Abstract
Background
Enhanced nonpharmaceutical interventions (NPIs) to prevent the Coronavirus Disease 2019 (COVID-19) have shown various levels of impact on common respiratory pathogens. We aimed to analyze the epidemiological changes seen in certain common respiratory viruses found in Taiwanese children (e.g., influenza virus, enterovirus, parainfluenza virus, adenovirus and respiratory syncytial virus (RSV)) after the implementation of public health measures, as well as interpret the possible meaning of these changes.
Methods
This retrospective observational study examined the viral isolation from children younger than 18 years at a medical center in central Taiwan during the period January 2015–December 2020, a time frame of six years. Viral isolations prior to the COVID-19 pandemic (January 2015–December 2019), along with those during the post-COVID-19 period (January–December 2020) were analyzed and compared.
Results
A total of 6899 throat swab samples were collected during the pre-pandemic period of 2015–2019, with 2681 of them having a positive result (38.86%). There were a total of 713 samples collected in 2020, with 142 of them showing positive results (19.92%). The overall positive rate of viral isolates significantly decreased in 2020 (p < 0.001). Declines in the isolation of the influenza virus, parainfluenza virus, adenovirus and enterovirus were observed. The RSV surprisingly became the leading isolate, with up to 47 (6.59%) instances in 2020, and showing an unusual peak in the winter of 2020. The rise began in September of 2020 and reached its plateau in November of that year.
Conclusions
Most respiratory viruses decreased under NPIs regarding SARS-CoV-2. However, the RSV outbreak in the winter of 2020 had shown the limitation of current NPIs. Possible explanations have been discussed in details and public preventive measures should be reinforced for RSV, particularly amongst people having young children both at home and in care centers.
Keywords: COVID-19 pandemic, Influenza virus, Respiratory syncytial virus, Epidemiology, Non-pharmaceutical intervention
Introduction
The Coronavirus Disease 2019 (COVID-19) pandemic has changed the way of life in almost every country on the planet. Prior to vaccines and effective antivirals becoming available, nonpharmaceutical interventions (NPIs) had been the most effective available form of control both locally and globally, as well as for the mitigation of COVID-19.1 These NPIs, including social distancing policies, the wearing of face masks, hand sanitation, lockdowns of certain scales in administrative districts, and travel restrictions, all provided a temporary containment of COVID-19 in several countries. There were many NPIs implemented in Taiwan in the year 2020. Individuals had to wear face masks in indoor public spaces, on public transportation and in health care facilities. Social distancing was also strictly required in populated public spaces. International border control was carried out through the requirement that all arriving passengers undergo a 14-day quarantine period, with active surveillance on body temperature and respiratory symptoms also being implemented.
Surveillance studies had previously demonstrated the potential benefits of NPIs on preventing the transmission of several common pathogens via the respiratory route.2 Surveillance data taken in 2020 from Korea and Japan showed a decrease in influenza activity as compared with previous seasons.3 , 4 Studies from France, Finland, and rural Alaska had also shown decreases in most acute respiratory infections in children.5, 6, 7 A significant reduction in hospital admissions for both non-influenza respiratory viruses and influenza was reported in Singapore.8 Systematic reviews have also considered the reduction of transmission associated with mask wearing for respiratory viruses.9 , 10 A Taiwan study in 2020 using figures taken from the National Health Insurance Research Database (NHIRD) showed significantly lower rates of common infections after Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) preventive measures were announced.11 A recent study in northern Taiwan using multiplex RT-PCR tests also showed a reduced average positivity rate of 11.2% in respiratory viruses after nationwide public health interventions were established.12
Despite many studies having had already revealed similar findings, the methods of population selection varied from study to study. The various study samples may have come from a database bank using codes of diagnosis, multiplex RT-PCR tests, viral cultures, serum tests and also various kinds of rapid antigen tests. Diagnoses may have been made clinically without microbiological evidence. Serum tests and rapid antigen tests cannot completely reflect true infections in time, while multiplex PCR is a relatively novel technique with limited universality. Amongst these methods, it is viral cultures which may provide consistent data homogeneity for longer periods of time.
We aimed to analyze the epidemiological changes in some common respiratory viral pathogens found in Taiwan after the application of public health measures, as well as interpret the possible meaning of these changes.
Materials and methods
We collected viral cultures and viral rapid antigen tests to analyze the prevalence of some common viruses during the 6-year period of January 2015 to December 2020. Viral isolations and the results of viral rapid antigen tests were collected retrospectively. The viral isolates for enterovirus, influenza virus, adenovirus, parainfluenza virus and RSV among children (≤18 years of age) were from the virology laboratory of Taichung Veterans General Hospital (TCVGH), a medical center in central Taiwan. Sources for the viral isolations came from throat swab samples taken in outpatient departments, during hospitalizations, in emergency departments and sentinel local clinics in the Taichung area. RSV rapid antigen tests performed during 2019–2020 were also collected. The RSV rapid antigen test using the BinaxNOW RSV card (Abbott Diagnostics Scarborough, Inc., ME, US), with 89% sensitivity and 100% specificity according to the product instructions, was processed at the laboratory of the Children's Medical Center of Taichung Veterans General Hospital. The use of these data involving human subjects was appropriately approved by the Institutional Review Board (IRB) of Taichung Veterans General Hospital (CE21094B). Open access to the Taiwan Centers for Disease Control (CDC) data was given, including the official website and the Disease Surveillance Weekly Report by the Epidemic Intelligence Center, and collected for comparison.
Statistical analysis
Hypothesis testing using the Chi-square test was performed to assess differences between the number of detected viruses in the 2 groups (i.e., 2015–2019 and 2020). A p value < 0.05 was considered statistically significant. Analysis was performed using IBM SPSS Statistics, Version 22.0 (IBM Corp., Armonk, NY, USA).
Results
During the period of 2015–2019, a total of 6899 throat swab samples from children younger than 18 years of age were collected, with 2681 samples showing positive results (38.86%). There were a total of 713 samples collected in 2020, with 142 of them revealing positive results (19.92%). The overall positive rate of viral isolates significantly decreased in 2020 (p < 0.001), as shown in Table 1 . The virus isolation rates of enterovirus, influenza virus, adenovirus, parainfluenza virus and RSV were 893 (12.94%), 852 (12.35%), 488 (7.07%), 291 (4.22%) and 157 (2.28%), respectively, during the period 2015 to 2019. The enterovirus and influenza virus were the most common viruses isolated during this period. Surprisingly, RSV became the leading isolate, with up to 47 (6.59%) cases in 2020. The percentages of adenovirus, enterovirus and parainfluenza virus all declined in 2020, while the isolation rate of influenza virus decreased by 7.86% in 2020.
Table 1.
Outcome of all viral isolations and ratio of each viral category.
| Outcome | 2015–2019 (n = 6,899) |
2020 (n = 713) |
p value |
||
|---|---|---|---|---|---|
| n | % | n | % | <0.001∗ | |
| Positive |
2681 |
(38.86%) |
142 |
(19.92%) |
|
| Viral Category |
<0.001∗ |
||||
| Enterovirus | 893 | (12.94%) | 25 | (3.51%) | |
| Influenza | 852 | (12.35%) | 32 | (4.49%) | |
| Adenovirus | 488 | (7.07%) | 27 | (3.79%) | |
| Parainfluenza | 291 | (4.22%) | 11 | (1.54%) | |
| RSV | 157 | (2.28%) | 47 | (6.59%) | |
| Negative | 4218 | (61.14%) | 571 | (80.08%) | |
Chi-Square test. ∗p < 0.001.
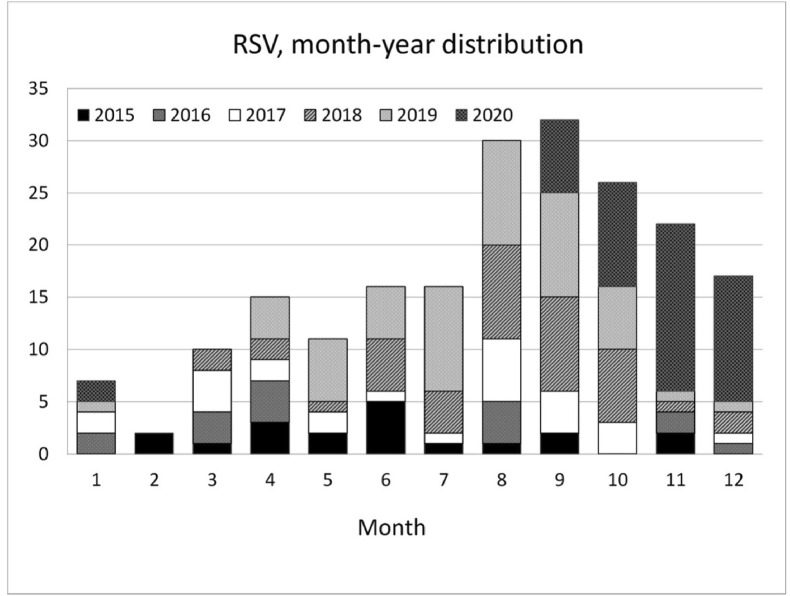
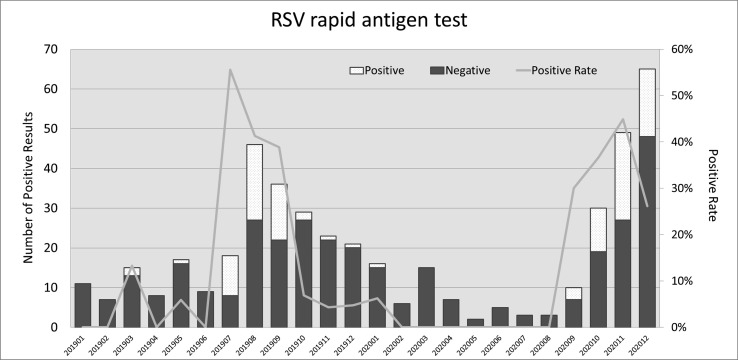
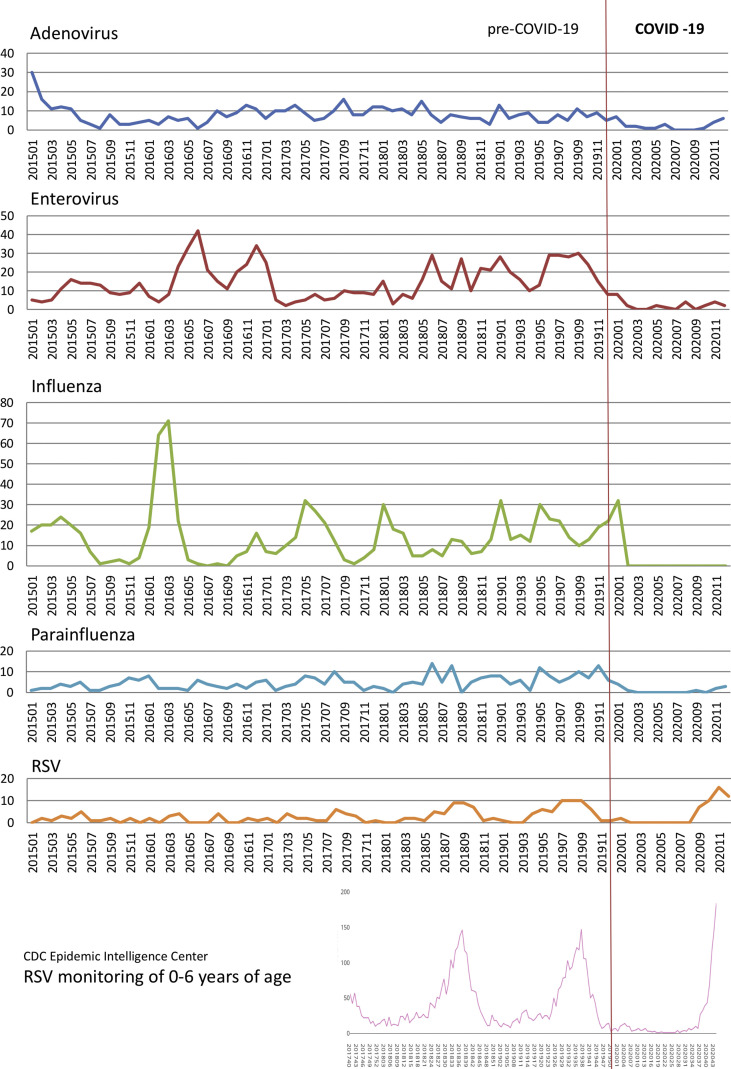
RSV showed an unusual increase during the winter of 2020 (Fig. 1 ). The rise began in September of 2020 and reached its plateau in November. Results from RSV rapid antigen tests performed during 2019–2020 are demonstrated in Fig. 2 . The peak month was August, 2019, while the peak positive rate was in July, 2019 (55.56%). In 2020, the most prevalent month was December, while the highest positive rate was in November (44.9%). The six-year trend for these five viruses is shown in Fig. 3 . We also collected the RSV isolation numbers from children under six years of age from the Epidemic Intelligence Center for comparison, revealing a sudden rise since the 43rd week, which was the fourth week in October of 2020.
Figure 1.
The month-year distribution of positive RSV isolates as seen in accumulated bar charts.
Figure 2.
The monthly number of positive results of RSV rapid antigen tests during the period of January 2019 to December 2020. A line chart of positive rates is also demonstrated.
Figure 3.
Six-year trend of adenovirus, enterovirus, influenza virus, parainfluenza virus and RSV, with RSV monitoring data taken from the Epidemic Intelligence Center, Taiwan CDC as a comparison. The vertical axis indicates the number of isolates.
Discussion
People in Taiwan had well-cooperated with public preventive measures, including strictly required face masks, social distancing, international border control with quarantine, and active surveillance on body temperature and respiratory symptoms. Hence SARS-CoV-2 did not cause serious community transmission in Taiwan in 2020. Most of the confirmed COVID-19 cases were imported from other countries and contained through hospital quarantines. However, a nosocomial outbreak occurred in January 2021 which was associated with a medical staff member who had cared for a confirmed imported case at a COVID-19 duty hospital in Taoyuan, showing that the persistent implementations of public health measures can never be overemphasized. Defining the effects which public health measures have on other common infections is essential for both the allocation and preparedness of health resources. The experience gained from the SARS pandemic during 2002–2003 showed that social distancing, face masks, hand sanitation, travel restrictions, quarantine policies and restricted hospital visits were all effective measures taken to prevent respiratory infections. It is expected that these methods of control will help in the prevention of all respiratory tract infections. Additionally, medical visits, hospital stays, and medical activities all declined, as we can see from the CDC surveillance system.13 In our study however, a decline was not observed in all the selected pathogens.
In a pediatric population, viral spread by an asymptomatic adult carrier remains an important factor. It had been demonstrated that an asymptomatic individual may shed the influenza virus, although studies have not conclusively determined if such people will effectively transmit influenza.14 Influenza virus transmission may be reduced through NPIs, particularly by following proper hand hygiene protocol and the wearing of face masks.15 , 16 Other viruses e.g., adenovirus, RSV, parainfluenza virus and enterovirus are usually carried by an asymptomatic individual.17, 18, 19 This phenomenon implies that there are differences in the natural reservoirs of common respiratory viruses. Both the SARS-CoV-2 and influenza virus are spread by infected persons who come in close contact with others. The seasonal parainfluenza virus, adenovirus, and RSV all supposedly originated from a human carrier. Following this rationale, less effectiveness surrounding the public measures e.g., face mask wearing and hand sanitation can be expected, as most people tend to take off their face masks and perform hand hygiene less often at home. This situation is particularly prominent for those people having infants and toddlers. Therefore, due to personal hygiene habits and close physical contact with these young individuals, the spread of these viruses will facilitate. Also, it has been proven that asymptomatic carriers excreting the enterovirus play a crucial role in the spread of hand-foot-mouth disease, with their “silent” presence helping to perpetuate enterovirus circulation in their community.20 The spread of the enterovirus is often seen amongst children having frequent and close contact in schools and child care centers. Social distancing contributes to a decline in its prevalence, as observed in our study. A decrease in visits to hospitals and local clinics also reduces any contact with other infected individuals.
An unexpected outbreak of bronchiolitis amongst children occurred from September 2020 to December 2020 in our hospital. A similar finding from the contract virological laboratory of Taiwan's CDC has shown a high rate of RSV isolation since September 2020. A study performed in Australia showed a significant reduction in RSV detection and admissions, emergency department visits and intensive care unit admissions due to acute bronchiolitis in New South Wales during the winter of 2020.21 This was quite a different finding when compared with our study. Also, a study in Singapore also showed an overall decrease in RSV incidence in 2020, as compared with 2019.22 These differences could be attributed to climate, culture, quarantine methods, personal protective equipment, hygiene and habits.
RSV infection is mainly presented as bronchiolitis in children younger than 3 years of age, causing endemics during the fall and winter seasons in temperate countries, as well as during the hot rainy seasons in tropical climates. A biennial pattern with hospitalization peaks in spring and fall due to RSV bronchiolitis in Taiwan had been reported in 2011, even though there is no significant seasonality in southern Taiwan.23 We also found that there was a seasonality of RSV bronchiolitis occurring during the period 2015–2020 (Fig. 3). The peak season for RSV infection is summer, with a usual decline being seen in winter, except in 2020 during the COVID-19 pandemic. Taichung is a city located in central Taiwan with a warm, humid subtropical climate. The rainy season is mostly during May–August, which corresponds to the epidemiological findings seen in tropical areas.24, 25, 26 These findings indicate there was a distinct rise in RSV incidence in the winter of 2020, despite the public preventive measures of COVID-19 having been implemented. Other than the asymptomatic carriage reasoning, a possible explanation could be the decreased prevalence of co-infections with other respiratory pathogens. In a Dutch community-based study, viral load was significantly associated with disease severity when RSV was the only pathogen detected, although there was no evident association seen in infants co-infected with RSV and another respiratory virus.27 The decreased incidence of other respiratory viruses may be attributed to an increased incidence of RSV being isolated on its own, which caused more patients to have severe symptoms. This may explain the phenomenon we observed in the winter of 2020. Another possible explanation is age-related. Family exposure usually occurs through an infected school age child, whom the parents believe has only a mild “cold”. Subsequently, other young children in the family then become secondarily infected. Studies have shown a positive correlation between an increase in family size with increasing incidence of disease.28 Young children have a difficulty in wearing face masks due to both their hyperactivity and the poor-fitting of face masks. The compliance of mask wearing in children may be profoundly underestimated as well. According to a review of current interventions for COVID-19 prevention in India,29 the selection of a properly fitted, effective mask may act as a significant landmark in controlling the spreadability of COVID-19 infection.
According to our analysis in this study, further in-home measures for preventing viruses with asymptomatic carriages, particularly RSV which affects young infants and children, should be enhanced. RSV is the most common etiologic agent amongst children with bronchiolitis in Taiwan.30 Other potential pathogens that cause bronchiolitis include parainfluenza virus, enterovirus D-68 and human metapneumovirus, as well as the less commonly seen rhinovirus, influenza virus and adenovirus. Infection control measures must be emphasized in postpartum nursing care centers, infant care centers and long-term care facilities. In addition to the pediatric population, RSV also causes many outbreaks amongst adults, particularly in the elderly living in long-term care facilities.31 Preventive strategies for hospital-acquired infections, including self-health monitoring of every medical personnel and adequate nurse–patient ratios, should be strictly implemented. As more medical resources begin placing much attention and effort on COVID-19 vaccines, the development of RSV vaccines should also catch up as well, along with the necessary effective antivirals. Incidences of influenza are expected to remain low under the current public preventative measures, although the adenovirus, enterovirus and parainfluenza virus are all less predictable. Outbreaks of the enterovirus are still possible due to asymptomatic gastro-intestinal viral shedding, non-enveloped property living quarters and high environmental tolerance. As the COVID-19 pandemic continues, all common pathogens should be intensively monitored and intervened with in time, in order to reduce the burden on health-care services.
Our study has some limitations, including the retrospective, single-centered nature of our analysis which involved cases with positive viral culture findings via the use of only throat swabs. The testing of viral culture is not mandatory and may not be performed in every case within the pediatric population. Additionally, the observed decrease in number of tests/positivity rate may be related to a decrease in the overall number of outpatient department visits, admissions and emergency department visits. Still, the reported decrease in viral infections transmitted via the respiratory route in several countries supports the generalizability of our observations. Also, the consistency of the public's compliance with NPIs could not be quantified. Furthermore, the period over which we studied the impact of NPIs was only within a year, so it remains to be seen whether community transmission of respiratory viruses has been averted in the upcoming years, particularly as restrictions are relaxed and the application of COVID-19 vaccinations has been completed.
Conclusion
Most respiratory viruses decreased under NPIs regarding SARS-CoV-2. However, the RSV outbreak in the winter of 2020 had shown the limitation of current NPIs. Possible explanations have been discussed in details and public preventive measures should be reinforced for RSV, particularly amongst people having young children both at home and in care centers.
Declaration of competing interest
The authors declare there are no conflicts of interest to disclose.
References
- 1.Cowling B.J., Aiello A.E. Public health measures to slow community spread of Coronavirus disease 2019. J Infect Dis. 2020;221(11):1749–1751. doi: 10.1093/infdis/jiaa123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oster Y., Michael-Gayego A., Rivkin M., Levinson L., Wolf D.G., Nir-Paz R. Decreased prevalence rate of respiratory pathogens in hospitalized patients during the COVID-19 pandemic: possible role for public health containment measures? Clin Microbiol Infect. 2021;27:811–812. doi: 10.1016/j.cmi.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee H., Lee H., Song K.H., Kim E.S., Park J.S., Jung J., et al. Impact of public health interventions on seasonal influenza activity during the SARS-CoV-2 outbreak in Korea. Clin Infect Dis. 2021;73(1):132–140. doi: 10.1093/cid/ciaa672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakamoto H., Ishikane M., Ueda P. Seasonal influenza activity during the SARS-CoV-2 outbreak in Japan. J Am Med Assoc. 2020;323(19):1969–1971. doi: 10.1001/jama.2020.6173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angoulvant F., Ouldali N., Yang D.D., Filser M., Gajdos V., Rybak A., et al. COVID-19 pandemic: impact caused by school closure and national lockdown on pediatric visits and admissions for viral and non-viral infections, a time series analysis. Clin Infect Dis. 2021;72(2):319–322. doi: 10.1093/cid/ciaa710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuitunen I., Artama M., Makela L., Backman K., Heiskanen-Kosma T., Renko M. Effect of social distancing due to the COVID-19 pandemic on the incidence of viral respiratory tract infections in children in Finland during early 2020. Pediatr Infect Dis J. 2020;39(12):e423–e427. doi: 10.1097/INF.0000000000002845. [DOI] [PubMed] [Google Scholar]
- 7.Nolen L.D., Seeman S., Bruden D., Klejka J., Desnoyers C., Tiesinga J., et al. Impact of social distancing and travel restrictions on non-COVID-19 respiratory hospital admissions in young children in rural Alaska. Clin Infect Dis. 2021;72(12):2196–2198. doi: 10.1093/cid/ciaa1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tan J.Y., Conceicao E.P., Sim X.Y.J., Wee L.E.I., Aung M.K., Venkatachalam I. Public health measures during COVID-19 pandemic reduced hospital admissions for community respiratory viral infections. J Hosp Infect. 2020;106(2):387–389. doi: 10.1016/j.jhin.2020.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Worby C.J., Chang H.H. Face mask use in the general population and optimal resource allocation during the COVID-19 pandemic. Nat Commun. 2020;11(1):4049. doi: 10.1038/s41467-020-17922-x. www.nature.com/naturecommunications [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jefferson T., Del Mar C., Dooley L., Ferroni E., Al-Ansary L.A., Bawazeer G.A., et al. Physical interventions to interrupt or reduce the spread of respiratory viruses: systematic review. Br Med J. 2009;339:b3675. doi: 10.1136/bmj.b3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee H.H., Lin S.H. Effects of COVID-19 prevention measures on other common infections, Taiwan. Emerg Infect Dis. 2020;26(10):2509–2511. doi: 10.3201/eid2610.203193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen A.P., Chu I.Y., Yeh M.L., Chen Y.Y., Lee C.L., Lin H.H., et al. Differentiating impacts of non-pharmaceutical interventions on non-coronavirus disease-2019 respiratory viral infections: hospital-based retrospective observational study in Taiwan. Influenza Other Respir Viruses. 2021;15(4):478–487. doi: 10.1111/irv.12858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National infectious surveillance system. https://nidss.cdc.gov.tw/Home/Index?op=2
- 14.Patrozou E., Mermel L.A. Does influenza transmission occur from asymptomatic infection or prior to symptom onset? Publ Health Rep. 2009;124(2):193–196. doi: 10.1177/003335490912400205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aiello A.E., Murray G.F., Perez V., Coulborn R.M., Davis B.M., Uddin M., et al. Mask use, hand hygiene, and seasonal influenza-like illness among young adults: a randomized intervention trial. J Infect Dis. 2010;201(4):491–498. doi: 10.1086/650396. [DOI] [PubMed] [Google Scholar]
- 16.Suess T., Remschmidt C., Schink S.B., Schweiger B., Nitsche A., Schroeder K., et al. The role of facemasks and hand hygiene in the prevention of influenza transmission in households: results from a cluster randomised trial; Berlin, Germany, 2009-2011. BMC Infect Dis. 2012;12:26. doi: 10.1186/1471-2334-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birger R., Morita H., Comito D., Filip I., Galanti M., Lane B., et al. Asymptomatic shedding of respiratory virus among an ambulatory population across seasons. mSphere. 2018;3(4):1–11. doi: 10.1128/mSphere.00249-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galanti M., Birger R., Ud-Dean M., Filip I., Morita H., Comito D., et al. Rates of asymptomatic respiratory virus infection across age groups. Epidemiol Infect. 2019;147(e176):1–6. doi: 10.1017/S0950268819000505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Munywoki P.K., Koech D.C., Agoti C.N., Bett A., Cane P.A., Medley G.F., et al. Frequent asymptomatic respiratory syncytial virus infections during an epidemic in a rural Kenyan household cohort. J Infect Dis. 2015;212(11):1711–1718. doi: 10.1093/infdis/jiv263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiao M.M.A., Apostol L.N., de Quiroz-Castro M., Jee Y., Roque V., Jr., Mapue M., 2nd, et al. Non-polio enteroviruses among healthy children in the Philippines. BMC Publ Health. 2020;20:167–172. doi: 10.1186/s12889-020-8284-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Britton P.N., Hu N., Saravanos G., Shrapnel J., Davis J., Snelling T., et al. COVID-19 public health measures and respiratory syncytial virus. Lancet Child Adolesc Health. 2020;4(11):e42–e43. doi: 10.1016/S2352-4642(20)30307-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wan W.Y., Thoon K.C., Loo L.H., Chan K.S., Oon L.L.E., Ramasamy A., et al. Trends in respiratory virus infections during the COVID-19 pandemic in Singapore, 2020. JAMA Netw Open. 2021;4(6):e2115973. doi: 10.1001/jamanetworkopen.2021.15973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chi H., Chang I.S., Tsai F.Y., Huang L.M., Shao P.L., Chiu N.C., et al. Epidemiological study of hospitalization associated with respiratory syncytial virus infection in Taiwanese children between 2004 and 2007. J Formos Med Assoc. 2011;110(6):388–396. doi: 10.1016/S0929-6646(11)60057-0. [DOI] [PubMed] [Google Scholar]
- 24.Meissner H.C., Anderson L.J., Pickering L.K. Annual variation in respiratory syncytial virus season and decisions regarding immunoprophylaxis with palivizumab. Pediatrics. 2004;114(4):1082–1084. doi: 10.1542/peds.2004-1300. [DOI] [PubMed] [Google Scholar]
- 25.Loscertales M.P., Roca A., Ventura P.J., Abacassamo F., Dos Santos F., Sitaube M., et al. Epidemiology and clinical presentation of respiratory syncytial virus infection in a rural area of southern Mozambique. Pediatr Infect Dis J. 2002;21(2):148–155. doi: 10.1097/00006454-200202000-00013. [DOI] [PubMed] [Google Scholar]
- 26.Stensballe L.G., Devasundaram J.K., Simoes E.A. Respiratory syncytial virus epidemics: the ups and downs of a seasonal virus. Pediatr Infect Dis J. 2003;22(2 Suppl):S21–S32. doi: 10.1097/01.inf.0000053882.70365.c9. [DOI] [PubMed] [Google Scholar]
- 27.Borchers A.T., Chang C., Gershwin M.E., Gershwin L.J. Respiratory syncytial virus–a comprehensive review. Clin Rev Allergy Immunol. 2013;45(3):331–379. doi: 10.1007/s12016-013-8368-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Handforth J., Friedland J.S., Sharland M. Basic epidemiology and immunopathology of RSV in children. Paediatr Respir Rev. 2000;1(3):210–214. doi: 10.1053/prrv.2000.0050. [DOI] [PubMed] [Google Scholar]
- 29.Pradhan D., Biswasroy P., Kumar Naik P., Ghosh G., Rath G. A review of current interventions for COVID-19 prevention. Arch Med Res. 2020;51(5):363–374. doi: 10.1016/j.arcmed.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen Y.W., Huang Y.C., Ho T.H., Huang C.G., Tsao K.C., Lin T.Y. Viral etiology of bronchiolitis among pediatric inpatients in northern Taiwan with emphasis on newly identified respiratory viruses. J Microbiol Immunol Infect. 2014;47(2):116–121. doi: 10.1016/j.jmii.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Falsey A.R., Walsh E.E. Respiratory syncytial virus infection in adults. Clin Microbiol Rev. 2000;13(3):371–384. doi: 10.1128/cmr.13.3.371-384.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]