Abstract
Background
The pathogenesis of Clostridioides difficile infection (CDI) involves a significant host immune response. Generally, corticosteroids act by suppressing the host inflammatory response, and their anti-inflammatory effects are used to treat gastrointestinal disorders. Although previous investigations have demonstrated mixed results regarding the effect of corticosteroids on CDI, we hypothesized that the anti-inflammatory effect of corticosteroids would decrease the risk of CDI in hospitalized patients.
Methods
This was a case–control study of hospitalized adults. The case population included patients diagnosed with primary CDI who received at least 1 dose of a high-risk antibiotic (cefepime, meropenem, or piperacillin-tazobactam) in the 90 days before CDI diagnosis. The control population included patients who received at least 1 dose of the same high-risk antibiotic but did not develop CDI in the 90 days following their first dose of antibiotic. The primary study outcome was the development of CDI based on receipt of corticosteroids.
Results
The final study cohort consisted of 104 cases and 153 controls. Those who received corticosteroids had a lower odds of CDI after adjusting for age, proton pump inhibitor use, and antibiotic days of therapy (odds ratio, 0.54; 95% CI, 0.30–0.97; P = .04). We did not observe an association between corticosteroid dose or duration and CDI.
Conclusions
We demonstrated a 46% relative reduction in the odds of developing CDI in patients who received corticosteroids in the past 90 days. We believe that our results provide the best clinical evidence to further support mechanistic studies underlying this phenomenon.
Keywords: antibiotic, Clostridium difficile, corticosteroid, diarrhea, steroid
Clostridioides difficile is a toxin-producing, spore-forming, anaerobic, gram-positive bacillus and the most common pathogen causing health care–associated infections in the United States [1]. The pathogenesis of C. difficile infection (CDI) involves a complex relationship between bacterial and host factors mediated primarily by toxins A and B. These toxins produce a significant host inflammatory response and damage the colonic epithelium [2]. This inflammatory cascade is comprised of the release of pro-inflammatory cytokines, activation of intestinal innate lymphoid cells, production of effector cytokines, and eventual neutrophil invasion of the colon. Several studies have suggested that an overactive immune response can have a detrimental effect on a host with CDI [3–6].
Corticosteroids blunt the host inflammatory response via multiple mechanisms [7, 8] and are used to treat other inflammatory gastrointestinal (GI) disorders, such as inflammatory bowel disease (IBD) [9–11]. Although the benefit of an anti-inflammatory agent in an inflammation-driven disease state makes intuitive sense, the immunosuppressive effects of corticosteroids could increase the likelihood of CDI in a susceptible patient population. It is worth noting that the anti-inflammatory effects of corticosteroids are quite rapid, while the immunosuppressive effects are not maximal until at least several weeks of dosing [7, 8, 12]. Numerous case–control studies have suggested that corticosteroid use increases a patient’s risk of developing CDI [13–19], while others have found corticosteroid use to be protective against the development of CDI [20, 21]. However, these studies have a number of limitations, including an inability to control for predisposing antibiotic use [13, 15, 19], lack of corticosteroid dosing documentation [13, 15–17, 19], changes in CDI diagnostic testing over time [17, 19, 20], and the inclusion of populations that limit generalizability (eg, IBD [14, 16, 18, 19] or hematopoietic stem cell transplant [21]).
Given this mechanistic understanding of CDI and corticosteroids, we hypothesized that the anti-inflammatory effects of corticosteroids would decrease the risk of CDI when studied in a generalizable population. To test this hypothesis, we conducted a case–control study examining the association between corticosteroid use and CDI risk.
METHODS
Study Population
This case–control study included hospitalized patients admitted to a single quaternary care hospital in the Texas Medical Center in Houston, Texas. The case population was derived from our ongoing cohort study of patients with CDI and included adults (age ≥18 years) consecutively diagnosed with primary CDI between October 2015 and August 2017 who received at least 1 dose of a high-risk antibiotic (cefepime, meropenem, or piperacillin-tazobactam) in the 90 days before CDI diagnosis. The control population consisted of adult patients who received at least 1 dose of the same high-risk antibiotic during the same time frame but did not develop clinically significant diarrhea or other symptoms that would warrant testing for C. difficile toxins in the 90 days following their first dose of antibiotic. Patients with a documented history of CDI were excluded.
Patient Consent
The study was approved by the University of Houston Committee for the Protection of Human Subjects with a waiver of informed consent (UH CPHS IRB study 00000128).
Covariates and Definitions
Electronic medical records (EMRs; Epic Systems Co., Verona, WI, USA) were reviewed retrospectively for demographic information, underlying comorbidities, medication administration records (MARs), laboratory data, and clinical outcomes. Charlson Comorbidity Index (CCI) scores were calculated using comorbidities documented on or before the date of hospital admission [22]. Options for residence before admission included home, skilled nursing facility, nursing home/long-term care facility, another hospital, or hospice, but were coded and reported as a binary variable of admission from home vs nonhome. Recent GI surgery was defined as any invasive GI procedure (ie, laparoscopic or open) in the 6 months before CDI diagnosis (cases) or last day of follow-up (controls). The 90-day study period refers to the 90 days preceding the date of the positive C. difficile toxin test for cases and the 90 days after initiation of a high-risk antibiotic for controls. The primary outcomes, corticosteroids and antibiotic days of therapy (DOT), were defined as the aggregate sum of days for which any amount of high-risk corticosteroid or antibiotic was administered within the 90-day study period. Our EMR’s interoperability platform, Care Everywhere, enabled us to collect medication use before hospital admission (cases) and following hospital discharge (controls) assuming the patient was seen in a facility within the Epic Network.
All corticosteroid doses were converted into prednisone equivalents [23, 24]. Daily doses were averaged for individuals receiving >1 steroid and/or >1 daily dose. Corticosteroid use was expressed as receipt (administration of at least 1 dose of any corticosteroid) within the 90-day study period, average daily dose (prednisone dose equivalent per day), and duration of therapy.
Multiple prespecified subgroup analyses were planned in those who received corticosteroids in order to assess whether an association between dose or duration and CDI risk existed. In these analyses, corticosteroid dose and duration were used as predictor variables while CDI remained the outcome variable. Furthermore, an additional subgroup analysis using the receipt of an “immunosuppressive dose” of corticosteroid as the predictor variable to investigate the potential for a ceiling effect was performed. An immunosuppressive dose of corticosteroid was defined as the receipt of ≥20 mg of prednisone or equivalent for ≥2 weeks [12].
Outcomes
The primary study outcome was the development of CDI. Patients were tested for CDI at the discretion of the treating physician and medical team. The CDI testing standard of care in our facility during the study time frame was a C. difficile nucleic acid amplification test (NAAT) in patients with unexplained and new-onset diarrhea (≥3 unformed stools in 24 hours). The Epic Care Everywhere Network was accessed to limit attrition bias by ensuring that outcome data were captured as completely as possible.
Statistical Analysis
For baseline characteristic comparison, binary variables were compared using the χ 2 test while continuous variables were compared using the Student t test or the Wilcoxon rank-sum test, depending on the distribution of the data for the given variable. A logistic regression model was developed modeling CDI diagnosis as a function of relevant patient covariates. To prevent overfitting the model, only covariates whose univariate Wald test had a P value <.20 were considered as candidates for the multivariable model. A stepwise backwards elimination procedure was performed by which variables with a P value >.05 were removed 1 at a time, and the partial likelihood ratio test was used to compare the new, smaller model with the old model. Variables that did not improve the model fit remained excluded. Subgroup analyses were completed using the same variables included in the multivariable model for the primary analysis. Odds ratios (ORs) and 95% CIs were calculated. All P values resulted from 2-sided tests, and outcomes were deemed statistically significant at P < .05. All statistical analyses were performed using STATA, version 15.1 (StataCorp LLC, College Station, TX, USA), and results of the subgroup analyses were visualized using the “forestplot” package in R, version 3.6.1 (The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patient Characteristics
A total of 313 patients were identified, with 56 excluded due to a history of CDI, leaving a final study cohort of 257 patients (104 cases and 153 controls) aged 65 ± 14 years (female, 43%; median CCI, 2). Demographic and baseline characteristics of the patients are shown in Table 1. More patients in the control arm received steroids (P = .02) at lower doses over a longer time period compared with cases. Conversely, more patients in the case arm had documented proton pump inhibitor (PPI) use (P < .001). Lastly, control patients had higher antibiotic DOT (P < .001).
Table 1.
Comparison of Patient Demographics, Comorbidities, and Medication Use
| Covariate | Cases | Controls | P Value |
|---|---|---|---|
| (n = 104) | (n = 153) | ||
| Age, mean (SD), y | 63.5 (16.8) | 66.4 (13.0) | .13 |
| Female, No. (%) | 53 (51.0) | 60 (39.2) | .06 |
| CCI, median (IQR) | 2 (1–4) | 3 (1–4) | .39 |
| SOT, No. (%) | 8 (7.7) | 24 (15.7) | .06 |
| Residence PTA, No. (%) | |||
| Home | 78 (75.0) | 112 (73.2) | .75 |
| Recent GI surgery, No. (%) | 15 (14.4) | 24 (15.7) | .78 |
| Corticosteroid use, No. (%) | 45 (43.3) | 89 (58.2) | .02 |
| Prednisone equivalent/d, median (IQR),a mg | 40 (25–86) | 27 (13–48) | .006 |
| ≥20 mg | 39 (86.7) | 58 (65.2) | |
| 10–19 mg | 3 (6.7) | 18 (20.2) | |
| 5–9 mg | 3 (6.7) | 7 (7.9) | |
| <5 mg | 0 (0.0) | 6 (6.7) | |
| Corticosteroid DOT, median (IQR)a | 5 (2–11) | 9 (4–35) | .01 |
| PPI use, No. (%) | 72 (69.2) | 52 (34.0) | <.001 |
| Antibiotic use, No. (%) | |||
| Cefepime | 67 (64.4) | 91 (59.5) | .42 |
| Meropenem | 41 (39.4) | 77 (50.3) | .09 |
| Piperacillin-tazobactam | 49 (47.1) | 80 (52.3) | .42 |
| Antibiotic DOT, median (IQR) | 6 (3–10) | 10 (6–19) | <.001 |
Abbreviations: CCI, Charlson Comorbidity Index; DOT, days of therapy; GI, gastrointestinal; IQR, interquartile range; PPI, proton pump inhibitor; PTA, prior to admission; SOT, solid organ transplantation.
aPatients without 90-day corticosteroid receipt were excluded (n = 134 included).
Clinical Outcomes
In the univariate analysis, the odds of CDI were lower among patients who received corticosteroids (Table 2). Receipt of corticosteroids remained protective in the multivariable model after adjusting for age, PPI use, and antibiotic DOT (OR, 0.54; 95% CI, 0.30–0.97; P = .04).
Table 2.
Univariate and Multivariable Analysis for Predictors of Clostridioides difficile Infection
| Covariate | Univariate Analysis | P Value | Multivariable Analysis | P Value |
|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | |||
| Corticosteroid use | 0.55 (0.33–0.91) | .02 | 0.54 (0.30–0.97) | .04 |
| Antibiotic DOT | 0.93 (0.90–0.96) | <.001 | 0.93 (0.91–0.96) | <.001 |
| Age | 0.99 (0.97–1.00) | .13 | 0.98 (0.96–0.99) | .03 |
| CCI | 0.94 (0.85–1.03) | .19 | ||
| PPI use | 4.37 (2.56–7.46) | <.001 | 4.58 (2.58–8.12) | <.001 |
| Female | 1.61 (0.97–2.66) | .06 | ||
| SOT | 0.45 (0.19–1.04) | .06 |
Abbreviations: CCI, Charlson Comorbidity Index; DOT, days of therapy; OR, odds ratio; PPI, proton pump inhibitor; SOT, solid organ transplantation.
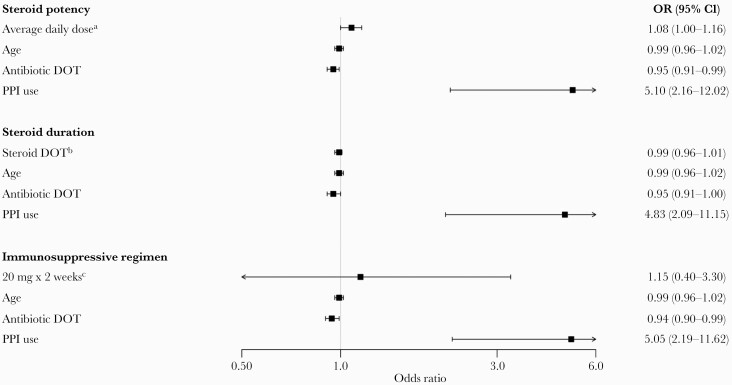
Our prespecified subgroup analyses were done in the 134 patients who received corticosteroids. The results of these analyses can be seen in Figure 1. Corticosteroid dose and duration did not increase the risk of development of CDI.
Figure 1.
Multivariable logistic regression subgroup analyses. aOR represents relative odds for every 10-mg increase in the average daily dose of corticosteroid (measured using prednisone equivalents). bOR represents relative odds for every 1 DOT increase in the corticosteroid duration of therapy. cIncludes patients who received ≥20 mg of prednisone or equivalent per day for ≥2 weeks (n = 27) vs those who did not (n = 107). Abbreviations: DOT, days of therapy; OR, odds ratio; PPI, proton pump inhibitor.
We conducted a post hoc analysis of all patients diagnosed with CDI to determine risk factors that may have mitigated the protective effects of corticosteroids (Table 3). A significantly higher proportion of patients who received corticosteroids and subsequently developed CDI had received solid organ transplantation in the past (P = .02). Although not reaching statistical significance, these patients also had numerically higher CCI scores and rates of PPI use.
Table 3.
Stratified Analysis of Corticosteroid Use Among Patients Diagnosed With Clostridioides difficile Infection
| Covariate | Steroids (n = 45) | No Steroids (n = 59) | P Value |
|---|---|---|---|
| Age, mean (SD), y | 62.1 (15.2) | 64.5 (18.1) | .47 |
| Female, No. (%) | 24 (53.3) | 29 (49.2) | .67 |
| CCI, median (IQR) | 3 (1–4) | 2 (1–4) | .12 |
| SOT, No. (%) | 7 (15.6) | 1 (1.7) | .02 |
| Residence PTA, No. (%) | |||
| Home | 33 (73.3) | 45 (76.3) | .73 |
| Recent GI surgery, No. (%) | 7 (15.6) | 8 (13.6) | .77 |
| PPI use, No. (%) | 34 (75.6) | 38 (64.4) | .22 |
| Antibiotic use, No. (%) | |||
| Cefepime | 28 (62.2) | 39 (66.1) | .68 |
| Meropenem | 20 (44.4) | 21 (35.6) | .36 |
| Piperacillin-tazobactam | 24 (53.3) | 25 (42.4) | .27 |
| Antibiotic DOT, median (IQR) | 6 (4–12) | 6 (3–10) | .31 |
| Albumin, mean (SD), g/dL | 2.9 (0.6) | 2.8 (0.6) | .38 |
Abbreviations: CCI, Charlson Comorbidity Index; DOT, days of therapy; GI, gastrointestinal; IQR, interquartile range; PPI, proton pump inhibitor; PTA, prior to admission; SOT, solid organ transplantation.
DISCUSSION
Although previous investigations have demonstrated mixed results regarding the effect of corticosteroids on CDI risk [13–21], we hypothesized that the anti-inflammatory effect of corticosteroids would decrease the risk of CDI in a generalizable population of hospitalized patients. This single-center, retrospective study demonstrated a 46% relative reduction in the odds of developing CDI in patients who received corticosteroids in the past 90 days, thereby supporting our hypothesis. Our long-term hypothesis is that an anti-inflammatory agent may aid in the prevention, severity, and treatment of CDI. We believe that the results of this study provide preliminary evidence for our long-term hypothesis and help to justify a drug development program targeting the host inflammatory response to CDI.
The anti-inflammatory effects of corticosteroids are not completely understood; however, the binding of corticosteroid to glucocorticoid receptors is known to lead to a decrease in the production of cytokines and chemokines [7]. Elevated cytokines have been associated with poor CDI outcomes including death in mice [3], prolonged symptomology in humans [4], and increased severity of disease in humans [5, 6]. Therefore, it is reasonable to hypothesize that administering an anti-inflammatory agent such as corticosteroid might abrogate CDI pathogenesis and lessen symptomatic disease severity. It is possible that corticosteroids reduce disease severity, resulting in subclinical disease and/or underdiagnosis. As cytokine and chemokine production relies on transcription, the effects of corticosteroids can persist even after discontinuation of the agent [7]. As these effects are cytokine/chemokine specific, further research will be needed to assess the pharmacologic activity of corticosteroids or other future anti-inflammatory agents in relation to the pathophysiology of CDI. Furthermore, glucocorticoid receptors are subject to saturation and are completely occupied following the administration of ~100–200 mg of prednisolone [7]. Thus, higher doses of corticosteroid will not be of benefit. Our study was designed with these pharmacokinetic (PK) and pharmacodynamic (PD) characteristics in mind. We measured corticosteroid use in the previous 90 days rather than 30 days and collected both dose and duration data. Not surprisingly, our findings support these PK/PD characteristics: We observed an association between receipt of corticosteroid in the past 90 days (regardless of time since administration) and CDI, but we did not observe an association between corticosteroid dose or duration and CDI.
A strength of this study was the ability to assess the effect of corticosteroid dose and duration of therapy, which has not previously been done. Another strength was the inclusion of a large population of hospitalized adults receiving broad-spectrum antibiotics without a history of CDI, thus controlling for a known predisposing risk factor of CDI. In addition, we controlled for antibiotic DOT, which is positively correlated with CDI risk [25]. The non-CDI control group was selected based on receipt of ≥1 of the targeted high-risk antibiotics. The control group had increased high-risk antibiotic DOT compared with the CDI case group. However, the control group was selected based on no subsequent occurrence of CDI, and thus the unexpected increase in antibiotic DOT in the control group was simply an artefact of the matching process. Three of the 7 previously mentioned case–control studies that concluded that corticosteroid use increases a patient’s risk of developing CDI were unable to adequately control for predisposing antibiotic use [13, 15, 19]. Our ability to control for these variables decreases the likelihood of residual confounding and increases the robustness of our findings.
Our study also has several limitations. First, the inherent disadvantages of a retrospective design were present, including attrition and misclassification bias. We attempted to minimize attrition bias by using the Epic Care Everywhere Network but cannot rule out the possibility that some patients in the control group were lost to follow-up. This may have led to the misclassification of a case patient as a control patient and an overestimation in the protective effect of corticosteroids. Patient who receive corticosteroids are inherently different than those who do not require them. Likewise, case–control studies cannot assess temporality, and controls may not have had the same baseline risk for CDI or corticosteroid use as the cases despite matching on the receipt of high-risk antibiotics. Additionally, although MAR data were used for medication exposure while inpatient, we relied on accurate documentation of before-admission and/or discharge medications in the EMR to capture exposures received outside of the hospital. We were unable to account for patient adherence (which would overestimate exposure) and the receipt of medications omitted from the before-admission and/or discharge medication lists in the EMR (which would underestimate exposure). However, as both groups would be equally affected by this, it is unlikely to have biased our conclusions.
In summary, corticosteroid use significantly reduced the odds of developing CDI in patients without a history of CDI who received cefepime, meropenem, or piperacillin-tazobactam. These results are hypothesis-generating and provide support for further investigation into the mechanism behind this phenomenon and future drug development targeting the CDI inflammatory host response.
Acknowledgments
Financial support. This study was funded in part by the Society of Infectious Diseases Pharmacists (SIDP) and the National Institutes of Health (NIH) National Institute of Allergy and Infectious Diseases (NIAID) U01AI124290-01.
Potential conflicts of interest. All authors: no reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Guarantor of the article. Kevin W. Garey accepts full responsibility for the conduct of this study. He had access to the data and had control of the decision to publish.
Author contributions. T.J.C. made substantial contributions to this work including the acquisition of the data, data interpretation and analysis, and drafting of the manuscript. He approved of the final draft submitted and agrees to be accountable for the accuracy and integrity of the work. A.J.G.-L. made substantial contributions to this work including the acquisition of the data, data interpretation and analysis, and drafting of the manuscript. She approved of the final draft submitted and agrees to be accountable for the accuracy and integrity of the work. M.F.W. made substantial contributions to this work including the conception and design of the study, acquisition of the data, and critical revision of the manuscript for important intellectual content. She approved of the final draft submitted and agrees to be accountable for the accuracy and integrity of the work. S.G.T. made substantial contributions to this work including the conception and design of the study, acquisition of the data, and critical revision of the manuscript for important intellectual content. She approved of the final draft submitted and agrees to be accountable for the accuracy and integrity of the work. F.S.A. made substantial contributions to this work including the acquisition of the data, data interpretation and analysis, and drafting of the manuscript. He approved of the final draft submitted and agrees to be accountable for the accuracy and integrity of the work. P.P. made substantial contributions to this work including the acquisition of the data and critical revision of the manuscript for important intellectual content. He approved of the final draft submitted and agrees to be accountable for the accuracy and integrity of the work. B.K.A. made substantial contributions to this work including the acquisition of the data and critical revision of the manuscript for important intellectual content. She approved of the final draft submitted and agrees to be accountable for the accuracy and integrity of the work. E.J.Z. made substantial contributions to this work including the conception and design of the study, acquisition of the data, data interpretation and analysis, and revision of the manuscript for important intellectual content. He approved of the final draft submitted and agrees to be accountable for the accuracy and integrity of the work. K.W.G. made substantial contributions to this work including the conception and design of the study, data interpretation and analysis, and drafting of the manuscript. He approved of the final draft submitted and agrees to be accountable for the accuracy and integrity of the work.
Ethics statement. The study was approved by the University of Houston Committee for the Protection of Human Subjects with a waiver of informed consent (IRB study 00000128).
References
- 1. Magill SS, O’Leary E, Janelle SJ, et al. ; Emerging Infections Program Hospital Prevalence Survey Team. Changes in prevalence of health care-associated infections in U.S. hospitals. N Engl J Med 2018; 379:1732–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abt MC, McKenney PT, Pamer EG. Clostridium difficile colitis: pathogenesis and host defence. Nat Rev Microbiol 2016; 14:609–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buonomo EL, Madan R, Pramoonjago P, et al. Role of interleukin 23 signaling in Clostridium difficile colitis. J Infect Dis 2013; 208:917–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. El Feghaly RE, Stauber JL, Deych E, et al. Markers of intestinal inflammation, not bacterial burden, correlate with clinical outcomes in Clostridium difficile infection. Clin Infect Dis 2013; 56:1713–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Czepiel J, Biesiada G, Brzozowski T, et al. The role of local and systemic cytokines in patients infected with Clostridium difficile. J Physiol Pharmacol 2014; 65:695–703. [PubMed] [Google Scholar]
- 6. Yu H, Chen K, Sun Y, et al. Cytokines are markers of the Clostridium difficile-induced inflammatory response and predict disease severity. Clin Vaccine Immunol 2017; 24:e00037–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Czock D, Keller F, Rasche FM, Häussler U. Pharmacokinetics and pharmacodynamics of systemically administered glucocorticoids. Clin Pharmacokinet 2005; 44:61–98. [DOI] [PubMed] [Google Scholar]
- 8. Rhen T, Cidlowski JA. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med 2005; 353:1711–23. [DOI] [PubMed] [Google Scholar]
- 9. Faubion WA Jr, Loftus EV Jr, Harmsen WS, et al. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology 2001; 121:255–60. [DOI] [PubMed] [Google Scholar]
- 10. Lichtenstein GR, Loftus EV, Isaacs KL, et al. ACG clinical guideline: management of Crohn’s disease in adults. Am J Gastroenterol 2018; 113:481–517. [DOI] [PubMed] [Google Scholar]
- 11. Ko CW, Singh S, Feuerstein JD, et al. ; American Gastroenterological Association Institute Clinical Guidelines Committee. AGA clinical practice guidelines on the management of mild-to-moderate ulcerative colitis. Gastroenterology 2019; 156:748–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Recommendations of the Advisory Committee on Immunization Practices (ACIP): use of vaccines and immune globulins for persons with altered immunocompetence. MMWR Recomm Rep 1993; 42:1–18. [PubMed] [Google Scholar]
- 13. Dhalla IA, Mamdani MM, Simor AE, et al. Are broad-spectrum fluoroquinolones more likely to cause Clostridium difficile-associated disease? Antimicrob Agents Chemother 2006; 50:3216–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schneeweiss S, Korzenik J, Solomon DH, et al. Infliximab and other immunomodulating drugs in patients with inflammatory bowel disease and the risk of serious bacterial infections. Aliment Pharmacol Ther 2009; 30:253–64. [DOI] [PubMed] [Google Scholar]
- 15. Furuya-Kanamori L, Stone JC, Clark J, et al. Comorbidities, exposure to medications, and the risk of community-acquired Clostridium difficile infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol 2015; 36:132–41. [DOI] [PubMed] [Google Scholar]
- 16. Razik R, Rumman A, Bahreini Z, et al. Recurrence of Clostridium difficile infection in patients with inflammatory bowel disease: the RECIDIVISM study. Am J Gastroenterol 2016; 111:1141–6. [DOI] [PubMed] [Google Scholar]
- 17. Messick CA, Hammel JP, Hull T. Risk factors that predict recurrent Clostridium difficile infections in surgical patients. Am Surg 2017; 83:653–9. [PubMed] [Google Scholar]
- 18. Sokol H, Lalande V, Landman C, et al. Clostridium difficile infection in acute flares of inflammatory bowel disease: a prospective study. Dig Liver Dis 2017; 49:643–6. [DOI] [PubMed] [Google Scholar]
- 19. Singh H, Nugent Z, Yu BN, et al. Higher incidence of Clostridium difficile infection among individuals with inflammatory bowel disease. Gastroenterology 2017; 153:430–438.e2. [DOI] [PubMed] [Google Scholar]
- 20. Wojciechowski AL, Parameswaran GI, Mattappallil A, Mergenhagen KA. Corticosteroid use is associated with a reduced incidence of Clostridium difficile-associated diarrhea: a retrospective cohort study. Anaerobe 2014; 30:27–9. [DOI] [PubMed] [Google Scholar]
- 21. Scardina TL, Kang Martinez E, Balasubramanian N, et al. Evaluation of risk factors for Clostridium difficile infection in hematopoietic stem cell transplant recipients. Pharmacotherapy 2017; 37:420–8. [DOI] [PubMed] [Google Scholar]
- 22. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40:373–83. [DOI] [PubMed] [Google Scholar]
- 23. Mager DE, Lin SX, Blum RA, et al. Dose equivalency evaluation of major corticosteroids: pharmacokinetics and cell trafficking and cortisol dynamics. J Clin Pharmacol 2003; 43:1216–27. [DOI] [PubMed] [Google Scholar]
- 24. Edsbäcker S, Andersson T. Pharmacokinetics of budesonide (Entocort EC) capsules for Crohn’s disease. Clin Pharmacokinet 2004; 43:803–21. [DOI] [PubMed] [Google Scholar]
- 25. Branch-Elliman W, O’Brien W, Strymish J, et al. Association of duration and type of surgical prophylaxis with antimicrobial-associated adverse events. JAMA Surg 2019; 154:590–8. [DOI] [PMC free article] [PubMed] [Google Scholar]