appendage clip exclusion experience using an epicardial linear clip device in patients with atrial fibrillation with high-risk of thromboembolic stroke and intolerance to oral anticoagulants.
Results
During the period from December 2017 to September 2020, we performed 42 robotics-assisted left atrial appendage clip exclusions in response to increased risk of bleeding in patients with atrial fibrillation and intolerance to oral anticoagulants. The average congestive heart failure, hypertension, age, diabetes, stroke, and vascular disease score was 5.2 ± 1.6 and hypertension, abnormal liver or kidney function, stroke, bleeding, labile international normalized ratio, elderly, drugs (aspirin, other antiplatelets, or anticoagulants) score was 4.5 ± 0.9. No patients died intraoperatively or within 30 days, or due to conversion to thoracotomy, intraoperative complications, or failure to apply the clip satisfactorily. The procedure was successfully completed despite pericardial adhesions in 2 patients with prior coronary bypass grafts and 3 with postpericarditis scars. Intraoperative transesophageal echocardiography was performed in 38 out of 42 patients; satisfactory exclusion with left atrial appendage stump <5 mm was confirmed in all. Average length of stay was 3.4 ± 3 days with 12 out of 42 patients discharged within 24 hours. Oral anticoagulants were discontinued in 41 out of 42 patients and no cases of 30-day stroke, myocardial ischemia, or new arrhythmias were observed. One case of hemothorax required thoracoscopy a day later. There was no reported thromboembolic stroke or transient ischemic attack at 12 months. One case of late lacunar stroke was due to in situ small intracranial vessel thrombosis without left atrial appendage thrombus on imaging.
Conclusions
Robotics-assisted left atrial appendage clip exclusion is a safe and feasible minimally invasive method for left atrial appendage management in patients with atrial fibrillation with intolerance to oral anticoagulants and increased risk of thromboembolic stroke.
Key Words: stroke prevention, atrial fibrillation, left atrial appendage, oral anticoagulant intolerance, robotic cardiac surgery
Abbreviations and Acronyms: AF, atrial fibrillation; CHA2DS2-VASc, congestive heart failure, hypertension, age, diabetes, stroke, vascular disease score; CTA, computerized tomographic angiography; DAPT, dual antiplatelets therapy; HAS-BLED, hypertension, abnormal liver or kidney function, stroke, bleeding, labile international normalized ratio, elderly, drugs (aspirin, other antiplatelets, or anticoagulants) score; LAA, left atrial appendage; LCX, left circumflex artery; OACs, oral anticoagulants; RLAAC, robotics-assisted left atrial appendage clip exclusion; TEE, transesophageal echocardiography; TES, thromboembolic stroke
Robotics-assisted left atrial appendage clipping as verified on transesophageal echocardiograph.
Central Message.
The use of a robotic surgical platform to minimize invasiveness of epicardial left atrial appendage exclusion with the AtriClip is feasible and safe.
Perspective.
The role of left atrial appendage (LAA) in atrial fibrillation associated stroke is well established. Epicardial clip exclusion of LAA provides an alternative to endocardial LAA closure in patients intolerant to oral anticoagulants. The less-invasive nature of robotics-assisted surgery is employed to improve outcomes of LAA epicardial exclusion. Robotic LAA exclusion appears to be feasible and safe.
See Commentaries on pages 69 and 71.
Thrombus formation in left atrial appendage (LAA) is a common source for thromboembolic stroke (TES) in atrial fibrillation (AF) and is believed to be responsible for about 90% of TES in nonvalvular–disease-associated AF and 50% of valvular–disease-associated AF.1 AF increases the incidence of TES by up to 5-fold.2 The influence of AF on TES incidence is compounded by additional risk factors that constitute the congestive heart failure, hypertension, age, diabetes mellitus, stroke or transient ischemic attack, vascular disease score (CHA2DS2-VASc).3 The use of oral anticoagulants (OACs) results in a significant decrease of TES relative incidence by 50% to 80% and is thus considered the standard of TES prevention in AF.4
Intolerance and complications of OACs pose a therapeutic dilemma. Thus, alternative approaches directed toward local management of LAA thrombus risk by endocardial occlusion, or epicardial exclusion, have been shown to result in reduction of TES incidence comparable to that of systemic OACs.5, 6, 7, 8, 9 The application of endocardial occlusion devices might be inappropriate for a number of reasons. Anatomical obstacles such as the size and shape of LAA, previous atrial septal repairs or devices, contraindications to transesophageal echocardiography (TEE), absolute contraindication to OACs—especially warfarin and/or dual antiplatelets therapy (DAPT)—which are typically administered after implantation of endocardial LAA occlusion Watchman device (Boston Scientific, Marlborough, Mass) all may be limiting reasons for endocardial closure. Epicardial exclusion of LAA with a parallel closure linear clip device, such as AtriClip (Atricure, Mason, Ohio) has been found to provide reproducible, consistent, and complete exclusion of LAA.10,11 The use of robotic surgical platform such as Intuitive Surgical da Vinci (Intuitive Surgical, Sunnyvale, Calif) facilitates minimally invasive procedures in the cardiothoracic anatomic environment. It allows precise application of an epicardial LAA exclusion device under a variety of conditions, independent of size, shape, or prior interventions. The epicardial route is also advantaged by the lack of requirements for a period of postimplant OACs and/or DAPT.
Our team demonstrated the proof of concept of using robotics-assisted surgery for standalone epicardial LAA exclusion with AtriClip on February 28, 2011 using da Vinci Si platform and AtriClip Pro (internal communication with Atricure, April 18, 2011). This series covers our recent experience with an updated technology over a period of 33 months, starting December 2017. This article seeks to demonstrate the feasibility and safety of robotics-assisted epicardial LAA exclusion with a clip (RLAAC).
Materials and Methods
Study Design and Patients
Pearl Independent Institutional Review Board (Indianapolis, Ind) approved the study protocol (#20-HOLM-10) and the publication of data. Patient written consent for the publication of the study data was waived by the institutional review board based on secondary research uses of data or specimens.
This is a retrospective analysis of pretreatment and posttreatment data and outcomes of 42 consecutive patients who underwent RLAAC at Health First Holmes Regional Medical Center, Melbourne, Fla, between December 2017 and September 2020. All procedures were performed by the same surgeon (T.A.) and 41 out of 42 cases by the same physician assistant (J.M.). All TEE examinations records were reviewed post hoc by the same TEE-credentialed cardiac anesthesiologist (J.Mc.G.).
Technology
Epicardial left atrial appendage clip: AtriClip Pro2 and AtriClip ProV. Both designs are suited for thoracoscopic procedures. The robotic surgical platform was da Vinci Xi.
Data Collection and Follow-up
All relevant data were retrospectively harvested from clinical records of study patients and stored in an Excel (Microsoft, Redmond, Wash) data base. TEE studies were re-read by a credentialed anesthesiologist blinded to prior official TEE reports. Outcome data were obtained from longitudinal review of each medical record and supplemented by telephone calls to the study patients.
Surgical Technique
All procedures were performed under general anesthesia with double-lumen endotracheal intubation. The patient is placed in a neutral supine position. For stand-alone RLAAC, the left lung is selectively deflated. Three robotic ports are placed on the left lateral aspect of the chest wall in the second, fourth, and sixth interspaces, and an accessory 12-mm thoracoscopic port is placed close to posterior axillary line in the sixth or seventh interspace for AtriClip introduction. Carbon dioxide insufflation at 6 to 10 mm Hg pressure is used. The robotic platform is docked with the patient cart parked on the right side of the patient. Using robotic instruments, the pericardial sac is opened in front of the left pulmonary veins and usually posterior to the phrenic nerve. The LAA, with the local anatomic landmarks are identified, including the left circumflex artery (LCX), both pulmonary veins, and left pulmonary artery. The ligament of Marshall is incised, if feasible, because it might facilitate a more complete closure of LAA. A pericardial traction stitch is placed on the anterior side of the pericardial incision and exteriorized anteriorly for exposure. The appropriately sized AtriClip is then introduced by the patient-side assistant. The surgeon then manipulates the LAA and the AtriClip for proper placement using nontraumatic robotic graspers and a modified Kittner gauze loaded on a robotic grasper. The robotic left-hand instrument is pushed gently medially toward the heart as the modified robotic Kittner is repeatedly used to assist the LAA to protrude more laterally through the clip. Once optimal position of the clip relative to LAA is achieved, the LCX is directly visualized as the clip is closed but kept in its applier and TEE multiple views performed and reviewed. The ST segments on electrocardiography monitor and the hemodynamic parameters are checked before the clip is released. A final detailed visual and TEE survey of LCX, clip position and any residual LAA portions is made. A soft chest tube is then left in the pleural space (Video 1).
Video 1.
Robotics-assisted left atrial appendage procedure steps. Video available at: https://www.jtcvs.org/article/S2666-2507(21)00486-7/fulltext.
TEE Protocol
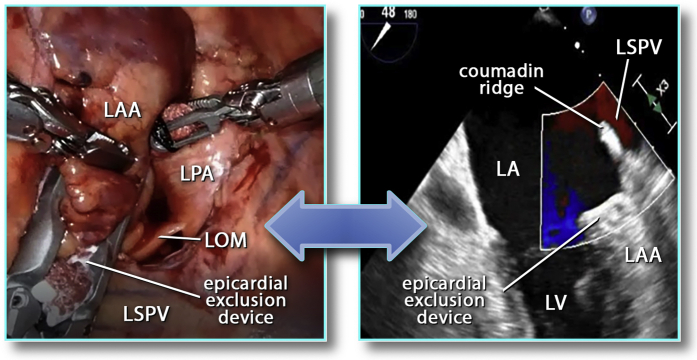
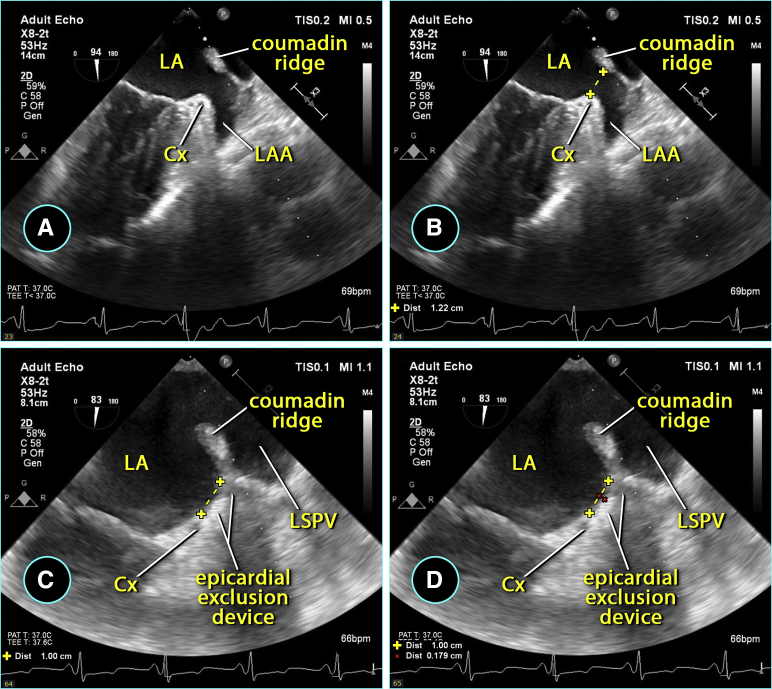
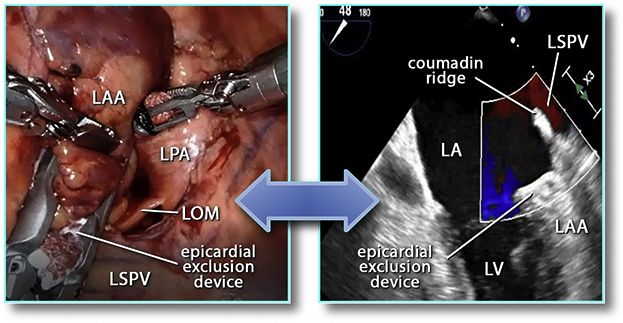
A convention to interpret TEE data was developed and consisted of 3 optimal 2-dimensional view planes and 3-dimensional reconstructions of LAA at 3 time points: before AtriClip application, with the clip applied but not released, and after the release of the clip. In addition, clots, smoke sign, LAA size and shape, color flow, and pulsed Doppler velocities were recorded. The positions of the LCX, the Coumadin ridge, and the reference counterpoint of LAA ostium on the lateral edge opposite LCX were determined. The depth of the postexclusion stump was measured as the height of a triangle, its base made of the line between LCX and that reference counterpoint on the lateral ridge, and its apex the deepest point of the stump on the best view plane after the release of the clip (Figures 1, 2, and 3).
Figure 1.
Robotics-assisted left atrial appendage clip exclusion being monitored on transesophageal echocardiography. LAA, Left atrial appendage; LPA, left pulmonary artery; LOM, ligament of Marshall; LSPV, left superior pulmonary vein; LA, left atrium; LV, left ventricle.
Figure 2.
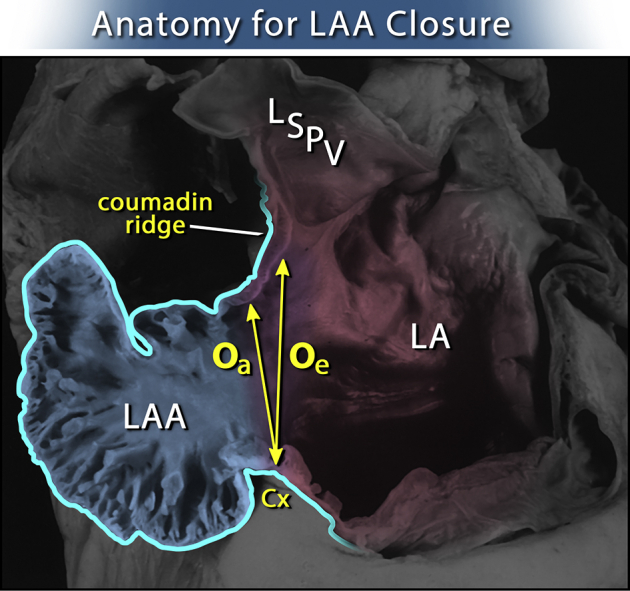
Measurements of the left atrial appendage (LAA) in an anatomical specimen. The echocardiographic orifice (Oe) is somewhat larger than the true anatomic orifice (Oa). Oe is measured from the circumflex artery (CX) to the junction of the left superior pulmonary vein (LSPV) entering the left atrium (LA), also known as the Coumadin Ridge. The true anatomic orifice is measured from the CX to the left atrial wall directly across it. Modified with permission from Elsevier.
Figure 3.
Method of determining true anatomic orifice of left atrial appendage (LAA) and measurement of closure stump depth during AtriClip (Atricure, Mason, Ohio) application, steps A to D. LA, Left atrium; Cx, circumflex artery; LSPV, left superior pulmonary vein.
Statistical Analysis
Continuous variables were expressed as averages and standard variations, or as absolute numbers with percentages using Excel statistical module. Values were rounded up to the first decimal.
Results
Between December 2017 and September 2020, a total of 42 RLAAC procedures were performed. The indication for the procedure was a need for primary management of LAA due to intolerance, or complications, of OACs. Left ventricle epicardial pacer lead placement was added for cardiac resynchronization indication in 3 patients.
Patient Characteristics and Clinical Profiles
Men comprised the majority of patients (27 out of 42; 64%). The average age was 76.5 ± 7.6 years.
CHA2DS2-VASc was 5.2 ± 1.6, with the lowest being 2 and the highest 9. Hypertension, abnormal liver or kidney function, stroke, bleeding, labile international normalized ratio, elderly, drugs (aspirin, other antiplatelets, or anticoagulants score) (HAS-BLED) was 4.5 ± 0.9. The body mass index was 26.5 ± 3.5 and the body surface area was 2 ± 0.2 m2. Paroxysmal AF was present in 17 out of 42 (40%), persistent AF in 7 out of 42 (17%) and permanent AF in 18 out of 42 (43%) of patients. Other preoperative baseline clinical data are summarized in Table 1. All of the patients were declared intolerant to OACs by the referring providers and not suitable for Watchman implantation (Tables 1 and 2).
Table 1.
Preoperative clinical profiles (N = 42)
| Preoperative profile | Patients |
|---|---|
| Congestive heart failure | 12 (29) |
| Hypertension | 38 (90) |
| Diabetes mellitus | 16 (38) |
| Stroke | 11 (26) |
| Transient ischemic attack | 5 (12) |
| Cerebrovascular disease | 21 (50) |
| Coronary artery disease | 22 (52) |
| Peripheral arterial disease | 10 (24) |
| Chronic lung disease | 15 (36) |
| Chronic kidney disease | 13 (31) |
| Intolerance to OACs | |
| Liver disease | 7 (17) |
| Gastrointestinal bleeding | 26 (62) |
| Central nervous bleeding | 6 (14) |
| Hematuria | 4 (10) |
| Macular degeneration, wet | 2 (5) |
| Other bleeding | 4 (10) |
| Anemia, nonspecific | 23 (55) |
| Excessive bruising | 6 (14) |
| Frequent falls | 8 (19) |
| Unstable INR | 3 (7) |
| Alcoholism | 5 (12) |
Values are presented as n (%). OACs, Oral anticoagulants; INR, International normalized ratio.
Table 2.
Indications for epicardial left atrial appendage exclusion (N = 42)
| Indication | Incidence |
|---|---|
| Watchman∗ procedure aborted | |
| LAA too small | 2 |
| LAA too large | 1 |
| Watchman∗ preplanning size mismatch | |
| LAA too small | 6 |
| LAA too large | 2 |
| TEE for Watchman∗ implant contraindicated | |
| Varices | 2 |
| Zenker's diverticulum | 1 |
| Esophageal strictures | 1 |
| Absolute oral anticoagulants contraindication | 27 |
LAA, Left atrial appendage; TEE, transesophageal echocardiography.
Boston Scientific, Marlborough, Mass.
Preoperative Use of Antiplatelet or Anticoagulant Agents
The majority (38 out of 42; 90%) were not taking OACs preoperatively due to absolute contraindications. Of those 38 patients, 16 were taking aspirin, 1 was taking clopidogrel, and 2 were taking both. Nineteen out of 42 (45%) were taking neither antiplatelet agents, nor OACs. Three of the remaining patients were taking apixaban and 1 was taking rivaroxaban and aspirin. None were taking warfarin. Review of the medical records revealed that 38 out of 42 (90%) were declared intolerant to warfarin.
Epicardial Versus Endocardial LAA Management
Three patients (out of 42; 7%) were referred to our cardiac surgery service for RLAAC after aborted Watchman procedures (LAA too small in 2 and too large in 1). Thirty-nine out of 42 were referred due to prerecognized contraindications or technical difficulties of Watchman implantation. Those included absolute contraindication to OACs in 27 out of 42 (warfarin is required for 6 weeks after Watchman implantation), LAA/Watchman size mismatch in 8 (LAA too small in 6 and too large in 2), or inability to use TEE in 4 out of 42 cases (2 varices: 1 Zenker's diverticulum, and 1 esophageal stricture) (Table 2).
Prior Cardiac Surgery and Pericardial Adhesions
Two patients had prior coronary artery bypass surgery, both with known left internal thoracic graft to anterior descending, and a saphenous graft to obtuse marginal coronaries. Computerized tomographic angiography (CTA) was used to plan surgery in both. As expected, pericardial adhesions were encountered in both and managed successfully. Unexpected pericardial adhesions due to unsuspected prior pericarditis were encountered in 3 other cases, and likewise successfully managed using robotic instrumentation.
Preoperative Imaging
CTA was performed in 30 out of 42 (71%), TEE in 15 out of 42 (36%) and both imaging modalities in 13 out of 42 (31%) of patients. Based on preoperative imaging, important findings of size and shape of LAA were anticipated in 15 out of 42 (36%) cases, including too small LAA in 7, too large LAA in 6, and multiple lobes in 2. In all of those instances, preoperative imaging was helpful to realize a successful RLAAC.
Intraoperative TEE
During RLAAC, TEE was utilized in 38 out of 42 (90%) of patients. As stated above, esophageal contraindications prevented TEE in 4 (1 Zenker's diverticulum, 2 varices, and 1 stricture). Intraoperative findings of distal small clots in LAA were documented in 2 cases but did not interfere with placement of LAA clip. We were encouraged to place AtriClip Pro-V in those 2 cases since the small clots were located distally and the open V-shaped design allowed minimal manipulation of the LAA. Echocardiographic smoke sign (spontaneous echocardiograph contrast) was documented in 10 out of 38 (26%) cases.
There was no evidence of any residual flow past the clip closure line in any of the 38 TEE cases. Completeness of LAA exclusion was quantified by measuring the depth of the LAA stump using the method detailed in the Methods section. It averaged 0.4 ± 0.6 mm.
AtriClip Details
Thirty-eight (out of 42) patients received AtriClip Pro2, 3 received ProV, and 1 patient received both models. The most common clip size was 35 mm (26 out of 43; 60%). Forty millimeters was used in 9 out of 43 cases, 45 mm in 7 out of 43 cases, and 50 mm in only 1 case. Clip sizing was primarily based on CTA and thoracoscopic appearance with the use of the provided ruler. The correlation between modalities was particularly good.
Intraoperative Adverse Events
We did not encounter any adverse events such as bleeding, arrhythmias, myocardial ischemia, cardiac or noncardiac injury, conversion to thoracotomy, stroke, or death during all our RLAAC procedures.
Postoperative Events
The length of stay was 3.4 ± 3 days, and chest tube drainage 2.3 ± 2 days. Twelve patients (out of 42; 29%) stayed <24 hours. Table 3 lists causes of length of stay beyond 24 hours. Thirty-one patients had no complications (31 out of 42; 74%), leaving 26% with at least 1 complication. We have considered any postoperative clinical event that factored in delaying discharge a complication, except for preexisting frailty. Table 4 summarizes postoperative complications. One patient was found to have hemothorax the next day postoperatively and required thoracoscopy only.
Table 3.
Causes of length of stay >24 hours (N = 42)
| Cause of stay >24 h | Incidence |
|---|---|
| Frailty | 11 (26) |
| Severe hyponatremia (<125 mEq/L) | 4 (10) |
| Gastrointestinal complications | 3 (7) |
| Tachycardia | 3 (7) |
| Added left ventricle pacer lead procedure | 3 (7) |
| Urinary retention | 3 (7) |
Values are presented as n (%).
Table 4.
Postoperative complications (N = 42)
| Complication | Incidence |
|---|---|
| Urinary retention | 5 (19) |
| Severe hyponatremia (<125 mEq/L) | 4 (10) |
| Gastrointestinal complications | 3 (7) |
| Acute on chronic renal insufficiency | 2 (5) |
| Late hemothorax | 1 (2) |
| Pneumonia | 1 (2) |
Values are presented as n (%).
Hyponatremia
Hyponatremia with sodium level <136 mEq/L was observed in 11 out of 42 (26%) patients, and not counted as a complication, having not played a major role in delaying discharge. However severe hyponatremia with sodium level <125 mEq/L happened in 4 out of 42 (10%) and was responsible for added length of stay in those 4 patients.
Late Postoperative Events
At the time of analysis of our experience, the follow-up range was 1 to 33 months, a median of 11 months, and an average ± standard deviation of 12.4 ± 10 months. Forty-one (98%) patients remained free of any OACs after RLAAC. Only one patient with a CHA2DS2-VASc score of 9 remained on apixaban per her other health care provider's judgment. Two patients experienced late gastrointestinal bleeding, despite being off OACs. Late deaths due to abdominal sepsis at 6 and 11 months were reported in 2 patients, plus 1 death at 12 months due to pancreatic cancer and 1 at 2 months due to liver failure.
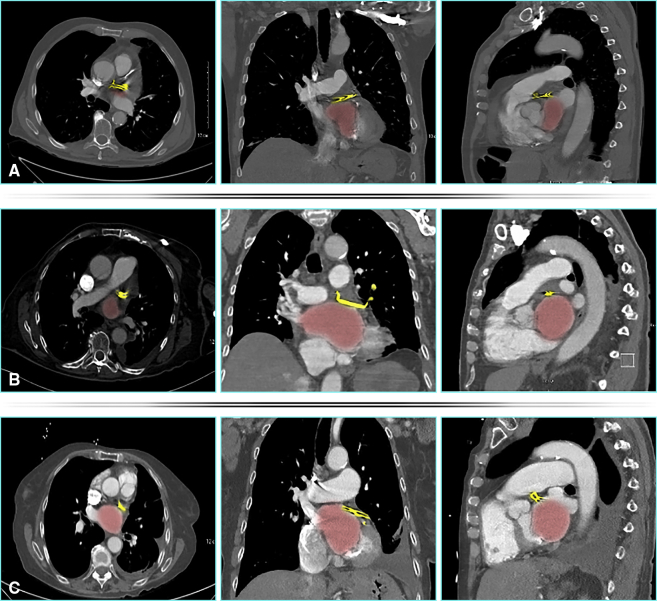
Late CTA was available for review in 3 out of 42 patients. Complete and stable exclusion of LAA was confirmed in all 3 without appreciable stump or flow into LAA (Figure 4). None of the patients underwent postoperative TEE.
Figure 4.
Postoperative computerized tomographic angiography imaging of 3 patients, A, B, and C, in transverse, coronal, and sagittal views from left to right showing no significant left atrial appendage stumps. Red indicates left atrium; yellow indicates atrial clip.
Postoperative Thromboembolic Events
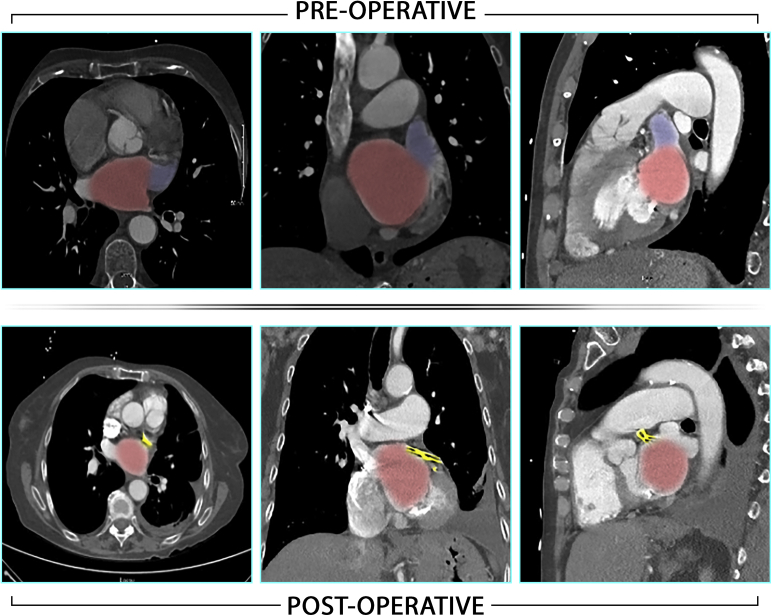
During the period of follow-up of 1 to 33 months, the absolute majority of patients were not found on clinical follow-up to have any transient ischemic attacks or strokes. Twenty-five (out of 42; 60%) patients completed 12 months' follow-up and none of them had any TES. One patient, with a CHA2DS2-VASc of 7 and HAS-BLED of 5, experienced a small cerebral lacunar infarct at 18 months postoperatively. CTA showed appropriate LAA exclusion without any stump or intra-atrial thrombus (Figure 5). No TEE was performed. Her stroke was believed to be due to carotid or intracranial atherosclerotic disease.
Figure 5.
Preoperative and postoperative computerized tomographic angiography views of the patient with a late stroke displaying exclusion of left atrial appendage without appreciable stump. Red indicates left atrium, blue indicates left atrial appendage, yellow indicates AtriClip (Atricure, Mason, Ohio).
Discussion
Our study presents a single-institution experience with RLAAC as a stand-alone procedure for reduction of bleeding in patients intolerant to OACs. To our knowledge, this series might represent the largest reported of its kind using robotic assistance. The importance of reproducible and effective closure of LAA is compounded by the reports of potential increase of TES after inconsistent and incomplete LAA closure attempts.12,13 Likewise, despite the success of endocardial LAA occlusion devices, such as Watchman, there remain important gaps in their ability to help every patient with a valid indication for nonanticoagulation management of LAA. A course of OACs, preferably warfarin, followed by DAPT, is required after endocardial occlusion device implantation. That requirement might temporarily increase the risk of bleeding after implantation, especially in patients with severe and unidentified sources of gastrointestinal bleeding or intracranial bleeding.14, 15, 16 Some LAA anatomical variations might also limit the pool of beneficiaries of endocardial LAA occluders. The development of our program was in response to some of those needs and challenges. The growing published support of epicardial linear LAA closure devices, particularly AtriClip,10 encouraged our expanded use of them in concomitant, and later on, in LAA stand-alone exclusion procedures. We graduated in its use from open chest to thoracoscopy then to a robotics-assisted technique. Once the proof of concept of minimizing invasiveness using the robotic platform was established, and with the evolution of both technologies, namely da Vinci Xi, AtriClip Pro2, and AtriClip ProV, we pivoted into the routine and preferred use of a robotic platform for epicardial LAA exclusion.
Reports of the use of robotic platforms for LAA interventions included right-sided approach for epicardial clip placement during concomitant valve surgery,17 port-access cardiopulmonary bypass assisted ligation,18 and endocardial suturing during robotic assisted mitral valve surgery.19 We believe that the left-sided approach provides optimal access from physiological and anatomical standpoints. It allows performance without cardiopulmonary support and maximal visualization of all relevant anatomy. It is minimally invasive and, most importantly, allows real-time assessment by TEE to ascertain satisfactory closure. The gradual progress from thoracoscopic to robotic technique promoted safety and feasibility. Among the advantages of a robotic platform is its improved visibility and maneuverability. Body size and habitus effects are less significant in robotic than thoracoscopic systems. Our standardized LAA exclusion protocol permitted consistent closure without overlooking any lobes or persistent flow and without any appreciable residual LAA stump, which all have been considered markers of inadequate LAA exclusion and associated with increased risk of TES.13 The length of surgical time decreased to <30 minutes in the majority of cases. In addition, hospital length of stay decreased with the majority of recent patients being discharged on the first day postoperatively.
The absence of any intraoperative complications, mortalities, or the need to convert to thoracotomy demonstrate procedural safety. All procedures were successfully completed, including reoperative procedures after coronary bypass surgery, or with adhesions after pericarditis. In fact, the 3-dimensional display and magnification and ergonomics of the robotic platform are matched very well with the low profile and articulation of AtriClip Pro2 and AtriClip ProV, allowing for safe application of the clip in the most difficult situations, such as reoperations, pericarditis adhesions, morbid obesity, LAA clots, and difficult anatomies. The size and shape of the LAA proved to be completely irrelevant to the quality of exclusion. The open V-shaped design of AtriClip ProV allowed safe application of the clip at the base of the LAA without disturbing small peripheral blood clots that were seen on intraoperative TEE in 2 patients. It was also used to improve closure after AtriClip Pro2 in 1 case.
With the exception of esophageal contraindications listed in the Results section, we implemented TEE in every case and relied heavily on it to ascertain complete LAA exclusion (Figures 1 and 3). We adopted a systematic approach to TEE during the procedure detailed in the Methods section. Our method of assessing the depth of LAA stump was based on identification of the true anatomic LAA ostium and corresponded to Watchman implantation guidelines (Figures 2, 3, and 6). Based on our method, all of our patients had an LAA stump ≤2 mm (Figures 1 and 3). We believe that the repeatedly published recommendations10,20,21 for a stump ≤10 mm have been based on measuring in relation to the tip of Coumadin ridge and may need to be modified. To ascertain satisfactory LAA closure and patency of LCX, we allowed for readjustment of the clip position before final release from its applier based on detailed TEE views and electrocardiography. Based on published evidence of stable permanent clip exclusion,10 we opted not to require any routine postoperative TEE or CTA. Due to the peculiar nature of indications for stand-alone LAA exclusion, at least 30% of patients have multiple morbid conditions and are very frail (Table 3). The length of stay is influenced primarily by those factors. Intraoperatively, the procedure remained safe.
Figure 6.
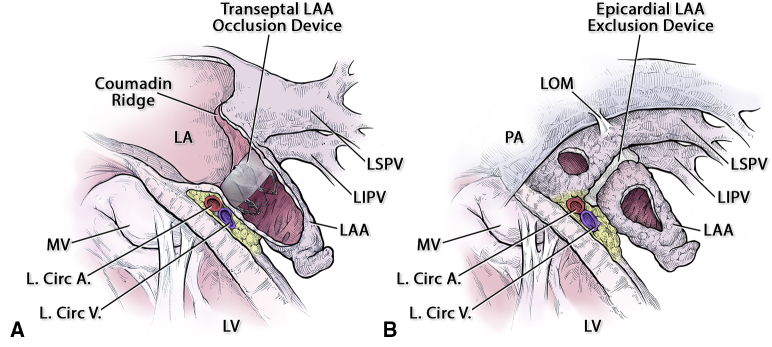
The 2 methods of left atrial appendage (LAA) closure share the same definition for LAA true anatomic orifice. A, Endocardial device. B, Epicardial device. LA, Left atrium; LSPV, left superior pulmonary vein; LIPV, left inferior pulmonary vein; MV, mitral valve; L. Circ A., left circumflex artery; L. Circ V., left circumflex vein; LV, left ventricle; LOM, ligament of Marshall; PA, pulmonary artery.
All patients, except 1 with the highest possible CHA2DS2-VASc of 9, stopped OACs after RLAAC in the case that they had taken any before the procedure. Postclip bleeding was reported in only 2 patients with gastrointestinal bleeding. None of the 25 patients who completed 12 months' follow-up had any transient ischemic attack or stroke. The 1 patient with a documented small lacunar stroke 18 months postoperatively had a CHA2DS2-VASc of 7. Her poststroke CTA showed no left atrial thrombus, no stump or flow into any residual LAA, and her stroke was believed to be due to a carotid or intracranial source (Figure 5). The absence of significant bleeding or stroke following RLAAC in this remarkably high average CHA2DS2-VASc population provides credibility to the concept of epicardial clip exclusion of LAA.
An interesting finding of mild-to-moderate hyponatremia in a quarter of patients did not influence length of stay but severe hyponatremia was seen in 10% of patients, especially in association with heart, renal failure, and frailty and contributed to extended length of stay. The success of developing an epicardial LAA exclusion program in our institution is greatly influenced by the heart team approach to LAA therapies and the mutual support between the endocardial device and epicardial device teams.
Limitations
The study lacks a comparative control group, such as thoracoscopic stand-alone LAA clip or endocardial device group. Our positive bias toward the use of robotic platform skewed our experience in recent years toward it. Another limitation is that it represents an isolated experience of 1 team/1 surgeon. Our current level of competencies is based on a long and slow learning curve that built on experience in complex thoracoscopic followed by robotic thoracic surgery and ablations. Our single-center experience might benefit from meta-analysis with other teams' similar experiences.
Conclusions
Epicardial left atrial appendage clip exclusion using a robotics-assisted technique is feasible and safe.
Conflict of Interest Statement
Dr Antaki has a consultant agreement with Atricure, Inc. All other authors reported no conflicts of interest.
The Journal policy requires editors and reviewers to disclose conflicts of interest and to decline handling or reviewing manuscripts for which they may have a conflict of interest. The editors and reviewers of this article have no conflicts of interest.
Acknowledgments
The authors thank Mr Mason Wiest for assisting in the production and editing of the figures.
Footnotes
Partial funding was provided by an educational grant from AtriCure, Inc.
Read at the American Association for Thoracic Surgery Surgical Treatment of Arrhythmias and Rhythm Disorders: A Virtual Learning Experience, October 30-31, 2020.
Supplementary Data
Robotics-assisted left atrial appendage procedure steps. Video available at: https://www.jtcvs.org/article/S2666-2507(21)00486-7/fulltext.
References
- 1.Blackshear J.L., Odell J.A. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg. 1996;61:755–759. doi: 10.1016/0003-4975(95)00887-X. [DOI] [PubMed] [Google Scholar]
- 2.Wolf P.A., Dawber T.R., Thomas H.E., Kannel W.B. Epidemiologic assessment of chronic atrial fibrillation and risk of stroke—The Framingham Study. Neurology. 1978;28:973–977. doi: 10.1212/wnl.28.10.973. [DOI] [PubMed] [Google Scholar]
- 3.Lip G.Y., Nieuwlaat R., Pisters R., Lane D.A., Crijns H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- 4.Petersen P., Godtfredsen J., Boysen G., Andersen E.D., Andersen B. Placebo-controlled, randomised trial of warfarin and Aspirin for prevention of thromboembolic complications in chronic atrial fibrillation: the Copenhagen AFASAK Study. Lancet. 1989;333:175–179. doi: 10.1016/s0140-6736(89)91200-2. [DOI] [PubMed] [Google Scholar]
- 5.Holmes D.R., Reddy V.Y., Turi Z.G., Doshi S.K., Sievert H., Buchbinder M. Percutaneous closure of the left atrial appendage vs. warfarin therapy for the prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009;374:534–542. doi: 10.1016/S0140-6736(09)61343-X. [DOI] [PubMed] [Google Scholar]
- 6.Reddy V.Y., Doshi S.K., Sievert H., Buchbinder M., Neuzil P., Huber K. Percutaneous left atrial closure for stroke prophylaxis in patients with atrial fibrillation: 2.3-year follow-up of the PROTECT AF (Watchman left atrial appendage system for embolic protection in patients with atrial fibrillation) trial. Circulation. 2013;127:720–729. doi: 10.1161/CIRCULATIONAHA.112.114389. [DOI] [PubMed] [Google Scholar]
- 7.Holmes D.R., Kar S., Price M.J., Whisenant B., Sievert H., Doshi S.K. Prospective randomized evaluation of the Watchman left atrial appendage closure device in patients with atrial fibrillation versus long term warfarin therapy: the PREVAIL trial. J Am Coll Cardiol. 2014;64:1–12. doi: 10.1016/j.jacc.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 8.Park-Hansen J., Holme S., Irmukhamedov A., Carranza C.L., Greve A.M., Al-Farra G. Adding left appendage closure to open heart surgery provides protection from ischemic brain injury six years after surgery independently of atrial fibrillation history: the LAACS randomized study. J Cardiothorac Surg. 2018;13:53–61. doi: 10.1186/s13019-018-0740-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caliskan E., Sahin A., Yilmaz M., Seifert B., Hinzpeter R., Alkadhi H. Epicardial left atrial appendage Atriclip occlusion reduces the incidence of stroke in patients with atrial fibrillation undergoing cardiac surgery. Europace. 2018;20:e105–e114. doi: 10.1093/europace/eux211. [DOI] [PubMed] [Google Scholar]
- 10.Ailawadi G., Gerdisch M., Harvey R., Hooker R.L., Damiano R.J., Jr., Salamon T. Exclusion of the left atrial appendage with a novel device: early results of a multicenter trial. J Thorac Cardiovasc Surg. 2011;142:1002–1009. doi: 10.1016/j.jtcvs.2011.07.052. [DOI] [PubMed] [Google Scholar]
- 11.Kurfirst V., Mokráček Čanádyová J., Frána R., Zeman P. Epicardial clip occlusion of the left atrial appendage during cardiac surgery provides optimal surgical results and long-term stability. Interact Cardiovasc Thorac Surg. 2017;25:37–40. doi: 10.1093/icvts/ivx065. [DOI] [PubMed] [Google Scholar]
- 12.Viles-Gonzalez J.F., Kar S., Douglas P., Dukkipati S., Feldman T., Horton R. The clinical impact of incomplete left atrial appendage closure with the Watchman Device in patients with atrial fibrillation: a PROTECT AF (percutaneous closure of the left atrial appendage versus Warfarin therapy for prevention of stroke in patients with atrial fibrillation) substudy. J Am Coll Cardiol. 2012;59:923–929. doi: 10.1016/j.jacc.2011.11.028. [DOI] [PubMed] [Google Scholar]
- 13.Kanderian A.S., Gillinov A.M., Pettersson G.B., Blackstone E., Klein A.L. Success of surgical left atrial appendage closure: assessment by transesophageal echocardiography. J Am Coll Cardiol. 2008;52:924–929. doi: 10.1016/j.jacc.2008.03.067. [DOI] [PubMed] [Google Scholar]
- 14.Akca F., Verberkmoes N.J., Verstraeten S.E., van Laar C., van Putte B.P., van Straten A.H.M. Is there an alternative treatment for patients intolerant to antiplatelet therapy if percutaneous left atrial appendage closure is considered? Neth Heart J. 2017;25:510–515. doi: 10.1007/s12471-017-0987-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gurol E.M. Nonpharmacological management of atrial fibrillation in patients at high intracranial hemorrhage risk. Stroke. 2018;49:247–254. doi: 10.1161/STROKEAHA.117.017081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Branzoli S., Marini M., Guarracini F., Pederzolli C., Pomarolli C., D'Onghia G. Epicardial standalone left atrial appendage clipping for prevention of ischemic stroke in patients with atrial fibrillation contraindicated for oral anticaogulation. J Cardiovasc Electrophysiol. 2020;31:2187–2191. doi: 10.1111/jce.14599. [DOI] [PubMed] [Google Scholar]
- 17.Lewis C., Stephens R., Horst V., Angelillo M., Tyndal C. Application of an epicardial left atrial appendage occlusion device by a robotic-assisted, right chest approach. Ann Thorac Surg. 2016;101:e177–e178. doi: 10.1016/j.athoracsur.2015.11.028. [DOI] [PubMed] [Google Scholar]
- 18.Kiaii B., McClure R.S., Skanes A., Ross I., Spouge A., Swinamer S. Robotic assisted left atrial appendage ligation for stroke reduction in chronic atrial fibrillation: a case report. Heart Surg Forum. 2006;9:e533–e535. doi: 10.1532/HSF98.20051148. [DOI] [PubMed] [Google Scholar]
- 19.Ward A.F., Applebaum R.M., Toyoda N., Fakiha A., Neuburger P.J., Ngai J. Totally endoscopic robotic left atrial appendage closure demonstrates high success rate. Innovations. 2017;12:46–49. doi: 10.1097/IMI.0000000000000330. [DOI] [PubMed] [Google Scholar]
- 20.Osmancik P., Budera P., Zdarska J., Herman D., Petr R., Fojt R. Residual echocardiographic and computed tomography findings after thoracoscopic occlusion of the left atrial appendage using the AtriClip PRO device. Interac Cardiovasc Thorac Surg. 2018;26:919–925. doi: 10.1093/icvts/ivx427. [DOI] [PubMed] [Google Scholar]
- 21.Caliskan E., Eberhard M., Falk V., Alkadhi H., Emmert M.Y. Incidence and characteristics of left atrial appendage stumps after device-enabled epicardial closure. Interac Cardiovasc Thorac Surg. 2019;29:663–669. doi: 10.1093/icvts/ivz176. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Robotics-assisted left atrial appendage procedure steps. Video available at: https://www.jtcvs.org/article/S2666-2507(21)00486-7/fulltext.