Key Points
Question
What is the risk of SARS-CoV-2 infection among children compared with adults during periods of increased SARS-CoV-2 circulation in the community?
Findings
In this cohort study of 1236 participants in 310 households conducted from September 2020 through April 2021 in New York City, New York, and selected counties in Utah, site-adjusted incidence rates per 1000 person-weeks were similar by age group: 6.3 for children aged 0 to 4 years, 4.4 for children aged 5 to 11 years, 6.0 for children aged 12 to 17 years, and 5.1 for adults (aged ≥18 years).
Meaning
In this study, children had similar risks of SARS-CoV-2 infection compared with adults.
This cohort study of households in selected Utah counties and New York City, New York, compares incidence rates and clinical characteristics of SARS-CoV-2 infection among adults and children and prospectively assesses infection risks.
Abstract
Importance
Data about the risk of SARS-CoV-2 infection among children compared with adults are needed to inform COVID-19 risk communication and prevention strategies, including COVID-19 vaccination policies for children.
Objective
To compare incidence rates and clinical characteristics of SARS-CoV-2 infection among adults and children and estimated household infection risks within a prospective household cohort.
Design, Setting, and Participants
Households with at least 1 child aged 0 to 17 years in selected counties in Utah and New York City, New York, were eligible for enrollment. From September 2020 through April 2021, participants self-collected midturbinate nasal swabs for reverse transcription–polymerase chain reaction testing for SARS-CoV-2 and responded to symptom questionnaires each week. Participants also self-collected additional respiratory specimens with onset of COVID-19–like illness. For children unable to self-collect respiratory specimens, an adult caregiver collected the specimens.
Main Outcomes and Measures
The primary outcome was incident cases of any SARS-CoV-2 infection, including asymptomatic and symptomatic infections. Additional measures were the asymptomatic fraction of infection calculated by dividing incidence rates of asymptomatic infection by rates of any infection, clinical characteristics of infection, and household infection risks. Primary outcomes were compared by participant age group.
Results
A total of 1236 participants in 310 households participated in surveillance, including 176 participants (14%) who were aged 0 to 4 years, 313 (25%) aged 5 to 11 years, 163 (13%) aged 12 to 17 years, and 584 (47%) 18 years or older. Overall incidence rates of SARS-CoV-2 infection were 3.8 (95% CI, 2.4-5.9) and 7.7 (95% CI, 4.1-14.5) per 1000 person-weeks among the Utah and New York City cohorts, respectively. Site-adjusted incidence rates per 1000 person-weeks were similar by age group: 6.3 (95% CI, 3.6-11.0) for children 0 to 4 years, 4.4 (95% CI, 2.5-7.5) for children 5 to 11 years, 6.0 (95% CI, 3.0-11.7) for children 12 to 17 years, and 5.1 (95% CI, 3.3-7.8) for adults (≥18 years). The asymptomatic fractions of infection by age group were 52%, 50%, 45%, and 12% among individuals aged 0 to 4 years, 5 to 11 years, 12 to 17 years, and 18 years or older, respectively. Among 40 households with 1 or more SARS-CoV-2 infections, the mean risk of SARS-CoV-2 infection among all enrolled household members was 52% (range, 11%-100%), with higher risks in New York City compared with Utah (80% [95% CI, 64%-91%] vs 44% [95% CI, 36%-53%]; P < .001).
Conclusions and Relevance
In this study, children had similar incidence rates of SARS-CoV-2 infection compared with adults, but a larger proportion of infections among children were asymptomatic.
Introduction
Differences have been observed in SARS-CoV-2 infection frequency and clinical presentation among children compared with adults since the earliest months of the COVID-19 pandemic. In early case series of SARS-CoV-2 infections,1,2,3,4 children accounted for a minority of cases, which raised questions about whether children were tested for SARS-CoV-2 less frequently than adults because of differences in clinical presentation, care seeking, or access to testing; had fewer opportunities for exposure because of prevention measures; or in fact were less susceptible to infection because of differences in baseline immune status. Multiple studies have now suggested that children are more likely than adults to have asymptomatic or atypical SARS-CoV-2 infections that may not meet COVID-19 case definitions.5,6,7 At the same time, seroprevalence,8,9,10 household,11,12,13 outbreak,14,15 and surveillance studies have now clearly established that children are susceptible to SARS-CoV-2. However, findings remain mixed with respect to the relative risk of infection among children compared with adults.16 Most studies that have drawn inferences about risk among children vs adults lacked systematic, longitudinal testing for both asymptomatic and symptomatic infections or relied on serologic markers of infection that may not always be present or sustained after asymptomatic infection.17,18 Both limitations might lead to underdetection of SARS-CoV-2 infection cases among children and the false conclusion that risk of infection is lower among children than adults.
Estimating and comparing SARS-CoV-2 community infection rates among children and adults could provide critical data to inform COVID-19 risk communication and prevention strategies, including COVID-19 vaccination policies for children if and when vaccines are available for younger age groups in the future. At the same time, accurate estimates of the asymptomatic fraction of infection and clearer characterization of COVID-19 symptom presentation among different age groups can inform infection prevention measures. The Coronavirus Household Evaluation and Respiratory Testing (C-HEART) study follows up households with 1 or more children aged 0 to 17 years in Utah and New York City, New York, with an intensive surveillance approach that includes weekly systematic molecular testing for both asymptomatic and symptomatic SARS-CoV-2 infections. Using interim data from this prospective cohort, we aimed to estimate and compare incidences of SARS-CoV-2 infections among children and adults, estimate cumulative household infection risk, and compare clinical features of infection by age during a period of increased SARS-CoV-2 circulation.
Methods
Ethical Review and Results Reporting
The study protocol was reviewed and approved by the University of Utah Institutional Review Board (IRB), which served as the central IRB for all study collaborators. The US Centers for Disease Control and Prevention IRB relied on the review of the University of Utah IRB. Columbia University IRB also reviewed and approved the study. The eMethods in the Supplement present additional details about consent and assent procedures. Study methods and findings are reported in accordance with the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.
Participants
The C-HEART cohort enrolled convenience samples of households with 1 or more children aged 0 to 17 years from New York City, and selected counties in Utah (Salt Lake, Weber, Davis, Box Elder, Cache, Tooele, Wasatch, Summit, Utah, and Iron). A convenience sampling approach was used to allow for rapid cohort enrollment from previous cohort studies19,20 and the broader community. Participants were enrolled during August 2020 through February 2021, and SARS-CoV-2 surveillance was conducted from September 2020 through August 2021. This analysis includes the results of surveillance conducted from September 2020 through April 15, 2021. Cohort surveillance began the week of September 6, 2020, in Utah and October 18, 2020, in New York City. The eMethods in the Supplement shows additional details of recruitment procedures and eligibility criteria.
Data Collection Procedures
At enrollment, a designated household reporter completed a telephone survey about the household composition. All individuals completed online surveys about their sociodemographic characteristics, including self-reported race and ethnicity, employment status (for adults [aged ≥18 years]), school attendance status (for children aged 4-17 years of age), childcare attendance status (for children aged 0-17 years), medical histories, and any diagnosis of SARS-CoV-2 infection before cohort enrollment. All data for children aged 0 through 17 years were collected from designated adult proxies (eg, the child’s parent or guardian). Adult proxies were asked to have children nearby to confirm responses with them before entering questionnaire data. Study data were collected and managed using Research Electronic Data Capture (REDCap) electronic data capture tools hosted at Vanderbilt University Medical Center. REDCap is a secure, web-based software platform designed to support data capture for research studies, providing (1) an intuitive interface for validated data capture, (2) audit trails for tracking data manipulation and export procedures, (3) automated export procedures for seamless data downloads to common statistical packages, and (4) procedures for data integration and interoperability with external sources.
Individuals were asked to self-collect midturbinate flocked nasal swabs in viral transport media every week, regardless of illness symptoms. For children unable to self-collect specimens, an adult caregiver collected respiratory specimens. Individuals were also contacted by text message or email every week to ascertain whether they had COVID-19–like illness symptoms or any other illness symptoms and whether children attended school or childcare outside the home during the preceding seven days. COVID-19–like illness was defined as 1 or more of the following: fever or feverishness, cough, shortness of breath, sore throat, diarrhea, muscle aches, chills, or change in taste or smell. At the onset of COVID-19–like illness symptoms, individuals were asked to self-collect and ship 3 additional respiratory specimens, including a midturbinate specimen using a flocked nasal swab in viral transport media, a midturbinate specimen using a foam swab in a dry sterile tube, and a saliva specimen in a dry sterile container. Collection of the 3 specimen types was done as part of a substudy to assess SARS-CoV-2 detection from different specimen types; available published data from other studies suggest that detection of SARS-CoV-2 from saliva samples is comparable with detection from nasopharyngeal swabs.21
The eMethods in the Supplement presents additional details about surveillance and illness follow-up. Data about laboratory-confirmed SARS-CoV-2 infections detected outside the study were also collected via monthly surveys completed by the designated household reporter. Once COVID-19 vaccines became available in the US, the weekly surveillance questionnaire for participants 18 years and older included additional questions every fourth week to collect information about COVID-19 vaccine receipt, vaccine type, and date of receipt.
Laboratory Testing and Reporting
All respiratory specimens were tested at the Marshfield Clinic Research Institute, Marshfield, Wisconsin, by reverse transcription–polymerase chain reaction (RT-PCR) for SARS-CoV-2 (eMethods in the Supplement). Test results that were positive for SARS-CoV-2 were returned to participants and reported to state or local public health authorities according to local requirements.
Outcomes of Interest and Study Definitions
The primary incident outcome of interest was any RT-PCR–confirmed SARS-CoV-2 infection, including both asymptomatic infections and symptomatic infections. Symptomatic SARS-CoV-2 infection was defined as a reported illness with 1 or more respiratory specimen types positive for SARS-CoV-2 by RT-PCR and report of any symptom asked about as part of the study during the 7 days before the first positive respiratory specimen through the last positive respiratory specimen.
Household level outcomes of interest included the proportion of households with 1 or more SARS-CoV-2 infections and the household SARS-CoV-2 infection risk among individuals in households with 1 or more SARS-CoV-2 infections. The household infection risk was defined as the cumulative proportion of all household members with SARS-CoV-2 infection detected within a 14-day period of the first infection(s) detected in each household, inclusive of the first infection(s), divided by the total number of individuals participating in the study in the affected household.
Clinical outcomes of interest included symptom frequencies, the duration of symptoms, and SARS-CoV-2 viral RNA detection from any respiratory specimen type, outpatient medically attended infection (including telemedicine and ambulatory care visits), and hospitalization. The eMethods in the Supplement provide additional study definitions.
Analytic Populations and Statistical Analysis
Individuals were considered fully enrolled if they met eligibility criteria, consented, and completed core questions on the enrollment questionnaire. Individuals who were fully enrolled and also participated in SARS-CoV-2 infection surveillance by submitting 1 or more respiratory specimens and did not have a self-reported diagnosis of COVID-19 with laboratory confirmation by either molecular or serologic testing prior to enrollment were included in incidence and infection analyses.
Incidence rates per 1000 person-weeks for each incident outcome were calculated with outcomes as the numerator and person-weeks at risk for events as the denominator (eMethods in the Supplement). In addition to calculating a crude overall incidence rate among the cohort, a mean of the age-adjusted incidence rates at the 2 study sites was calculated to estimate the mean risk of infection between the 2 sites. Incidence rate ratios comparing rates among pediatric age groups (0-4 years, 5-11 years, and 12-17 years) with adults (≥18 years) were calculated using site-adjusted incidence rates. The asymptomatic fraction of infection was calculated by age group by dividing incidence rates of asymptomatic infection by rates of any infection. The eMethods in the Supplement provide additional methods for incidence calculations and sensitivity analyses.
Among individuals with incident SARS-CoV-2 infections, the frequency of symptoms and features of SARS-CoV-2 infection were compared among children 0 to 17 years and adults after initial exploratory analysis indicated that findings among children aged 0 to 4 years, 5 to 11 years, and 12 to 17 years were similar (eTable 1 in the Supplement). Median duration of SARS-CoV-2 detection by RT-PCR was estimated among all individuals with infections and by age group using nonparametric survival analyses after accounting for interval censoring. Fisher exact, χ2, or Wilcoxon rank sum tests were used to test for statistical significance, as appropriate. We calculated 95% CIs using standard formulas, assuming a binomial distribution. Analyses were conducted in SAS version 9.4 (SAS Institute).
Results
Cohort Enrollment
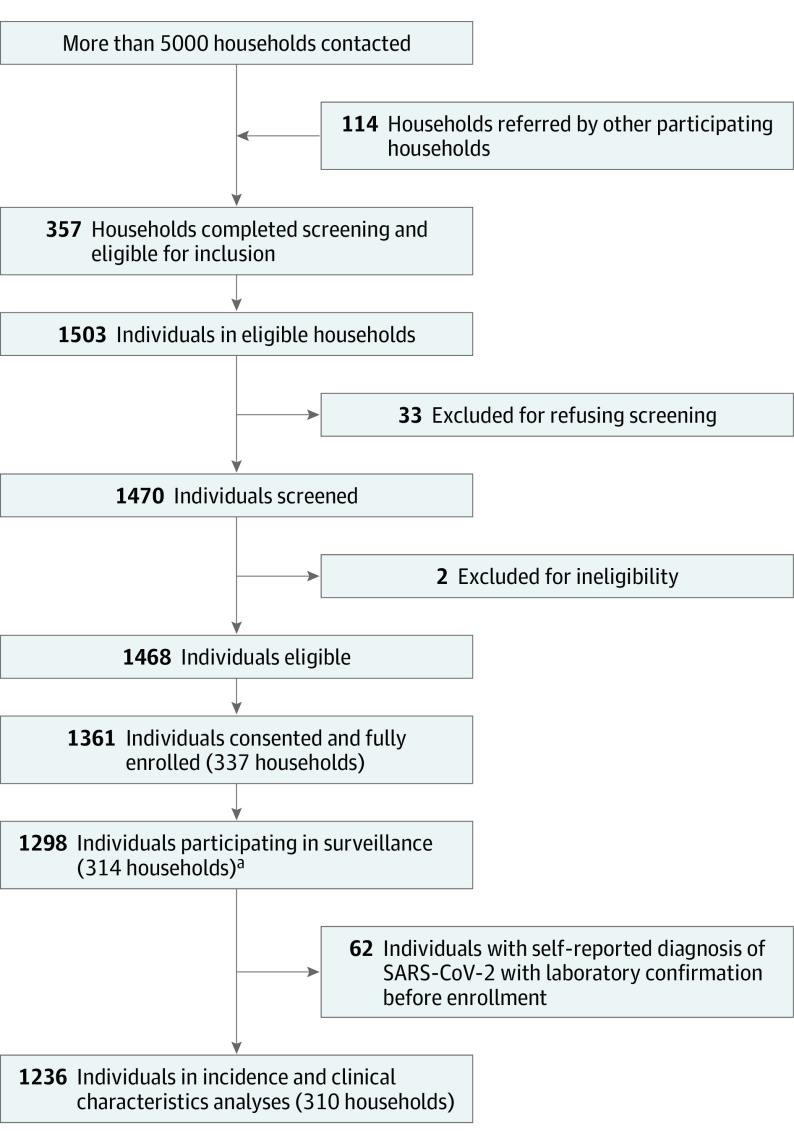
From August 2020 through February 2021, 1361 of 1468 individuals (91%) in 337 households consented to participation and were fully enrolled (Figure 1). Among 1361 individuals who were fully enrolled, 1298 individuals (95%) contributed 1 or more respiratory specimens to SARS-CoV-2 surveillance. After excluding 62 individuals who reported a diagnosis of laboratory-confirmed SARS-CoV-2 infection before cohort enrollment, 1236 were included in analyses of incidence and clinical characteristics of infection.
Figure 1. Cohort Recruitment, Screening, Consent, and Surveillance Participation, Coronavirus Household Evaluation and Respiratory Testing Cohort in Utah and New York City, New York.
aSurveillance participation was defined as submitted at least 1 weekly or acute illness respiratory sample.
Household and Participant Characteristics
The 310 households of participants included in analyses had a median of 4 (IQR, 3-5) household members (range, 2-10), with a median of 2 (IQR, 2-2) adults, and 2 (IQR, 1-3) children (Table 1). Overall, 146 households (47%) reported a household income level of $100 000 or more per year, and only 22 households (7%) reported an income level below the poverty line.
Table 1. Baseline Characteristics of 310 Households Enrolled in the Coronavirus Household Evaluation and Respiratory Testing Study.
| Characteristic | No. (%) | ||
|---|---|---|---|
| All households (N = 310) | New York City, New York (n = 121) | Utah (n = 189) | |
| Type of home | |||
| Single-family house (detached or attached to other houses) | 182 (59) | 1 (1) | 181 (96) |
| Apartment building | 122 (39) | 115 (95) | 7 (4) |
| Other | 1 (<1) | 0 (0) | 1 (1) |
| Missing/declined | 5 (2) | 5 (4) | 0 (0) |
| Household income, $ | |||
| <25 000 | 15 (5) | 13 (11) | 2 (1) |
| 25 000 to <50 000 | 29 (9) | 20 (17) | 9 (5) |
| 50 000 to <75 000 | 44 (14) | 18 (15) | 26 (14) |
| 75 000 to <100 000 | 47 (15) | 14 (12) | 33 (17) |
| ≥100 000 | 146 (47) | 39 (32) | 107 (57) |
| Missing/declined | 29 (9) | 17 (14) | 12 (6) |
| Household income below the poverty linea | |||
| Yes | 22 (7) | 18 (15) | 4 (2) |
| No | 259 (84) | 86 (71) | 173 (92) |
| Missing/declined | 29 (9) | 17 (14) | 12 (6) |
| Median (IQR) | |||
| No. of household members | 4 (3-5) | 4 (3-4) | 4 (4-5) |
| No. of household members aged ≥18 y | 2 (2-2) | 2 (2-2) | 2 (2-2) |
| No. of household members aged <18 y | 2 (1-3) | 2 (1-2) | 2 (2-3) |
| Persons/room in the house | 0.8 (0.5-1.0) | 1.0 (0.8-1.3) | 0.6 (0.4-0.7) |
| Persons/sleeping room in the house | 1.5 (1.3-1.7) | 1.8 (1.3-2.0) | 1.4 (1.2-1.5) |
Calculated based on US Federal Poverty Guidelines available at https://aspe.hhs.gov/poverty-guidelines.
Among the 1236 individuals included in analyses, 176 (14%) were aged 0 to 4 years, 313 (25%) were aged 5 to 11 years, 163 (13%) were aged 12 to 17 years, and 584 (47%) were 18 years or older (Table 2). Among all individuals, 281 (23%) identified as Hispanic, and 881 (71%) identified as White and non-Hispanic. Among adults, 331 (57%) received 1 or more doses of a COVID-19 vaccine during the study period (110 of 584 partially vaccinated [19%] and 221 of 584 fully vaccinated [38%]). Among the 652 children aged 0 to 17 years, 462 (71%) attended childcare (n = 129 [20%]) and/or school (n = 416 [64%]) outside the home at some point during cohort participation. Demographic characteristics of individuals in the study and the 2 source populations in Utah and New York City are shown in eTable 2 in the Supplement.
Table 2. Baseline Characteristics of 1236 Individuals Enrolled in the Coronavirus Household Evaluation and Respiratory Testing Studya.
| Characteristic | No. (%) | ||
|---|---|---|---|
| All participants (N = 1236) | New York City, New York (n = 414) | Utah (n = 822) | |
| Age group, y | |||
| 0-4 | 176 (14) | 58 (14) | 118 (14) |
| 5-11 | 313 (25) | 98 (24) | 215 (26) |
| 12-17 | 163 (13) | 49 (12) | 114 (14) |
| 18-29 | 38 (3) | 17 (4) | 21 (3) |
| 29-39 | 242 (20) | 79 (19) | 163 (20) |
| 40-49 | 230 (19) | 80 (19) | 150 (18) |
| 50-59 | 58 (5) | 26 (6) | 32 (4) |
| ≥60 | 16 (1) | 7 (2) | 9 (1) |
| Sex/gender | |||
| Male | 576 (47) | 182 (44) | 394 (48) |
| Female | 656 (53) | 230 (56) | 426 (52) |
| Nonbinary/third gender | 4 (<1) | 2 (<1) | 2 (<1) |
| Race and ethnicityb | |||
| Black, Non-Hispanic | 18 (1) | 16 (4) | 2 (<1) |
| Hispanic | 281 (23) | 234 (57) | 47 (6) |
| White, Non-Hispanic | 881 (71) | 139 (34) | 742 (90) |
| Missing/declined | 9 (1) | 7 (1) | 2 (<1) |
| Other, Non-Hispanic | 47 (4) | 18 (4) | 29 (4) |
| ≥1 Medical conditionc | 383 (31) | 121 (29) | 262 (32) |
| Asthma | 177 (14) | 53 (13) | 124 (15) |
| Medical conditions besides asthma | 262 (21) | 79 (19) | 183 (22) |
| Adults (n = 584) | |||
| Highest educational level | |||
| Less than high school graduation | 33 (6) | 30 (14) | 3 (1) |
| High school graduation | 49 (8) | 25 (12) | 24 (6) |
| Some college or technical school | 87 (15) | 34 (16) | 53 (14) |
| College graduation | 402 (69) | 109 (52) | 293 (78) |
| Missing/declined | 13 (2) | 11 (5) | 2 (<1) |
| Pregnant, No./total No. of female participants aged 13-50 yd | 9/349 (3) | 2/125 (2) | 7/224 (3) |
| Employment and telework status | |||
| Employed, teleworking | 180 (29) | 45 (22) | 135 (35) |
| Employed, not teleworking | 242 (41) | 69 (33) | 173 (45) |
| Not employed | 141 (24) | 79 (38) | 62 (17) |
| Missing/declined | 21 (3) | 16 (8) | 5 (1) |
| Receipt of COVID-19 vaccine during study participatione | |||
| Partially vaccinated | 110 (19) | 31 (15) | 79 (21) |
| Fully vaccinated | 221 (38) | 71 (34) | 150 (40) |
| Did not receive vaccine | 251 (43) | 107 (51) | 144 (38) |
| Unknown status | 2 (<1) | 0 | 2 (1) |
| Fully vaccinated status by month, No/.total No. of individuals fully vaccinatede | |||
| January | 25/221 (11) | 1/71 (1) | 24/150 (16) |
| February | 67/221 (30) | 19/71 (27) | 48/150 (32) |
| March | 69/221 (31) | 18/71 (25) | 51/150 (34) |
| April | 58/221 (26) | 32/71 (45) | 26/150 (17) |
| Unknown month | 2/221 (1) | 1/71 (1) | 1/150 (1) |
| Children (n = 652) | |||
| Childcare outside the home during cohort participation | 129/652 (20) | 42/205 (20) | 87/447 (19) |
| School outside the home during cohort participation | 416/652 (64) | 79/205 (39) | 337/447 (75) |
All characteristics were ascertained at enrollment only unless otherwise specified.
Race and ethnicity are based on participant self-report. Participants who self-identified as Hispanic are categorized as Hispanic, regardless of self-reported race. Participants who self-identified as non-Hispanic are categorized based on their self-reported races. The category of other, non-Hispanic includes participants who self-identified as non-Hispanic and Asian, Native Hawaiian/Pacific Islander, or multiple races.
Based on questionnaires that asked about the following medical conditions: asthma; chronic lung diseases other than asthma; chronic metabolic diseases, such as diabetes types 1 and 2 and thyroid disease; blood disorders, such as thalassemia and sickle cell disease; hypertension; cardiovascular diseases; bladder or kidney diseases; liver diseases; immunocompromising conditions; neurologic or neuromuscular diseases; and rheumatologic conditions. Among the 1236 participants in this analysis, 17 reported having immunocompromising conditions, including 15 adults and 2 children.
The denominator excludes individuals with unknown status (n = 3).
COVID-19 vaccination status was based on self-reporting. Vaccination information was only collected among adults during the period included in this analysis. Fully vaccinated was defined as having received 2 doses of vaccines with a recommended 2-dose series or 1 dose of a vaccine for which only a single dose was recommended. Partially vaccinated was defined as receipt of 1 dose of vaccines with a recommended 2-dose series.
SARS-CoV-2 Incidence and Household Infection Rates
Among the 1236 individuals included in analyses, 443 (36% [95% CI, 33%-39%]) reported 1 or more episodes of symptomatic illness and submitted a respiratory sample for testing, and 94 of 1236 (8% [95% CI, 6%-9%]) had incident episodes of RT-PCR–confirmed SARS-CoV-2 infection. There were no cases of SARS-CoV-2 infection detected solely outside of the study without detection by study surveillance. There were also no SARS-CoV-2 infections among participants after vaccination against COVID-19. As of April 15, 2021, individuals in cohort surveillance were followed up for 21 465 person-weeks (mean [SD] per individual, 17 [9] person-weeks). The median swab submission adherence rate, defined as the proportion of surveillance weeks for each participant in which they submitted a swab specimen, was 96% (IQR, 90%-100%). The median time from flocked nasal swab collection to testing at the central laboratory was 2 (IQR, 2-3) days. The eFigure in the Supplement shows SARS-CoV-2 infections detected among the cohort and through local surveillance during the study period.
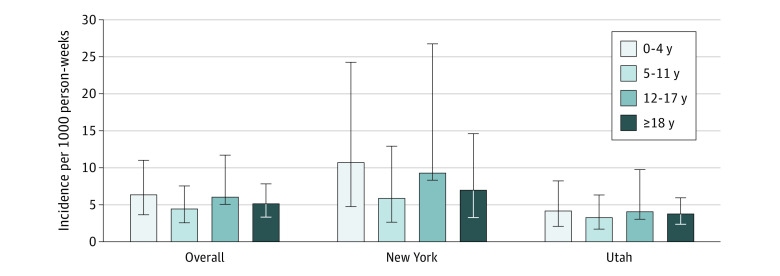
The mean age-adjusted incidence rate of SARS-CoV-2 infection across the 2 study sites was 5.8 (95% CI, 3.1-8.4) per 1000 person-weeks for all infections (eTable 3 in the Supplement). Incidence of any SARS-CoV-2 infection was higher among the New York City cohort compared with the Utah cohort overall (7.7 vs 3.8 per 1000 person-weeks; eTable 3 in the Supplement). Site-adjusted SARS-CoV-2 infection incidence rates per 1000 person-weeks by age group were 6.3 (95% CI, 3.6-11.0) for children 0 to 4 years, 4.4 (95% CI, 2.5-7.5) for children 5 to 11 years, 6.0 (95% CI, 3.0-11.7) for children 12 to 17 years, and 5.1 (95% CI, 3.3-7.8) for adults (Figure 2; eTables 3 and 4 in the Supplement). The incidence rate ratios comparing children aged 0 to 4 years, 5 to 11 years, and 12 to 17 years with adults were 1.2 (95% CI, 0.8-2.0; P = .34), 0.9 (95% CI, 0.5-1.4; P = .55), and 1.2 (95% CI, 0.6-2.3; P = .63), respectively. The age-adjusted mean incidence rates of symptomatic and asymptomatic SARS-CoV-2 infections across the 2 study sites were 3.5 (95% CI, 2.4-4.6) and 2.1 (95% CI, 0.8-3.3), respectively, resulting in an overall asymptomatic fraction of 36%. The asymptomatic fraction of infection by age group was 52%, 50%, 45%, and 12% among individuals aged 0 to 4 years, 5 to 11 years, 12 to 17 years, and 18 years or older, respectively.
Figure 2. SARS-CoV-2 Infection Incidences per 1000 Person-Weeks by Site and Age in Utah and New York City, New York, From September 2020 Through April 2021 (N = 1236).
Error bars denote 95% CIs. All incidence estimates are adjusted for household clustering using negative-binomial models with generalized estimating equations assuming independent correlation structure. Overall age-stratified incidence estimates are also adjusted for site.
Among the 310 households participating in surveillance as of April 15, 2021, 40 (13% [95% CI, 9%-17%]) included 1 or more persons with SARS-CoV-2 infection during the study period. Among the 40 households with SARS-CoV-2 infections, 17 (43%) had a single case of infection and 23 (58%) had at least 2 cases of infection occurring within 14 days of each other, including 7 households (18%) in which all household members were infected (household size range, 3-7 individuals). Thirty of 40 households (75%) with SARS-CoV-2 infections included an infection in a child aged 0 to 17 years, whereas 28 of 40 (70%) included an infection in an adult. The mean household infection risk among individuals in affected households was 52% (range, 11%-100%). Mean household infection risks were higher in New York City compared with Utah (80% [95% CI, 64%-91%] vs 44% [95% CI, 36%-53%]; P < .001).
Characteristics of SARS-CoV-2 Infection Episodes
Among the 94 individuals with incident SARS-CoV-2 infections from 40 households, 16 (17%) were aged 0 to 4 years, 21 (22%) were aged 5 to 11 years, 14 (15%) were aged 12 to 17 years, and 43 (46%) were 18 years or older. Overall, 65 individuals (69%) had symptomatic infection and 29 (31%) were asymptomatic throughout their infections. Among 52 symptomatic infections with information about symptom onset dates, 15 (27%) were first detected while individuals were still presymptomatic. The median duration of SARS-CoV-2 RNA detection by RT-PCR was 14 (IQR, 6-15) days, based on weekly and acute illness swab collection. Among 38 individuals with symptomatic illness with information available about illness onset and end dates, the median duration of symptoms was 14 (IQR, 7-22) days. Fifteen infections (15 of 65 symptomatic infections [23%] or 15 of all 94 infections [16%]) resulted in outpatient medical care, and 2 resulted in hospitalizations; no one died.
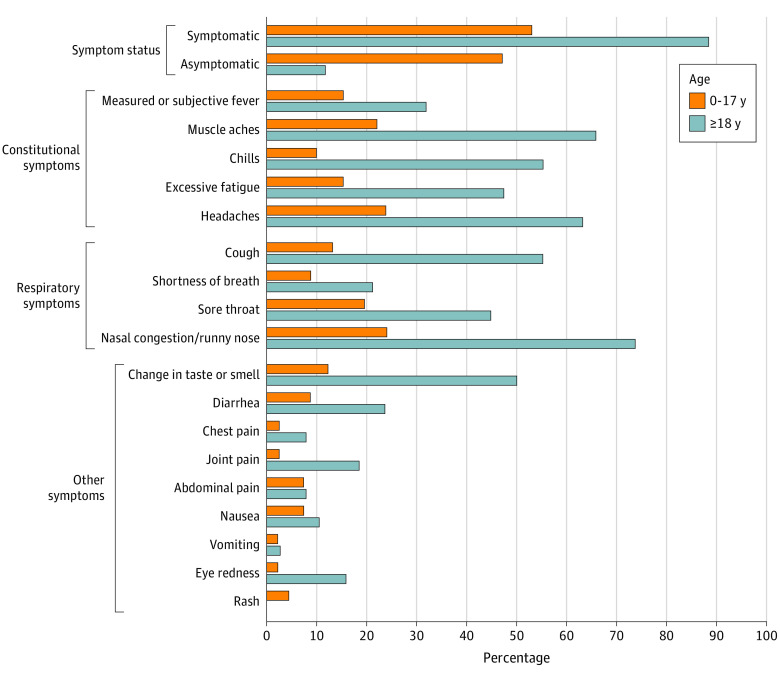
Compared with infections among adults, a larger proportion of infections among children aged 0 to 17 years were asymptomatic (12% [95% CI, 4%-25%] vs 47% [95% CI, 33%-62%]; P < .001), but there was no difference in the duration of SARS-CoV-2 RNA detection between the 2 age groups (median days: 14 vs 13; P = .50). After excluding individuals with symptomatic infection without information about specific symptoms (n = 10), measured or subjective fever was infrequent among both children and adults (15% [95% CI, 6%-29%] vs 32% [95% CI, 18%-49%], respectively; Figure 3).
Figure 3. SARS-CoV-2 Infection Symptom Status and Symptom Frequency Among Children vs Adults (N = 84).
This figure includes 84 individuals with SARS-CoV-2 infection who had complete information about symptom status and/or specific symptoms. Questionnaires for children younger than 24 months excluded chills, muscle aches or body aches, change in taste or smell, sore throat, joint pain, nausea, abdominal pain, headache, or chest pain because these symptoms can be difficult for caregivers to identify in younger children who are nonverbal or less verbal; infections in children younger than 24 months are excluded from analyses of these symptoms in the age group 0 to 17 years. No child younger than 24 months had increased fussiness (data not shown). There were significant differences (P < .05) between children and adults in the frequency of being symptomatic, being asymptomatic, having muscle aches, having chills, having excessive fatigue, having headaches, having a cough, having a sore throat, having nasal congestion/runny nose, experiencing a change in taste or smell, having joint pain, and having eye redness.
Discussion
Among a cohort of households with adults and children followed up during periods of high virus circulation in Utah and New York City, incidence rates of SARS-CoV-2 infection at the 2 sites were 3.8 and 7.7 per 1000 person-weeks, respectively, equating to a 0.4% to 0.8% risk of infection per week among study households. Adults and children of all ages had similar risks of SARS-CoV-2 infection, but approximately half of SARS-CoV-2 infections among children were asymptomatic compared with a much smaller fraction among adults. Measured and subjective fever were infrequent symptoms among both adults and children, indicating that screening for SARS-CoV-2 infection based on fever is likely to miss a large proportion of infections. Among households with 1 or more individuals with SARS-CoV-2 infection, the mean household infection risk was 52% (range, 11%-100%), suggesting that households remain a common site for transmission of SARS-CoV-2. The mean household infection risk was higher in New York City compared with Utah, which may be attributable to differences by site in household crowding or preventive behaviors, community transmission of SARS-CoV-2, or circulating virus lineages.
To date, data on the community incidence rate of SARS-CoV-2 infections in the US are sparse. Our study provides infection incidence rates and symptom profiles among adults and children using a standard approach that allows direct comparison across age groups. Our findings suggest that children and adults have similar incidence rates of SARS-CoV-2 infection, underscoring the need for rapid evaluation of vaccine efficacy and safety in children to expand vaccine indications to younger age groups. We also found that SARS-CoV-2 infections were more frequently asymptomatic in children compared with adults, highlighting the need for additional data on risk of SARS-CoV-2 transmission from persons with asymptomatic infection, including children. To date, published data22,23,24,25 about the risk of SARS-CoV-2 infection transmission from individuals without symptoms and the comparative risk of transmission from children vs adults remain mixed. Additional analyses from this household cohort are planned to examine transmission from children vs adults by estimating secondary infection risks using probabilistic modeling approaches to assign primary case status in households.
Studies that start with identification of infections based on surveillance or medical testing of individuals with illness symptoms are likely to underestimate the true number of infections because asymptomatic and non–medically attended infections are underascertained.26 In this study, all household members were prospectively tested using a standard approach that reduces the potential bias toward identifying symptomatic cases. Overall SARS-CoV-2 infection incidence rates in our study are consistent with modeled estimates for the US during February through December 2020 that attempt to account for underascertainment of infections that are not identified by testing (4.6-6.2 per 1000 person-weeks for all ages combined), but incidence rates among children aged 0 through 4 years in our study were higher than modeled estimates for this age group (2.7-3.8 per 1000 person-weeks for children aged 0-4 years).27
Limitations
Several limitations should be considered when interpreting study findings. First, individuals who participate in studies that require intensive follow-up likely differ from the general population in their attitudes toward public health and science, which may in turn influence behaviors associated with infection risk. If cohort participants were more likely to adhere to COVID-19 prevention practices, then incidence rates from this study may underestimate community rates of infection. Reporting of positive SARS-CoV-2 test results to participants may also have influenced household infection prevention behaviors, resulting in reduced secondary transmission, although reporting lagged behind specimen collection by at least a few days in all cases. Second, persons of certain racial and ethnic backgrounds and low-income households were underrepresented in this cohort, and incidence rates may not be generalizable to those populations if infection risks vary by race, ethnicity, or income level because of inequities in social determinants of health. Third, it is possible that some illness symptoms experienced by children with SARS-CoV-2 infection went unidentified because symptom information was collected from adult caregivers. However, the same limitation applies to any setting where children are screened for SARS-CoV-2, since screening of young children relies on the observation of adult caregivers.
Conclusion
This study quantifies incidence rates and household risk of SARS-CoV-2 infection among a cohort of households in 2 areas of the US that were heavily affected by SARS-CoV-2 circulation from September 2020 through April 2021. Incidence rates of SARS-CoV-2 infection were similar among children and adults. A larger fraction of SARS-CoV-2 infections in children were asymptomatic and would likely have gone undetected without study testing, supporting hypotheses that SARS-CoV-2 infections among children have been substantially underascertained during the COVID-19 pandemic. It remains unclear how risk of SARS-CoV-2 infection among adults and children will evolve with increasing COVID-19 vaccine uptake among adults and increasing circulation of SARS-CoV-2 variants of concern. Our findings suggest that SARS-CoV-2 infection prevention strategies, such as handwashing, masking, physical distancing, and COVID-19 vaccination should target children in addition to adults to both mitigate individual health outcomes for children and reduce the overall burden of SARS-CoV-2 infection in the community.
eMethods.
eReferences.
eTable 1. Symptom status and frequencies by age group among individuals with SARS-CoV-2 infection
eTable 2. Baseline Characteristics of C-HEART Participants compared to New York City and Utah State Populations
eTable 3. SARS-CoV-2 Infection Episodes and Incidences per 1,000 Person-Weeks by Site, Age Group, and Symptom Status, Utah and New York City, New York
eTable 4. SARS-CoV-2 Infection Episodes and Incidences per 1,000 Person-Weeks by Age Group among Participants without Full Vaccination Against COVID-19, Utah and New York City, New York
eFigure. Number of SARS-CoV-2 infection cases detected in the C-HEART cohort and reported to local health departments by week, Health and Human Services Protect Public Data Hub
References
- 1.Ladhani SN, Amin-Chowdhury Z, Davies HG, et al. COVID-19 in children: analysis of the first pandemic peak in England. Arch Dis Child. 2020;105(12):1180-1185. doi: 10.1136/archdischild-2020-320042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dawood FS, Ricks P, Njie GJ, et al. Observations of the global epidemiology of COVID-19 from the prepandemic period using web-based surveillance: a cross-sectional analysis. Lancet Infect Dis. 2020;20(11):1255-1262. doi: 10.1016/S1473-3099(20)30581-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Epidemiology Working Group for NCIP Epidemic Response, Chinese Center for Disease Control and Prevention . [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China]. Zhonghua Liu Xing Bing Xue Za Zhi. 2020;41(2):145-151. [DOI] [PubMed] [Google Scholar]
- 4.CDC COVID-19 Response Team . Coronavirus disease 2019 in children—United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(14):422-426. doi: 10.15585/mmwr.mm6914e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siebach MK, Piedimonte G, Ley SH. COVID-19 in childhood: transmission, clinical presentation, complications and risk factors. Pediatr Pulmonol. 2021;56(6):1342-1356. doi: 10.1002/ppul.25344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han MS, Choi EH, Chang SH, et al. Clinical characteristics and viral RNA detection in children with coronavirus disease 2019 in the republic of Korea. JAMA Pediatr. 2021;175(1):73-80. doi: 10.1001/jamapediatrics.2020.3988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Council of State and Territorial Epidemiologists . Update to the standardized surveillance case definition and national notification for 2019. novel coronavirus disease (COVID-19). Published June 22, 2020. Accessed April 28, 2021. https://cdn.ymaws.com/www.cste.org/resource/resmgr/ps/positionstatement2020/Interim-20-ID-02_COVID-19.pdf
- 8.Hobbs CV, Drobeniuc J, Kittle T, et al. ; CDC COVID-19 Response Team . Estimated SARS-CoV-2 seroprevalence among persons aged <18 years—Mississippi, May-September 2020. MMWR Morb Mortal Wkly Rep. 2021;70(9):312-315. doi: 10.15585/mmwr.mm7009a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gudbjartsson DF, Helgason A, Jonsson H, et al. Spread of SARS-CoV-2 in the Icelandic population. N Engl J Med. 2020;382(24):2302-2315. doi: 10.1056/NEJMoa2006100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pollán M, Pérez-Gómez B, Pastor-Barriuso R, et al. ; ENE-COVID Study Group . Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396(10250):535-544. doi: 10.1016/S0140-6736(20)31483-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bi Q, Wu Y, Mei S, et al. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis. 2020;20(8):911-919. doi: 10.1016/S1473-3099(20)30287-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg ES, Dufort EM, Blog DS, et al. ; New York State Coronavirus 2019 Response Team . COVID-19 testing, epidemic features, hospital outcomes, and household prevalence, New York State—March 2020. Clin Infect Dis. 2020;71(8):1953-1959. doi: 10.1093/cid/ciaa549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grijalva CG, Rolfes MA, Zhu Y, et al. Transmission of SARS-COV-2 infections in households—Tennessee and Wisconsin, April-September 2020. MMWR Morb Mortal Wkly Rep. 2020;69(44):1631-1634. doi: 10.15585/mmwr.mm6944e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lopez AS, Hill M, Antezano J, et al. Transmission dynamics of COVID-19 outbreaks associated with child care facilities—Salt Lake City, Utah, April-July 2020. MMWR Morb Mortal Wkly Rep. 2020;69(37):1319-1323. doi: 10.15585/mmwr.mm6937e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szablewski CM, Chang KT, Brown MM, et al. SARS-CoV-2 transmission and infection among attendees of an overnight camp—Georgia, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(31):1023-1025. doi: 10.15585/mmwr.mm6931e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Viner RM, Mytton OT, Bonell C, et al. Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults: a systematic review and meta-analysis. JAMA Pediatr. 2021;175(2):143-156. doi: 10.1001/jamapediatrics.2020.4573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lei Q, Li Y, Hou HY, et al. Antibody dynamics to SARS-CoV-2 in asymptomatic COVID-19 infections. Allergy. 2021;76(2):551-561. doi: 10.1111/all.14622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Long QX, Tang XJ, Shi QL, et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat Med. 2020;26(8):1200-1204. doi: 10.1038/s41591-020-0965-6 [DOI] [PubMed] [Google Scholar]
- 19.Stockwell MS, Reed C, Vargas CY, et al. MoSAIC: mobile surveillance for acute respiratory infections and influenza-like illness in the community. Am J Epidemiol. 2014;180(12):1196-1201. doi: 10.1093/aje/kwu303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.University of Utah . Utah Children's Project. Accessed March 21, 2021. https://healthcare.utah.edu/clinicaltrials/trial.php?id=FP00004007
- 21.Hanson KE, Caliendo AM, Arias CA, et al. IDSA guidelines on the diagnosis of COVID-19: molecular diagnostic testing. Updated December 23, 2020. Accessed September 1, 2021. https://www.idsociety.org/practice-guideline/covid-19-guideline-diagnostics/
- 22.Madewell ZJ, Yang Y, Longini IM Jr, Halloran ME, Dean NE. Household transmission of SARS-CoV-2: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(12):e2031756. doi: 10.1001/jamanetworkopen.2020.31756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim J, Choe YJ, Lee J, et al. Role of children in household transmission of COVID-19. Arch Dis Child. 2021;106(7):709-711. doi: 10.1136/archdischild-2020-319910 [DOI] [PubMed] [Google Scholar]
- 24.Posfay-Barbe KM, Wagner N, Gauthey M, et al. COVID-19 in children and the dynamics of infection in families. Pediatrics. 2020;146(2):e20201576. doi: 10.1542/peds.2020-1576 [DOI] [PubMed] [Google Scholar]
- 25.Lee B, Raszka WV Jr. COVID-19 transmission and children: the child is not to blame. Pediatrics. 2020;146(2):e2020004879. doi: 10.1542/peds.2020-004879 [DOI] [PubMed] [Google Scholar]
- 26.Reese H, Iuliano AD, Patel NN, et al. Estimated incidence of coronavirus disease 2019 (COVID-19) illness and hospitalization—United States, February-September 2020. Clin Infect Dis. 2021;72(12):e1010-e1017. doi: 10.1093/cid/ciaa1780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention . Estimated COVID-19 burden. Updated July 27, 2021. Accessed September 24, 2021. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/burden.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eReferences.
eTable 1. Symptom status and frequencies by age group among individuals with SARS-CoV-2 infection
eTable 2. Baseline Characteristics of C-HEART Participants compared to New York City and Utah State Populations
eTable 3. SARS-CoV-2 Infection Episodes and Incidences per 1,000 Person-Weeks by Site, Age Group, and Symptom Status, Utah and New York City, New York
eTable 4. SARS-CoV-2 Infection Episodes and Incidences per 1,000 Person-Weeks by Age Group among Participants without Full Vaccination Against COVID-19, Utah and New York City, New York
eFigure. Number of SARS-CoV-2 infection cases detected in the C-HEART cohort and reported to local health departments by week, Health and Human Services Protect Public Data Hub