Abstract
Caenorhabditis elegans, a free-living nematode, is an animal model that has been extensively employed in a variety of research fields, including in the study of obesity. Its favorable features include its compact size, short life cycle, large brood size, easy handling, low cost, availability of complete genetic information, 65% conserved human diseases-associated genes, relatively easy genetic manipulation, and research using Caenorhabditis elegans does not require approvals by the Institutional Animal Care and Use Committee. These advantages make Caenorhabditis elegans a great in vivo model for life science research including obesity research. In this review, we provide graphic overviews of Caenorhabditis elegans’ basic anatomy, growth conditions, routes of compound delivery, and fat metabolism, both synthesis and degradation pathways, including major signaling pathways involved. Our aim is to provide an overview for researchers interested in applying C. elegans as an in vivo model for the screening and identification of anti-obesity bioactive compounds prior to testing in vertebrate animal models.
Keywords: Caenorhabditis elegans, Fat metabolism, Obesity
Highlights
The basic anatomy of C. elegans includes a pharynx, intestine, gonad, and cuticle
Ingestion and diffusion are available to deliver compounds to C. elegans
The de novo fatty acid synthesis pathway in C. elegans is compared to the mammals
Fatty acid β-oxidation in C. elegans is comparable to that in the mammals
1. Introduction
Caenorhabditis elegans is a eukaryotic, multi-organ, transparent nematode that lives in the interstitial water of soil and survives by feeding on microbes. It is a small animal with a transparent body, ∼1 mm adult length, and a short lifecycle of ∼3 days. It consists of 4 larval stages (L1-L4) and adulthood, with a total longevity of around 2–3 weeks at 20 °C (Shen et al., 2018b). In the lab it can be easily maintained in solid or liquid medium with non-pathogenic bacteria, E. coli OP50, as a food source. C. elegans has two natural sexes, hermaphrodite (XX) and male (XO). The hermaphrodite can reproduce ∼300 progenies (∼0.1% male) via self-fertilization, which allows easy generation of genetically-identical progeny. It has a known genetic sequence, with >65% of its genes having homologs with genes associated with human disease. Genes of C. elegans can be easily manipulated using numerous genetic tools, such as CRISPR-Cas9 gene editing and genome-scale RNA interference (RNAi). In addition, a large number of mutant strains are available at low cost from the Caenorhabditis Genetic Center funded by the National Institutes of Health (NIH). Thus, C. elegans can be used in many research areas employing genetic modifications for rapid, large-scale, high-throughput screenings to discover drugs and bioactive compounds. This review will graphically summarize key points for the application of C. elegans to research, with a particular focus on obesity research.
1.1. Anatomical structure of C. elegans
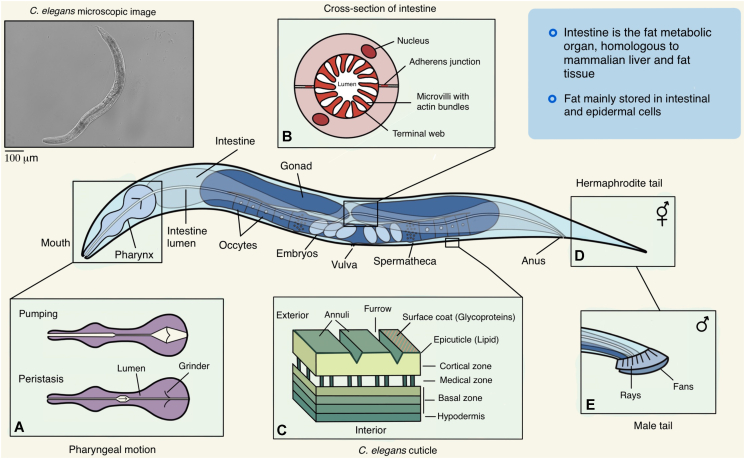
Fig. 1 summarizes key features of the anatomy of C. elegans. It has a simple anatomy consisting of a small number of tissues and organs. The C. elegans intestine, one of its major organs, functions as the fat metabolic organ, which is homologous to the mammalian liver and fat tissue (Altun and Hall, 2003). Accumulated fat in C. elegans, mainly in hypodermal and intestinal cells (Zheng and Greenway, 2012), can be easily quantified via chemical/biochemical or dye-based methods as previous summarized (Shen et al., 2018a).
Fig. 1.
Anatomy of adult Caenorhabditis elegans. The basic anatomy of C. elegans includes a mouth, pharynx, intestine, gonad, and collagenous cuticle. (A) The mouth has 6 symmetrical lips that form a 1–3 μm circular cavity to deliver the food to the feeding organ: pharynx. The pharynx is a neuromuscular pump with three functional parts (the corpus, the isthmus, and the terminal bulb) with pumping and peristalses motions; pumping is responsible for getting the food into the worm, concentrating the bacteria in the anterior isthmus, and crushing the bacteria for further digestion, while the isthmus peristalsis carries the bacteria to the grinder in the terminal bulb, grinds up the bacteria, and passes the debris back into the intestine (Avery and You, 2012; Song and Avery, 2013). (B) The intestine is comprised of 20 large epithelial cells that are positioned as bilaterally symmetric pairs to form a long tube with a central lumen (Altun and Hall, 2003). Two intestinal cells are firmly linked by adherens junctions at their apical borders and form the intestinal lumen between their opposed apical microvilli-containing surfaces (McGhee, 2007). (C) In young adults, the cuticle is ∼0.5 μm thick and has 4 major layers (epicuticle, cortical, medial, and basal). The epicuticle layer contains lipids covered by a thin glycoprotein-rich surface coat. Below it is the cortical zone, which consists of cuticulin and collagens. The medial zone is a fluid-filled space with collagenous struts that connect the cortical and basal zones (Wolkow et al., 2017). (D & E) The hermaphrodite tail tapers to a thin point, while the male tail is blunt-ended and bears a copulatory apparatus.
1.2. Compound uptake routes in C. elegans
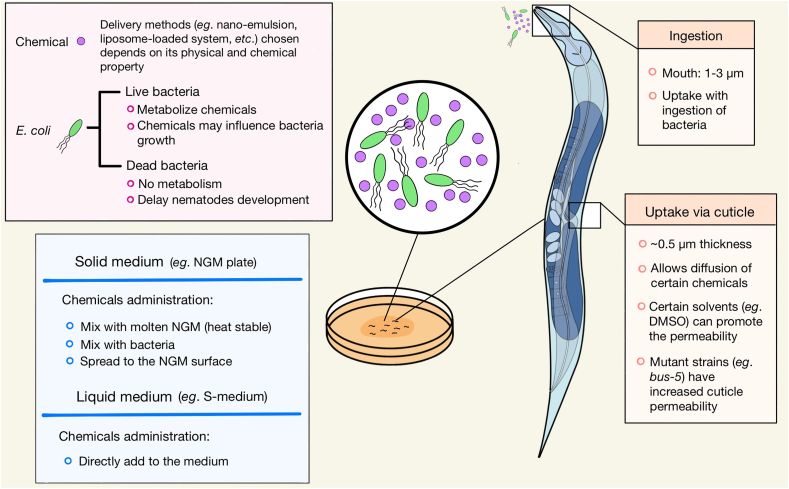
C. elegans uptakes chemicals via two major routes: ingestion and cuticle diffusion (Fig. 2). Ingestion occurs with food intake and is associated with the feeding behavior. It is affected by satiety, the food source, and/or food availability, and is regulated by the nervous system. C. elegans feeding begins by sucking in liquid containing suspended bacteria, trapping bacteria in the pharynx, and finally expelling liquid. The other route for a compound update is diffusion via cuticle. The C. elegans cuticle is synthesized and secreted by underlying epithelial cells, and then re-established by molting at the end of each developmental stage (Page and Johnstone, 2007). As a multifunctional exoskeleton, the C. elegans cuticle is essential to body shape, integrity, ensuring motility, and protecting the nematode from the external environment. However, it can be a barrier for the absorption of many compounds (Bulterijs and Braeckman, 2020). Therefore, mutants with increased cuticle permeability, such as bus-5, can be used for the increased chemical diffusion into the body (Shen et al., 2018a; Xiong et al., 2017).
Fig. 2.
Routes of compound uptake in Caenorhabditis elegans. Based on physical and chemical properties, chemicals can be dissolved in amphipathic solvents, aqueous buffer, or via different delivery systems. There are two major routes: ingestion through food intake, influenced by many factors regulating feeding behaviors, and diffusion via cuticle, determined by the cuticle permeability to the tested chemical. Solvents such as DMSO or mutant strains (bus-5) with reduced cuticle thickness and increased permeability can improve compound delivery (Shen et al., 2018a; Xiong et al., 2017).
Based on these two major routes of chemical uptake and the physical and chemical properties of target chemicals, various delivery methods can be applied to C. elegans (Fig. 2). Tested chemicals can be either mixed with the molten nematode growth medium (NGM) plate (∼65 °C for heat-stable compounds), added onto the surface of the NGM plate, mixed with E. coli, or added in the liquid S-medium. Water-soluble chemicals can be easily administrated using any of these methods. Amphipathic solvents, such as dimethyl sulfoxide (DMSO), are commonly used to dissolve lipophilic compounds using a final DMSO concentration of less than 0.6% without adversely influencing the health of the nematodes (Bulterijs and Braeckman, 2020). Alternatively, delivery methods that generate bacteria mimics were developed to increase drug uptake via ingestion. These include nano-emulsion (Colmenares et al., 2016) or liposome-loaded (Shibamura et al., 2009) systems to deliver lipophilic or hydrophilic compounds, respectively.
The use of live vs. dead E. coli should be considered when delivering compound given that live E. coli may metabolize target chemicals. This could lower the dose delivered to the nematodes and/or lead to potential effects by those metabolites. However, dead E. coli could potentially slow the growth of the worms at developmental stages or adversely influence health (Lenaerts et al., 2008; Saul et al., 2009). According to a prior report (Zheng et al., 2013), mixing target compounds with a molten NGM plate or adding dead E. coli to a liquid growing medium produces the largest drug absorption efficiency, and is therefore considered to be the optimum method in screening studies.
1.3. de novo fat synthesis in C. elegans
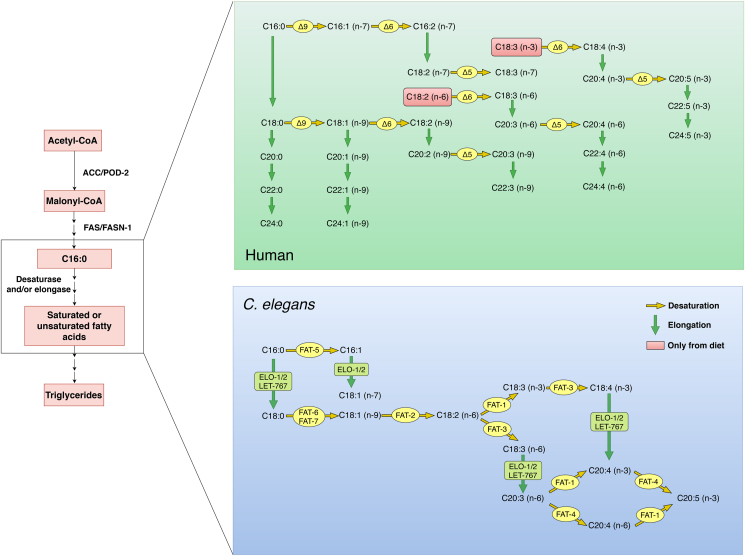
C. elegans, as a cholesterol auxotroph organism, needs to obtain a small amount of cholesterol from its diet for its survival. It is suggested that cholesterol is not a required component of C. elegans cell membranes, but rather a necessity for sterol-based signaling. The other fat metabolic pathways of mammals are well conserved in C. elegans. Counterparts of a number of proteins/enzymes central to regulating lipid metabolism, including fat synthesis (Fig. 3) and breakdown (Fig. 4), appear to have similar functions in C. elegans. As shown in Fig. 3, C. elegans has homologs of enzymes for lipogenesis. With three Δ9 desaturases (FAT-5, FAT-6, and FAT-7) and elongases, C. elegans does not rely on dietary supplies of essential fatty acids (C18:2 n-6 and C18:3 n-3 for humans) as it has the set of desaturases and elongases necessary for the synthesis of these fatty acids (Shen et al., 2018a). As with mammals, inhibition of Δ9 desaturases in C. elegans is associated with reduced overall fat accumulation (Yue et al., 2019).
Fig. 3.
Fat synthesis pathway in Caenorhabditis elegans versus human.C. elegans obtains fatty acids from its bacterial diet or via de novo synthesis, and the related genes are functionally conserved in mammals. The de novo synthesis starts by converting acetyl-CoA to malonyl-CoA by acetyl-CoA carboxylase (ACC/POD-2 in C. elegans), which is further catalyzed by fatty acid synthase (FAS/FASN-1 in C. elegans) to form fatty acids with different lengths, mainly palmitic acid (C16:0). Palmitic acid can either be incorporated into triglyceride/phospholipids or serve as the substrate that undergoes desaturation and/or elongation to generate a variety of fatty acids. Compared with human, there are three Δ9 desaturases, FAT-5, FAT-6, and FAT-7, other desaturases, FAT-1 to FAT-4, elongases, ELO-1 and ELO-2, and 3-ketoacyl-CoA reductase LET-767 in C. elegans. Notably, C. elegans can de novo synthesize all its polyunsaturated fatty acids from palmitic acid, whereas mammals need to obtain linoleic acid (C18:2 n-6) and linolenic acid (C18:3 n-3) from the diet (Shen et al., 2018a).
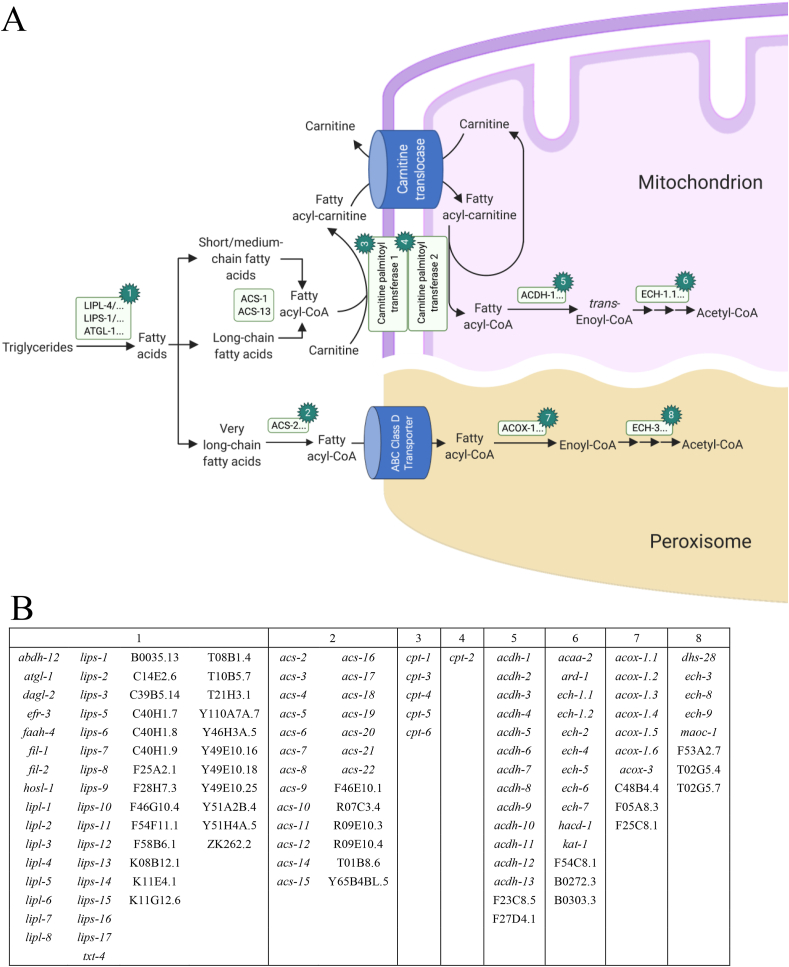
Fig. 4.
Fat breakdown pathway in Caenorhabditis elegans.C. elegans fats are stored predominantly as triglycerides. (A) In C. elegans fat metabolism, lipases (1) break TAGs to free fatty acids. Acyl-CoA synthetases (2) convert fatty acids into fatty acyl-CoA. Fatty acyl-CoA generated from short, medium, or long-chain fatty acid is converted to fatty acyl-carnitine by carnitine palmitoyl transferase 1 (3), which can be transported to the inner mitochondrial membrane by carnitine translocase. Then carnitine palmitoyl transferase 2 (4) converts fatty acyl-carnitine into fatty acyl-CoA and carnitine. Carnitine can get through the mitochondrial membrane to be recycled. Fatty acyl-CoA enters mitochondrial β-oxidation via acyl-CoA dehydrogenases (5) followed by trifunctional enzymes (6). The very-long-chain fatty acyl-CoA crosses the peroxisome via ABC class D transporter and enters peroxisomal β-oxidation after acyl-CoA oxidases (7) and the trifunctional enzymes (8) (Gilst et al., 2005; Haataja et al., 2011; Ranawade et al., 2018). (B) Gene encoding enzymes for 1–8 are listed.
1.4. Fat breakdown pathways in C. elegans
In eukaryotes, it is commonly accepted that mitochondria break down long, medium, and short-chain fatty acids, while peroxisomes are responsible for breaking down very-long-chain fatty acids (Watts and Ristow, 2017). Similarly, fat breakdown occurs via peroxisomes or mitochondria β-oxidation in C. elegans (Fig. 4) (Shen et al., 2018a). Although the relative proportions of fat oxidation occurring in mitochondria or peroxisomes have not been clearly characterized in C. elegans, genetic screens have indicated that peroxisomal oxidation is important for C. elegans in breaking down fats in large lipid droplets (Watts and Ristow, 2017). In addition, peroxisomal fat oxidation is required for the biosynthesis of ascaroside pheromones, which are signaling molecules that control dauer formation, lifespan, and behavior in this model animal (Park and Paik, 2017).
1.5. Fat metabolism related signaling pathways in C. elegans
As in mammals, the balance between fat storage and utilization relies on a variety of complex transcriptional, translational, and post-translational regulatory mechanisms. Of these processes, transcriptional regulation has been studied the most in C. elegans. Many key transcriptional regulators have been reported to regulate lipid metabolism in C. elegans by targeting genes involved in the regulatory pathways of lipogenesis, desaturation, and fatty acid oxidation. CEBP-2, the homolog of human CCAAT/enhancer-binding proteins, positively regulates acyl-CoA synthetase (ACS-2) and enoyl-CoA hydratase (ECH-1). SBP-1, the homolog of a sterol regulatory element-binding protein in mammals, positively regulates fatty acid desaturases (FAT-6/7), fatty acid synthase (FASN-1), and polarity and osmotic sensitivity defect (POD-2, homolog of acetyl-CoA carboxylase). AAK-2, the homolog of mammal AMP-activated protein kinase, positively regulates adipose triglyceride lipase (ATGL-1), while inhibiting FAT-2. NHR-49, the homolog of mammal peroxisome proliferator-activated receptors, positively regulates FAT-5/6/7, ACS-2, and ECH-1. NHR-64 is the homolog of mammal hepatocyte nuclear factor 4, and positively regulates FAT-5/6/7 and ACS-2, while negatively regulating POD-2. TUB-1, the homolog of tubby in mammals, positively regulates 3-ketoacyl-CoA thiolase (KAT-1) (Liang et al., 2010; Shen et al., 2018b).
2. Conclusion
C. elegans has been widely used in research areas of aging, neurodegenerative disorder, and genetics. In this review, we provide overviews of C. elegans as a model animal in obesity study for researchers interested in applying C. elegans as an in vivo model for identification of anti-obesity bioactives as well as large genetic and pharmacological screens that are not possible with vertebrate animals.
In addition to what we summarized in this review, C. elegans can be used to determine the neuroendocrine control, such as the oxygen sensing, population density sensing and food sensing that may contribute to overall balance of fat accumulation (Srinivasan, 2020). Moreover, C. elegans shows food preference behavior, which is regulated by various neurological signals comparable to those in humans (Engleman et al., 2018). Lastly, humanized C. elegans with target human genes and/or receptors can be easily developed, which can provide highly translatable results to humans. However, there is currently lack of understanding on direct comparison of doses between C. elegans and other species, which needs to be addressed in the near future. In conclusion, C. elegans is a valuable tool for the many research fields, including obesity, for fundamental understanding of mechanisms and used as a model prior to testing other vertebrate animals.
CRediT authorship contribution statement
Yiren Yue: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. Sida Li: Conceptualization, Visualization, Writing – original draft, Writing – review & editing. Peiyi Shen: Conceptualization. Yeonhwa Park: Conceptualization, Project administration, Supervision, Funding acquisition, Writing – review & editing.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This material is based upon work supported in part by the National Institute of Food and Agriculture, U.S. Department of Agriculture, the Massachusetts Agricultural Experiment Station, and the Department of Food Science, at the University of Massachusetts Amherst, under project number MAS00556.
References
- Altun Z.F., Hall D.H. WormAtlas hermaphrodite handbook-Alimentary system-Intestine. Wormatlas. 2003 doi: 10.3908/wormatlas.1.4. [DOI] [Google Scholar]
- Avery L., You Y.J. C. elegans feeding. Wormbook. 2012;1–23 doi: 10.1895/wormbook.1.150.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulterijs S., Braeckman B.P. Phenotypic screening in C. elegans as a tool for the discovery of new geroprotective drugs. Pharmaceuticals. 2020;13:164. doi: 10.3390/ph13080164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmenares D., Sun Q., Shen P., Yue Y., McClements D.J., Park Y. Delivery of dietary triglycerides to Caenorhabditis elegans using lipid nanoparticles: nanoemulsion-based delivery systems. Food Chem. 2016;202:451–457. doi: 10.1016/j.foodchem.2016.02.022. [DOI] [PubMed] [Google Scholar]
- Engleman E.A., Steagall K.B., 2nd, Bredhold K.E., Breach M., Kline H.L., Bell R.L., Katner S.N., Neal-Beliveau B.S. Caenorhabditis elegans show preference for stimulants and potential as a model organism for medications screening. Front. Physiol. 2018;9:1200. doi: 10.3389/fphys.2018.01200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilst M.R.V., Hadjivassiliou H., Jolly A., Yamamoto K.R. Nuclear hormone receptor NHR-49 controls fat consumption and fatty acid composition in C. elegans. PLoS Biol. 2005;3:e53. doi: 10.1371/journal.pbio.0030053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haataja T.J.K., Koski M.K., Hiltunen J.K., Glumoff T. Peroxisomal multifunctional enzyme type 2 from the fruitfly: dehydrogenase and hydratase act as separate entities, as revealed by structure and kinetics. Biochem. J. 2011;453:771–781. doi: 10.1042/BJ20101661. [DOI] [PubMed] [Google Scholar]
- Lenaerts I., Walker G.A., Hoorebeke L.V., Gems D., Vanfleteren J.R. Dietary restriction of Caenorhabditis elegans by axenic culture reflects nutritional requirement for constituents provided by metabolically active microbes. J. Gerontology Ser. A: Biol. Sci. Med. Sci. 2008;63:242–252. doi: 10.1093/gerona/63.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang B., Ferguson K., Kadyk L., Watts J.L. The role of nuclear receptor NHR-64 in fat storage regulation in Caenorhabditis elegans. PLoS One. 2010;5 doi: 10.1371/journal.pone.0009869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee J. The C. elegans intestine. Wormbook. 2007;1–36 doi: 10.1895/wormbook.1.133.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page A.P., Johnstone I.L. The cuticle. Wormbook. 2007;1–15 doi: 10.1895/wormbook.1.138.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S., Paik Y.K. Genetic deficiency in neuronal peroxisomal fatty acid β-oxidation causes the interruption of dauer development in Caenorhabditis elegans. Sci. Rep.-uk. 2017;7:9358. doi: 10.1038/s41598-017-10020-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranawade A., Mallick A., Gupta B.P. PRY-1/Axin signaling regulates lipid metabolism in Caenorhabditis elegans. PLoS One. 2018;13 doi: 10.1371/journal.pone.0206540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul N., Pietsch K., Menzel R., Stürzenbaum S.R., Steinberg C.E. Catechin induced longevity in C. elegans: from key regulator genes to disposable soma. Mech. Ageing Dev. 2009;130:477–486. doi: 10.1016/j.mad.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Shen P., Yue Y., Park Y. A living model for obesity and aging research: Caenorhabditis elegans. Crit. Rev. Food Sci. Nutr. 2018;58:741–754. doi: 10.1080/10408398.2016.1220914. [DOI] [PubMed] [Google Scholar]
- Shen P., Yue Y., Zheng J., Park Y. Caenorhabditis elegans: a convenient in vivo model for assessing the impact of food bioactive components on obesity, aging, and alzheimer’s disease. Annu. Rev. Food Sci. Technol. 2018;9:1–22. doi: 10.1146/annurev-food-030117-012709. [DOI] [PubMed] [Google Scholar]
- Shibamura A., Ikeda T., Nishikawa Y. A method for oral administration of hydrophilic substances to Caenorhabditis elegans: effects of oral supplementation with antioxidants on the nematode lifespan. Mech. Ageing Dev. 2009;130:652–655. doi: 10.1016/j.mad.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Song B., Avery L. The pharynx of the nematode C. elegans: a model system for the study of motor control. Worm. 2013;2 doi: 10.4161/worm.21833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan S. Neuroendocrine control of lipid metabolism: lessons from C. elegans. J. Neurogenet. 2020;34:1–7. doi: 10.1080/01677063.2020.1777116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts J.L., Ristow M. Lipid and carbohydrate metabolism in Caenorhabditis elegans. Genetics. 2017;207:413–446. doi: 10.1534/genetics.117.300106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkow C., Herndon L.A., Hall D.H. Wormatlas; 2017. WormAtlas Aging Handbook - the Aging Cuticle. [DOI] [Google Scholar]
- Xiong H., Pears C., Woollard A. An enhanced C. elegans based platform for toxicity assessment. Sci. Rep. 2017;7:1–11. doi: 10.1038/s41598-017-10454-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y., Shen P., Chang A.L., Qi W., Kim K.H., Kim D., Park Y. trans-Trismethoxy resveratrol decreased fat accumulation dependent on fat-6 and fat-7 in Caenorhabditis elegans. Food Funct. 2019;10:4966–4974. doi: 10.1039/c9fo00778d. [DOI] [PubMed] [Google Scholar]
- Zheng J., Greenway F. Caenorhabditis elegans as a model for obesity research. Int. J. Obes. 2012;36:186–194. doi: 10.1038/ijo.2011.93. [DOI] [PubMed] [Google Scholar]
- Zheng S.Q., Ding A.J., Li G.P., Wu G.S., Luo H.-R. Drug absorption efficiency in Caenorhbditis elegans delivered by different methods. PLoS One. 2013;8 doi: 10.1371/journal.pone.0056877. [DOI] [PMC free article] [PubMed] [Google Scholar]