Abstract
Background
Valganciclovir is the most commonly used antiviral for cytomegalovirus (CMV) prophylaxis in solid organ transplant recipients. However, there are limited clinical outcomes-supported data available to guide valganciclovir dosing in patients on hemodialysis (HD). This study aimed to assess the safety of our institution’s current dosing strategy of valganciclovir 450 mg 3 times weekly post-HD.
Methods
This was a single-center retrospective review of all adult nonkidney transplant recipients between May 2016 and June 2018. Patients with end-stage renal disease requiring HD for >28 days posttransplant receiving valganciclovir 450 mg 3 times weekly post-HD were matched with non-HD patients receiving valganciclovir prophylaxis dosed per renal function. The primary endpoints were incidence of leukopenia, neutropenia, and thrombocytopenia while on valganciclovir prophylaxis.
Results
A total of 465 nonkidney transplants were performed during the study period, with 37 patients included in the HD group who were matched to 111 control patients in the non-HD group. Liver transplant recipients comprised 84% and 72% of each group, with none being CMV D+/R−. The rates of leukopenia (51.4% vs 51.4%, P = 1.00), severe neutropenia (absolute neutrophil count <500 cells/µL, 15.8% vs 14.0%, P = .85), and thrombocytopenia (24.3% vs 20.7%, P = .64) were similar in both HD and non-HD groups. There were no cases of CMV infection while on valganciclovir prophylaxis in either group.
Conclusions
Valganciclovir 450 mg 3 times weekly was found to have similar rates of leukopenia, neutropenia, thrombocytopenia, and CMV infection in comparison to valganciclovir dosed per renal function in non-HD transplant recipients.
Keywords: cytomegalovirus, hemodialysis, prophylaxis, solid organ transplant, valganciclovir
Use of valganciclovir 450 mg 3 times weekly in nonrenal solid organ transplant recipients on hemodialysis who are not at high risk for CMV resulted in similar rates of safety outcomes compared with a matched cohort of non-hemodialysis transplant recipients.
Cytomegalovirus (CMV) is the most common viral infection after solid organ transplant (SOT) and is associated with significant morbidity and mortality [1]. The incidence of CMV infection varies by type of organ transplanted, serostatus of both donor and recipient, prophylaxis strategy used, and magnitude of immunosuppression. The 1-year incidence of CMV infection has been well described across all organs: intermediate-risk liver transplant recipients receiving valganciclovir 450 mg daily for prophylaxis for 3 months developed CMV infection within 1 year at a rate of 8% [2]; heart transplant recipients receiving valganciclovir 900 mg daily for prophylaxis for 6 months experienced CMV infection at a rate of 11% [3]; lastly, lung transplant recipients receiving valganciclovir prophylaxis for 6 to 12 months reported an incidence of CMV infection of 33% [4]. In all of these studies, valganciclovir dosing was adjusted for renal function.
Valganciclovir (VGCV) is the most commonly used antiviral for CMV prophylaxis posttransplant, although there are currently minimal data regarding dosing strategies and outcomes in patients requiring renal replacement, and even fewer data in patients with end-stage renal disease (ESRD) requiring hemodialysis (HD) [5, 6]. The package insert does not recommend use of VGCV in patients requiring HD due to the risk of hematologic toxicity [7]. Current Transplantation Society guidelines published in 2018 recommend VGCV 100 mg given post-HD; however, this recommendation is based on pharmacokinetic data rather than clinical outcomes [8]. In addition, this dosing regimen requires the use of an oral solution, which can be associated with significant cost and accessibility issues.
The current dosing strategy used for CMV prophylaxis at our institution is VGCV 450 mg administered orally 3 times weekly after HD. Although this dosing strategy allows for the use of VGCV tablets, there is concern for potential overdosing, which could lead to higher rates of adverse effects such as cytopenia. In the absence of clear clinical outcomes-supported dosing, this study aimed to assess the safety of VGCV 450 mg 3 times weekly for CMV prophylaxis in nonkidney solid organ transplant recipients on HD.
METHODS
Patient Selection
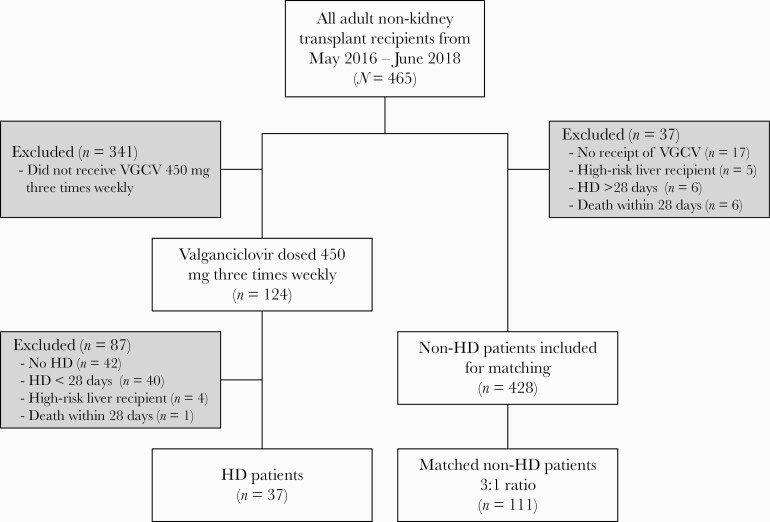
We performed a single-center retrospective review of all adult liver, heart, and lung transplant recipients between May 2016 and June 2018. Exclusion criteria were as follows: receipt of a kidney or multiorgan transplant that included a kidney, CMV high-risk liver recipients, those requiring hemodialysis for fewer than 28 days, or death within 28 days of transplant. Kidney transplant recipients were excluded based on the assumption that patients would no longer require long-term dialysis. High-risk liver recipients were excluded due to institutional protocol requiring intravenous ganciclovir for 3 months posttransplant. Cytomegalovirus high-risk lung, heart, or multiorgan recipients were included if appropriate based on other inclusion and exclusion criteria. Patients with ESRD requiring HD for >28 sequential days posttransplant (HD cohort) were identified through orders for VCGV dosed 450 mg 3 times weekly, with HD status confirmed through electronic medical record review. Propensity score matching between the HD and non-HD cohorts was performed based on a ratio of non-HD to HD at 3:1 with caliper of 1 and using the following matching criteria: age at transplant (in years), gender, transplanted organ (liver, heart, lung, and heart/liver), and recipient CMV risk (low, moderate, and high risk) [8]. The one-to-many propensity score-matching approach was used to increase the precision of statistical testing. The ratio of 3:1 was chosen because matching at a higher ratio did not significantly improve the precision of the analysis [9, 10].
Immunosuppression, Prophylaxis, and Posttransplant Monitoring
Our institution’s induction immunosuppression protocol was as follows: steroid induction in heart transplant recipients with consideration for use of basiliximab in cases of pretransplant renal dysfunction, steroid induction in liver transplant recipients, and basiliximab induction in lung transplant recipients. Lymphocyte-depleting antibodies were not routinely used for induction at our center for nonkidney transplant recipients. The initial maintenance immunosuppression regimen typically consisted of tacrolimus, mycophenolate, and prednisone for all organ types. The target mycophenolate dosing was 1500 mg twice daily in heart transplant recipients and 1000 mg twice daily in liver and lung transplant recipients.
Heart and lung transplant recipients received Aspergillus prophylaxis with azole antifungals. Liver transplant recipients received fungal prophylaxis stratified by their preoperative Model for End-Stage Liver Disease (MELD) score; Aspergillus-active agents were used in patients at highest risk, systemic Candida prophylaxis was used for those at moderate risk, and topical Candida prophylaxis was used for those at lowest risk.
Pneumocystis jirovecii prophylaxis was administered for 12 months in heart and liver transplant recipients and lifelong in lung transplant recipients. Sulfamethoxazole-trimethoprim was the preferred agent and was dosed as 1 double-strength tablet (800-160 mg) 3 times weekly for all patients. This dose was adjusted to 1 single-strength tablet (400–80 mg) 3 times weekly in patients requiring dialysis.
For CMV prophylaxis, VGCV was dosed at 450 mg twice daily for heart and lung transplant recipients with creatinine clearance (CrCl) ≥60 mL/minute, 450 mg once daily for CrCl 40–59 mL/minute, 450 mg every other day for CrCl 25–39 mL/minute, and 450 mg 3 times weekly for CrCl <25 mL/minute or receiving HD. In liver transplant recipients, VGCV was dosed at 450 mg once daily for patients with CrCl ≥40 mL/minute, 450 mg every other day for CrCl 25–39 mL/minute, and 450 mg 3 times weekly for CrCl <25 mL/minute or receiving HD. Duration of VCGV prophylaxis was 12 months in CMV moderate-risk lung recipients, lifelong in CMV high-risk lung recipients, and 3 months in heart, liver, and heart/liver recipients. Serum CMV viral load was monitored at the following intervals per institutional protocol: monthly through month 6 in liver recipients; week 1 and months 1, 3, 6, 9, and 12 in lung recipients; and weekly through week 4, then biweekly through week 12, then monthly through month 12 in heart recipients. Cytomegalovirus viral loads were also checked when clinically warranted. Ganciclovir levels were not obtained at our institution.
Safety Definitions
Leukopenia was defined as a white blood cell (WBC) count <3.5 × 103 cells/µL. Neutropenia was defined as an absolute neutrophil count (ANC) <1500 cells/µL with the following subtypes: mild (ANC 1000–1500 cells/µL), moderate (ANC 500–999 cells/µL), and severe (ANC <500 cells/µL). Thrombocytopenia was defined as a platelet (PLT) count <100 × 103 cells/µL. Laboratory data were collected in all patients at 1, 3, 6, 9, and 12 months posttransplant.
Infection Definitions
Cytomegalovirus terminology was defined in accordance with the Definitions of Cytomegalovirus Infection and Disease in Transplant Patients for Use in Clinical Trials [11]. Cytomegalovirus infection was defined as quantifiable detection (>300 copies/mL) of viral deoxyribonucleic acid in plasma via a polymerase chain reaction assay. Cytomegalovirus disease was defined according to the organ-specific criteria for CMV hepatitis, CMV gastrointestinal disease, etc.
Outcomes and Statistical Analysis
The primary endpoints were safety outcomes (ie, leukopenia, neutropenia, and thrombocytopenia) within 1 year posttransplant in patients with ESRD requiring HD receiving CMV prophylaxis with VGCV 450 mg 3 times weekly. Secondary outcomes within 1 year posttransplant included rate of CMV infection while on VGCV prophylaxis, overall rate of CMV infection (regardless of whether the patient was on VGCV prophylaxis), type of CMV infection, CMV resistance, and recurrent CMV infections within 1 year posttransplant. In addition to laboratory markers of cytopenia, administration of granulocyte colony-stimulating factors (G-CSF) and adjustments to VGCV therapy were collected. Data collection and reporting were approved by the Institutional Review Board at Houston Methodist Hospital (protocol number 0002285).
Patient characteristics were reported as frequencies and proportions for categorical variables and as median and interquartile range (IQR) for continuous variables. Differences across groups were determined by χ 2 or Fisher’s exact tests for categorical variables and Kruskal-Wallis test for continuous variables as appropriate. Linear mixed modeling was used to assess the mean change over time of laboratory results. The mean change over time of the laboratory results was also depicted by line plots. Post hoc marginal pairwise comparisons were performed to determine the adjusted means (95% confidence intervals) of changes of each continuous variable from month 1 to month 12. Missing data were assessed for missing completely at random (MCAR) and covariate-dependent missingness (CDM) using the Little’s χ 2 test [12]. All of the analyses were performed on Stata version 16.1 (StataCorp LLC, College Station, TX). A P < .05 was considered statistically significant.
RESULTS
Baseline Characteristics
A total of 465 heart (n = 85), lung (n = 135), liver (n = 238), and multiorgan transplants (n = 7) were performed at our institution during the study period. Thirty-seven patients were included in the HD group who were matched to 111 control patients in the non-HD group (Figure 1). Baseline characteristics are reported in Table 1. The non-HD group experienced a longer median duration of VGCV prophylaxis compared with the HD group (98 days vs 90 days, P = .01). CMV serostatus is reported in Table 2. The majority of patients in both groups were liver transplant recipients and were CMV immunoglobulin donor seropositive/recipient seropositive (D+/R+). Incidentally, there were no CMV high-risk (D+/R−) patients of any organ type included in either group.
Figure 1.
STROBE diagram. HD, hemodialysis; VGCV, valganciclovir.
Table 1.
Baseline Characteristics
| Characteristics | HD Patients | Non-HD Patients | P value |
|---|---|---|---|
| Total number of patients, n | 37 | 111 | |
| Male gender, n (%) | 23 (62.2) | 63 (56.8) | .56 |
| Age at time of transplant in years, median (IQR) | 63 (53, 68) | 60 (50, 66) | .38 |
| Type of transplant, n (%) | .47 | ||
| Liver | 31 (83.8) | 80 (72.1) | |
| Heart | 2 (5.4) | 11 (9.6) | |
| Lung | 4 (10.8) | 17 (14.9) | |
| Heart/Liver | 0 (0.0) | 3 (2.7) | |
| Duration of VGCV PPX in days, median (IQR) | 90 (75, 123) | 98 (90, 119) | .01 |
Abbreviations: HD, hemodialysis; IQR, interquartile range; PPX, prophylaxis; VGCV, valganciclovir.
Table 2.
CMV Serostatus
| HD Patients | Non-HD Patients | |||||||
|---|---|---|---|---|---|---|---|---|
| CMV Serostatusb | Liver (n = 31) | Heart (n = 4) | Lung (n = 2) | Liver (n = 80) | Heart (n = 17) | Lung (n = 11) | Heart/Liver (n = 3) | P valuea |
| Donor +/Recipient – | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | .61 |
| Donor +/Recipient + | 22 (71.0) | 4 (100) | 2 (100) | 60 (75.0) | 5 (29.4) | 7 (63.6) | 2 (66.7) | |
| Donor –/Recipient + | 6 (19.3) | 0 (0.0) | 0 (0.0) | 13 (16.3) | 7 (41.2) | 1 (9.1) | 1 (33.3) | |
| Donor –/Recipient – | 3 (9.7) | 0 (0.0) | 0 (0.0) | 7 (8.8) | 5 (29.4) | 3 (27.3) | 0 (0.0) | |
aOverall P value comparing HD vs. non-HD patients.
bAll data expressed as n (%).
Abbreviations: CMV, cytomegalovirus; HD, hemodialysis.
Safety
The incidence of leukopenia and thrombocytopenia across all patients on VGCV prophylaxis was 51.4% and 21.6%, respectively (Table 3). The rates of leukopenia (51.4% vs 51.4%, P = 1.00), severe neutropenia (ANC <500 cells/µL, 15.8% vs 14.0%, P = .85), and thrombocytopenia (24.3% vs 20.7%, P = .64) were similar in both HD and non-HD groups, respectively. There was also no difference in duration of either leukopenia or thrombocytopenia. There were no differences in the frequency or duration that VGCV prophylaxis was held for leukopenia or thrombocytopenia between groups. A small proportion of patients received G-CSF support; however, there was no difference observed between groups (13.5% in HD group vs 11.7% in non-HD group, P = .77).
Table 3.
Outcomes
| Endpoints | Total (n = 148) | HD Patients(n = 37) | Non-HD Patients(n = 111) | P value |
|---|---|---|---|---|
| Leukopenia on VGCV prophylaxis, n (%) | 76 (51.4) | 19 (51.4) | 57 (51.4) | 1.00 |
| ANC 1000 –1500 cells/µL, n (%) | 16 (21.1) | 4 (21.1) | 12 (21.1) | 0.99 |
| ANC 500 – 999 cells/µL, n (%) | 14 (18.4) | 4 (21.1) | 10 (17.5) | 0.79 |
| ANC < 500 cells/µL, n (%) | 11 (14.5) | 3 (15.8) | 8 (14.0) | 0.85 |
| Thrombocytopenia on VGCV prophylaxis, n (%) | 32 (21.6) | 9 (24.3) | 23 (20.7) | 0.64 |
| Leukopenia or thrombocytopenia on VGCV prophylaxis, n (%) | 82 (55.4) | 22 (59.5) | 60 (54.1) | 0.57 |
| CMV infection on VGCV PPX, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA |
| Overall CMV infection at 1 year, n (%) | 23 (15.5) | 6 (16.2) | 17 (15.3) | 0.90 |
| Type of CMV infection, n (% total infections) | ||||
| CMV viremia | 19 (82.6) | 5 (83.3) | 14 (82.4) | 0.96 |
| CMV syndrome | 3 (13.0) | 1 (16.7) | 2 (11.8) | 0.76 |
| CMV disease | 1 (4.3) | 0 (0.0) | 1 (5.9) | 0.54 |
| Time to infection post-PPX in days, median (IQR) | 47 (28, 78) | 41 (24, 61) | 52 (30, 78) | 0.33 |
| Duration of CMV infection in days, median (IQR) | 23 (13, 42) | 19 (7, 21) | 29 (16, 42) | 0.18 |
| Peak CMV viral load in copies/mL, median (IQR) | 1326 (564, 10667) | 3283 (797, 10667) | 835 (564, 2077) | 0.53 |
| VGCV held for leukopenia or thrombocytopenia, n (%) | 12 (8.1) | 5 (13.5) | 7 (6.3) | 0.16 |
| Number of days VGCV was held, median (IQR) | 11 (5, 20) | 6 (3, 10) | 14 (5, 45) | 0.12 |
| Receipt of G-CSF on VGCV PPX, n (%) | 18 (12.2) | 5 (13.5) | 13 (11.7) | 0.77 |
| Number of doses of G-CSF given on VGCV PPX, median (IQR) | 2 (1, 3) | 2 (1, 3) | 2 (1, 3) | 0.84 |
Abbreviations: ANC, absolute neutrophil count; CMV, cytomegalovirus; G-CSF, granulocyte colony stimulating factor; HD, hemodialysis; IQR, interquartile range; NA, not applicable; PPX, prophylaxis; VGCV, valganciclovir.
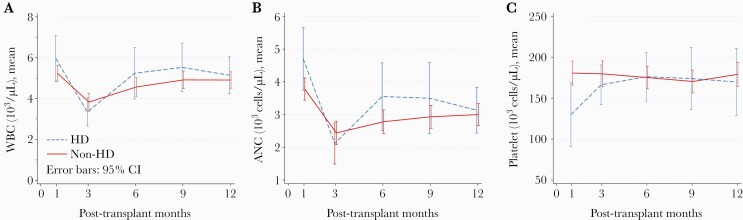
Laboratory data at 1, 3, 6, 9, and 12 months posttransplant are presented in Table 4. Median estimated glomerular filtration rate in the non-HD cohort were 55 mL/minute (IQR, 40–80), 67 mL/minute (IQR, 53–89), 69 mL/minute (IQR, 55–82), 61 mL/minute (IQR, 49–79), and 67 mL/minute (IQR, 48–80) for months 1, 3, 6, 9, and 12, respectively. Platelet counts were found to be significantly lower in the HD group at 1 month posttransplant (96.5 × 103 cells/µL vs 174.0 × 103 cells/µL, P < .001). The mean change over time in WBC count, ANC, and PLT count in both groups is illustrated in Figure 2. There were no significant differences in these parameters between the HD and non-HD groups. Absolute neutrophil count declined significantly in both groups between months 1 and 12 (−1539 cells/µL in the HD group vs −753 cells/µL in the non-HD group, P = .10).
Table 4.
Laboratory Data at Specified Time Points Post-Transplanta
| Laboratory Parameters | HD Patients (n = 37) | Non-HD Patients (n = 111) | p-valueb |
|---|---|---|---|
| 1 Month | |||
| Lowest WBC count | 4.9 (3.6, 7.5) | 5.0 (3.9, 6.5) | 0.72 |
| ANC | 3661 (2461, 6710) | 3539 (2650, 4565) | 0.19 |
| Lowest PLT count | 97 (57, 154) | 174 (115, 240) | <0.001 |
| Highest SCr | 3.5 (2.8, 5.1) | 1.5 (1.1, 2.0) | <0.001 |
| 3 Months | |||
| Lowest WBC count | 3.0 (2.1, 3.8) | 3.2 (2.4, 4.8) | 0.20 |
| ANC | 1629 (1320, 2380) | 2003 (1130, 3145) | 0.20 |
| Lowest PLT count | 154 (112, 216) | 177 (122, 214) | 0.36 |
| Highest SCr | 2.9 (2.1, 5.2) | 1.2 (1.0, 1.6) | <0.001 |
| 6 Months | |||
| Lowest WBC count | 4.4 (3.3, 6.1) | 4.0 (3.0, 5.8) | 0.32 |
| ANC | 2712 (2163, 4635) | 2442 (1585, 3600) | 0.12 |
| Lowest PLT count | 153 (134, 200) | 173 (127, 217) | 0.78 |
| Highest SCr | 2.2 (1.6, 3.2) | 1.1 (1.0, 1.5) | <0.001 |
| 9 Months | |||
| Lowest WBC count | 4.8 (3.8, 6.9) | 4.5 (3.4, 6.3) | 0.38 |
| ANC | 2730 (1900, 3930) | 2670 (1742, 3840) | 0.57 |
| Lowest PLT count | 164 (121, 202) | 170 (111, 222) | 0.82 |
| Highest SCr | 2.2 (1.5, 3.6) | 1.2 (1.0, 1.6) | <0.001 |
| 12 Months | |||
| Lowest WBC count | 4.6 (3.1, 6.3) | 4.4 (3.4, 6.2) | 0.80 |
| ANC | 2582 (2012, 3538) | 2679 (1768, 3676) | 0.86 |
| Lowest PLT count | 152 (106, 224) | 170 (141, 208) | 0.31 |
| Highest SCr | 2.0 (1.5, 3.9) | 1.2 (1.0, 1.6) | <0.001 |
aAll data are expressed in median (IQR).
bObtained from the Kruskal Wallis test.
Abbreviations: ANC, absolute neutrophil count (cells/µL); HD, hemodialysis; IQR, interquartile range; PLT, platelets (103 cells/µL); SCr, serum creatinine (mg/dL); WBC, white blood cell (103 cells/µL).
Figure 2.
Mean change over time in hematologic parameters from months 1–12. (A) Mean change in white blood cell count (103 cells/μL). Hemodialysis (HD): −0.83 (−1.73 to .08), P = .07. Non-HD: −0.36 (−0.87 to 0.16), P = .17. Non-HD vs HD: P = .31. (B) Mean change in absolute neutrophil count ([ANC] cells/μL). HD: −1540 (−2324 to −755), P < .001. Non-HD: −753 (−1201 to −304), P = .001. Non-HD vs HD: P = .11. (C): Mean change in platelet count (103 cells/μL). HD: 39 (11–66), P = .01. Non-HD: −5 (−20 to 11), P = .58. Non-HD vs HD: P = .24. CI, confidence interval; WBC, white blood cell count.
Efficacy
Zero patients in either group developed CMV infection while on VGCV prophylaxis (Table 3). The overall rate of CMV infection at 1 year was similar (16.2% in HD group vs 15.3% in non-HD group, P = .90). In both groups, the majority of CMV infections was classified as CMV viremia. The 1 patient that developed CMV disease was a liver transplant recipient in the non-HD group diagnosed with CMV hepatitis. There were no resistant or recurrent CMV infections observed in either group.
DISCUSSION
Valganciclovir 450 mg 3 times weekly post-HD for CMV prophylaxis was associated with similar rates of leukopenia, neutropenia, and thrombocytopenia in comparison to VGCV dosed per renal function in non-HD patients.
The overall rate of leukopenia on VGCV prophylaxis observed in this study across both groups was higher than the approximate 20%–30% incidence reported in previous studies, primarily in kidney transplant recipients [13, 14]. This could be explained by the large number of liver transplant recipients included in this study, who often develop leukopenia as a result of end-stage liver disease. In general, this leukopenia persists for a prolonged period of time posttransplant [15]. The incidence of neutropenia on VGCV prophylaxis, which is more clinically relevant than leukopenia alone, was approximately 35% lower across both groups than the incidence of leukopenia. The 14.5% total incidence of severe neutropenia observed in this study is higher than the 7.8% reported in a previous study of kidney transplant recipients [13]. This difference may also be attributable to the large number of liver transplant recipients included in this study.
Valganciclovir dosed 450 mg 3 times weekly in solid organ transplant recipients on HD was similar with regard to prevention of CMV infection as valganciclovir dosed per renal function in non-HD transplant recipients. However, because our cohort did not include any D+/R− recipients, efficacy of this dosing regimen cannot be directly inferred in a high-risk population. The overall rate of CMV infection observed at 1 year in this study is comparable to that reported in the literature [2, 4]. Another small retrospective study evaluating VGCV 450 mg twice or 3 times weekly in nonkidney transplant recipients on HD reported a 1-year CMV viremia rate of 7.7%, although it was not reported whether any of those cases occurred specifically while on VGCV prophylaxis [16]. There are otherwise limited available data evaluating clinical outcomes associated with VGCV use in patients on HD.
The patients in the HD group had a significantly lower PLT count at 1 month than the non-HD group, although the etiology and clinical significance of this difference is unclear. The HD group experienced a statistically significant mean increase in PLT count of 39 × 103 cells/µL between months 1 and 12, which is a relatively small change and may lack clinical significance. The significant decline in ANC observed in both groups between months 1 and 12 is of unclear clinical significance, although the decreasing maintenance steroid doses after transplant could explain this phenomenon because steroid-induced leukocytosis is predominantly driven by an increase in neutrophils [17]. In addition to changes in maintenance steroid doses, medications such as mycophenolate and sulfamethoxazole-trimethoprim could also contribute to this decline in ANC over time.
Additional information may be gained from utilization of ganciclovir levels to determine the ganciclovir exposure associated with various VGCV doses in patients requiring renal replacement therapy. A small study published in 2002 evaluated the pharmacokinetics of ganciclovir and VGCV in patients with renal impairment and included 6 patients with ESRD on long-term HD. After a single dose of VGCV 900 mg, the ganciclovir mean area under the concentration-time curve from time 0 extrapolated to infinity (area under the curve [AUC]0-∞) was 407 ± 83 hour × µg/mL in the patients on HD, approximately 15 times higher than the 28.1 ± 5.8 hour × µg/mL that was observed in the patients with a baseline creatinine clearance >70 mL/minute. The authors suggested that a dose-adjustment scheme can be derived by reducing the VGCV dose in relation to the increased AUC in renal impairment [18].
A prospective pharmacokinetic study of 10 SOT patients receiving continuous veno-venous hemodialysis was conducted to determine whether VGCV 450 mg every 24 hours could achieve ganciclovir trough concentrationsr ≥0.6 mcg/mL, which has been suggested as an efficacy target for CMV prophylaxis [19]. Valganciclovir 450 mg every 24 hours was found to produce ganciclovir troughs ≥0.6 mcg/mL in 80% of patients, with a median trough of 2.27 mcg/mL. Neutropenia, defined as ANC <1000 cells/µL, did not occur during the study. Significant thrombocytopenia, defined as PLT count <50 × 103 cells/µL, occurred in 60% of patients, 83% of which were liver transplant recipients, which could partially explain this finding. Future studies of VGCV 450 mg 3 times weekly in SOT patients on HD may benefit from inclusion of pharmacokinetic data to help guide conclusions regarding the balance of safety and efficacy with this dosing strategy.
This study has several limitations, including those inherent to retrospective designs. The actual incidence of adverse effects may be higher than observed due to laboratory data being collected only at 1, 3, 6, 9, and 12 months posttransplant. However, the assessment for missing laboratory data using the Little’s χ 2 test for MCAR and CDM had nonsignificant P values (.73 and .69, respectively), which suggest that the missing values could be completely at random and do not influence the outcome.
The lack of CMV D+/R− patients in this study limits applicability of these results to the high-risk population. In addition, the large number of liver transplant recipients included in this study may potentially limit generalizability to other organ types. Another limitation is that data regarding the use of other medications that may affect hematologic parameters, such as mycophenolate and sulfamethoxazole-trimethoprim, were not collected; therefore, the occurrence of leukopenia and thrombocytopenia cannot be solely attributed to VGCV. Future studies would benefit from inclusion of data regarding other medications, including any dose adjustments made for leukopenia or thrombocytopenia. Lastly, one notable limitation is the small sample size, which may not rule out the possibility of Type II error. However, given the absence of clear clinical outcomes-supported literature on the VGCV dosing for CMV prophylaxis in nonkidney solid organ transplant recipients on HD, findings from our longitudinal follow-up would be useful for future larger studies.
Future studies directly comparing the VGCV guideline-recommended dose of 100 mg 3 times weekly to 450 mg 3 times weekly in the HD population would allow us to more definitively determine whether patients receiving 450 mg 3 times weekly actually experience higher rates of adverse hematologic effects and assess the true efficacy of a prophylaxis regimen based upon pharmacokinetic simulations versus a more practical regimen. Valganciclovir 450 mg 3 times weekly allows for use of oral tablets and is a more practical regimen that is often less costly for patients than the oral solution, which would be required to administer the guideline-recommended dose of 100 mg 3 times weekly.
CONCLUSIONS
In conclusion, valganciclovir 450 mg 3 times weekly in solid organ transplant recipients on HD exhibited similar safety and efficacy outcomes to a comparable cohort of non-HD transplant recipients. Although the rate of leukopenia on VGCV prophylaxis was relatively high in this study compared to that observed in previous studies, the rates of leukopenia, neutropenia, and thrombocytopenia were similar between groups, and this is potentially explained by the high number of liver transplant recipients included. Further prospective research is required to definitively identify the optimal dose of valganciclovir in the solid organ, nonrenal transplant HD population.
Acknowledgments
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Balfour HH Jr. Cytomegalovirus: the troll of transplantation. Arch Intern Med 1979; 139:279–80. [DOI] [PubMed] [Google Scholar]
- 2. Khan S, Sullivan T, Ali M, et al. Low-dose valganciclovir for cytomegalovirus prophylaxis in intermediate-risk liver transplantation recipients. Liver Transpl 2018; 24:616–22. [DOI] [PubMed] [Google Scholar]
- 3. Doesch AO, Repp J, Hofmann N, et al. Effects of oral valganciclovir prophylaxis for cytomegalovirus infection in heart transplant patients. Drug Des Devel Ther 2012; 6:289–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hammond SP, Martin ST, Roberts K, et al. Cytomegalovirus disease in lung transplantation: impact of recipient seropositivity and duration of antiviral prophylaxis. Transpl Infect Dis 2013; 15:163–70. [DOI] [PubMed] [Google Scholar]
- 5. Czock D, Scholle C, Rasche FM, et al. Pharmacokinetics of valganciclovir and ganciclovir in renal impairment. Clin Pharmacol Ther 2002; 72:142–50. [DOI] [PubMed] [Google Scholar]
- 6. Wiltshire H, Paya CV, Pescovitz MD, et al. ; Valganciclovir Solid Organ Transplant Study Group. Pharmacodynamics of oral ganciclovir and valganciclovir in solid organ transplant recipients. Transplantation 2005; 79:1477–83. [DOI] [PubMed] [Google Scholar]
- 7. VALCYTE [package insert]. Nutley, NJ: Roche Laboratories Inc.; 2001. [Google Scholar]
- 8. Kotton CN, Kumar D, Caliendo AM, et al. ; The Transplantation Society International CMV Consensus Group. The Third International Consensus Guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation 2018; 102:900–31. [DOI] [PubMed] [Google Scholar]
- 9. Rassen JA, Shelat AA, Myers J, et al. One-to-many propensity score matching in cohort studies. Pharmacoepidemiol Drug Saf 2012; 21(Suppl 2):69–80. [DOI] [PubMed] [Google Scholar]
- 10. Kang M, Choi S, Koh I. The effect of increasing control-to-case ratio on statistical power in a simulated case-control snp association study. Genomics Inf 2009; 7:148–51. [Google Scholar]
- 11. Ljungman P, Boeckh M, Hirsch HH, et al. ; Disease Definitions Working Group of the Cytomegalovirus Drug Development Forum. Definitions of cytomegalovirus infection and disease in transplant patients for use in clinical trials. Clin Infect Dis 2017; 64:87–91. [DOI] [PubMed] [Google Scholar]
- 12. Little RJA. A test of missing completely at random for multivariate data with missing values. J Am Stat Assoc 1988; 83:1198–202. [Google Scholar]
- 13. Brum S, Nolasco F, Sousa J, et al. Leukopenia in kidney transplant patients with the association of valganciclovir and mycophenolate mofetil. Transplant Proc 2008; 40:752–4. [DOI] [PubMed] [Google Scholar]
- 14. Liang X, Famure O, Li Y, Kim SJ. Incidence and risk factors for leukopenia in kidney transplant recipients receiving valganciclovir for cytomegalovirus prophylaxis. Prog Transplant 2018; 28:124–33. [DOI] [PubMed] [Google Scholar]
- 15. Alraddadi B, Nierenberg NE, Price LL, et al. Characteristics and outcomes of neutropenia after orthotopic liver transplantation. Liver Transpl 2016; 22:217–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang U, Yang A, Dong M, Busque S. Safety and effectiveness of valganciclovir for cytomegalovirus prophylaxis in solid organ transplant patients on hemodialysis [abstract]. Am J Transplant 2013; 13. [Google Scholar]
- 17. Shoenfeld Y, Gurewich Y, Gallant LA, Pinkhas J. Prednisone-induced leukocytosis. Influence of dosage, method and duration of administration on the degree of leukocytosis. Am J Med 1981; 71:773–8. [DOI] [PubMed] [Google Scholar]
- 18. Czock D, Scholle C, Rasche FM, et al. Pharmacokinetics of valganciclovir and ganciclovir in renal impairment. Clin Pharmacol Ther 2002; 72:142–50. [DOI] [PubMed] [Google Scholar]
- 19. Jarrell AS, Crow JR, Strout SE, et al. Valganciclovir dosing for cytomegalovirus prophylaxis in solid-organ transplant recipients on continuous veno-venous hemodialysis. Clin Infect Dis 202173:101–6. doi: 10.1093/cid/ciaa537. [DOI] [PubMed] [Google Scholar]