Significance
Neurons have long been considered the main active cell type within the circuitry of the enteric nervous system (ENS), giving rise to a neurocentric paradigm that has tended to overlook the role of enteric glia as key regulators of gut motility. Understanding how simple synapse-level interactions give rise to complex, network-level behaviors remains a fundamental problem in neurogastroenterology. We show that enteric glia and neurons interact in a cell- and network-specific manner and that enteric glia display functional heterogeneity based on selective signaling with particular neuron subtypes and circuits belonging to overlapping ascending, descending, and circumferential pathways of the ENS. Enteric glia thus function as logic gates to modify neural network activity through purinergic and cholinergic mechanisms.
Keywords: autonomic, glia, gastrointestinal, enteric nervous system, neuroglia
Abstract
Glia in the central nervous system exert precise spatial and temporal regulation over neural circuitry on a synapse-specific basis, but it is unclear if peripheral glia share this exquisite capacity to sense and modulate circuit activity. In the enteric nervous system (ENS), glia control gastrointestinal motility through bidirectional communication with surrounding neurons. We combined glial chemogenetics with genetically encoded calcium indicators expressed in enteric neurons and glia to study network-level activity in the intact myenteric plexus of the proximal colon. Stimulation of neural fiber tracts projecting in aboral, oral, and circumferential directions activated distinct populations of enteric glia. The majority of glia responded to both oral and aboral stimulation and circumferential pathways, while smaller subpopulations were activated only by ascending and descending pathways. Cholinergic signaling functionally specifies glia to the descending circuitry, and this network plays an important role in repressing the activity of descending neural pathways, with some degree of cross-inhibition imposed upon the ascending pathway. Glial recruitment by purinergic signaling functions to enhance activity within ascending circuit pathways and constrain activity within descending networks. Pharmacological manipulation of glial purinergic and cholinergic signaling differentially altered neuronal responses in these circuits in a sex-dependent manner. Collectively, our findings establish that the balance between purinergic and cholinergic signaling may differentially control specific circuit activity through selective signaling between networks of enteric neurons and glia. Thus, enteric glia regulate the ENS circuitry in a network-specific manner, providing profound insights into the functional breadth and versatility of peripheral glia.
The nervous system controls essential body functions through neuronal circuits that display exquisite temporal and spatially precise regulation. An emerging body of evidence suggests that glia, which respond to neurotransmitters and release substances that modulate neurotransmission (1, 2), are responsible for at least some of this precision. Glial recruitment by synaptic activity was once considered a general phenomenon that reflects metabolic demands in active neural networks (3, 4). While this may be partially true, more recent data show that signaling in networks of neurons and glia is unexpectedly precise. Astrocytes in particular have emerged as circuit-specific cells that tune neurotransmission on a synapses-by-synapse basis (5–8). These observations provide strong support for the concept that, rather than acting in a purely supportive role, glia serve integral regulatory functions in synaptic circuits. Yet, whether the circuit-specific attributes of astrocytes represent highly evolved functions that emerge in certain circuits in the brain or reflect a more general role of glia in synaptic signaling is not understood.
The enteric nervous system (ENS) is the largest and most complex division of the peripheral nervous system. “Brain-like” neurocircuits composed of enteric neurons and glia are intrinsic to the intestine and function to control reflexive activities of the digestive tract such as patterns of intestinal movement (9). The neuronal component of the enteric circuitry is well described, and the characteristics of intrinsic sensory neurons (primary afferent neurons), interneurons, and motor neurons that compose enteric motor circuits are known (10). The basic organization of enteric neurons into functional circuits that control peristalsis is also understood and involves activation of intrinsic primary afferent neurons, which recruit ascending excitatory and descending inhibitory pathways that evoke contractions above and relaxations below the point of stimulation, respectively (10). Although less understood, enteric glia are involved in enteric motor circuits and modulate reflex strength through bidirectional communication with enteric neurons (11–13). During reflexive activities, enteric neurons recruit enteric glia (11, 12), which release transmitters that influence neurotransmission (13, 14). Enteric glia are both necessary (14) and sufficient (13) to control the strength of enteric motility reflexes, but whether subpopulations of enteric glia are devoted to specific synaptic pathways is unknown. Enteric glia are localized to synapses and are postsynaptic to enteric neurons, which places them in an optimal site for regulating the flow of information within the ENS (11, 12, 15). How these interactions ultimately modulate activity within distinct branches of the ENS reflex circuitry remains unclear.
The polarized arrangement of enteric synaptic pathways and the ability of isolated preparations to maintain their integrity offers an ideal system for studying the specificity of glial interactions in an integrated yet tractable network. Here, we leveraged this system to test the hypothesis that enteric glia function in a circuit-specific manner. To this end, we evoked activity in overlapping but distinct circuits and recorded calcium (Ca2+) responses elicited in the enteric neuron–glial networks involved using genetically encoded Ca2+ indicators. We combined this approach with glial chemogenetics to assess whether enteric glia exert specific effects on separate neural pathways and used selective drugs to manipulate cholinergic and purinergic transmitter systems. Our data show that subpopulations of enteric glia are committed to specific ascending or descending neural networks that control intestinal motility. A large subpopulation of glia activated by circumferential pathway stimulation likely represent glial recruitment by intrinsic primary afferent neurons. Furthermore, glial activation exerts differential effects on nearby overlapping functional circuit pathways. The dedication of glia to a particular enteric neurocircuit depends, at least in part, on cholinergic and purinergic transmitter systems that constrain subsets of glia to a particular pathway, effects that are sex dependent. Therefore, bidirectional enteric glia–neuron signaling selectively occurs between specific populations of enteric glia and neurons within distinct functional networks.
Results
The ENS controls intestinal motility through neuronal circuitry composed of polarized ascending excitatory and descending inhibitory nerve pathways (16) (Fig. 1A). Enteric glia sense (11, 12) and modulate (13, 14) enteric neuron activity, but whether they do so in a circuit-specific manner is unclear. To determine whether enteric glia display selectivity in their responsiveness to neurons associated with ascending, descending, or circumferential pathways, we used focal electrical stimulation to activate overlapping synaptic pathways and recorded responses in associated neuron–glia networks of Wnt1Cre;GCaMP5g-tdT mice. We tested the selectivity of glia-to-neuron signaling by combining this approach with glial chemogenetics in Wnt1Cre;GCaMP5g-tdT;GFAP-hM3Dq mice, which express a modified Gq/11 protein-coupled DREADD (designer receptor exclusively activated by designer drug) that is activated by clozapine-N-oxide (CNO).
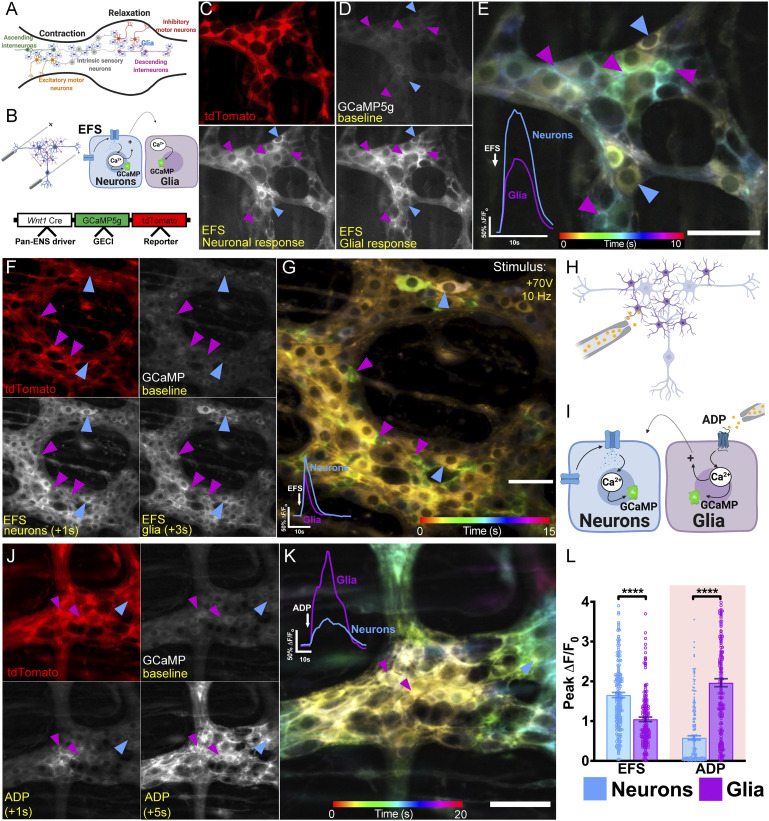
Fig. 1.
Bidirectional enteric neuron–glia communication. (A) The ENS circuitry controlling gastrointestinal motility is composed of ascending and descending networks of neurons and glia. Intrinsic sensory neurons initiate reflex motility, synapsing onto neurons belonging to ascending and descending pathways. The descending circuitry promotes relaxation, while the ascending circuitry promotes contraction. (B) Experimental paradigm demonstrating neuron-to-glia communication (Top Inset) and genotypic schematic demonstrating expression of a genetically encoded Ca2+ indicator (GECI) in ENS cells under the control of the Wnt1 neural-crest promoter (Bottom Inset). (C) tdTomato expression is predominantly glial in this model (magenta arrowheads) (SI Appendix, Fig. S1). (D) GCaMP signals at baseline and following EFS, which evokes a fast Ca2+ response in neurons (blue arrowheads) that is followed by glial responses (magenta arrowheads). (E) Temporal color-coding of enteric neuron and glial Ca2+ responses to +100 V EFS applied at 20 Hz. Note that neuronal responses are yellow, indicating early activation, and glial responses are green-blue, indicating later responses. Traces show averaged responses of neurons and glia to EFS within the ganglion. Glial responses are activated by neurons. (F) Stimulating the plexus with +70 V EFS (at 10 Hz), a physiological stimulus intensity, achieves a similar Ca2+ response profile but mitigates tissue hyperexcitability. (G) Color-coded temporal projection depicting EFS-mediated Ca2+ responses at +70 V. Blue arrowheads indicate neurons, and magenta arrowheads indicate glia. Representative tracings showing fold change in average neuronal and glial fluorescence in 40 cells taken from a single ganglion, with similar results obtained in all animals (Bottom Left Inset). (H) Experimental paradigm showing glia-to-neuron communication. (I) ADP directly activates enteric glia and can indirectly recruit enteric neurons. (J) Localized ADP application causes a dramatic glial Ca2+ response (magenta arrowheads). (K) Color-coded temporal projection of the ADP response, which propagates along the glial network (magenta arrowheads) and is accompanied by a neuronal recruitment phase (blue arrowheads). Representative Ca2+ tracings showing neuronal and glial fluorescence in 40 cells taken from a single ganglion, with similar results obtained in all animals (Top Left Inset). (L) Summary of average peak Ca2+ responses in neurons and glia consequent to stimulation with EFS or ADP. Bars depict means ± SEM (****P < 0.0001, unpaired Mann–Whitney U test).
Bidirectional Enteric Neuron–Glial Communication.
Before addressing the question of enteric glial network specificity, we determined the potential breadth of glial recruitment by enteric neurons. For these experiments, we used electrical field stimulation (EFS) as a broad neuronal stimulus and recorded evoked Ca2+ responses in networks of enteric neurons and glia in samples of myenteric plexus from the colons of Wnt1Cre;GCaMP5g-tdT mice (Fig. 1A). This mouse line expresses the genetically encoded Ca2+ indicator GCaMP5g in all enteric neurons and glia while allowing differentiation of neurons and glia based on tdTomato expression, which is high in glia and nearly undetectable in neurons (Fig. 1 B and C and SI Appendix, Figs. S1 and S2A). Immunolabeling experiments with antibodies against peripherin for enteric neurons and GFP for GCaMP5g show that all peripherin immunopositive neurons express GCaMP5g despite undetectable tdTomato fluorescence (SI Appendix, Fig. S1). It is unlikely that the low tdTomato expression in neurons is related to fluorescent protein–induced neuronal apoptosis since immunolabeling experiments with an antibody specific to the N terminus of the activated isoform of the proapoptotic factor Bax (Bax-NT) (17, 18) were comparable in wild-type and Wnt1Cre;GCaMP5g-tdT myenteric neurons (SI Appendix, Fig. S3). Therefore, this model allows recording from all subtypes of myenteric neurons and glia without complications from ongoing apoptotic processes.
Using an initial EFS pulse of 3 s, we observed a broad activation of neurons and glia within myenteric ganglia (Fig. 1 D and E). Unlike enteric neurons, the majority of enteric glia do not express voltage-sensitive sodium (19) or voltage-gated Ca2+ channels (19, 20). Enteric glia consequently do not possess the molecular machinery required to directly respond to EFS. Instead, enteric glia require intact intercellular signaling with neurons to undergo Ca2+ responses indicating that the effect of EFS on glia is indirect (12). Under physiological conditions, enteric glial background currents are dominated by large, passive outward potassium channel conductances that oppose electrical excitability within these cells (20). As such, even though a small minority of cells express sodium channels, these weak currents are only unmasked during states of stress in which intracellular pH drops aberrantly low and is not sufficient to generate transient electrical excitation as would be expected in an action potential (19). Thus, under these conditions, 31.3% of myenteric glia were recruited by neuronal activity. However, the peak neuronal Ca2+ response was extreme (ΔF/Fo = 3.5 ± 0.2; n = 22 cells), indicating that this stimulus is excessive and may push the system to the limits of physiological activity. In subsequent experiments, we titrated the EFS stimulus, identifying a 1-s pulse as an optimal stimulus that evoked responses that were still robust but which were more in line with physiological activity. Under these conditions, EFS elicited a 1.7 ± 0.1–fold increase in neuronal Ca2+ levels and activated 94.4 ± 4.4% of all studied neurons (n = 216 cells from six mice; Fig. 1 F and G). The onset of the neuronal Ca2+ response was immediate in all neurons visualized within the field of view, with peak responses occurring within 1 to 2 s following the application of EFS. Neuronal depolarization was followed immediately by a phase of glial recruitment that typically occurred ∼1 to 2 s after the onset of EFS. Glial Ca2+ responses were characterized by a 1.1 ± 0.1–fold increase in Ca2+, and 88.6 ± 3.4% of all glia were recruited by EFS (n = 182 cells from six mice). Consistent with findings from previous work (21), these data suggest that most enteric glia in the myenteric plexus can be recruited by neuronal activity.
We conducted similar experiments to assess the ability of enteric glia to recruit enteric neurons by stimulating enteric glia with adenosine diphosphate (ADP) and assessing Ca2+ responses in glia and neurons (Fig. 1 H and I). ADP stimulates robust Ca2+ responses in all myenteric glia in the mouse colon (22) and constitutes a well-known signaling pathway that culminates in gliotransmitter release through connexin-43 hemichannels (23). A subpopulation of myenteric neurons also expresses the P2ry1 receptor gene, but its functional relevance in neurons is not understood since mouse myenteric neurons in cell culture do not exhibit direct, P2Y1 receptor–dependent responses to ADP (22, 24) Therefore, ADP is a reasonably specific glial agonist in the myenteric plexus of the mouse colon. In agreement, focal application of ADP triggered an immediate 2.0 ± 0.1–fold increase in glial Ca2+ levels (Fig. 1 J and K). Most of the effect of ADP was restricted to glia; however, ADP also triggered responses in 42.5 ± 9.0% of all neurons within the ganglia. In these cases, the peak neuronal responses to ADP (ΔF/Fo = 0.6 ± 0.1) were substantially smaller in magnitude than their glial counterparts but exhibited overall similar kinetics best appreciated through the spatiotemporal projections that allow for a finer resolution of staggered Ca2+ responses (Fig. 1L). These data agree with prior studies and suggest activating glial Ca2+ signaling modulates activity in enteric neurons (13, 25).
Functionally Specified Networks of Neurons and Glia Control Intestinal Reflexes.
The results to this point show that enteric glia are responsive to neuronal activity in enteric motor circuits that control intestinal motility. The gut motor reflex comprises overlapping neural programs in which ascending pathways promote contractions, while descending pathways promote relaxation. In addition, circumferentially oriented neural pathways formed by projections that course orthogonally to the ascending–descending circuit axis also modulate neurocircuit activity. In general, fast synaptic communication between enteric neurons is mediated by cholinergic, nitrergic, or purinergic signaling pathways (26). A blockade of cholinergic signaling prevents contraction of orally situated gut segments (27), while inhibition of neuronal nitric oxide synthase abolishes relaxation of aborally situated gut segments (27, 28). This suggests that different neurotransmitter systems are at least somewhat functionally coupled to ascending or descending pathways even if their associations are not absolute. Thus, the polarized arrangement of the ENS provides an ideal paradigm for testing whether enteric glia distinguish between activity in nearby neural pathways. We tested this concept using focal tract stimulation (FTS) to independently activate ascending, descending, and circumferential pathways traveling through individual myenteric ganglia (Fig. 2 A and H) (29) and assessed the neuron–glial networks recruited under each condition.
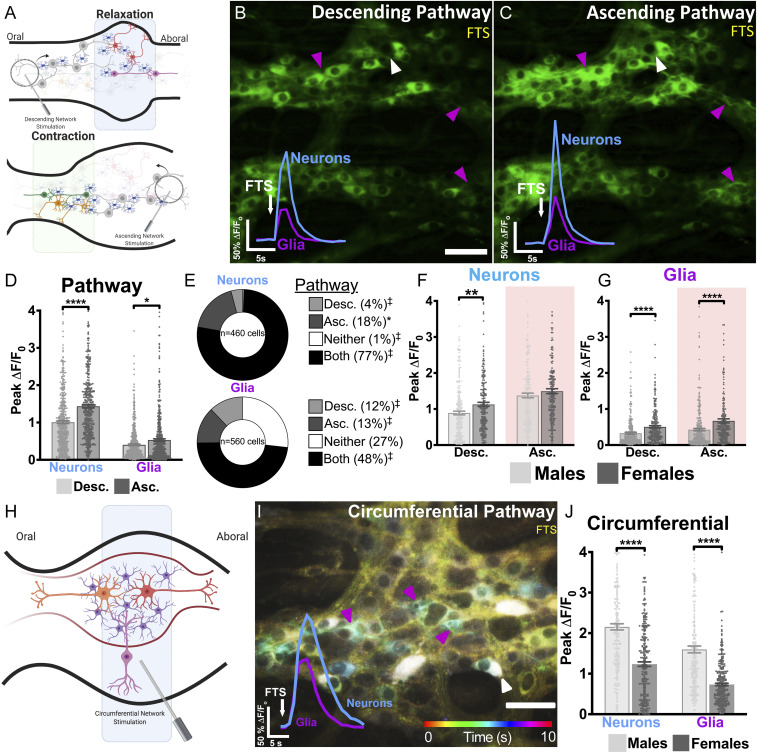
Fig. 2.
Functionally specified networks of neurons and glia in myenteric neurocircuits. (A) Schematic diagram illustrating how the peristaltic reflex was experimentally manipulated using a concentric bipolar focal tract stimulator. Stimulating ganglia from the oral direction activates the descending neural circuitry associated with gut relaxation (Top). Applying the same stimulus from the aboral side activates the ascending circuit pathway controlling gut contraction (Bottom). Although not explicitly shown here, some populations of neurons and glia are functionally associated with both pathways. (B and C) Representative images depicting Ca2+ responses provoked by descending or ascending FTS. Descending and ascending FTS stimulate distinct populations of neurons (white arrowhead) and glia (magenta arrowhead). Representative time courses (Bottom Left Insets) demonstrate the average Ca2+ response evoked in the same population of neurons and glia. (D) Compared with the descending pathway, ascending synaptic activity categorically provokes larger Ca2+ responses in both neurons and glia. (E) Ascending and descending FTS recruit functionally distinct subpopulations of neurons and glia. Some neurons and glia are activated by both ascending and descending FTS (“Both”), whereas fewer neurons are exclusively activated by ascending (“Asc.”) or descending (“Desc.”) FTS alone. If randomly distributed between these functional classes, ∼25% of cells would be associated with each category. ‡P < 0.0001 and *P < 0.05 by two-tailed χ2 test comparing observed values with these expected proportions. (F and G) In the descending pathway, both neurons and glia are more robustly activated in females than males. In the ascending pathway, glia but not neurons are more robustly activated in females than males. (H) Schematic diagram showing how the circumferential pathway was activated by placing a focal tract stimulator on fiber tracts leading to the ganglion in the circumferential axis. (I) Color-coded temporal image displays Ca2+ responses in myenteric neurons and glia over time (10 s) triggered by circumferential FST. Traces show averaged responses of neurons and glia to c-FST within the ganglion. (J) Sex differences in overall Ca2+ response magnitudes in the pool of enteric neurons and glia recruited by the circumferential pathway. Bars represent means ± SEM (*P < 0.05, **P < 0.01, and ****P < 0.0001, two-tailed unpaired Mann–Whitney U test).
Like EFS, FTS evoked an immediate-onset Ca2+ response in neurons that peaked within 1 s and was accompanied by a glial recruitment phase that peaked 1 to 3 s following application of FTS. In general, stimulating ascending pathways elicited more robust Ca2+ responses in both neurons (∼44% higher) and glia (∼13% higher) compared with the stimulation of descending pathways (Fig. 2 B–D, n = 460 neurons and n = 560 glia from 11 mice, comprising 5 females). The differences in Ca2+ response magnitudes between ascending and descending circuits were also associated with differences in the total numbers of neurons and glia activated within each pathway. Quantification of the number of neurons and glia recruited by FTS revealed that 77% of neurons were activated by both ascending and descending pathways, 18% were uniquely activated by ascending pathways, and only 4% were exclusively activated by descending pathways (Fig. 2E). Only 1% of neurons failed to respond to the stimulation of either ascending or descending pathways. Glial recruitment also differed between ascending and descending pathways. Nearly half (48%) of all glia were recruited by both ascending and descending pathways, while different, nonoverlapping subsets were specifically recruited by ascending (13%) and descending (12%) pathways (Fig. 2E). A fraction of glia (27%) failed to respond to pathway stimulation in either direction. Thus, nonoverlapping subpopulations of myenteric glia are functionally committed to either ascending or descending neural pathways. These basic rules of glial and neuronal pathway selectivity were constant in males and females, but the magnitudes of glial and neuronal Ca2+ responses differed between male and female mice (Fig. 2 F and G, n = 227 and n = 233 for neurons and n = 240 and n = 320 for glia from five females and six males, respectively).
We reasoned that the large population of glia that responded to stimulation in either direction could be explained by responses to multipolar intrinsic primary afferent neurons, while glia responding to unipolar interneurons projecting in ascending and descending directions underlie the fractions specific to those pathways. Intrinsic primary afferent neurons send projections that are organized in a circumferential direction and can be preferentially activated by stimulating circumferentially oriented fiber tracts (Fig. 2H) (30). Circumferential FTS (c-FTS) lead to a 1.7-fold greater calcium response in male neurons (n = 229 from three mice) compared with females (n = 275 from four mice, P < 0.0001; Fig. 2 I and J). In addition, glial responses to cFTS were 2.2-fold greater in males (n = 220 from three mice) compared with females (n = 312 from four mice, P < 0.0001). Despite these marked sex differences in overall Ca2+ response magnitudes, cFTS recruited equal proportions of glia in both male (36.8 ± 5.6%, n = 220 from three mice) and female mice (35.0 ± 6.7%, n = 307 from four mice; P = 0.789). The pool of glia recruited by this circumferential pathway is comparable in size to the fraction that responded to both ascending and descending pathways. Neuronal recruitment, however, was greater in males (63.9 ± 2.5%, n = 229 from three mice) compared with females (37.2 ± 8.1%, n = 143 from four mice; P = 0.01), suggesting that even though neuronal and glial Ca2+ responses are generally more robust in males, this only appears to affect neuronal but not glial recruitment. Therefore, sex governs the magnitude of signals transduced by pathways, although the portions of neurons and glia associated with the distinct pathways are similar in males and females.
Cholinergic and Purinergic Transmitters Dictate Functional Neuron–Glial Networks.
Fast excitatory synaptic transmission in the myenteric plexus is mediated by ACh and purines (31). In neurons, these transmitters contribute to the fast component of neurotransmission through activation of ionotropic nicotinic and purinergic receptors (32, 33), whereas glial responses to synaptic activity are driven through metabotropic M3 and P2Y1 receptors (23, 25). Cholinergic signaling mediates ascending excitatory pathway activity and is abolished by nicotinic receptor blockade, which does not affect descending neuronal pathway responses (34). In contrast, destruction of descending fiber tracts abolishes purinergic neuronal activity (34), highlighting broad roles for cholinergic and purinergic transmission in the motor circuitry of the gut (35). Given that our data show that ascending and descending circuits involve functionally specified networks of neurons and glia, we tested whether specific transmitters (i.e., ACh and purines) dictate glial pathway specificity.
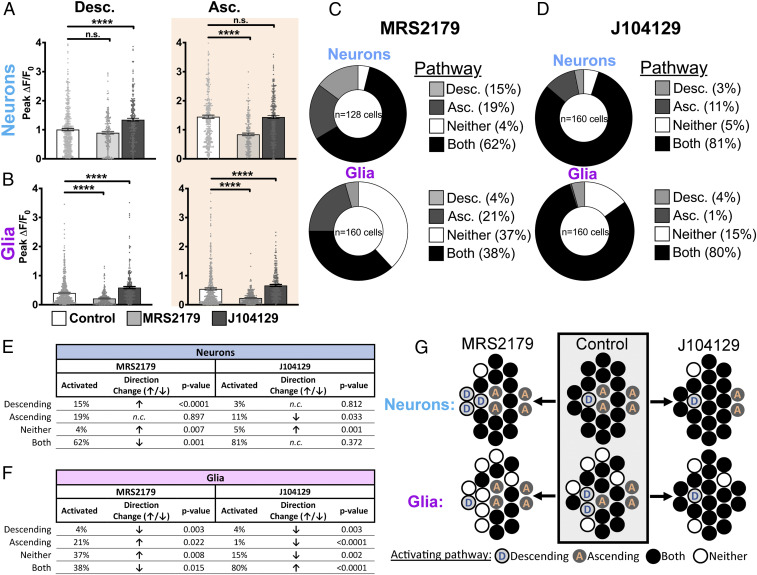
We first tested the effects of blocking purinergic neuron–glia communication using the P2Y1 receptor antagonist, MRS2179 (1 µM) (23, 36). MRS2179 specifically and robustly inhibited ascending neural circuits, reducing peak neuronal responses to FTS by ∼40% (P < 0.0001; n = 460 control versus n = 181 MRS2179; Fig. 3A) without affecting the magnitude of neuronal responses in descending pathways (P = 0.079). While a given cell can be recruited by one or both pathways, it is unclear whether P2Y1 blockade impacts ascending-only and dually recruitable (“Both”) neurons in the same way. As such, we studied alterations in neuronal responses at a network-level and found that blocking glial P2Y1 receptors increased the proportion of neurons activated within descending circuits from 4 to 15% relative to controls (Fig. 3 C and E; P < 0.0001) but not within ascending circuits (19 versus 18% in control, P = 0.897). Given the parallel reduction in neuronal recruitment by both pathways (62 versus 77% in control, P = 0.001), our findings indicate that MRS2179 selectively abolishes the ascending component of neurons normally wired to both pathways. This effect is consistent with the expansion in the pool of FTS-unresponsive glia (“Neither”) from 27% (control) to 37% (P = 0.008) (Fig. 3C). Interestingly, cFTS recruits ∼37% of glia under drug-free conditions, possibly encompassing the initial FTS-unresponsive population of glia and the additional 10% unmasked in the presence of MRS2179. Together, these findings indicate that glial recruitment by purines functions to enhance ascending elements of the neural networks involved in gut motility (summarized in Fig. 3G).
Fig. 3.
Purinergic and cholinergic signaling differentially regulate ascending and descending divisions of the myenteric circuitry. (A, Left) Under baseline conditions, descending FTS provokes an increase in neuronal Ca2+ levels that is largely unaffected by the purinergic blocker MRS2179 but is augmented by the M3 cholinergic blocker J104129. (Right) J104129 does not affect the magnitude of the neuronal response evoked by ascending FTS, whereas MRS2179 reduces this response by nearly 40% compared with controls (black versus white bars). (B, Left) Glial Ca2+ responses triggered by descending FTS are reduced by MRS2179 and augmented by J104129. (Right) Glial recruitment consequent to ascending FTS is attenuated by MRS2179 and augmented by J104129. All values represent means ± SEM. (C) In the presence of MRS2179, ∼15% of all responsive neurons were activated exclusively by descending FTS, while only 4% of glia were recruited by the same stimulus. In contrast, 19% of neurons were activated exclusively by ascending FTS, while 21% of glia were recruited by ascending FTS in the presence of MRS2179. (D) In the presence of J104129, 3% of neurons were activated by descending FTS, while 4% of glia were recruited by the same stimulus. Ascending FTS activated 11% of neurons within a myenteric ganglion in the presence of J104129, while 1% of glia were recruited under this condition. (E and F) Summary table comparing proportions of neurons that are activated (E) and glia that are activated and recruited (F) by directional FTS in the presence of MRS2179 or J104129. (G) Summary diagram recapitulating how cholinergic or purinergic blockade alters neuronal and glial population recruitment dynamics within each circuit pathway. MRS2179 reduces neuronal recruitment by ascending pathways, which expands the pool of neurons associated with the descending circuitry (white → blue circles). J104129 bolsters neuron recruitment within the descending pathway, expanding the pool of neurons functionally affiliated with both pathways (orange → white circles). n.s., not significant. ****P < 0.0001.
Next, we tested how perturbing glial activation by ACh affects ascending and descending neuron–glia networks. ACh is the predominant excitatory neurotransmitter in the ENS, and glial responses to ACh are mediated through M3 mAChR receptors (25). Therefore, we used the M3 mAChR antagonist J104129 (100 nM) to block glial recruitment without affecting fast cholinergic neurotransmission, which relies on nicotinic receptors. J104129 increased peak Ca2+ responses in neurons associated with descending pathways by 35% (P < 0.0001; n = 460 controls versus n = 239 J104129; Fig. 3 A, D, and E) without affecting neuronal responses in ascending pathways (P = 0.896). Similarly, J104129 increased the magnitude of glial Ca2+ responses triggered by descending pathways by 45% (n = 240 to 560) and ascending pathways by 22% (n = 240 to 480, P < 0.0001; Fig. 3 B, D, and F). Cholinergic blockade also altered neuronal and glial population recruitment dynamics. In the presence of J104129, 4% of glia were recruited by the descending pathway alone compared with 12% in controls (P = 0.003), whereas 1% were recruited by the ascending circuit compared with 13% in control conditions (P < 0.0001). These changes were accompanied by an increase in the number of glia that were responsive to both ascending and descending pathways from 48% under control conditions to 80% in the presence of J104129 (P < 0.0001). These results indicate that cholinergic transmission via the glial M3 receptor serves to constrain glial recruitment within the descending pathway and that loss of this inhibitory regulation permits glia to be activated by both ascending and descending circuits (Fig. 3G).
Glial Activation Designates Myenteric Neurons to Ascending or Descending Divisions of the Motor Reflex.
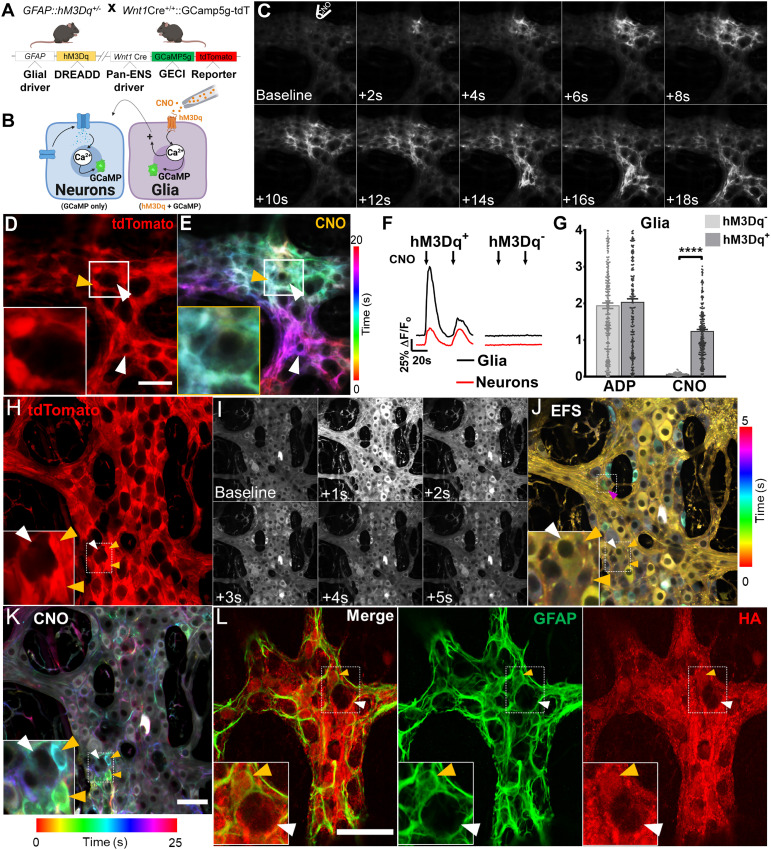
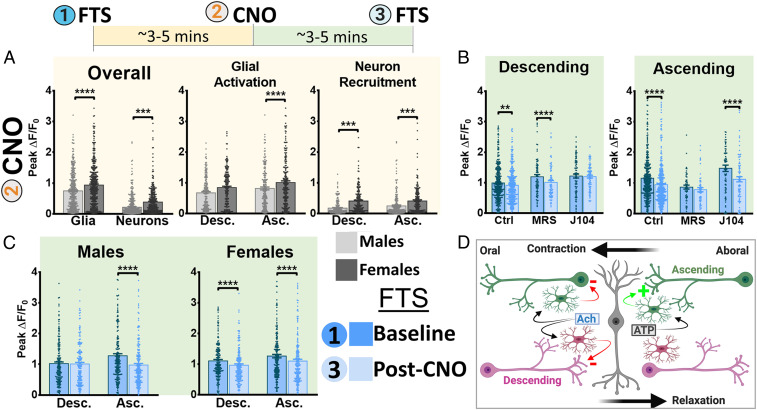
Our data show that endogenous cholinergic and purinergic transmitter systems regulate specific motor pathways through glial signaling. However, it is possible that some of the observed effects could be due to M3 or P2Y1 receptor expression by enteric neurons. To circumvent this, we developed a chemogenetic mouse model that allows selective activation of enteric glia and asked how glial activation affects neurons in ascending and descending motor pathways. To this end, we crossed the Wnt1Cre;GCaMP5g-tdT mice used in the experiments above with GFAP::hM3Dq mice (13) to yield triple-transgenic Wnt1Cre;GCaMP5g-tdT;GFAP::hM3Dq mice in which the chemogenetic actuator hM3Dq is expressed by enteric glia (Fig. 4 A and B), and GCaMP5g-tdT is expressed by both neurons and glia. Ca2+ imaging and immunohistochemistry data confirm that hM3Dq receptor expression was confined mainly to cell bodies and processes of enteric glia expressing tdTomato and GFAP (glial fibrillary acidic protein) (Fig. 4 C–E and L) and that focal CNO application produced robust Ca2+ responses in these same cells (ΔF/Fo = 1.2 ± 0.1, n = 219; Fig. 4 E–G), activating 69.9 ± 11% of all cells (13, 37). In this initial subset of experiments, CNO administration was accompanied by a robust Ca2+ response in glia and a smaller and fast neuronal response consistent with rapid activation of neuronal voltage-gated calcium channels following their stimulation (ΔF/Fo = 0.4 ± 0.03, n = 229; Fig. 4F), indicating that glia-to-neuron communication pathways remain preserved in this model. The initial response rate to CNO was characterized by a change in fluorescence intensity of 0.9 ± 0.1 ΔF/s in glia compared with 0.2 ± 0.1 ΔF/s in neurons, suggesting CNO evoked greater and faster changes in the intracellular calcium concentration in glia than neurons. Glial responses to CNO were a near perfect fit to classical GPCR response models, while neuronal responses showed a weaker correlation (CNO R-square = 0.6940 and 0.9661 for neurons and glia, respectively) (38), likely reflecting the direct effects of CNO on glia and subsequent effects on neurons. Subsequent glial responses to serial CNO application were lower likely due to the brief drug washout period. Similarly, the magnitudes of neuron and glial Ca2+ responses triggered by CNO were larger in females than males (n = 357 glia from four males versus 430 glia from four females; n = 356 neurons from four males versus 390 neurons from four females; P < 0.0001; Fig. 5A), indicating that the putative differences in Ca2+ handling dynamics observed in control animals are preserved. Next, we assessed the functional implications of glial activation on neuronal responses in ascending and descending pathways and investigated the role of cholinergic and purinergic transmission in this process. Peak Ca2+ responses to FTS in neurons wired in descending pathways were reduced by activating glia with CNO (8% overall reduction in paired responses, n = 335; P = 0.0012), suggesting that glial cholinergic signaling physiologically represses neuronal activity within the descending circuitry (Fig. 5 B, Left and SI Appendix, Fig. S5C). The inhibitory effect of glia on neurons in descending pathways was unaffected by purinergic blockade (P < 0.0001) but was abolished by J104129 (P = 0.69), suggesting that the subtle repressive effect of enteric glia on the descending pathway is mediated, in part, by M3 cholinergic signaling.
Fig. 4.
Effects of glial chemogenetic activation on myenteric networks. (A) Schematic diagram demonstrating mouse genotype. (B) Enteric glia are directly activated by direct application of CNO to the ganglion. (C) Still-frame montage depicting propagation of the CNO-induced Ca2+ response throughout the glial cell network, which is accompanied by eventual recruitment of enteric neurons within the vicinity. (D) tdTomato expression is highest in glia (SI Appendix, Fig. S1). (E) Color-coded temporal responses evoked by focal application of CNO overlap with glial tdTomato signals, confirming functional expression of the DREADD receptor in enteric glial cells (yellow arrowhead) but not enteric neurons (white arrowhead). Glial activation by CNO can nevertheless recruit neurons (lower white arrowhead), demonstrating intact glia-to-neuron recruitment pathways. (F) Representative tracings depict average Ca2+ response evoked by CNO in enteric glia (black tracings) and neurons (red tracings) in DREADD mice (Left) compared with DREADD-null littermates (Right). Time courses shown are typical of the response evoked by CNO in enteric glia. (G) Peak Ca2+ response evoked by glial agonists in DREADD-positive (hM3Dq+) mice and DREADD-null littermates (hM3Dq−). (H) In situ expression of native tdTomato signals within the myenteric plexus of DREADD mice, imaged by confocal fluorescence microscopy. (I) Time-lapse montage of EFS-induced Ca2+ responses. (J) Color-coded temporal projection of responses in I, with the topmost box depicting glial recruitment by neurons (magenta arrowhead) and preservation of neuro–glial communication pathways in this model. (Inset) Neuronal response to EFS (white arrowhead) and the absence of a response in glia (orange arrowheads). (K) Color-coded temporal responses to bath-applied CNO in enteric glia. CNO responses overlap with tdTomato signals and are absent in adjacent neurons located nearby. (L) Immunohistochemical staining of a fixed myenteric plexus from a DREADD mouse. The plexus was probed for GFAP expression and hemagglutinin (HA; red channel), which is primarily expressed in GFAP+ enteric glia (orange arrowheads; green channel). By comparison, enteric neurons (white arrowheads) express substantially lower levels of HA. ****P < 0.0001.
Fig. 5.
Reciprocal modulation of enteric glial and neuronal activity varies with ENS circuitry. (A, Top Inset) Myenteric ganglia were stimulated with either ascending or descending FTS before and after CNO application. (Bottom Left) Unpaired CNO-induced effects on glial activation and neuronal recruitment in male and female mice. CNO provokes a more robust Ca2+ response in female glia compared with male glia. Similarly, the magnitude of the neuronal Ca2+ response evoked by CNO-induced glial activation is greater in females than in males. (Bottom Middle and Bottom Right) Glial and neuronal CNO responses are further substratified according to circuit pathway. In females but not males, glia associated with the ascending pathway are more robustly activated by CNO. For females, neuronal recruitment by CNO is categorically more robust in both ascending and descending pathways. (B) Paired neuronal Ca2+ responses evoked by descending and ascending pathway activation prior to and following CNO treatment. Under control conditions, glial stimulation with CNO attenuates neuronal responses in both pathways. The CNO-induced reduction in neuronal recruitment is more pronounced in the ascending pathway than in the descending pathway. (Left) Pretreatment with the M3 cholinergic antagonist, J104129, abolishes this effect in the descending pathway. (Right) Pretreatment with the selective P2Y1 antagonist, MRS2179, preferentially blocks CNO-induced reductions in neuronal responses in the ascending pathway. (C) Substratifying the effects of CNO on neuronal pathway responses reveals a sex dependence in the effect of glial activation on neuronal responses. (Left) In males, glial activation with CNO attenuates the neuronal Ca2+ response in the ascending circuitry without affecting descending pathway responses. (Right) In females, glial activation by CNO attenuates neuronal responses in both network pathways. (D) Mechanistic paradigm highlighting pathway-specific roles of glial purinergic and cholinergic signaling. Neuronal recruitment within the descending circuitry is subject to glial inhibitory control in an M3-dependent manner. As such, a cholinergic blockade with J104129 unleashes neuronal recruitment by descending synaptic stimuli. Neurons formerly recruited by the ascending circuitry are subsequently activated by both pathways (see increase in baseline response in B, Left. In contrast, glial purinergic signaling preferentially bolsters neuronal responses within the ascending circuitry. Abrogating P2Y1 activation with MRS2179 uncouples neurons from this pathway (see decrease in baseline response in B, Right, allowing for unopposed activation by the descending circuitry). These effects are concordant with alterations in population recruitment dynamics described in Fig. 3G.
Paired neuronal responses to FTS were also reduced in the ascending pathway following glial activation with CNO (by 19%; P < 0.0001; n = 366, Fig. 5 B, Right), suggesting that cholinergic signaling from the descending pathway exerts cross-inhibitory effects on ascending circuits (Fig. 5 B and D). J104129 prevents inhibition of the descending but not ascending network, suggesting that glial cholinergic signaling is necessary for repression of one pathway but redundant in the other (post-CNO ΔF/Fo = 1.1 ± 0.1, P < 0.0001, and n = 80). Baseline neuronal activity was 25% lower in the presence of MRS2179 compared with control conditions (P = 0.0002; n = 53), demonstrating that purinergic signaling plays an important role in bolstering neuronal recruitment in the ascending circuit. Interestingly, glial stimulation with CNO in the presence of MRS2179 did not further repress neuronal Ca2+ responses compared with effects of CNO alone (P = 0.11). This “floor effect,” which was noted previously (Fig. 3A), explains why CNO-mediated glial activation could not further attenuate neuronal responses in the ascending circuit and suggests that loss of glial purinergic signaling is sufficient to abolish a large component of ascending neural circuit excitability (Fig. 5 B and D).
In keeping with the repressive effects of glial cholinergic signaling on neural circuit activity, J104129 augmented CNO-driven glial Ca2+ responses by 25% (n = 159; P < 0.0001; SI Appendix, Fig. S5B), whereas MRS2179 reduced CNO-driven glial Ca2+ responses by nearly half (n = 148; P < 0.0001). The decreased glial responsiveness in the presence of MRS2179 might reflect a reduction in feed-forward glial network recruitment through purinergic P2Y1 activation (12), while the increased responses observed in the presence of J104129 is consistent with loss of glial repressive activity at the network level (14).
The effects of CNO on each pathway highlight a regulatory role for glial cholinergic signaling in the ENS, particularly with regards to the modulation of descending circuit activity. Our initial findings demonstrate that some of the network-specific effects of glia are sex dependent (Fig. 2 F, G, and I), although differences in Ca2+ response magnitudes did not amount to differences in the number of glia or neurons recruited between males and females. In anticipation of additional possible sex differences, we further substratified the effects of CNO on neuronal activation within each pathway by sex. The repressive effect of cholinergic activation was absent within the descending pathway in males (P = 0.723; n = 182) but persisted in females (n = 193; P < 0.0001), suggesting that the descending cholinergic circuitry in particular is subject to sex-dependent effects. Taken together, these data demonstrate that glial activation through purinergic or cholinergic mechanisms alters neuronal recruitment in ascending and descending pathways and that these neurotransmitters serve opposing roles in the myenteric circuitry (Fig. 5D).
Discussion
Bidirectional communication between enteric glia and neurons occurs in synaptic pathways in the gut, but how neuron–glia communication affects intestinal motor function by shaping network-level activity is not understood. Here, we show that different sets of enteric glia are recruited by ascending, descending, and circumferential neural pathways and that glial activation has differential reciprocal effects that are neural pathway specific. Enteric glia and neurons interact in a cell- and network-specific manner, and enteric glia display functional heterogeneity based on selective signaling with neuronal subtypes belonging to overlapping ascending, descending, and circumferential motility pathways of the ENS. These observations suggest that some enteric glial cells, like astrocytes (5–8), are circuit-specific cells.
Although the properties of separate enteric neuronal subtypes are generally well characterized (16), very little is known about how these neuronal subtypes and enteric glia interact within organized networks to coordinate gastrointestinal motility. As such, decoding how enteric glia modulate neural circuit activity in the ENS is a critical step toward formulating a comprehensive model of how gastrointestinal motor circuits control gut motility. Glia that exhibit specific responses to ascending or descending circuit pathways are likely responding to unipolar interneurons, while glia responding to both pathways are likely recruited by intrinsic primary afferent neurons. Intrinsic primary afferent neurons possess multipolar projections, some which are circumferentially arranged (30). Using circumferential pathway stimulation, a large component of enteric glia could be activated, suggesting that the glial population responsive to both ascending and descending circuit activity might in fact be those wired to intrinsic primary afferent neurons within the myenteric circuitry (30). Additionally, one striking observation that also emerged from our study is that ascending synaptic activity provoked more robust Ca2+ responses than descending synaptic activity (29). Although cholinergic signaling is by no means constrained to the ascending circuit pathway, the ascending circuitry primarily utilizes acetylcholine as an excitatory neurotransmitter to activate targets located in orally situated ganglia (16, 26). In contrast, neurotransmission within the descending neural circuitry is more varied in that it also involves nitrergic (39) and purinergic release (26, 34, 35). The recruitment of these inhibitory transmitter systems might therefore constitute a major effector function of cholinergic signaling within the descending pathway and account for the lower Ca2+ response in this circuit. However, ascending tract stimulation also recruited greater numbers of neurons and glia, which is consistent with a prior report showing relatively greater neuronal recruitment following stimulation of ascending pathways in vivo (29). For this reason, we suspect that neuro–glial wiring of ascending and descending circuits is fundamentally different. While our data ultimately support both possibilities, the salient interpretation remains that some enteric glia are functionally dedicated to specific microcircuits within the ENS.
Patterns of intestinal motility are controlled by synaptic signaling within the ENS, mediated in part by cholinergic and purinergic neurotransmitters (32, 35). Purines and ACh are responsible for fast synaptic transmission between enteric neurons (35) and neuron-to-glia communication in myenteric motor networks (12, 25). Our data show that the neuron-to-glia arm of cholinergic and purinergic transmission functions to establish motor networks and regulate their activity. Purines released by enteric neurons act on P2Y1 receptors expressed by neighboring enteric glia (12, 40), and mechanisms downstream of glial activation regulate colonic contractility (14). This effect is mediated 5through specific positive interactions of ascending neural networks and restrictive effects that constrain activity within the descending pathway. It should be noted that the decrease in the magnitude of ascending neuronal Ca2+ responses observed in the presence of purinergic blockade is seemingly at odds with population recruitment dynamics. However, this apparent discrepancy can be reconciled by the fact that the main effect of MRS2179 is a selective reduction in neuronal recruitment within the ascending circuitry, which accounts for the reduction in neurons recruited by “Both” pathways; this latter recruitment, in turn, can only be accomplished by descending pathways. Since ascending signaling is abolished by MRS2179 but descending signaling proceeds unperturbed, the result is an apparent increase in the proportion of neurons associated with the descending circuitry that does not reflect a bona fide change in the number of neurons wired to this pathway. These data likely explain the observed decrease in neurogenic contractions and colonic motility under conditions in which intercellular glial signaling mediated by connexin-43 is perturbed (14). Neuronal ACh release and glial recruitment during synaptic communication also enhance colonic contractility (25) but apparently do so through inhibitory effects. Here, the actions of ACh at glial M3 receptors constrain glial recruitment within the descending pathway and dampen activity in descending networks. Thus, disrupting either purinergic or cholinergic mechanisms of glial recruitment can lead to widespread changes in neural networks. Together, these observations show that receptor-dependent glial signaling promotes intestinal motility by enhancing ascending motor pathways, dampening descending motor pathways, and restricting the neural network recruited during a stimulus.
Our gain-of-function DREADD model enabled us to activate enteric glia and thereby dissect the glia-to-neuron arm of the enteric motor reflex. We found that CNO was sufficient to directly recruit glia and, thus, neurons through circuit-specific interactions that were differentially mediated by glial purinergic and cholinergic signaling. Glial recruitment remains spatially restricted between neighboring populations of glia and neurons at neuro–glial units (40). Although enteric glia are coupled by gap junctions (41), the propagation of Ca2+ waves through this pathway is mainly observed under pathological conditions (40). The fact that Ca2+ responses evoked by local CNO application exclusively propagated in the direction of drug perfusion confirms that glial recruitment was due to a direct effect of DREADD receptor activation rather than junctional coupling. It is therefore likely that the unique spatial arrangement of glia at synaptic foci, where neuro–glial units are formed, is responsible for the integration of discrete cellular responses into complex regulatory programs at a network level.
While the consistent result of glial activation appears to be a global reduction in neuronal activity, how this effect is achieved within each pathway is governed by the neurotransmitters involved. In the presence of MRS2179, neuronal responses in the ascending pathway are reduced to such an extent that glial activation fails to attenuate neuronal responses any further. This “floor effect” is absent in the descending neural circuit, where glial activation with CNO continues to repress neuronal responses. This further highlights a glial purinergic bias in the ascending pathway. In contrast, glial repression of descending neural activity is abolished by J104129 but preserved within the ascending pathway. This highlights a glial cholinergic bias in the descending pathway, though it is unclear why cross-inhibition of the ascending pathway is unaffected. As is the case for hippocampal astrocytes, however, it is possible that enteric glia decode some frequency or amplitude domain of neuronal firing in mounting specific interactions with the ascending circuitry (42). Alternatively, it is also possible that glial cross-inhibition of the ascending neural circuitry is orchestrated through synaptically distinct networks that are altogether different from those that mediate repression of the descending circuitry (Fig. 5D).
Functional gastrointestinal and motility disorders display a strong female bias (43, 44), and enteric glial ablation models show sex-dependent effects on motility (45). Our data suggest that these effects could be due to intrinsic functional differences in female glia and neurons. The factors responsible for driving these differences are not yet clear but hormones could be responsible. Similar positive effects of hormones on cholinergic neurotransmission are observed in the rat forebrain, where estrogen promotes choline acetyltransferase expression (46, 47). Recent single-cell transcription data also show that expression of the type 1 estrogen receptor (ESR1) is enriched in intrinsic primary afferent neurons (IPANs) and possibly ascending excitatory interneurons (24). IPANs drive activity in ascending and descending reflex pathways as well as activity in the corresponding population of glia that responded to both pathways and likely correspond to the neuronal population that responded to stimuli in either direction in this study. Estrogen-dependent effects on IPANs could, therefore, have large effects on neuron and glial activity in enteric motor pathways.
Our data nevertheless indicate that enteric glial cholinergic signaling modulates some degree of activity within the ascending neural network of both male and female mice. This suggests that even though certain aspects of neurocircuit communication differ in a sex-dependent manner (i.e., descending circuit regulation; Fig. 5 C, Left), the motility effects of glial cholinergic transmission controlled by the ascending pathway may be more conserved. In such a case, pathology affecting the ascending circuit might compromise motility similarly between males and females while those affecting the descending circuit might manifest with sex-dependent alterations in motility. In line with this finding, studies that altered glial function through pharmacological (48) or chemogenetic (25) means rather than ablation demonstrated motility effects in both males and females. Furthermore, the process of glial ablation (49) depletes glia in a nonuniform manner (45) and may drive compensatory remodeling events in surrounding neurons that are poorly understood but otherwise amenable to the effects of sex hormones per se (50, 51).
Our data show that circuit-specific enteric glia regulate neural pathways that control intestinal motility. We postulate that cholinergic recruitment of glia represses the descending circuit pathway and that its concurrent and exclusive cross-inhibition of the ascending circuitry can protect against states of ENS hyperactivation. Circumferential pathway activation of IPANs likely recruits a functionally distinct subset of glia that is otherwise indistinguishable from those recruited by both ascending and descending circuit pathways. We suspect that glial P2Y1 activation positively modulates ascending neural circuit activity and that this can offset the inhibitory effects of purinergic signaling, which dominates the descending neural pathway (35). The complexity that emerges at the network level far outstrips what can be gleaned from a neurocentric description of the ENS circuitry in which neurons are thought to act in isolation. This elegant “division of labor” between cholinergic and purinergic signaling provides valuable insights into how two distinct cell populations can form a connectome (52) in which shared neurotransmitters orchestrate complex physiological processes such as motility.
Materials and Methods
Mouse Models.
All animal experiments were conducted in accordance with the standards established by the NIH Guide for the Care and Use of Laboratory Animals (53) and were approved by the Michigan State University Institutional Animal Care and Use Committee.
Male and female mice were used between 10 to 14 wk of age for all experiments. Mice expressing the genetically encoded Ca2+ sensor, GCaMP5g, in enteric neurons and glia were generated by breeding B6;129S6-Polr2aTn(pb-CAG-GCaMP5g,-tdTomato)Tvrd/J mice (The Jackson Laboratory, stock no. 024477; RRID: IMSR_JAX:024477) with B6.Cg-E2f1Tg(Wnt1-cre)2Sor/J mice (The Jackson Laboratory, stock no. 022501; RRID: IMSR_JAX:022501). The offspring, herein referred to as Wnt1Cre;GCaMP5g-tdT mice, were used for experiments. This line was crossed with GFAP::hM3Dq+/− mice (gift from Ken McCarthy, University of North Carolina at Chapel Hill, NC; RRID: MMRRC_042286-UNC) to generate triple-transgenic Wnt1Cre;GCaMP5g-tdT;GFAP::hM3Dq mice (see Fig. 4 for functional and immunohistochemical characterization) (25). Mouse genotypes were verified commercially by Transnetyx, Inc. (SI Appendix, Materials & Methods).
Tissue Isolation, Dissection, Fixation, and Preparation.
Whole-mount circular muscle myenteric plexus (CMMP) preparations, used for live-cell imaging experiments, were obtained by microdissecting the mucosa and longitudinal muscle to expose the myenteric plexus. Tissue orientation was carefully maintained during the dissection process. The resulting CMMP preparation maintains most of the functional connectivity in the myenteric plexus as well as connections with target tissue (smooth muscle) (SI Appendix, Materials & Methods).
Field and FTS.
EFS was used to drive broad neuronal depolarization within enteric networks. In these experiments, tissues were stimulated with +100 V voltage (20 Hz) or with +70 V voltage (10 Hz) using two platinum wires positioned on either side of the tissue preparation. FTS was used to stimulate interganglionic myenteric nerve bundles in experiments designed to assess ascending, descending, and circumferential synaptic pathways. In these experiments, a custom-assembled tungsten bipolar electrode (World Precision Instruments Inc.) was placed on an interganglionic nerve bundle (54) located a distance of one field of view (FOV) immediately oral, aboral, or lateral to the ganglion of interest (SI Appendix, Materials & Methods).
Local Drug Application.
Filamented borosilicate glass microelectrodes were back filled with drug-containing Kreb’s buffer. Drugs were then puffed onto the surface of ganglia using very slight positive pressure applied with a 1-mL syringe connected to a pipette holder. During recordings, enteric glial responses were evoked with either ADP (3 mM) or CNO (100 µM), which were locally applied (SI Appendix, Materials & Methods).
Ca2+ Imaging Studies.
Myenteric ganglia were visualized through a 20× wide-field water-immersion objective lens (Olympus XLUMPLFLN20xW, 1.0 numerical aperture) on an upright Olympus BX51WI fixed-stage microscope (Center Valley). Ganglia were identified based on their plexiform appearance and high expression of the fluorescent tdTomato reporter in resident glial cells. Illumination for fluorescence imaging was supplied by a DG4 Xenon light source (Sutter Instrument) (SI Appendix, Materials & Methods).
Data Analysis and Statistics.
Recordings were analyzed using Fiji software (NIH) as previously described (55). The tdT channel image obtained at the outset of the experiment was used to confirm the identity and location of glial cells for all experiments and to generate corresponding regions of interest (ROIs). Glial ROIs were autodetected using the resultant binary masks and were more specific for glial cells (SI Appendix, Figs. S4 and S6A). We confirmed the functional validity of this approach by comparing the magnitude of the peak Ca2+ responses evoked by application of ADP (SI Appendix, Fig. S6B), a potent agonist of the P2Y1 receptor, which is highly expressed on enteric glia (14, 36). Neuronal ROIs were manually selected after the exclusion of autogenerated glial ROIs based on morphology and responsiveness to electrical stimuli as depicted in spatiotemporal response maps (Fig. 1E and SI Appendix, Fig. S4). Final data values were quantified as fold change in mean cellular fluorescence intensity relative to baseline florescence intensity (ΔF/Fo) within a given cell. An analysis of Ca2+ response time-course kinetics was conducted according to the equation describing the rise and fall to baseline time-course profile (38). For population recruitment studies evaluating the proportions of neurons or glia activated by FTS, a positive response was defined as a peak fluorescence value >5 SDs above the average baseline Ca2+ level measured within a given cell for a fixed recording duration.
Consistent with prior functional studies on population responses, sample size refers to the number of cells that responded under given experimental conditions, and the number of mice utilized is also indicated where appropriate (56). Only cells recorded within the FOV were used for analysis. For all experiments, ∼30 to 40 neurons and a comparable number of glia were studied per ganglion that was imaged, with at least one to two ganglia utilized per mouse under each recording condition (SI Appendix, Materials & Methods).
Supplementary Material
Acknowledgments
B.D.G. receives support from Grants R01DK103723 and R01DK120862 from the National Institute of Diabetes and Digestive and Kidney Diseases of the NIH. The content is solely our responsibility and does not necessarily represent the official views of the NIH.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2025938118/-/DCSupplemental.
Data Availability
All study data are included in the article and/or SI Appendix.
References
- 1.Gundersen V., Storm-Mathisen J., Bergersen L. H., Neuroglial transmission. Physiol. Rev. 95, 695–726 (2015). [DOI] [PubMed] [Google Scholar]
- 2.Gulbransen B. D., Enteric glia. Colloq. Ser. Neuroglia Biol. Med. Physiol. Dis. 1, 1–70 (2014). [Google Scholar]
- 3.Colón-Ramos D. A., Margeta M. A., Shen K., Glia promote local synaptogenesis through UNC-6 (netrin) signaling in C. elegans. Science 318, 103–106 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christopherson K. S., et al., Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell 120, 421–433 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Martín R., Bajo-Grañeras R., Moratalla R., Perea G., Araque A., Circuit-specific signaling in astrocyte-neuron networks in basal ganglia pathways. Science 349, 730–734 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Martin-Fernandez M., et al., Synapse-specific astrocyte gating of amygdala-related behavior. Nat. Neurosci. 20, 1540–1548 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chai H., et al., Neural circuit-specialized astrocytes: Transcriptomic, proteomic, morphological, and functional evidence. Neuron 95, 531–549.e9 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu X., et al., Reducing astrocyte calcium signaling in vivo alters striatal microcircuits and causes repetitive behavior. Neuron 99, 1170–1187.e9 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spencer N. J., Hu H., Enteric nervous system: Sensory transduction, neural circuits and gastrointestinal motility. Nat. Rev. Gastroenterol. Hepatol. 17, 338–351 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fung C., Vanden Berghe P., Functional circuits and signal processing in the enteric nervous system. Cell. Mol. Life Sci. 77, 4505–4522 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Broadhead M. J., Bayguinov P. O., Okamoto T., Heredia D. J., Smith T. K., Ca2+ transients in myenteric glial cells during the colonic migrating motor complex in the isolated murine large intestine. J. Physiol. 590, 335–350 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gulbransen B. D., Sharkey K. A., Purinergic neuron-to-glia signaling in the enteric nervous system. Gastroenterology 136, 1349–1358 (2009). [DOI] [PubMed] [Google Scholar]
- 13.McClain J. L., Fried D. E., Gulbransen B. D., Agonist-evoked Ca2+ signaling in enteric glia drives neural programs that regulate intestinal motility in mice. Cell. Mol. Gastroenterol. Hepatol. 1, 631–645 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McClain J., et al., Ca2+ responses in enteric glia are mediated by connexin-43 hemichannels and modulate colonic transit in mice. Gastroenterology 146, 497–507.e1 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gabella G., Fine structure of the myenteric plexus in the guinea-pig ileum. J. Anat. 111, 69–97 (1972). [PMC free article] [PubMed] [Google Scholar]
- 16.Furness J. B., The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 9, 286–294 (2012). [DOI] [PubMed] [Google Scholar]
- 17.Berens H. M., Tyler K. L., The proapoptotic Bcl-2 protein Bax plays an important role in the pathogenesis of reovirus encephalitis. J. Virol. 85, 3858–3871 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hetz C., et al., Bax channel inhibitors prevent mitochondrion-mediated apoptosis and protect neurons in a model of global brain ischemia. J. Biol. Chem. 280, 42960–42970 (2005). [DOI] [PubMed] [Google Scholar]
- 19.Hanani M., et al., Patch-clamp study of neurons and glial cells in isolated myenteric ganglia. Am. J. Physiol. Gastrointest. Liver Physiol. 278, G644–G651 (2000). [DOI] [PubMed] [Google Scholar]
- 20.Broussard D. L., Bannerman P. G. C., Tang C.-M., Hardy M., Pleasure D., Electrophysiologic and molecular properties of cultured enteric glia. J. Neurosci. Res. 34, 24–31 (1993). [DOI] [PubMed] [Google Scholar]
- 21.Delvalle N. M., et al., Communication between enteric neurons, glia, and nociceptors underlies the effects of tachykinins on neuroinflammation. Cell. Mol. Gastroenterol. Hepatol. 6, 321–344 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gomes P., et al., ATP-dependent paracrine communication between enteric neurons and glia in a primary cell culture derived from embryonic mice. Neurogastroenterol. Motil. 21, 870–e62 (2009). [DOI] [PubMed] [Google Scholar]
- 23.Brown I. A. M., McClain J. L., Watson R. E., Patel B. A., Gulbransen B. D., Enteric glia mediate neuron death in colitis through purinergic pathways that require connexin-43 and nitric oxide. Cell. Mol. Gastroenterol. Hepatol. 2, 77–91 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeisel A., et al., Molecular architecture of the mouse nervous system. Cell 174, 999–1014.e22 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delvalle N. M., Fried D. E., Rivera-Lopez G., Gaudette L., Gulbransen B. D., Cholinergic activation of enteric glia is a physiological mechanism that contributes to the regulation of gastrointestinal motility. Am. J. Physiol. Gastrointest. Liver Physiol. 315, G473–G483 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costa M., Brookes S. J. H., Hennig G. W., Anatomy and physiology of the enteric nervous system. Gut 47, iv15–iv19 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tonini M., Costa M., A pharmacological analysis of the neuronal circuitry involved in distension-evoked enteric excitatory reflex. Neuroscience 38, 787–795 (1990). [DOI] [PubMed] [Google Scholar]
- 28.Grider J. R., Jin J. G., Distinct populations of sensory neurons mediate the peristaltic reflex elicited by muscle stretch and mucosal stimulation. J. Neurosci. 14, 2854–2860 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith-Edwards K. M., et al., Extrinsic primary afferent neurons link visceral pain to colon motility through a spinal reflex in mice. Gastroenterology 157, 522–536.e2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brookes S. J. H., Song Z. M., Ramsay G. A., Costa M., Long aboral projections of Dogiel type II, AH neurons within the myenteric plexus of the guinea pig small intestine. J. Neurosci. 15, 4013–4022 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.LePard K. J., Messori E., Galligan J. J., Purinergic fast excitatory postsynaptic potentials in myenteric neurons of guinea pig: Distribution and pharmacology. Gastroenterology 113, 1522–1534 (1997). [DOI] [PubMed] [Google Scholar]
- 32.Galligan J. J., LePard K. J., Schneider D. A., Zhou X., Multiple mechanisms of fast excitatory synaptic transmission in the enteric nervous system. J. Auton. Nerv. Syst. 81, 97–103 (2000). [DOI] [PubMed] [Google Scholar]
- 33.Decker D. A., Galligan J. J., Cross-inhibition between nicotinic acetylcholine receptors and P2X receptors in myenteric neurons and HEK-293 cells. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G1267–G1276 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Johnson P. J., Shum O. R., Thornton P. D. J., Bornstein J. C., Evidence that inhibitory motor neurons of the guinea-pig small intestine exhibit fast excitatory synaptic potentials mediated via P2X receptors. Neurosci. Lett. 266, 169–172 (1999). [DOI] [PubMed] [Google Scholar]
- 35.LePard K. J., Galligan J. J., Analysis of fast synaptic pathways in myenteric plexus of guinea pig ileum. Am. J. Physiol. 276, G529–G538 (1999). [DOI] [PubMed] [Google Scholar]
- 36.Gulbransen B. D., et al., Activation of neuronal P2X7 receptor-pannexin-1 mediates death of enteric neurons during colitis. Nat. Med. 18, 600–604 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agulhon C., et al., Modulation of the autonomic nervous system and behaviour by acute glial cell Gq protein-coupled receptor activation in vivo. J. Physiol. 591, 5599–5609 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoare S. R. J., Tewson P. H., Quinn A. M., Hughes T. E., Bridge L. J., Analyzing kinetic signaling data for G-protein-coupled receptors. Sci. Rep. 10, 12263 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gould T. W., Swope W. A., Heredia D. J., Corrigan R. D., Smith T. K., Activity within specific enteric neurochemical subtypes is correlated with distinct patterns of gastrointestinal motility in the murine colon. Am. J. Physiol. Gastrointest. Liver Physiol. 317, G210–G221 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boesmans W., et al., Structurally defined signaling in neuro-glia units in the enteric nervous system. Glia 67, 1167–1178 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maudlej N., Hanani M., Modulation of dye coupling among glial cells in the myenteric and submucosal plexuses of the guinea pig. Brain Res. 578, 94–98 (1992). [DOI] [PubMed] [Google Scholar]
- 42.Covelo A., Araque A., Neuronal activity determines distinct gliotransmitter release from a single astrocyte. eLife 7, e32237 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oshima T., Miwa H., Epidemiology of functional gastrointestinal disorders in Japan and in the world. J. Neurogastroenterol. Motil. 21, 320–329 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang F.-Y., et al., Prevalence of functional gastrointestinal disorders in Taiwan: Questionnaire-based survey for adults based on the Rome III criteria. Asia Pac. J. Clin. Nutr. 21, 594–600 (2012). [PubMed] [Google Scholar]
- 45.Rao M., et al., Enteric glia regulate gastrointestinal motility but are not required for maintenance of the epithelium in mice. Gastroenterology 153, 1068–1081.e7 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gibbs R. B., Fluctuations in relative levels of choline acetyltransferase mRNA in different regions of the rat basal forebrain across the estrous cycle: Effects of estrogen and progesterone. J. Neurosci. 16, 1049–1055 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singer C. A., McMillan P. J., Dobie D. J., Dorsa D. M., Effects of estrogen replacement on choline acetyltransferase and trkA mRNA expression in the basal forebrain of aged rats. Brain Res. 789, 343–346 (1998). [DOI] [PubMed] [Google Scholar]
- 48.Nasser Y., et al., Role of enteric glia in intestinal physiology: Effects of the gliotoxin fluorocitrate on motor and secretory function. Am. J. Physiol. Gastrointest. Liver Physiol. 291, G912–G927 (2006). [DOI] [PubMed] [Google Scholar]
- 49.Bush T. G., et al., Fulminant jejuno-ileitis following ablation of enteric glia in adult transgenic mice. Cell 93, 189–201 (1998). [DOI] [PubMed] [Google Scholar]
- 50.D’Errico F., et al., Estrogen receptor β controls proliferation of enteric glia and differentiation of neurons in the myenteric plexus after damage. Proc. Natl. Acad. Sci. U.S.A. 115, 5798–5803 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ro S., Hwang S. J., Muto M., Jewett W. K., Spencer N. J., Anatomic modifications in the enteric nervous system of piebald mice and physiological consequences to colonic motor activity. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G710–G718 (2006). [DOI] [PubMed] [Google Scholar]
- 52.Swanson L. W., Lichtman J. W., From Cajal to connectome and beyond. Annu. Rev. Neurosci. 39, 197–216 (2016). [DOI] [PubMed] [Google Scholar]
- 53.National Research Council , Guide for the Care and Use of Laboratory Animals (National Academies Press, Washington, DC, ed. 8, 2011). [Google Scholar]
- 54.Johnson S. M., Katayama Y., Morita K., North R. A., Mediators of slow synaptic potentials in the myenteric plexus of the guinea-pig ileum. J. Physiol. 320, 175–186 (1981). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schindelin J., et al., Fiji: An open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McClain J. L., Gulbransen B. D., The acute inhibition of enteric glial metabolism with fluoroacetate alters calcium signaling, hemichannel function, and the expression of key proteins. J. Neurophysiol. 117, 365–375 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All study data are included in the article and/or SI Appendix.