Graphical abstract
Keywords: New Psychoactive Substances, Hair analysis, Extraction, Mass spectrometry, LOD, Identification
Highlights
-
•
The detection of 280 NPS has been reported to be enabled through hair analysis.
-
•
The LODs/ LOQs for these NPS are as low as pg/mg of hair.
-
•
The NPS hair concentrations in clinical/forensic samples are considerably higher than the respective LOD.
-
•
Untargeted-mass spectroscopic detection techniques could advance NPS hair analysis.
-
•
NPS hair analysis could become the tool to monitor the extent of NPS use worldwide.
Abstract
In this review article, we performed an overview of extraction and chromatographic analysis methods of NPS in hair from 2007 to 2021, evaluating the limit of detection (LOD), limit of quantification (LOQ), limit of reporting (LOR), and limit of identification (LOI) values reported for each NPS. Our review aimed to highlight the limitations of modern hair analytical techniques, and the prerequisites for the proper evaluation and use of analytical results in relation to the objectives of NPS hair analysis. In the selected studies the detection of a total of 280 NPS was reported. The detected NPS belonged to seven classes: synthetic cannabinoids with 109 different substances, synthetic opioids with 58, cathinones with 50, phenethylamines with 34, other NPS with 15, tryptamines with ten, and piperazines with four substances. The NPS hair analysis of real forensic/ clinical cases reported the detection of only 80 NPS (out of the 280 targeted), in significantly higher levels than the respective LODs. The analytical protocols reviewed herein for NPS hair analysis showed continuously growing trends to identify as many NPS as possible; the extraction methods seem to have a limited potential to improve, while the various mass spectroscopic techniques and relevant instrumentation provide an enormous field for development and application. Hair is a biological indicator of the past chronic, sub-chronic, and, even, in certain cases, acute exposure to xenobiotics. Therefore, future research in the field could progress NPS hair analysis and aim the monitoring of NPS expansion and extent of use in the community.
1. Introduction
Over the last few years, new recreational psychotropic substances, have been synthesized and flooded the illicit drug market, being identified under different labels e.g. “legal highs,” “research chemicals,” or “designer drugs”. Nowadays, they are known as “Novel Psychoactive Substances” (NPS). Initially, NPS were designed to mimic the effects of internationally controlled illegal drugs while being structurally different to not be controlled under the Misuse of Drugs Act 1971 [1]. Currently, there are more than 1000 NPS belonging to defined groups, e.g. synthetic cannabinoids, phenethylamines, cathinones, piperazines, plant-based substances, and miscellaneous substances including hallucinogens, synthetic opioids, and synthetic benzodiazepines [1].
NPS have become a worldwide health problem due to the vast variety of novel substances available, their ambiguous legal situation and ability to pass undetected routine toxicological, immunochemical tests, their rapid adaptation to legal restrictions, and their unidentified, in many cases, adverse effects [2,3]. Most of these products are more pharmacologically potent and hazardous than classical drugs of abuse [4]. Meanwhile, several fatal and acute intoxication cases have been accredited to this diverse group of compounds [[5], [6], [7]].
Hair analysis can be used in biomonitoring of toxicants and it is the method of choice for assessing retrospective evaluation of the past, chronic, sub-chronic, and, even, in certain cases, acute exposure to xenobiotics [8]. The past detection window can exceed up to several months or even years, while segmental hair analysis has been used to provide information on the history and state of drug abuse of the tested individual [8,9]. While generally, the biomonitoring of particular toxicants applied in clinical studies [10,11], hair analysis was performed, specifically, to study the exposure of certain populations to pesticides and other organic pollutants [[12], [13], [14], [15]] and, the prevalence of NPS among drug users or addicts [9,[16], [17], [18]]. We are of opinion that when large populations would be subjected to NPS hair analysis, then the relevant gathered results would provide epidemiological data on the NPS trends and extent of use in the community.
In this review article, we performed an overview of extraction and chromatographic analysis methods of NPS in hair from 2007 to 2021, evaluating the limit of detection (LOD), limit of quantification (LOQ), limit of reporting (LOR), and limit of identification (LOI) values of each NPS. Our review aimed to highlight the limitations of modern hair analytical techniques, and the prerequisites for the proper evaluation and use of analytical results in relation to the objectives of the analysis.
The following keywords: “New Psychoactive Substances” or “NPS” and “hair” and “analytical methods” and “pre-treatment methods” or “extraction methods” and “phenethylamines” or “piperazines” or “synthetic cannabinoids” or “cathinones” or “synthetic opioids”, or “tryptamines”, were used to search the databases Scopus and PubMed. Information of interest of this review were found in 45 reports which were selected for further study.
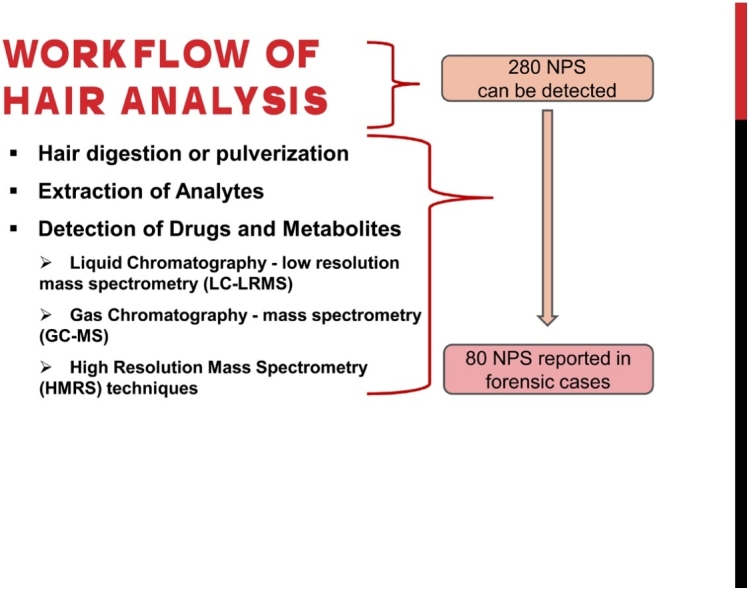
2. Extraction and detection methods for the determination of NPS in hair
In the selected studies the detection of a total of 280 NPS was reported, for the different hair analysis protocols. The detected NPS belonged to seven classes: phenethylamines (2C-X series, other phenethylamines), piperazines, synthetic cathinones (SCa), synthetic cannabinoids (SCs, categorized in the subclasses of benzoylindoles, naphthoylindoles, phenylacetylindoles, naphthoypyrroles, other SCs), synthetic opioids (SO), tryptamines, and other NPS classes. Synthetic cannabinoids dominated the other categories with 109 different substances, followed by synthetic opioids with 58, cathinones with 50, phenethylamines with 34, other NPS with 15, tryptamines with ten, and piperazines with four substances. The overview of methods of extraction, analysis and detection to determine NPS in hair are exhibited in Table 1, Table 2, Table 3, Table 4, Table 5.
Table 1.
Selected parameters of hair analysis for Synthetic Phenethylamines and Piperazines.
| NPS | Extraction Method | Method of Analysis | LOD (pg/mg) |
LOQ (pg/mg) |
LOI/ LOR (pg/mg) |
Concentrations- Clinical/forensic samples (pg/mg) | References |
|---|---|---|---|---|---|---|---|
| PHENETHYLAMINES (2C-x-series) | |||||||
| 2 C-B | MeOH/ HCL 0.25 M at 50 °C | GC/MS | 4 | 20 | [19] | ||
| MeOH at 55 °C | LC-MS/MS | 6.2 | 12 | [20] | |||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 50 | -/100 | [21] | |||
| Enzymatic Digestion, LLE neutral and basic with DEE/EA | LC-MS/MS | 80 | [22] | ||||
| 2 C-D | Enzymatic Digestion, LLE neutral and basic with DEE/EA | LC-MS/MS | 50 | [22] | |||
| 2 C–E | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | ||
| Enzymatic Digestion, LLE neutral and basic with DEE/EA | LC-MS/MS | 20 | [22] | ||||
| 2 C–I | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | ||
| Enzymatic Digestion, LLE neutral and basic with DEE/EA | LC-MS/MS | 80 | [22] | ||||
| 2 C-G | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | ||
| 2 C-N | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 50 | -/100 | [21] | ||
| 2 C–P | MeOH at 55 °C | LC-MS/MS | 1 | 2 | [20] | ||
| Enzymatic Digestion, LLE neutral and basic with DEE/EA | LC-MS/MS | 50 | [22] | ||||
| 2 C–T | Enzymatic Digestion, LLE neutral and basic with DEE/EA | LC-MS/MS | 100 | [22] | |||
| 2C–T-4 | Acid digestion at 45 °C, PLE, SPE/C18 cartridge | LC-HRMS | 10 | 50 | [23] | ||
| Me-EPHE | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | ||
| MXP | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| N-EPHE | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 50 | -/100 | [21] | ||
| PE | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 50 | -/100 | [21] | ||
| PS-EPHE | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | ||
| 25B-NBOMe | MeOH at 55 °C | LC-MS/MS | 4.1 | 8.2 | [20] | ||
| 25C-NBOMe | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| MeOH at 55 °C | LC-MS/MS | 1.5 | 3 | [20] | |||
| 25H-NBOMe | MeOH at 55 °C | LC-MS/MS | 1 | 2 | [20] | ||
| 25I-NBOMe | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| MeOH at 55 °C | LC-MS/MS | 1.5 | 3 | [20] | |||
| OTHER PHENETHYLAMINES- AMPHETAMINE TYPE | |||||||
| Butylone (bk-MBDB) |
SPE/Bond Elute Certify I | LC-MS/MS | 0.8 | 1 | [24] | ||
| MeOH at 55 °C | LC-MS/MS | 3.7 | 7.4 | [20] | |||
| 0.1 M HCOOH at 45 °C | LC-MS/MS | 5 | 20 | [25] | |||
| MeOH/ACN/aq. HCOONH4 at 40 °C | LC-MS/MS | 8 | 25 | [26] | |||
| MeOH, MeOH/HCL 33 % | LC-MS/MS | 10 | [27] | ||||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | |||
| DMA | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | ||
| MDEA | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| PLE, SPE/C18 cartridge | LC-MS-MS | 1 | 4.5 | [28] | |||
| MeOH/ HCL 0.25 M at 50 °C | GC/MS | 24 | 80 | 100−25,000 | [19] | ||
| MPHP | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| M3® reagent at 100 °C | LC-MS/MS | 5 | 20 | [29] | |||
| MXE | MeOH at 55 °C | LC-MS/MS | 1 | 2 | 7.7−27 | [20] | |
| Acid digestion at 45 °C, PLE, SPE/C18 cartridge | LC-HRMS | 3 | 10 | [23] | |||
| Incubation at 95 °C, LLE with Hept/EA, DCM/Isopropanol | LC-HRMS- Orbitrap | 5 | 5/- | [30] | |||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | |||
| PMA | 0.1 M HCOOH at 45 °C | LC-MS/MS | 5 | 10 | [25] | ||
| MeOH at 55 °C | LC-MS/MS | 8.8 | 18 | [20] | |||
| Incubation at 45 °C, SPE/MCX® Oasis cartridge | LC-MS/MS | 10 | 50 | [31] | |||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | |||
| MeOH/ HCL 1% | GC/MS | 250 | 500 | 20,100 | [32] | ||
| PMMA | MeOH at 55 °C | LC-MS/MS | 1.3 | 2.6 | [20] | ||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | |||
| PPMA | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | ||
| 4-FA | MeOH at 55 °C | LC-MS/MS | 1.6 | 3.2 | [20] | ||
| 0.1 M HCOOH at 45 °C | LC-MS/MS | 2 | 5 | [25] | |||
| MeOH, MeOH/HCL 33 % | LC-MS/MS | 10 | [27] | ||||
| 4-FMA | MSPE: MeOH/ HCL 0.1 M at 60 °C, SPE/ C18 | LC-MS/MS | 2 | 10 | [33] | ||
| 4-MTA | 0.1 M HCOOH at 45 °C | LC-MS/MS | 2 | 20 | [25] | ||
| Incubation at 45 °C, SPE/MCX® Oasis cartridge | LC-MS/MS | 20 | 50 | [31] | |||
| 5-APB | M3® reagent at 100 °C | LC-MS/MS | 5 | 20 | [29] | ||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | |||
| 5-EAPB | M3® reagent at 100 °C | LC-MS/MS | 5 | 20 | [29] | ||
| 5-MAPB | MeOH at 55 °C | LC-MS/MS | 4.6 | 9.2 | [20] | ||
| 6-APB | M3® reagent at 100 °C | LC-MS/MS | 5 | 20 | 70 | [29] | |
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | |||
| MeOH at 55 °C | LC-MS/MS | 17 | 35 | [20] | |||
| 6-MAPB | M3® reagent at 100 °C | LC-MS/MS | 5 | 20 | [29] | ||
| PIPERAZINES | |||||||
| Benzylpiperazine | 0.1 M HCOOH at 45 °C | LC-MS/MS | 5 | 20 | [25] | ||
| MeOH, MeOH/HCL 33 % | LC-MS/MS | 10 | [27] | ||||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 50 | -/100 | [21] | |||
| mCPP | MeOH at 55 °C | LC-MS/MS | 3 | 6 | [20] | ||
| 0.1 M HCOOH at 45 °C | LC-MS/MS | 5 | 20 | [25] | |||
| MSPE: MeOH/ HCL 0.1 M at 60 °C, SPE/ C18 | LC-MS/MS | 5 | 10 | 3,411.4- >4000 | [33] | ||
| Incubation at 95 °C, LLE with Hept/EA, DCM/Isopropanol | LC-HRMS- Orbitrap | 5 | 500/- | [30] | |||
| MeOH, MeOH/HCL 33 % | LC-MS/MS | 10 | [27] | ||||
| Incubation at 45 °C, SPE/MCX® Oasis cartridge | LC-MS/MS | 10 | 50 | [31] | |||
| MeOH/ACN/aq. HCOONH4 at 40 °C | LC-MS/MS | 25 | 37 | [26] | |||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 50 | -/100 | [21] | |||
| MSPE: Basic digestion at 50 °C, SPE/MCX® cartridges | GC-MS | – | LLOQ: 50 | [34] | |||
| MeOPP | MeOH/ACN/aq. HCOONH4 at 40 °C | LC-MS/MS | 29 | 46 | [26] | ||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 50 | -/100 | [21] | |||
| MSPE: Basic digestion at 50 °C, SPE/MCX® cartridges | GC-MS | – | LLOQ:50 | [34] | |||
| TFMPP | MSPE: MeOH/ HCL 0.1 M at 60 °C, SPE/ C18 | LC-MS/MS | 1 | [33] | |||
| MeOH/ACN/aq. HCOONH4 at 40 °C | LC-MS/MS | 9 | 24 | [26] | |||
| MeOH, MeOH/HCL 33 % | LC-MS/MS | 10 | [27] | ||||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 50 | -/100 | [21] | |||
| MSPE: Basic digestion at 50 °C, SPE/MCX® cartridges | GC-MS | – | LLOQ: 50 | [34] | |||
Table 2.
Selected parameters of hair analysis for Synthetic Cathinones.
| NPS | Extraction Method | Analysis Method | LOD | LOQ | LOI/ LOR | Concentrations- Clinical/forensic samples (pg/mg) | References |
|---|---|---|---|---|---|---|---|
| CATHINONES | |||||||
| pg/mg | |||||||
| Amfepramone | MeOH at 55 °C | LC-MS/MS | 4 | 8 | [20] | ||
| MeOH/ACN/aq. HCOONH4 at 40 °C | LC-MS/MS | 13 | 40 | [26] | |||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 50 | -/100 | [21] | |||
| a-PBP | Basic digestion, SPE/Extrelut column | LC-MS | 0.02 ng/ 10-mm | 0.05 ng/10- mm |
[35] | ||
| MeOH/ACN/aq. HCOONH4 at 40 °C | LC-MS/MS | 6 | 17 | [26] | |||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | |||
| α-PHP | SPE/Bond Elute Certify I | LC-MS/MS | 0.1 | 1 | 4700.0/ 0−2.5 cm3600.0/ 2.5 −5 cm |
[24] | |
| a- PPP | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | ||
| MeOH/ACN/aq. HCOONH4 at 40 °C | LC-MS/MS | 13 | 25 | α-PPP < LOQ | [26] | ||
| a-PVP | Basic digestion, SPE/Extrelut column | LC-MS | 0.02 ng/ 10mm | 0.05 ng/ 10-mm | 0.8−1.2 ng/10- mm |
[35] | |
| SPE/Bond Elute Certify I | LC-MS/MS | 0.3 | 1 | 52.8/ 0−2.5 cm 24.4/ 2.5 −5 cm |
[24] | ||
| MeOH at 55 °C | LC-MS/MS | 2 | 4 | [20] | |||
| Acid digestion at 45 °C, PLE, SPE/C18 cartridge | LC-HRMS | 5 | 10 | [23] | |||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | |||
| MeOH/ACN/aq. HCOONH4 at 40 °C | LC-MS/MS | 17 | 24 | α-PVP < LOQ | [26] | ||
| a-PVT | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | ||
| Benzedrone | MeOH/ACN/aq. HCOONH4 at 40 °C | LC-MS/MS | 45 | 80 | benzedrone < LOQ 150 |
[26] | |
| Buphedrone | SPE/Bond Elute Certify I | LC-MS/MS | 1 | 5 | [24] | ||
| M3® reagent at 100 °C | LC-MS/MS | 2 | 20 | [29] | |||
| MeOH at 55 °C | LC-MS/MS | 4.2 | 8.4 | [20] | |||
| MeOH/ACN/aq. HCOONH4 at 40 °C | LC-MS/MS | 23 | 40 | [26] | |||
| Bupropion | MeOH/ACN/aq. HCOONH4 at 40 °C | LC-MS/MS | 17 | 18 | [26] | ||
| Buthylone | M3® reagent at 100 °C | LC-MS/MS | 2 | 20 | [29] | ||
| Cathinone | MeOH/ HCL 0.25 M at 50 °C | GC/MS | 3 | 20 | [19] | ||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | |||
| MeOH/ACN/aq. HCOONH4 at 40 °C | LC-MS/MS | 23 | 55 | 100−1,270 390 (pubic hair) |
[26] | ||
| Dibutylone | MeOH/ACN/aq. HCOONH4 at 40 °C | LC-MS/MS | 14 | 23 | [26] | ||
| Diethylcathinone | M3® reagent at 100 °C | LC-MS/MS | 2 | 20 | [29] | ||
| Dimethylcathinone | M3® reagent at 100 °C | LC-MS/MS | 2 | 20 | [29] | ||
| Ethcathinone | M3® reagent at 100 °C | LC-MS/MS | 2 | 20 | [29] | ||
| SPE/Bond Elute Certify I | LC–MS/MS | 2.3 | 5 | 11.0/ 0−2.5 cm | [24] | ||
| MeOH at 55 °C | LC-MS/MS | 3.1 | 6.2 | [20] | |||
| 0.1 M HCOOH at 45 °C | LC-MS/MS | 20 | 20 | [25] | |||
| Ethylone | SPE/Bond Elute Certify I | LC-MS/MS | 0.1 | 1 | [24] | ||
| 0.1 M HCOOH at 45 °C | LC-MS/MS | 2 | 5 | [25] | |||
| M3® reagent at 100 °C | LC-MS/MS | 2 | 20 | [29] | |||
| MeOH/ACN/aq. HCOONH4 at 40 °C | LC-MS/MS | 7 | 12 | [26] | |||
| MeOH, MeOH/HCL 33 % | LC-MS/MS | 10 | [27] | ||||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | |||
| Eutylone | MeOH/ACN/aq. HCOONH4 at 40 °C | LC-MS/MS | 16 | 23 | [26] | ||
| Heliomethylamine | MeOH/ACN/aq. HCOONH4 at 40 °C | LC-MS/MS | 7 | 8 | [26] | ||
| MDBC | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | ||
| MDMC | MSPE: MeOH/ HCL 0.1 M at 60 °C, SPE/ C18 | LC-MS/MS | 1 | 2 | [33] | ||
| 0.1 M HCOOH at 45 °C | LC-MS/MS | 2 | 20 | [25] | |||
| M3® reagent at 100 °C | LC-MS/MS | 2 | 20 | [29] | |||
| MeOH at 55 °C | LC-MS/MS | 3.2 | 6.4 | 28 | [20] | ||
| MeOH, MeOH/HCL 33 % | LC-MS/MS | 10 | [27] | ||||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | |||
| MeOH/ACN/aq. HCOONH4 at 40 °C | LC-MS/MS | 12 | 34 | [26] | |||
| MDPPP | MeOH/ACN/aq. HCOONH4 at 40 °C | LC-MS/MS | 7 | 22 | [26] | ||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | |||
| MDPV | Basic digestion, SPE/Extrelut column | LC-MS | 0.02 ng/ 10-mm |
0.05 ng/10-mm | 16−22 ng/10-mm | [35] | |
| MSPE: MeOH/ HCL 0.1 M at 60 °C, SPE/ C18 | LC-MS/MS | 0.2 | 2 | [33] | |||
| Acid/basic digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.5 | LLOQ: 1 | 1000 | [36] | ||
| Acid digestion at 45 °C, PLE, SPE/C18 cartridge | LC-HRMS | 0.5 | 8 | [23] | |||
| SPE/Bond Elute Certify I | LC-MS/MS | 0.5 | 1 | [24] | |||
| Incubation at 95 °C, LLE with Hept/EA, DCM/Isopropanol | LC-HRMS- Orbitrap | 1 | 5/- | [30] | |||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | |||
| 0.1 M HCOOH at 45 °C | LC-MS/MS | 2 | 5 | 50 | [25] | ||
| MeOH at 55 °C | LC-MS/MS | 2 | 4 | [20] | |||
| M3® reagent at 100 °C | LC-MS/MS | 2 | 20 | [29] | |||
| MeOH, MeOH/HCL 33% | LC-MS/MS | 10 | [27] | ||||
| MeOH/ACN/aq. HCOONH4 at 40 °C | LC-MS/MS | 10 | 23 | 20−800 | [26] | ||
| Mephtetramine | ACN/H20/ TFA at 40 °C | LC–HRMS-Orbitrap | 50 | 200 | [37] | ||
| Metamfepramone | MeOH/ACN/aq. HCOONH4 at 40 °C | LC-MS/MS | 7 | 10 | metamfepramone < LOQ 10 |
[26] | |
| Methcathinone or ephedrone (MC) | SPE/Bond Elute Certify I | LC-MS/MS | 1 | 5 | 1600.0/ 0−2.5 cm695.6/ 2.5 −5 cm |
[24] | |
| M3® reagent at 100 °C | LC-MS/MS | 2 | 20 | [29] | |||
| MeOH, MeOH/HCL 33 % | LC-MS/MS | 10 | [27] | ||||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | |||
| MeOH/ HCL 0.25 M at 50 °C | GC/MS | 11 | 40 | [19] | |||
| MeOH/ACN/aq. HCOONH4 at 40 °C | LC-MS/MS | 15 | 29 | [26] | |||
| Methylbuphedrone | MeOH/ACN/aq. HCOONH4 at 40 °C | LC-MS/MS | 15 | 46 | [26] | ||
| MOPPP | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| MeOH/ACN/aq. HCOONH4 at 40 °C | LC-MS/MS | 6 | 18 | 10 | [26] | ||
| MPBP | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| MeOH/ACN/aq. HCOONH4 at 40 °C | LC-MS/MS | 9 | 23 | [26] | |||
| Naphyrone or naphthylpyrovalerone (NPV) | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| M3® reagent at 100 °C | LC-MS/MS | 2 | 20 | [29] | |||
| SPE/Bond Elute Certify I | LC-MS/MS | 2.5 | 5 | [24] | |||
| MeOH/ACN/aq. HCOONH4 at 40 °C | LC-MS/MS | 6 | 18 | [26] | |||
| 0.1 M HCOOH at 45 °C | LC-MS/MS | 10 | 20 | [25] | |||
| N-ethylcathinone (EC) | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | ||
| MeOH/ACN/aq. HCOONH4 at 40 °C | LC-MS/MS | 16 | 44 | [26] | |||
| N,N-DMC | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | ||
| Penthedrone | M3® reagent at 100 °C | LC-MS/MS | 2 | 20 | [29] | ||
| Pentedrone | SPE/Bond Elute Certify I | LC-MS/MS | 0.4 | 1 | 198.4/ 0−2.5 cm586.2/ 2.5 −5 cm |
[24] | |
| 0.1 M HCOOH at 45 °C | LC-MS/MS | 2 | 20 | [25] | |||
| MeOH at 55 °C | LC-MS/MS | 3.9 | 7.8 | [20] | |||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | |||
| MeOH/ACN/aq. HCOONH4 at 40 °C | LC-MS/MS | 18 | 39 | 7340 | [26] | ||
| Penthylone | M3® reagent at 100 °C | LC-MS/MS | 2 | 20 | [29] | ||
| Pentylone | SPE/Bond Elute Certify I | LC-MS/MS | 0.1 | 1 | [24] | ||
| 0.1 M HCOOH at 45 °C | LC-MS/MS | 2 | 20 | [25] | |||
| MeOH/ACN/aq. HCOONH4 at 40 °C | LC-MS/MS | 8 | 23 | [26] | |||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | |||
| PMMC | SPE/Bond Elute Certify I | LC-MS/MS | 0.2 | 1 | [24] | ||
| MSPE: MeOH/ HCL 0.1 M at 60 °C, SPE/ C18 | LC-MS/MS | 1 | 2 | [33] | |||
| 0.1 M HCOOH at 45 °C | LC-MS/MS | 2 | 20 | [25] | |||
| MeOH/ACN/aq. HCOONH4 at 40 °C | LC-MS/MS | 4 | 18 | [26] | |||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | |||
| Pyrovalerone | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| MeOH/ACN/aq. HCOONH4 at 40 °C | LC-MS/MS | 8 | 14 | [26] | |||
| 2-FMC | Acid digestion at 45 °C, PLE, SPE/C18 cartridge | LC-HRMS | 4 | 50 | [23] | ||
| 2-Methoxymethcathinone | Acid digestion at 45 °C, PLE, SPE/C18 cartridge | LC-HRMS | 7 | 20 | [23] | ||
| 3,4-DMMC | SPE/Bond Elute Certify I | LC-MS/MS | 0.3 | 1 | 2800.0/ 0−2.5 cm572.6/ 2.5 −5 cm |
[24] | |
| M3® reagent at 100 °C | LC-MS/MS | 2 | 20 | [29] | |||
| 0.1 M HCOOH at 45 °C | LC-MS/MS | 5 | 20 | [25] | |||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | |||
| MeOH/ACN/aq. HCOONH4 at 40 °C | LC-MS/MS | 17 | 43 | [26] | |||
| 3-FMC | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | ||
| MeOH/ACN/aq. HCOONH4 at 40 °C | LC-MS/MS | 31 | 35 | [26] | |||
| 3-MMC | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | ||
| MeOH/ TFA at 45 °C, after pulver. | LC-HRMS-Orbitrap | 20 | 100 | 25.800 | [38] | ||
| 4-BMC | MeOH/ACN/aq. HCOONH4 at 40 °C | LC-MS/MS | 52 | 95 | 2730 | [26] | |
| 4-FMC | SPE/Bond Elute Certify I | LC-MS/MS | 1 | 5 | 41.1/ 0−2.5 cm45.6/ 2.5 −5 cm |
[24] | |
| M3® reagent at 100 °C | LC-MS/MS | 2 | 20 | [29] | |||
| 0.1 M HCOOH at 45 °C | LC-MS/MS | 5 | 10 | [25] | |||
| MSPE: MeOH/ HCL 0.1 M at 60 °C, SPE/ C18 | LC-MS/MS | 5 | 10 | [33] | |||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | |||
| 4-FPP | Acid digestion at 45 °C, PLE, SPE/C18 cartridge | LC-HRMS | 7 | 30 | [23] | ||
| 4-MBu | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | ||
| 4-MEC | SPE/Bond Elute Certify I | LC-MS/MS | 0.4 | 1 | 2200.0/ 0−2.5 cm591.0/ 2.5 −5 cm |
[24] | |
| Acid/basic digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.5 | LLOQ: 1 | 30,000 | [36] | ||
| M3® reagent at 100 °C | LC-MS/MS | 2 | 20 | [29] | |||
| MeOH at 55 °C | LC-MS/MS | 3 | 6 | [20] | |||
| 0.1 M HCOOH at 45 °C | LC-MS/MS | 5 | 20 | < LOQ; 26 | [25] | ||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | |||
| MeOH/ACN/aq. HCOONH4 at 40 °C | LC-MS/MS | 11 | 33 | [26] | |||
| 4-MMC | MSPE: MeOH/ HCL 0.1 M at 60 °C, SPE/ C18 | LC-MS/MS | 1 | 2 | [33] | ||
| SPE/Bond Elute Certify I | LC-MS/MS | 1 | 5 | 6200.0/ 0−2.5 cm1500.0/ 2.5 −5 cm |
[24] | ||
| 0.1 M HCOOH at 45 °C | LC-MS/MS | 2 | 20 | [25] | |||
| M3® reagent at 100 °C | LC-MS/MS | 2 | 20 | [29] | |||
| MeOH at 55 °C | LC-MS/MS | 2.4 | 4.8 | 50−59 | [20] | ||
| Enzymatic digestion, LLE with chloroform/EtOH/DEE | LC-MS/MS | 2.5 | 5 | 21.11 | [39] | ||
| Acid digestion at 45 °C, PLE, SPE/C18 cartridge | LC-HRMS | 4 | 10 | [23] | |||
| Incubation at 95 °C, LLE with Hept/EA, DCM/Isopropanol | LC-HRMS- Orbitrap | 5 | 50/- | [30] | |||
| MeOH/ACN/aq. HCOONH4 at 40 °C | LC-MS/MS | 7 | 10 | 220−3.500 mephedrone < LOQ |
[26] | ||
| MeOH, MeOH/HCL 33 % | LC-MS/MS | 10 | [27] | ||||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | |||
| Neutral digestion at 40 °C, LLE with EA | GC-MS | 80 | 200 | 200−313,20 | [40] | ||
| 4-Methylnorephedrine | Enzymatic digestion, LLE with chloroform/EtOH/DEE | LC-MS/MS | 5 | 10 | [39] | ||
Table 3.
Selected parameters of hair analysis for Synthetic Cannabinoids.
| NPS | Extraction Method | Method of Analysis | LOD (pg/mg) | LOQ (pg/mg) | LOI/ LOR (pg/mg) | Concentrations- Clinical/forensic samples (pg/mg) | References |
|---|---|---|---|---|---|---|---|
| CANNABINOIDS: Benzoylindoles | |||||||
| AM-2233 | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| M3® reagent at 100 °C | LC-MS/MS | 5 | 25 | [29] | |||
| MeOH at 45 °C | LC-MS/MS | 10 | 20 | [25] | |||
| AM-694 | EtOH | LC-MS/MS | 0.5 | 0.5 | [41] | ||
| Basic digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.8 | 2.6 | [42] | |||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | |||
| M3® reagent at 100 °C | LC-MS/MS | 5 | 25 | [29] | |||
| MeOH at 45 °C | LC-MS/MS | 10 | 20 | 30 | [25] | ||
| Enzymatic Digestion, LLE neutral and basic with DEE/EA | LC-MS/MS | 20 | [22] | ||||
| RCS-4 | EtOH | LC-MS/MS | 0.5 | 0.5 | [41] | ||
| Basic digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.7 | 2.3 | [42] | |||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | |||
| M3® reagent at 100 °C | LC-MS/MS | 5 | 25 | [29] | |||
| MeOH at 45 °C | LC-MS/MS | 10 | 20 | [25] | |||
| Enzymatic Digestion, LLE neutral and basic with DEE/EA | LC-MS/MS | 100 | [22] | ||||
| RCS4−2-methoxy | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| RCS4-C-4 | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| RCS-4 ortho isomer | EtOH | LC-MS/MS | 0.5 | 0.5 | [41] | ||
| WIN 48.098 | EtOH | LC-MS/MS | 0.5 | 0.5 | [41] | ||
| Basic digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.7 | 2.3 | [42] | |||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | |||
| MeOH at 45 °C | LC-MS/MS | 5 | 20 | [25] | |||
| Naphthoylindoles | |||||||
| AKB-48 | MeOH at 38 °C | LC-MS/MS | Range 0.1 to 10 |
Range 0.1–20 |
[43] | ||
| AM-1220 azepane | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | ||
| AM-1220 | Basic digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.4 | 1.3 | 1,3 | [42] | |
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | |||
| AM-1241 | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | ||
| AM-1248 | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| AM-2201 | MeOH at 38 °C | LC-MS/MS | 0.05 | 0.1 | 1.7−739,0 | [44] | |
| Acid digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.35 | 1 | [45] | |||
| Basic digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.7 | 2.3 | [42] | |||
| EtOH | LC-MS/MS | 0.5 | 0.5 | [41] | |||
| M3® reagent at 100 °C | LC-MS/MS | 1 | 10 | [29] | |||
| MeOH at 45 °C | LC-MS/MS | 10 | 10 | [25] | |||
| Enzymatic Digestion, LLE neutral and basic with DEE/EA | LC-MS/MS | 20 | [22] | ||||
| Basic digestion at 90 °C, LLE with hexane/EA | GC/MS | 1000 | 1000 | 5516 | [46] | ||
| AM-2201 N-4-OH M | MeOH at 38 °C | LC-MS/MS | 0.05 | 0.1 | 0.4 | [44] | |
| AM-2232 | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | ||
| PX-1 (derivative of AM2201) | M3® reagent at 100 °C | LC-MS/MS | 5 | 25 | [29] | ||
| AM-2201 N-6-OHindole M | MeOH at 38 °C | LC-MS/MS | 0.05 | 0.1 | 0,2−3,1 | [44] | |
| BB-22 (analog of JWH 018) | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| Acid digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 3 | 10 | [45] | |||
| EAM-2201 | Acid digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.35 | 1 | [45] | ||
| JWH-007 | Basic digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.2 | 0.7 | [42] | ||
| EtOH | LC-MS/MS | 0.5 | 0.5 | [41] | |||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | |||
| M3® reagent at 100 °C | LC-MS/MS | 3 | 25 | [29] | |||
| MeOH at 45 °C | LC-MS/MS | 5 | 10 | [25] | |||
| JWH-015 | EtOH | LC-MS/MS | 0.5 | 0.5 | [41] | ||
| Basic digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.6 | 2 | [42] | |||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC–MS/MS | 1 | -/100 | [21] | |||
| MeOH at 45 °C | LC-MS/MS | 2 | 10 | [25] | |||
| Enzymatic Digestion, LLE neutral and basic with DEE/EA | LC-MS/MS | 500 | [22] | ||||
| JWH-018 | MeOH at 38 °C | LC-MS-MS | 0.05 | 0.1 | 10−1700 | [47] | |
| Basic digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.18 | 0.59 | 0,6−70,5 | [48] | ||
| Acid digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.35 | 1 | [45] | |||
| EtOH | LC-MS/MS | 0.5 | 0.5 | 5.1−5.7 | [41] | ||
| Basic digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.9 | 3 | 3.1−17.3 | [42] | ||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC–MS/MS | 1 | -/100 | [21] | |||
| M3® reagent at 100 °C | LC-MS/MS | 3 | 25 | [29] | |||
| Incubation at 95 °C, LLE with Hept/EA, DCM/Isopropanol | LC-HRMS- Orbitrap | 5 | 50/- | 0.8–70.5 | [30] | ||
| MeOH at 45 °C | LC-MS/MS | 5 | 10 | Case 1: 20, Case 2: 90 Case 3: traces below LOQ |
[25] | ||
| Enzymatic Digestion, LLE neutral and basic with DEE/EA | LC-MS/MS | 500 | [22] | ||||
| MeOH at 38 °C | LC-MS/MS | 0.4−59.2 | [44] | ||||
| JWH-018 N-COOH M | MeOH at 38 °C | LC-MS-MS | 0.05 | 0.1 | [47] | ||
| MeOH at 38 °C | LC-MS/MS | 0.2−1.1 | [44] | ||||
| JWH-018 N-4-OH M | MeOH at 38 °C | LC-MS-MS | 0.05 | 0.1 | [47] | ||
| JWH-018 N-(5-OH) M | MeOH at 38 °C | LC-MS-MS | 0.05 | 0.1 | 3−85 | [47] | |
| Acid digestion at 95 °C, LLE with hexane/EA | UPLC-MS/MS | 0.35 | 1 | [45] | |||
| Acid digestion at 45 °C, PLE, SPE/C18 cartridge | LC-HRMS | 7 | 30 | [23] | |||
| MeOH at 38 °C | LC-MS/MS | 0.3−37.2 | [44] | ||||
| JWH-018 adamantyl | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC–MS/MS | 1 | -/100 | [21] | ||
| THJ 018 (analog of JWH 018) | M3® reagent at 100 °C | LC-MS/MS | 3 | 25 | [29] | ||
| JWH-019 | Acid digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.35 | 1 | [45] | ||
| EtOH | LC-MS/MS | 0.5 | 0.5 | [41] | |||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | |||
| Basic digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 1 | 3.3 | 3.8−4.1 | [42] | ||
| M3® reagent at 100 °C | LC-MS/MS | 3 | 25 | [29] | |||
| MeOH at 45 °C | LC-MS/MS | 5 | 20 | [25] | |||
| Basic digestion at 90 °C, LLE with hexane/EA | GC/MS | 50 | 100 | 4996 | [46] | ||
| JWH-073 | MeOH at 38 °C | LC-MS-MS | 0.05 | 0.1 | 2−55 | [47] | |
| Basic digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.1 | 0.33 | 0.5−413.3 | [48] | ||
| Acid digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.35 | 1 | [45] | |||
| EtOH | LC-MS/MS | 0.5 | 0.5 | 0.7−3.2 | [41] | ||
| Basic digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.5 | 1.6 | 1.6−50.5 | [42] | ||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | |||
| MeOH at 45 °C | LC-MS/MS | 10 | 20 | Case 1: below LOQ, Case 2: 2.100 Case 3: traces below LOQ |
[25] | ||
| Enzymatic Digestion, LLE neutral and basic with DEE/EA | LC-MS/MS | 500 | [22] | ||||
| MeOH at 38 °C | LC-MS/MS | 0.1−0.8 | [44] | ||||
| JWH-073−4-N(OHbutyl) | Acid digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.35 | 1 | [45] | ||
| JWH-073 N-3-OH M | MeOH at 38 °C | LC-MS-MS | 0.05 | 0.1 | [47] | ||
| JWH-073 N-COOH M | MeOH at 38 °C | LC-MS-MS | 0.05 | 0.1 | [47] | ||
| MeOH at 38 °C | LC-MS/MS | 0.3 | [44] | ||||
| JWH-073 N-4-OH M | MeOH at 38 °C | LC-MS-MS | 0.1 | 0.1 | [47] | ||
| JWH073 4-methylnapthyl | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| JWH073 N-(3-methylbutyl) | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| JWH-081 | Incubation at 45 °C, SPE/MCX® Oasis cartridge | LC-MS/MS | 0.5 | 0.5 | 1st segment: 78 3rd segment: 1100 |
[31] | |
| Basic digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.6 | 2 | 8.0−194 | [42] | ||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | |||
| Acid digestion at 45 °C, PLE, SPE/C18 cartridge | LC-HRMS | 3 | 10 | [23] | |||
| M3® reagent at 100 °C | LC-MS/MS | 3 | 25 | [29] | |||
| MeOH at 45 °C | LC-MS/MS | 5 | 20 | Case 1: 470 Case 3: traces below LOQ |
[25] | ||
| Basic digestion at 90 °C, LLE with hexane/EA | GC/MS | 100 | 100 | 5.533 | [46] | ||
| JWH-098 | M3® reagent at 100 °C | LC-MS/MS | 3 | 25 | [29] | ||
| MeOH at 45 °C | LC-MS/MS | 5 | 20 | [25] | |||
| JWH-122 | MeOH at 38 °C | LC-MS/MS | 0.05 | 0.1 | 0.1- 402.0 | [44] | |
| Acid digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.35 | 1 | 200/ 0−2 cm450/ 2−4 cm430/4 −6 cm |
[45] | ||
| EtOH | LC-MS/MS | 0.5 | 0.5 | [41] | |||
| Basic digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.9 | 3 | 7,4−2,800 | [42] | ||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | |||
| M3® reagent at 100 °C | LC-MS/MS | 3 | 20 | [29] | |||
| MeOH at 45 °C | LC-MS/MS | 10 | 20 | [25] | |||
| Basic digestion at 90 °C, LLE with hexane/EA | GC/MS | 100 | 100 | 5366 | [46] | ||
| Enzymatic Digestion, LLE neutral and basic with DEE/EA | LC-MS/MS | 500 | [22] | ||||
| JWH-122 N-(4-pentenyl) | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| JWH-122 N-5-OH | MeOH at 38 °C | LC-MS/MS | 0.05 | 0.1 | 0.1- 3.5 | [44] | |
| JWH-200 | Basic digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.02 | 0.07 | [48] | ||
| Acid digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.35 | 1 | [45] | |||
| Basic digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.4 | 1.3 | [42] | |||
| EtOH | LC-MS/MS | 0.5 | [41] | ||||
| MeOH at 45 °C | LC-MS/MS | 5 | 10 | [25] | |||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | |||
| Acid digestion at 45 °C, PLE, SPE/C18 cartridge | LC-HRMS | 1 | 5 | [23] | |||
| Enzymatic Digestion, LLE neutral and basic with DEE/EA | LC-MS/MS | 20 | [22] | ||||
| JWH-210 | MeOH at 38 °C | LC-MS/MS | Range 0.1 to 10 |
Range 0.1–20 |
0.06−7.6 | [43] | |
| EtOH | LC-MS/MS | 0.5 | 0.5 | 0.5−5.2 | [41] | ||
| Basic digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.7 | 2.3 | 2.3−5.1 | [42] | ||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC–MS/MS | 1 | -/100 | [21] | |||
| M3® reagent at 100 °C | LC-MS/MS | 3 | 25 | [29] | |||
| Enzymatic Digestion, LLE neutral and basic with DEE/EA | LC-MS/MS | 10 | [22] | ||||
| JWH-398 | Basic digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.3 | 1 | [42] | ||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC–MS/MS | 1 | -/100 | [21] | |||
| M3® reagent at 100 °C | LC-MS/MS | 3 | 25 | [29] | |||
| EtOH | LC-MS/MS | 5 | 5 | [41] | |||
| MeOH at 45 °C | LC-MS/MS | 5 | 10 | [25] | |||
| MAM-2201 | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| MeOH at 38 °C | LC-MS/MS | 0.05 | 0.1 | 0.2−276.0 | [44] | ||
| MAM-2201 N-4-OH M | MeOH at 38 °C | LC-MS/MS | 0.05 | 0.1 | [44] | ||
| MAM 2201 N-(5-pentanoic acid) -potential phase 1 metabolite of JWH 122 | MeOH at 38 °C | LC-MS/MS | 0.05 | 0.1 | [44] | ||
| Acid digestion at 45 °C, PLE, SPE/C18 cartridge | LC-HRMS | 5 | 40 | [23] | |||
| WIN 55, 212−2 | EtOH | LC-MS/MS | 0.5 | 0.5 | [41] | ||
| Basic digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.8 | 2.6 | [42] | |||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC–MS/MS | 1 | -/100 | [21] | |||
| Acid digestion at 45 °C, PLE, SPE/C18 cartridge | LC-HRMS | 8 | 30 | [23] | |||
| 5 F-AKB48 | MeOH at 38 °C | LC-MS/MS | Range 0.1 to 10 |
Range 0.1–20 |
[43] | ||
| Acid digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 3 | 10 | [45] | |||
| M3® reagent at 100 °C | LC-MS/MS | 5 | 30 | [29] | |||
| 5 F NNEI-2 (analog of JWH 018) | M3® reagent at 100 °C | LC-MS/MS | 5 | 30 | [29] | ||
| Phenylacetylindoles | |||||||
| JWH-203 | EtOH | LC-MS/MS | 0.5 | 0.5 | [41] | ||
| Basic digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.7 | 2.3 | [42] | |||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC–MS/MS | 1 | -/100 | [21] | |||
| M3® reagent at 100 °C | LC-MS/MS | 3 | 25 | [29] | |||
| Enzymatic Digestion, LLE neutral and basic with DEE/EA | LC-MS/MS | 50 | [22] | ||||
| JWH-250 | Basic digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.04 | 0.13 | 1.5−729.4 | [48] | |
| Acid digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.35 | 1 | [45] | |||
| Basic digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.5 | 1.6 | 4.8−83.4 | [42] | ||
| EtOH | LC-MS/MS | 0.5 | 0.5 | 0.5−24 | [41] | ||
| Acid digestion at 45 °C, PLE, SPE/C18 cartridge | LC-HRMS | 1 | 9 | [23] | |||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC–MS/MS | 1 | -/100 | [21] | |||
| Enzymatic Digestion, LLE neutral and basic with DEE/EA | LC-MS/MS | 10 | [22] | ||||
| MeOH at 45 °C | LC-MS/MS | 10 | 10 | [25] | |||
| Basic digestion at 90 °C, LLE with hexane/EA | GC/MS | 50 | 100 | 5.320 | [46] | ||
| JWH-251 | MeOH at 45 °C | LC-MS/MS | 10 | 20 | [25] | ||
| EtOH | LC-MS/MS | 0.5 | 0.5 | [41] | |||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | |||
| Basic digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.3 | 1 | [42] | |||
| M3® reagent at 100 °C | LC-MS/MS | 3 | 25 | [29] | |||
| JWH-302 | M3® reagent at 100 °C | LC-MS/MS | 2 | 25 | [29] | ||
| RCS-8 | EtOH | LC-MS/MS | 0.5 | 0.5 | [41] | ||
| Basic digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.9 | 3 | [42] | |||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | |||
| M3® reagent at 100 °C | LC-MS/MS | 5 | 30 | [29] | |||
| MeOH at 45 °C | LC-MS/MS | 5 | 10 | [25] | |||
| Naphthoylpyrroles | |||||||
| JWH-030 | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| MeOH at 45 °C | LC-MS/MS | 10 | 10 | [25] | |||
| JWH-147 | M3® reagent at 100 °C | LC-MS/MS | 3 | 25 | [29] | ||
| MeOH at 45 °C | LC-MS/MS | 10 | 20 | [25] | |||
| JWH-307 | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| Basic digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 1.3 | 4.3 | [42] | |||
| MeOH at 45 °C | LC-MS/MS | 10 | 20 | [25] | |||
| OTHER CANNABINOIDS | |||||||
| AB-CHMINACA | MeOH | LC-MS/MS | 0.1 | LLOQ: 2.5 | ∼40−1850 | [49] | |
| MeOH at 38 °C | LC-MS/MS | 0.5 | 2 | 2.2−1512.0 | [50] | ||
| MeOH at 38 °C | LC-MS/MS | Range 0.1 to 10 |
Range 0.1–20 |
2.5−15300.0 | [43] | ||
| M3® reagent at 100 °C | LC-MS/MS | 5 | 25 | [29] | |||
| AB-CHMINACA M1A | MeOH at 38 °C | LC-MS/MS | Range 0.1 to 10 |
Range 0.1–20 |
18.3 (1 case) | [43] | |
| AB-CHMINACA M2 | MeOH at 38 °C | LC-MS/MS | 1 | 5 | [50] | ||
| MeOH at 38 °C | LC-MS/MS | Range 0.1 to 10 |
Range 0.1 to 20 |
0.5−35.1 | [43] | ||
| AB-CHMINACA M3A | MeOH at 38 °C | LC-MS/MS | 2.5 | 5 | [50] | ||
| AB-CHMINACA M4 | MeOH at 38 °C | LC-MS/MS | Range 0.1 to 10 |
Range 0.1–20 |
59.8 | [43] | |
| MeOH at 38 °C | LC-MS/MS | 2.5 | 5 | [50] | |||
| AB-CHMINACA M5A | MeOH at 38 °C | LC-MS/MS | 10 | 50 | [50] | ||
| AB-CHMINACA M6 | MeOH at 38 °C | LC-MS/MS | 2.5 | 5 | [50] | ||
| AB-CHMINACA M7 | MeOH at 38 °C | LC-MS/MS | 2.5 | 10 | [50] | ||
| AB-CHMINACA Valine (METABOLITE) |
MeOH | LC-MS/MS | 0.1 | ∼100−450 | [49] | ||
| AB-FUBINACA | MeOH at 38 °C | LC-MS/MS | Range 0.1 to 10 |
Range 0.1–20 |
[43] | ||
| Acid digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 3 | 10 | [45] | |||
| M3® reagent at 100 °C | LC-MS/MS | 5 | 25 | [29] | |||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | |||
| AB PINACA | MeOH at 38 °C | LC-MS/MS | Range 0.1 to 10 |
Range 0.1–20 |
[43] | ||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | |||
| ADB FUBINACA | M3® reagent at 100 °C | LC-MS/MS | 5 | 25 | [29] | ||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | |||
| ADB-PINACA | Acid digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.35 | 1 | [45] | ||
| APICA | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| APINACA | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| APP FUBINACA (analog of AB-FUBINACA) | M3® reagent at 100 °C | LC-MS/MS | 5 | 25 | 50 | [29] | |
| A-834,735 | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| CB-13 | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| M3® reagent at 100 °C | LC-MS/MS | 5 | 30 | [29] | |||
| MeOH at 45 °C | LC-MS/MS | 10 | 20 | [25] | |||
| CP47, 497-C8 | M3® reagent at 100 °C | LC-MS/MS | 5 | 30 | [29] | ||
| Basic digestion at 90 °C, LLE with hexane/EA | GC/MS | 50 | 500 | 5.300 | [46] | ||
| CUMYL 5 F PINACA | M3® reagent at 100 °C | LC-MS/MS | 5 | 25 | [29] | ||
| CUMYL-PEGACLONE | M3® reagent at 100 °C | LC-MS/MS | 5 | 25 | [29] | ||
| HU-210 | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| Basic digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 3 | 9.9 | [48] | |||
| Basic digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 24 | 80 | [42] | |||
| STS-135 | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| JWH-016 | M3® reagent at 100 °C | LC-MS/MS | 3 | 25 | [29] | ||
| MeOH at 45 °C | LC-MS/MS | 5 | 10 | [25] | |||
| JWH-020 | Basic digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.2 | 0.7 | [42] | ||
| EtOH | LC-MS/MS | 0.5 | 0.5 | [41] | |||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | |||
| JWH-022 | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| JWH-072 | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| JWH-175 | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| JWH-176 | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | ||
| JWH-182 | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| JWH-201 | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| MeOH at 45 °C | LC-MS/MS | 2 | 10 | [25] | |||
| JWH-213 | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| JWH-412 | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| MMB 2201 | M3® reagent at 100 °C | LC-MS/MS | 5 | 25 | [29] | ||
| PB-22 | MeOH at 38 °C | LC-MS/MS | Range 0.1 to 10 |
Range 0.1–20 |
[43] | ||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | |||
| PB-22 5-OH-pentyl | MeOH | LC-MS/MS | 0.5 | ∼0−450 | [49] | ||
| Pravadoline | M3® reagent at 100 °C | LC-MS/MS | 5 | 25 | [29] | ||
| P X 2 (analog of 5-fluoro AB-PINACA) | M3® reagent at 100 °C | LC-MS/MS | 5 | 25 | [29] | ||
| UR-144 | MeOH at 38 °C | LC-MS/MS | 0.01 | 0.2 | 0.4−1.6 | [51] | |
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | |||
| M3® reagent at 100 °C | LC-MS/MS | 1 | 10 | 100 | [29] | ||
| Acid digestion at 45 °C, PLE, SPE/C18 cartridge | LC-HRMS | 6 | 20 | [23] | |||
| Basic digestion at 90 °C, LLE with hexane/EA | GC/MS | 50 | 500 | [46] | |||
| UR-144 N-4-OH M | MeOH at 38 °C | LC-MS/MS | 0.01 | 0.2 | 1−25.3 | [51] | |
| UR-144 N (5 Cl-pentyl) | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| UR-144 N-COOH M | MeOH at 38 °C | LC-MS/MS | 0.01 | 0.2 | 0.2−7.9 | [51] | |
| UR-144 N-5-OH M | MeOH at 38 °C | LC-MS/MS | 0.01 | 0.2 | 0.2−39.7 | [51] | |
| URB-754 | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | ||
| XLR-11 N-4-OH M | MeOH at 38 °C | LC-MS/MS | 0.2 | 0.2 | [51] | ||
| XLR-11 | MeOH at 38 °C | LC-MS/MS | 0.01 | 0.2 | 0.8−5350 | [51] | |
| Acid digestion at 45 °C, PLE, SPE/C18 cartridge | LC-HRMS | Range 0.1 to 10 |
Range 0.1–20 |
[23] | |||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | |||
| 5 CL AB PINACA | M3® reagent at 100 °C | LC-MS/MS | 5 | 25 | [29] | ||
| 5 F-AB-PINACA | Acid digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 8 | 25 | [45] | ||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | |||
| 5-F ADB | M3® reagent at 100 °C | LC-MS/MS | 5 | 25 | [29] | ||
| 5 F-APINACA | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | ||
| 5-fluoro PB-22 | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| Acid digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 3 | 10 | [45] | |||
| MeOH | LC-MS/MS | 10 | LLOQ: 1 | ∼200−1900 | [49] | ||
| 5 F-PB-22 3-carboxyindole | MeOH | LC-MS/MS | 10 | ∼200−800 | [49] | ||
Table 4.
Selected parameters of hair analysis for Synthetic Opioids.
| NPSs | Extraction Method | Method of Analysis | LOD (pg/mg) | LOQ (pg/mg) |
LOI/ LOR (pg/mg) |
Concentrations- Clinical/forensic samples (pg/mg) | References |
|---|---|---|---|---|---|---|---|
| SYNTHETIC OPIOIDS | |||||||
| AH-7921 | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| Acetyl fentanyl | M3® at 100 °C, SPE/Prime HLB cartridges | LC-MS/MS | 0.004 | 0.012 | [52] | ||
| MeOH at 55oC | LC-MS-MS | 0.2 | 0.6 | [53] | |||
| Acid digestion at 45oC, SPE/BondElute CertifyI | LC-MS/MS | 0.2 | 0.5 | 1.0−1.4 (post-mortem cases) | [54] | ||
| Acid digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.3 | 1 | 1.0/0−2 cm | [55] | ||
| MeOH at 55 °C | LC-MS/MS | Range 0.1−0.3 | LOQ-3200 | [56] | |||
| MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 0.5 | 2 | [57] | |||
| MeOH at 55 °C | UHPLC-QTOF-HRMS | 0.6 | 1.2 | LOQ-230 | [58] | ||
| M3® reagent at 100 °C | LC-MS/MS | 1 | 2 | [29] | |||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | |||
| Acetyl norfentanyl | M3® at 100 °C, SPE/Prime HLB cartridges | LC-MS/MS | 0.003 | 0.011 | [52] | ||
| Acid digestion at 45oC, SPE/BondElute CertifyI | LC-MS/MS | 0.2 | 0.5 | [54] | |||
| M3® reagent at 100 °C | LC-MS/MS | 1 | 2 | [29] | |||
| MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 2.5 | 2 | [57] | |||
| Acrylfentanyl | Acid digestion at 45oC, SPE/BondElute CertifyI | LC-MS/MS | 0.2 | 0.5 | [54] | ||
| MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 0.5 | 2 | [57] | |||
| MeOH at 55 °C | UHPLC-QTOF-HRMS | 0.6 | 1.2 | LOQ | [58] | ||
| Alfentanil | M3® at 100 °C, SPE/Prime HLB cartridges | LC-MS/MS | 0.005 | 0.017 | [52] | ||
| MeOH at 55oC | LC-MS-MS | 0.1 | 0.3 | [53] | |||
| Acid digestion at 45oC, SPE/BondElute CertifyI | LC-MS/MS | 0.2 | 0.5 | [54] | |||
| MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 0.5 | 2 | [57] | |||
| M3® reagent at 100 °C | LC-MS/MS | 1 | 2 | [29] | |||
| α-Methylfentanyl | MeOH at 55 °C | UHPLC-QTOF-HRMS | 0.5 | 1.0 | [58] | ||
| MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 2.5 | 5 | [57] | |||
| Benzoylfentanyl | MeOH at 55 °C | LC-MS/MS | 621/4460/5870: proximal to distal hair sections (3 cm length each) |
[59] | |||
| Butyryl fentanyl | M3® at 100 °C, SPE/Prime HLB cartridges | LC-MS/MS | 0.005 | 0.015 | [52] | ||
| Acid digestion at 45oC, SPE/BondElute CertifyI | LC-MS/MS | 0.2 | 0.5 | 2.0 (drug users hair samples) | [54] | ||
| MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 0.5 | 2 | [57] | |||
| MeOH at 55 °C | UHPLC-QTOF-HRMS | 0.6 | 1.2 | 54 | [58] | ||
| M3® reagent at 100 °C | LC-MS/MS | 1 | 2 | 380 | [29] | ||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | |||
| Butyrylfentanyl Carboxy Metabolite |
M3® at 100 °C, SPE/Prime HLB cartridges | LC-MS/MS | 0.006 | 0.02 | [52] | ||
| Acid digestion at 45oC, SPE/BondElute CertifyI | LC-MS/MS | 0.2 | 0.5 | [54] | |||
| M3® reagent at 100 °C | LC-MS/MS | 1 | 2 | [29] | |||
| Butyryl Norfentanyl | M3® at 100 °C, SPE/Prime HLB cartridges | LC-MS/MS | 0.006 | 0.018 | [52] | ||
| Acid digestion at 45oC, SPE/BondElute CertifyI | LC-MS/MS | 0.2 | 0.5 | [54] | |||
| M3® reagent at 100 °C | LC-MS/MS | 1 | 2 | 160 | [29] | ||
| b-Hydroxy-3-Methylfentanyl | MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 1 | 5 | [57] | ||
| b-Hydroxyfentanyl | M3® at 100 °C, SPE/Prime HLB cartridges | LC-MS/MS | 0.005 | 0.014 | [52] | ||
| Acid digestion at 45oC, SPE/BondElute CertifyI | LC-MS/MS | 0.2 | 0.5 | [54] | |||
| M3® reagent at 100 °C | LC-MS/MS | 1 | 2 | [29] | |||
| MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 1 | 5 | [57] | |||
| b-Hydroxythiofentanyl | M3® at 100 °C, SPE/Prime HLB cartridges | LC-MS/MS | 0.005 | 0.014 | [52] | ||
| Acid digestion at 45oC, SPE/BondElute CertifyI | LC-MS/MS | 0.2 | 0.5 | [54] | |||
| MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 1 | 5 | [57] | |||
| Carfentanil | MeOH at 55oC | LC-MS-MS | 0.2 | 0.6 | [53] | ||
| M3® at 100 °C, SPE/Prime HLB cartridges | LC-MS/MS | 0.006 | 0.019 | [52] | |||
| M3® reagent at 100 °C | LC-MS/MS | 1 | 2 | [29] | |||
| MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 0.5 | 2 | [57] | |||
| MeOH at 55 °C | LC-MS/MS | Range 0.1−0.3 | LOQ-1.5 | [56] | |||
| Acid digestion at 45oC, SPE/BondElute CertifyI | LC-MS/MS | 0.2 | 0.5 | 1.2 (drug users hair samples) | [54] | ||
| Acid digestion at 95 °C, LLE with hexane/EA | LC–MS/MS | 0.8 | 2.5 | 9−12 Months after the Overdose/ S. B: 2−4 cm = 3.0 and S. A: 0−2 cm = 2.5 |
[55] | ||
| MeOH at 55 °C | UHPLC-QTOF-HRMS | 0.8 | 1.6 | [58] | |||
| MeOH at 55 °C | LC-MS/MS | 54/114/166: (from proximal to distal hair section - 3 cm length each) | [59] | ||||
| Cis-3-Methylfentanyl | MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 1 | 5 | [57] | ||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | |||
| Cis-3-Methyl Norfentanyl | M3® reagent at 100 °C | LC-MS/MS | 1 | 2 | [29] | ||
| Cyclopropyl Fentanyl | M3® at 100 °C, SPE/Prime HLB cartridges | LC-MS/MS | 0.006 | 0.019 | [52] | ||
| MeOH at 55 °C | UHPLC-QTOF-HRMS | 0.7 | 1.4 | 4.7 | [58] | ||
| M3® reagent at 100 °C | LC-MS/MS | 1 | 2 | [29] | |||
| MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 1 | 5 | [57] | |||
| Cyclopropyl Norfentanyl | M3® at 100 °C, SPE/Prime HLB cartridges | LC-MS/MS | 0.006 | 0.018 | [52] | ||
| M3® reagent at 100 °C | LC-MS/MS | 1 | 2 | [29] | |||
| Despropionyl para-fluorofentanyl |
M3® at 100 °C, SPE/Prime HLB cartridges | LC-MS/MS | 0.005 | 0.015 | [52] | ||
| Acid digestion at 45oC, SPE/BondElute CertifyI | LC-MS/MS | 0.2 | 0.5 | [54] | |||
| M3® reagent at 100 °C | LC-MS/MS | 1 | 2 | [29] | |||
| Fentanyl | M3® at 100 °C, SPE/Prime HLB cartridges | LC-MS/MS | 0.003 | 0.013 | 2540−2,800 | [52] | |
| MeOH at 55oC | LC-MS-MS | 0.1 | 0.3 | 3−6 | [53] | ||
| MeOH at 55 °C | LC-MS/MS | Range 0.1−0.3 | LOQ-8600 | [56] | |||
| Acid digestion at 45oC, SPE/BondElute CertifyI | LC-MS/MS | 0.2 | 0.5 | 8.3−12.8 (post-mortem cases) | [54] | ||
| Acid digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.3 | 1 | 9−12 Months after the Overdose/ S. B:2−4 cm = 760 and S. A: 0−2 cm = 620 |
[55] | ||
| MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 0.5 | 2 | 8.02 | [57] | ||
| MeOH at 55 °C | UHPLC-QTOF-HRMS | 0.6 | 1.2 | LOQ–1400 | [58] | ||
| M3® reagent at 100 °C | LC-MS/MS | 1 | 2 | 2800−3200 | [29] | ||
| Fentanyl-D5 | Acid digestion at 45oC, SPE/BondElute CertifyI | LC-MS/MS | 0.2 | 0.5 | [54] | ||
| Furanyl Fentanyl | M3® at 100 °C, SPE/Prime HLB cartridges | LC-MS/MS | 0.005 | 0.016 | [52] | ||
| MeOH at 55oC | LC-MS-MS | 0.1 | 0.3 | 44 | [53] | ||
| MeOH at 55 °C | LC-MS/MS | Range 0.1−0.3 | LOQ-590 | [56] | |||
| Acid digestion at 45oC, SPE/BondElute CertifyI | LC-MS/MS | 0.2 | 0.5 | 136.7−195.8 (post-mortem cases) | [54] | ||
| MeOH at 55 °C | UHPLC-QTOF-HRMS | 0.6 | 1.2 | LOQ-6300 | [58] | ||
| Acid digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.8 | 2.5 | 9−12 Months after the Overdose/ S. B: 2−4 cm = 500 and S. A: 0−2 cm = 310 |
[55] | ||
| M3® reagent at 100 °C | LC-MS/MS | 1 | 2 | [29] | |||
| MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 1 | 5 | [57] | |||
| Furanylethyl Fentanyl | M3® at 100 °C, SPE/Prime HLB cartridges | LC-MS/MS | 0.003 | 0.014 | [52] | ||
| M3® reagent at 100 °C | LC-MS/MS | 1 | 2 | [29] | |||
| Furanyl Norfentanyl | Basic digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.003 | 0.012 | [42] | ||
| Acid digestion at 45oC, SPE/BondElute CertifyI | LC-MS/MS | 0.2 | 0.5 | [54] | |||
| M3® reagent at 100 °C | LC-MS/MS | 1 | 2 | [29] | |||
| Hydrocodone | MeOH at 55oC | LC-MS-MS | 0.1 | 0.3 | 13−71 | [53] | |
| MeOH at 55 °C | LC-MS/MS | Range 0.1−0.3 | LOQ-12,600 | [56] | |||
| Isobutyryl Fentanyl | MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 2.5 | 5 | [57] | ||
| Methoxyacetyl fentanyl | M3® at 100 °C, SPE/Prime HLB cartridges | LC-MS/MS | 0.005 | 0.016 | [52] | ||
| M3® reagent at 100 °C | LC-MS/MS | 1 | 2 | [29] | |||
| MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 0.5 | 2 | [57] | |||
| Acid digestion at 45oC, SPE/BondElute CertifyI | LC-MS/MS | 0.5 | 1 | 259.9−479.6 (post-mortem cases) | [54] | ||
| Acid digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.3 | 1 | 9−12 Months after the Overdose/ S. B: 2−4 cm = 600 and S. A: 0–2 cm = 500 |
[55] | ||
| Methoxyacetyl Norfentanyl |
Acid digestion at 45oC, SPE/BondElute CertifyI | LC-MS/MS | 0.5 | 1 | 17.1- 32.7 (post-mortem cases) | [54] | |
| MeOH at 55oC | LC-MS/MS | 0.005 | 0.016 | [53] | |||
| M3® reagent at 100 °C | LC-MS/MS | 1 | 2 | [29] | |||
| N-Desmethyl U-47,700 | MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 0.5 | 2 | [57] | ||
| Norcarfentanil | MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 0.5 | 2 | [57] | ||
| Norfentanyl | MeOH at 55oC | LC-MS-MS | 0.1 | 0.3 | [53] | ||
| M3® at 100 °C, SPE/Prime HLB cartridges | LC-MS/MS | 0.005 | 0.015 | 15.1−149 | [52] | ||
| MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 1 | 5 | [57] | |||
| MeOH at 55 °C | LC-MS/MS | Range 0.1−0.3 | LOQ-320 | [56] | |||
| Acid digestion at 45oC, SPE/BondElute CertifyI | LC-MS/MS | 0.2 | 0.5 | [54] | |||
| MeOH at 55 °C | UHPLC-QTOF-HRMS | 1.2 | 2.4 | 3.5−600 | [58] | ||
| Ocfentanil | Acid digestion at 45oC, SPE/BondElute CertifyI | LC-MS/MS | 0.2 | 0.5 | 0.9 (drug users hair samples) 4.1−11.1 (post-mortem cases) |
[54] | |
| MeOH at 55 °C | UHPLC-QTOF-HRMS | 0.4 | 0.8 | [58] | |||
| MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 0.5 | 2 | [57] | |||
| Oxycodone | MeOH at 55oC | LC-MS-MS | 1.5 | 4.5 | 13−780 | [53] | |
| MeOH at 55 °C | LC-MS/MS | 1.5 | LOQ-25,700 | [56] | |||
| para/ortho-Fluorofentanyl | MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 2.5 | 5 | [57] | ||
| PFBF | Acid digestion at 45oC, SPE/BondElute CertifyI | LC-MS/MS | 0.2 | 0.5 | [54] | ||
| MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 2.5 | 5 | [57] | |||
| Phenylacetyl fentanyl | M3® at 100 °C, SPE/Prime HLB cartridges | LC-MS/MS | 0.005 | 0.015 | [52] | ||
| Acid digestion at 45oC, SPE/BondElute CertifyI | LC-MS/MS | 0.5 | 1 | [54] | |||
| M3® reagent at 100 °C | LC-MS/MS | 1 | 2 | [29] | |||
| Remifentanil acid | MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 2.5 | 5 | [57] | ||
| Remifentanil | MeOH at 55oC | LC-MS-MS | 0.3 | 0.9 | [53] | ||
| Acid digestion at 45oC, SPE/BondElute CertifyI | LC-MS/MS | 0.2 | 0.5 | [54] | |||
| MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 0.5 | 2 | [57] | |||
| Sufentanil | M3® at 100 °C, SPE/Prime HLB cartridges | LC-MS/MS | 0.006 | 0.019 | [52] | ||
| MeOH at 55oC | LC-MS-MS | 0.3 | 0.9 | [53] | |||
| MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 0.5 | 2 | S1: 0−3 cm: 183.91, S2: 3−6 cm: 131.68,S3: 6 −9 cm: 31.48 |
[57] | ||
| Acid digestion at 45oC, SPE/BondElute CertifyI | LC-MS/MS | 0.5 | 1 | [54] | |||
| THFF | Acid digestion at 45oC, SPE/BondElute CertifyI | LC-MS/MS | 0.2 | 0.5 | 1.3 (drug users hair samples) | [54] | |
| MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 0.5 | 2 | [57] | |||
| Thiofentanyl | MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 2.5 | 5 | [57] | ||
| Tramadol | MeOH at 55oC | LC-MS-MS | 0.1 | 0.3 | 2.0−3,700 | [53] | |
| MeOH at 55 °C | LC-MS/MS | Range 0.1−0.3 | LOQ-34,700 | [56] | |||
| M3® reagent at 100 °C | LC-MS/MS | 5 | 10 | 12,300−15,000 | [29] | ||
| Trans-3-Methylfentanyl | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 2.5 | 5 | [57] | |||
| Trans-3-Methylnorfentanyl | Acid digestion at 45oC, SPE/BondElute CertifyI | LC-MS/MS | 0.2 | 0.5 | 35.9 (In subject S29 the metabolite of 3-methyl fentanyl was identified. Unfortunately, due to the lack of reference standard, the presence of parent drug was not confirmed/ Positive results of hair samples collected from drug users hair samples)) | [54] | |
| M3® reagent at 100 °C | LC-MS/MS | 1 | 2 | [29] | |||
| U-47,700 | MeOH at 55oC | LC-MS-MS | 0.1 | 0.3 | [53] | ||
| MeOH at 55 °C | LC-MS/MS | Range 0.1−0.3 | LOQ-420 | [56] | |||
| MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 0.5 | 2 | [57] | |||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | |||
| MeOH at 55 °C pulverization | LC-MS/MS | 5700 | [60] | ||||
| U-48,800 | MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 0.5 | 2 | [57] | ||
| U-51,754 | MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 0.5 | 2 | [57] | ||
| U-50,488 | MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 1 | 5 | [57] | ||
| Valeryl fentanyl | MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 2.5 | 5 | [57] | ||
| Valeryl fentanyl carboxy metabolite |
M3® at 100 °C, SPE/Prime HLB cartridges | LC-MS/MS | 0.007 | 0.021 | [52] | ||
| Acid digestion at 45oC, SPE/BondElute CertifyI | LC-MS/MS | 0.5 | 1 | [54] | |||
| M3® reagent at 100 °C | LC-MS/MS | 1 | 2 | [29] | |||
| W-18 | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 2.5 | 5 | [57] | |||
| 3- fluorofentanyl | Incubation at 95 °C, LLE with Hept/EA, DCM/Isopropanol | LC-HRMS- Orbitrap | 1 | [30] | |||
| 3(meta)-fluorofentanyl | Acid digestion at 95 °C, LLE with hexane/EA | LC-MS/MS | 0.8 | 2.5 | 9–12 Months after the Overdose/ Segment B: 2−4 cm = 80 and Segment A: 0–2 cm = 25 |
[55] | |
| 3-Methylthiofentanyl | MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 1 | 5 | [57] | ||
| 4-ANPP | M3® at 100 °C, SPE/Prime HLB cartridges | LC-MS/MS | 0.006 | 0.018 | 10.4−11.2 | [52] | |
| MeOH at 55oC | LC-MS-MS | 0.1 | 0.3 | 1−2 | [53] | ||
| MeOH at 55 °C | LC-MS/MS | Range 0.1−0.3 | LOQ-1400 | [56] | |||
| Acid digestion at 45oC, SPE/BondElute CertifyI | LC-MS/MS | 0.2 | 0.5 | [54] | |||
| MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 0.5 | 2 | [57] | |||
| MeOH at 55 °C | UHPLC-QTOF-HRMS | 0.7 | 1.4 | 1.4−230 | [58] | ||
| M3® reagent at 100 °C | LC-MS/MS | 1 | 2 | 7 | [29] | ||
| 4-Fluorobutyrfentanyl | MeOH at 55 °C | UHPLC-QTOF-HRMS | 0.2 | 0.4 | 5.2−180 | [58] | |
| MeOH at 55 °C | LC-MS/MS | 4/152/719: proximal to distal hair sections (3 cm length each) |
[59] | ||||
| 4-Fluoroisobutyryl fentanyl | MeOH/ ACN/ Acetate NH4 pulverization | LC-MS/MS | 2.5 | 5 | [57] | ||
Table 5.
Selected parameters of hair analysis for Synthetic Tryptamines and other NPS.
| NPSs | Extraction Method | Method of Analysis | LOD (pg/mg) | LOQ (pg/mg) | LOI/LOR (pg/mg) | Concentrations- Clinical/forensic samples (pg/mg) | References |
|---|---|---|---|---|---|---|---|
| TRYPTAMINES | |||||||
| AcO DMT | M3® reagent at 100 °C | LC-MS/MS | 2 | 6 | [29] | ||
| DMT | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC–MS/MS | 10 | -/100 | [21] | ||
| 4-AcO-DIPT | M3® reagent at 100 °C | LC-MS/MS | 2 | 6 | [29] | ||
| 4-OH DET | M3® reagent at 100 °C | LC-MS/MS | 2 | 6 | [29] | ||
| 5-MeO-AMT | M3® reagent at 100 °C | LC-MS/MS | 2 | 6 | 70 | [29] | |
| 5-MeO-DALT | Incubation at 95 °C, LLE with Hept/EA, DCM/Isopropanol | LC-HRMS- Orbitrap | 1 | 50/- | [30] | ||
| M3® reagent at 100 °C | LC-MS/MS | 2 | 6 | [29] | |||
| 5-MeO-DiPT | aq.HCOOH pulverization at 4 °C | LC-MS/MS | 0.05 | LLOQ: 0.1 | 0.2−7532.5 | [61] | |
| 5-MeO-DPT | M3® reagent at 100 °C | LC-MS/MS | 2 | 6 | [29] | ||
| 5-MeO-DMT | MeOH at 60 °C | LC-MS/MS, LC-HRMS | 25 | LLOQ: 100 | 1990−3390 | [62] | |
| 5-MeO-MIPT | M3® reagent at 100 °C | LC-MS/MS | 2 | 6 | [29] | ||
| Other NPS | |||||||
| Benzoylecgonine | PLE, SPE/C18 cartridge | LC-MS-MS | 1 | 3.5 | [28] | ||
| Incubation at 95 °C, LLE with Hept/EA, DCM/Isopropanol | LC-HRMS- Orbitrap | 5 | 50/- | [30] | |||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 50 | -/100 | [21] | |||
| Deschloroketamine | Aq.HCOOH 0.1 M at 40 °C | LC-MS/MS | 10 | 50 | [63] | ||
| Aq.HCOOH 0.1 M at 40 °C | LC-HRMS | 50 | [63] | ||||
| Diphenidine | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC–MS/MS | 1 | -/100 | [21] | ||
| MeOH at 55 °C | LC-MS/MS | 3.4 | [20] | ||||
| EPH | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | ||
| Ketamine | PLE, SPE/C18 cartridge | LC-MS-MS | 2.5 | 8 | [28] | ||
| Incubation at 95 °C, LLE with Hept/EA, DCM/Isopropanol | LC-HRMS- Orbitrap | 5 | 50/- | [30] | |||
| M3® reagent at 100 °C | LC-MS/MS | 5 | 12 | 80- 27,300 | [29] | ||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | |||
| MeOH/ HCL 1% | GC/MS | 500 | 500 | [32] | |||
| Mescaline | PLE, SPE/C18 cartridge | LC-MS-MS | 3.7 | 13 | [28] | ||
| MeOH/ HCL 0.25 M at 50 °C | GC/MS | 9 | 40 | [19] | |||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 50 | -/100 | [21] | |||
| Methylphenidate | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | ||
| Methoxpropamine | Aq.HCOOH 0.1 M at 40 °C | LC-MS/MS | 10 | 50 | [63] | ||
| Aq.HCOOH 0.1 M at 40 °C | LC-HRMS | 50 | [63] | ||||
| MPA | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | ||
| Incubation at 95 °C, LLE with Hept/EA, DCM/Isopropanol | LC-HRMS- Orbitrap | 50 | 50/- | [30] | |||
| Norketamine | M3® reagent at 100oC | LC-MS/MS | 5 | 12 | 40−8400 | [29] | |
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 10 | -/100 | [21] | |||
| MeOH/ HCL 0.25 M at 50 °C | GC/MS | 21 | 80 | 0.34 | [19] | ||
| MeOH/ HCL 1% | GC/MS | 250 | 500 | [32] | |||
| PCP | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 1 | -/100 | [21] | ||
| PLE, SPE/C18 cartridge | LC-MS-MS | 1.5 | 2.4 | [28] | |||
| 2-fluoro-deschlotoketamine | Aq.HCOOH 0.1 M at 40oC | LC-MS/MS | 10 | 50 | [63] | ||
| Aq.HCOOH 0.1 M at 40 °C | LC-HRMS | 50 | [63] | ||||
| MeOH at 60 °C | LC-MS/MS, LC-HRMS | 25 | LLOQ: 100 | [62] | |||
| 3-MeO-PCP | MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 50 | -/100 | [21] | ||
| 3-methoxyeticyclidine (3-MeO-PCE) | MeOH at 60 °C | LC-MS/MS, LC-HRMS | 25 | LLOQ: 100 | 1610- 3610 | [62] | |
| 4-MeO-PCP | MeOH at 55 °C | LCMS/MS | 9 | 1.8 | [20] | ||
| MeOH/ HCL 0.1 M at 40 °C, pulverization | LC-MS/MS | 50 | -/100 | [21] | |||
Generally, the analytical methodology consisted of the following steps: hair decontamination from external contaminants, hair digestion or pulverization and analytes extraction from a hair amount ranging between 10−100 mg. The hair decontamination procedures used were washing with: (i) organic solvents, such as methanol [20,21,23,24,32,34,43,44,47,50,51,53,54,56,58,60], ethanol [35], acetone [19,22,[25], [26], [27],37,38,41,46,49,57,61,63], hexane [27], petroleum ether [41,49], dichloromethane [20,21,23,24,[28], [29], [30], [31],33,34,36,39,40,42,45,48,[52], [53], [54], [55], [56],[58], [59], [60],62], isopropanol [23,28], isooctane [38,52]; (ii) sodium dodecyl sulfate solution [35,46]; (iii) non-ionic surfactant and emulsifier (TWEEN 80) [25,37,63]; and (iv) water of variable analytical grade: distilled [19,25,32,35,37,43,44,46,47,50,51,63], deionized [31,34,41,49], ultra-pure [36,45,55,57] and not specified type of water [22,23,26,26,27,30,59,62].
Analytes’ extraction from hair, which followed hair digestion or pulverization, was achieved by either single step methanol extraction [20,25,27,43,44,47,[49], [50], [51],53,56,[58], [59], [60],62], or acidified methanol extraction [19,21,32,49], or ethanol extraction [41], or liquid-liquid extraction (LLE) with various mixtures of organic solvents [22,25,30,36,39,40,42,45,46,48,55,61,63], or extraction with aqueous buffers of organic solvents (methanol/ acetonitrile/ ammonium formate) [26], or (methanol/ acetonitrile/ trifluoroacetic acid (TFA)) [37] or (methanol/ TFA) [38] or (methanol/ acetonitrile/ ammonium acetate) [57], or solid phase extraction (SPE) on various cartridges [24,29,31,52,54] or mixed-mode solid phase extraction (MSPE) [33,34] or pressurized liquid extraction (PLE) [23,28,35], assisted in many cases by mild heat of the samples [[19], [20], [21], [22], [23],25,26,29,34,36,39,40,[42], [43], [44], [45],47,48,[50], [51], [52], [53], [54], [55], [56],[58], [59], [60],62,63]. Most of the reports presented the concurrent detection of several NPS from different chemical classes [[19], [20], [21], [22], [23],[25], [26], [27], [28], [29], [30], [31],33,46,62] and others focused on the analysis of just one NPS class [24,32,34,[43], [44], [45],[47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61],63]. Most of the reviewed methodologies used liquid chromatography coupled to low resolution mass spectrometry [21,22,[24], [25], [26], [27], [28], [29],31,33,35,36,39,41,[43], [44], [45],[47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57],[60], [61], [62]] followed by gas chromatography-mass spectrometry [19,32,34,40,46], and more recently by techniques coupled to high resolution mass spectrometry techniques (HMRS) [23,30,37,38,58,59,62,63] for the detection of drugs and metabolites. The selection and application of the appropriate NPS extraction method from hair was intimately bound to the properties of the chemical examined, the sensitivity of the detection instrument, and the hair amount. It is generally accepted that a rapid and efficient extraction is essential for forensic laboratories and the justice timeline, allowing the minimization of false-negative results and the maximum sensitivity of detection (lower LODs/LOQs).
From analytical point of view, the LOD and LOQ are defined with strict and widely accepted criteria [64]. All but one of the reviewed manuscripts reported LODs and LOQs at the level of nanogram or picogram NPS per milligram of hair, while the exception attained NPS levels at nanogram per 10-mm hair segment [35]. In addition, the limit of reporting (LOR) was another relevant parameter defined as the concentration for reporting positive samples, aiming to discriminate the active drug incorporation during consumption from the deposition of NPS on hair during external exposure [21]. LOR values have been set (at the level of 100 pg/mg of hair) being at least 10fold higher than the respective LODs for most of the 132 NPS analysed. It is obvious that such a value can only be set arbitrarily. Moreover, the limit of identification (LOI) has been also utilized in one study and defined as the lowest analyte concentration that could be correctly identified by the screening software [30] and it was equal to up to a hundred times higher than the respective LODS for the 10 NPS applied. The efforts to set LOR or LOI values to report NPS in hair are indicative of the concerns about the subsequent proper interpretation of the hair analysis results and to discriminate positive hair samples due to NPS active use from passive exposure.
2.1. Determination of synthetic phenethylamines and piperazines in hair
Synthetic phenethylamines that share a common phenethylamine moiety are considered to be a noteworthy group of legal highs [1]. Psychedelic phenethylamines such as 2C (2C-x) have methoxy groups on the two and five positions of a benzene ring, and various lipophilic substituents at position four. NBOMe (or 25X-NBOMe) is another class containing an N-(2-methoxy) benzyl substituent. Additionally, other phenethylamines, such as PMMA, include designer drugs from the amphetamine class, which hold serotonergic effects.
2C-x series: A total of five studies have been carried out for the determination of eighteen 2 C-x in hair. LC–MS/MS [[20], [21], [22]] assays have mainly been utilized, while GC/MC [32] and LC-HRMS [23] have been used to determine only 2 substances. The relative data are presented in Table 1.
Three of these studies [[21], [22], [23]] have been engaged with the simultaneous analysis of phenethylamines with other NPS classes. The dominant extraction method applied is acidified methanol with various HCL concentrations (0.1 M [21] or 0.25 M [19]), to define 12 2C-x. LLE with a diethyl ether-ethyl acetate mixture was used to define 6 analytes, before their LC–MS/MS analysis [22] resulting to a higher LOD for 2 C-B, 2 C–E, and 2 C–I, compared to their extraction with acidified methanol [21].
The LODs achieved for 25C-NBOMe and 25I-NBOMe were comparable, after methanol [20] or acidified methanol extraction [21]. Markedly, 2 C–P provided an admittedly low LOD after methanol extraction [20], comparing to that obtained after other LLEs [22].
The most elaborated extraction method used was a PLE followed by SPE to determine 2 C–T-4 by LC-HRMS/MS analysis. The respective LOD attained was comparable to that achieved for other NPS of this class detected with LC-HRMS [23].
Unexpectedly, the LODs achieved with GC–MS methods after extraction with acidified methanol were lower in most cases than the respective with LC–MS methods.
Other Synthetic Phenethylamines: A total of fourteen studies have been interpreted and the relative data are presented in Table 1, including 16 different amphetamine type-phenethylamines in hair. Detection methods included: LC–MS/MS [20,21,[24], [25], [26], [27], [28], [29],31,33], GC/MS [19,32], and LC-HRMS [23,30].
Ten of these studies [21,23,[25], [26], [27], [28], [29], [30], [31],33], report on their simultaneous analysis with NPS from other classes.
The principal extraction method applied was acidified methanol with various HCL concentrations (either 0.1 M [21] or 0.25 M [19] or 1% [32]), to define 10 substances. Single-step methanol extraction was used before the LC–MS/MS analysis of seven compounds (PMA, PMMA, MXE, 4-FA, 6-APB, butylone, and 5-MAPB) resulting in LODs comparable to those achieved with acidified methanol. However, acidified methanol [27] was less effective than the extraction with methanol [20], for the extraction of 4-FA and butylone.
Obviously, the concentration of HCl in methanol for extraction had a variable effect on the respective LODs of different NPS analysed with LC–MS/MS or GC/MS (e.g. extraction of PMA and MDEA with 1% or 0.25 M HCl in methanol [19,32] before GC/MS analysis, resulted in an excessively high LOD, as compared to that achieved with 0.1 M HCl in methanol and LC–MS/MS analysis [21]).
Various SPE protocols were applied to extract PMA, 4MTA, MXE, MDEA, 4FMA from hair [23,24,28,30,31,33] resulting in LODs comparable to other simpler protocols and detection with LC–MS or HRMS. Only the extraction of butylone with SPE resulted in considerably lower LOD compared to other applied extractions.
Synthetic Piperazines: Piperazines belong to a broad class of chemical compounds that have been designed to replicate the effects of ecstasy. Piperazines may act as central nervous system stimulants and can produce hallucinogenic or toxic effects similar to amphetamine and other sympathomimetics [1].
A total of nine studies report the determination of four piperazines in hair, using LC–MS/MS [20,21,[25], [26], [27],31,33], GC–MS [34], and LC-HRMS [30]. The relative data are presented in Table 1. Only two of these studies [20,34], report analysis of piperazines alone. Overall, the highest LODs for the four piperazines were achieved after extraction with acidified methanol and LC–MS/MS analysis [21]. Other LLE protocols, with methanol as the main solvent, resulted in comparable LODs. GC–MS methods were less sensitive, in terms of LLOQs, than LC–MS methods for mCPP, TFMPP, and MeOPP [34].
2.2. Determination of synthetic cathinones (SCa) in hair
Synthetic cathinones are stimulants, which belong to a category of drugs frequently recognised as bath salts [1,65]. These synthetic substances are chemical analogs of cathinone, the active stimulant of the khat plant, which act as monoamine release or reuptake inhibitors and have similar effects to amphetamines. In general, the polarity of these substances is increased by the β-keto group if compared to related amphetamines.
A total of 17 studies have been carried out for the determination of 50 SCa in human hair by LC–MS/MS [20,21,[24], [25], [26], [27],29,33,35,36,39], GC–MS [19,40], and LC-HRMS [23,30,37,38]. The relative data are presented in Table 2. Eleven of these studies [19,21,23,[25], [26], [27],29,30,33,38], have been engaged with the simultaneously analysis of cathinones with other NPS classes.
As expected, methanol, alone or mixed with other organic solvents or aqueous hydrochloric solutions, was the most used solvent for cathinones extraction from hair, since they are holding dissociation constants in the basic range [66]. The most effective extraction mixture seems to be MeOH/ACN/H2O plus ammonium formate, and acidified methanol (0.1 M) were used to extract 31 [26] and 28 [21] cathinones, respectively.
Remarkably, the LOD of 3-MMC in pubic hair after extraction with methanol: TFA, and LC-HRMS-Orbitrap analysis [38] was higher than the respective LODs achieved by acidified MeOH and LC–MS/MS analysis [21]. Generally, SPE and LLE methods were proved to be equally effective by different low or high resolution LC–MS methods. As expected, higher LOD was achieved during GC–MS analysis [40] for 4-MMC, compared to that obtained with different extraction methods and LC–MS/MS analysis and detection [20,21,[24], [25], [26], [27],29,33,39].
2.3. Determination of synthetic cannabinoids in hair
Synthetic cannabinoids (SCs) are among the most popular NPS that display high-affinity binding to the CB1 and CB2 cannabinoid receptors and demonstrate a pharmacological profile like trans-Δ9 -tetrahydrocannabinol (THC) [1,67]. They hold hallucinogenic, hypnotic, and/ or sedative effects. Seventeen studies reported the presence of 109 synthetic cannabinoids in hair, using LC–MS/MS [21,22,25,29,[41], [42], [43], [44], [45],47,48,50,51], GC/MS [46], LC-HRMS [23,30]. The relative data are presented in Table 3. Seven of these studies [[21], [22], [23],25,29,30,46] have been engaged with the simultaneous analysis of cannabinoids with other NPS classes.
From chemical point of view, the majority of SCs molecules consist of 22–26 carbon atoms being highly lipophilic [2]. They are soluble in solvents with low polarity (e.g. isooctane) as well as in methanol, ethanol, acetonitrile, ethyl acetate, acetone and other medium polar organic solvents while their solubility in water is low [1].
Benzoylindoles: A total of six studies were conducted for the determination of seven benzoylindoles in hair by LC–MS methods [21,22,25,29,41,42].
The most frequent extraction method applied was acidified methanol (0.1 M HCl) [21], being the most effective compared to methanol alone [25] or with other mixtures of organic solvents [22,41,42] used for LLE of NPS from hair, except for WIN48.098 [25].
Naphthoylindoles: A total of 14 studies have been conducted for the determination of 42 naphthoylindoles in hair [[21], [22], [23],25,29,30,41,[43], [44], [45], [46], [47], [48]]. The principal extraction method applied is MeOH/ 0.1 M HCL, to define 22 of them [21]. Methanol [44,47] was the most efficient extraction method for most naphthoindoles than with other organic solvents, or SPE methods. However, the LODs of JWH-398, AM1220, WIN 55, 212−2, MAM-2201 N (5-pentanoic acid), and JWH-018N-(5−OH), after LLE with various solvents [41,42], were comparable to those attained with methanol extraction [25,44] or acidified methanol [21].
As expected, higher LODs were achieved during GS-MS analysis [46] for determination of AM2201, JWH-081, and JWH-019, after LLE with a mixture of hexane: ethyl acetate (9:1), compared to those obtained either with a mixture of hexane/ethyl acetate (1/1, v/v) [45] or with n-hexane/ethyl acetate 90:10 (v/v) [42] and ethanol extraction [41], during LC–MS/MS.
Phenylacetylindoles, Naphthoylpyrroles, Other Synthetic Cannabinoids: A total of 10 studies have been conducted for the determination of 5 phenylacetylindoles in hair [[21], [22], [23],25,29,41,42,45,46,48]. The main extraction method applied is acidified methanol (0.1 M), with acceptable efficiency for most of the analytes, since the respective LODs were lower or comparable to those achieved after extraction with other LLE protocols [25,41,42,46,48].
Single-step methanol extraction [25] was the main hair extraction method used for JWH-030, JWH-147 and JWH-307, although it provided higher LODs compared to that obtained after LLE with other solvent combinations [29,42] or acidified methanol [21].
A total of 13 studies were performed in hair for the determination of 52 SCs (not included in the previous classes) using LC–MS/MS [21,25,29,[41], [42], [43],45,[48], [49], [50], [51]], GC–MS [46], and LC-HRMS [23]. The relative data are presented in Table 3.
The foremost extraction method applied is MeOH/ 0.1 M HCL, to define 26 of them [21], resulting in LODs comparable to those achieved with different solvents and extraction protocols [23,25,29,[41], [42], [43],45,[48], [49], [50], [51]]. Once again, LLE with hexane/ ethyl acetate before GC–MS analysis has obtained the highest LODs for the respective SCs [46].
2.4. Determination of Synthetic Opioids in hair
Synthetic opioids (SOs) act on the same brain targets as naturally occurring drugs of the opium poppy plant (e.g., morphine, heroin, and codeine) to produce analgesic (pain relief) effects [68]. The design of some SOs (e.g., methadone and fentanyl) progressed from therapeutic use to the clandestine synthesis of new fentanyl derivatives for the illicit market. Various fentanyl analogs (e.g., acetyl, furanyl-fentanyl, and carfentanyl) have shown particularly hazardous pharmacological effects [69,70].
A total of 12 studies have been conducted for the determination of 58 synthetic opioids, using LC–MS/MS [21,29,[52], [53], [54], [55], [56], [57],59,60], and LC-HRMS assays [30,58]. The relative data are presented in Table 4.
Extraction with MeOH/ACN/ammonium acetate was mainly utilized for the determination of 37 SOs, achieving comparable and considerably low LODs for 11 of them [57].
Generally, similar LODs were achieved for the determination of several SOs, after extraction with different organic solvents [53,56,58,60], or combinations of organic solvents [21,29,30,55,57], or SPE [54].
Notable, one SPE protocol [52] resulted in considerably lower LODs for the tested analytes than those achieved with LLEs protocols indicating that improvements in SPE matrices could result in more efficient extractions and lower LODs.
2.5. Determination of synthetic tryptamines and other NPS in hair
Over the last few years, synthetic tryptamine analogs (STs) show a growing demand among drug users. STs can offer increased potencies compared to natural tryptamines as a result of a functional group modification, specifically from decarboxylation of the amino acid tryptophan [71].
A total of five studies have been conducted for the determination of ten tryptamines in hair using LC–MS/MS [21,29,61], and LC-HRMS assays [30,62]. The relative data are presented in Table 5. Three of these studies [21,29,30,62], performed simultaneously analysis of tryptamines with other NPS classes.
The LODs were comparable for the different tryptamines determined. Interestingly, methanol was not the main solvent for the extraction of tryptamines from hair. The different extraction protocols applied resulted in comparable LODS for the different compounds analysed.
A total of nine studies have been conducted for the determination of 15 other NPS in hair, using LC–MS/MS [20,21,28,63], GC–MS [19,32], and LC-HRMS assays [30,63]. The relative data are presented in Table 5. Overall, the leading extraction method applied was again acidified methanol (0.1 M) [21].
3. Aspects of NPS hair extraction
The different applied hair extraction procedures have focused on isolating from the hair matrix, certain NPS, of the same or different classes, with the highest possible efficiency. It is known that the keratinized hair shaft has a complex, multi-compartment structure and the drug incorporation in hair is a function of the acidity/basicity of the compound, its lipophilicity, and its affinity to melanin [72]. On the other hand, the main factors that influence drug incorporation in hair, affect the drug extraction from hair, as well [73]. Therefore, the extraction procedure poses several issues most relevant to the complexity of this matrix.
Of main concern to the toxicologists is the removal of the external contaminations from hair, consisting of organic and inorganic chemicals that have been deposited to the hair shafts, by applying appropriate washing steps. The hair washing steps are usually applied according to the suggested guidelines which states further that external contamination must be considered to interpret findings, while researchers should evaluate the efficiency of washing procedures [73,74]. Nevertheless, some of the reviewed studies herein, reported extensive washing procedures, and others none, indicating the different viewpoints for the necessity of this step, especially in respect to differentiate the active drug use from external drug deposition onto hair.
Several extraction methods have been applied to isolate NPS from hair (such as methanolic extraction, LLE, or SPE, in ultrasonic and/or heating blocks, under different conditions), depending on the chemical properties of the analytes. The dominant extraction method applied for the determination of most NPS classes was acidified methanol (with various concentrations of hydrochloride). This preference should be attributed, firstly, to its ability to extract from hair very diverse classes of NPS, and secondly, to the simplicity of the relevant procedures, as compared to SPE, or other LLEs. However, the extraction with acidified methanol presents the disadvantages of yielding lower drug recoveries compared to other procedures, and of resulting in a high degree of contamination from hair matrix (matrix effect) [72,73]. In fact, matrix decontamination is one of the main limitations in hair testing, with an impact on extraction efficiencies and on the LODs/LOQs of the analytes. Although not within the scope of this review, we comment that the extraction efficiencies of the various NPS analysis methodologies, as expressed by precision and accuracy, were considered satisfactory [75].
Our review data depicted on Table 1, Table 2, Table 3, Table 4, Table 5 have revealed that NPS of the same chemical class had similar LODs and range of identification when extracted from hair with the same medium and detected with the same method, as expected. Additionally, our data made apparent that the LODs, LOQs and ranges of various NPS groups determined in hair, with different analysis protocols, were comparable (being all in the low pg/mg level). These data indicate that modern NPS hair analysis ensures high selectivity and sensitivity of detection of different analytes. It is worth mentioning that different NPS with very diverse chemical structures were efficiently extracted from hair, with acidified methanol; proving acidified methanol as a generic extractor.
Last but not least finding of our review was that the NPS hair levels measured in real forensic/ clinical cases were significantly higher than the respective LODs. The NPS hair analysis of real cases reported the detection of 80 NPS (out of the 280 targeted NPS) with the respective developed methods for NPS hair analysis (only five of the reviewed publications don’t report application in real cases [21,23,28,31,37]). These findings indicate that the existing methods enable adequate identification and measurement of these compounds in hair from possible NPS (ab)users. However, it should be underlined that one of the reviewed studies had set LOR as the cut-off analyte concentration that can discriminate passive exposure from active incorporation of NPS in hair (it was set at the level 100 pg/mg of hair and being at least 10fold higher than the respective LODs); subsequently cases with NPS concentrations higher than the predefined LOR were considered positive [21]. Although this LOR value was set arbitrarily, the relevant consideration is in accordance with the recently expressed concerns on the possibility of misinterpretations of the very low drug concentrations in hair [76]. Specifically, for NPS more concerns could arise from the absence of specific guidelines for their analysis, the absence of official cut-offs to discriminate consumption from contamination, and from their unknown pharmacology which probably would make necessary the establishment of lower cut-offs in certain cases. Probably, future research could advance the progress of more efficient extraction mixtures or micro extraction methods of diverse NPS from hair [[77], [78], [79]]. Undoubtedly, an improved universal extraction protocol of NPS from hair will advance the aim of studying the epidemiology of NPS among drug abusers.
4. Aspects of the NPS detection methods
The most crucial issue on NPS hair analysis is the accurate compound detection and identification. The objective measures of identity are assured by the various available mass spectrometry techniques which enable the definitive identification of analytes, reducing to a minimum, or ideally, eliminating, the number of false-positive and false-negative identifications. NPS hair analysis was exclusively performed by MS techniques, after a chromatographic (LC or GC) separation.
All but one reviewed methods herein, concerned targeted analysis of NPS in hair (mainly on low resolution mass spectrometers, LRMS) allowing the detection of few to several NPS of one class or of different classes (up to 132 NPS which is the largest number of NPS so far [21]. The “targeted” analysis strategy achieves the definitive identification and confirmation of an unknown analyte, by “fitting” selected MS data (m/z values of molecular ions, relative abundances of fragment ions, etc.) and relevant chromatographic parameters (such as the retention time of the analyte) with, either the MS data of a reference standard analysed under the same conditions as the unknown, in accordance with the suggested up to date relevant guidelines, or the data form MS/MS spectral libraries. Nevertheless, a serious limitation of the targeted NPS identification with LRMS is the lack of verified reference standards for newly identified NPS and metabolites.
One the other hand, the HRMS screening approach can overcome this limitation and therefore it became gradually more popular than LRMS for comprehensive drug screening, including NPS analysis. Worth mentioning advantages of HRMS, including high resolution and high mass accuracy, both of which increase the confidence of compound identification, and selectivity of the method, allowing the confirmation of the NPS molecular formula; even in identifying the minor mass differences that are often present in NPS molecules [[80], [81], [82]]. Furthermore, HRMS could prove beneficial in identifying structural clusters of potential novel toxic metabolites of NPS [[83], [84], [85]].
However, comprehensive HRMS-based screening and confirmatory methods for NPS hair analysis are reported to a limited number of studies, mainly because they need standardized spectral libraries for screening and identification of compounds present in a sample [23,30,38]. Some attempts [23,86] succeeded to construct MS/MS spectral libraries including large numbers of NPS and metabolites. Initially, Montesano and colleagues described the development of a broad screening technique for NPS that included the use of an in-house MS/MS spectral library for 300 NPS and known metabolites [23]. More recently, the development of a comprehensive compound database for 875 unique chemical entities considered as possible NPS, in addition to a full HRMS MS/MS spectral library for 252 of these compounds was reported [86].
Although these recent trends in NPS analysis, presently, HRMS instrumentation does not seem to be a replacement for standard LRMS which are a commonplace worldwide for routine basic toxicology applications. Routine toxicology procedures need to fulfill standard guidelines which are not set yet in the forensic field for not targeted analyses provided by the HRMS instruments. However, we are of opinion that hair analysis by HRMS could be advantageous for conducting any NPS and metabolite identification, in the aim to study the prevalence and spread of NPS use in the community.
5. Conclusions
Initially, NPS hair analysis was performed by using the established analytical methodologies for drugs of abuse hair analysis which were validated as suggested by official organizations. However, the quickly changing chemical structures of new NPS delivered in the illegal drug markets, the unknown pharmacology of new NPS, and the possibility some of them to have high potency, which could lead to emergency hospitalizations and/or deaths has challenged their analysis in biological specimens. Therefore, the improvement of analytical strategies, and the development of alternative and innovative analytical methodologies became a necessity. The relative research has focused, mainly, on improving the NPS extraction from hair and, the detection techniques of extracted analytes.
The analytical protocols reviewed herein for NPS hair analysis showed continuously growing trends to identify as many NPS as possible; the extraction methods seem to have a limited potential to improve, while the various mass spectroscopic techniques and relevant instrumentation used for NPS detection and identification provide an enormous field for development and application. Future research in the field could progress NPS hair analysis and aim the monitoring of NPS expansion and extent of use worldwide.
Authors’ statement
The authors declare that they have contributed to the manuscript as follows:
-
•
DF has written the original manuscript according to VB’s suggestions;
-
•
VB has designed the manuscript, supervised the writing and wrote the critical discussion of the subject.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Dr. Aristidis Tsatsakis
References
- 1.United Nations Office on Drugs and Crime . 2021. February 2021 – UNODC EWA: Share of NPS Stimulants and Synthetic Cannabinoids Remains Stable While Opioids Continue to Increase.https://www.unodc.org/LSS/Announcement/Details/d45a4db1-4f64-447e-818c-35ce7a2e2278 [Google Scholar]
- 2.European Monitoring Centre for Drugs and Drug Addiction . 2021. European Drug Report 2021: Trends and Developments.https://www.emcdda.europa.eu/publications/edr/trends-developments/2021_en [Google Scholar]
- 3.Meader N., Mdege N., McCambridge J. The public health evidence-base on novel psychoactive substance use: scoping review with narrative synthesis of selected bodies of evidence. J. Public Health Oxf. (Oxf) 2018;40:e303–e319. doi: 10.1093/pubmed/fdy016. [DOI] [PubMed] [Google Scholar]
- 4.Logan B.K., Mohr A.L.A., Friscia M., Krotulski A.J., Papsun D.M., Kacinko S.L., Ropero-Miller J.D., Huestis M.A. Reports of adverse events associated with use of novel psychoactive substances, 2013-2016: a review. J. Anal. Toxicol. 2017;41(7):573–610. doi: 10.1093/jat/bkx031. [DOI] [PubMed] [Google Scholar]
- 5.Kraemer M., Boehmer A., Madea B., Maas A. Death cases involving certain new psychoactive substances: a review of the literature. Forensic Sci. Int.l. 2019;298:186–267. doi: 10.1016/j.forsciint.2019.02.021. [DOI] [PubMed] [Google Scholar]
- 6.Specka M., Kuhlmann T., Sawazki J., Bonnet U., Steinert R., Cybulska-Rycicki M., Eich H., Zeiske B., Niedersteberg A., Schaaf L., Scherbaum N. Prevalence of novel psychoactive substance (NPS) use in patients admitted to drug detoxification treatment. Front. Psychiatry. 2020;11:569. doi: 10.3389/fpsyt.2020.00569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schifano F., Napoletano F., Chiappini S., Guirguis A., Corkery J.M., Bonaccorso S., Ricciardi A., Scherbaum N., Vento A. New/emerging psychoactive substances and associated psychopathological consequences. Psychol. Med. 2021;51:30–42. doi: 10.1017/S0033291719001727. [DOI] [PubMed] [Google Scholar]
- 8.Boumba V.A., Ziavrou K.S., Vougiouklakis T. Hair as a biological Indicator of drug use, drug abuse or chronic exposure to environmental toxicants. Int. J. Toxicol. 2006:143–163. doi: 10.1080/10915810600683028. [DOI] [PubMed] [Google Scholar]
- 9.Gryczynski J., Schwartz R.P., Mitchell S.G., O’Grady K.E., Ondersmac S.J. Hair drug testing results and self-reported drug use among primary care patients with moderate-risk illicit drug use. Drug Alcohol Depend. 2014;141:44–50. doi: 10.1016/j.drugalcdep.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akiyama Y., Sherwood N. Systematic review of biomarker findings from clinical studies of electronic cigarettes and heated tobacco products. Toxicol. Rep. 2021;8:282–294. doi: 10.1016/j.toxrep.2021.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willson C. The clinical toxicology of caffeine: a review and case study. Toxicol. Rep. 2018;5:1140–1152. doi: 10.1016/j.toxrep.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hernández A.F., Lozano-Paniagua D., González-Alzaga B., Kavvalakis M.P., Tzatzarakis M.N., López-Flores I., Aguilar-Garduño C., Caparros-Gonzalez R.A., Tsatsakis A.M. Lacasaña M.BIomonitoring of common organophosphate metabolites in hair and urine of children from an agricultural community. Environ. Int. 2019;131(104997) doi: 10.1016/j.envint.2019.104997. Epub 2019 Jul 27. PMID: 31362151. [DOI] [PubMed] [Google Scholar]
- 13.Katsikantami I., Tzatzarakis M.N., Karzi V., Stavroulaki A., Xezonaki P., Vakonaki E., Alegakis A.K., Sifakis S., Rizos A.K. Tsatsakis AM.BIomonitoring of bisphenols A and S and phthalate metabolites in hair from pregnant women in Crete. Sci. Total Environ. 2020;10(712):135651. doi: 10.1016/j.scitotenv.2019.135651. Epub 2019 Nov 20. PMID: 31810691. [DOI] [PubMed] [Google Scholar]
- 14.Barmpas M., Vakonaki E., Tzatzarakis M., Sifakis S., Alegakis A., Grigoriadis T., Sodré D.B., Daskalakis G., Antsaklis A., Tsatsakis A. Organochlorine pollutants’ levels in hair, amniotic fluid and serum samples of pregnant women in Greece. A cohort study. Environ. Toxicol. Pharmacol. 2020;73(103279) doi: 10.1016/j.etap.2019.103279. Epub 2019 Oct 16.PMID: 31704585. [DOI] [PubMed] [Google Scholar]
- 15.Karzi V., Tzatzarakis M.N., Vakonaki E., Alegakis T., Katsikantami I., Sifakis S., Rizos A., Tsatsakis A.M. Biomonitoring of bisphenol A, triclosan and perfluorooctanoic acid in hair samples of children and adults. J. Appl. Toxicol. 2018;38(8):1144–1152. doi: 10.1002/jat.3627. [DOI] [PubMed] [Google Scholar]
- 16.Larabi I.A., Fabresse N., Etting I., Nadour L., Pfau G., Raphalen J.H., Philippe P., Edel Y., Alvarez J.C. Prevalence of New Psychoactive Substances (NPS) and conventional drugs of abuse (DOA) in high risk populations from Paris (France) and its suburbs: a cross sectional study by hair testing (2012-2017) Drug Alcohol Depend. 2019;204:107508. doi: 10.1016/j.drugalcdep.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 17.Salomone A., Vincenti M., Gerace E. Interpretation of NPS results in real hair samples. Toxicol. Anal. Clin. 2017;29(1):4–10. doi: 10.1016/j.toxac.2016.12.008. [DOI] [Google Scholar]
- 18.Cuypers E., Flanagan R.J. The interpretation of hair analysis for drugs and drugs metabolites. Clin. Toxicol. Phila. (Phila) 2018;56(2):90–100. doi: 10.1080/15563650.2017.1379603. [DOI] [PubMed] [Google Scholar]
- 19.Kim J.Y., Jung K.S., Kim M.K., Lee J.I., In M.K. Simultaneous determination of psychotropic phenylalkylamine derivatives in human hair by gas chromatography/mass spectrometry. Rapid Commun. Mass Spectrom. 2007;21(11):1705–1720. doi: 10.1002/rcm.3010. [DOI] [PubMed] [Google Scholar]
- 20.Salomone A., Gazzilli G., Di Corcia D., Gerace E., Vincenti M. Determination of cathinones and other stimulant, psychedelic, and dissociative designer drugs in real hair samples. Anal. Bioanal. Chem. 2016;408(8):2035–2042. doi: 10.1007/s00216-015-9247-4. [DOI] [PubMed] [Google Scholar]
- 21.Boumba V.A., Di Rago M., Peka M., Drummer O.H., Gerostamoulos D. The analysis of 132 novel psychoactive substances in human hair using a single step extraction by tandem LC/MS. Forensic Sci. Int. 2017;279:192–202. doi: 10.1016/j.forsciint.2017.08.031. [DOI] [PubMed] [Google Scholar]
- 22.Montenarh D., Hopf M., Warth S., Maurer H.H., Schmidt P., Ewald A.H. A simple extraction and LC-MS/MS approach for the screening and identification of over 100 analytes in eight different matrices. Drug Test. Anal. 2015;7(3):214–240. doi: 10.1002/dta.1657. [DOI] [PubMed] [Google Scholar]
- 23.Montesano C., Vannutelli G., Massa M., Simeoni M.C., Gregori A., Ripani L., Compagnone D., Curini R., Sergi M. Multi-class analysis of new psychoactive substances and metabolites in hair by pressurized liquid extraction coupled to HPLC-HRMS. Drug Test. Anal. 2017;9(5):798–807. doi: 10.1002/dta.2043. [DOI] [PubMed] [Google Scholar]
- 24.Freni F., Bianco S., Vignali C., Groppi A., Moretti M., Osculati A.M.M., Morini L. A multi-analyte LC-MS/MS method for screening and quantification of 16 synthetic cathinones in hair: application to postmortem cases. Forensic Sci. Int. 2019;298:115–120. doi: 10.1016/j.forsciint.2019.02.036. [DOI] [PubMed] [Google Scholar]
- 25.Strano-Rossi S., Odoardi S., Fisichella M., Anzillotti L., Gottardo R., Tagliaro F. Screening for new psychoactive substances in hair by ultrahigh performance liquid chromatography-electrospray ionization tandem mass spectrometry. J. Chromatogr. A. 2014;1372C:145–156. doi: 10.1016/j.chroma.2014.10.106. [DOI] [PubMed] [Google Scholar]
- 26.Niebel A., Krumbiegel F., Hartwig S., Parr M.K., Tsokos M. Detection and quantification of synthetic cathinones and selected piperazines in hair by LC-MS/MS. Forensic Sci. Med. Pathol. 2020;16(1):32–42. doi: 10.1007/s12024-019-00209-z. [DOI] [PubMed] [Google Scholar]
- 27.Rust K.Y., Baumgartner M.R., Dally A.M., Kraemer T. Prevalence of new psychoactive substances: a retrospective study in hair. Drug Test. Anal. 2012;4(6):402–408. doi: 10.1002/dta.1338. [DOI] [PubMed] [Google Scholar]
- 28.Sergi M., Napoletano S., Montesano C., Iofrida R., Curini R., Compagnone D. Pressurized-liquid extraction for determination of illicit drugs in hair by LC-MS-MS. Anal. Bioanal. Chem. 2013;405(2-3):725–735. doi: 10.1016/j.chroma.2015.06.021. [DOI] [PubMed] [Google Scholar]
- 29.Mannocchi G., Di Trana A., Tini A., Zaami S., Gottardi M., Pichini S., Busardò F.P. Development and validation of fast UHPLC-MS/MS screening method for 87 NPS and 32 other drugs of abuse in hair and nails: application to real cases. Anal. Bioanal. Chem. 2020;412(21):5125–5145. doi: 10.1007/s00216-020-02462-6. [DOI] [PubMed] [Google Scholar]
- 30.Fabresse N., Larabi I.A., Stratton T., Mistrik R., Pfau G., Lorin de la Grandmaison G., Etting I., Grassin Delyle S., Alvarez J.C. Development of a sensitive untargeted liquid chromatography-high resolution mass spectrometry screening devoted to hair analysis through a shared MS2 spectra database: a step toward early detection of new psychoactive substances. Drug Test. Anal. 2019;11(5):697–708. doi: 10.1002/dta.2535. [DOI] [PubMed] [Google Scholar]
- 31.Imbert L., Dulaurent S., Mercerolle M., Morichon J., Lachâtre G., Gaulier J.M. Development and validation of a single LC-MS/MS assay following SPE for simultaneous hair analysis of amphetamines, opiates, cocaine and metabolites. Forensic Sci. Int. 2014;234:132–138. doi: 10.1016/j.forsciint.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Jang M., Yang W., Jeong S., Park S., Kim J. A fatal case of paramethoxyamphetamine poisoning and its detection in hair. Forensic Sci. Int. 2016;266:e27–e31. doi: 10.1016/j.forsciint.2016.06.030. [DOI] [PubMed] [Google Scholar]
- 33.Lendoiro E., Jiménez-Morigosa C., Cruz A., Páramo M., López-Rivadulla M., de Castro A. An LC-MS/MS methodological approach to the analysis of hair for amphetamine-type-stimulant (ATS) drugs, including selected synthetic cathinones and piperazines. Drug Test. Anal. 2017;9(1):96–105. doi: 10.1002/dta.1948. [DOI] [PubMed] [Google Scholar]
- 34.Barroso M., Costa S., Dias M., Vieira D.N., Queiroz J.A., López-Rivadulla M. Analysis of phenylpiperazine-like stimulants in human hair as trimethylsilyl derivatives by gas chromatography-mass spectrometry. J. Chromatogr. A. 2010;1217(40):6274–6280. doi: 10.1016/j.chroma.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 35.Namera A., Urabe S., Saito T., Torikoshi-Hatano A., Shiraishi H., Arima Y., Nagao M. A fatal case of 3,4-methylenedioxypyrovalerone poisoning: coexistence of a-pyrrolidinobutiophenone and a-pyrrolidinovalerophenone in blood and/or hair. Forensic Toxicol. 2013;31:338–343. doi: 10.1007/s11419-013-0192-7. [DOI] [Google Scholar]
- 36.Alvarez J.C., Etting I., Abe E., Villa A., Fabresse N. Identification and quantification of 4-methylethcathinone (4-MEC) and 3,4-methylenedioxypyrovalerone (MDPV) in hair by LC-MS/MS after chronic administration. Forensic Sci. Int. 2017;270:39–45. doi: 10.1016/j.forsciint.2016.11.028. [DOI] [PubMed] [Google Scholar]
- 37.Odoardi S., Mestria S., Biosa G., Arfè R., Tirri M., Marti M., Strano Rossi S. Metabolism study and toxicological determination of mephtetramine in biological samples by liquid chromatography coupled with high-resolution mass spectrometry. Drug Test. Anal. 2021 doi: 10.1002/dta.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Frison G., Frasson S., Zancanaro F., Tedeschi G., Zamengo L. Detection of 3-methylmethcathinone and its metabolites 3-methylephedrine and 3-methylnorephedrine in pubic hair samples by liquid chromatography-high resolution/high accuracy Orbitrap mass spectrometry. Forensic Sci. Int. 2016;265:131–137. doi: 10.1016/j.forsciint.2016.01.039. [DOI] [PubMed] [Google Scholar]
- 39.Shah S.A., Deshmukh N.I., Barker J., Petróczi A., Cross P., Archer R., Naughton D.P. Quantitative analysis of mephedrone using liquid chromatography tandem mass spectroscopy: application to human hair. J. Pharm. Biomed. Anal. 2012;61:64–69. doi: 10.1016/j.jpba.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 40.Martin M., Muller J.F., Turner K., Duez M., Cirimele V. Evidence of mephedrone chronic abuse through hair analysis using GC/MS. Forensic Sci. Int. 2012;218(1-3):44–48. doi: 10.1016/j.forsciint.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 41.Hutter M., Kneisel S., Auwärter V., Neukamm M.A. Determination of 22 synthetic cannabinoids in human hair by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2012;903:95–101. doi: 10.1016/j.jchromb.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 42.Salomone A., Luciano C., Di Corcia D., Gerace E., Vincenti M. Hair analysis as a tool to evaluate the prevalence of synthetic cannabinoids in different populations of drug consumers. Drug Test. Anal. 2014;6(1–2):126–134. doi: 10.1002/dta.1556. [DOI] [PubMed] [Google Scholar]
- 43.Cho B., Cho H.S., Kim J., Sim J., Seol I., Baeck S.K., In S., Shin D.H., Kim E. Simultaneous determination of synthetic cannabinoids and their metabolites in human hair using LC-MS/MS and application to human hair. Forensic Sci. Int. 2020;306:110058. doi: 10.1016/j.forsciint.2019.110058. [DOI] [PubMed] [Google Scholar]
- 44.Kim J., Park Y., Park M., Kim E., Yang W., Baeck S., Lee S., Han S. Simultaneous determination of five naphthoylindole-based synthetic cannabinoids and metabolites and their deposition in human and rat hair. J. Pharm. Biomed. Anal. 2015;102:162–175. doi: 10.1016/j.jpba.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 45.Larabi I.A., Riffi M., Fabresse N., Etting I., Alvarez J.C. Validation of an UPLC-MS/MS method for the determination of sixteen synthetic cannabinoids in human hair. Application to document chronic use of JWH-122 following a non-fatal overdose. Toxicol. Anal. Clin. 2019;31:283–292. doi: 10.1016/j.toxac.2019.10.005. [DOI] [Google Scholar]
- 46.Anzillotti L., Calò L., Giacalone M., Banchini A., Cecchi R. Determination of methadone and eight new psychoactive substances in hair samples by gas Chromatography/Mass spectrometry. J.Forensic Sci. and Med. 2020;4(4):184–191. doi: 10.4103/jfsm.jfsm_22_18. [DOI] [Google Scholar]
- 47.Kim J., In S., Park Y., Park M., Kim E., Lee S. Deposition of JWH-018, JWH-073 and their metabolites in hair and effect of hair pigmentation. Anal. Bioanal. Chem. 2013;405(30):9769–9778. doi: 10.1007/s00216-013-7423-y. [DOI] [PubMed] [Google Scholar]
- 48.Salomone A., Gerace E., D’Urso F., Di Corcia D., Vincenti M. Simultaneous analysis of several synthetic cannabinoids, THC, CBD and CBN, in hair by ultra-high performance liquid chromatography tandem mass spectrometry. Method validation and application to real samples. J. Mass Spectrom. 2012;47(5):604–610. doi: 10.1002/jms.2988. [DOI] [PubMed] [Google Scholar]
- 49.Franz F., Angerer V., Hermanns-Clausen M., Auwärter V., Moosmann B. Metabolites of synthetic cannabinoids in hair--proof of consumption or false friends for interpretation? Anal. Bioanal. Chem. 2016;408(13):3445–3452. doi: 10.1007/s00216-016-9422-2. [DOI] [PubMed] [Google Scholar]
- 50.Sim J., Cho H.S., Lee J., In S., Kim E. Determination of AB-CHMINACA and its metabolites in human hair and their deposition in hair of abusers. J. Pharm. Biomed. Anal. 2017;140:162–168. doi: 10.1016/j.jpba.2017.03.041. [DOI] [PubMed] [Google Scholar]
- 51.Park M., Yeon S., Lee J., In S. Determination of XLR-11 and its metabolites in hair by liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2015;114:184–189. doi: 10.1016/j.jpba.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 52.Busardò F.P., Carlier J., Giorgetti R., Tagliabracci A., Pacifici R., Gottardi M., Pichini S. Ultra-high-Performance liquid chromatography-tandem mass spectrometry assay for quantifying fentanyl and 22 analogs and metabolites in whole blood, urine, and hair. Front. Chem. 2019;7:184. doi: 10.3389/fchem.2019.00184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salomone A., Palamar J.J., Bigiarini R., Gerace E., Di Corcia D., Vincenti M. Detection of fentanyl analogs and synthetic opioids in real hair samples. J. Anal. Toxicol. 2019;43(4):259–265. doi: 10.1093/jat/bky093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Freni F., Moretti M., Radaelli D., Carelli C., Osculati A.M.M., Tronconi L., Vignali C., Morini L. Determination of fentanyl and 19 derivatives in hair: application to an Italian population. J. Pharm. Biomed. Anal. 2020;189 doi: 10.1016/j.jpba.2020.113476. [DOI] [PubMed] [Google Scholar]
- 55.Larabi I.A., Martin M., Fabresse N., Etting I., Edel Y., Pfau G., Alvarez J.C. Hair testing for 3‑fluorofentanyl, furanylfentanyl, methoxyacetylfentanyl, carfentanil, acetylfentanyl and fentanyl by LC-MS/MS after unintentional overdose. Forensic Toxicol. 2020;38:277–286. doi: 10.1007/s11419-019-00502-0. [DOI] [Google Scholar]
- 56.Salomone A., Bigiarini R., Palamar J.J., McKnight C., Vinsick L., Amante E., Di Corcia D., Vincenti M. Toward the interpretation of positive testing for fentanyl and its analogs in real hair samples: preliminary considerations. J. Anal. Toxicol. 2020;44(4):362–369. doi: 10.1093/jat/bkz102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Qin N., Shen M., Xiang P., Wen D., Shen B., Deng H., Qiang H., Song F., Shi Y. Determination of 37 fentanyl analogues and novel synthetic opioids in hair by UHPLC-MS/MS and its application to authentic cases. Sci. Rep. 2020;10(1):11569. doi: 10.1038/s41598-020-68348-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Salomone A., Di Corcia D., Negri P., Kolia M., Amante E., Gerace E., Vincenti M. Targeted and untargeted detection of fentanyl analogues and their metabolites in hair by means of UHPLC-QTOF-HRMS. Anal. Bioanal. Chem. 2021;413(1):225–233. doi: 10.1007/s00216-020-02994-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gaulier J.-M., Richeval c., Phanithavong M., Brault S., Allorge D., Dumestre-Toulet V. A case report of carfentanil-related fatality in France. Toxicol. Anal. Clin. 2019;31(4):323–331. doi: 10.1016/j.toxac.2019.01.002. [DOI] [Google Scholar]
- 60.Gerace E., Salomone A., Luciano C., Di Corcia D., Vincenti M. First case in Italy of fatal intoxication involving the new opioid U-47700. Front. Pharmacol. 2018;9:747. doi: 10.3389/fphar.2018.00747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang R., Xiang P., Yu Z., Shi Y. Application of hair analysis to document illegal 5-methoxy-N,N-dissopropyltrptamine (5-MeO-DiPT) use. Forensic Sci. Int. 2019;304:109972. doi: 10.1016/j.forsciint.2019.109972. [DOI] [PubMed] [Google Scholar]
- 62.Gicquel T., Richeval C., Mesli V., Gish A., Hakim F., Pelletier R., Cornez R., Balgairies A., Allorge D., Gaulier J.-M. Fatal intoxication related to two new arylcyclohexylamine derivatives (2F-DCK and 3-MeO-PCE) Forensic Sci. Int. 2021;324:110852. doi: 10.1016/j.forsciint.2021.110852. [DOI] [PubMed] [Google Scholar]
- 63.Mestria S., Odoardi S., Biosa G., Valentini V., Di Masi G., Cittadini F., Strano-Rossi S. Method development for the identification of methoxpropamine, 2-fluoro-deschloroketamine and deschloroketamine and their main metabolites in blood and hair and forensic application. Forensic Sci. Int. 2021;323 doi: 10.1016/j.forsciint.2021.110817. [DOI] [PubMed] [Google Scholar]
- 64.Armbruster D.A., Pry T. Limit of blank, limit of detection and limit of quantitation. Clin. Biochem. Rev. 2008;(Suppl 1):S49–S52. [PMC free article] [PubMed] [Google Scholar]
- 65.Soares J., Costa V.M., Bastos M.L., Carvalho F., Capela J.P. An updated review on synthetic cathinones. Arch.Toxicol. 2021 doi: 10.1007/s00204-021-03083-3. [DOI] [PubMed] [Google Scholar]
- 66.Glicksberg L., Winecker R., Miller C., Kerrigan S. Postmortem distribution and redistribution of synthetic cathinones. Forensic Toxicol. 2018;36:291–303. doi: 10.1007/s11419-018-0403-3. [DOI] [Google Scholar]
- 67.Alves V.L., Gonçalves J.L., Aguiar J., Teixeira H.M., Câmara J.S. The synthetic cannabinoids phenomenon: from structure to toxicological properties. A review. Crit. Rev. Toxicol. 2020;50(5):359–382. doi: 10.1080/10408444.2020.1762539. [DOI] [PubMed] [Google Scholar]
- 68.Karila L., Marillier M., Chaumette B., Nicolas F., Benyamina A. New Synthetic Opioids: part of a new addiction landscape. Neurosci. Biobehav. Rev. 2019;106:133–140. doi: 10.1016/j.neubiorev.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 69.Jones C.M., Einstein E.B., Compton W.M. Changes in synthetic opioid involvement in drug overdose deaths in the United States, 2010-2016. JAMA. 2018;319(17):1819–1821. doi: 10.1001/jama.2018.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brunetti P., Pirani F., Carlier J., Giorgetti R., Busardò F.P. A 2017-2019 update on acute intoxications and fatalities from illicit fentanyl and analogs. J. Anal. Toxicol. 2021;45(6):537–554. doi: 10.1093/jat/bkaa115. [DOI] [PubMed] [Google Scholar]
- 71.Malaca S., Fabrizio Lo Faro A., Tamborra A., Pichini S., Busardò F.P., Huestis M.A. Toxicology and analysis of psychoactive tryptamines. Int. J. Mol. Sci. 2020;21(23):9279. doi: 10.3390/ijms21239279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pragst F., Balikova M.A. State of the art in hair analysis for detection of drug and alcohol abuse. Clin. Chim. Acta. 2006;370(1–2):17–49. doi: 10.1016/j.cca.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 73.Mantinieks D., Gerostamoulos D., Wright P., Drummer O. The effectiveness of decontamination procedures used in forensic hair analysis. Forensic Sci. Med. Pathol. 2018;3:349–357. doi: 10.1007/s12024-018-9994-6. [DOI] [PubMed] [Google Scholar]
- 74.Cooper G.A.A., Kronstrand R., Kintz Pl. Society of Hair Testing, Society of Hair Testing guidelines for drug testing in hair. Forensic Sci. Int. 2012;218:20–24. doi: 10.1016/j.forsciint.2011.10.024. [DOI] [PubMed] [Google Scholar]
- 75.Kyriakou C., Pellegrini M., García-Algar O., Marinelli E., Zaami S. Recent trends in analytical methods to determine new psychoactive substances in hair. Curr. Neuropharmacol. 2017;15(5):663–681. doi: 10.2174/1570159x15666161111112545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Drummer O.H., Gerostamoulos D., LeBeau M.A., Pragst F. Concerns on the misinterpretation of very low drug concentrations in hair. J. Anal. Toxicol. 2020;44(9):e6–e8. doi: 10.1093/jat/bkaa078. [DOI] [PubMed] [Google Scholar]
- 77.Ferreira C., Paulino C., Quintas A. Extraction procedures for hair forensic toxicological analysis: a mini-review. Chem. Res. Toxicol. 2019;32(12):2367–2381. doi: 10.1021/acs.chemrestox.9b00301. [DOI] [PubMed] [Google Scholar]
- 78.Birk L., Santos S.O., Eller S., Merib J.O., Oliveira T.F. Determinations of new psychoactive substances in biological matrices with focus on microextraction techniques: a review of fundamentals and state-of-the-art extraction methods. Forensic Toxicol. 2021;39:350–367. doi: 10.1007/s11419-021-00582-x. [DOI] [Google Scholar]
- 79.Da Costa B.R.B., Wilson J.R.S.Junior, Maximiliano I.F., Gomens N.C., Freitas B.T., De Martinis B.S. Application of microextraction techniques in alternative biological matrices with focus on forensic toxicology: a review. Bioanalysis. 2021;13(1):45–64. doi: 10.4155/bio-2020-0241. [DOI] [PubMed] [Google Scholar]
- 80.Reman D., Wissenbach D.K., Peters F.T. Recent advances of liquid chromatography-(tandem) mass spectrometry in clinical and forensic toxicology- an update. Clin. Biochem. 2016;49:1051–1071. doi: 10.1016/j.clinbiochem.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 81.Meyer M.R., Maurer H.H. Review: LC coupled to low- and high-resolution mass spectrometry for new psychoactive substance screening in biological matrices – Where do we stand today? Anal. Chim. Acta. 2016;927:13–20. doi: 10.1016/j.aca.2016.04.046. [DOI] [PubMed] [Google Scholar]
- 82.Pasin D., Cawley A., Bidny S., Fu S. Current applications of high-resolution mass spectrometry for the analysis of new psychoactive substances: a critical review. Anal. Bioanal. Chem. 2017;409:5821–5836. doi: 10.1007/s00216-017-0441-4. [DOI] [PubMed] [Google Scholar]
- 83.Limban C., Nuţă D.C., Chiriţă C., Negres S., Arsene A.L., Goumenou M., Karakitsios S.P., Tsatsakis A.M., Sarigiannis D.A. The use of structural alerts to avoid the toxicity of pharmaceuticals. Toxicol. Rep. 2018;5:943–953. doi: 10.1016/j.toxrep.2018.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fabregat-Safont D., Sancho J.V., Hernández F., Ibáñez M. The key role of mass spectrometry in comprehensive research on new psychoactive substances. J. Mass Spectrom. 2021;56(7):e4673. doi: 10.1002/jms.4673. [DOI] [PubMed] [Google Scholar]
- 85.Di Trana A., Brunetti P., Giorgetti R., Marinelli E., Zaami S., Busardo F.P., Carlier J. In silico prediction, LC-HRMS/MS analysis, and targeted/untargeted data-mining workflow for the profiling of phenylfentanyl in vitro metabolites. Talanta. 2021;235:122740. doi: 10.1016/j.talanta.2021.122740. [DOI] [PubMed] [Google Scholar]
- 86.Seither J.Z., Hindle R., Arroyo-Mora L.E., DeCaprio A.P. Systematic analysis of novel psychoactive substances. I. Development of a compound database and HRMS spectral library. J. Forensic Chem. Toxicol. 2018;9:12–20. doi: 10.1016/j.forc.2018.03.003. [DOI] [Google Scholar]


