Abstract
Introduction
Acne vulgaris is a common, chronic disease. One of the most commonly encountered complications of acne is permanent atrophic scarring. Treatment of atrophic scars includes fillers, dermabrasion, laser resurfacing, microneedling and peelings and it is often difficult to treat. In our double-blind randomized controlled trial (RCT), we investigated the synergistic effect of microneedling with the application of trichloroacetic acid, kojic acid and hydrogen peroxide in the treatment of atrophic acne scars.
Aim
To assess the clinical effectiveness and patients’ quality-of-life (HRQoL) after three types of atrophic post-acne scar treatment, namely microneedling alone (MN) vs chemical peeling alone (CP) vs. a combination of microneedling and chemical peeling (MN + CP).
Material and methods
A total of 120 patients were enrolled into the study following strict inclusion/exclusion criteria and randomized into the three treatment groups – MN, CP (a combination of trichloroacetic acid, kojic acid and hydrogen peroxide), and MN + CP. According to a preapproved protocol, each patient underwent four treatment sessions, each spread 20 days apart. Both pre- and post-treatment clinical status (using the Goodman-Baron scale; two expert raters blinded to the treatment used) and patients’ HRQoL (using the Dermatology Life Quality Index) were assessed.
Results
During the 5-month recruitment period, a total of 120 patients were approached and agreed to take part in the study (94 females – 78.3% and 26 males) (mean age of 30.14 ±3.64 years; range: 18–45 years). Only in the MN + CP group there was a statistically significant improvement according to the G-B scale post-treatment (2.87 ±0.83 vs. 2.03 ±1.16 respectively; p = 0.0005). Patients in all three treatment groups experienced a statistically significant improvement in their HRQoL post-treatment (all p’s < 0.05).
Conclusions
A combination of microneedling and chemical peeling produces the best, objectively measured effects in the treatment of atrophic post-acne scars. All examined treatments, even if not producing a clinically significant treatment outcome, improve patients’ HRQoL.
Keywords: acne, microneedling, PRX-T33, scar, quality of life
Introduction
Acne vulgaris is a common, chronic disease [1], most often affecting young adults from around age 11, with a peak incidence around the age of 16 [2], and persisting in some individuals into their 20s or even 30s [3]. The majority of acne vulgaris cases can be classified as mild (~85%), however about 15% of the afflicted develop the severe form of acne which can result in various forms of scarring [1, 2]. Acne is almost always associated with a high psycho-social burden, which often leads to the development of anxiety disorders or depression [4]. Studies show that patients with acne display a severe decrease in their health-related quality-of-life (HRQoL), comparable to that of patients with other severe chronic diseases such as diabetes, arthritis, and asthma [5, 6].
One of the most commonly encountered complications of acne is permanent atrophic scarring, which severely impacts patients’ HRQoL and is often difficult to treat [7]. Through an inflammatory process, acne leads to damage of the sebaceous follicles, impairs their healing and finally results in scar formation [8]. It has been demonstrated that patients with a tendency to develop post-acne scarring have an altered cell inflammatory profile, which results in a prolonged inflammatory response. It is this tendency that leads to collagen destruction and is associated with atrophic scar development [9]. Scar formation is mostly encountered in moderate and severe inflammatory forms of acne but some mild form can also result in this complication [10]. Acne scarring significantly impairs patients HRQoL, leading to loss of confidence, decreased social functioning, and poor emotional well-being [11].
Treatment options for early acne comprise antibiotics, retinoids, and benzoyl peroxide in monotherapy or a combination of the previously listed substances and chosen systemic medication such as antiandrogens and isotretinoin [12, 13]. Treatment of atrophic scars includes corrective procedures such as fillers, dermabrasion, laser resurfacing, and microneedling [14]. To date topical therapy and ablative modalities such as dermabrasion, peelings, and lasers were the main focus of atrophic scar therapy but in recent years newer, regenerative medicine methods, such as microneedling have gained popularity [15]. Ablative procedures base their effectiveness on partial removal of the epidermis to trigger the growth of new skin to replace scars [16]. This may result in papillary dermis fibrosis, irregular pigmentation, and even excessive scarring [17, 18]. On the other hand, percutaneous collagen induction reaches the papillary and reticular dermis in a purely mechanical way, minimizing the disruption of the epidermis and leading to scarless wound healing [19]. However, microneedling being a fairly new treatment modality still lacks strong clinical evidence to fully support its use in the treatment of atrophic post-acne scars [15]. This is the “call for action”, which has been echoed in several papers [15, 20]. This existing knowledge gap led us to design and execute this double-blind randomized controlled trial (RCT) to examine the possible synergistic effect of the use of microneedling and a combination of trichloroacetic acid, kojic acid and hydrogen peroxide in the treatment of post-acne atrophic scars.
Aim
Thus, the aim of this double-blind RCT was to assess the clinical effectiveness and patients’ quality-of-life after three types of atrophic post-acne scar treatment, namely microneedling alone vs chemical peeling alone (a combination of trichloroacetic acid, kojic acid and hydrogen peroxide) vs. a combination of microneedling and chemical peeling.
Material and methods
This double-blind RCT was conducted between August and December 2016. A total of 120 patients were enrolled into the study, with 118 (98.3%) completing the entire course of treatment.
Inclusion and exclusion criteria
The following patient inclusion criteria were used: (1) presence of post-acne atrophic scars grade 2–4 according to Goodman and Baron (G-B) [21] located on the face, (2) age 18 and older, and (3) written, informed consent to participate in the study. Study exclusion criteria were: (1) active facial skin acne, (2) confirmed tendency to form keloid scars, (3) impaired blood clotting (including ongoing anticoagulant treatment), (4) oral glucocorticoid use, (5) active facial skin infection, (6) pregnancy and lactation, and (7) lack of consent to participate in the study.
Randomization and blinding
Each of the patients recruited for this study was randomized into one of the three treatment groups – 1) microneedling alone, 2) chemical peeling alone, 3) combination of microneedling and chemical peeling. Patient randomization was performed by a computer algorithm which also allowed to maintain age and gender balance between the three treatment groups.
This RCT was a double-blind study, i.e. the patients did not know to which treatment group they have been assigned and the assessing dermatologists were blinded to the procedures performed (the assessing dermatologists were not the ones treating the patients). Before starting the treatment, each patient had been informed that he/she is entering a double-blind RCT with three treatment arms, and that he/she will be assigned to one of the treatment arms and will only be informed which treatment arm it was after the study finishes. If at the end of the study the patient was not satisfied with the overall cosmetic result, he/she was offered complimentary treatment using the treatment arm showing the best results in this trial. The exact way of patient blinding during the treatment procedures is described in “Treatment procedures and outcome evaluation” (see below).
Treatment procedures and outcome evaluation
Each patient underwent four treatment sessions. The treatment sessions were each performed 20 days apart. During each treatment session, the patients’ eyes were covered to prevent them seeing the procedures performed. Each patient was treated as he/she would be qualified for the microneedling and chemical peeling treatment group.
“Placebo-microneedling” was performed using a “placebo-dermapen” (“placebo-dermapen” had the same shape, size and colour as a normal dermapen but was equipped with blunt needles that did not pierce the patient’s skin). The use of the “placebo-dermapen” was pretested on 10 patients (5 males and 5 females). Each patient, during a routine microneedling procedure, was blindfolded and anaesthetic cream was applied on their face as described in Appendix 1. Each patient had “real” microneedling performed on one half of their face, and “placebo-microneedling” on the other half, for 10 min each. After this procedure the patients were asked whether they felt any differences in the procedure being performed on their face. Only 1 in 10 patients told he felt a difference but was not able to tell on which half of his face the “placebo dermapen” was used.
Microneedling and chemical peeling treatment group (MN + CP group)
Each session included microneedling performed using an automatic dermapen (Dermapen, Revisage, Poland) and the use of a combination of trichloroacetic acid (33%), kojic acid (5%) and hydrogen peroxide (5%) (product name PRX-T33; WIQOmed; Italy). A detailed description of the treatment protocol is attached as Appendix 1. Each treatment session in each patient was performed by the same physician (specialist in dermatology and aesthetic medicine; with 10 years of experience in treating skin disorders).
Chemical peeling treatment group (CP group)
For patients qualified to chemical peeling treatment alone, the treatment protocol followed the same steps as described in Appendix 1 with the exception of step 7 (microneedling) where the dermapen was replaced with the “placebo-dermapen”. The patient had his/hers eyes covered and anaesthetic cream applied and thus did not feel the difference between microneedling and “placebo-microneedling”.
Microneedling treatment group (MN group)
For patients qualified to microneedling treatment alone, the treatment protocol followed the same steps as described in Appendix 1 with the exception of steps 5, 6 and 8 (application of chemical peeling) where the chemical peeling tested was replaced with the placebo peel (pH 2.0 hydrochloric acid in polyethylene glycol vehicle 45M). The patient had his/hers eyes covered and anaesthetic cream applied and thus did not feel the difference between either application.
Regardless of the treatment group, before each treatment session each patient received a detailed, oral and written explanation of the entire treatment (procedures to be conducted, their potential risks and benefits). After each treatment session the patient was instructed to use silicone gel on their facial skin during the night and the NoScar cream (AZ Medica, Poland) during the day throughout the entire duration of the treatment (all four treatment sessions, the intervals between them, and 20 days after the last treatment session). Additionally each patient was instructed that for the duration of the entire treatment he or she should use, for the treated skin, an SPF50 cream, avoid sunlight exposure, and should not use sauna or the swimming pool.
One day before the first treatment session and 30 days after the last treatment session, each patient facial skin was assessed by two independent physicians (specialists in dermatology, each with 10 years of experience in treating skin disorders; the assessing dermatologists were not the ones treating the patients and were blinded to the procedures performed) using the G-B scale [21]. At the same two time-points each patient face was photographed, in both cases in the same lighting conditions. In addition, 1 day before the first treatment session and 30 days after the last treatment session each patient filled in the Polish version of the Dermatology Life Quality Index (DLQI) [22], a ten-question questionnaire used to measure the impact of skin disease on the patients’ HRQoL.
Ethics
The protocol of this study has been approved by the Bioethical Committee at the Medical University of Lodz, registry number RNN/112/16/KE. Each patient gave their informed and written consent to participate in this study. The study has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Statistical analysis
To analyse the data, descriptive statistics (mean, standard deviation, percentage distribution) were used. To compare the differences in scale scores between two groups, the U Mann-Whitney test was used (due to the non-normal distribution of analysed data). To compare scores between three groups, the ANOVA test (with Tukey’s HSD post-hoc test) was used. Correlations between patients’ age, clinical outcome and changes in HRQoL were performed using Spearman’s rank-order correlation (due to the non-normal distribution of analysed data).
Agreement between the two assessors using the G-B scale (pre- and post-treatment) was assessed using Cohen’s k coefficient.
The significance level was set at p < 0.05. Statistical analysis was conducted using computer software Statistica 10.0 PL by StatSoft Poland.
Results
During the 5-month recruitment period, a total of 120 patients were approached and agreed to take part in the study (94 females – 78.3% and 26 males) (mean age of 30.14 ±3.64 years; range: 18–45 years). Further information on the three treatment groups is presented in Table 1.
Table 1.
Treatment groups’ characteristics, clinical outcomes and health-related quality-of-life
| Parameter | MN + CP (n = 38) | CP alone (n = 41) | MN alone (n = 40) | P-value (MN + CP vs. CP) | P-value (MN + CP vs. MN) | P-value (CP vs. MN) | P-value (ANOVA for three groups comparison) |
|---|---|---|---|---|---|---|---|
| Number of males and females | 10 M/28 F | 8 M/33 F | 10 M/30 F | – | – | – | – |
| Mean age (SD) | 30.65 (9.08) | 28.45 (5.23) | 31.33 (7.54) | 0.39 | 0.91 | 0.19 | 0.19 |
| G-B before Tx | 2.87 (0.83) | 2.50 (1.03) | 2.88 (0.98) | 0.20 | 1.00 | 0.18 | 0.13 |
| G-B after Tx | 2.03 (1.16) p = 0.0005 | 2.28 (0.95) p = 0.32 | 2.55 (0.92) p = 0.13 | 0.52 | 0.07 | 0.46 | 0.08 |
| DLQI before Tx | 13.42 (6.05) | 6.60 (4.19) | 10.30 (6.36) | < 0.0001 | 0.04 | 0.01 | < 0.0001 |
| DLQI after Tx | 7.63 (5.15) p < 0.0001 | 4.63 (3.79) p = 0.03 | 7.30 (5.46) p = 0.03 | 0.02 | 0.95 | 0.04 | 0.01 |
MN – microneedling, CP – chemical peeling, G-B – Goodman and Baron scale, DLQI – Dermatology Life Quality Index, Tx – treatment, M – males, F – females. Numbers in brackets give standard deviation. The p-values given in the “after Tx” cells refer to the pre- and post-treatment comparison. Bold marks statistical significance.
In the MN + CP group, out of the 40 patients, 38 (95%) completed the study. Two females did not complete the study due to facial skin discoloration which occurred after the patients did not follow the post-treatment instructions of using SPF50 cream and avoiding sun exposure. Apart from the 2 patients, no major treatment-related side effects were noted in any of the patients during this trial. In the CP and in the MN groups all the patients completed the study.
Cohen’s k coefficient, as a measure of agreement between the two assessors using the G-B scale, was 0.90–0.91 and 0.93–0.94 for pre- and post-treatment assessment, respectively for the three treatment groups, showing excellent agreement between the assessing physicians.
Clinical outcomes (Goodman and Baron scale)
An in-depth description of the clinical outcomes is presented in Tables 1 and 2. Overall, only in the MN + CP group there was a statistically significant improvement according to the G-B scale post-treatment (2.87 ±0.83 vs. 2.03 ±1.16, respectively; p = 0.0005) (Table 1, Figure 1). This was similar for the female only subgroup where only MN + CP treatment led to a statistically significant improvement according to the G-B scale post-treatment (2.86 ±0.83 vs. 1.89 ±1.11, respectively; p = 0.0005) (Table 2). However, no treatment led to an improvement according to the G-B scale post-treatment in the male subgroup (all p’s > 0.05) (Table 2).
Table 2.
Clinical outcomes and health-related quality-of-life results sub-analysis according to gender
| Parameter | MN + CP (n = 38) | CP alone (n = 41) | MN alone (n = 40) | P-value (MN + CP vs. CP) | P-value (MN + CP vs. MN) | P-value (CP vs. MN) | P-value (ANOVA for three groups comparison) |
|---|---|---|---|---|---|---|---|
| Number of males and females | 10 M/28 F | 8 M/33 F | 10 M/30 F | – | – | – | – |
| Females: | |||||||
| Mean age (SD) | 31.13 (9.56) | 28.33 (5.09) | 31.60 (8.00) | 0.33 | 0.97 | 0.21 | 0.19 |
| G-B before Tx | 2.86 (0.83) | 2.42 (1.02) | 2.87 (1.02) | 0.19 | 1.00 | 0.16 | 0.11 |
| G-B after Tx | 1.89 (1.11) p = 0.0005 | 2.21 (0.95) p = 0.39 | 2.50 (0.92) p = 0.15 | 0.43 | 0.06 | 0.48 | 0.07 |
| DLQI before Tx | 12.89 (6.26) | 6.58 (4.29) | 10.70 (6.44) | 0.0001 | 0.31 | 0.01 | < 0.0001 |
| DLQI after Tx | 7.07 (5.04) p = 0.0003 | 4.67 (3.92) p = 0.06 | 7.27 (5.46) p = 0.03 | 0.13 | 0.99 | 0.09 | 0.06 |
| Males: | |||||||
| Mean age (SD) | 29.00 (6.94) | 29.00 (5.85) | 30.5 (5.83) | 1.00 | 0.85 | 0.87 | 0.83 |
| G-B before Tx | 2.90 (0.83) | 2.86 (0.99) | 2.90 (0.83) | 1.00 | 1.00 | 1.00 | 0.99 |
| G-B after Tx | 2.40 (1.20) p = 0.29 | 2.57 (0.90) p = 0.55 | 2.70 (0.90) p = 0.65 | 0.93 | 0.78 | 0.96 | 0.81 |
| DLQI before Tx | 14.90 (5.11) | 6.71 (3.69) | 9.10 (5.97) | 0.006 | 0.05 | 0.06 | 0.006 |
| DLQI after Tx | 9.20 (5.15) p = 0.023 | 4.43 (3.06) p = 0.20 | 7.40 (5.46) p = 0.56 | 0.11 | 0.68 | 0.40 | 0.13 |
MN – microneedling, CP – chemical peeling, G-B – Goodman and Baron scale, DLQI – Dermatology Life Quality Index, Tx – treatment, M – males, F – females. Numbers in brackets give standard deviation. The p-values given in the “after Tx” cells refer to the pre- and post-treatment comparison. Bold marks statistical significance.
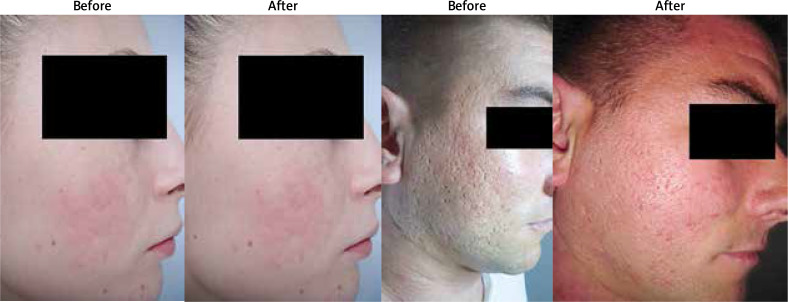
Figure 1.
Patient photographs pre- and post-treatment with microneedling and chemical peeling in the treatment of atrophic post-acne scars
In the MN + CP group, out of 30 females, 25 (83.3%) experienced an improvement of facial skin look post-treatment, measured by the G-B scale, by one or more grades. Five (16.7%) female patients did not experience any improvement of facial skin look post-treatment (4 of which were G-B grade 4). Out of the 10 males, 5 (50%) experienced an improvement of facial skin look post-treatment, measured by the G-B scale. Five (50%) male patients did not experience any improvement of facial skin look post-treatment (3 of which were G-B grade 4).
In the CP group, out of the 33 females, 8 (24.2%) experienced an improvement of facial skin look post-treatment, measured by the G-B scale, by one or more grades. Twenty-five (75.8%) female patients did not experience any improvement of facial skin look post-treatment. Out of the 8 males, 2 (25%) experienced an improvement of facial skin look post-treatment, measured by the G-B scale. Six (75%) male patients did not experience any improvement of facial skin look post-treatment. Lack of improvement post-treatment in the CP group affected both females and males with G-B grades ranging from 1 to 4.
In the MN group, out of the 30 females, 11 (36.7%) experienced an improvement of facial skin look post-treatment, measured by the G-B scale, by one or more grades. Nineteen (63.3%) female patients did not experience any improvement of facial skin look post-treatment. Out of the 10 males, 2 (20%) experienced an improvement of facial skin look post-treatment, measured by the G-B scale. Eight (80%) male patients did not experience any improvement of facial skin look post-treatment. Lack of improvement post-treatment in the CP group affected both females and males with G-B grades ranging from 1 to 4.
HRQoL
An in-depth description of the patients’ HRQoL is presented in Tables 1 and 2. Overall, patients in all three treatment groups experienced a statistically significant improvement in their HRQoL post-treatment (all p’s < 0.05) (Table 1). When assessing gender subgroups, females experienced a statistically significant improvement in their HRQoL only after MN + CP (12.89 ±6.26 vs. 7.07 ±5.04; p = 0.0003) and MN (10.70 ±6.55 vs. 7.27 ±5.46; p = 0.03) treatment. CP treatment was on the verge of statistical significance (6.58 ±4.29 vs. 4.67 ±3.92; p = 0.06). In the male subgroup, only MN + CP treatment led to a statistically significant improvement in HRQoL post-treatment (14.90 ±5.11 vs. 9.20 ±5.15; p = 0.023).
In the MN + CP group, all 40 patients, with the exception of 1 female and 1 male (G-B grade 3 and 4 respectively), experienced an improvement in their HRQoL post-treatment.
In the CP group, 2 females’ (G-B grade 1 and 3, both with no change post-treatment) HRQoL did not change after treatment.
In the MN group, 2 patients’ (female G-B grade 2 and male G-B grade 4 both with no change post-treatment) HRQoL did not change after treatment, and 1 male’s HRQoL (G-B grade 4 with no change post-treatment) worsened (minor change from 4 to 5 points).
Correlations between clinical outcome and changes in HRQoL
MN + CP treatment group
When correlating DLQI and G-B pre-treatment scores, there were statistically significant, moderately strong positive correlations for the overall patient group (R = 0.51; p = 0.0011), as well as for female patients (R = 0.50; p = 0.0067), but statistically insignificant for male (R = 0.54; p = 0.11) patients.
When correlating DLQI and G-B post-treatment scores, there were statistically significant, strong positive correlations for the overall patient group (R = 0.80; p < 0.00001), as well as for female (R = 0.77; p < 0.00001) and male (R = 0.86; p = 0.0014) patients.
CP treatment group
When correlating DLQI and G-B pre-treatment scores, there were statistically significant, moderately strong positive correlations for the overall patient group (R = 0.55; p < 0.00001), as well as for female patients (R = 0.58; p < 0.00001), but statistically insignificant for male (R = 0.65; p = 0.08) patients.
When correlating DLQI and G-B post-treatment scores, there were statistically significant, strong positive correlations for the overall patient group (R = 0.60; p < 0.00001), as well as for female (R = 0.60; p = 0.0002) and male (R = 0.69; p = 0.06) patients.
MN treatment group
When correlating DLQI and G-B pre-treatment scores, there were statistically significant, moderately strong positive correlations for the overall patient group (R = 0.54; p = 0.0003), as well as for female patients (R = 0.62; p = 0.0003), but statistically insignificant for male (R = 0.24; p = 0.50) patients.
When correlating DLQI and G-B post-treatment scores, there were statistically significant, strong positive correlations for the overall patient group (R = 0.58; p < 0.00001), as well as for female (R = 0.64; p = 0.0001) and male (R = 0.41; p = 0.24) patients.
Discussion
Post-acne atrophic scars are a major dermatological challenge, for which numerous treatment options, varying in their skin-related invasiveness, have been devised [14, 15]. This dermatological problem has a major impact on patients’ HRQoL, leading to numerous psychological problems including decreased self-confidence, with potential decreased employability and embarrassment [23, 24], as well as possible negative perception by the society [25]. The above warrants further research into existing and novel treatment options for post-acne atrophic scars, including microneedling with or without adjuvant treatments. This is why the aim of this prospective cohort study was to assess the clinical effectiveness and patients’ quality-of-life after atrophic post-acne scar treatment using microneedling and a combination of trichloroacetic acid, kojic acid and hydrogen peroxide.
The present study has shown that, taking into account all treated patients, only microneedling with adjuvant treatment using PRX-T33 produces a clinically significant improvement, as measured by the Goodman and Baron scale. A more in-depth analysis according to gender has revealed that no treatment from the three tested treatment types managed to produce clinically significant results in males. However, a more qualitative analysis (based on a case-by-case improvement of G-B scores post treatment) of male results has shown that MN + CP treatment produced the best effects from the three tested treatment types. When assessing patients’ HRQoL, taking into account all treated patients, all treatments managed to produce a significant improvement in HRQoL. This highlights the importance of proper patient-doctor communication (as to the potential, beneficial treatment outcomes, regardless of treatment), as well as the significance of patients’ wants and beliefs towards the potential treatment success. However, again a more in-depth analysis according to gender has revealed that MN + CP treatment managed to produce a clinically significant result in male patients.
It is also important to note that no major treatment-related side effects were noted during this study (regardless of the treatment used), which further proves that microneedling with adjuvant treatment using PRX-T33 is a safe treatment option for post-acne atrophic scars.
This study has two main limitations. Firstly, the balance of males and females in each of the treatment groups favours females. This could partially explain the statistically insignificant results obtained in the male subgroup. This imbalance was caused by the fact that this was a single-centre RCT and thus was subjected to certain recruitment restrictions. However, the authors of this study, through proper planning and randomization achieved an almost perfect gender balance among the three treatment groups. Secondly, this RCT reports only early post-treatment outcomes. Reporting long-term or even medium-term outcomes would certainly add value to the study, however it was not possible due to the fact that this RCT was carried out in a rural area. This and the negative attitude of patients towards follow-up prohibited us from reporting long-term results. However, this fact does not diminish the value of the short-term results reported in this paper. A potential third limitation is the fact that an analysis of treatment effect according to age was not possible as the majority of treated patients were young adults (approximately 25–30 years of age). However, patients at such an age were chosen on purpose to create a more homogeneous group for clinical assessment.
Conclusions
Combination of microneedling and chemical peeling produces the best, objectively measured effects in the treatment of atrophic post-acne scars. Using the tested treatments it is easier to produce clinically beneficial results in women than in men. All examined treatments, even if not producing a clinically significant treatment outcome, improve patients’ HRQoL. However, when assessing males and females separately, only the MN + CP treatment managed to improve HRQoL in males. The authors of this double-blind RCT recommend using microneedling and chemical peeling together to produce the best possible results when treating atrophic post-acne scars.
Conflict of interest
The authors declare no conflict of interest.
Appendix 1.
Full treatment protocol (microneedling and chemical peeling)
1. This protocol describes a single session of microneedling and chemical peeling
2. Facial skin disinfection and drying
3. Application of the anaesthetic cream (Emla prescription cream, 5% lidocaine and 5% prilocaine) on the area planned for treatment for approximately 30–45 min (until full anaesthetic effect is obtained)
4. Removal of the anaesthetic cream
5. Re-disinfection and re-drying of the skin
6. Manual application of PRX-T33 acid (nitrile gloves used only) through massaging the entire skin area planned for treatment, until full PRX-T33 absorption occurs
7. Re-application of step 5 three times (to apply a total of 3 layers of PRX-T33; each layer applied 10 min apart)
8. Twenty minutes after the last application of PRX-T33 microneedling is performed with the use of an automatic dermapen (Dermapen, Revisage, Poland) on the facial skin area planned for treatment. Needle penetration depth was set to 2 mm. Microneedling lasts 20 min
9. Re-application of PRX-T33 (additional single layer) immediately after the completion of microneedling. This layer is left for 20 min
10. Washing of the entire treated skin area with running water for approximately 5 min
11. Application of saline-soaked swabs on the treated skin area for 10 min. Next, application of an ice compress on the same skin area for 10 min
References
- 1.Gollnick H, Finlay A, Shear N. Global Alliance to Improve Outcomes in Acne. Can we define acne as a chronic disease? If so, how and when? Am J Clin Dermatol. 2008;9:279–84. doi: 10.2165/00128071-200809050-00001. [DOI] [PubMed] [Google Scholar]
- 2.Dréno B, Poli F. Epidemiology of acne. Dermatology. 2003;206:7–10. doi: 10.1159/000067817. [DOI] [PubMed] [Google Scholar]
- 3.Bhate K, Williams HC. Epidemiology of acne vulgaris. Br J Dermatol. 2012;168:474–85. doi: 10.1111/bjd.12149. [DOI] [PubMed] [Google Scholar]
- 4.Barnes L, Levender M, Fleiscer A, et al. Quality of life measures for acne patients. Dermatol Clin. 2012;30:293–300. doi: 10.1016/j.det.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 5.Tasoula E, Chalikias J, Danopoulou I, et al. The impact of acne vulgaris on quality of life and psychic health in young adolescents in Greece. Results of a population survey. An Bras Dermatol. 2012;87:862–9. doi: 10.1590/S0365-05962012000600007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newton J, Mallon E, Klassen A, et al. The quality of life in acne: a comparison with general medical conditions using generic questionnaires. Br J Dermatol. 1999;140:672–6. doi: 10.1046/j.1365-2133.1999.02768.x. [DOI] [PubMed] [Google Scholar]
- 7.Magnani LR, Schweiger ES. Fractional CO2 lasers for the treatment of atrophic acne scars: a review of the literature. J Cosmet Laser Ther. 2014;16:48–56. doi: 10.3109/14764172.2013.854639. [DOI] [PubMed] [Google Scholar]
- 8.Holland DB. The role of inflammation in the pathogenesis of acne and acne scarring. Semin Cutan Med Surg. 2005;24:79–83. doi: 10.1016/j.sder.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Sobanko JF, Alster TS. Management of acne scarring, part I: a comparative review of laser surgical approaches. Am J Clin Dermatol. 2012;13:319–30. doi: 10.2165/11598910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 10.Layton AM, Henderson C, Cunliffe W. A clinical evaluation of acne scarring and its incidence. Clin Exp Dermatol. 1994;19:303–8. doi: 10.1111/j.1365-2230.1994.tb01200.x. [DOI] [PubMed] [Google Scholar]
- 11.Brown BC, McKenna SP, Siddhi K, et al. The hidden cost of skin scars: quality of life after skin scarring. J Plast Reconstr Aesthet Surg. 2008;61:1049–58. doi: 10.1016/j.bjps.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 12.Gollnick H, Bettoli V, Lambert J, et al. A consensus-based practical and daily guide for the treatment of acne patients. J Eur Acad Dermatol Venereol. 2016;30:1480–90. doi: 10.1111/jdv.13675. [DOI] [PubMed] [Google Scholar]
- 13.Zaenglein A, Pathy A, Schlosser B, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74:945–73. doi: 10.1016/j.jaad.2015.12.037. [DOI] [PubMed] [Google Scholar]
- 14.Waibel J, Rudnick A. Current trends and future considerations in scar treatment. Semin Cutan Med Surg. 2015;34:13–6. doi: 10.12788/j.sder.2015.0129. [DOI] [PubMed] [Google Scholar]
- 15.Lisa R, Henk H, Ali P, et al. Microneedling: where do we stand now? A systematic review of the literature. J Plast Reconstr Aesthet Surg. 2018;71:1–14. doi: 10.1016/j.bjps.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 16.Dover JS, Hruza G. Lasers in skin resurfacing. Aust J Dermatol. 2000;41:72–85. doi: 10.1046/j.1440-0960.2000.00399.x. [DOI] [PubMed] [Google Scholar]
- 17.Aust MC, Reimers K, Repenning C, et al. Percutaneous collagen induction: minimally invasive skin rejuvenation without risk of hyperpigmentation-fact or fiction? Plast Reconstr Surg. 2008;122:1553–63. doi: 10.1097/PRS.0b013e318188245e. [DOI] [PubMed] [Google Scholar]
- 18.McCrudden MTC, McAlister E, Courtenay AJ, et al. Microneedle applications in improving skin appearance. Exp Dermatol. 2015;24:561–6. doi: 10.1111/exd.12723. [DOI] [PubMed] [Google Scholar]
- 19.Aust MC, Reimers K, Kaplan HM, et al. Percutaneous collagen induction-regeneration in place of cicatrisation? J Plast Reconstr Aesthet Surg. 2011;64:97–107. doi: 10.1016/j.bjps.2010.03.038. [DOI] [PubMed] [Google Scholar]
- 20.Hou A, Cohen B, Haimovic A, et al. Microneedling: a comprehensive review. Dermatol Surg. 2017;43:321–39. doi: 10.1097/DSS.0000000000000924. [DOI] [PubMed] [Google Scholar]
- 21.Goodman GJ, Baron JA. Postacne scarring: a qualitative global scarring grading system. Dermatol Surg. 2006;32:1458–66. doi: 10.1111/j.1524-4725.2006.32354.x. [DOI] [PubMed] [Google Scholar]
- 22.Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI): a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210–6. doi: 10.1111/j.1365-2230.1994.tb01167.x. [DOI] [PubMed] [Google Scholar]
- 23.Kaminaka C, Uede M, Matsunaka H, et al. Clinical evaluation of glycolic acid chemical peeling in patients with acne vulgaris: a randomized, double-blind, placebo-controlled, split-face comparative study. Dermatol Surg. 2014;40:314–22. doi: 10.1111/dsu.12417. [DOI] [PubMed] [Google Scholar]
- 24.Tan J, Thiboutot D, Gollnick H, et al. Development of an atrophic acne scar risk assessment tool. J Eur Acad Dermatol Venereol. 2017;31:1547–54. doi: 10.1111/jdv.14325. [DOI] [PubMed] [Google Scholar]
- 25.Dréno B, Tan J, Kang S, et al. How people with facial acne scars are perceived in society: an online survey. Dermatol Ther (Heidelb) 2016;6:207–18. doi: 10.1007/s13555-016-0113-x. [DOI] [PMC free article] [PubMed] [Google Scholar]