Abstract
PCR-restriction fragment length polymorphism analysis (PRA) of the hsp65 gene present in all mycobacteria was used in the present investigation to characterize Mycobacterium leprae. Bacilli were extracted and purified from different organs from experimentally infected armadillos and nude mice (Swiss mice of nu/nu origin). A total of 15 samples were assayed in duplicate, and the results were compared with those obtained for a total of 147 cultivable mycobacteria representing 34 species. Irrespective of its origin or viability, M. leprae strains from all the samples were uniformly characterized by two fragments of 315 and 135 bp upon BstEII digestion and two fragments of 265 and 130 bp upon HaeIII digestion. PRA is a relatively simple method and permits the conclusive identification of M. leprae to the species level.
PCR-restriction fragment length polymorphism analysis (PRA), which relies on the amplification of a 439-bp portion of the hsp65 gene present in all mycobacteria (5, 9, 10), followed by two distinct digestions of the PCR product, now offers a rapid and easy alternative that permits the rapid identification of mycobacteria without the need for specialized equipment (2, 12, 13). Recently, we have proposed a modified PRA algorithm with characteristic BstEII and HaeIII fragment lengths as a means for the rapid identification of a total of 34 mycobacterial species (2). In the present investigation, we attempted to use the same methodology to investigate if it was possible to conclusively identify Mycobacterium leprae, which is nonidentifiable by routine methodologies (11) and which remains the only noncultivable mycobacterial species.
The M. leprae bacteria were extracted and purified from lepromas, livers, and spleens from experimentally infected armadillos (five animals) and footpads, lymph nodes, livers, and spleens from Swiss nu/nu mice (five animals) as reported earlier (3, 7). The infected armadillo and nude mouse tissues were kindly provided by Y. Robin, Pasteur Institute of French Guyana, and C. C. Guelpa-Lauras, Medical Faculty of Pitié-Salpêtrière Hospital, Paris, France, respectively. Briefly, the infected tissues were manually ground with a mortar and pestle in the presence of sterilized sand. The mixture was centrifuged at 800 × g and 4°C to remove the larger fragments of tissue, which were treated as described above two to three times to further extract the bacteria. The pooled supernantants were then centrifuged successively at 800 and 3,000 × g to remove the sand plus tissue debris and the larger bacterial clumps, respectively. The bacilli from the supernatant obtained after centrifugation at 3,000 × g were recovered by centrifugation at 10,000 × g and 4°C, thoroughly washed to remove the tissue debris, treated with 4% (vol/vol) H2SO4 for 10 min at room temperature, neutralized with NaOH, and washed three times. The purity of the bacteria at this step was controlled by Ziehl-Neelsen staining and electron microscopy, and the absence of any microbiological contamination was confirmed by plating the bacterial suspension on the usual growth media (7). The number of bacteria was determined by counting the bacteria in 10 fields of 0.02 mm2 and obtaining an average, and the proportion of solid-staining bacteria, expressed as a morphological index, was determined as reported previously (14). The purified bacteria were also characterized by the presence of tuberculostearic acid and phenolic glycolipid-1, as described earlier (7). The bacteria obtained were suspended in small aliquots and were frozen at −80°C. For some experiments, the bacterial suspensions were irradiated with gamma radiation at 106 rads.
A total of 15 M. leprae samples were assayed in duplicate, and the results that were obtained (Table 1) were compared with those obtained for a total of 147 cultivable mycobacteria comprising 43 reference strains and 104 clinical isolates representing 34 species for which an algorithm has recently been described (2). Bacterial DNA was prepared by a microbead method as reported earlier (2), and 5 μl of the supernatant containing the crude DNA extract was used for PCR with the primers Tb11 (5′-ACCAACGATGGTGTGTCCAT) and Tb12 (5′-CTTGTCGAACCGC-ATACCCT) by the procedure described by Telenti et al. (13). The PCR amplified a 439-bp fragment of the gene encoding for the 65-kDa heat shock protein. The amplification product was subjected to BstEII (Promega, Madison, Wis.) and HaeIII (BioLabs, Inc., Beverly, Mass.) enzyme digestions, and the fragments were electrophoresed on a 4% (wt/vol) NuSieve 3:1 agarose gel (FMC Bioproducts, Rockland, Maine) as reported previously (2). A 100-bp ladder (Pharmacia Biotech, Uppsala, Sweden) served as an external molecular size marker and was added after every sixth lane of the gel to reduce migration-related errors. The fragments were visualized by ethidium bromide staining to observe the fluorescence, videotaped by using a camera and Gel-Analyst software (Gel-Analyst and Video-copy; Bioprobe Systems, Montreuil, France), and stored in the TIFF format. The Taxotron software package (Institut Pasteur, Paris, France) coupled to a Power MacIntosh 7200/90 (Apple Computers, Cupertino, Calif.) was used to convert the migration file into molecular mass data file by the Schaeffer and Sederoff method reported previously (2). As indicated previously (13), restriction fragments shorter than 60 bp were not taken into account because they are suspected to be primer or primer-dimer bands. The sensitivity of the PRA method for M. leprae detection was assayed by adjusting the bacterial suspensions to an optical density at 650 nm (OD650) of 0.15 (about 1 mg/ml) and performing serial dilutions that were subjected to both acid-fast microscopy and PRA in parallel. Because M. leprae is a noncultivable species, to have an idea of the sensitivity of the PRA method in terms of viable bacterial counts, similar experiments were also performed in parallel with a clinical isolate (isolate 97096) of Mycobacterium tuberculosis.
TABLE 1.
Characterization of M. leprae obtained from experimentally infected armadillos and nude mice
| Origin | Batch (organ) | No. of globia | No. of bacilli/g of tissue | Presence of:
|
PRA fragment size (bp) after digestion withb:
|
||
|---|---|---|---|---|---|---|---|
| Phenolic glycolipid | Tuberculostearate | BstEII | HaeIII | ||||
| Armadillo | AV (leproma)c | +5 | 4.0 × 1010 | + | + | 315/135 | 265/130 |
| Armadillo | AV (liver) | +5 | 9.0 × 1010 | + | + | 315/135 | 265/130 |
| Armadillo | AV (spleen)c | +5 | 7.0 × 1010 | + | + | 315/135 | 265/130 |
| Armadillo | AP(leproma) | +5 | 1.1 × 1011 | + | + | 315/135 | 265/130 |
| Armadillo | AP (liver)c | +2 | 1.6 × 1010 | + | + | 315/135 | 265/130 |
| Armadillo | AP (spleen) | +5 | 4.7 × 1010 | + | + | 315/135 | 265/130 |
| Armadillo | AJ (leproma) | +1 | 7.7 × 109 | + | + | 315/135 | 265/130 |
| Armadillo | AU (spleen)c | +4 | 5.2 × 1010 | + | + | 315/135 | 265/130 |
| Armadillo | A10 (leproma) | +4 | 3.6 × 1011 | + | + | 315/135 | 265/130 |
| Armadillo | A10 (liver) | 0 | 4.0 × 107 | − | − | 315/135 | 265/130 |
| Armadillo | A10 (spleen) | +3 | 2.7 × 1010 | + | + | 315/135 | 265/130 |
| Nude miced | L1 (foot pad)ce | +5 | 3.2 × 1010 | NDf | ND | 315/135 | 265/130 |
| Nude mice | L2 (lymph node) | +1 | 8.7 × 107 | ND | ND | 315/135 | 265/130 |
| Nude mice | L3 (liver) | 0 | 4.7 × 106 | ND | ND | 315/135 | 265/130 |
| Nude mice | L4 (spleen) | 0 | 4.0 × 103 | ND | ND | 315/135 | 265/130 |
The numbers of globi were counted in a smear of 100 mm2 (made with 5 μl of bacterial suspension) on a field of 0.02 mm2.
The values for the PRA fragments are mean values that varied within a range of ±5 bp.
These samples were also used in parallel after being irradiated with 106 rads. Similar PRA results were found for samples containing viable or irradiated organisms.
For nude mice, the infected organs from five animals inoculated with bacilli obtained from a single patient were pooled.
The sample was also run in parallel as a partially purified bacterial preparation that contained significant amounts of host tissues, and PRA results were similar to those obtained for the purified bacterial suspension.
ND, not done.
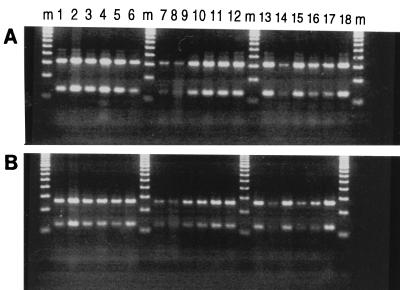
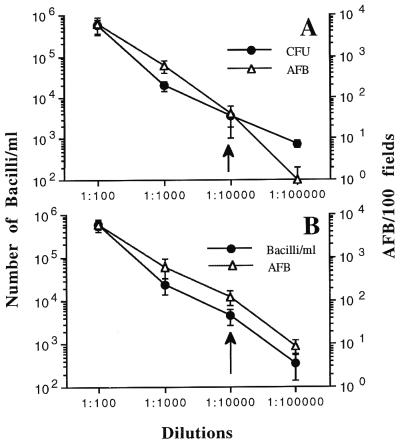
As summarized in Fig. 1 and Table 1, all of the M. leprae suspensions, irrespective of their origins, were uniformly characterized by two fragments of 315 and 135 bp upon BstEII digestion and two fragments of 265 and 130 bp upon HaeIII digestion. When subjected to PRA analysis, gamma-irradiated M. leprae (five samples; Table 1) gave similar results, indicating that this method is not affected by bacterial viability. Similarly, the limit of sensitivity of PRA for the detection of M. leprae was nearly identical to that for the detection of M. tuberculosis (Fig. 2); all the samples initially adjusted to contain 1 mg of bacteria/ml (OD650 of 0.15) gave a positive PRA result up to a dilution of 10−4, which corresponded to about (3.5 ± 2.5) × 103 CFU/ml for M. tuberculosis (about 4 ± 2 acid-fast bacilli [AFB]/10 fields) and (4.70 ± 1.95) × 103 bacilli/ml (about 12.5 ± 4.5 AFB/10 fields) for M. leprae. Because 100 μl of bacterial suspension mixed with an equal amount of TE (Tris-EDTA) buffer was used for DNA extraction and only 5 μl of this suspension was finally used in the amplification reaction (2), it may be presumed that the minimal number of bacteria needed for a positive PRA result under our experimental conditions was about 8 to 13 bacilli for both M. tuberculosis and M. leprae.
FIG. 1.
PRA gel after BstEII (A) and HaeIII (B) digestion of a 439-bp DNA fragment amplified from M. leprae bacilli isolated from various samples. Lanes m, molecular weight marker; lanes 1 to 5, samples from armadillos AV, AP, AJ, AU, and A10, respectively, calibrated to a 1:100 dilution; lanes 6 to 8, samples from nude mouse L1 calibrated to 1:100, 1:1,000, and 1:10,000 dilutions, respectively; lanes 9 and 10, a sample from nude mouse L1 that was purified to remove all host tissues and calibrated to a 1:100 dilution compared to the dilution of the same sample run in parallel as a partially purified preparation containing a significant amount of host tissues; lanes 11 to 13, samples from armadillos AU, AV, and AP, respectively, that were calibrated to 1:100 dilutions and that were gamma irradiated with 106 rads; lanes 14 and 15, a gamma-irradiated preparation from nude mouse L2 at 1:1,000 and 1:100 dilutions, respectively; lanes 16 to 18, gamma-irradiated preparations from nude mice L4, L3, and L1, respectively, at a 1:100 dilutions. See the text and Table 1 for details.
FIG. 2.
Sensitivity of PRA method for M. tuberculosis (A) and M. leprae (B) detection. PRA was performed with bacterial suspensions that were initially adjusted to an OD650 of 0.15 (about 1 mg/ml) and that were serially diluted 10-fold. The serially diluted suspensions were subjected to both acid-fast microscopy and PRA in parallel. The numbers of CFU of M. tuberculosis per milliliter were determined by plating the suspensions on 7H11 agar medium and counting the bacterial colonies after 21 days of incubation at 37°C. Due to the inability of M. leprae to grow in vitro, the number of bacilli per milliliter for various 10-fold dilutions was estimated by measuring the bacterial count in the initial sample by acid-fast microscopy, as indicated in the text and Table 1, and then by assuming that the actual number of bacilli in each of the dilutions was successively decreased 10-fold.
Because the armadillos were inoculated with bacilli from patients of different geographical origins (Africa, South America, and Europe), the identical restriction profiles for M. leprae from all samples tested in this investigation suggest that M. leprae is characterized by a specific PRA profile with bands of 315 and 135 bp upon BstEII digestion and 265 and 130 bp upon HaeIII digestion, which is in agreement with the published sequence of the M. leprae 65-kDa antigen (5). When these results were compared with those obtained for a total of 147 cultivable mycobacteria that included 34 mycobacterial species studied recently by Devallois et al. (2), the PRA profile of M. leprae upon BstEII digestion matched that for the group that at present comprises only two other species, namely, Mycobacterium haemophilum and Mycobacterium chelonaeI. However, on the basis of the results obtained by HaeIII digestion, M. leprae (fragments of 265 and 130 bp) was unequivocally discriminated from both M. chelonaeI (which has a single fragment of 210 bp) and M. haemophilum (which has two fragments of 175 and 115 bp). It should also be emphasized that the M. leprae-specific PRA pattern was easily detected in five parallel samples that were exposed to 106 rads of gamma irradiation, as well as in an M. leprae-infected sample from the footpads of nude mice that was only partially purified and that contained significant amounts of host tissue (Table 1; Fig. 1). Hence, we conclude that the primers Tb11 and Tb12 used in this investigation selectively amplified the 439-bp portion of the mycobacterial hsp65 gene, irrespective of M. leprae viability, and that the presence of host tissue did not significantly interfere with the PRA results. This information may be important in a clinical setting when this methodology is used for the conclusive identification of M. leprae.
M. leprae is a noncultivable mycobacterium, and consequently, the routine diagnosis of leprosy is still based on the demonstration of at least two of the following observations: a characteristic skin lesion, loss of sensation, a thickened nerve, or the presence of AFB in smears of skin lesions (11). Considering that the results of examinations such as smears and biopsy may be erroneous, Talhari (11) recently concluded that, “employing diagnostic tools that are currently available, it is nearly impossible to state with certainty whether or not the patient has leprosy, and one is faced with a difficult choice—to treat for leprosy without a definitive diagnosis, or to follow the course of the patient for a number of months while withholding treatment; neither is a very satisfactory alternative.” In this context, the rapid diagnosis of leprosy by molecular methods has found a renewed interest among various scientific groups, and recently described methods include a variety of techniques such as amplification of M. leprae-specific repetitive sequences (15), in situ hybridization (1), nested-primer gene amplification of a 347-bp product from a bacterial genomic library (6), amplification of a 360-bp fragment of the 18-kDa protein gene (8), or reverse transcription-PCR targeting the 16S rRNA of M. leprae (4).
In the context of the information presented above, it was interesting that the PRA methodology, which is easily applicable in a clinical microbiology setting, was able to identify a variety of mycobacteria in a single experiment (2, 17, 18). On the basis of the results of the present study, which determined the PRA profiles of M. leprae from different geographical areas inoculated into two experimental hosts (a total of 15 M. leprae-infected samples were tested), we suggest that the methodology that we used is capable of identifying M. leprae from any source or geographical origin by uniformly providing fragments of 315 and 135 bp upon BstEII digestion and 265 and 130 bp upon HaeIII digestion. We conclude that PRA is particularly useful for the positive identification of M. leprae, which still remains the only noncultivable mycobacterium. Finally, the unique PRA profile obtained for all M. leprae-infected samples tends to confirm the genetic homogeneity of M. leprae species.
Acknowledgments
We are grateful to the “Délégation Générale au Réseau International des Instituts Pasteur et Instituts Associés,” Institut Pasteur, Paris, France, and the Fondation Française Raoul Follereau, Paris, France, for financial support.
REFERENCES
- 1.Arnoldi, J., C. Schluter, M. Duchrow, L. Hubner, M. Ernst, A. Teske, H. D. Flad, J. Gerdes, and E. C. Böttger. Species-specific assessment of Mycobacterium leprae in skin biopsies by in situ hybridization and polymerase chain reaction. Lab. Invest. 66:618–623. [PubMed]
- 2.Devallois A, Goh K S, Rastogi N. Rapid identification of mycobacteria to species level by PCR-restriction fragment length polymorphism analysis of the hsp65 gene and proposition of an algorithm to differentiate 34 mycobacterial species. J Clin Microbiol. 1997;35:2969–2973. doi: 10.1128/jcm.35.11.2969-2973.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fréhel C, Rastogi N. Mycobacterium leprae surface components intervene in the early phagosome-lysosome fusion inhibition event. Infect Immun. 1987;55:2916–2921. doi: 10.1128/iai.55.12.2916-2921.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurabachew M, Wondimu A, Ryon J J. Reverse transcriptase-PCR detection of Mycobacterium leprae in clinical specimens. J Clin Microbiol. 1998;36:1352–1356. doi: 10.1128/jcm.36.5.1352-1356.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehra V, Sweetser D, Young R A. Efficient mapping of protein antigenic determinants. Proc Natl Acad Sci USA. 1986;83:7013–7017. doi: 10.1073/pnas.83.18.7013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plikaytis B B, Gelber R H, Shinnick T M. Rapid and sensitive detection of Mycobacterium leprae using a nested-primer gene amplification assay. J Clin Microbiol. 1990;28:1913–1917. doi: 10.1128/jcm.28.9.1913-1917.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rastogi N, Cadou S, Hellio R. Differential handling of bacterial antigens in macrophages infected with Mycobacterium leprae as studied by immunogold labelling of ultrathin sections. Int J Lepr. 1991;59:278–291. [PubMed] [Google Scholar]
- 8.Scollard D M, Gillis T P, Williams D L. Polymerase chain reaction assay for the detection and identification of Mycobacterium leprae in patients in the United States. Am J Clin Pathol. 1998;109:642–646. doi: 10.1093/ajcp/109.5.642. [DOI] [PubMed] [Google Scholar]
- 9.Shinnick T M. The 65-kilodalton antigen of Mycobacterium tuberculosis. J Bacteriol. 1987;169:1080–1088. doi: 10.1128/jb.169.3.1080-1088.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shinnick T M, Vodkin M H, Williams J C. The Mycobacterium tuberculosis 65-kilodalton antigen is a heat shock protein which corresponds to common antigen and to the Eschericha coli GroEL protein. Infect Immun. 1988;56:446–451. doi: 10.1128/iai.56.2.446-451.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talhari S. Diagnosis, classification and prognosis. Int J Lepr Other Mycobact Dis. 1996;64(Suppl. 1):S13–S15. [PubMed] [Google Scholar]
- 12.Taylor T B, Patterson C, Hale Y, Safranek W W. Routine use of PCR-restriction fragment length polymorphism analysis for identification of mycobacteria growing in liquid media. J Clin Microbiol. 1997;35:79–85. doi: 10.1128/jcm.35.1.79-85.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Telenti A, Marchesi F, Balz M, Bally F, Böttger E C, Bodmer T. Rapid identification of mycobacteria to the species level by polymerase chain reaction and restriction enzyme analysis. J Clin Microbiol. 1993;31:175–178. doi: 10.1128/jcm.31.2.175-178.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waters M F R, Rees R J W. Changes in the morphology of Mycobacterium leprae in patients under treatment. Int J Lepr Other Mycobact Dis. 1962;30:266–277. [PubMed] [Google Scholar]
- 15.Yoon K H, Cho S N, Lee M K, Abalos R M, Cellona R V, Fajardo T T, Jr, Guido L S, Dela Cruz E C, Walsh G P, Kim J D. Evaluation of polymerase chain reaction amplification of Mycobacterium leprae-specific repetitive sequences in biopsy specimens from leprosy patients. J Clin Microbiol. 1993;31:895–899. doi: 10.1128/jcm.31.4.895-899.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]