Abstract
Background: The t:slim X2™ insulin pump with Control-IQ® technology from Tandem Diabetes Care is an advanced hybrid closed-loop system that was first commercialized in the United States in January 2020. Longitudinal glycemic outcomes associated with real-world use of this system have yet to be reported.
Methods: A retrospective analysis of Control-IQ technology users who uploaded data to Tandem's t:connect® web application as of February 11, 2021 was performed. Users age ≥6 years, with >2 weeks of continuous glucose monitoring (CGM) data pre- and >12 months post-Control-IQ technology initiation were included in the analysis.
Results: In total 9451 users met the inclusion criteria, 83% had type 1 diabetes, and the rest had type 2 or other forms of diabetes. The mean age was 42.6 ± 20.8 years, and 52% were female. Median percent time in automation was 94.2% [interquartile range, IQR: 90.1%–96.4%] for the entire 12-month duration of observation, with no significant changes over time. Of these users, 9010 (96.8%) had ≥75% of their CGM data available, that is, sufficient data for reliable computation of CGM-based glycemic outcomes. At baseline, median percent time in range (70–180 mg/dL) was 63.6 (IQR: 49.9%–75.6%) and increased to 73.6% (IQR: 64.4%–81.8%) for the 12 months of Control-IQ technology use with no significant changes over time. Median percent time <70 mg/dL remained consistent at ∼1% (IQR: 0.5%–1.9%).
Conclusion: In this real-world use analysis, Control-IQ technology retained, and to some extent exceeded, the results obtained in randomized controlled trials, showing glycemic improvements in a broad age range of people with different types of diabetes.
Keywords: Continuous glucose monitoring, Automated insulin dosing, Closed-loop control, Time in range
Introduction
Diabetes mellitus is one of the best quantified human conditions: elaborate models describe the action of the metabolic system, real-time signals, such as continuous glucose monitoring (CGM), are readily available, insulin delivery can be automated, and advancing artificial pancreas (AP) technology is increasingly capable of controlling blood glucose fluctuations in a patient's natural environment. Consequently, in the past 30 years, the treatment of diabetes progressed from occasional clinical encounters and review of hemoglobin A1C (HbA1c) once in several months, through daily insulin adjustments using episodic self-monitoring, to fine-tuning of treatment decisions driven by CGM and automated closed-loop control (CLC), a.k.a. the AP.1
In 2004–2007, the first attempts to automate glucose control using CGM and Continuous Subcutaneous Insulin Infusion (CSII) were made with Proportional Integral Derivative (PID) and Model Predictive Control (MPC) algorithms.2–4 Over the subsequent decade, a variety of algorithms were evaluated, with the bulk falling into either PID, MPC, or Fuzzy Logic paradigms5: Between 2016 and 2020, PubMed included >120 publications per year related to engineering and clinical testing of various AP systems. Systematic reviews and meta-analyses are now available1,5,6–9 and an international consensus was reached on the use of glycemic control metrics for the interpretation of CGM and AP data.10,11
In 2016, a letter to JAMA announced the first pivotal trial of a new hybrid closed-loop system—the MiniMed 670G (Medtronic, Dublin, Ireland).12 This first study did not have a control group, focusing solely on the safety of the system, which was subsequently approved by the Food and Drug Administration (FDA) for clinical use. In the years that followed, a number of long-term randomized clinical trials were reported, assessing AP safety and efficacy for 3–10 months.13–17
In 2018–2020, the subject of this article—the new advanced hybrid closed-loop system t:slim X2™ insulin pump with Control-IQ® technology from Tandem Diabetes Care, based on a control algorithm developed at the University of Virginia, was tested in two randomized controlled trials (RCTs) comparing Control-IQ with sensor-augmented pump (SAP) therapy. Both studies were part of the International Diabetes Closed-Loop (iDCL) Trial sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK): NCT03563313 randomized n = 168 participants ages 14 years and older to Control-IQ versus SAP, all of whom completed the 6-month trial. The study met all of its predefined primary and secondary outcomes, resulting in 11% increase in the time within 70–180 mg/dL, reduction in the time <70 mg/dL by 0.9% without any severe hypoglycemic episodes, and reduction of HbA1c by 0.3% on Control-IQ compared with SAP.18 NCT03844789 randomized n = 101 participants ages 6–13 years old and achieved outcomes similar to those in adults: Control-IQ compared with SAP resulted in 11% increase in the time within 70–180 mg/dL, 0.4% reduction in the time <70 mg/dL without severe hypoglycemic episodes, and reduction of HbA1c by 0.4%.18 These trials led to the FDA clearance of this system for clinical use children and adults, ages 6 and up.
In this article, we report data from 1-year of real-world use of t:slim X2 with Control-IQ technology in >9000 users with diabetes, who wore the system for 12 months as part of their regular clinical treatment.
Research Design and Methods
The Tandem t:slim X2 with Control-IQ technology was commercially released in the United States in January 2020. This advanced hybrid closed-loop system comprises a t:slim X2 insulin pump with an embedded model-based CLC algorithm, an integrated Dexcom G6 CGM, and an infusion set.
Algorithm action
Control-IQ technology uses CGM and delivered insulin data to predict glucose levels 30 minutes ahead and adjust insulin delivery accordingly, including automatic correction boluses. If sensor glucose values are predicted to drop <112.5 mg/dL, basal insulin is reduced, and when predicted to be <70 mg/dL, basal insulin delivery is stopped. If sensor glucose values are predicted to be >160 mg/dL, basal insulin is increased. If sensor glucose values are predicted to be >180 mg/dL, Control-IQ calculates an automatic correction bolus with a target of 110 mg/dL and delivers 60% of that value. Automatic corrections are allowed up to once per hour as needed. Different treatment targets apply when a Sleep or Exercise Activity is enabled, with for example a gradual tightening of glycemic control during Sleep to 120 mg/dL. If the CGM connection has been lost or stopped for 20 min or longer, Control-IQ will stop automatically adjusting insulin delivery and the pump returns to the active Personal Profile settings until the connection is restored. Once restored, Control-IQ will resume automatically.
Indications
Control-IQ technology is a prescription device embedded in the software of the t:slim X2 insulin pump and indicated for people ages 6 years and up. Individuals new to Tandem pumps must train with a Certified Diabetes Education Specialist to onboard to Control-IQ technology. For people already using an in-warranty Tandem t:slim X2 pump, starting Control-IQ technology begins with a remote software update, after completion of online educational training. As of publication >100,000 people with diabetes have on-boarded to this system.
Study population
We performed a retrospective analysis of users of Control-IQ technology in the United States who had descriptive data available in Tandem's Customer Relations Management database and had uploaded their glycemic data—either through Tandem's t:connect uploader or through the mobile app—to Tandem's t:connect web application as of February 11, 2021. The data extracted for analysis were de-identified. Participants consented to the use of their data for research purposes as part of their onboarding to Tandem when initiating their t:connect account. No IRB approval was sought for this retrospective analysis. Data from users who were ages 6 years and above, had at least 12 consecutive months of data available on Control-IQ, and had at least 2 weeks of CGM data with ≥75% CGM availability before Control-IQ initiation were included in the analysis. Any Control-IQ user with data uploaded in t:connect that did not meet these criteria were excluded.
Outcome metrics
Percent time in closed-loop automation was calculated as the percentage of the total basal rates delivered by the pump, in 5-min increments, which were decided by the Control-IQ algorithm. Descriptive data were organized according to diabetes type, age, gender, time since diabetes diagnosis, glucose management indicator, previous Tandem pump software versions, and time in Control-IQ technology automation. Glycemic outcomes were calculated for all participants who had at least 2 weeks of CGM data with ≥75% CGM availability before Control-IQ initiation and 1 year of CGM data with ≥75% CGM availability after Control-IQ initiation.
The outcomes were calculated by participant, per time period of interest (2 weeks, 1 month, 3 months, 6 months, and 1 year), and the median and interquartile range (IQR) across subjects are reported. CGM values outside the valid ranges of 40–400 mg/dL were filtered out, with glucose value <40 mg/dL and >400 mg/dL being saturated at 40 mg/dL and 400 mg/dL, respectively. No CGM interpolation was performed. Glycemic outcomes are reported regardless of closed-loop status. Outcomes were analyzed using Wilcoxon signed rank test and are reported as median (quartiles). The study was not statistically powered and tests are not corrected for multiple comparisons.
Distribution are presented as either boxplots (a graphical representation including median, mean, quartiles, and extremes for a given outcome) or violin plots, the combination of boxplots with the addition of a rotated kernel density estimation step,19 the kernel estimation being saturated at 0% and 100%.
Results
Overall, 9451 users met the inclusion criteria, of which 83% had type 1 diabetes (T1DM) and the rest had type 2 (T2DM) or other forms of diabetes; and 52% were female (Table 1). Mean age for the sample was 41.9 ± 20.8 years, almost 99% were using Basal-IQ technology (a predictive low glucose suspend system embedded in the t:slim X2 pump) at baseline. After filtering for availability of CGM (≥75% CGM use for the 2 weeks preceding and for the 12 months after Control-IQ technology initiation) 9010 users remained, or ∼95% of the original set.
Table 1.
Baseline Attributes of System Users with At Least 12 Months of Consecutive Control-IQ Technology Software Data Available
| N | 9451 |
|---|---|
| Age | 41.9 ± 20.8, minimum 6 years; maximum 91 years |
| Gender | Female: 52% (n = 4905) |
| Male: 48% (n = 4540) | |
| eA1c | 7.3% |
| Diabetes type | Type 1 diabetes: 83% (n = 7813) |
| Type 2 diabetes: 4% (n = 378) | |
| Not self-reported: 13% (n = 1260) | |
| Diabetes duration | Type 1 diabetes: 21.84 ± 20.6 |
| Type 2 diabetes: 20.76 ± 10.3 | |
| Prior insulin pump software | Tandem t:slim X2 pump with Dexcom G5 Mobile CGM: 1.26% (n = 119) |
| Tandem t:slim X2 pump with Basal-IQ technology: 98.74% (n = 9332) |
Data presented as mean ± SD or % (n)
CGM, continuous glucose monitoring; eA1c, estimated hemoglobin A1C.
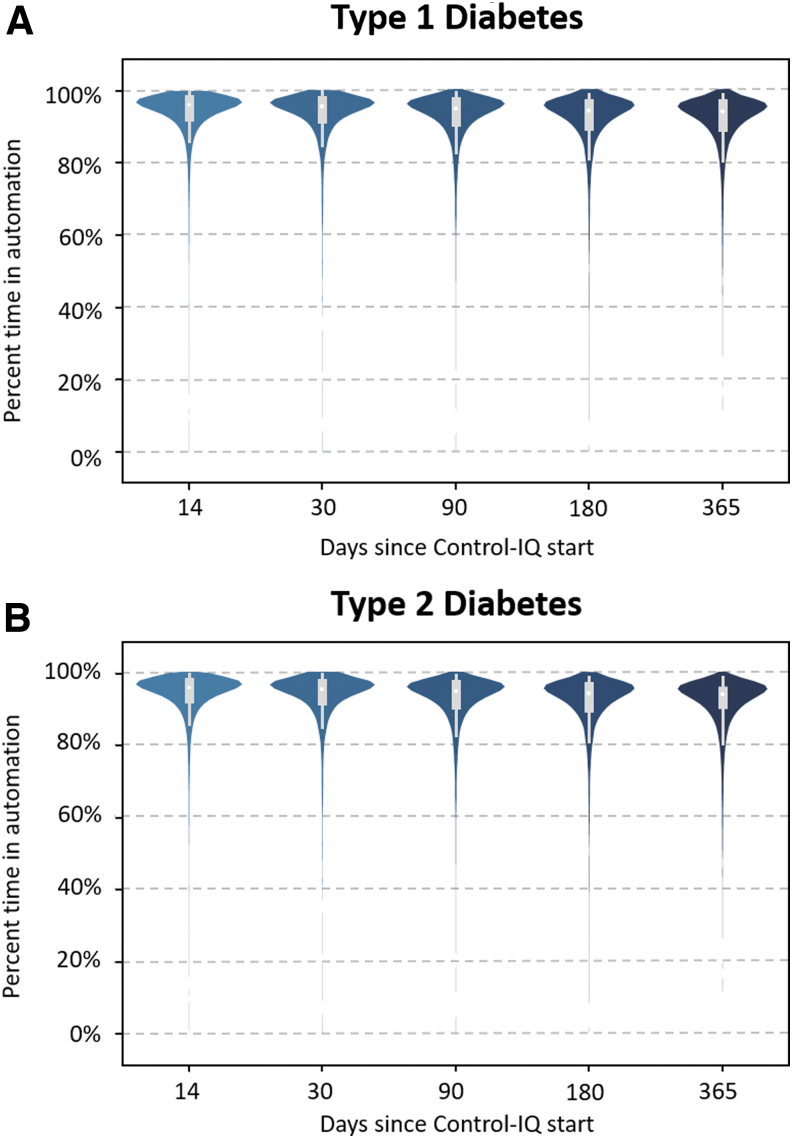
Median percent time in automation was 94.2% [IQR: 90.1%–96.4%] for the entire 12-month duration with no significant changes over time (first 3 months: 95.1%, first 6 months: 94.5%). High automation was observed for both types of diabetes (Fig. 1), and in all age groups with three quarters of each subpopulation above ∼90% automation (Supplementary Fig. S1 and Supplementary Table S1), although adolescents (14–18 years) showed a slightly lower time in automation than the rest of the population (92.5% [88.3–95.0]).
FIG. 1.
Violin graph representing percent time in automation over 365 days of Control-IQ (CIQ) technology use by type of diabetes (A: T1DM, B: T2DM).
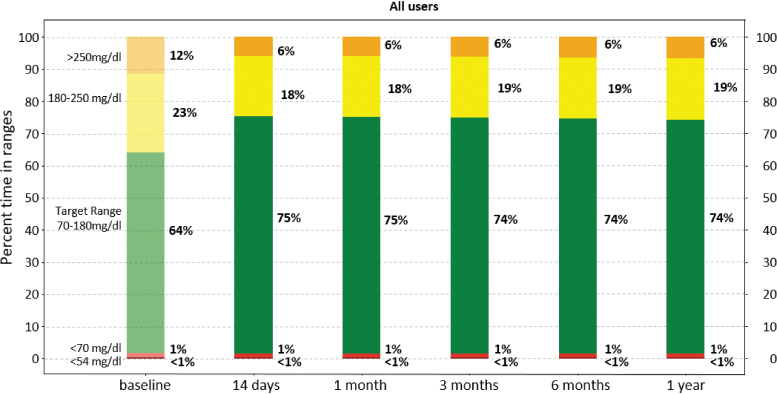
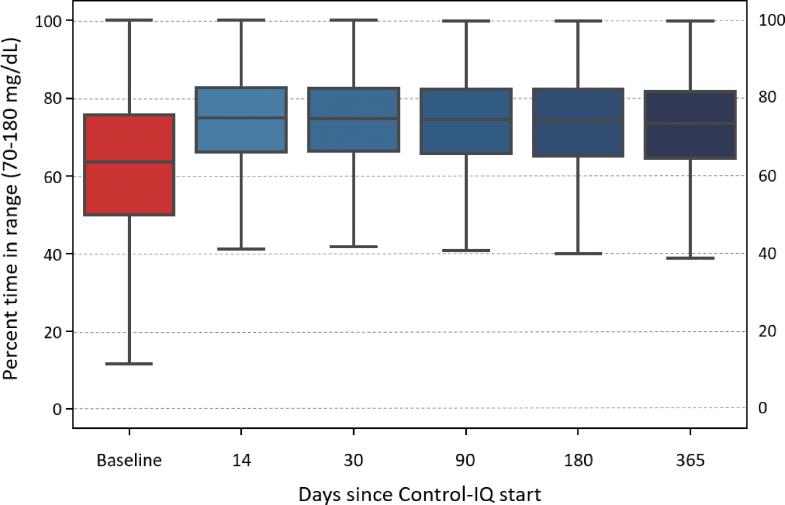
Glycemic control improved rapidly (within 2 weeks) after initiation of Control-IQ and was maintained for the entirety of the study period (Fig. 2). Specifically, median percent time in range (TIR) (70–180 mg/dL) was 63.6 (IQR: 49.9%–75.6%) at baseline and increased to 73.6% (IQR: 64.4%–81.8%) for 12 months of Control-IQ use (P < 0.001) with no significant degradation in time (Fig. 3). Median percent time <70 mg/dL remained consistent at ∼1% (IQR: 0.5%–1.9%, Fig. 4), whereas median percent time <54 mg/dL statistically increased from 0.1% [0.0–0.3] to 0.15% [0.06–0.3]. Mean glucose decreased from 167.5 ± 30.7 mg/dL at baseline to 154.3 ± 20.7 mg/dL for the 12 months after Control-IQ initiation. These improvements were seen for both people with T1DM and T2DM, with median TIR improvements of +10.3% and +8.1%, respectively. Table 2 summarizes the main glycemic outcomes.
FIG. 2.
Consensus CGM outcomes for baseline and Control-IQ use over time. CGM, continuous glucose monitoring.
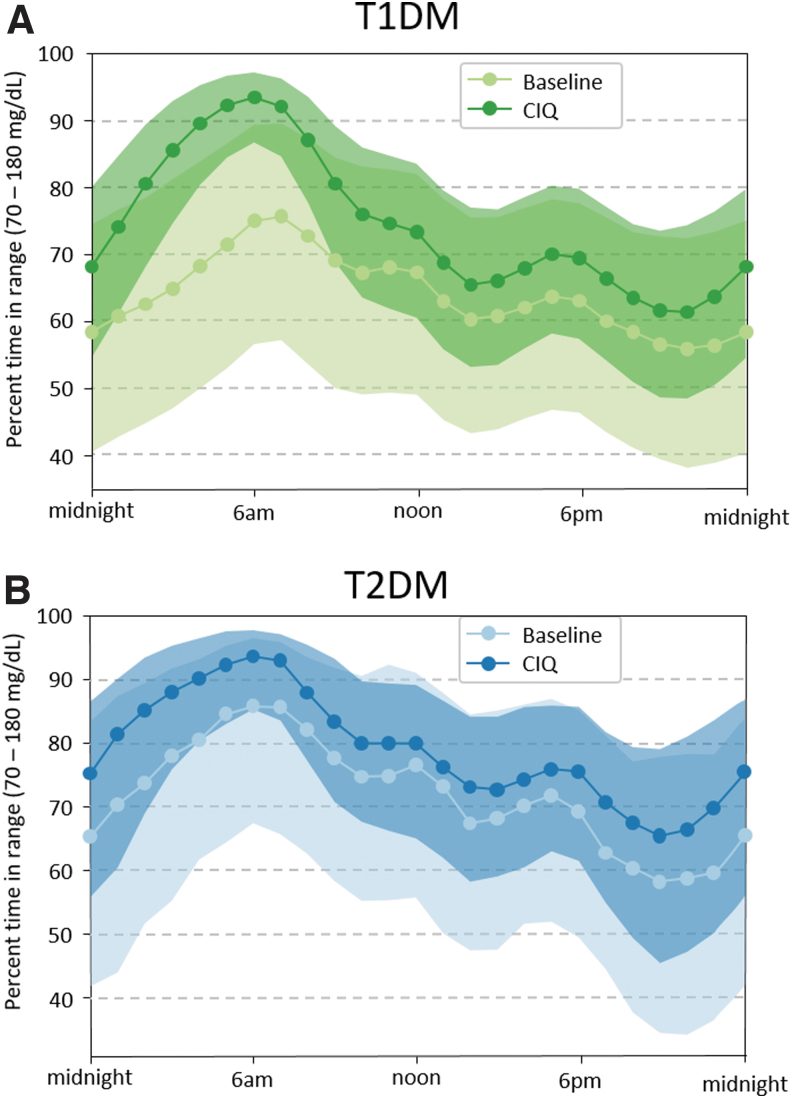
FIG. 3.
Median (line and markers) and quartiles (envelop) of Time In Range (TIR, 70–180 mg/dL) over time of day by types of Diabetes (A: T1DM, B: T2DM) for Baseline and 1 year of CIQ use.
FIG. 4.
Overall distribution of Time In Range (TIR, 70-180mg/dL) at baseline and over time of CIQ use. Horizontal line shows median and filled rectangle represent the 50% of data within the first and third quartiles, whiskers represent minimum and maximum values.
Table 2.
Comparison of Glycemic Outcomes for Users Who Had ≥75% Continuous Glucose Monitoring Use (Baseline vs. 12-Month Use of Control-IQ)
| Baseline (Basal-IQ) | 12-mth control-IQ use | P | |
|---|---|---|---|
| All users | |||
| No. of participants | 9010 | 9010 | |
| Mean sensor glucose [mg/dL] | 164 (146–185) | 152 (140–166) | <0.001 |
| Sensor time <54 mg/dL [%] | 0.10 (0.00–0.30) | 0.15 (0.06–0.30) | <0.001 |
| Sensor time 54–70 mg/dL [%] | 0.8 (0.3–1.8) | 0.9 (0.4–1.6) | 0.053 |
| Sensor TIR [%] | 63.6 (50.0–75.7) | 73.6 (64.5–81.8) | <0.001 |
| Sensor time 180–250 mg/dL [%] | 25.1 (18.0–31.1) | 19.7 (14.2–24.3) | <0.001 |
| Sensor time >250 mg/dL [%] | 8.1 (2.9–16.7) | 4.6 (1.9–9.5) | <0.001 |
| Coefficient of variation [%] | 33.7 (30.0–37.6) | 32.9 (29.5–36.3) | <0.001 |
| GMI | 7.2 (6.8–7.7) | 6.9 (5.6–7.3) | <0.001 |
| T1DM users | |||
| No. of participants | 7813 | 7813 | |
| Mean sensor glucose [mg/dL] | 163 (141–190) | 151 (134–170) | <0.001 |
| Sensor time <54 mg/dL [%] | 0.01 (0.00–0.35) | 0.02 (0.00–0.4) | <0.001 |
| Sensor time 54–70 mg/dL [%] | 0.9 (0.3–1.9) | 0.9 (0.5–1.7) | 0.123 |
| Sensor TIR [%] | 63.2 (49.8–75.1) | 73.5 (64.4–81.6) | <0.001 |
| Sensor time 180–250 mg/dL [%] | 25.2 (18.2–31.0) | 19.7 (14.3–24.2) | <0.001 |
| Sensor time >250 mg/dL [%] | 8.3 (3.1–16.9) | 4.7 (2.0–9.6) | <0.001 |
| T2DM users | |||
| No. of participants | 378 | 378 | |
| Mean sensor glucose [mg/dL] | 158 (138–184) | 150 (136–169) | <0.001 |
| Sensor time <54 mg/dL [%] | 0.00 (0.0–0.07) | 0.04 (0.01–0.10) | <0.001 |
| Sensor time 54–70 mg/dL [%] | 0.2 (0.0–0.6) | 0.2 (0.0–0.6) | 0.337 |
| Sensor TIR [%] | 69.9% (55.1–82.6) | 78.0% (66.2–86.1) | <0.001 |
| Sensor time 180–250 mg/dL [%] | 23.9 (14.6–32.0) | 19.0 (12.4–25.5) | <0.001 |
| Sensor time >250 mg/dL [%] | 3.6 (0.7–10.4) | 2.3 (0.8–6.7) | <0.001 |
Data are expressed as median (IQR) unless otherwise specified.
GMI, glucose management indicator; IQR, interquartile range; T1DM, type 1 diabetes; T2DM, type 2 diabetes; TIR, time in range.
Most of these improvements for T1DM users were driven by profound TIR increase at night, reaching a median >90% between 4 and 7 am, which is a reflection of the control algorithm design; in T2DM users the improvements over baseline were similar throughout the day, with a peak >90% by 4 am (Fig. 5).
FIG. 5.
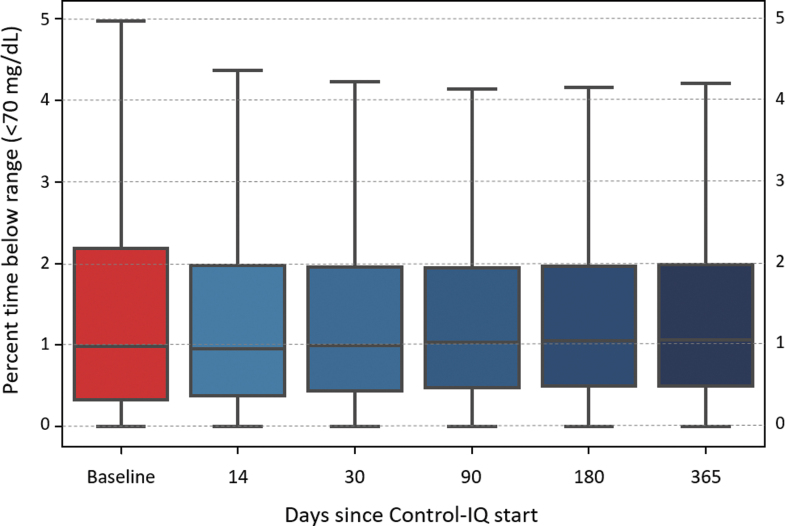
Overall distribution of Time Below Range (TBR, <70mg/dL) at baseline and over time of CIQ use. Horizontal line shows median and filled rectangle represent the 50% of data within the first and third quartiles, whiskers represent minimum and maximum values.
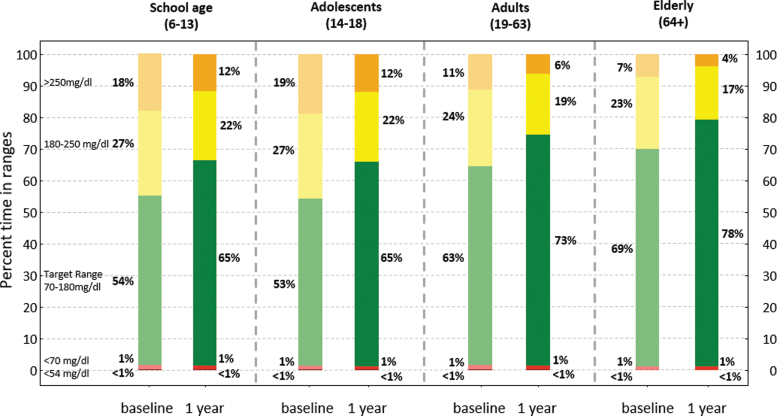
These outcomes were seen across age groups (from +9 in elderly users to +13 for adolescents) (Fig. 6). We observed larger improvements in populations with lower baseline TIR and interpatient variability was clearly reduced (Supplementary Figs. S2 and S3).
FIG. 6.
Consensus CGM outcomes for baseline and 12-months Control-IQ use broken down by age groups.
Discussion
This uniquely large data set of AP real-world use confirmed the glycemic control improvement noted during the system pivotal trials18,20: in a sample of >9000 patients with diabetes, TIR improved by ∼10 percentage points in people with T1DM (62% vs. 72%, or ∼2.5 h/day improvement) from baseline compared with 11 percentage points for both RCTs. Both RCTs had extension studies (total of 6 months use for pediatric and 18 months for adults) that showed maintenance of this improvement: +9 and +10 percentage points for adolescents/adults and children, respectively.21,22 Exposure to hypoglycemia remained low throughout our analysis period with median time below range (TBR) ∼1%; this compares favorably with the published RCTs with TBR equal to ∼1.6% for both. Although there was a statistically significant increase (due to the very large sample size) in time <54 mg/dL, from 0.1% to 0.15% median, this increase was irrelevant clinically (equivalent to <1 min/day).
The TIR improvement (and the maintenance of TBR) appeared within the first 2 weeks of use and were maintained throughout the 1-year analysis, showing consistency of glycemic control outside of any clinical trial structure. These results were achieved after consistently high use of closed loop: from 95% median time in closed loop for the first 2 weeks to 94% by year's end. The smaller T2DM sample (n = 378) also showed an improvement of 8 percentage points from 67% to 75% average TIR (median 70% vs. 78%) with very low hypoglycemia exposure: median TBR of 0.3%.
Other observational studies in real-world clinical use have reported glycemic outcomes and CLC use for commercial AP systems in T1DM (although not yet in T2DM). In 2019, Akturk et al. presented data from 127 patients of a single U.S. clinic transitioning from SAP therapy to AP and followed for 6 months.23 TIR improved ∼11 percentage points (from 59.5% to 70.1%) with TBR of 2.2% (although improved from 3.2% at baseline). Time in closed loop was ∼77% at 3 months, and 73% at 6 months with a significant decline in men only.
Berget et al. presented outcomes from a 6-month analysis of 92 children and young adults starting the same AP system at a single U.S. clinic: TIR improved almost 7 percentage points maintaining TBR at ∼3% (no change between baseline and AP use)24 whereas use of closed loop (a.k.a. auto mode) was only 65.5% at month 1 and 51.2% by month 6. A large proportion of the studied patients (n = 28, 30%) discontinued use of closed loop by 6 months, confirming the results presented in Lal et al.25 in a smaller (n = 68) but broader (9–61 years old) population.
It is important to note that the population studied in Berget et al. is particularly challenging and although the age range was represented in our analysis, we do not present outcomes by this specific subpopulation; nonetheless the school-aged children and adolescent subpopulation outcomes reported in this study compare favorably with Berget et al. In a similar analysis, Petrovski et al. presented data from 12 months of AP use in a single clinic in Qatar.26 In this pediatric observational study following a 30 participants prospective study of AP use in patients originally using multiple daily injections (MDI), TIR improved from 47% at baseline to 56.3% (Manual model) to 73% for 12 months of AP use, maintaining a high (compared with Berget et al.) closed-loop use of 86% at 12 months.
Limitations of our analysis include (1) the absence of HbA1c measurements, which limited our capacity to assess glycemic control to only CGM-based outcomes; (2) the relatively recent commercial approval of Control-IQ, biasing our sample toward early adopters, and (3) our requirement for a baseline CGM period before AP initiation. The latter, though it allowed for a more thorough characterization of our population, also likely biased our sample toward patients already comfortable with the use of technology in their diabetes management, with almost 99% prior Basal-IQ users. Finally, the nature of a retrospective observational study (especially the absence of a control group) does not allow to allocate the presented improvement solely to system use, but only to characterize glycemic control on the studied population after 12 months of use.
Conclusions
In this article, we present analysis of data from the t:connect database (Tandem Diabetes Care) of Control-IQ technology users, who had available CGM data before initiation of Control-IQ technology and then had 12 consecutive months of real-life Control-IQ technology use. More than 9000 individuals met the preset CGM availability thresholds, generating nearly a billion data points that were included in this analysis. The results of this large-scale exploration of the real-world use of Control-IQ technology confirm the conclusions reached by the two pivotal trials of this system, in adolescents/adults and children, respectively: the use of Control-IQ technology increased time in the 70–180 mg/dL range by >10 percentage points from baseline.
This increase was evident after the first 2 weeks of use and was sustained throughout the entire year. In parallel, the frequency of CGM readings <70 mg/dL was kept low at ∼1% throughout the year. Time in closed loop was high throughout the year, with median use at 94%. We can, therefore, conclude that Control-IQ technology is well accepted and sustainably used by people over a broad age range, with varying degrees of baseline glycemic control, and different types of diabetes, achieving results that exceed the criteria for quality of glycemic control set forth by the International Consensus on TIR.11
Supplementary Material
Acknowledgments
The authors thank Tandem Diabetes Care, Inc., for providing the de-identified data underlying this article, as well as the contributions of Tandem employees Steph Habif EdD, MS, Alexandra Constantin, PhD, and Harsimran Singh, PhD, for their assistance in collecting, analyzing, and interpreting the data, as well as their participation in drafting this article. Tandem Diabetes Care placed no restrictions regarding submission of this article for publication; no Tandem funding was provided for this article.
Author Disclosure Statement
M.D.B. reports research support from Dexcom, Inc., Arecor Ltd., Novo Nordisk A/S, and Tandem Diabetes Care, Inc; M.D.B. reports consulting fees from Dexcom, Inc., Arecor, and Adocia. B.P.K. reports research support handled by the University of Virginia from Dexcom, Inc., Novo Nordisk A/S, and Tandem Diabetes Care, Inc; M.D.B. and B.P.K. report patent royalties, managed by the University of Virginia, from Johnson & Johnson, Sanofi, and Dexcom.
Funding Information
This study was funded internally by the University of Virginia.
Supplementary Material
References
- 1. Kovatchev BP: A century of diabetes technology: signals, models, and artificial pancreas control. Trends Endocrinol Metab 2019;30:432–444 [DOI] [PubMed] [Google Scholar]
- 2. Hovorka R, Chassin LJ, Wilinska ME, et al. : Closing the loop: the ADICOL experience. Diabetes Technol Ther 2004;6:307–318 [DOI] [PubMed] [Google Scholar]
- 3. Steil GM, Rebrin K, Darwin C, et al. : Feasibility of automating insulin delivery for the treatment of Type 1 Diabetes. Diabetes 2006;55:3344–3350 [DOI] [PubMed] [Google Scholar]
- 4. Dassau E, Zisser H, Harvey R, et al. : “Clinical Evaluation of a Personalized Artificial Pancreas.” Diabetes Care 2013;36:801–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Doyle III FJ, Huyett LM, Lee JB, et al. : Closed-Loop Artificial Pancreas Systems: engineering the Algorithms. Diabetes Care 2014;37:1191–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weisman A, Bai JW, Cardinez M, et al. : Effect of artificial pancreas systems on glycaemic control in patients with type 1 diabetes: a systematic review and meta-analysis of outpatient randomised controlled trials. Lancet Diabetes Endocrinol 2017;5:501–512 [DOI] [PubMed] [Google Scholar]
- 7. Kovatchev BP: The artificial pancreas in 2017: the year of transition from research to clinical practice. Nature Reviews Endocrinology 2018;14:74–76 [DOI] [PubMed] [Google Scholar]
- 8. Bekiari E, Kitsios K, Thabit H, et al. : Artificial pancreas treatment for outpatients with type 1 diabetes: systematic review and meta-analysis. BMJ 2018;361:k1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Karageorgiou V, Papaioannou TG, Bellos I, et al. : Effectiveness of artificial pancreas in the non-adult population: a systematic review and network meta-analysis. Metabolism 2019;90:20–30 [DOI] [PubMed] [Google Scholar]
- 10. Kovatchev BP: Metrics for glycaemic control—from HbA1c to continuous glucose monitoring. Nature Rev Endocrinol 2017;13:425–436 [DOI] [PubMed] [Google Scholar]
- 11. Danne T., Nimri R, Battelino T, et al. : International Consensus on Use of Continuous Glucose Monitoring. Diabetes Care 2017;40:1631–1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bergenstal RM et al.: Safety of a hybrid closed-loop insulin delivery system in patients with type 1 diabetes. JAMA 2016;316:1407–1408 [DOI] [PubMed] [Google Scholar]
- 13. Dassau E, Pinsker JE, Kudva YC, et al. : Twelve-Week 24/7 Ambulatory Artificial Pancreas With Weekly Adaptation of Insulin Delivery Settings: effect on Hemoglobin A1c and Hypoglycemia. Diabetes Care 2017;40:1719–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tauschmann M, Thabit H, Bally L, et al. : for the APCam11 Consortium. Closed-loop insulin delivery in suboptimally controlled type 1 diabetes: a multicentre, 12-week randomised trial. Lancet 2018;392:1321–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Benhamou PY, Franc S, Reznik Y, et al. : on behalf of the DIABELOOP WP7 Trial Investigators. Closed-loop insulin delivery in adults with type 1 diabetes in real-life conditions: a 12-week multicentre, open-label randomised controlled crossover trial. Lancet Digital Health 2019;1:e17–e25 [DOI] [PubMed] [Google Scholar]
- 16. Kovatchev B, Anderson SM, Raghinaru D, et al. : for the iDCL Trial Research Group. Randomized controlled trial of mobile closed-loop control. Diabetes Care 2020;43:607–615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kovatchev BP, Kollar L, Anderson SM, et al. : Evening and overnight closed-loop control versus 24/7 continuous closed-loop control for type 1 diabetes: a randomized crossover trial. Lancet Digital Health 2020;2:e64–e73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brown SA, Kovatchev BP, Raghinaru D, et al. : for the iDCL Trial Research Group. Six-month randomized, multicenter trial of closed-loop control in type 1 diabetes. N Engl J Med 2019;381:1707–1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hintze JL, Nelson RD: Violin plots: a box plot-density trace synergism. The American Statistician 1998;52:181–184 [Google Scholar]
- 20. Breton MD, Kanapka LG, Beck RW, et al. : for the iDCL Trial Research Group. A Randomized Trial of Closed-Loop Control in Children with Type 1 Diabetes. N Engl J Med 2020;383:836–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brown SA, Buckingham B, Kudva YC, et al. : for the IDCL Trial Group. 18-Month Use of Closed-Loop Control (CLC): a Randomized, Controlled Trial. 80th Virtual Scientific Sessions of the American Diabetes Association, June 2020. [Google Scholar]
- 22. Kanapka LG, Wadwa RP, Breton MD, et al. : Extended Use of the Control-IQ Closed-Loop Control System in Children With Type 1 Diabetes. Diabetes Care 2021;44:473–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Akturk HK, Giordano D, Champakanath A, et al. : Long-term real-life glycaemic outcomes with a hybrid closed-loop system compared with sensor-augmented pump therapy in patients with type 1 diabetes. Diabetes, Obesity and Metabolism 2020;22:583–589 [DOI] [PubMed] [Google Scholar]
- 24. Berget C, Messer LH, Vigers T, et al.: Six months of hybrid closed loop in the real-world: an evaluation of children and young adults using the 670G system, Pediatric Diabetes 2019;21:310–318 [DOI] [PMC free article] [PubMed]
- 25. Lal RA, Basina M, Maahs DM, et al. : One Year Clinical Experience of the First Commercial Hybrid Closed-Loop System. Diabetes Care 2019;42:2190–2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Petrovski G, Al Khalaf F, Campbell J, et al. : One-year experience of hybrid closed-loop system in children and adolescents with type 1 diabetes previously treated with multiple daily injections: drivers to successful outcomes. Acta Diabetologica 2021;58:207–2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.