Significance
The extent to which pre-Columbian societies in Amazonia occupied and significantly altered the tierra firme (nonflooded, nonriverine) forest environment is an enduring question and the subject of a current debate. Our research addresses these issues in tierra firme forests of northeastern Peru. We present phytolith and charcoal data indicating the forests were not cleared, farmed, or otherwise significantly altered in prehistory. Frequencies of hyperdominant palm species did not increase through time, indicating prehistoric human exploitation contributed little to the species’ disproportionate abundance in the modern flora. Our data indicate that forest resurgence and fire decrease upon the tragic consequences of European contact were not so widespread as to have been principal contributors to the onset of the “Little Ice Age.”
Keywords: Amazonia, vegetation history, phytoliths, charcoal
Abstract
This paper addresses an important debate in Amazonian studies; namely, the scale, intensity, and nature of human modification of the forests in prehistory. Phytolith and charcoal analysis of terrestrial soils underneath mature tierra firme (nonflooded, nonriverine) forests in the remote Medio Putumayo-Algodón watersheds, northeastern Peru, provide a vegetation and fire history spanning at least the past 5,000 y. A tree inventory carried out in the region enables calibration of ancient phytolith records with standing vegetation and estimates of palm species densities on the landscape through time. Phytolith records show no evidence for forest clearing or agriculture with major annual seed and root crops. Frequencies of important economic palms such as Oenocarpus, Euterpe, Bactris, and Astrocaryum spp., some of which contain hyperdominant species in the modern flora, do not increase through prehistoric time. This indicates pre-Columbian occupations, if documented in the region with future research, did not significantly increase the abundance of those species through management or cultivation. Phytoliths from other arboreal and woody species similarly reflect a stable forest structure and diversity throughout the records. Charcoal 14C dates evidence local forest burning between ca. 2,800 and 1,400 y ago. Our data support previous research indicating that considerable areas of some Amazonian tierra firme forests were not significantly impacted by human activities during the prehistoric era. Rather, it appears that over the last 5,000 y, indigenous populations in this region coexisted with, and helped maintain, large expanses of relatively unmodified forest, as they continue to do today.
More than 50 y ago, prominent scholars argued that due to severe environmental constraints (e.g., poor natural resources), prehistoric cultures in the Amazon Basin were mainly small and mobile with little cultural complexity, and exerted low environmental impacts (1, 2). Contentious debates ensued and have been ongoing ever since. Empirical data accumulated during the past 10 to 20 y have made it clear that during the late Holocene beginning about 3,000 y ago dense, permanent settlements with considerable cultural complexity had developed along major watercourses and some of their tributaries, in seasonal savannas/areas of poor drainage, and in seasonally dry forest. These populations exerted significant, sometimes profound, regional-scale impacts on landscapes, including with raised agricultural fields, fish weirs, mound settlements, roads, geometric earthworks called geoglyphs, and the presence of highly modified anthropic soils, called terra pretas or “Amazonian Dark Earths” (Fig. 1) (e.g., refs. 3–15).
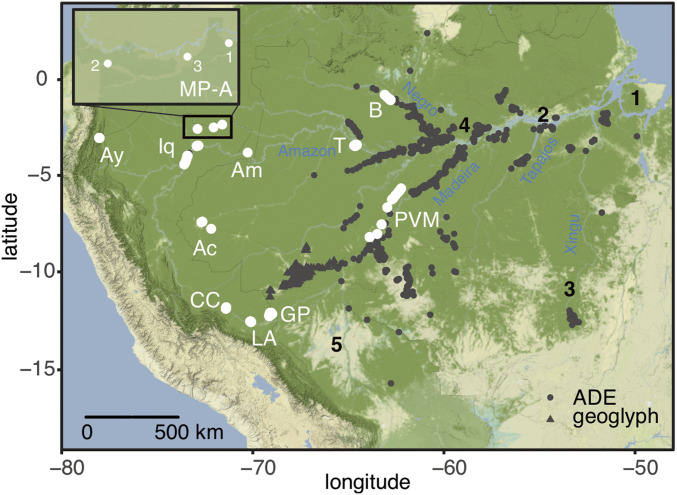
Fig. 1.
Location of study region (MP-A) and other Amazonian sites discussed in the text. River names are in blue. The black numbers represent major pre-Columbian archaeological sites with extensive human alterations (1, Marajó Island; 2, Santarém; 3, Upper Xingu; 4, Central Amazon Project; 5, Bolivian sites) (3, 5–10, 14, 15). ADE, terra preta locations (e.g., refs. 19 and 20); triangles are geoglyph sites (6, 8). The white circles are terrestrial soil locations previously studied by Piperno and McMichael (29, 31–33, 54) (Ac, Acre; Am, Amacayacu; Ay, Lake Ayauchi; B, Barcelos; GP, lakes Gentry-Parker; Iq, Iquitos to Nauta; LA, Los Amigos; PVM, Porto Velho to Manaus; T, Tefe).
An important, current debate that frames this paper centers not on whether some regions of the pre-Columbian Amazon supported large and complex human societies, but rather on the spatial scales, degrees, and types of cultural impacts across this continental-size landscape. Some investigators drawing largely on available archaeological data and studies of modern floristic composition of selected forests, argue that heavily modified “domesticated” landscapes were widespread across Amazonia at the end of prehistory, and these impacts significantly structure the vegetation today, even promoting higher diversity than before (e.g., refs. 14–21). It is believed that widespread forms of agroforestry with planted, orchard-like formations or other forest management strategies involving the care and possible enrichment of several dozens of economically important native species have resulted in long-term legacies left on forest composition (e.g., refs. 14–22). Some (20) propose that human influences played strong roles in the enrichment of “hyperdominant” trees, which are disproportionately common elements in the modern flora (sensu ref. 23). Some even argue that prehistoric fires and forest clearance were so spatially extensive that post-Columbian reforestation upon the tragic consequences of European contact was a principal contributor to decreasing atmospheric CO2 levels and the onset of the “Little Ice Age” (24, 25).
However, modern floristic studies are often located in the vicinity of known archaeological sites and/or near watercourses (26). Many edible trees in these studies are early successional and would not be expected to remain as significant forest elements for hundreds of years after abandonment. Historic-period impacts well-known in some regions to have been profound have been paid little attention and may be mistaken for prehistoric legacies (26–28). Moreover, existing phytolith and charcoal data from terrestrial soils underneath standing tierra firme forest in some areas of the central and western Amazon with no known archaeological occupations nearby exhibit little to no evidence for long-term human occupation, anthropic soils, agriculture, forest clearing or other significant vegetation change, or recurrent/extensive fires during the past several thousand years (Fig. 1) (29–33). Even such analyses of terrestrial soils of lake watersheds in western Amazonia known to have been occupied and farmed in prehistory revealed no spatially extensive deforestation of the watersheds, as significant human impacts most often occurred in areas closest to the lakes (Fig. 1) (34). Furthermore, vast areas have yet to be studied by archaeologists and paleoecologists, particularly the tierra firme forests that account for 95% of the land area of Amazonia.
To further inform these issues, we report here a vegetation and fire history spanning 5,000 y derived from phytolith and charcoal studies of terrestrial soils underneath mature tierra firme forest in northeastern Peru. Phytoliths, the silica bodies produced by many Neotropical plants, are well preserved in terrestrial soils unlike pollen, and are deposited locally. They can be used to identify different tropical vegetational formations, such as old-growth forest, early successional vegetation typical of human disturbances including forest clearings, a number of annual seed and root crops, and trees thought to have been cultivated or managed in prehistory (e.g., refs. 29–33 and 35).
The Study Region, Its Ecology, and Peoples
The Medio Putumayo-Algodón (MP-A) study area lies in a remote region in northeastern-most Peru (Fig. 1 and SI Appendix, Fig. S1). In 2016, a team of researchers led by the Field Museum’s Rapid Biological and Social Inventory Program (FMRI) visited the previously unexplored area to characterize its flora, fauna, soils, and geology, together with its potential for future conservation efforts (36). The region borders three existing protected areas to the south, forming part of a corridor of conserved area and indigenous lands. Annual precipitation is about 3,000 mm and vegetational zones include floodplain, tierra firme forests, peatland forests, and palm swamps. A number of indigenous communities live on the banks of the Putumayo and Algodón rivers (SI Appendix, Fig. S1). Team members visited four of these, establishing collaborative relationships for documenting social organization and natural resource use, and for long-term participation in conservation efforts (37). Today, the communities practice subsistence agriculture in chacras and house gardens along the riverbanks, fish, and hunt bushmeat, mainly peccaries and paca (38) (SI Appendix, Supplementary Text 1). No archaeological work has been carried out in the area. Ceramics found at the edge of a large oxbow lake located north of one of the FMRI’s study campsites, camp 1, indicate human activities there at an unknown time.
The study region currently houses an intact and diverse tierra firme forest on poor soils and free of roads, large-scale deforestation, and other anthropogenic disturbances. The terrain is rolling, with gentle slopes and terraces and few to no steep slopes. In this forest, the FMRI surveyed vegetation, carried out tree inventories, and collected multiple soil cores for phytolith and charcoal analysis at three campsites: Quebrada Bufeo (camp 1), Medio Algodón (camp 2), and Bajo Algodón (camp 3) (SI Appendix, Fig. S1) (Methods). About 1,300 vascular plants and 550 tree species were recorded during the inventory and it is estimated that over 3,000 vascular plants and 1,900 tree species occur in the entire region (39, 40). The FMRI rarely found adult rubber trees (Hevea spp.) and no old trees scarred for rubber extraction were seen, suggesting that the forest around these campsites had not been subject to severe effects of the rubber boom that took place in this region from AD 1850–1920. The vegetation surveys and tree inventories provide an important interpretive framework for the phytolith analyses.
Results
Vegetational History and Chronology.
Phytolith and charcoal analyses were carried out on a total of 10 soil cores: four from camp 2, and three each from camps 1 and 3 (Methods). Direct 14C dating of phytolith assemblages consisting of all phytoliths present in selected soil levels was carried out (SI Appendix, Table S1). A date of 4950–4647 cal BP from the deepest soils at 60–80 cm b.s. (below the soil surface) from camp 2 indicates the bottom of the sequence there and likely elsewhere reaches to about the last 5,000 y (hereafter, all centimeter depths are b.s.). In the uppermost 30 cm combined from camps 1 and 2, dates are 2154–1941 and 454–300 cal BP, respectively. Phytoliths combined from 30–60 cm at camp 1 returned an age of 2952–2780 cal BP, while dates from 40–70 cm combined were 2863–2751 cal BP at camp 2 and 3255–3025 cal BP at camp 3 (SI Appendix, Supplementary Text 2, for further discussion of phytolith dates). Phytoliths from lower-dated levels are older than those above, indicating stratigraphic and chronological separation. As individual 10-cm levels had to be combined to achieve sufficient phytolith carbon for a 14C determination, we can expect the middle and lower of the 10-cm levels combined for dating to contain phytoliths older than those above. Phytolith ages from MP-A are consistent with 14C determinations from terrestrial soils in other regions of Amazonia, where the upper 20–30 cm of soil typically yielded ages from the last 2,000–2,500 y and determinations from 60–80 cm were 5,000–7,000 y old (29, 31). Surface or “pinch” soils collected at MP-A (Methods) also allow us to discriminate modern and recent historical events—likely not more than the past 100 to 200 y—from those of the pre-Columbian period.
For the most robust identification and interpretation of vegetational and agricultural history, phytoliths from the lowland Neotropics are segregated into two size classes by analyzing the silt and sand fractions of soils separately (29, 31, 32, 35). In the MP-A silt fractions (5- to 50-µm-sized phytoliths), spheroidal phytoliths from arboreal species (exclusive of palms, discussed below) and shrubs indicative of closed, mature forest dominate the records of all localities, accounting for >70–90% of the sum (Fig. 2; Methods). Native annual crops that can be identified with phytoliths were absent; i.e., maize (Zea mays L.), squashes (Cucurbita spp.), bottle gourd [Lagenaria siceraria (Molina) Standl.], manioc (Manihot esculenta Crantz), lerén [Goeppertia allouia (Aubl.) Borchs. and S. Suárez, formerly Calathea allouia Lindl.], and arrowroot (Maranta arundinacea L.). Phytoliths from the Old World introductions rice (Oryza sativa L.), banana (Musa paradisiaca L.), and sugar cane (Saccharum officinarum L.) were also absent, indicating they were not grown on the tierra firme forest during the past 400–500 y.
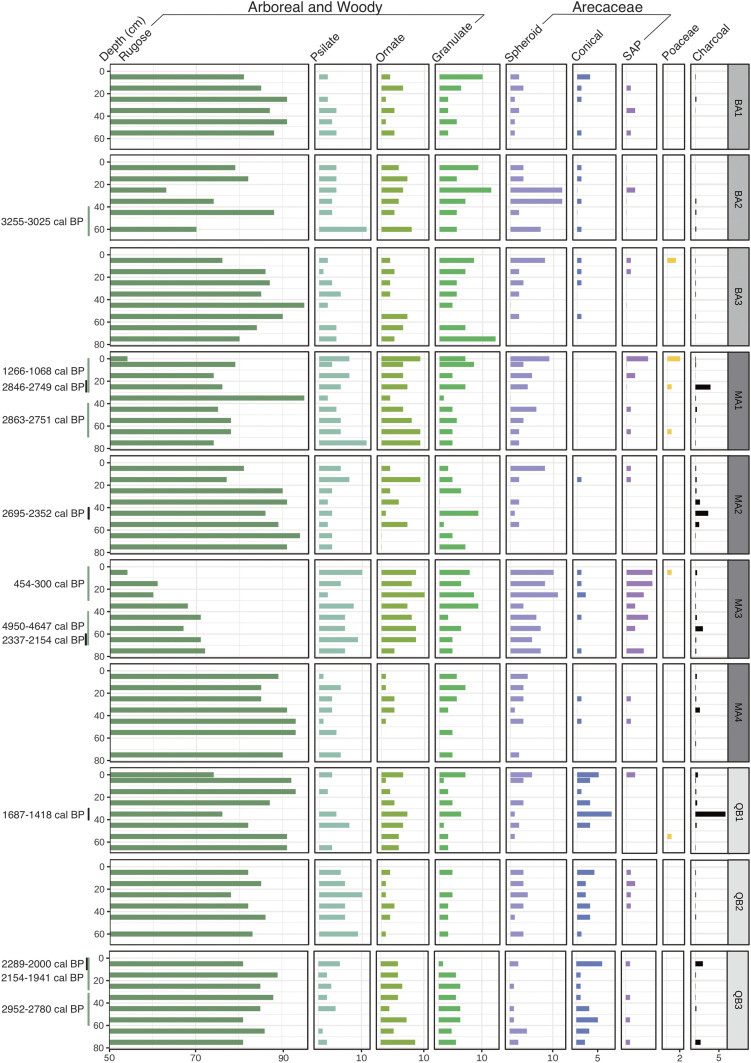
Fig. 2.
Phytolith percentages from MP-A fine silt fractions. QB, Quebrada Bufeo (camp 1); MA, Medio Algodón (camp 2); and BA, Bajo Algodón (camp 3). The black bars on the y axis are charcoal dates and the category charcoal at the top represents charcoal volume (in cubic millimeters per cubic centimeter). If multiple charcoal samples fitted within a single depth-interval of one phytolith sample, then charcoal concentrations were averaged. The green bars on the y axis are phytolith dates. See also SI Appendix, Supplementary Text 3 for further description of phytolith types and frequencies.
Grasses, along with other taxa of weedy, disturbed growth associated with human disturbance and forest clearings, such as Heliconia and the Cyperaceae, are often absent from the bottom to top of the sequences, even upon extended scanning of thousands of phytoliths per slide (Fig. 2). When observed they were often single occurrences in one or two levels of a core sequence. The rare grasses present were predominantly bamboos along with types that occur across the family and are common in bamboos. Forest understory herbs from the Costaceae, Marantaceae, and Zingiberaceae were rare to absent throughout the time spans of the records (SI Appendix, Supplementary Text 3A).
Spheroidal rugose, psilate, and ornate phytoliths that dominate the assemblages derive predominantly from a number of trees and shrubs in the flora (Fig. 2 and SI Appendix, Figs. S2 and S3 and Supplementary Text 3B). The Chrysobalanaceae, one of the dominant tree families at MP-A, likely contributed the significant majority of the rugose forms, as the family produces them in high number, including genera common in the tree inventories such as Couepia, Licania, Leptobalanus, Moquilea, Hymenopus, and Parinariopsis (the latter four genera were formerly grouped in the genus Licania) (Dataset S1). The phytoliths are produced far less frequently outside of the Chrysobalanaceae in a number of woody genera of mostly trees in the MP-A flora. They are as follows: Aspidosperma (Apocynaceae); Dodecastigma and Hevea (Euphorbiaceae); Eschweilera (Lecythidaceae); Huberodendron, Matisia, Ochroma, Pachira, and Pseudobombax (Malvaceae); Vismia (Hypericaceae); and Sorocea (Moraceae) (35, 41–43). Eschweilera and Aspidosperma, both with hyperdominant species, are much more common in the forest than the other non-Chrysobalananceae genera and can have been expected to contribute many of the non-Chrysobalanaceae rugose spheroids to the soils. These two genera are characterized by having slow-growing tree species, also suggesting past vegetation stability and little past human-mediated disturbance.
The spheroidal ornate category encompasses phytoliths from a diverse array but limited number of woody plants at MP-A, nearly all of them trees (SI Appendix, Fig. S3). Psilate spheroids are commonly found in the same genera as rugose and ornate forms, and also in a significant number of other woody taxa. Ornate and psilate forms are produced in far lower frequencies in plants than are rugose types, accounting for their lower percentages in the soils (41). Granulate spheroids are mainly found in trunk wood and twigs of a number of genera at MP-A (41) (see SI Appendix, Supplementary Text 3B for further identification and explanation of the spheroidal types). Largely unvarying frequencies of the different spheroids throughout the sequences point to a stable forest structure and diversity.
The Arecaceae (palms), prolific and diagnostic phytolith producers, are among the 10 most speciose families in the MP-A modern flora (40). Palm phytolith frequencies across the sampled localities range from 1 to 20% and are often <10% (Fig. 2). The spheroidal palm subtype is found in genera such as Attalea, Phytelephas, and Lepidocaryum reported today from the MP-A region. Judging from the tree inventories, the majority—at least from the surficial samples—likely derived from Attalea spp. (Dataset S1). Palm phytolith percentages sometimes vary in a core sequence, and at most sampling locales there is no clear trend for an increase through time. Of particular interest are two palm species in the MP-A region, Oenocarpus bataua Mart. and Euterpe precatoria Mart., both major human dietary items in the present and past (44), and also hyperdominants (23). The two palms contribute a subtype of spheroidal phytolith present in a few other genera, but that can be discriminated from them on the basis of their much larger sizes (32, 41, 45) (SI Appendix, Fig. S4 and Supplementary Text 3C). O. bataua also has phytoliths larger than in the two other Oenocarpus species reported from the region, O. minor and O. mapora. Euterpe precatoria is the only species of the genus reported from the MP-A region and is found there mainly on poorly drained soils, not in the tierra firme forest. One individual of E. precatoria was identified in the tree inventory, at camp 1, for example (Dataset S1). O. bataua, on the other hand, is among the five most common trees in the inventories and the most common palm by far.
The phytoliths in question are most conservatively identified in the soils as Oenocarpus and/or Euterpe spp., and are present in nearly all sampling levels from every locality (SI Appendix, Tables S2–S4). However, mean and maximum phytolith size rule out O. mapora and O. minor, and although the species, O. bacaba, has a phytolith size overlapping that of O. bataua (41), it is not reported from the MP-A region. In light of these factors and the ecological preference of E. precatoria, it is likely that O. bataua contributed the significant majority of the phytoliths to the records, with the possibility that E. precatoria is also present. Notably, as would pertain to both species, there is no trend for an increase through time of the phytoliths in subsurface levels (SI Appendix, Tables S2–S4). This indicates prehistoric populations, if shown in future research to have been present in the area, did not increase the frequencies of these palms through management or cultivation.
A comparison of the tree inventory data and soil phytolith frequencies demonstrates that phytoliths effectively track variations in palm frequencies on a landscape (see refs. 32, 46, and 47 for similar findings). In the inventories, individuals of O. bataua number 16/ha at camp 1, 50/ha at camp 2, and 31/ha at camp 3 (Dataset S1). Camp 2 also had the highest frequencies of Oenocarpus/Euterpe phytoliths, both in the surficial sample with a percentage of 5% and in its core 3, where its percentages often reached 4–6%. As discussed above, most phytoliths likely can be attributed to O. bataua. Taking into account the camp 1 surficial soil with 2% of these phytoliths, it can be suggested that low to rare frequencies (e.g., <1–1%) found in all subsurface levels from every core except camp 2, core 3, represent O. bataua densities of not more and probably often considerably less than 16/ha that were stable through time.
Of considerable interest also is the conical palm phytolith subtype that may derive from a number of genera in the region including Astrocaryum, Bactris, Iriartea, and Socratea. Two genera contain important dietary palms present at the MP-A; Bactris gasipaes Kunth (pehibaye or peach palm), a species domesticated in Amazonia and well-spread in prehistory, and Astrocaryum chambira Burret. Two other species at the MP-A are hyperdominants, A. murumuru Mart. and A. jauari. Mart. The phytoliths these taxa produce show no trend for an increase through time (Fig. 2), again providing no evidence that prehistoric cultures exploited the palms to the extent of causing increases in their abundances.
Considerable information on forest composition and change through time also derives from the sand fractions of soils that contain phytoliths of a size of 50–250 µm mainly derived from arboreal and other woody growth (SI Appendix, Supplementary Text 4A). The Asteraceae, excellent herbaceous indicators of human disturbance, and Trema micrantha, a common tree of early secondary woody growth including fallows, also produce abundant, diagnostic sand-sized forms, providing further insight into vegetation disturbance history. Results indicate a diversity of taxa present in the vegetation inventory are represented throughout the sequences (SI Appendix, Fig. S5). They include trees from the genera Protium (Burseraceae), Tapura and/or Stephanopodium (Dichapetalaceae), Hirtella (Chrysobalanaceae), and Guatteria, Oxandra, and Unonopsis (Annonaceae) (SI Appendix, Figs. S6–S10). Elongate and baculate phytoliths occur in a small number of unrelated genera of trees, shrubs, and vines present today at the MP-A, such as Amanoa (Phyllanthaceae), Mabea (Euphorbiaceae), Protium, Stephanopodium, Licania, Hirtella, and Brosimum (Moraceae), and Mendoncia (Acanthaceae) (SI Appendix, Figs. S11–S13) (SI Appendix, Supplementary Text 4B). Due to the overlap of phytolith morphologies in different taxa and presence of more than one type in the same species, more than one phytolith of the elongate and baculate types may be present from the same taxon and/or multiple taxa may be represented. A number of phytolith types from presently unknown woody taxa are also present throughout the sequences (SI Appendix, Figs. S14–S17). Absent from the records is Trema micrantha and nearly absent are the Asteraceae, which occurred at frequencies of 1% at 0–10 cm at camp 1, core 3, and <1% at 0–10 cm at camp 3, core 3. The taxonomic diversity indicated by these records does not appear to increase or decrease through time. Rather, various taxa are maintained throughout the sequences indicating stable forest structure and composition.
Fire History.
Fire was relatively rare at MP-A (Fig. 2), and 14C ages were obtained from all charcoal fragments large enough for dating (SI Appendix, Table S1). At camp 1, fires occurred from 2300–2000 and 1700–1400 cal y BP. At camp 2, fires occurred between ca. 2800–2100 cal BP, and at camp 3, a single date of 2000–1800 cal BP could be obtained. The fires around MP-A do not appear to have been recurrent or synchronous across the three camps and none are evidenced in the last 1,400 y.
Because phytolith dates are obtained from combined stratigraphic levels and do not represent single points in time as do charcoal ages from discrete fragments in a single level, it is often unrealistic to expect close dating conformity between charcoal and phytoliths from the same soil levels. Charcoal fragments may also move in soils to a greater extent than phytoliths, which chemically bind to clays (see SI Appendix, Supplementary Text 2 for more information). The combined charcoal and phytolith records suggest that fires were not of sufficient intensity to cause shifts in the vegetation.
Discussion
As in other regions of western Amazonia and in the central Amazon where terrestrial soils from underneath tierra firme forest were studied with phytolith and charcoal records (Fig. 1) (29–32), there is no evidence over a 5,000-y period for slash-and-burn or slash-and-mulch cultivation using major annual seed and root crops, or cultural activities of other kinds resulting in significant forest clearings. Adding to these findings is the absence of anthropic soils and material cultural remains such as ceramics and stone tools in our studied soils (Methods). Our vegetational and fire history from this previously unstudied, remote region joins the increasing body of evidence that deforestation and fires during the prehistoric period and subsequent vegetation recovery upon European Contact were not so widespread and intense as to have contributed significantly to decreasing atmospheric CO2 levels and the onset of the Little Ice Age (e.g., refs. 29–33 and 48–53). Our research provides additional evidence that tierra firme forest in some areas of the western and central Amazon was significantly less impacted by pre-Columbian populations than locations along rivers and their tributaries (Fig. 1) (29–32). Even our sampled locations a few kilometers from floodplain habitats at the MP-A revealed no evidence for significant cultural influences.
Central to recent arguments is the degree to which forms of forest management such as planting, protecting, and enriching preferred species were practiced by prehistoric populations across Amazonia, and the extent to which such activities contributed to current forest diversity and tree species hyperdominance (e.g., refs. 15–22 and 32). Our data lack evidence for significant alterations in forest structure or diversity due to human manipulation, as the same array of woody taxa were maintained throughout the sequences. The Arecaceae, with well-utilized examples today and in the past in Amazonia, are among the most prolific and diagnostic of phytolith producers, and particularly well-placed to address these issues. Our data indicate that phytoliths likely from Oenocarpus bataua and possibly Euterpe precatoria, along with those that could possibly derive from Bactris gasipaes and utilized or hyperdominant Astrocaryum spp., showed no trend for increase through time. Phytolith studies of other terrestrial soils in western and central Amazonia indicated the same pattens for these genera and species (29, 32, 54).
It appears that in some areas of the Amazon modern palm hyperdominance should not be attributed, at least not principally, to pre-Columbian human activities. Rather, explanations may better be sought in ecological and evolutionary factors that promote competitive influences; for example, edaphic conditions and negative density-dependent interactions associated with herbivory and chemical diversity/similarity in chemical compounds (55, 56). Also, studies of Eschweilera coriacea (DC.) S.A. Mori (Lecythidaceae), a widely distributed hyperdominant and little-utilized species that is among the 10 most abundant in Amazonia including at MP-A, indicated it possesses greater genetic heterogeneity than nonhyperdominant congenerics (57). This is predictive of a higher adaptive potential and population size for E. coriacea. In addition to their dietary and other favorable qualities, palms and other trees may have been well-exploited because they were among the most common that people encountered. Multifactorial historical and evolutionary studies of hyperdominants will better inform the reasons for their abundance in the modern flora.
Of course, subtle manipulation strategies involving few tree species and relatively little increase of individuals from a given species might go undetected. We also recognize the limitations of our data, in that the management of some major economic fruit and nut species would be “silent,” as they do not leave informative phytolith records (35, 41). Examples include the avocado (Persea americana L.), Brazil nut (Bertholletia excelsa Bonpl.), cacao (Theobroma cacao L.), soursop (Annona muricata L.) and other Annona spp., cashew nut (Anacardium occidentale L.), guaba (Inga edulis Mart.), guava (Psidium guajava L.), genipa (Genipa americana L.), and achiote (Bixa orellana L.). However, in searching for a past forest management imprint for them at MP-A, a clue for the past 100 y and possibly more may be found in the vegetation surveys, in which the avocado, cashew nut, guaba, soursop, guava, genipa, and achiote were not observed in the tierra firme forest (much of northern Peru is outside of the known geographic distribution of the Brazil nut) (Dataset S1) (39, 40). Today, the trees, along with Bactris gasipaes—also not observed in the surveys—are cultivated at the MP-A riverine settlements, with the possible exception of guava that is commonly grown today along riverbanks in other areas of western Amazonia (38). This pattern is possibly due to the richer riverine soils or simply factors of convenience. The life spans of most of these trees are probably at least 100 y, as few tropical tree species are found to live <100 y and many live 200 to 300 y (58, 59). All could be expected to be observed today in the forests we studied if they were present and enriched to any extent there during those past time frames. Anacardium, Genipa, and Annona are genera that can survive wild in closed forests like at MP-A, and E. precatoria, a long-lived palm species, is rare today in the tierra firme forest.
Today in Amazonia and the Neotropics at large, the management of major and other economic tree species typically occurs in fallows or home gardens (60, 61), which would have left tell-tale signs in our records. This point is buttressed by the lack of cultural artifacts and anthropic soils in the studied samples. These circumstances may present an analog for the deeper past at MP-A, whereby most significant tree manipulation that led to an increased abundance of those species and significantly altered forest structure took place near settlements that existed along the riverbank and other areas of high fertility, such as the shores of oxbows. The latter activities are perhaps foreshown by the ceramics found at the oxbow shore north of camp 1. Future research in the region should focus on prehistoric- and historic-period changes in those locations, including with palynological studies of the oxbow.
We do not question that in some regions of Amazonia floristic composition may at least in part be a legacy of prehistoric influences, particularly for areas near watercourses including tributaries, or in the vicinity of prehistoric occupations (Fig. 1). However, as shown for the Brazil nut growing in an interfluve along the Madeira river and Attalea speciosa Mart. ex Spreng (a highly utilized palm today) in an area of Maranhão state, Brazil, large stands thought to be a legacy of prehistoric nut manipulation and anthropogenic landscapes, respectively, are historic period phenomena (27, 28). Modern vegetation may also be reflecting cumulative prehistoric–historic period effects. These and other factors highlight how considerably more empirical data on forest history are needed before generalizations about the degree of domestication of the Amazonian landscape during the prehistoric era can be proposed.
Finally, the problems addressed in this research are of considerable importance to a diversity of scholarly disciplines in addition to archaeology and anthropology, bearing also on conservation and sustainability science, tropical ecology, and climate change. All are extremely important to modern indigenous societies who utilize the natural resources of their environments. Moreover, our data indicate the activities of present and past societies in the MP-A have not strongly altered the community composition and structure of the species-diverse forests over perhaps thousands of years of utilization. Rather, these societies were a positive force in maintaining forest integrity and biodiversity. It is also the case that good sustainable land use and conservation policies require adequate knowledge of past anthropogenic and natural impacts on the Amazonian ecosystem together with its responses, and should not assume the forests were once resilient in the face of significant past disturbance. It may take years of work before a scientific consensus is achieved on the issues considered here, but we expect our data will be an important contribution to it.
Methods
Soil cores were collected and tree inventories were carried out ∼4–5 km south of the floodplain of the Putumayo river at camp 1; ∼2 km north of the Algodón floodplain and ∼15 km south of the Putumayo floodplain at camp 2; and 1–2 km north of the Algodón floodplain and 1–2 km south of the Putumayo floodplain at camp 3 (SI Appendix, Fig. S1). Soil cores from camps 1, 2, and 3, were, respectively, a minimum of about 11, 7, and 13 km from the nearest indigenous communities and their agricultural plots. The closest soil core from camp 1 to the Cocha Bufeo oxbow lake shore with sherds on the surface was 4 km. Soil samples at each camp were taken along a 5 × 2,000-m transect in which all free-standing trees ≥10 cm dbh were inventoried (Dataset S1). Broader surveys of vegetation and flora were also carried out at each campsite (39, 40). Soils were collected with a 10-cm diameter AMS Soil Sampling hand auger; were 200–250 m apart from each other; reached a depth of 80 cm; and were sampled in 10-cm increments. Corresponding surface samples were taken with “pinches” of the uppermost ≤1 cm of soil directly underfoot at ∼10 locations along the transects and placed in the same bag for averaging.
At the laboratory, all soils were screened through mesh of various sizes and no artifacts such as ceramics, stone tools, or stone tool-making debitage were found. No anthropic soils (terra preta or terra mulata [the latter modified brown earths thought to be relictual prehistoric fields (7)]) were uncovered. Phytoliths and charcoal were analyzed by standard techniques (35) (SI Appendix, Supplementary Text 2) and phytoliths were identified with our modern reference collection of over 2,000 tropical species and other literature (42, 43, 47). A minimum of 300 phytoliths from the silt fractions were counted. To search for the presence of rare phytolith types extended scanning was carried out. During the scans, frequencies of the less common types (palm, ornate, psilate, and granulate spheroids) continued to be noted to ensure the most accurate percentages.
Supplementary Material
Acknowledgments
This work was supported by grants from the Wenner-Gren Foundation for Anthropological Research (9648), the NSF (1821816), and European Research Council Starting Grant (StG 853394), and by the Smithsonian Tropical Research Institute, Panama; Smithsonian National Museum of Natural History, Washington, DC; The Gordon and Betty Moore Foundation; and the Field Museum’s Grainger Bioinformatics Center. We thank Irene Holst and Graciela Quijano for excellent technical assistance.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. P.R. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2022213118/-/DCSupplemental.
Data Availability
All data are included in the article and SI Appendix.
References
- 1.Meggers B. J., Environmental limitations on the development of culture. Am. Anthropol. 56, 801–824 (1954). [Google Scholar]
- 2.Meggers B. J., Amazonia: Man and Culture in a Counterfeit Paradise (Smithsonian Institution Press, Washington, DC, 1971). [Google Scholar]
- 3.Erickson C. L., An artificial landscape-scale fishery in the Bolivian Amazon. Nature 408, 190–193 (2000). [DOI] [PubMed] [Google Scholar]
- 4.Heckenberger M. J., Who is Amazonia? The “salt of the matter” for indigenous sustainability. Environ. Res. Lett. 8, 041007 (2013). [Google Scholar]
- 5.Neves E. G., Petersen J., “Political economy and pre-Columbian landscape transformations in central Amazonia” in Time and Complexity in Historical Ecology: Studies in the Neotropical Lowlands, Balée W., Erickson C. L., Eds. (Columbia University Press, New York, 1991), pp. 279–310. [Google Scholar]
- 6.Pärssinen M., Schaan D., Ranzi A., Pre-Columbian geometric earthworks in the upper Purús: A complex society in Western Amazonia. Antiquity 83, 1084–1095 (2009). [Google Scholar]
- 7.Arroyo-Kalin M., The Amazonian formative: Crop domestication and anthropogenic soils. Diversity (Basel) 2, 473–504 (2010). [Google Scholar]
- 8.Schaan D., et al., New radiometric dates for pre-Columbian (2000–700 BP) earthworks in western Amazonia, Brazil. J. Field Archaeol. 37, 132–142 (2012). [Google Scholar]
- 9.Woods W., et al., Eds., Amazonian Dark Earths: Wim Sombroek’s Vision (Springer, Berlin, 2012). [Google Scholar]
- 10.Whitney B. S., Dickau R., Mayle F. E. J., Soto J. D., Iriarte J., Pre-Columbian landscape impact and agriculture in the Monumental Mound region of the Llanos de Moxos, lowland Bolivia. Quat. Res. 80, 207–217 (2013). [Google Scholar]
- 11.Iriarte J., et al., The origins of Amazonian landscapes: Plant cultivation, domestication and the spread of food production in tropical South America. Quat. Sci. Rev. 248, 106582 (2020). [Google Scholar]
- 12.Denevan W. M., The pristine myth revisited. Geogr. Rev. 101, 576–591 (2011). [Google Scholar]
- 13.de Souza J. G., et al., Pre-Columbian earth-builders settled along the entire southern rim of the Amazon. Nat. Commun. 9, 1125 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heckenberger M. J., et al., Amazonia 1492: Pristine forest or cultural parkland? Science 301, 1710–1714 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Erickson C. L., “Amazonia: “The historical ecology of a domesticated landscape” in The Handbook of South American Archaeology, Silverman H., Isbell W. H., Eds. (Springer, New York, 2008), pp. 157–183. [Google Scholar]
- 16.Balée W., Contingent diversity on anthropic landscapes. Diversity (Basel) 2, 163–181 (2010). [Google Scholar]
- 17.Balée W., Cultural Forests of the Amazon: A Historical Ecology of People and Their Landscapes (University of Alabama Press, Tuscaloosa, AL, 2013). [Google Scholar]
- 18.Clement C. R., et al., The domestication of Amazonia before European conquest. Proc. Biol. Sci. 282, 20150813 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levis C., et al., Historical human footprint on modern tree species composition in the Purus-Madeira interfluve, central Amazonia. PLoS One 7, e48559 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Levis C., et al., Persistent effects of pre-Columbian plant domestication on Amazonian forest composition. Science 355, 925–931 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Ferreira M. J., Levis C., Iriarte J., Clement C. R., Legacies of intensive management in forests around pre-columbian and modern settlements in the Madeira-Tapajós interfluve, Amazon. Acta Bot. Bras. 33, 212–220 (2019). [Google Scholar]
- 22.Watling J., et al., Impact of pre-Columbian “geoglyph” builders on Amazonian forests. Proc. Natl. Acad. Sci. U.S.A. 114, 1868–1873 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.ter Steege H., et al., Hyperdominance in the Amazonian tree flora. Science 342, 1243092 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Dull R. A., et al., The Columbian encounter and the Little Ice Age: Abrupt land use change, fire, and greenhouse forcing. Ann. Assoc. Am. Geogr. 100, 755–771 (2010). [Google Scholar]
- 25.Koch A., Brierley C., Maslin M. M., Lewis S. L., Earth system impacts of the European arrival and great dying in the Americas after 1492. Quat. Sci. Rev. 207, 13–36 (2019). [Google Scholar]
- 26.McMichael C. H., Feeley K. J., Dick C. W., Piperno D. R., Bush M. B., Comment on “Persistent effects of pre-Columbian plant domestication on Amazonian forest composition.” Science 358, 1–2 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Forline L. C., Putting history back into historical ecology: Some perspectives on the recent human ecology of the Brazilian Amazon. J. Ecol. Anthropol. 12, 69–74 (2008). [Google Scholar]
- 28.Scoles R., Gribel R., Population structure of Brazil nut (Bertholletia excelsa, Lecythidaceae) stands in two areas with different occupation histories in the Brazilian Amazon. Hum. Ecol. 39, 455–464 (2011). [Google Scholar]
- 29.McMichael C. H., et al., Sparse pre-Columbian human habitation in western Amazonia. Science 336, 1429–1431 (2012). [DOI] [PubMed] [Google Scholar]
- 30.Piperno D. R., Becker P., Vegetational history of a site in the central Amazon Basin derived from phytolith and charcoal records from natural soils. Quat. Res. 45, 202–220 (1996). [Google Scholar]
- 31.Piperno D. R., McMichael C. H., Bush M. B., Amazonia and the Anthropocene: What was the spatial extent and intensity of human landscape modification in the Amazon Basin at the end of prehistory? Holocene 25, 1588–1597 (2015). [Google Scholar]
- 32.Piperno D. R., McMichael C. H., Bush M. B., Finding forest management in prehistoric Amazonia. Anthropocene 26, 100211 (2019). [Google Scholar]
- 33.Bush M. B., et al., Anthropogenic influence on Amazonian forests in prehistory: An ecological perspective. J. Biogeogr. 42, 2277–2288 (2015). [Google Scholar]
- 34.McMichael C. H., et al., Spatial and temporal scales of pre-Columbian disturbance associated with western Amazonian lakes. Holocene 22, 131–141 (2012). [Google Scholar]
- 35.Piperno D. R., Phytoliths: A Comprehensive Guide for Archaeologists and Paleoecologists (Altamira, Lanham, MD, 2006). [Google Scholar]
- 36.Pitman N., Ed. et al., Perú: Medio Putumayo-Algodón, (Rapid Inventories Biological and Social Report 28, The Field Museum, Chicago, IL, 2016). [Google Scholar]
- 37.Alvira Reyes D., et al., “Communities visited: Sociocultural assets and quality of life” in Perú: Medio Putumayo-Algodón, Pitman N., Ed. et al. (Rapid Biological and Social Inventories Report 28, The Field Museum, Chicago, IL, 2016), pp. 329–345. [Google Scholar]
- 38.Alvira Reyes D., et al., “Traditional ecological knowledge and natural resource use and management” in Perú: Medio Putumayo-Algodón, Pitman N., Ed. et al. (Rapid Biological and Social Inventories Report 28, The Field Museum, Chicago, IL, 2016), pp. 346–359, 498–508. [Google Scholar]
- 39.Ríos Paredes M. A., et al., “Flora” in Perú: Medio Putumayo-Algodón, Pitman N., Ed. et al. (Rapid Biological and Social Inventories Report 28, The Field Museum, Chicago, IL, 2016), pp. 284–291, 372–431. [Google Scholar]
- 40.Torres-Montenegro L. A.et al., “Vegetation” in Perú: Medio Putumayo-Algodón, Pitman N., Ed. et al. (Rapid Biological and Social Inventories Report 28, The Field Museum, Chicago, IL, 2016), pp. 276–284, 372–431. [Google Scholar]
- 41.Piperno D. R., McMichael C. H., Phytoliths in modern plants from Amazonia and the Neotropics at large: Implications for vegetation history reconstruction. Quat. Int. 565, 54–74 (2020). [Google Scholar]
- 42.Watling J., et al., Phytoliths from native plants and surface soils from the Upper Madeira river, SW Amazonia, and their potential for paleoecological reconstruction. Quat. Int. 550, 85–110 (2020). [Google Scholar]
- 43.Pearsall D. M., et al., Phytoliths in the Flora of Ecuador: The University of Missouri Online Phytolith Database. https://phytolith.missouri.edu/. Accessed 6 January 2021.
- 44.Morcote-Ríos G., Raz L., Giraldo-Cañas D., Calvo C. F., Terras pretas de Índio of the Caquetá-Japurá river (Colombian Amazonia). J. Soc. Anthro. Lowland S. Am. 11, 30–39 (2013). [Google Scholar]
- 45.Morcote-Ríos G., Bernal R., Raz L., Phytoliths as a tool for archaeobotanical, palaeobotanical and palaeoecological studies in Amazonian palms. Bot. J. Linn. Soc. 182, 348–360 (2016). [Google Scholar]
- 46.Piperno D. R., Phytolith Analysis: An Archaeological and Geological Perspective (Academic Press, Orlando, FL, 1988). [DOI] [PubMed] [Google Scholar]
- 47.Dickau R., et al., Differentiation of neotropical ecosystems by modern soil phytolith assemblages and its implications for palaeoenvironmental and archaeological reconstructions. Rev. Palaeobot. Palynol. 193, 15–37 (2013). [Google Scholar]
- 48.Bush M. B., et al., Holocene fire and occupation in Amazonia: Records from two lake districts. Philos. Trans. R. Soc. Lond. B Biol. Sci. 362, 209–218 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kelly T. J., et al., Continuous human presence without extensive reductions in forest cover over the past 2500 years in an aseasonal Amazonian rainforest. J. Quat. Sci. 33, 369–379 (2018). [Google Scholar]
- 50.Maezumi S. Y., et al., The legacy of 4,500 years of polyculture agroforestry in the eastern Amazon. Nat. Plants 4, 540–547 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Power M. J., et al., Climatic control of the biomass-burning decline in the Americas after AD 1500. Holocene 23, 3–13 (2013). [Google Scholar]
- 52.Stocker B. D., Yu Z., Massa C., Joos F., Holocene peatland and ice-core data constraints on the timing and magnitude of CO2 emissions from past land use. Proc. Natl. Acad. Sci. U.S.A. 114, 1492–1497 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Urrego D. H., et al., Holocene fires, forest stability and human occupation in south-western Amazonia. J. Biogeogr. 40, 521–533 (2013). [Google Scholar]
- 54.Heijink B. M., et al., Holocene increases in palm abundances in north‐western Amazonia. J. Biogeogr. 47, 698–711 (2020). [Google Scholar]
- 55.Forrister D. L., Endara M.-J., Younkin G. C., Coley P. D., Kursar T. A., Herbivores as drivers of negative density dependence in tropical forest saplings. Science 363, 1213–1216 (2019). [DOI] [PubMed] [Google Scholar]
- 56.Wetzel W. C., Whitehead S. R., The many dimensions of phytochemical diversity: Linking theory to practice. Ecol. Lett. 23, 16–32 (2020). [DOI] [PubMed] [Google Scholar]
- 57.Heurertz M., et al., The hyperdominant tropical tree Eschweilera coriacea (Lecythidaceae) shows higher genetic heterogeneity than sympatric Eschweilera species in French Guiana. Plant Ecol. Evol. 153, 67–81 (2020). [Google Scholar]
- 58.Brienen R. J. W., Zuidema P. A., Lifetime growth patterns and ages of Bolivian rain forest trees obtained by tree ring analysis. J. Ecol. 94, 481–493 (2006). [Google Scholar]
- 59.Brienen R. J. W., Schöngart J., Zuidema P. A., “Tree rings in the tropics: Insights into the ecology and climate sensitivity of tropical trees” in Tropical Tree Physiology, Goldstein G., Santiago L. S., Eds. (Springer, Cham, Switzerland, 2016), vol. 6, pp. 439–461. [Google Scholar]
- 60.Miller R. P., Nair P. K. R., Indigenous agroforestry systems in Amazonia: From prehistory to today. Agrofor. Syst. 66, 151–164 (2006). [Google Scholar]
- 61.Peters C. M., “Precolumbian silviculture and indigenous management of Neotropical forests” in Imperfect Balance: Landscape Transformations in the Precolumbian Americas, Lentz D. L., Ed. (Columbia University Press, New York, 2000), pp. 203–224. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are included in the article and SI Appendix.