Abstract
Numerous studies have demonstrated the clinical value of continuous glucose monitoring (CGM) in type 1 diabetes and type 2 diabetes populations. However, the eligibility criteria for CGM coverage required by the Centers for Medicare & Medicaid Services (CMS) ignore conclusive evidence that supports CGM use in various diabetes populations that are currently deemed ineligible. This article discusses the limitations and inconsistencies of the CMS eligibility criteria relative to current scientific evidence and proposes workable solutions to address this issue and improve the safety and care of all individuals with diabetes.
Keywords: Continuous glucose monitoring, Centers for Medicare & Medicaid Services, Insurance coverage, Type 1 diabetes, Type 2 diabetes
Introduction
Among individuals ≥65 years, the prevalence of diabetes has now reached over 30%.1–4 In the recent Centers for Disease Control & Prevention 2020 report, it was found that an additional 7.3 million adults who met laboratory criteria for diabetes were not aware of their condition.1
The increasing prevalence in the United States continues to be a significant and growing health issue. However, the concern is greatest among racial and ethnic minority populations, in which the prevalence of diabetes and its debilitating complications is significantly higher than the broader white population.5 According to the latest estimates, the prevalence of diagnosed diabetes is highest within the Native American (14.7%), Hispanic American (12.5%), and non-Hispanic black American (11.7%) populations compared with non-Hispanic white (7.5%) populations.1,5
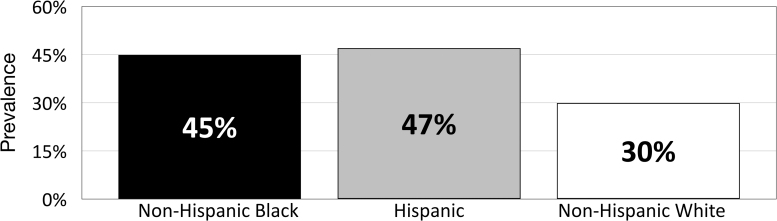
These disparities are most notable among the Medicare diabetes population. Key findings from a 2017 report from Centers for Medicare & Medicaid Services (CMS) revealed that diabetes prevalence was higher among non-Hispanic black (30.0%) and Hispanic beneficiaries (26.7%) compared with non-Hispanic white beneficiaries (18.0%), but that significantly lower percentages of black (65.2%) and Hispanic (64.3%) than white (79.4%) beneficiaries are aware that Medicare helps pay for diabetes testing supplies and education.6 A 2018 survey by the Kaiser Family Foundation reported that the prevalence of diabetes was notably higher within non-Hispanic black and Hispanic Medicare populations compared with the non-Hispanic white population (Fig. 1).3
FIG. 1.
Diabetes prevalence within the Medicare population by race/ethnicity.3
It is important to note that these statistics are from government agency reports, which include comprehensive descriptions of the various scientific methodologies used to generate the data. However, there appears to be disconnection between how science is used to assess a problem but not utilized in solving problems.
Although the medical community traditionally relies on high-quality scientific evidence when developing clinical guidelines for managing diabetes and other conditions, many regulatory agencies and public and private insurers tend to ignore the evidence and take a different path when establishing coverage eligibility criteria for medications and medical devices. This is particularly apparent in the current eligibility criteria for use of personal continuous glucose monitoring (CGM), which deny millions of Americans access to this proven technology.
This article discusses the limitations and inconsistencies of the CMS eligibility criteria relative to current scientific evidence and proposes workable solutions to address this issue and improve the safety and care of all individuals with diabetes.
Evidence Supporting CGM Use in Various Diabetes Populations
Type 1 diabetes
The clinical efficacy of CGM has been demonstrated for over a decade in numerous studies of individuals with type 1 diabetes (T1D) regardless of insulin delivery method.7–23 Benefits of CGM use in this population include reductions in HbA1c,7,9,11,17,18,24–28 fewer severe hypoglycemia events,25,26,29 increased time within target glucose range (TIR),11,18,19,30 and reductions in time below range.11,18 Large observational registry and database studies have also shown an association between CGM use and significant reductions in hospitalizations for severe hypoglycemia and diabetic ketoacidosis (DKA)25,26,29,31 For example, in the RESCUE trial, a multicenter prospective observational cohort study of T1D adults (n = 515) treated with insulin pump therapy, switching from self-monitoring of blood glucose (SMBG) to CGM during the 12-month observation period, was associated with significant reductions in the number of patients hospitalized for severe hypoglycemia, a decrease of 73% (from 11.9% to 3.2%),29 as well as DKA-related hospitalizations decreased by 80% (from 4.6% to 1.1%).
Problematic hypoglycemia regardless of treatment regimen
Problematic hypoglycemia has been well-documented in individuals with T1D and type 2 diabetes (T2D) who are treated with intensive insulin therapy, and recent studies have also reported problematic hypoglycemia in T2D patients who are treated with less intensive insulin regimens or no insulin.32–34 This is particularly concerning among older patients, who are at significantly higher risk for severe hypoglycemia compared with younger patients due to their age, diabetes duration, insulin therapy duration, glucose variability, and higher prevalence of impaired hypoglycemia awareness.35–40 Early and recent studies, utilizing CGM documentation, have demonstrated an increased risk of severe hypoglycemia among patients ≥65 years treated with less intensive insulin regimens or oral antidiabetic medications.16,41,42 Importantly, as reported by Weinstock et al., the risk of severe hypoglycemia is not associated with HbA1c or mean glucose measured by SMBG.35 Recent studies have shown that use of CGM compared with SMBG significantly reduces glycemic variability,17 a risk factor for severe hypoglycemia,43–45 and the time spent in hypoglycemia21,22 among T2D adults treated with intensive insulin therapy.
In a recent study by Pratley et al., 203 older adults (≥60 years) were randomized to CGM or SMBG use.46 At 6 months, CGM use was associated with decreases in severe hypoglycemia compared with SMBG, showing significant reductions in severe hypoglycemia incidence rates (per 100 person-years) compared with SMBG (1.9 vs. 22.4, respectively, P = 0.02). CGM use was also associated with reductions in the percentage of time spent <70 mg/dL (from 5.1% to 2.7%) versus increases with SMBG use (from 4.7% to 4.9%), P < 0.001. Considering that the average cost for a hypoglycemia-related hospitalization among Medicare beneficiaries is estimated at >$10,000,47 the 10-fold decrease in severe hypoglycemia incidence rates reported by Pratley is notable. It is therefore reasonable to suggest that CGM use would significantly reduce health care costs while improving the safety of T2D patients treated with less intensive insulin regimens, particularly in older patients with frequent severe hypoglycemia or impaired hypoglycemia awareness.
Apart from the acute clinical outcomes resulting from severe hypoglycemia events, these episodes also impact patients' willingness to adhere to their prescribed therapy, which can result in suboptimal glycemic control and increased risk of long-term complications.48,49 An international survey of 27,585 diabetes patients found that 25.8% to 46.7% of people with T2D reduced their insulin dosages in response to hypoglycemia.50 CGM use has been shown to reduce hypoglycemia fear and increase patient confidence in avoiding/treating hypoglycemia.7,48 This is particularly relevant in patients with problematic hypoglycemia.
Pregnancy
The CONCEPTT trial assessed the clinical impact of CGM use versus SMBG within a cohort of 325 women with T1D who were pregnant (≤13 weeks gestation) or planning to become pregnant.51 Significant increases in time in target range with CGM compared with SMBG use (68% vs. 61%; P = 0 · 0034, respectively) were observed. CGM users also experienced improved fetal outcomes, including lower incidence of large for gestational age (P = 0.0210), fewer neonatal intensive care admissions lasting more than 24 h (P = 0.0157), fewer incidences of neonatal hypoglycemia (P = 0 · 0250), and shorter length of hospital stay (P = 0 · 0091).
Chronic kidney disease
Although few studies of CGM use in patients with advanced chronic kidney disease (CKD) have been conducted, Joubert et al. demonstrated a strong association between iterative CGM and frequent treatment changes and improved glycemic control without increased risk of hypoglycemia in diabetes patients on chronic dialysis.52 The value of CGM has also been shown in monitoring and managing glycemic levels in nondiabetic patients with end-stage renal disease who are undergoing dialysis.53 A recent analysis of the T1D Exchange registry data set found that fewer participants using CGM experienced an adverse renal outcome compared with those with no history of CGM use.54 An added benefit of CGM use in this population is that it provides additional data regarding glycemic status via the Glucose Management Indicator, which is a more reliable method of monitoring long-term glycemic control compared to HbA1c.55
Telemedicine
Numerous meta-analyses and systematic reviews of randomized controlled trials have demonstrated that the addition of telemedicine and telemonitoring interventions in patients with T1D and T2D results in reductions in HbA1c,56–61 incidence of severe hypoglycemic events,60 diabetes-related distress,62 and improvements in medication adherence.63 A 2020 systematic review and meta-analysis of remote monitoring and telehealth technologies in patients with diabetes reported significant reductions in HbA1c levels.57 Importantly, a subgroup analysis showed that remote patient monitoring is effective for patients who are residents of cities, especially when using monitoring software (e.g., Dexcom Clarity, Abbott LibreView, Medtronic CareLink) as a component of the intervention.
Use of digital diabetes technologies that transmit and present CGM data in a standardized report, such as the Ambulatory Glucose Profile (AGP), support analysis of patient glucose data to inform treatment decisions. When shared with the patient, the AGP results were found to be an effective basis for education, helping achieve better understanding of glycemic variability and increasing involvement in diabetes self-management.64 Most recently, use of remotely monitored CGM data as a component of a comprehensive telemedicine program showed statistically significant HbA1c reductions (P < 0.001) in a cohort to 594 T2D adults treated with less intensive insulin therapy or noninsulin medications.15
Current CMS Eligibility Criteria for CGM Coverage
On January 12, 2017, CMS initiated coverage for use of CGM among insulin-treated diabetes beneficiaries who met the following eligibility criteria: (1) diagnosis of diabetes; (2) documentation of frequent SMBG (defined as testing ≥4 times daily); (3) treatment with intensive insulin therapy (defined as ≥3 insulin injections per day or use of a Medicare-covered insulin pump); (4) frequent adjustment of insulin dosages based on blood glucose measurements; (5) face-to-face consultation with clinician before initiating CGM; and (6) follow-up face-to-face clinical consultations every 6 months. However, despite the demonstrated clinical benefits of CGM, many Medicare beneficiaries with diagnosed diabetes do not meet these eligibility criteria and are thus denied access to CGM technology.65 Most clinicians who care for persons with diabetes are unfamiliar with the criteria and documentation required to obtain a CGM for eligible patients, further limiting use by patients treated in primary care and clinic settings.
Recommended Changes to Current Eligibility Criteria
Eliminate SMBG frequency requirement
Requiring SMBG frequency of ≥4 times daily is not only overly restrictive but also medically unfounded and should not be included in Medicare coverage criteria. In the Ruedy study, 52% of the CGM users reported SMBG frequency of <4 tests per day at baseline, with no association between HbA1c reductions and baseline SMBG frequency.17 A similar absence of association between previous SMBG frequency and positive clinical outcomes with CGM use has been observed in other large, randomized trials.10,21,31
In the DIAMOND T2D study, the mean self-reported SMBG frequency for the CGM and SMBG groups was 3.3 and 3.2, respectively, at baseline.10 At 6 months, the mean change in HbA1c was significantly greater in the CGM group (−1.0) compared with SMBG users (−0.6%), P = 0.005. No association between baseline SMBG frequency and CGM outcomes was observed. Similarly, post hoc analysis of data from the REPLACE study showed no association between prior SMBG frequency (<4 × vs. ≥4 × daily) and outcomes.21
Findings from a recent retrospective claims that data analyses have also shown no association between prior SMBG frequency and reductions in acute diabetes events (ADE) associated with CGM use.31 In the analysis, a cohort of 12,521 individuals with T1D and T2D experienced reductions in ADE from 0.245 to 0.132 events/patient-year (P < 0.001), with similar reductions observed in patients testing <4 and ≥4 times per day.31 Importantly, many Medicare beneficiaries are unable to meet the ≥4 times per day fingerstick testing requirement due to limited dexterity or restrictions on the number of strips allowed by Medicare.66 Although ≥4 times per day fingerstick testing is required for coverage, Medicare only covers 100 test strips per month (∼3 strips/day) unless clinicians are willing to provide additional documentation supporting more frequent testing.
Eliminate intensive insulin regimen requirements for T2D
Studies have demonstrated that use of CGM by T2D patients confers significant reductions in HbA1c levels,10,13–15,17,24,67,68 significant increases in percent time in range (defined as glucose values between 70 and 180 mg/dL, %TIR),10,17 significant decreases in percent time below range (defined as glucose values <70 mg/dL, %TBR),21,22 and significant reductions in diabetes-related hospitalizations31,69 regardless of insulin regimen. Although a substantial number of T2D Medicare beneficiaries are treated with less-intensive insulin regimens, they are at much higher risk for severe hypoglycemia than younger patients.16,35,36,42
Eliminate requirement for frequent insulin dosage adjustments based on glucose values
The requirement for a documented history of frequent insulin dosage adjustment based on SMBG values is unrealistic and burdensome for both health care providers and patients, and there is no evidence demonstrating its value as a predictor of successful CGM use. Moreover, this requirement ignores the safety features of CGM, which include the automated alarms and alerts that warn patients of current or impending hypoglycemia/hyperglycemia, enabling them to take immediate remedial action. Importantly, several instances of U-500- and premixed insulin-related errors have been reported, resulting in severe hypoglycemia.70 These insulin preparations are generally administered only once or twice daily, which means that patients using these medications are not currently eligible for CGM use, denying them an important safety device. Finally, the specific wording of the requirement for “injecting” insulin fails to address other options for insulin administration (e.g., insulin infusion using a pump and inhaled insulin).
Include telemedicine as an option for clinical consultations
Apart from the fact that FDA labeling for current CGM systems does not require in-person training, numerous studies have shown that use of telemedicine consults confers significant glycemic56–61 and psychosocial62,63 benefits. Moreover, the successful utilization of telemedicine consults during the COVID-19 pandemic71–75 highlights the clinical value and utility of this approach to health care delivery. It is hoped that this temporary allowance by CMS will continue after the pandemic ends, and that Congress will include all patients who choose this option, not just those in rural communities. Because many older patients may not have access or unable to use more advanced telemedicine technologies, remote consultations via telephone must be an option for meeting the 6-month consult requirement for verifying continued CGM use.
Streamlined and standardized documentation requirements for obtaining coverage
Because obtaining CMS coverage for CGM places an unwarranted burden on clinicians and office staff who must gather and submit substantial documentation,76 many clinicians are unwilling or unable to meet the documentation requirements. This, in turn, can be detrimental to patient care. In a 2017 survey by the American Medical Association, 92% of the 1000 physicians surveyed reported that prior authorizations delay patient treatment and negatively impact clinical outcomes.77 Importantly, 78% of respondents reported that the documentation requirements associated with obtaining preauthorizations sometimes (57%), often (19%), or always (2%) result in their patients discontinuing their prescribed therapy. This is evidenced by the apparent racial/ethnic disparities relative to CGM eligibility within the Medicare population. A recent analysis of 2018 Medicare data found that the majority (69%) of insulin-treated beneficiaries do not qualify for CGM coverage under the current ≥4x daily blood glucose testing requirement.65 However, the percentage of ineligible non-Hispanic black (≥74%) and Hispanic (≥75%) beneficiaries is notably higher than observed in non-Hispanic white (68%) beneficiaries.
Provide clear guidance to Durable Medical Equipment suppliers
Beneficiary access to CGM is further hindered by CMSs lack of clarity in providing guidance to Durable Medical Equipment (DME) suppliers for determining the medical necessity for CGM in many of the coverage claims they receive. As a result, claims are often rejected by suppliers to avoid potential financial penalties that could be imposed by CMS.
Proposed Eligibility Criteria for CGM Coverage
To expand access to all individuals who would benefit from CGM, streamline clinician documentation requirements and clarify DME supplier guidance, the panel recommends that CMS modify its eligibility requirements to include all Medicare beneficiaries who meet any one of the first four criteria below, and who also meet the fifth criterion:
-
1.
Diagnosed with T1D.
-
2.
Diagnosed with T2D and treated with any insulin regimen.
-
3.
Diagnosed with T2D and documented problematic hypoglycemia regardless of diabetes therapy. This would include a history of at least one of the following conditions: Level 2 (moderate) hypoglycemia, characterized by glucose levels ≤54 mg/dL; Level 3 (severe) hypoglycemia, characterized by physical/mental dysfunction requiring third-party assistance; or nocturnal hypoglycemia
-
4.
Advanced CKD at risk for hypoglycemia.
-
5.
In-person or telemedicine consultation with the prescribing health care provider before CGM initiation and every 6 months thereafter while continuing CGM therapy. (Coverage for telemedicine consults should be available for all patients regardless of geographic location.)
Table 1 presents the link between these proposed criteria and their supporting evidence.
Table 1.
Proposed Eligibility Criteria and Supporting Evidence
| Criterion | Supporting evidence |
|---|---|
| 1. Diagnosed with T1D. | CGM use confers: Significant reductions in HbA1c.7,9,11,17,18,24–28 Significant reductions in severe hypoglycemia events.25,26,29 Significant increases in %TIR.11,18,19,30 Significant decreases in %TBR.11,18 Significant reductions in diabetes-related hospitalizations.25,26,29,31 Significant improvements in treatment satisfaction with less diabetes distress25,27,78 |
| 2. Diagnosed with T2D and treated with any insulin therapy. | CGM use confers: Significant reductions in HbA1c.10,13–15,17,24,67,68,79 Significant increases in %TIR.10,17 Significant decreases in %TBR.21,22 Significant reductions in diabetes-related hospitalizations.31,69 |
| 3. Diagnosed with T2D and documented problematic hypoglycemia regardless of diabetes therapy. This would include a history of at least one of the following conditions: Level 2 (moderate) hypoglycemia, characterized by glucose levels ≤54 mg/dL. Level 3 (severe) hypoglycemia—characterized by physical/mental dysfunction requiring third-party assistance. Nocturnal hypoglycemia. |
Older diabetes patients are at increased hypoglycemia risk: T2D patients treated with antihyperglycemic medications (e.g., insulin, sulfonylureas) are at higher risk for hypoglycemia than those treated with nonhypoglycemia medications (e.g., metformin).16 T2D patients ≥65 years treated with basal insulin (typically one injection per day) are at increased risk for severe hypoglycemia.42 A key driver of hypoglycemia risk is impaired hypoglycemia awareness.35,70 CGM use confers: Significant reductions in diabetes-related hospitalizations, including severe hypoglycemia events.31,69 Significant reductions in hypoglycemia fear and increases patient confidence in avoiding/treating hypoglycemia,7,80 thereby supporting treatment adherence.48,49 |
| 4. CKD. | CGM use facilitates: More frequent treatment changes and improved glycemic control without increased risk of hypoglycemia.52 Effective monitoring and managing glycemic levels in non-diabetic patients with ESRD undergoing dialysis.53 |
| 5. In-person or telemedicine consultation with the prescribing health care provider before CGM initiation and every 6 months thereafter while continuing CGM therapy. | Use of telemedicine consults: Significantly reduces HbA1c.56–61 Reduces the incidence of severe hypoglycemic events.60 Significantly reduces diabetes-related distress.62 Significantly improves medication adherence.63 Effectively addresses the obstacles caused by the COVID-19 pandemic.71–75 Are more effective for patients who are residents of cities and using the websites as their intervention method.57 Use of downloaded CGM data into standardized reports: Supports patient education.64 Enhances patient engagement in their self-management.64 |
%TIR = percentage of time in glucose range (70–180 mg/dL); %TBR = percentage of time below target ranges (<70 mg/dL, <54 mg/dL).
CGM, continuous glucose monitoring; CKD, chronic kidney disease; ESRD, end-stage renal disease; T1D, type 1 diabetes; T2D, type 2 diabetes.
Conclusion
A substantial and growing body of evidence clearly demonstrates the clinical benefits of CGM in individuals with T1D and T2D regardless of their current therapy and prior glucose monitoring frequency.21,31,67–69,79,81,82 The medically unfounded Medicare eligibility criteria for CGM coverage and lack of clear guidance to DME suppliers deny access to CGM among a substantial population of Medicare beneficiaries with diagnosed diabetes. To the extent that Medicare's coverage criteria are adopted by Medicaid or commercial payers, these policies have a negative ripple effect on access to CGM.
Restricted access to CGM is particularly concerning within populations of patients with racial and ethnic diversity. As reported by Taylor et al. in an early study of racial and ethnic disparities in diabetes care, non-Hispanic black patients had a 25% lower odds ratio for achieving HbA1c levels of <8.0% and 58% higher odds ratio of sustaining HbA1c levels at >9.0%.83 An analysis of unpublished Medicare data revealed that a notably higher percentage of non-Hispanic black (>75%) and Hispanic (>75%) insulin-treated beneficiaries are ineligible for CGM based on their current SMBG frequency. Importantly, although an early study by Groeneveld et al. found that there were inconsistent differences between non-Hispanic black and non-Hispanic white patients in their openness to using innovative technology,84 a recent study of 300 young adult T1D patients found notably lower use of CGM among non-Hispanic black (28%) and Hispanic (37%) patients compared with non-Hispanic white (71%) patients.85
Many researchers add a qualifier at the end of their published study reports, explaining how additional studies are needed to further understand their findings and/or support their conclusions. For this report, no such qualifier is needed. Given the demonstrated clinical benefits and lower rates of diabetes-related hospitalizations associated with CGM use, limiting access to CGM technology achieves neither cost-efficiencies nor clinical efficacies. We believe our evidence-based recommendations for modifying current eligibility criteria both streamline the administrative processes for documenting medical necessity, expand access to CGM and improve the safety of our most vulnerable diabetes population.
Authors' Contributions
R.J.G., C.G.P., and J.B.M. wrote the article. G.A., A.L.C., D.F.K., C.J.L., and G.E.U. provided input on the draft. All authors reviewed the final draft and approved its submission.
Author Disclosure Statement
R.J.G. has received unrestricted research support to Emory for investigator-initiated studies from Novo Nordisk and Dexcom, Inc., and consulting fees from Abbott Diabetes Care, Sanofi, Novo Nordisk, Eli Lilly, BI, and Valeritas. R.J.G. is partially supported by research grants from NIH/NIDDK P30DK11102 and 1K23DK123384-01. C.G.P. has received consulting fees from Abbott Diabetes Care, CeQur SA, Dexcom, Inc., Onduo, Roche Diabetes Care; and Novo Nordisk. G.A. has received research support from AstraZeneca, Dexcom, Eli Lilly, Insulet, Novo Nordisk, and is a consultant for Dexcom and Insulet. A.L.C. has received research support from UnitedHealthcare, Abbott, Dexcom, Eli Lilly, Insulet, Medtronic, Novo Nordisk, and Sanofi, and is a consultant for Medtronic and Insulet, with all financial support going to his institution. In addition, A.L.C. has a patent, Treatment of Hypoglycemia Unawareness with Intranasal Insulin, pending to HealthPartners Institute. D.F.K. has served on advisory boards and/or speaker bureaus for Dexcom and Abbott Diabetes Care, and her institution has received research support from Dexcom. C.J.L. reports grants from Abbott Diabetes, Dexcom, Insulet and Senseonics paid to her institution, non-financial device support from Dexcom and Abbott Diabetes, service as a consultant for Sanofi, Eli Lilly and Dexcom, and other support from Novo Nordisk outside the submitted work. G.E.U. is partly supported by research grants from the NIH/NATS UL1 TR002378 and 1P30DK111024-05, and P30DK111024-05S and has received unrestricted research support (to Emory University) from Astra Zeneca, Novo Nordisk, and Dexcom. J.B.M. reports consulting fees from Bayer, Boehringer Ingelheim, Lilly, Metavant, and Salix as well as research funding grants from Dexcom, Medtronic, and Novo Nordisk.
Funding Information
Dexcom, Inc., provided funding for editorial assistance in developing this article.
References
- 1. Centers for Disease Control and Prevention (CDC): National Diabetes Statistics Report, 2020: Estimates of diabetes and Its Burden in the United States. https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf (accessed February29, 2020)
- 2. Centers for Disease Control and Prevention: Diabetes Prevalence and Incidence Among Medicare Beneficiaries—United States, 2001–2015. https://www.cdc.gov/mmwr/volumes/68/wr/mm6843a2.htm (accessed February26, 2021)
- 3. Kaiser Family Foundation: Racial and Ethnic Health Inequities and Medicare. February 2021. http://files.kff.org/attachment/Report-Racial-and-Ethnic-Health-Inequities-and-Medicare.pdf (accessed February25, 2021)
- 4. Teigland C, Pulungan Z, Shah T, et al. : As It Grows, Medicare Advantage Is Enrolling More Low-Income and Medically Complex Beneficiaries. The Commonwealth Fund May 13, 2020. https://www.commonwealthfund.org/publications/issue-briefs/2020/may/medicare-advantage-enrolling-low-income-medically-complex (accessed February26, 2021)
- 5. U.S. Food & Drug Administration: Fighting Diabetes' Deadly Impact on Minorities. April 10, 2020. https://www.fda.gov/consumers/consumer-updates/fighting-diabetes-deadly-impact-minorities (accessed February7, 2021)
- 6. Centers for Medicare & Medicaid Services: Racial and Ethnic Disparities in Diabetes Prevalence, Self-Management, and Health Outcomes among Medicare Beneficiaries. Volume 6, 2017. https://www.cms.gov/About-CMS/Agency-Information/OMH/research-and-data/information-products/data-highlights/disparities-in-diabetes-prevalence (accessed February3, 2021)
- 7. Lind M, Polonsky W, Hirsch IB: Continuous glucose monitoring vs conventional therapy for glycemic control in adults with type 1 diabetes treated with multiple daily insulin injections: the GOLD randomized clinical trial. JAMA 2017;317:379–387 [DOI] [PubMed] [Google Scholar]
- 8. Aleppo G, Ruedy KJ, Riddlesworth TD, et al. : REPLACE-BG: a randomized trial comparing continuous glucose monitoring with and without routine blood glucose monitoring in adults with well-controlled type 1 diabetes. Diabetes Care 2017;40:538–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Beck RW, Riddlesworth T, Ruedy K, et al. : Effect of continuous glucose monitoring on glycemic control in adults with type 1 diabetes using insulin injections: the DIAMOND randomized clinical trial. JAMA 2017;317:371–378 [DOI] [PubMed] [Google Scholar]
- 10. Beck RW, Riddlesworth TD, Ruedy K, et al. : Continuous glucose monitoring versus usual care in patients with type 2 diabetes receiving multiple daily insulin injections: a randomized trial. Ann Intern Med 2017;167:365–374 [DOI] [PubMed] [Google Scholar]
- 11. Šoupal J, Petruželková L, Grunberger G, et al. : Glycemic outcomes in adults with T1D are impacted more by continuous glucose monitoring than by insulin delivery method: 3 years of follow-up from the COMISAIR study. Diabetes Care 2020;43:37–43 [DOI] [PubMed] [Google Scholar]
- 12. Beck RW, Riddlesworth TD, Ruedy K, et al. : Effect of initiating use of an insulin pump in adults with type 1 diabetes using multiple daily insulin injections and continuous glucose monitoring (DIAMOND): a multicentre, randomised controlled trial. Lancet Diabetes Endocrinol 2017;5:700–708 [DOI] [PubMed] [Google Scholar]
- 13. Vigersky RA, Fonda SJ, Chellappa M, et al. : Short- and long-term effects of real-time continuous glucose monitoring in patients with type 2 diabetes. Diabetes Care 2012;35:32–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Majithia AR, Kusiak CM, Lee AA, et al. : Glycemic outcomes in adults with type 2 diabetes participating in a continuous glucose monitor–driven virtual diabetes clinic: prospective trial. J Med Internet Res 2020;22:e21778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bergenstal RM, Layne JE, Zisser H, et al. : Remote application and use of real-time continuous glucose monitoring by adults with type 2 diabetes in a virtual diabetes clinic. Diabetes Technol Ther 2021;23:128–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gehlaut RR, Dogbey GY, Schwartz FL, et al. : Hypoglycemia in type 2 diabetes—more common than you think: a continuous glucose monitoring study. J Diabetes Sci Technol 2015;9999–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ruedy KJ, Parkin CG, Riddlesworth TD, Graham C; for the DIAMOND Study Group: Continuous glucose monitoring in older adults with type 1 and type 2 diabetes using multiple daily injections of insulin: results from the DIAMOND trial. J Diabetes Sci Technol 2017;11:1138–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Šoupal J, Petruželková L, Flekač M, et al. : Comparison of different treatment modalities for type 1 diabetes, including sensor-augmented insulin regimens, in 52weeks of follow-up: a COMISAIR study. Diabetes Technol Ther 2016;18:532–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bolinder J, Antuna R, Geelhoed-Duijvestijn P, et al. : Novel glucose-sensing technology and hypoglycemia in type 1 diabetes: a multicentre, non-masked, randomised controlled trial. Lancet 2016;388:2254–2263 [DOI] [PubMed] [Google Scholar]
- 20. Oskarsson P, Antuna R, Geelhoed-Duijvestijn P, et al. : Impact of flash glucose monitoring on hypoglycaemia in adults with type 1 diabetes managed with multiple daily injection therapy: a pre-specified subgroup analysis of the IMPACT randomised controlled trial. Diabetologia 2018;61:539–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haak T, Hanaire H, Ajjan R, et al. : Use of flash glucose sensing technology for 12months as a replacement for blood glucose monitoring in insulin-treated type 2 diabetes. Diabetes Ther 2017;8:573–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haak T, Hanaire H, Ajjan R, et al. : Flash glucose-sensing technology as a replacement for blood glucose monitoring for the management of insulin-treated type 2 diabetes: a multicenter, open-label randomized controlled trial. Diabetes Ther 2017;8:55–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. The Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group: Continuous glucose monitoring and intensive treatment of type 1 diabetes. N Engl J Med 2008;359:1464–1476 [DOI] [PubMed] [Google Scholar]
- 24. Kröger J, Fasching P, Hanaire H: Three European retrospective real-world chart review studies to determine the effectiveness of flash glucose monitoring on HbA1c in adults with type 2 diabetes. Diabetes Ther 2020;11:279–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Charleer S, De Block C, Van Huffel L, et al. : Quality of life and glucose control after 1 year of nationwide reimbursement of intermittently scanned continuous glucose monitoring in adults living with type 1 diabetes (FUTURE): a prospective observational real-world cohort study. Diabetes Care 2020;43:389–397 [DOI] [PubMed] [Google Scholar]
- 26. Fokkert M, van Dijk P, Edens M, et al. : Improved well-being and decreased disease burden after 1-year use of flash glucose monitoring (FLARE-NL4). BMJ Open Diabetes Res Care 2019;7:e000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tyndall V, Stimson RH, Zammitt NN, et al. : Marked improvement in HbA1c following commencement of flash glucose monitoring in people with type 1 diabetes. Diabetologia 2019;62:1349–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Paris I, Henry C, Pirard F, et al. : The new FreeStyle Libre Flash Glucose Monitoring System improves the glycaemic control in a cohort of people with type 1 diabetes followed in real-life conditions over a period of one year. Endocrinol Diab Metab 2018;1:e00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Charleer S, Mathieu C, Nobels F, et al. : Effect of continuous glucose monitoring on glycemic control, acute admissions, and quality of life: a real-world study. Clin Endocrinol Metab 2018;103:1224–1232 [DOI] [PubMed] [Google Scholar]
- 30. van Beers CA, DeVries JH, Kleijer SJ, et al. : Continuous glucose monitoring for patients with type 1 diabetes and impaired awareness of hypoglycaemia (IN CONTROL): a randomised, open-label, crossover trial. Lancet Diabetes Endocrinol 2016;4:893–902 [DOI] [PubMed] [Google Scholar]
- 31. Hirsch IB, Kerr MSD, Roberts GJ, et al. : Utilization of continuous glucose monitors is associated with reduction in inpatient and outpatient emergency acute diabetes events regardless of prior blood test strip usage. Diabetes 2020;Suppl 1:875-P [Google Scholar]
- 32. Matsuoka A, Hirota Y, Takeda A, et al. : Relationship between glycated hemoglobin level and duration of hypoglycemia in type 2 diabetes patients treated with sulfonylureas: a multicenter cross-sectional study. J Diabetes Investig 2020;11:417–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dunkley, AJ, Fitzpatrick C, Gray LJ, et al. : Incidence and severity of hypoglycaemia in type 2 diabetes by treatment regimen: a UK multisite 12-month prospective observational study. Diabetes Obes Metab 2019;21:1585–1595 [DOI] [PubMed] [Google Scholar]
- 34. Lipska KJ, Yao X, Herrin J, et al. : Trends in drug utilization, glycemic control, and rates of severe hypoglycemia, 2006–2013. Diabetes Care 2017;40:468–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weinstock RS, DuBose SN, Bergenstal RM, et al. : Risk factors associated with severe hypoglycemia in older adults with type 1 diabetes. Diabetes Care 2016;39:603–610 [DOI] [PubMed] [Google Scholar]
- 36. Bremer JP, Jauch-Chara K, Hallschmid M, et al. : Hypoglycemia unawareness in older compared with middle-aged patients with type 2 diabetes. Diabetes Care 2009;32:1513–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Punthakee Z, Miller ME, Launer LJ, et al. : Poor cognitive function and risk of severe hypoglycemia in type 2 diabetes: post hoc epidemiologic analysis of the ACCORD trial. Diabetes Care 2012;35:787–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Giorda CB, Ozzello A, Gentile S, et al. : Incidence and risk factors for severe and symptomatic hypoglycemia in type 1 diabetes. Results of the HYPOS-1 study. Acta Diabetol 2015;52:845–853 [DOI] [PubMed] [Google Scholar]
- 39. Cariou B, Fontaine P, Eschwege E, et al. : Frequency and predictors of confirmed hypoglycaemia in type 1 and insulin-treated type 2 diabetes mellitus patients in a real-life setting: results from the DIALOG study. Diabetes Metab 2015;41:116–125 [DOI] [PubMed] [Google Scholar]
- 40. Seaquist ER, Anderson J, Childs B, et al. : Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013;36:1384–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Munshi MN, Segal AR, Suhl E, et al. : Frequent hypoglycemia among elderly patients with poor glycemic control. Arch Intern Med 2011;171:362–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Hollander PA, Kiljanski J, Spaepen E, Harris CJ: Risk of clinically relevant hypoglycaemia in patients with type 2 diabetes self-titrating insulin glargine U-100. Diabetes Obes Metab 2019;21:2413–2421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Monnier L, Colette C, Wojtusciszyn A, et al. : Toward defining the threshold between low and high glucose variability in diabetes. Diabetes Care 2017;40:832–838 [DOI] [PubMed] [Google Scholar]
- 44. Kovatchev B: Glycemic variability: risk factors, assessment, and control. J Diabetes Sci Technol 2019;13:627–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kovatchev BP, Cox DJ, Gonder-Frederick LA, et al. : Assessment of risk for severe hypoglycemia among adults with IDDM: validation of the low blood glucose index. Diabetes Care 1998;21:1870–1875 [DOI] [PubMed] [Google Scholar]
- 46. Pratley RE, Kanapka LG, Rickels MR, et al. : Effect of continuous glucose monitoring on hypoglycemia in older adults with type 1 diabetes; a randomized clinical trial. JAMA 2020;323:2397–2406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Goyal R, Sura S, Mehta H: Direct medical costs of hypoglycemia hospitalizations in the United States. In: Poster Session Presented at: ISPOR 20th Annual European Congress. Glasglow, Scotland, November 4–8, 2017
- 48. Barnard K, Thomas S, Royle P, et al. : Fear of hypoglycaemia in parents of young children with type 1 diabetes: a systematic review. BMC Pediatr 2010;10:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Haugstvedt A, Wentzel-Larsen T, Graue M, et al. : Fear of hypoglycaemia in mothers and fathers of children with type 1 diabetes is associated with poor glycaemic control and parental emotional distress: a population-based study. Diabet Med 2010;27:72–78 [DOI] [PubMed] [Google Scholar]
- 50. Khunti K, Alsifri S, Aronson R, et al. : Impact of hypoglycaemia on patient-reported outcomes from a global, 24-country study of 27,585 people with type 1 and insulin-treated type 2 diabetes. Diabetes Res Clin Pract 2017;130:121–129 [DOI] [PubMed] [Google Scholar]
- 51. Feig DS, Donovan LE, Corcoy R, et al. : Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet 2017;390:2347–2359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Joubert M, Fourmy C, Henri P, et al. : Effectiveness of continuous glucose monitoring in dialysis patients with diabetes: the DIALYDIAB pilot study. Diabetes Res Clin Pract 2015;107:348–354 [DOI] [PubMed] [Google Scholar]
- 53. Sobngwi E, Ashuntantang G, Ndounia E, et al. : Continuous interstitial glucose monitoring in nondiabetic subjects with end-stage renal disease undergoing maintenance haemodialysis. Diabetes Res Clin Pract 2010;90:22–25 [DOI] [PubMed] [Google Scholar]
- 54. McGill JB, Wu M, Pop-Busui R, et al. : Risk factors for adverse kidney disease outcomes in type 1 diabetes: results from the T1D Exchange Clinic Network. J Diabetes Complications 2019;33:107400. PMID: 31279735 [DOI] [PubMed] [Google Scholar]
- 55. Bergenstal RM, Beck RW, Close KL, et al. : Glucose Management Indicator (GMI): a new term for estimating A1C from continuous glucose monitoring. Diabetes Care 2018;41:2275–2280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Faruque LI, Wiebe N, Ehteshami-Afshar, et al.: Effect of telemedicine on glycated hemoglobin in diabetes: a systematic review and meta-analysis of randomized trials. CMAJ 2017;189:E341–E364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Salehi S, Olyaeemanesh A, Mobinizadeh M, et al. : Assessment of remote patient monitoring (RPM) systems for patients with type 2 diabetes: a systematic review and meta-analysis. J Diabetes Metab Disord 2020;19:115–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Tchero H, Kangambega P, Briatte C, et al. : Clinical effectiveness of telemedicine in diabetes mellitus: a meta-analysis of 42 randomized controlled trials. Telemed J E Health 2019;25:569–583 [DOI] [PubMed] [Google Scholar]
- 59. Wang X, Shu W, Du J, et al. : Mobile health in the management of type 1 diabetes: a systematic review and meta-analysis. BMC Endocr Disord 2019;19:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Charpentier G, Benhamou PY, Dardari D, et al. : The Diabeo software enabling individualized insulin dose adjustments combined with telemedicine support improves HbA1c in poorly controlled type 1 diabetic patients: a 6-month, randomized, open-label, parallel-group, multicenter trial (TeleDiab 1 Study). Diabetes Care 2011;34:533–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Dixon RF, Zisser H, Layne JE, et al. : A Smartphone-Based Type 2 Diabetes Clinic Using Video Endocrinology Consultations and CGM. J Diabetes Sci Technol 2020;14:908–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Polonsky WH, Layne JE, Parkin CG, et al. : Impact of participation in a virtual diabetes clinic on diabetes-related distress in individuals with type 2 diabetes. Clin Diabetes 2020;38:357–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lee WC, Balu S, Cobden D, et al. : Medication adherence and the associated health-economic impact among patients with type 2 diabetes mellitus converting to insulin pen therapy: an analysis of third-party managed care claims data. Clin Ther 2006;28:1712–1725 [DOI] [PubMed] [Google Scholar]
- 64. Matthaei S: Assessing the value of the Ambulatory Glucose Profile in clinical practice. Br J Diabetes Vasc Dis 2014;14:148–152 [Google Scholar]
- 65. National Minority Quality Forum (NMQF). Unpublished Data [Google Scholar]
- 66. Pfützner J, Hellhammer J, Musholt P, et al. : Evaluation of dexterity in insulin-treated patients with type 1 and type 2 diabetes mellitus. J Diabetes Sci Technol 2011;5:158–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Miller E, Brandner L, Wright E: HbA1c reduction after initiation of the FreeStyle Libre® System in type 2 diabetes patients on long-acting insulin or non-insulin therapy. Diabetes 2020;Suppl 1:84-LB [Google Scholar]
- 68. Wright E, Kerr MSD, Reyes I, et al. : HbA1c reduction associated with a FreeStyle Libre® system in people with type 2 diabetes not on bolus insulin therapy. Diabetes 2020;Suppl 1:78-LB-P. [Google Scholar]
- 69. Miller E, Kerr MSD, Roberts GJ, et al. : FreeStyle Libre® system use associated with reduction in acute diabetes events and all-cause hospitalizations in patients with type 2 diabetes without bolus insulin. Diabetes 2020;Suppl 1:85-LB [Google Scholar]
- 70. ISMP: Problems associated with the use of U-500 insulin syringes. ISMP Medication Safety Alert! 2017;22:1.4 [Google Scholar]
- 71. Keesara S, Jonas A, Schulman K: Covid-19 and health care's digital revolution. N Engl J Med 2020. [Epub ahead of print]; DOI: 10.1056/NEJMp2005835 [DOI] [PubMed] [Google Scholar]
- 72. Galindo RG, Aleppo G, Klonoff DC, et al. : Implementation of continuous glucose monitoring in the hospital: emergent considerations for remote glucose monitoring during the COVID-19 pandemic. J Diabetes Sci Technol 2020. [Epub ahead of print]; DOI: 10.1177/1932296820932903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Jones MS, Goley AL, Alexander BE, et al. : Inpatient transition to virtual care during COVID-19 pandemic. Diabetes Technol Ther 2020;22:444–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Centers for Disease Control and Prevention: Coronavirus Disease 2019 (COVID-19). Using Telehealth to Expand Access to Essential Health Services during the COVID-19 Pandemic. June 10, 2020. https://www.cdc.gov/coronavirus/2019-ncov/hcp/telehealth.html (accessed June26, 2020)
- 75. Peters AL, Garg SK: The silver lining to COVID-19: avoiding diabetic ketoacidosis admissions with telehealth. Diabetes Technol Ther 2020;22:449–453 [DOI] [PubMed] [Google Scholar]
- 76. Huynh P, Toulouse A, Hirsch IB: One-year time analysis in an academic diabetes clinic: quantifying our burden. Endocr Pract 2018;24:489–491 [DOI] [PubMed] [Google Scholar]
- 77. American Medical Association (AMA): 2017 AMA Prior Authorization Physician Survey. https://www.ama-assn.org/sites/ama-assn.org/files/corp/media-browser/public/arc/prior-auth-2017.pdf (accessed March1, 2021)
- 78. Al Hayek AA, Al Dawish MA: The potential impact of the FreeStyle Libre Flash glucose monitoring system on mental well-being and treatment satisfaction in patients with type 1 diabetes: a prospective study. Diabetes Ther 2019;10:1239–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Manning JP, Halford J, Sulik B, et al. : Use of continuous glucose monitoring is acceptable and potentially beneficial in older T2DM patients treated with basal insulin therapy: a pilot study. Infusystems USA 2014;11:1–5 [Google Scholar]
- 80. Ólafsdóttir AF, Polonsky W, Bolinder J, et al. : A randomized clinical trial of the effect of continuous glucose monitoring on nocturnal hypoglycemia, daytime hypoglycemia, glycemic variability, and hypoglycemia confidence in persons with type 1 diabetes treated with multiple daily insulin injections (GOLD-3). Diabetes Technol Ther 2018;20:274–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Roussel R, Bruno Guerci B, Vicaut E, et al. : Dramatic drop in ketoacidosis rate after FreeStyle Libre™ system initiation in type 1 and type 2 diabetes in France, especially in people with low self-monitoring of blood glucose (SMBG): a nationwide study. Diabetes 2020;Suppl 1:68-OR [Google Scholar]
- 82. Anderson JE, Gavin JR, Kruger DF: Current eligibility requirements for CGM coverage are harmful, costly, and unjustified. Diabetes Technol Ther 2020;22:169–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Taylor YJ, David ME, Mahabaleshwarkar R, Spencer MD: Racial/ethnic disparities in diabetes care and outcomes: a mixed methods study. JHDRP 2018;11. https://digitalscholarship.unlv.edu/cgi/viewcontent.cgi?article=1748&context=jhdrp (accessed November13, 2020)
- 84. Groeneveld PW, Sonnad SS, Lee AK, et al. : Racial differences in attitudes toward innovative medical technology. J Gen Intern Med 2006;21:559–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Agarwal S, Schechter C, Gonzalez, Long JA: Racial–ethnic disparities in diabetes technology use among young adults with type 1 diabetes. Diabetes Technol Ther 2020. [Epub ahead of print]; DOI: 10.1089/dia.2020.0338 [DOI] [PMC free article] [PubMed] [Google Scholar]